Abstract
Patient engagement often starts after research funding is secured with little or no involvement of patients in the proposal development phase. This paper compares three levels of patient engagement and describes patients’ early engagement in research proposal development process and its contemporary relevance to clinical and translational research. Authentic patient engagement is illustrated using an example of an ongoing pragmatic clinical trial. The paper also addresses key patient considerations and questions that have an impact on the proposal development. The final section presents strategies to overcome challenges to the patients’ early engagement in research proposal development approach from the perspectives of both patients and researchers. Although the examples are from comparative effectiveness research, strategies discussed can be applied to all clinical and translational research.
Keywords: clinical and translational research, comparative effectiveness research, patient involvement, patient participation, proposal writing, research design, research development
“Before becoming a patient researcher, I thought about clinical trials even less than how little I thought about gravity. I knew they existed and probably were a good and useful thing but didn’t really have much to do with my life.” Many patients feel alienated from the scientific inquiry process, amplifying distrust and lack of interest in research [1–3]. Researchers are more readily recognizing the importance of patient involvement in clinical and translational research. Patient engagement can be continuous, from prioritization and framing of research questions to protocol development, and from selection of clinically relevant outcomes to dissemination and implementation of findings [4]. Positive implications of patient engagement in research include practicality (e.g., more relevant research questions), recruitment (e.g., increased enrollment and attrition), data interpretation (e.g., more meaningful analyses) and dissemination (e.g., more understandable outcomes) [5–7]. Furthermore, patient engagement will be of great significance as healthcare delivery models are transitioning more toward ‘value-based medicine’ - a concept that promotes new ways to deliver medical care and to evaluate care delivery, based on actual results experienced by the patient [8,9]. It would seem logical, should this concept of ‘value-based medicine’ be more and more prominent in care evaluation to engage patients more broadly in the current research since it will determine and shape future medical care delivery.
Reviews of the spectrum of patient engagement in research showed engagement can and should start early, and especially during the protocol development phase [5,10,11]. Domecq and colleagues performed a systematic review of patient engagement in research, showing that levels or methods of engagement varied [5]. The review documented that the vast majority of studies only engaged patients through focus groups, interviews or surveys, not as study team members. Moreover, there is no substantial evidence of involving patients in the study design or process. Out of 142 studies included in the review, only four included patients in study board or investigator meetings. Patients were typically invited to participate only during the project preparation phase [5]. Since then, the engagement of patients in research has been expanding in depth and breadth thanks to the Patient-Centered Outcomes Research Institute (PCORI) efforts to improve the quality and relevance of evidence available to make better-informed health decisions [12]. Nonetheless, several challenges and barriers still impede meaningful engagement of patients in the research process [13].
Although previous papers have examined and reported the value of patient engagement, there remains limited discussion of patient involvement in the early stages of the research cycle prior to the protocol development stage [5,8,14]. Lavallee and colleagues demonstrated that patient engagement often starts after research funding was secured with little or no involvement in the development of proposal for funding [14]. In this paper, we compare different levels of engagement and describe the effect of patients’ early engagement in research proposal development (PEER-PD) on the development of a research proposal and its contemporary relevance to clinical and translational research (Figure 1). We illustrate practical, authentic ways to involve patients using an example of a pragmatic clinical trial and discuss strengths and challenges of this approach. Although we use examples from comparative effectiveness research, strategies discussed in this paper can also be applied to the basic, clinical and translational sciences. We adopt PCORI’s definition of the ‘patient’ to include ‘patients (those with lived experience), family members, caregivers, and organizations that represent the population of interest in a particular study’ [15].
Figure 1.
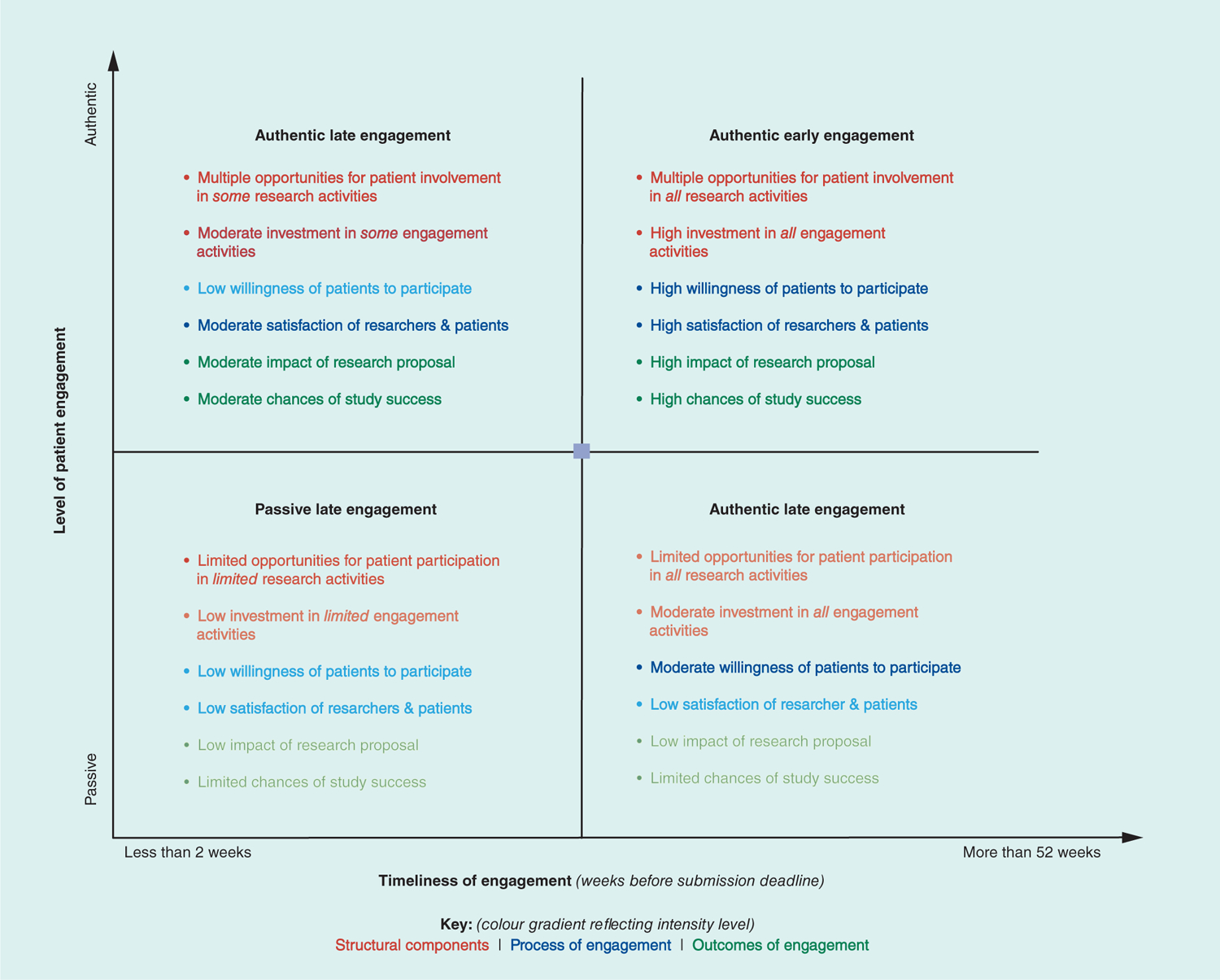
Key outcomes of patient early engagement in research proposal development in relation to the level and timeliness of engagement.
