Abstract
Objective
To expand the genetic spectrum of hereditary spastic paraparesis by a treatable condition and to evaluate the therapeutic effects of biotin supplementation in an adult patient with biotinidase deficiency (BD).
Methods
We performed exome sequencing (ES) in a patient with the clinical diagnosis of complex hereditary spastic paraparesis. The patient was examined neurologically, including functional rating scales. We performed ophthalmologic examinations and metabolic testing.
Results
A 41-year-old patient presented with slowly progressive lower limb spasticity combined with optic atrophy. He was clinically diagnosed with complex hereditary spastic paraparesis. The initial panel diagnostics did not reveal the disease-causing variant; therefore, ES was performed. ES revealed biallelic pathogenic variants in the BTD gene leading to the genetic diagnosis of BD. BD is an autosomal recessive metabolic disorder causing a broad spectrum of neurologic symptoms, optic atrophy, and dermatologic abnormalities. When treatment is initiated in time, symptoms can be prevented or reversed by biotin supplementation. After diagnosis in our patient, biotin supplementation was started. One year after the onset of therapy, symptoms remained stable with slight improvement of sensory deficits.
Conclusions
These findings expand the genetic spectrum of the clinical diagnosis of complex hereditary spastic paraparesis by a treatable disease. Today, most children with BD should have been identified via newborn screening to start biotin supplementation before the onset of symptoms. However, adult patients and those born in countries without newborn screening programs for BD are at risk of being missed. Therapeutic success depends on early diagnosis and presymptomatic treatment.
Biotinidase deficiency (BD) is an autosomal recessive inborn error of metabolism characterized by developmental retardation, epilepsy, metabolic acidosis, optic atrophy, hearing impairment, skin rash, and alopecia if untreated. Treatment with oral biotin supplementation prevents clinical symptoms given presymptomatic diagnosis and early treatment.1 The worldwide incidence is reported to be approximately 1:25,000–1:60,000; however, in regions were consanguinity is frequent, the incidence is suspected to be much higher.2
Infants who are identified via newborn screening remain asymptomatic under biotin supplementation. However, the large number of patients who were not yet included in a newborn screening program might remain undiagnosed because of unspecific symptoms. These include myelopathy-causing limb weakness or spastic paresis. Visual impairment caused by optic atrophy was reported in all adult cases. Reports of late-onset patients are rare.1,3,4 Hereditary spastic paraplegia (HSP) is defined as progressive lower limb spasticity and paresis. The presence of additional features such as neuropathy, ataxia, optic atrophy, and mental retardation is summarized as complex HSP. The genetic heterogeneity of HSP has grown in the past decade, with more than 100 disease genes described to date.5
Here, we report an adult case with a clinical diagnosis of complex HSP and the genetic diagnosis of BD, thus expanding the genetic spectrum of HSP.
Methods
Probands and samples
The subject gave written informed consent for the collection, storage, and publication of clinical data, blood samples, photographs, and genetic testing. This study was conducted according to the Declaration of Helsinki (2013) and approved by the local ethics committee of the Technical University Munich (#5360/12S). The patient was neurologically assessed using the Spastic Paraplegia Rating Scale (SPRS) and the Scale for the Assessment and Rating of Ataxia (SARA). A complete ophthalmologic examination was performed. Multimodal imaging included SD-OCT (SD-OCT on SPECTRALIS® HRA + OCT, Heidelberg Engineering, Heidelberg, Germany) and ultrawide field photography performed on the Optos system (Dunfermline, UK). Metabolic investigations (acylcarnitines in dried blood spots, organic acids in urine, and biotinidase activity in serum) were performed at the Dietmar Hopp Metabolic Center, Center for Pediatric and Adolescent Medicine, University Hospital Heidelberg. Biotinidase activity in serum was determined as previously described.7
Exome sequencing
Exome sequencing (ES) and variant prioritization was performed as previously described5 using a SureSelect Human All Exon Kit 60Mb, V6 (Agilent, Santa Clara, CA), for exome enrichment. Libraries were sequenced on an Illumina NovaSeq6000 system (Illumina, San Diego). Confirmatory Sanger sequencing was performed according to the standard procedures. Primer sequences are available on request.
Data availability
The authors declare that all relevant data are published within the article or is available on request from the corresponding author.
Results
Clinical findings
The 41-year-old male patient from a nonconsanguineous German family presented with a syndrome of spastic paraparesis, optic atrophy, and ataxia. First symptoms reportedly manifested at the age of 5 years with hyperopia. Later that year, he developed subacute exercise intolerance and gait disturbances because of spasticity and muscle weakness. The sudden onset was followed by progressive visual impairment within 2 weeks. No preceding incident or illness was reported. Left-predominant optic atrophy was diagnosed at the age of 6 years. Symptoms of ataxia manifested later with occasional falls and fine motor difficulties. Since then, symptoms of ataxia and spastic paraparesis have remained relatively stable, whereas the visual impairment deteriorated.
