Abstract
Objective:
As an alternative to estrogen therapy, the efficacy of an estrogen receptor (ER) β-selective phytoestrogenic (phyto-β-SERM) formulation to regulate climacteric symptoms and decline in brain responses associated with ovarian hormone loss in menopause was assessed.
Methods:
A phyto-β-SERM formulation-containing diet was compared to a commercial soy extract diet and a phytoestrogen-free base/control diet in an ovariectomized (OVX) mouse model of human menopause. Two treatment studies were conducted: (1) a 2-month study assessed effects of experimental diets on tail skin temperature as a model of menopausal hot flashes, and (2) a 9-month study assessed long-term impact of the diets on overall health, hair thinning/loss, spatial working memory and associated protein expression in the hippocampus.
Results:
The phyto-β-SERM diet prevented OVX-induced menopause-like changes including the rise in skin temperature, hair thinning/loss, and deficit in spatial memory function, and reversed OVX-induced decline in expression of hippocampal proteins involved in neural plasticity and β-amyloid degradation/clearance. The soy extract diet had no effect or exacerbated OVX-induced changes.
Conclusions:
Overall, the phyto-β-SERM diet induced physical and neurological responses comparable to ovary-intact mice, suggesting the therapeutic potential of the phyto-β SERM formulation for prevention/alleviation of climacteric symptoms and decline in brain responses induced by ovarian hormone loss which provides the basis for further work in menopausal women.
Keywords: Estrogen Receptor β, Phytoestrogen, Menopause, Hot Flashes, Hair Loss, Cognition
INTRODUCTION
In light of the substantial risks of estrogen therapy (ET),1-2 research has focused on alternative approaches that would achieve the effectiveness of ET for menopausal symptoms without significant adverse effects.3-4
One avenue of development has centered on a group of plant-derived structural analogs of mammalian estrogens, known as phytoestrogens.5 These non-steroidal estrogenic compounds can bind at weak to moderate affinities to estrogen receptors (ERs) and exert estrogenic or antiestrogenic activities.6-7 The recent rapidly expanded interest in phytoestrogens can be attributed, in large part, to the publicized health-promoting benefits of soy foods that are rich in phytoestrogens and regularly consumed in Asian countries.8 For instance, cross-national epidemiological studies revealed a 2.5-fold lower prevalence rate of Alzheimer’s disease (AD) in Japan and China than in North America and Europe,8 and which could have been largely attributed to the high dietary intake of phytoestrogens from soy foods, 20 to 80 mg/d in Asian populations as compared to less than 1 mg phytoestrogens/d in Western populations.9-10 In particular, recent statistics shows that only 25% of Japanese and 18% of Chinese menopausal women experience hot flashes as compared to 85% of North American and 70% of European women.11 In addition, breast and prostate cancer rates in Asia have been much lower than Western countries.12-14
These observational studies, however, lack confirmation from well-controlled randomized trials. Results from seven out of eight human studies published in 2000-2007 that sought to determine the impact of soy extracts on cognitive function in postmenopausal women are highly variable.8 The efficacy of soy extracts to treat menopausal hot flashes has been another widely studied area which has yielded similar inconsistent findings.15 The discrepancy between the results of observational studies which focused on soy-derived foods and interventional studies which investigated efficacy of soy-derived extracts, could be due to constitutive differences between the natural forms of soy foods and pharmacological preparations of soy extracts.8
In recent years, soy extract products have proliferated, most of which are advertised as dietary supplements targeting menopausal symptoms. These supplements are not regulated by the FDA and their composition and efficacy are highly variable. An area of particular concern has been the lack of standardization regarding the preparation and processing of soy extracts. An analysis of multiple commercial soy extract supplements revealed an abundance of constituents of unknown origin.16 Some of the newly formed substances derived from the processing of extraction could counteract positive properties.3,8
We sought to develop a phytoestrogenic formulation that can serve as an effective and safe alternative to estrogen therapy. As part of the preclinical analyses, we conducted a comparative study of both neural and reproductive responses induced by a group of phytoestrogenic compounds when used singly or in combination.17 Results of these analyses indicated that both the ERα/β binding profile and neural activities associated with individual compounds were modifiable when used in combination. In particular, we found that even the most potent individual compound induced only modest neural activity which could be modified, either positively or negatively, when combined with other phytoestrogens.17 These earlier analyses led to the discovery of a formulation composed of genistein, daidzein and equol, which was 83-fold more selective for ERβ than ERα and neuroprotective, while devoid of feminizing activity as seen with natural ovarian estrogens.17 Thus we refer to this formulation as a phytoestrogenic ERβ-selective modulator (SERM) formulation or phyto-β-SERM formulation.17
The present study was undertaken to investigate the efficacy of chronic daily dietary intake of the phyto-β-SERM formulation compared to a soy extract supplement diet and a phytoestrogen-free base/control diet on preventing physical and neurological changes associated with ovarian hormone loss in mouse models of human menopause.
METHODS
Custom diets
Three rodent diets were custom manufactured by Harlan Laboratories (Madison, WI). The composition of each diet is listed in Table 1. The Base/Control Diet was prepared from Teklad Global 16% Protein Rodent Diet (Harlan Laboratories), which was ground and repelleted. This diet has a fixed formula and is nutritionally balanced containing 16% protein and 3.6% fat that support the growth and maintenance of rodents, and does not contain alfalfa or soybean meal, thus minimizing the levels of natural phytoestrogens. The Phyto-β-SERM Diet was prepared by adding equal parts of genistein, daidzein and equol (LC Laboratories, Woburn, MA) to the base diet. A total of 100 mg (genistein, daidzein and equol) was added per 1000 g diet. This diet would deliver to a mouse a daily intake of 0.25 mg added phyto-β-SERM formulation (genistein, daidzein and equol), or 10 mg/kg (BW) mouse per day, assuming a 25 g mouse eating 2.5 g diet per day. The Soy Extract Diet was prepared by adding a commercial soy extract product, Healthy Women® Soy Extract Supplement (Amazon, Seattle, WA), to the base diet. Total addition sums to 100 mg (genistein/genistin, daidzein/daidzin and glycitein/glycitin) per 1000 g diet.16 Similarly, this diet would deliver to a mouse a daily intake of 0.25 mg added total phytoestrogens (genistein/genistin, daidzein/daidzin and glycitein/glycitin), or 10 mg/kg (BW) mouse per day, assuming a 25 g mouse eating 2.5 g diet per day.
