Developmental and epileptic encephalopathy (DEE) is a spectrum of neurodevelopmental conditions in which psychomotor delay or regression arises in association with frequent epileptic activity. In the past decade, molecular genetics studies showed that DEE is caused by environmental insults and by genetic factors; several de novo pathogenic variants were also identified.1
Recently, it was reported that de novo cyclin-dependent kinase 19 (CDK19 [MIM: 614720]) gene missense variants cause DEE in the early infantile period, such as infantile spasms (IS).2 The clinical characteristics of patients with CDK19 mutations consist of global developmental delay, intellectual disability, hypotonia, early onset epilepsy (including IS), and facial dysmorphism. Functional analyses have revealed that pathogenic CDK19 mutations cause the loss of the important function of CDK19 during neurodevelopment or synapse formation/function and underlie a syndromic neurodevelopmental disorder. Conversely, detailed biochemical and metabolic profiles have not been reported, and the mechanism underlying refractory epilepsy remains unknown.
Here, we report a case of DEE with a pathogenic CDK19 variant exhibiting distinct CSF abnormalities.
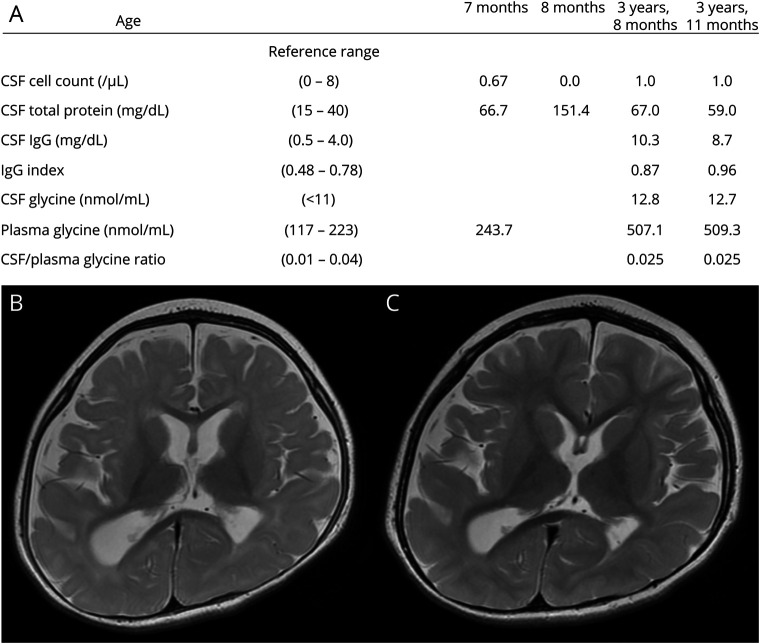
The index case is a 7-year-old girl born to nonconsanguineous healthy parents after an uneventful delivery. She exhibited global developmental delay since early infancy and onset of epileptic spasms and tonic seizures at 6 months of age. The diagnosis of IS was confirmed by hypsarrhythmia on interictal EEG. Biochemical and metabolic studies identified an elevated total protein content in the CSF (hyperproteinorrachia) (figure, A). At 12 months of age, her height, weight, and head circumference were 80.3 cm (99th percentile), 10.0 kg (88th percentile), and 44.6 cm (33rd percentile), respectively. She had distinct facial dysmorphism characterized by hypertelorism, a prominent nose with a bulbous tip, and a large mouth with widely spaced teeth. Neurologic assessment revealed severe developmental delay accompanied by global hypotonia. She was unable to control her head or track objects. Brain MRI revealed brain atrophy and white matter abnormalities (figure, B). Her epilepsy was refractory to conventional antiepileptic drugs and ketogenic diet. Brain atrophy and white matter abnormalities persisted in MRI at 3 years 7 months (figure, C). The CSF examinations performed at 3 years 8 months and at 3 years 11 months reproduced hyperproteinorrachia. Moreover, moderate elevation of glycine concentration in the CSF and plasma were identified (figure, A). The CSF/plasma glycine ratio was normal, and genetic testing for GLDC and AMT, the responsible genes for nonketotic hyperglycinemia (NKH), was negative. Oral dextromethorphan administration was initiated at 4 years of age but was unsuccessful. Finally, a de novo missense variant of CDK19 [NM_015076:c.94T>C, p.(Tyr32His)] that was pathogenic in a previous report2 was identified using trio-based whole-exome sequencing, as previously described.3 At 7 years of age, she continued to present seizures and remained profoundly disabled.