Authentic patient engagement: be genuine
There are several levels of patient engagement in clinical and translational research. Passive engagement - the most common - involves patients as study participants (i.e., human subjects) and is often researcher-driven and unidirectional [5]. A more advanced approach involves patients in the research process as consultants or advisors. Investigators seek input from patients - through focus groups, interviews or surveys - on research material and protocols already developed by researchers. Patients often perceive this as tokenistic engagement [5]. The most advanced level of engagement is active participation that achieves authentic engagement where the patient is a true partner in research [5]. With authentic engagement, the patient is a co-investigator and co-developer of the research, is a joint decision-maker and is encouraged to undertake initiatives that lead to patient-generated research [16]. Authentic engagement requires building long-term partnerships with patients and their communities, including capacity building and enabling and facilitating shared leadership. This requires significant effort, time and resources. We expand on the PCORI engagement rubric [15] and 10-STEP engagement continuum framework [4] by offering concrete examples that can serve to guide and/or ‘pulse-check’ when developing a research proposal (Table 1).
Table 1.
Examples of three levels of patient engagement along the 10-step research continuum†.
| Steps in research continuum† | Passive patient engagement - patient is a study participant | Tokenistic patient engagement - patient is an advisor | Authentic patient engagement - patient is a co-developer | |
|---|---|---|---|---|
| Planning the study (preparing to do research) | (1) Topic selection (2) Prioritization |
Researchers distribute surveys, database/EMR studies, patient registries, reviewing needs assessments | Focus groups, key informant interviews, patient/community forums, advisory boards - to identify understudied topics relevant to patients/communities | Building capacity/skills and trust within patient population(s) of interest (e.g., engaging with the patient/community early on and discussing ongoing research to inform/educate both the researcher and the patient/community members). Relevant and needed topics (and their urgency) become clear through ongoing dialogue |
| (3) Framing the question | Research question is shared with patients/advocacy organizations, after proposal has been completed | Research question is presented to patient groups for feedback on whether its language is culturally, linguistically and layperson appropriate | Researchers and patient partners co-develop the question, seek feedback from their respective communities, regroup and frame the research question in a way that is relevant, feasible and useful to the population of interest | |
| Conducting the study (doing research) | (4) Selection of comparators and outcomes | Comparators and outcomes are only explained to patients during study recruitment | Research symposia/workgroups are planned to get feedback from patients not necessarily directly involved in the research project | Patient partners perform needs-assessments within their communities and identify and prioritize alternative treatment options and outcomes of interest, bring findings back to research team for a joint selection process |
| (5) Creation of conceptual framework (6) Analysis plan |
Conceptual framework and analysis plan may be influenced by information gleaned from patient advocacy organizations and care provider input | Patients provide feedback on whether or not the framework reflects their personal experiences | Partners work together to ensure the framework is relevant to patients, and that data analysis plan gathers information that will ensure the project is feasible and balances the burden on participants with data needs | |
| (7) Recruitment and data collection | Researchers use social media (e.g., Facebook, Twitter) as outreach to recruit study participants and allow electronic data capture to make it convenient | Patient representatives who have participated in trials are asked to review the study protocol and provide feedback on the patient burden (e.g., not realistic/too high) | Patients identify potential challenges in data collection based on their experiences. Researchers and patients co-develop best practices for data collection and the data collection tool(s). Patients pilot test survey items and survey modality (e.g., phone vs online) | |
| Disseminating the study results (delivering solutions) | (8) Reviewing and interpreting results | Researchers share the final results with patients and/or patient organizations after all analyses and interpretation are complete | Researchers present preliminary findings to patient focus groups and elicit feedback on whether or not the information is relevant to and understandable by the patient community; patients are asked if results are surprising | Researchers discuss findings with patient partners and provide both context and potential ‘real-world’ effect of research results. Patients provide feedback on the conclusions being drawn by researchers, a ‘reality-check’ on the impact of implementing findings |
| (9) Translation | Researchers share findings with health systems and care providers. Patients and caregivers benefit from new findings that increase treatment options | Researchers ask patient advisory board members to ‘translate’ the results into lay terms and/or in the language(s) spoken by the population of interest | Experienced patient partners lead the translation of research findings into key communication points, understanding potential barriers within the patient population of interest, develop dissemination plan in consultation with researchers/care providers | |
| (10) Dissemination/ implementation | Research findings are made publicly available by sharing publications with or presenting results to advocacy organizations. Patients may be asked to complete surveys after research findings have been implemented | Patient representatives disseminate findings to communities directly, in presentation format and by working with researchers on blog posts, publications and flyers. Patients give ongoing feedback on the implementation phase of the project in a series of focus groups | Patients and academic researchers co-present/co-author findings across different settings (e.g., journal manuscripts, conferences, community events, etc.). Patients are co-PIs (or Pis) on dissemination proposals and are close collaborators in securing funding to effectively share results with patient populations/communities. Researchers and patients co-lead implementation plans, particularly where health system changes will be put into place. Researcher/patient partners work together to evaluate the project and identify best practices for improvement, generalizability and identify emerging topics for additional research projects | |
Continuous patient engagement in comparative effectiveness research.
Reproduced with permission from [4]
co-PI: Co-principal investigator; EMR: Electronic medical records; PI: Principal investigator.
Identifying patient co-investigators
The criteria for selecting patients representation is dependent on the specific nature and needs of the proposed investigation. For example, if the proposed study is a clinical trial comparing two forms of breast cancer therapies, then selected patient representation would include breast cancer patients and cancer survivors, as well as family members. Irrespective of the ‘type’ of patients engaged; however, authentic engagement requires developing relationships with patients and/or patient advocacy organizations early, and not simply to serve the needs of a single research endeavor. Building meaningful partnerships requires investing significant time to build trust, co-develop effective communication and conflict resolution plans, practice bidirectional learning and establish shared goals [5,11,15].