Table 1.
Diet composition.
| Custom Diets | Formula (g/Kg Diet) | Selected Nutrient Informationb | Additional Notes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diet Base (2016, Teklad Global 16% Protein Rodent Diet)a |
Phytoestrogen Supplement |
Protein | Carbohydratec | Fat | |||||
| %by Weight |
% Kcal fromd |
%by Weight |
% Kcal fromd |
%by Weight |
% Kcal fromd |
||||
| Control Diet (TD.00217) |
1000 | 0 | 16.2 | 21.0 | 52.8 | 68.5 | 3.6 | 10.5 | The 2016 Teklad Global 16% Protein Rodent Diet was ground and repelleted. |
| Phyto-β-SERM Diet (TD.07260)e |
999.9001 | Genistein: 0.0333 Daidzein: 0.0333 Equol: 0.0333 |
16.2 | 21.0 | 52.8 | 68.5 | 3.6 | 10.5 | Equal parts of genistein, daidzein and equol were added to the 2016 Teklad Global 16% Protein Rodent Diet. Total addition sums to 100 mg added phytoestrogens per 1000 g diet. |
| Soy Extract Diet (TD.07261)e,f |
998.5244 | Healthy Women® Soy Extract Supplement: 1.4756 |
16.2 | 21.1 | 52.7 | 68.6 | 3.5 | 10.3 | Healthy Women® Soy Extract Supplement was added to the 2016 Teklad Global 16% Protein Rodent Diet. The supplement contains about 6.8% phytoestrogens (genistein/genistin, daidzein/daidzin, glycitein/glycitin). Total addition sums to approximately 100 mg added phytoestrogens per 1000 g diet. |
2016 is a fixed formula and nutritionally balanced diet containing 16% protein and 3.6% fat which supports rodent growth and maintenance. 2016 does not contain alfalfa or soy meal, thus minimizing the occurrence of natural phytoestrogens.
Values are calculated from ingredient analysis or manufacturer data.
Estimated digestible carbohydrate
Kcal/g = 3.1
Dose was designed to deliver to mice a daily intake of 0.25 mg added phytoestrogens, or 10 mg/kg (BW) mouse per day, assuming a 25 g mouse eating 2.5 g diet per day
Phytoestrogen content was based on the analysis by Kenneth et al.16
The phyto-β-SERM and soy extract diets were designed to deliver to mice a total amount of added phytoestrogens that is biologically equivalent to a daily intake of 50 mg in humans. The conversion of human dose to mouse equivalent dose was based on the equivalent surface area dosage conversion factor from human to mouse:18 50 mg/60 kg (BW, human) x 12 (human to mouse conversion factor) = 10 mg/kg (BW, mouse). This human dose is the estimated average amount of phytoestrogens that Asians regularly ingest from dietary consumption of soy foods,9 and the recommended daily serving dose for many commercial soy extract supplements sold to women in the US, including the one tested in this study.16
Animals and treatment
The use of animals and treatment were approved by the Institutional Animal Care and Use Committee at the University of Southern California. As outlined in Table 2, two separate studies were conducted, in both of which, adult female 129/C57BL/6 mice were ovariectomized (OVX) or underwent a sham operation and immediately fed one of the three custom diets prepared above. The 2-month treatment study (repeated in two independent experiments conducted at different times), starting in 6-month-old mice, was designed to evaluate the thermal regulation of the diets. Throughout the treatment, mouse tail skin temperature (TST) and rectal temperature (RT) were recorded every other day. The 9-month treatment study (not repeated), starting in 3-month-old mice, was designed to evaluate the long-term impact of the diets on both the physical and neurological changes associated with menopause. Throughout the treatment, mouse food intake and body weigh were recorded 1-2 times every week, and physical appearances were photographed 1-2 times every two weeks. After 8.5-month treatment, 2 weeks before the treatment was ended, a cognition-behavioral test of spatial working memory function, Y-Maze, was administered. At the time of sacrifice, uteri were excised, trimmed of fat and connective tissue, and a wet weight was recorded. Uteri were further processed into total RNA samples and analyzed by quantitative real-time RT-PCR for changes in expression levels of genes associated with proliferation. Brain tissues were collected and dissected into hippocampus, cortex and cerebellum. Hippocampus were further processed into total protein samples and analyzed by Western blot for changes in expression levels of proteins associated with learning and memory.
Table 2.
Experimental overview.
| Animals | Diets | Duration | Start Age | End Age | Measurements | |
|---|---|---|---|---|---|---|
| Study 1 | Sham-OVX & OVX mice | Control Diet; Phyto-β-SERM Diet; Soy Extract Diet | 2 months | 6-month-old | 8-month-old | TST & RT – recorded every other day |
| Study 2 | Sham-OVX & OVX mice | Control Diet; Phyto-β-SERM Diet; Soy Extract Diet | 9 months | 3-month-old | 12-month-old | Food intake & body weight – recorded 1-2 times every week |
| General health & appearance – photographed 1-2 times every two weeks | ||||||
| Y-Maze – conducted 2 weeks prior to sacrifice | ||||||
| Protein expression in hippocampus – tissues collected at sacrifice | ||||||
| Uterine weight – recorded at sacrifice | ||||||
| Gene expression in uteri – tissues collected at sacrifice |
TST and RT measurement
TST and RT were recorded in a temperature-controlled test room with the experimenter blind to treatment conditions. Mice were first acclimated to handling and experimental apparatuses over a period of 2 weeks. Recordings were performed at 1500 h of the light phase of the light/dark cycle. TST was recorded with a small rodent infrared thermometer (Model No. IRB152, Braintree Scientific, Braintree, MA 02185), which was placed at the dorsal surface of the tail approximately 1 cm from the base of the tail. Over the course of 2 min, four readings at a 30-sec interval were recorded and the average of the last two readings was reported as the final TST. Following the TST measurement, RT was recorded with an animal rectal probe designed for use in mice (Model No. RET-3, Braintree Scientific) attached to a MicroTherma 2 type “T” thermometer (Model No. TW2-193, Braintree Scientific).