Figure. CSF profiles and brain MRI in the patient with a CDK19 variant.
Biochemical and metabolic profiles in chronological order (A). The elevated total protein content in the CSF (hyperproteinorrachia) persisted, whereas a moderate elevation of glycine in the CSF and plasma was identified in the examinations performed subsequently. MRI axial T2-weighted sequences were acquired at 1 year 0 month (B) and 3 years 7 months (C). Nonprogressive brain atrophy was concomitant with white matter abnormalities that were suggestive of delayed myelination.
Our case exhibited the clinical presentation of DEE with CDK19 mutation and was characterized by global developmental delay, severe intellectual disability, profound hypotonia, early onset refractory epilepsy, and facial dysmorphism.2 Tall stature was also identified as another physical feature encountered in our case and 1 of the 3 patients in the previous report. Of note, these 2 cases with accelerated growth harbored the same missense variant, c.94T>C [p.Tyr32His], suggesting a genotype-phenotype correlation.2
Distinct biochemical and metabolic abnormalities, i.e., hyperproteinorrachia and elevated glycine in the CSF and plasma, were also detected. The mechanism through which the CDK19 variant leads to the CSF abnormalities remains unknown. Although CDK19 and its paralog CDK8 are involved in transcription,4 it is not reported to date that the genes involved in glycine cleavage are regulated by CDK19/CDK8. On the other hand, it is important to elucidate the implication of these CSF abnormalities in the intractability of epilepsy in patients with a CDK19 variant. In particular, glycine serves as an excitation modulator of N-methyl-d-aspartate (NMDA)-type glutamatergic receptors, and excessive activation of NMDA receptors by abnormally accumulated glycine in NKH results in neuronal damage and DEE.5 A recent study reported that early intervention was critical for the successful management of NKH by specific treatments, such as the oral administration of dextromethorphan, an NMDA receptor antagonist.6 In other DEE cases with a CDK19 variant, a complete metabolic assessment, including the analysis of amino acids in the CSF, and when elevated CSF glycine is reproduced in early infancy, the investigation of the possibility of adopting a specific treatment option are advised.
In summary, this second report of DEE with recurrent CDK19 mutation expands its clinical spectrum and suggests that further investigations including biochemical and metabolic assessments are necessary to elucidate the pathophysiology of this specific DEE.
Acknowledgment
The authors are indebted to the patient and her parents. The authors would also like to thank Enago (enago.jp) for the English language review.
Appendix. Authors
Study funding
This work was supported by AMED under the grant numbers JP19lk0201069 (to M. Kato), JP20ek0109486, JP20dm0107090, JP20ek0109301, JP20ek0109348, and JP20kk0205012 (to N. Matsumoto); JSPS KAKENHI under the grant numbers JP 20K08236 (to M. Kato), JP20K08164 (to T. Mizuguchi), and JP17H01539 (to N. Matsumoto); MHLW Research program on rare and intractable diseases, Grant number JPMH20FC1039 (to M. Kato); Intramural Research Grants for Neurologic and Psychiatric Disorders of NCNP under the grant numbers 30-6 (to M. Kato and N. Matsumoto) and 30-7 (N. Matsumoto); and the Takeda Science Foundation (to T. Mizuguchi and N. Matsumoto).
Disclosure
The authors report no disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.He N, Lin ZJ, Wang J, et al. Evaluating the pathogenic potential of genes with de novo variants in epileptic encephalopathies. Genet Med 2019;21:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung H, Mao X, Wang H, et al. De novo variants in CDK19 are associated with a syndrome involving intellectual disability and epileptic encephalopathy. Am J Hum Genet 2020;106:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakamoto M, Kouhei D, Haniffa M, et al. A novel ITPA variant causes epileptic encephalopathy with multiple-organ dysfunction. J Hum Genet 2020;65:751–757. [DOI] [PubMed] [Google Scholar]
- 4.Galbraith MD, Donner AJ, Espinosa JM. CDK8: a positive regulator of transcription. Transcription 2010;1:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krawiec C, Goyal A. Nonketotic Hyperglycinemia. Treasure Island: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 6.Bjoraker KJ, Swanson MA, Coughlin CR, et al. Neurodevelopmental outcome and treatment efficacy of benzoate and dextromethorphan in siblings with attenuated nonketotic hyperglycinemia. J Pediatr 2016;170:234–239. [DOI] [PubMed] [Google Scholar]