Effective identification and establishment of early connections with patients includes contacting and meeting with patient/community advocacy groups (e.g., National Health Council), faith centers (e.g., churches, mosques, synagogues) and community service providers (e.g., libraries, transportation, schools, recreation centers, safety-net providers). Clinical and Translational Science Award [17] centers’ community engagement staff can also identify resources and potential partners. Importantly, talking with other researchers with experience in community-based participatory research, community-partnered participatory research and PCOR will provide helpful guidance on effective partnering with patients and communities [18–20].
Attempting to develop relationships on an accelerated timeline (i.e., days or weeks before a proposal deadline) is not only less effective for engaging patients but is potentially damaging to relationships. Thoughtful planning and respecting the time of patient partners is critical to the success of collaboration. Authentic engagement prioritizes relationships with patient partners. Researchers can demonstrate this by working to identify meeting times that work best for patient partners, budgeting to cover travel to/from meetings, parking, food and providing access for those needing special accommodation (e.g., virtual meetings, developing materials for variable literacy and numeracy skills).
Planning the study (preparing to do research)
Researchers often consider engaging patients in research when requests for proposals are issued and not earlier. Preparing to submit a proposal for funding - including topic selection, prioritization and framing the research question - will be most meaningful and effective if undertaken up to a year in advance of application submission. The depth of engagement depends on the project itself, but the key is to realize that authentic, patient-engaged research requires an investment of time and resources.
A critical step in planning is viewing the relationship with patient partners as a meeting of equals with different areas of expertise. Active listening, flexibility and genuine interest in dismantling the implicit and perceived power imbalance between academicians and nonacademics will build and deepen relationships. This can be achieved through a series of training and capacity-building workshops addressed to each of the two audiences separately and collectively [21,22]. Ensuring that patients have a clear, realistic understanding of the commitment, benefits and challenges of participating on a research team is critical to project success.
Conducting the study (doing research)
Authentic engagement requires that team leaders are clear and transparent regarding their shared vision. Budgeting for patient partners’ active participation and providing appropriate resources and support for patient partners facilitates more meaningful contributions [23]. Beginning the engagement process early positions teams to move forward with shared goals and offers opportunities for partners to conduct research in their communities and influence the project as colleagues. Not only will they be better prepared to do the research, but their research partners will have grown accustomed to listening and seriously considering the patient perspective. Authentically engaged teams, having been involved in the development of the proposal, have a more intimate grounding in the aims and investment in how the study is conducted and how data are analyzed and shared, strengthening the study impact.
Disseminating study results (delivering solutions)
Engagement of patients and patient advocacy organizations to support and inform dissemination and implementation of results helps translate scientific evidence into community practice. As subject-matter experts, patients and advocates know how best to communicate and share information with their communities. Disseminating study results and lessons learned from engaged patients can inform and encourage other researchers and patients to work together in similar ways. Finally, projects that are conceptualized with patient partners are focused on outcomes that matter to patients [11].
Patients’ early engagement in research proposal development
A detailed description of the PEER-PD approach and its application to clinical and translational research is illustrated with a current pragmatic clinical trial with expansion of the original proposed study to demonstrate additional hypothetical differences among three levels of engagement (Table 2). The Radiation Comparative Effectiveness Study (RadComp) is a nationwide clinical trial funded by PCORI to compare the effectiveness of the two US FDA-approved radiation therapies for the treatment of breast cancer [24,25].
Table 2.
Selected examples† of three levels of patients early engagement in research proposal development in the write-up of research proposal.
| The anatomy of a research proposal | Guiding question(s) | Passive patient engagement - patient is a study participant | Tokenistic patient engagement - patient is an advisor | Authentic patient engagement - patient is a co-developer |
|---|---|---|---|---|
| Study title and tag line | In less than 15 words, what is this study about? What is the catchphrase or slogan that clarify the thought behind this study? | Pragmatic randomized trial of proton vs photon therapy for patients with nonmetastatic breast cancer: a radiotherapy comparative effectiveness consortium trial | Pragmatic clinical trial of proton vs photon therapy for patients with breast cancer | Comparing two types of radiation treatment for patients with breast cancer The RadComp study: a study at the heart of breast cancer treatment |
| Rationale for proposed research/context | What is the research area? What is known? What is the gap in knowledge? What is the critical need? | Nearly 3 million women are living with breast cancer in the USA. Radiotherapy plays a major role in the treatment of breast cancers. Radiotherapy carries increased risks of cardiovascular morbidity and mortality; survivors who receive radiotherapy have at least a twofold increased risk of cardiovascular death. Thus, success of cancer therapy has led to survivorship burden. Patients live longer but suffer from toxic consequences of treatment | Proton therapy, by reducing the volume of heart and lungs exposed to radiation in the treatment of breast cancer, has the potential to lessen the morbidity of radiation therapy compared with photon therapy, now the predominant radiation treatment in the USA. However, proton therapy is more expensive and has yet to be shown to improve health outcomes for patients with breast cancer | Patients we have engaged expressed the three rationales for this PCORI proposal: (1) the hope that proton therapy could lessen the burden of radiation-related toxicity; (2) the frustration that evidence describing the benefits and harms of proton therapy for breast cancer is lacking; and (3) the expectation that our study would examine heart problems, cancer control and quality of life |
| ‘Big Picture’ | What is the solution? What is the long-term goal and potential impact? | Radiotherapy is an important component of curative treatment for locally advanced breast involving lymph nodes within the axilla (the cavity in the underarm) | A broad range of stakeholders (patients, payers, providers, manufacturers, researchers and policy makers) have called for randomized trial level evidence on the clinical benefits and harms of proton therapy, a promising but expensive cancer treatment, for patients with breast cancer | With this study, we hope to better understand the best available technologies for breast cancer to help patients live a longer, healthier life |
| Specific Aims | What is your objective? | Assess the effectiveness of proton vs photon therapy in reducing major cardiovascular events, defined as myocardial infarction, coronary revascularization, hospitalization for heart failure, arrhythmia, or unstable angina or atherosclerotic heart disease or other cardiac death | Assess the effectiveness of proton vs photon therapy in improving patient-reported body image and function and other measures of HRQOL | “I don’t only want to know about dying from heart problems from the radiation. I want to know about the heart problems that I would have to live with and the impact on my quality of life after cancer treatment” - Breast Cancer Survivor, Mayo Clinic |