Y-Maze cognition-behavioral test
Y-Maze with three identical arms that are evenly spaced with an arm length of 35 cm, arm height of 10 cm and lane width of 5 cm (Model No. 60180, Stoelting, Wood Dale, IL), was used. Test was conducted in a temperature-controlled test room with accurate configuration of spatial visual cues. The experimenter was blind to treatment conditions. In the one-trial test of spontaneous alteration behavior (SAB), mice were allowed to move freely within the Maze for 5 min. The total number and the order of arm entries were recorded. Alteration is defined as successive entries into the three arms in overlapping triplet sets as described previously.19-20 The percent alteration is calculated as the ratio of actual to possible alterations: (the total number of arm entries − 2) x 100. The two-trial recognition test consisted of two trials separated by an intertrial interval. In the first acquisition trial, one arm of the Maze was closed and mice were allowed to explore the two other arms for 10 min. During the 5 h of interval, mice were housed in their home cages located in a room other than the test room. In the second retention trial, mice had free access to all three arms, and were allowed to explore the Maze for 5 min. The first arm entered, the number of entries into each arm and the amount of time spent in the novel arm were recorded. The number of visits in the novel arm is calculated as a percentage of the total number of visits in all three arms during the first 2 min of the second trial.
qRT-PCR
Total RNA samples were extracted from mouse uteri using the PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA). RNA quantity and quality were analyzed using the Experion RNA StdSens Analysis Kit on an Experion Automated Electrophoresis System (Bio-Rad, Hercules, CA). cDNA was synthesized using the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA), on a MyCycler Thermal Cycler (Bio-Rad). Taqman real-time qRT-PCR reactions were performed on 50 ng cDNA samples mixed with the TaqMan Universal PCR Master Mix 2X (Applied Biosystems) and Taqman gene expression assays for Ki67 (assay ID: Mm01278617_m1; Applied Biosystems) and PCNA (assay ID: Mm00448100_g1; Applied Biosystems). β-actin was used as the endogenous control gene (Assay ID: 4352933E; Applied Biosystems). Fluorescence was detected on a ABI 7900HT Fast Real-Time PCR System equipped with the Sequence Detection System Software Version 2.3 (Applied Biosystems). Data were analyzed using the RQ Manager Version 1.2 and DataAssist Version 2.0 (Applied Biosystems). Relative gene expression levels or fold changes relative to the control group were calculated by the 2−ΔΔCt method.21
Western blot
Total protein samples were extracted from mouse hippocampus as previously described.17 20-40 μg of protein samples were loaded per lane and separated by electrophoresis on a 10-12% SDS-PAGE. Proteins were then electrotransferred to PVDF membranes and probed with primary antibodies against brain-derived neurotrophic factor (BDNF; 1:250, Chemicon, Temecula, CA), synaptophysin (SYP; 1:8000, Millipore, Billerica, MA), postsynaptic density protein 95 (PSD-95; 1:1000, Chemicon), apolipoprotein (ApoE; 1:2000, Chemicon), insulin-degrading enzyme (IDE; 1:1000, Calbiochem, San Diego, CA), and neprilysin (NEP; 1:250, Chemicon), at 4°C overnight, and then with HRP-conjugated secondary antibodies (1:5000~20000, Vector Laboratories, Burlingame, CA). β-tubulin (1: 5000, Abcam, Cambridge, MA) was used as the loading control. Bands were visualized with a TMB peroxidase kit (Vector Laboratories) or by chemiluminescence using an ECL detection kit (Amersham, Piscataway, NJ). Relative intensities of the immunoreactive bands were captured by Molecular Imager ChemiDoc XRS+ System (Model No. 170-8251, Bio-Rad, Hercules, CA) and quantitated by Quantity One Analysis Software, Version 4.6.4 (Model No. 170-9600, Bio-Rad).
Statistical analyses
Data are presented as group means ± S.E.M. Statistically significant differences between groups were determined by one-way analyses of variance (ANOVA) followed by Student-Newman-Keuls pairwise multiple comparison post-hoc tests.
RESULTS
Phyto-β-SERM diet, not soy extract diet, prevented OVX-induced rise in TST
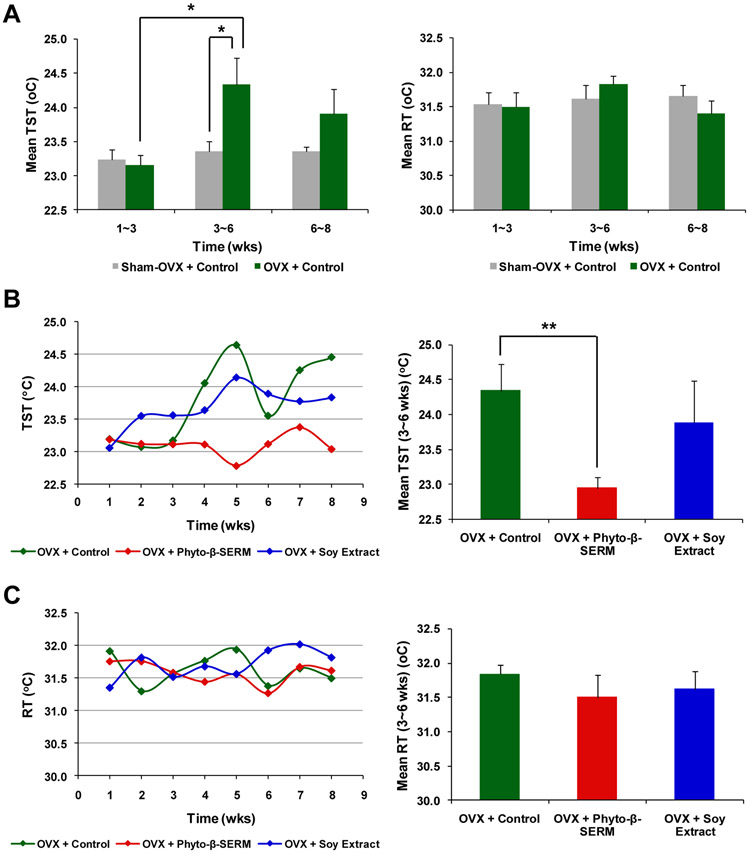
The 2-month treatment study revealed that when compared to sham-OVX mice treated with the control diet that showed no significant changes in TST throughout the treatment, OVX mice under the same diet exhibited a significant rise in TST, the surrogate marker of hot flashes, at 3-6 weeks following OVX (Fig. 1A (left); * P < 0.05 compared to OVX mice at 1-3 weeks and Sham-OVX at 3-6 weeks). OVX-induced rise in TST at 3-6 weeks was prevented by the phyto-β-SERM diet, but not by the soy extract diet (Fig. 1B; ** P < 0.01 compared to OVX mice treated with the control diet). In comparison with TST, RT, the indicator of the core body temperature, did not show significant changes with either the estrogen (Fig. 1A (right)) or dietary status (Fig. 1C) throughout the same observational period (3-6 weeks).