Extensive diagnostics in the first year after onset revealed normal results in electroencephalogram, lumbar puncture, scintigraphy of the brain, cerebral computed tomography (CT) scan, electrophysiologic testing, lymph node biopsy, skin biopsy, and muscle biopsy. Neuropsychological testing revealed an IQ of 110. At the age of 15 years, the patient was suspected to suffer from early onset MS, but cerebral magnetic resonance imaging, cerebral CT, somatosensory evoked potential of the tibial nerve, and liquor examination did not reveal any abnormalities to support this.
During early adulthood, the gait disturbance was rather stable, whereas optic atrophy was deteriorating. Referral to further neurologic examination and genetic testing was suggested during a regular ophthalmologic check-up.
Initial gene panel sequencing for HSP had revealed an unknown heterozygous variant of uncertain significance c.688G>A (p.Asp230Asn) in the ATL1-gene that was discussed as potentially causal for autosomal dominant SPG3A. The variant was excluded from further consideration because the same variant was found in the healthy mother by Sanger sequencing.
At presentation, the patient's major complaint was chronic lower back pain as a consequence of spasticity.
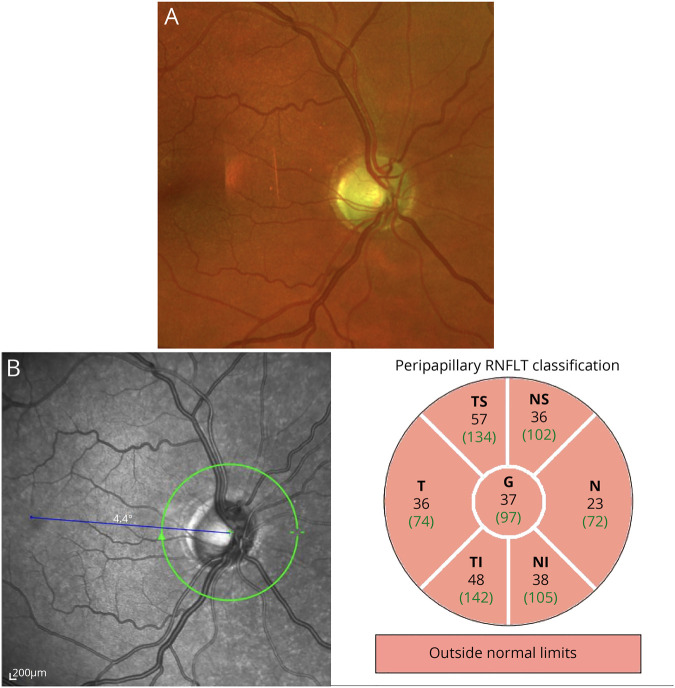
Physical examination revealed severe left-predominant visual impairment, weakness of hip flexion and hip abduction, spasticity of the lower limbs including hyperreflexia, and a positive Babinski sign. Pallesthesia at both ankles was significantly reduced, whereas no other sensory deficits were observed. He showed gait ataxia and mild ataxia of the extremities and dysdiadochokinesia, resulting in a SARA score of 2 of 40.6 No skin rash or alopecia could be observed. His SPRS score was 8 of 52.7 The ophthalmologic examination revealed an optic atrophy resulting in reduced visual acuity and bilateral central scotoma on both eyes. On fundoscopy, a sharp and pale optic disc with a physiologic excavation of 0.4 and a subtle peripapillary atrophy was seen. The findings of an optic atrophy could be confirmed by multimodal imaging (figure).
Figure. Ophthalmologic findings.
(A) Ultrawide field photography performed on the Optos system (Dunfermline, UK) showed a sharp and pale optic disc with a cup-disc-ratio of 0.4 and a subtle peripapillary atrophy. (B) The examination of the retinal nerve fiber layer on optical coherence tomography (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany) showed a pronounced loss of nerve fibers in all quadrants.
After the diagnosis but before the start of biotin supplementation, organic acids in urine were normal and acylcarnitine profile in dried blood spots showed elevation of C5OH-carnitine (1.14 µmol/L, N < 0.68). MRT imaging of cortex and spine again did not show any abnormalities (Supplemental figures 1–3, links.lww.com/NXG/A328). Subsequently, treatment with biotin supplementation was initiated and well tolerated. At follow-up visits 3, 6 months, and 1 year after the onset of therapy, a slight improvement of pallesthesia was observed. No other significant changes in neurologic examination, functional rating scales (SARA, SPRS), or patient-reported symptoms could be noted. The patient did not show any deterioration of symptoms and remains on treatment.