| What is your hypothesis (expected outcome)? | We hypothesize that proton therapy, as part of multimodality curative treatment for locally advanced breast cancer, reduces major cardiovascular events, is noninferior in cancer control and improves HRQOL compared with photon therapy, the current standard treatment | We hypothesize that proton therapy, as part of multiple-regimen treatment for breast cancer, reduces cardiovascular events, can control cancer, and improves quality of life compared with photon therapy, the current standard treatment | It is possible that the new breast cancer therapy (proton therapy) can control cancer while reducing treatment side effects (particularly heart-related) and improving general well-being | |
| What will you do to test the hypothesis? | Build critical infrastructure to develop predictive models of the association between radiotherapy dose distributions and major cardiovascular events or quality of life after radiation treatment, which will help patients and physicians identify who benefits most from proton or photon therapy | Identify the impact of proton vs photon therapy on physical, mental and social HRQOL in breast cancer, which will be a major contribution to address a critical patient-centric evidence gap | Engage patients and other essential stakeholders in the design and conduct of large scale pragmatic clinical trials of a promising, but expensive, medical technology, a first-of-its-kind effort which will, irrespective of outcome, inform future efforts to conduct holistic, patient-centric, pragmatic comparative effectiveness research as part of a learning healthcare system. The RadComp study will be conducted at medical centers around the country to compare two types of standard care radiation therapy: PHoton Therapy (pronounced ‘fo-tahn’) and PRoton Therapy (pronounced ‘pro-tahn’) - to find out which type of radiation is best for treating breast cancer | |
| Significance | What is the scientific premise? What is the importance of proposed research? | Interpreting the sparse evidence available is problematic because of the absence of trials of sufficient size and statistical power to assess key clinical outcomes, failure to adjust for known confounders, and substantial selection effects | Patients with breast cancer considering photon or proton therapy make treatment decisions in the context of extremely sparse comparative effectiveness evidence, and then may live for years with clinically burdensome treatment-related morbidity that affects their quality of life and engagement in activities of living. Our study results will be directly relevant to many thousands of patients who confront these difficult treatment decisions every day | “Of course I would want to know if proton therapy will improve the skin burn or my tiredness after radiation. But, I would be more motivated to participate in a big study if I knew we would learn whether proton therapy could avoid causing problems with my heart. That would help me weigh whether the long-term benefits of radiation outweigh the long-term side effects” - Breast Cancer Survivor, Rochester, MN |
| Innovation | Does your research employ novel methods? Is your hypothesis innovative? Does your research bring together novel expertise or resources? | While we hypothesize that the radiation dose characteristics of proton therapy translate into measurable clinical benefits for breast cancer, this has yet to be shown in a randomized study. The RadComp trials aim to address this evidence gap through real-world, patient-centered PRCTs for patients with locally advanced breast | Our goal is to generate new knowledge about how a promising, but expensive, cancer treatment (proton therapy) compares with its alternatives while ensuring that our approach reflects ‘real world’ clinical practice, identifies subgroups of patients that might benefit more from proton therapy, and helps patients and physicians apply our findings to their own lived experience | To increase enrollment and retention of eligible patients, we will use multifaceted innovative engagement and recruitment approaches and employ social networking techniques in collaboration with leading national breast cancer and patient advocacy organizations |
| Approach | What is the study design/strategy? | A total of 1716 breast cancer patients will be recruited at 22 centers within 48 months (3-month ramp up period, minimum 6 months of clinical follow-up, additional 3-month period for analysis) | The RadComp Coordinating Center, The PATIENTS Program, Executive Committee and Stakeholder Advisory Committee will work together to develop recruitment strategies | Reduced patient research visits and opened at more community sites. Patient partners will co-develop informed consent documents in a patient-friendly language. Partnerships with national advocacy partners and advocates will promote recruitment of participants through co-development of recruitment materials and outreach |
| What are the study procedures/methods? | We anticipate patient contact via clinical visit, RadComp online portal, or by mail or telephone throughout the study period | Clinical research coordinators will facilitate patient-reported outcomes assessment as needed and clinician reported adverse events reporting in accordance with protocol procedures | We will conduct an extensive communications campaign with RadComp participants and consortium members which will include monthly news-briefs and quarterly newsletters and weekly posts to a selected online interface for quick digital information sharing and two way communication | |
| How are you going to analyze the data? | The primary HRQOL end points will be the BCTOS cosmesis and arm function scores for patients with breast cancer. […] After estimating the effect of the intervention on each end point, additional covariates will be evaluated to select those most related to selected end point. Likelihood ratio tests willbe used to assess the contribution of each variable across hierarchical models | The analyses and key research findings will be reported to the Stakeholder Advisory Committee to solicit their feedback and comments and allow our patient and stakeholder advisors to ask questions, provide feedback and fully comprehend the study findings | Focus groups will be conducted by The PATIENTS Program as part of the patient engagement process to address recruitment and retention and to seek guidance on how best to provide feedback to patients throughout the study. This information will inform an iterative process of refining talking points, providing feedback to RadComp centers and devising effective strategies to overcome identified barriers | |
| What is your dissemination strategy? | The research team anticipates at least three manuscript submissions and abstracts will be submitted to at least five national conferences and annual meetings, where we can present our study findings and recommendations | In the spirit of community-engaged research, we propose both scientific and lay publications, some co-developed with members of the project’s Stakeholder Advisory Committee, via peer-reviewed journals and online interfaces, such as the RadComp and advocacy partner websites | Planned activities include focus groups to provide real-time bidirectional learning on recruitment and retention, assistance with co-developing lay summaries and patient informational ‘give backs’ throughout the study, and identification of dissemination portals and outlets | |
| How are you going to address potential problems? What is the alternative strategy? | We recognize the potential for some patients to refuse their assigned treatment but expect cross-over to be minimal | Adherence data will be monitored carefully by the DSMB | Intensive patient engagement efforts aimed at robust informed consent will minimize treatment assignment refusal. Careful construction of study materials will present both therapies as viable, the US FDA-approved therapies; not representing one over another. The RadComp coordinating team is available by phone and email to address concerns |
The examples in this table were adapted from the RadComp Study and are for illustrative purposes only; they may not necessarily reflect the exact language used in the original funded proposal.
BCTOS: Breast cancer treatment outcome scale; DSMB: Data and safety monitoring board; HRQOL: Health-related quality of life; PATIENTS: PATient-centered involvement in evaluating the effectiveness of treatments; PCORI: Patient-centered outcomes research institute; PRCTs: Pragmatic randomized clinical trials; RadComp: Radiation comparative effectiveness study.