FIG. 1.
Phyto-β-SERM diet prevented OVX-induced rise in TST, a surrogate marker of hot flashes. Six-month-old female mice were OVX or sham-OVX and fed one of the three test diets: a phytoestrogen-free control diet, a phyto-β-SERM formulation-containing diet or a commercial soy extract-containing diet, for 8 weeks. Throughout the treatment, mouse TST and RT were recorded every other day. Data are presented as group mean ± S.E.M.; N / group = 7-8. * P < 0.05 and **P < 0.01.
Phyto-β-SERM diet, not soy extract diet, prevented OVX-induced abnormalities in hair growth
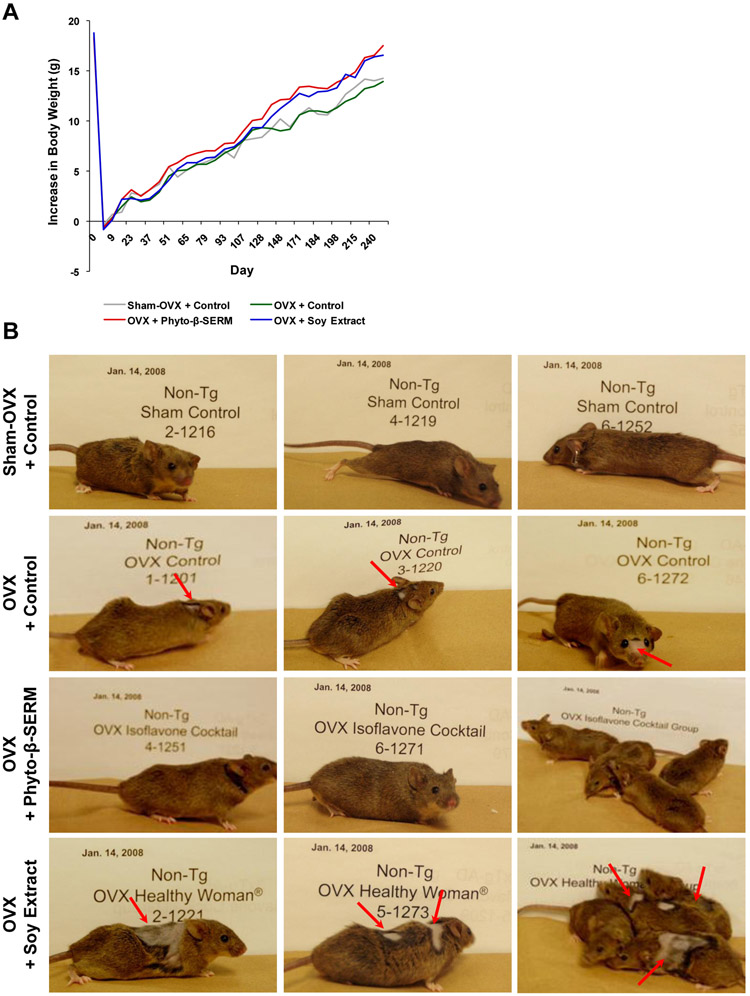
The 9-month treatment study revealed that although there appeared a positive effect from the two phytoestrogen-supplemented diets on the growth of mice, none of the three test diets induced a significant change in the body weight of treated mice (Fig. 2A). However, mice fed different diets exhibited profound differences in their physical appearances (Fig. 2B). When compared with sham-OVX mice treated with the control diet, OVX mice treated with the same diet exhibited an abnormal hair thinning/loss which was particularly noted around the forehead and neck (Fig. 2B). The abnormality in hair growth appeared even worse in OVX mice treated with the soy extract diet (Fig. 2B). In contrast, OVX mice treated with the phyto-β-SERM diet looked no different from sham-OVX mice under the control diet (Fig. 2B).
FIG. 2.
Phyto-β-SERM diet prevented OVX-induced abnormalities in hair growth. Three-month-old female mice were OVX or sham-OVX and fed one of the three test diets: a phytoestrogen-free control diet, a phyto-β-SERM formulation-containing diet or a commercial soy extract-containing diet, for 9 months. Throughout the treatment, mouse food intake and body weight were recorded, and physical appearances were photographed, 1-2 times a week. Photographs were taken after 6-month treatment, when mice were 9-month-old.