Molecular findings
Sequencing of 6 genes related to optic atrophy and 53 HSP genes did not detect (likely) pathogenic variants. Consequently, ES identified 2 heterozygous missense variants in BTD (NM_000060.4), which were classified as pathogenic according to the ACMG guidelines8: c.184G>A, p.(Val62Met) and c.832C>G, p. (Leu278Val). Segregation analysis by Sanger sequencing in the parents confirmed compound heterozygosity.
The diagnosis of profound BD was confirmed by a reduced enzyme activity of <2% in serum.
Conclusion
Our patient with the diagnosis of BD expands the genetic spectrum of HSP by a treatable differential diagnosis. This warrants an urgency to increase awareness and amend the genetic diagnostics for HSP to identify these cases.
Our patient could not yet benefit from a newborn screening program. At presentation at the age of 41 years, complex HSP was clinically diagnosed because of the symptom complex of early onset and slow progressive spastic paraparesis, ataxia pallhypesthesia without significant fluctuation and optic atrophy. No corresponding neuroradiologic abnormalities were found. Deficits in sensitivity were limited to the posterior column. In routine panel diagnostic for HSP, BD is not commonly tested and only ES facilitated a definite genetic diagnosis.
To date, the patient's symptoms only improved marginally under biotin supplementation. Generally, the supplementation with biotin is reported to improve symptoms, especially alopecia and skin rash. However, degeneration of the sensory cells, such as retinal and inner ear cells, can only be reversed if therapy is started few months after the onset of visual impairment.1
Newborn screening for BD was implemented nationwide in Germany only in 2002, implying that any patient born before or in a country without newborn screening for BD is at risk of harboring an undiagnosed but potentially treatable BD. In Europe, as of 2015, screening for BD had been implemented in 11 other countries.9
Owing to the establishment of the newborn screening program, it is expected that undiagnosed adult patients with BD will become rarer. However, BD should be considered in adult patients with HSP and—given the high migration rate from countries without newborn screening programs—in those groups even in children. As early diagnosis is crucial for recovery and prevention of symptoms, we recommend screening patients with unclear spastic paresis and optic atrophy for this treatable condition by measuring biotinidase enzyme activity.
Acknowledgment
The authors thank the patient and her family members who participated in this study.
Glossary
- BD
Biotinidase deficiency
- ES
exome sequencing
- HSP
hereditary spastic paraplegia
- SARA
Scale for the Assessment and Rating of Ataxia
- SPRS
Spastic Paraplegia Rating Scale
Appendix. Authors
Study funding
This work was supported by a German Federal Ministry of Education and Research (BMBF, Bonn, Germany) grant to the German Network for Mitochondrial Disorders (mitoNET 01GM1906A).
Disclosure
None to report. Go to Neurology.org/NG for full disclosure.
References
- 1.Deschamps R, Savatovsky J, Vignal C, et al. . Adult-onset biotinidase deficiency: two individuals with severe, but reversible optic neuropathy. J Neurol Neurosurg Psychiatry 2018;89:1009–1010. [DOI] [PubMed] [Google Scholar]
- 2.Gramer G, Nennstiel-Ratzel U, Hoffmann GF. 50 Jahre Neugeborenenscreening in Deutschland. Monatsschrift Kinderheilkunde 2018;166:987–993. [Google Scholar]
- 3.Baykal T, Gokcay G, Gokdemir Y, et al. . Asymptomatic adults and older siblings with biotinidase deficiency ascertained by family studies of index cases. J Inherit Metab Dis 2005;28:903–912. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz S, Serin M, Canda E, et al. . A treatable cause of myelopathy and vision loss mimicking neuromyelitis optica spectrum disorder: late-onset biotinidase deficiency. Metab Brain Dis 2017;32:675–678. [DOI] [PubMed] [Google Scholar]
- 5.Wagner M, Osborn DPS, Gehweiler I, et al. . Bi-allelic variants in RNF170 are associated with hereditary spastic paraplegia. Nat Commun 2019;10:4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz-Hubsch T, du Montcel ST, Baliko L, et al. . Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006;66:1717–1720. [DOI] [PubMed] [Google Scholar]
- 7.Schule R, Holland-Letz T, Klimpe S, et al. . The Spastic Paraplegia Rating Scale (SPRS): a reliable and valid measure of disease severity. Neurology 2006;67:430–434. [DOI] [PubMed] [Google Scholar]
- 8.Richards S, Aziz N, Bale S, et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therrell BL, Padilla CD, Loeber JG, et al. . Current status of newborn screening worldwide: 2015. Semin Perinatol 2015;39:171–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all relevant data are published within the article or is available on request from the corresponding author.