The RadComp study team has conducted multiple patient pre-engagement and engagement activities that have fundamentally informed the study questions, study design and dissemination plans (Appendix 1). Pre-engagement activities allowed patient and stakeholder partners to ask questions, provide feedback and fully comprehend the nature of the research and partnership relationships. Engagement activities have included several conference calls with Stakeholder Advisory Committee members (see Appendix 2) to co-develop the study design and questions, define their roles and assist with early engagement of a broader set of patients who also influenced the RADCOMP trial design. To further understand the patient perspective and the outcomes most meaningful to patients, the study team interviewed more than a dozen patient advisors individually and in small groups, leveraging the work of The PATIENTS Program at the University of Maryland. This was followed by an in-person stakeholder engagement conference on the campus of the National Cancer Institute, with representation from patients, payers, vendors, clinicians and researchers, government representatives and professional societies, in which the study team gained important insights on the formulation of the research questions, study designs, study implementation plans and other key characteristics of comparative effectiveness research.
TIP: Offer opportunities for patient to make the first and last suggestions during planning meetings; always start and end with the patient voice.
Study title & abstract
The title should be brief yet comprehensive, to help people understand the nature and importance of the work. Patients can help choose language that ensures the title is understandable by the public and not solely by those in the field. Similarly, the abstract should succinctly describe major aspects of the proposed work, providing an overview of key information in the proposal. Often, funders use the abstract to make preliminary decisions about the proposal. The abstract is also used to provide a brief description of the project in annual reports, presentations and public dissemination. Therefore, it is vital that the abstract be in plain language, and that all information is understandable to all stakeholders. Patients are uniquely advantaged to judge whether the abstract meets these criteria.
TIP: Work with patients to develop an attractive tagline and a #hashtag for the study.
Specific aims
The specific aims section often includes a rationale for proposed work, long-term research goals, study objective, hypotheses and specific aims. Identifying evidence-based aims and hypotheses is important as is identifying patient-informed aims. Studying questions of interest to patients makes the research more meaningful, boosts patients’ interest, and augments policy relevance. Patient-derived aims may or may not be of clinical or theoretical priority to clinical investigators, yet it is important to acknowledge their significance to patients, and that patients have a central, unique role in identifying aims that are of interest and relevant to their experience. For example, a clinical investigator’s priority might be to compare two forms of radiotherapy (proton vs photon) in reducing major cardiovascular events (e.g., myocardial infarction). Although this comparative effectiveness question is of obvious clinical relevance, patients may be more concerned about the pain associated with each of the two comparators at the time of therapy or the impact on quality of life and day-to-day activities. Therefore, both clinician/researcher-derived and patient-derived questions could and should reach the final round of aims. Patients can also orient researchers to real-life scenarios, presenting a litmus test for postulated hypotheses. Moreover, patients can testify, based on their experiences, if the aims are feasible and attainable.
TIP: Make points as direct as possible. Avoid jargon and overcomplicated language. Minimize the use of unfamiliar acronyms.
The study significance section enables the reader to position the proposed research problem in a context of common knowledge and to understand how its findings will advance the field. It is key to make sure it sounds compelling to the reviewer as well as to the key beneficiary of its outcome - the patient. Patients can frame the research problem from their perspective and may also contribute to the significance of the research by identifying how the proposed work affects them; patient researchers can provide practical answers to questions about the importance of the study, or how the findings from the proposed research will impact their quality of life.
The innovation section explains how the planned work seeks to shift current research or clinical practice paradigms. It is imperative to demonstrate how the proposed design and expected outcomes are novel and meaningfully advance the field. In patient-centered research, the focus is on investigating research questions that are meaningful to both patients and researchers. Patients may not be familiar enough with the most recent research evidence or clinical practice in the field of study to judge its innovation, but patients can help describe how the current proposed work differs from previous research in which they were involved or their clinical experiences. More importantly, patients can have vital role in brainstorming and suggesting innovative ideas in relation to patient participation in the study (e.g., recruitment, retention and how to elicit patient-reported information).
Research strategy
The study approach section details the implementation and evaluation of the project and includes a description of methods and analyses, potential difficulties and limitations, alternative approaches and a timeline or work plan. Patient-centeredness methodology is demonstrated by evidence of authentic engagement in determining study design and methodology across its different dimensions. Patient researchers, as co-developers of the study approach, can focus the research strategy around patients’ values, beliefs and experiences. They can also identify preferences for one approach or method compared with others. Patients can orient investigators to anything related to their direct involvement in the research process. Patients can also advise on the most appropriate or efficient data collection method (e.g., in-person vs online surveys), frequency of data collection and provide guidance on incentives for participation and minimizing loss to follow-up. Patients can also help the investigators identify potential problems and develop alternative solutions.
TIP: Patients’ journey can add value to the research, and has the potential to generate ideas for new research questions.
The budget and justification section describes financial aspects of the project, presenting and justifying all expenses required to achieve study aims and objectives. Patient partners provide expertise like any other expert on the study team and their contributions should be fairly recognized and compensated. Patients partners may not necessarily be involved in all budget items (e.g., investigator percentage (%) efforts), but they should be particularly involved in items related to patients participation. At face value, this may appear to be trivial and of less importance to other aspects of the proposal (e.g., study outcomes or recruitment efforts). But, we argue that patients can and should have valuable input on certain budget items (e.g., participants’ compensation or incentives) and key personnel (e.g., what stakeholder profile should be included in the study). For example, if patients advise that online follow-up for a specific study design and needs may be more favorable over face-to-face visit but the investigators fail to provide the appropriate incentive (say Amazon vs Target gift cards), then follow-up might be less appealing despite being online as per the patients’ engagement advice. Therefore, we believe this should be a comprehensive engagement effort.
The key personnel section provides an overview of the human resources of the project and includes a list of key research personnel and their expertise. Patient engagement in the ‘doing’ aspect is as crucial as involving patients in the proposal-writing phase. Having an interdisciplinary, collaborative team that includes a variety of researcher and patient expertise and experiences demonstrates the commitment to a cooperative research environment. It is important that patients and key stakeholders - co-selected with patients - are active members of the research team, not just advisors [26]. Patients can help investigators identify key stakeholders and personnel to be involved in the research study (e.g., community leaders and recruiters).
The patient perspective
Patients have key considerations that inform whether they will join a research study. It is important to hear and address these considerations early on, as the impact of the patient perspective on the questions can inform proposal development. Some of the considerations important to patients include:
Are you developing this concept in such a way that advocacy organizations could be involved in outreach to their communities for patient engagement?