Phyto-β-SERM diet, not soy extract diet, promoted spatial working memory
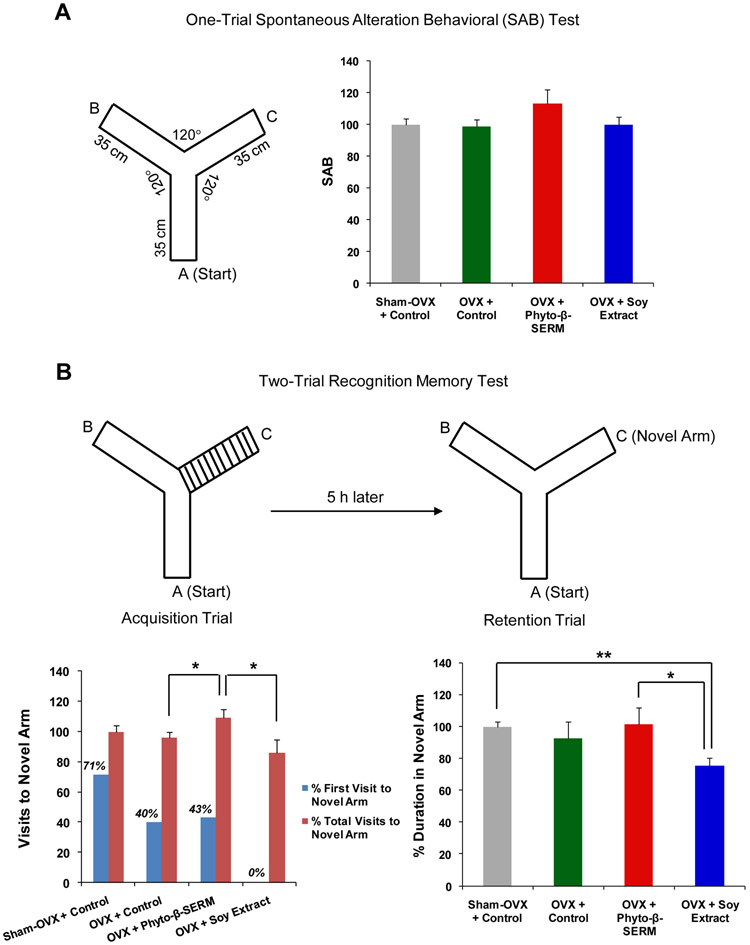
Two weeks before the end of the 9-month study, mice were subjected to a cognition-behavioral test, Y-Maze, which is based upon the natural tendency of mice to explore a novel arm rather than a familiar one when both are presented simultaneously to assess the hippocampus-dependent spatial working memory function. The one-trial SAB test was designed to assess the normal navigational behavior of mice when they were free of stress and allowed to explore all three arms of the Maze. Success in this test was reflected by a high rate of alternation indicating that mice remembered which arm was entered last. Compared to the SAB test, the two-trial recognition test was designed to assess a higher level of cognitive complexity that involves a time delay between the learning and recognition process. In brief, mice were first allowed to explore the Maze that had one arm closed. Following a 5-h interval, mice were then brought back to the Maze and allowed to explore freely all three arms. The choice to explore the novel arm (the arm closed in the learning process), as reflected by the first entry, the number of entries and amount of time spent in the novel arm, indicates the greater learning and recognition capacity. Data shown in Fig. 3A indicated that, in the one-trial test, there were no significant differences in SAB between groups, although it appeared that OVX mice fed the phyto-β-SERM diet performed slightly better than OVX mice fed the control diet (Fig. 3A).
FIG. 3:
Phyto-β-SERM diet promoted spatial working memory function. Three-month-old female mice were OVX or sham-OVX and fed one of the three test diets: a phytoestrogen-free control diet, a phyto-β-SERM formulation-containing diet or a commercial soy extract-containing diet, for 9 months. After 8.5-month treatment, a cognitive behavioral test of spatial working memory function, Y-Maze, was administered. In the one-trial test of SAB, the total number and the order of arm entries were recorded. In the two-trial recognition memory test that consisted of an acquisition trial followed by a retention trial, the first entry, the number of entries into each arm and the amount of time spent in the novel arm were recorded. Data are presented as percent of phytoestrogen-free diet-treated sham-OVX control group and expressed as group mean ± S.E.M.; N / group = 5-7. * P < 0.05 and ** P < 0.01.
Similar to the one-trial test, the two-trial test also failed to detect a significant difference in % visits to the novel arm and % duration in the novel arm between sham-OVX control and OVX control mice. However, the sham-OVX mice performed better than OVX mice in choosing the novel arm as their first entry choice (Fig. 3B; 71% compared to 40%). Further, a significant improvement in performance, as indicated by the % total visits to the novel arm, was observed in OVX mice treated with the phyto-β-SERM diet as compared to OVX mice treated with the control diet (Fig. 3B; * P < 0.05). By contrast, OVX mice treated with the soy extract diet exhibited a decline in performance, as indicated by the % first visit to the novel time, when compared to OVX control mice (Fig. 3B; 0% compared to 40%). Moreover, OVX mice treated with the phyto-β-SERM diet performed significantly better than OVX mice treated with the soy extract diet, as indicated by the % total visits to the novel arm (Fig. 3B; * P < 0.05) and the % duration in the novel arm (Fig. 3B; * P < 0.05). And, when compared to sham-OVX control mice, OVX mice treated with the soy extract diet performed significantly poorer as indicated by the % duration in the novel arm (Fig. 3B; ** P < 0.01), while the performance of those OVX mice treated with the phyto-β-SERM diet did not show significant differences (Fig. 3B).
Phyto-β-SERM diet, not soy extract diet, promoted expression of proteins involved in neural plasticity and β-amyloid (Aβ) degradation/clearance in the hippocampus
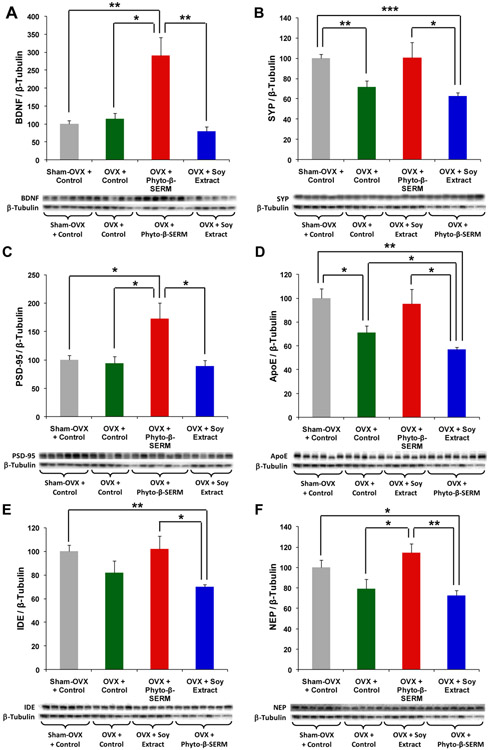
Hippocampal brain tissues collected from the 9-month study were analyzed for expression of proteins involved in neural plasticity including the neurotrophic factor, BDNF, pre-synaptic protein, SYP, and post-synaptic protein, SPD-95, as well as proteins involved in the catabolic degradation/clearance of Aβ in the brain including ApoE, IDE and NEP. Western blot data shown in Fig. 4 indicated that OVX induced a decline in a subset of proteins. Among the first panel of neurotrophic and synaptic proteins, OVX induced a significant reduction in the expression of the pre-synaptic protein, SYP (Fig. 4B; ** P < 0.01 between sham-OVX control and OVX control mice), whereas it had no effect on the expression of BDNF (Fig. 4A) and the post-synaptic protein, PSD-95 (Fig. 4C). By comparison, OVX appeared to exert a greater impact on the expression of proteins involved in Aβ degradation/clearance, as demonstrated by a significant deficit in the expression of ApoE (Fig. 4D; * P < 0.05 between sham-OVX control and OVX control mice), and a notable trend of reduction in the expression of IDE (Fig. 4E) and NEP (Fig. 4F), in OVX control mice as compared to sham-OVX control mice. The phyto-β-SERM diet reversed OVX-induced deficits in the expression of proteins including SYP (Fig. 4B), ApoE (Fig. 4D), IDE (Fig. 4E) and NEP (Fig. 4F; * P < 0.05 compared to OVX control mice), although most of the effects were not statistically significant. Moreover, the phyto-β-SERM diet enhanced the expression of PSD-95 (Fig. 4C; * P < 0.05 compared to both sham-OVX and OVX control mice) and induced the greatest effect on the expression of BDNF (Fig. 4A; * P < 0.05 compared to OVX control mice and ** P < 0.01 compared to sham-OVX control mice). By contrast, when compared to OVX control mice, the soy extract diet either had no effect on the expression of proteins including BDNF (Fig. 4A), SYP (Fig. 4B), PSD-95 (Fig. 4C), IDE (Fig. 4E) and NEP (Fig. 4F), or exerted a negative impact such as on the expression of ApoE (Fig. 4D; * P < 0.05 compared to OVX control mice). Moreover, the expression levels of all six proteins in OVX mice treated with the soy extract diet were significantly lower than the levels expressed in OVX mice treated with the phyto-β-SERM diet (Fig. 4A-4F; * P < 0.05 and ** p < 0.01). And, when compared to sham-OVX control mice, the expression levels of the majority of proteins in OVX mice treated with the soy extract diet were significantly lower (Fig. 4B & 4D-4F; * P < 0.05, ** P < 0.01 and *** P < 0.001), whereas, the levels of those same proteins in OVX mice treated with the phyto-β-SERM diet were not significantly different from the levels expressed in sham-OVX control mice (Fig. 4B & 4D-4F).
FIG. 4:
Phyto-β-SERM diet promoted expression of hippocampal proteins involved in neural plasticity and Aβ degradation/clearance. Three-month-old female mice were OVX or sham-OVX and fed one of the three test diets: a phytoestrogen-free control diet, a phyto-β-SERM formulation-containing diet or a commercial soy extract-containing diet, for 9 months. At the time of sacrifice, brain tissues were collected and dissected; hippocampus were processed and analyzed by Western blot for changes in expression levels of proteins associated with learning and memory. Data are presented as percent of phytoestrogen-free diet-treated sham-OVX control group and expressed as group mean ± S.E.M.; N / group = 5-7. * P < 0.05, ** P < 0.01 and *** P < 0.001.
Phyto-β-SERM and soy extract diets had no impact on uterine growth
For the 9-month study, uterine weight and mRNA expression levels of proliferation markers Ki67 and PCNA were assessed as indicators of impact on uterine growth. Data shown in Table 3 revealed that compared to sham-OVX control mice, OVX induced an approximately 50% reduction in uterine weight (* P < 0.05 between sham-OVX control and OVX control mice). Treatment of OVX mice with either the phyto-β-SERM diet or the soy extract diet did not induce a significant change in uterine weight as compared to OVX mice treated with the control diet (* P < 0.05 compared to sham-OVX control mice). Similarly, neither the phyto-β-SERM diet nor the soy extract diet induced a significant change in expression of either Ki67 or PCNA gene as compared to OVX control mice (Table 3). No significant differences in either uterine weight or gene expression were observed among the three OVX groups: OVX + Control, OVX + Phyto-β-SERM and OVX + Soy Extract (Table 3).
Table 3.
Phyto-β-SERM diet had no impact on uterine growth.
| Treatment Group | Uterine Weight (Wet) |
Uterine Gene Expression | |||
|---|---|---|---|---|---|
| Ki67 | PCNA | ||||
| Average Delta (Ct) |
Fold Change |
Average Delta (Ct) |
Fold Change |
||
| Sham-OVX + Control | 100.00 ± 11.11 | ---- | |||
| OVX + Control | 47.07 ± 18.29* | 9.80 ± 0.42 | 1.00 | 5.34 ± 0.59 | 1.00 |
| OVX + Phyto-β-SERM | 53.49 ± 9.00* | 10.39 ± 2.32 | 0.67 | 5.90 ± 0.23 | 0.68 |
| OVX + Soy Extract | 45.61 ± 18.47* | 9.17 ± 1.01 | 1.55 | 4.65 ± 0.65 | 1.61 |
Three-month-old female mice were OVX or sham-OVX and fed one of the three test diets: a phytoestrogen-free control diet, a phyto-β-SERM formulation-containing diet or a commercial soy extract-containing diet, for 9 months. At the time of sacrifice, uteri were excised, trimmed of fat and connective tissue, and a wet weight was recorded. Uteri were further processed and analyzed by quantitative real-time RT-PCR for changes in expression levels of genes associated with proliferation. Uterine weight is presented as percent of phytoestrogen-free diet-treated sham-OVX control group and expressed as group mean ± S.E.M.; N / group = 5-7
P < 0.05 compared to Sham-OVX + Control. Uterine gene expression average delta (Ct) is expressed as group mean ± S.E.M.; N / group = 5; fold change is relative to phytoestrogen-free diet-treated OVX control group (set as 1.00). Gene expression for the Sham-OVX + Control group was undetermined due to the poor integrity of total RNA samples (Relative Quality Indicator (RQI) < 5). There are no significant differences in either uterine weight or gene expression between OVX + Control, OVX + Phyto-β-SERM and OVX + Soy Extract groups.
DISCUSSION
Clinically, menopausal hot flashes result primarily from the gradual cessation of the ovarian estrogen production at menopause,22-23 and manifest as a transient increase in skin temperature and profuse sweating.24-25 In preclinical research, the elevation of TST in OVX mice or rats has been widely used as a model for menopausal hot flashes.26-27 In agreement with the findings from others,26-27 we observed a time-dependent significant change in TST, but not in RT, in response to OVX in our mouse model. We further observed that the phyto-β-SERM diet, but not the soy extract diet, prevented OVX-induced rise in TST throughout the same time course. These observations support the therapeutic potential of the phyto-β-SERM formulation for preventing/treating menopausal hot flashes. Soy extract-based products, however, could have no benefit as they are publicized.