Are you developing reasonable participation requirements?
Are you including transparent explanations of the side effects and potential risks of the intervention?
Have you developed the proposal in such a way that content (e.g., consent forms, recruitment flyers) is understandable and meaningful to patients?
Are you including plans for whether the proposed intervention (if effective) will continue to be available to patients?
Are you incorporating plans for long-term, post-trial follow-up? If not, have you explained why?
Are you considering whether the proposed study will be available to patients who do not have an immediate support system?
Are you including a quality of life component?
Have you explained how the study population will represent the affected population?
Have you developed a communication plan to update patients on the study status and findings?
Having said that, we acknowledge that there is heterogeneity in patients’ preferences and choices, hence the call for involving more than one patient and from varied backgrounds and experiences. Further, during the engagement process, patients are usually asked to reflect on the possibility of having other preferences and choices. For instance, patients receiving cancer treatment may have discussions with other patients with similar or different experiences about their choices and can present a wider spectrum in the discussion.
Advantages & challenges of PEER-PD
Early engagement of patients in research comes with both advantages and challenges that should be carefully considered. The inclusion of patients as partners in the proposal development ensures that it is more likely to address issues important to patients and that the proposed intervention is more likely to be seen by patients as acceptable. Patients have a unique understanding of a proposal’s relevance to the population and a clear vision of what is acceptable to ask of patients. Furthermore, the patient viewpoint may illuminate new questions and avenues of research. Finally, engaging patients brings the researcher closer to the impact their work has on people.
That said, investigators need to be cognizant of the challenges and potential strategies to overcome them (Table 3). The challenges to engage patients in research proposal development are not particularly different from challenges facing patient engagement endeavors in later stages of research let alone other types of community engagement, many of which has been previously discussed in community-based participatory research, community-partnered participatory research and PCOR literature [5,7,12,13,20,23,27]. However, in this article we highlight the main challenges that we think are relevant to the proposal development stage, with distinction between challenges from different perspectives - researchers’ perspective and that of patients. The initial challenge is setting up a program that includes patients as partners in the research to elicit patients’ input on ideas and proposed research questions and ultimately advance this partnership on projects prior to their inception. This means extending oneself outside of the familiar modes of scientific collaboration to build inclusive, meaningful relationships with members of the community. A second challenge is providing training to the engaged patients. Some patients will have a strong understanding of proposal development, but others will have to learn the basics, including a new vocabulary. Another important challenge is maintaining a delicate balance between using ‘easy-to-understand’ language and preserving the ‘scientific sounding’ language, which might be perceived to be superior by some reviewers. Investigators should be true to their authentic engagement activities and simplifying the language used to explain what they are doing and how they are doing it. At the same time, they should be cognizant of maintaining the scientific rigor of both approach and language used in writing up the proposal. A more logistical but essential challenge is facilitating the partners’ participation by underwriting costs such as transportation and, when necessary, housing and meals. Finally, it is important to recognize and address the reluctance of researchers to engage the patient in the proposal process. Some may see only potential barriers and be discouraged by the necessary investment of time and resources to the inclusion process, without seeing the knowledge depth and richness resulting from authentic engagement.
Table 3.
Challenges to engaging patients in research proposal development and strategies to overcome these challenges.
| Challenges to patient engagement | Strategies to overcome the challenges |
|---|---|
| From a patient perspective | |
| Time-intensive: proposal writing is a long tedious process and may include several meetings and correspondences | Be upfront with patients about how long the process is and how many meetings are anticipated. In addition, you can schedule meetings after regular business hours to accommodate for those who work and meet more frequently |
| Lack of patient experience in proposal writing/Disparate viewpoints and vocabulary | Reassure patients that they are not expected to be experts in the clinical and technical aspects. Rather, they are experts in their own experiences and values. In addition, during discussion of complex clinical and technological aspects, investigators should use lay simplified terms to explain the concepts to patients and make sure that all in the room are on the same page |
| Expensive process: patient participation will add proportionally high costs to the patient (e.g., transportation, opportunity costs, etc.) | Compensate participants for their participation and efforts, in par with other ‘experts’ involved in the study |
| Cultural barriers: lack of trust in research institutions | Trust needs to be earned. Patients should be treated with the same respect as the other expert at the table with the understanding that patients expertise is different from and complementary to researchers’ expertise. Further, long-term partnership (that ensures benefits for both parties involved) is a key in preserving and growing the trust |
| Knowing about the opportunity: patients may not always be aware of the available research proposals opportunities or knowledgeable about the research subject | Advertise potential research opportunities using various media outlets and in a variety of locations that are readily accessible to patients |
| From a researcher’s perspective | |
| Time-intensive: it takes time and effort to identify patients and community partners | Appropriate presubmission planning: incorporate enough time in the planning process to identify and develop productive relationships with patient stakeholders. Developed partnerships can (and should) be sustained for future collaborations |
| Lack of researcher’s experience in patient engagement | Researchers may participate in training activities that are focused on patient engagement and principles of PCOR. Alternatively, they may subcontract with third party entities that have track record in patient engagement to help the researchers in patient engagement activities |
| Meaningful engagement adds costs to the project and maybe resource-intensive | Some funding agencies can provide funding to support patient and other stakeholders’ engagement activities. Examples of funding opportunities available that particularly fund the work of building partnerships include PCORI’s Engagement Awards and the Robert Wood Johnson Foundation’s Culture of Health awards. Institutional support can also be achieved through protected time for clinical investigators and their staff to do the work, an increase in funding for partnership building between clinical researchers and patient/community partners, and rewarding those investigators who do this most effectively when evaluating their work for recognition/promotion |
| Some populations (e.g., children, inmates, low SES) may be more challenging to engage | Certain activities (e.g., developing relationships with certain groups, providing collaborating communities with adequate feedback, etc.) ought to be reflected in project timelines and acknowledged by academic institutions. Allocate added costs incurred for certain activities (e.g., translation services or bilingual staff, supplementary data collection locations and/or times, culturally appropriate materials, staff training) as separate budget item to be funded |
| Selection process: identification of representative and appropriate patients | Purposive sampling and snow-bowling technique maybe helpful to identify potential patients and patient advocates to be engaged in the research proposal development |
| Institutional barriers (e.g., policies that hinder or delay patient engagement activities) and logistics (e.g., difficulty having meetings with patients) | Academic institutions should be aware of barriers to patient engagement activities, and try to address those challenges and streamline the process to ease the way for PCOR. Also, researchers and patients should meet half-way in terms of logistical barriers (e.g., alternate in convenient meeting locations and timings). Researchers should show flexibility in meeting locations that are convenient for all participants in the process, including patients |
Conclusion
It is well to remember the words of Robert Moses: “I have no fear of change as such and, on the other hand, no liking for it merely for its own sake”. As humans, we both fear and anticipate change. That dichotomy applies to the introduction of patient engagement to the research process. Although there are challenges to PEER-PD, none is insurmountable and the benefits of adding the patient voice to the development of concepts and protocols outweigh the challenges. In the patient’s own words: “Now, I recognize and appreciate that research studies and their results aren’t just abstract good and useful things; rather, in significant ways they define and extend my life, enriching my ability to be a contributing member of society and part of a rich community network. Sometimes, it’s a medicine I take; other times, it’s a lifestyle change I’ve made because a carefully constructed study has given me new, life-enhancing options”.
Executive summary.
Patient engagement in research should start early, especially during the protocol development phase.
A review of the spectrum of engagement showed that patient engagement often starts after funding is secured with little or no involvement in the development of the proposal.
This paper compares different levels of engagement and describe the effect of patients’ early engagement in research proposal development (PEER-PD) on the write-up of a proposal and its contemporary relevance to clinical and translational research.
Authentic patient engagement: be genuine
Patients have key considerations that inform whether they will join a research study. Therefore, it is important to hear and address these considerations early on, as the impact of the patient perspective on the questions can inform proposal development.
- Levels of patient engagement in clinical and translational research:
- Passive engagement involves patients as study participants and is often researcher-driven and unidirectional.
- Tokenistic engagement is a more advanced approach that involves patients in the research process as consultants or advisors where investigators seek input on research material and protocols already developed.
- Authentic engagement is the most advanced level of engagement where the patient is a true partner in research: the patient is a co-investigator and co-developer of the research, is a joint decision-maker, and is encouraged to undertake initiatives that lead to patient-generated research.
Patients’ early engagement in research proposal development
Authentic engagement is illustrated using an example of an ongoing pragmatic clinical trial, addressing key patient considerations and questions that have an impact on the proposal development.
- Selected PEER-PD techniques and impact are presented for different components of the proposal anatomy.
- Study Title and Abstract. Patients can help choose language that ensures the title is understandable by the lay public and are uniquely advantaged to make sure the abstract is clear and all information is understandable to all stakeholders.
- Specific Aims. Patients can identify questions of interest to them making the research more meaningful, boosts patients’ interest and augments policy relevance. Patients can also frame the research problem from their perspective and identify how the proposed work affects them.
- Research Strategy. Patient researchers can focus the research strategy around patients’ values, beliefs and experiences. Patients can orient investigators to anything related to their direct involvement in the research process and advise on the most appropriate or efficient data collection method, frequency of data collection and provide guidance on incentives for participation and minimizing loss to follow-up.
Advantages & challenges of PEER-PD
Although there are challenges to PEER-PD, none is insurmountable and the benefits of adding the patient voice to the development of concepts and protocols outweigh the challenges.
Strategies to overcome challenges to the PEER-PD approach are presented from the perspectives of both patients and researchers.
Conclusion
PEER-PD ensures that the proposal is more likely to address issues of importance to patients.
Patient viewpoint can open new avenues of questions and research and brings the researcher closer to the reality of the impact on people.
Although the examples are from comparative effectiveness research, strategies discussed can be applied to all clinical and translational research.
Financial & competing interests disclosure
This project was funded by the Agency for Healthcare Research and Quality (AHRQ) (grant number R24HS022135) and Patient-Centered Outcomes Research Institute (PCORI) (award number PCS-1403-12804). The views, statements and opinions presented in this work are solely the responsibility of the authors and do not necessarily represent the views of the AHRQ or PCORI, its Board of Governors or Methodology Committee. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Appendix 1: Pre-engagement activities of radcomp study.
Table A1.
Selected activities of patient early engagement in research proposal development for the RadComp study.
| Pre-engagement activity | Impact |
|---|---|
| Seven conference calls with individual patients and investigators |
|
| One focus group with patients and patient advocates |
|
| Multi-stakeholder conference at the National Cancer Institute |
|
| Conference calls with the Center for Medicare and Medicaid Services |
|
| Patient engagement infrastructure: weekly calls with patient advisors |
|
| The PATIENTS Program External Advisory Board Calls |
|
CMS: Center for Medicare and Medicaid Services; HRQOL: Health-related quality of life; NCI: National Cancer Institute.
Appendix 2: Stakeholder advisory committee members.
Carole Baas, PhD. Dr Baas is a survivor of breast cancer with a background in medicine and engineering. Her training as a biomedical engineer, as an intermediary between the engineering and medical communities, is very similar to her work in research advocacy, where she serves as a much-needed conduit of information and expertise between the lay public and scientists.
Joanne Buzaglo, PhD, Cancer Support Community. Dr Buzaglo is the Vice President, Research and Training at the Cancer Support Community. Dr Buzaglo is a behavioral health researcher and clinical psychologist with extensive experience in the development and evaluation of theoretically guided interventions designed to help patients cope with the complex challenges associated with cancer.
Cynthia Chauhan, MSW. Ms Chauhan is a breast cancer survivor. She has been an active research advocate for 14 years and an active support advocate for almost 12 years. Her experience as a support advocate and a research advocate at the local, regional and national levels is augmented by her professional experience as a social worker.
Andrea Denicoff, RN, MS, ANP, National Institutes of Health. Ms Denicoff is the head of the NCI National Clinical Trial Network. She has done significant research into overcoming barriers to successful enrollment of participants in clinical trials.
Michael Kolodziej, MD, Aetna. Dr Kolodziej is the National Medical Director, Oncology for Aetna and the former Medical Director for Oncology Services for the US Oncology. He has published several manuscripts and given several presentations on cost of cancer care, oncology reimbursement reform, and use of evidence based treatment to enhance value.
Donna-Lee Lista, BS. Ms Lista is a nonsmoking lung cancer survivor and advocate. She has worked with LCFA-Lung Cancer Foundation of America, LCA-Lung Cancer Alliance and she is part of the FREE TO BREATHE organization in Philadelphia, PA, formerly the-National Lung Cancer Partnership.
Holly Massett, PhD, National Institutes of Health. Dr Massett is the Senior Behavioral Science Analyst for the Clinical Trials Operations and Informatics Branch of the Cancer Therapy Evaluation Program in the Division of Cancer Treatment and Diagnosis at the National Cancer Institute. She oversees the evaluation of several early and late phase clinical trial network systems within the NCI Cancer Therapy Evaluation Program.