Androgenic hair thinning/loss is another commonly noted climacteric symptom that occurs in approximately one-third of menopausal women. Estrogen/progesterone therapy is probably the most common systemic form of treatment for androgenic hair loss for women in menopause (http://www.americanhairloss.org/women_hair_loss/treatment.asp). Moreover, research indicate that ERβ is the predominant ER and the main mediator of estrogen action in human skin and hair follicles, where ERβ may play a regulatory role on androgen receptor expression leading to an alteration on hair growth.28 In the present study, our observation of the regional hair thinning/loss around the forehead and neck of OVX mice resembles clinical characteristics in menopausal women (http://www.pioneerthinking.com/hairloss.html), indicating that the hair abnormalities observed in these mice are directly associated with estrogen deficiency induced by OVX. OVX mice treated with the phyto-β-SERM diet were indistinguishable from ovary intact mice. In striking contrast, OVX mice treated with the soy extract diet exhibited significant hair thinning/loss that was equal to or exceeded that of OVX control mice. These observations provide further support for the therapeutic potential of the phyto-β-SERM formulation, but not the soy extract, in the intervention of climacteric symptoms, including the change in hair growth.
In addition to the commonly noted symptoms such as hot flashes and hair thinning/loss, cognitive decline and an increased risk for developing AD have also been associated with menopause.29 Although we failed to detect a significant decline in performance in OVX control mice when compared to sham-OVX control mice on the Y-Maze, which, we speculate, could be due to the relative simplicity of this task as compared to other tasks such as Morris water maze that involves a more complex learning network,30 we did observe a positive effect from the phyto-β-SERM diet in both the one-trial SAB and two-trial recognition test. And, OVX mice treated with the phyto-β-SERM diet performed significantly better than those treated with the soy extract diet.
Furthermore, we analyzed hippocampal brain tissues collected from these mice and compared the expression levels of a panel of proteins involved in neural plasticity, and another panel of proteins involved in Aβ degradation/clearance in the brain. Western blot data indicated that OVX appeared to have a greater impact on proteins involved in Aβ degradation/clearance, as evidenced by a significant deficit in the expression of ApoE and a notable trend of reduction in the expression of IDE and NEP observed in OVX control mice as compared to sham-OVX control mice. Moreover, OVX induced a significant decline in the expression of pre-synaptic protein, SYP, but not BDNF and PSD-95. Regardless of the differences between sham-OVX and OVX, the phyto-β-SERM diet either reversed OVX-induced deficit in the expression of proteins such as SYP, ApoE, IDE and NEP, or enhanced the expression of proteins such as BDNF and PSD-95. Taking both the cognition-behavioral and hippocampal protein expression data together, it can be concluded that the phyto-β-SERM formulation, but not the soy extract, may have a neurotherapeutic benefit regardless of the menopausal status.
It should be particularly noted that the brain-derived neurotrophic factor, BDNF, exhibited the most robust response to the phyto-β-SERM diet. As a small dimeric protein, BDNF is structurally related to NGF, but appears to have a greater expression and wider distribution in the CNS, with the greatest concentration found in the hippocampal formation.31 A large body of evidence indicates that BDNF plays a key role in promoting neuronal survival and differentiation in developing brain.32 In mature brain, BDNF regulates synaptogenesis and synaptic plasticity, and solidifies memory formation and storage.33-34 BDNF is abundant in brain regions vulnerable to neurodegenerative diseases such as AD, including hippocampus, cerebral cortex and amygdala complex.31 And, the expression of BDNF mRNA and protein in the above brain regions is reduced by AD, and its reduction could further impair synaptic function and cognition.35 Moreover, in the forebrain, co-localization of ERs, BDNF and its high-affinity membrane tyrosine receptor kinase B, as well as the fact that the BDNF gene contains an estrogen-sensitive response element, suggest potential interactions between estrogen and BDNF.36-37 Studies have demonstrated that estrogen-BDNF interactions and estrogen regulation of BDNF, potentially through transcription, are essential for estrogen-mediated protection of neuronal viability and cognitive function from neurodegenerative insults.38-41 We demonstrate here, for the first time, a close link between ERβ and BNDF in the hippocampal formation, an association that was previously observed in auditory neurons.42 This finding, together with the behavioral data, adds additional evidence supporting the postulate that activation of ERβ leads to an improvement in neural plasticity and cognitive function that is at least partially mediated by ERβ-induced increase in BDNF.
Induction of proliferative responses and risk of reproductive cancers has been a major concern to women who receive the currently available forms of ET.8 Our previous study has demonstrated that an acute treatment with the phyto-β-SERM formulation did not affect the uterine growth of treated animals.17 The present observation that a 9-month exposure to the phyto-β-SERM diet did not induce a significant change in either uterine weight or expression of proliferation markers including Ki67 and PCNA further confirms the lack of estrogenic proliferative property from the phyto-β-SERM formulation, suggesting that unlike ET, the phyto-β-SERM formulation does not pose a risk for reproductive cancers.
What could have potentially contributed to the differences in responses induced by the two phytoestrogen-enriched diets, the phyto-β-SERM diet in comparison with the soy extract diet? As discussed earlier, although both diets contain the same amount of commonly known phytoestrogens, the compositional complexity of two diets is significantly different (Table 4). In general, for the soy-derived extract preparations, since addition and/or deletion could occur during the extraction process, the constitutive composition of soy extracts could be quite variant from that present in the natural form of soy products, for instance, soy foods consumed in Asian countries. Therefore, research data generated from soy extracts could be not comparable with the observation resulted from the epidemiological studies on soy foods. The compositional change in soy extracts could have a consequence on the overall synergy and associated health impact present in natural soy products. For example, the unknown substances generated from the extraction process could pose undesirable effects counteracting the favorable health-giving properties of other substances. It can be conceived that these variations make the safety and efficacy of soy extract products nearly unpredictable.8,16 By comparison, a rationally designed formulation with a clearly defined composition and synergy could have greatly contributed to the therapeutic efficacy of the phyto-β-SERM formulation shown in the present study.