Cassandra McCullough, MBA, The Association of Black Cardiologists, Inc. Ms McCullough is the Interim CEO and Executive Director of the Association of Black Cardiologists, Inc. She and her colleagues at the ABC recognize the need to bring special attention to the adverse impact of cardiovascular disease on African American, including cardiac complications that arise when patients receive radiation therapy as treatment for breast and lung cancer.
C Daniel Mullins, PhD, (Chair) University of Maryland. Dr Mullins is Professor and Chair of the Pharmaceutical Health Services Research Department. He received one of the first PCORI contracts to assist the PCORI Methodology Committee develop its standards for patient and stakeholder engagement. He was awarded one of the first two AHRQ-funded R24 PCOR Infrastructure Development Program grants to develop the PATient-centered Involvement in Evaluating effectiveNess of TreatmentS (PATIENTS) Program.
Maureen Rigney, LICSW, Lung Cancer Alliance. In her role as Director of Community and Support Services at Lung Cancer Alliance, Ms Rigney speaks to those touched by lung cancer every day. This allows her a unique perspective and understanding of the needs of the lung cancer community. As a result, she is able to provide the insights she has gained over the past 9 years as it relates to the experience of individuals living with lung cancer and their loved ones.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Corbie-Smith G, Stephen BT, George DM St.. Distrust, race, and research. Arch. Intern. Med 162(21), 2458–2463 (2002). [DOI] [PubMed] [Google Scholar]
- 2.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 104(2), e16–e31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SB, Zak A, Iversen MD, Polletta VL, Shadick NA, Solomon DH. Participation in clinical research registries: a focus group study examining views from patients with arthritis and other chronic illnesses. Arthritis Care Res. 68(7), 974–980 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Mullins CD, Abdulhalim AM, Lavallee DC. Continuous patient engagement in comparative effectiveness research. JAMA 307(15), 1587–1588 (2012). [DOI] [PubMed] [Google Scholar]; •• Presents a 10-step process for Comparative Effectiveness Research (CER) projects and describes how patient engagement can guide CER toward patient-centered outcomes research and offers suggestions for the process and purpose of patient engagement across the 10 steps.
- 5.Domecq JP, Prutsky G, Elraiyah T et al. Patient engagement in research: a systematic review. BMC Health Serv. Res 14(1), 89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses best ways to identify patient representatives and to engage them in designing and conducting research with overview of observed benefits and barriers
- 6.Nilsen ES, Myrhaugh HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice and patient information material. Cochrane Database Syst. Rev 3, CD004563 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewlett S, Wit MD, Richards P et al. Patients and professionals as research partners: challenges, practicalities, and benefits. Arthritis Care Res. 55(4), 676–80 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Chernew ME, Rosen B, Fendrick AM. Value-based insurance design. Health Affairs 26(2), w195–w203 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Bae JM. Value-based medicine: concepts and application. Epidemiol. Health 37, e2015014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Concannon TW, Fuster M, Saunders T et al. A systematic review of stakeholder engagement in comparative effectiveness and patient-centered outcomes research. J. Gen. Intern. Med 29(12), 1692–1670 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brett J, Staniszewska S, Mockford C et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 17(5), 637–650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PCORI (Patient-Centered Outcomes Research Institute). The value of engagement, (2018). https://www.pcori.org/about-us/our-programs/engagement/value-engagement; • Reiterates the value of patient engagement and the future of science engagement
- 13.Ellis LE, Kass NE. Patient engagement in patient-centered outcomes research: challenges, facilitators, and actions to strengthen the field. J. Comp. Eff. Res 6(4), 363–373 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Lavallee DC, Gore JL, Lawrence SO et al. Initiative to Support Patient Involvement in Research (INSPIRE): findings from Phase I interviews. (2016). http://www.becertain.org/sites/default/files/INSPIRE%20Phase%20I%20Report%20Final%202016.09.30.pdf
- 15.PCORI (Patient-Centered Outcomes Research Institute). Engagement rubric for applicants. (2016). https://www.pcori.org/sites/default/files/Engagement-Rubric.pdf [Google Scholar]; • Provides guidance to PCORI awardees and those planning or conducting research, merit reviewers, engagement/program officers, and interested patients, caregivers, patient/caregiver organizations and other stakeholders, regarding engagement in the conduct of research
- 16.PCORI (Patient-Centered Outcomes Research Institute). Financial compensation of patients, caregivers, and patient/caregiver organizations engaged in PCORI-funded research as engaged research partners. (2015). https://www.pcori.org/sites/default/files/PCORI-Compensation-Framework-for-Engaged-Research-Partners.pdf
- 17.NIH (National Institutes of Health) - National Center for Advancing Translational Sciences. Clinical and Translational Science Award Program. (2018). https://ncats.nih.gov/ctsa
- 18.De Las Nueces D, Hacker K, DiGirolamo A, Hicks LS. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Serv. Res 47(3pt2), 1363–1386 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sofolahan-Oladeinde Y, Mullins CD, Baquet CR. Using community-based participatory research in patient-centered outcomes research to address health disparities in under-represented communities. J. Comp. Eff. Res 4(5), 515–523 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Jones L, Wells K. Strategies for academic and clinician engagement in community-participatory partnered research. JAMA 297(4), 407–410 (2007). [DOI] [PubMed] [Google Scholar]
- 21.PCORI (Patient-Centered Outcomes Research Institute). What we mean by engagement. (2018). https://www.pcori.org/engagement/what-we-mean-engagement
- 22.CIHR (Canadian Institutes of Health Research). Strategy for patient-oriented research (SPOR) SUPPORT unit training and capacity development opportunities. (2018). http://www.cihr-irsc.gc.ca/e/50896.html
- 23.Johnson DS, Bush MT, Brandzel S, Wernli KJ. The patient voice in research - evolution of a role. Res. Involv. Engagem 2, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekelman J Comparing two types of radiation treatments for patients with breast cancer - the RadComp study. Patient-Centered Outcomes Research Institute (PCOR), (2018). https://www.pcori.org/research-results/2015/comparing-two-types-radiation-treatment-patients-breast-cancer-radcomp-study [Google Scholar]
- 25.MacDonald SM. Proton therapy for breast cancer: getting to the heart of the matter. Int. J. Radiat. Oncol. Biol Phys 95(1), 46–48 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Ladeji O Multi-stakeholder engagement in health services research. J. Comp. Eff. Res 7(6), 517–521 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Bonevski B, Randell M, Paul C et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med. Res. Methodol 14(1), 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