Table 4.
Phyto-β-SERM vs soy extract formulations.
| Phyto-β-SERM Formulation | Soy Extract Formulation | |
|---|---|---|
| Compositional Complexity | Rationally designed and has a standardized composition | Varied in compositional complexity and are not standardized in composition |
| Phytoestrogens/Equol | Genistein, daidzein and equol | Genistein/genistin, daidzein/daidzin, glycitein/glycitin, and possibly other structurally similar chemicals; w/o equol |
| ERα/β Specificity | ERβ agonism | ERα/β agonism & antagonism |
| Efficacy/Safety | ERβ-medicated estrogenic activities; ERα-mediated proliferative effect on the reproductive system is minimal | Unpredictable |
In addition to the differences in the compositional complexity and related therapeutic effectiveness, the phyto-β-SERM formulation could also potentially offer two more clinical advantages over soy extract products (Table 4). The first advantage is associated with the high selectivity for ERβ by the phyto-β-SERM formulation. Numerous studies including our own have indicated that although both ERα and ERβ mediates estrogen-induced neuroprotection,43-44 ERβ could be more involved in estrogen regulation of neural development and synaptic plasticity.45 An ERβ-selective therapy could also potentially minimize ERα-mediated feminizing and proliferative responses associated with elevated risks for reproductive cancers in women, and could be much safer even with a long-term administration than a non-selective ET.45 The second advantage is associated with the presence of equol in the phyto-β-SERM formulation. Unlike genistein and daidzein, equol is not of direct plant origin, yet can be exclusively produced through the metabolism of daidzein catalyzed by intestinal microbial flora following the intake of soy products.5 Wide inter-individual variations in equol-producing phenotype exist across human populations. Approximately 20-35% of Western adults are equol-producers as compared to 55-60% in Asian populations.46-49 Research has suggested that the equol-producing phenotype could serve as a critical modulator of human response to phytoestrogen treatment.50-52 Therefore, inclusion of equol in the phyto-β-SERM formulation could potentially benefit both equol-producers and non-producers.
CONCLUSIONS
The present study indicates the therapeutic potential of the phyto-β-SERM formulation for prevention/alleviation of menopause-related climacteric symptoms. The clearly defined composition and synergy, the ERβ selectivity, and the inclusion of equol, make the phyto-β-SERM formulation a compellingly promising phytoestrogen-based alternative to estrogen therapy for future studies in humans.
Acknowledgments
This work was supported by grants from the Alzheimer’s Association (to L.Z.) and Bensussen Translational Research Fund (to R.D.B.). The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the united states. N Engl J Med 2007;356:1670–1674. [DOI] [PubMed] [Google Scholar]
- 2.Hammond CB. Women's concerns with hormone replacement therapy-compliance issues. Fert Steril 1994;62:157S–160S. [PubMed] [Google Scholar]
- 3.Zhao L, Brinton RD. In search of estrogen alternatives for the brain In: Hogervorst E, Henderson VW, Gibbs RB, Brinton RD, editors. Hormones, cognition and dementia: State of the art and emergent therapeutic strategies. Cambridge: University Press, 2009, p93–100 [Google Scholar]
- 4.Zhao L, O'Neill K, Brinton RD. Selective estrogen receptor modulators (serms) for the brain: Current status and remaining challenges for developing neuroserms. Brain Res Brain Res Rev 2005;49:472–493. [DOI] [PubMed] [Google Scholar]
- 5.Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am J Clin Nutr 1984;40:569–578. [DOI] [PubMed] [Google Scholar]
- 6.Dixon RA. Phytoestrogens. Annu Rev Plant Biol 2004;55:225–261. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KD. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 1998;68:1333S–1346S. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Brinton RD. Whi and whims follow-up and human studies of soy isoflavones on cognition. Expert Rev Neurother 2007;7:1549–1564. [DOI] [PubMed] [Google Scholar]
- 9.Rice MM, LaCroix AZ, Lampe JW, et al. Dietary soy isoflavone intake in older japanese american women. Public Health Nutr 2001;4:943–952. [DOI] [PubMed] [Google Scholar]
- 10.de Kleijn MJ, van der Schouw YT, Wilson PW, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the united states: The framingham study(1-4). J Nutr 2001;131:1826–1832. [DOI] [PubMed] [Google Scholar]
- 11.Maskarinec S The effect of phytoestrogens on hot flashes. Nutr Bytes 2003;9. [Google Scholar]
- 12.Ziegler RG. Phytoestrogens and breast cancer. Am J Clin Nutr 2004;79:183–184. [DOI] [PubMed] [Google Scholar]
- 13.Henderson BE, Bernstein L. The international variation in breast cancer rates: An epidemiological assessment. Breast Cancer Res Treat 1991;18:S11–17. [DOI] [PubMed] [Google Scholar]
- 14.Goetzl MA, Van Veldhuizen PJ, Thrasher JB. Effects of soy phytoestrogens on the prostate. Prostate Cancer Prostatic Dis 2007;10:216–223. [DOI] [PubMed] [Google Scholar]
- 15.Lethaby AE, Brown J, Marjoribanks J, et al. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev 2007:CD001395. [DOI] [PubMed] [Google Scholar]
- 16.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 2001;131:1362S–1375S. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology 2009;150:770–783. [DOI] [PubMed] [Google Scholar]
- 18.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemotherapy Reports 1966;50:219–244. [PubMed] [Google Scholar]
- 19.Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of alzheimer's disease. J Neurosci 2006;26:13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King DL, Arendash GW. Behavioral characterization of the tg2576 transgenic model of alzheimer's disease through 19 months. Physiol Behav 2002;75:627–642. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 22.Abe T, Furuhashi N, Yamaya Y, et al. Correlation between climacteric symptoms and serum levels of estradiol, progesterone, follicle-stimulating hormone, and luteinizing hormone. Am J Obstet Gynecol 1977;129:65–67. [DOI] [PubMed] [Google Scholar]
- 23.Erlik Y, Meldrum DR, Judd HL. Estrogen levels in postmenopausal women with hot flashes. Obstet Gynecol 1982;59:403–407. [PubMed] [Google Scholar]
- 24.Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: An hypothesis of flush mechanism. Clin Endocrinol (Oxf) 1985;22:293–312. [DOI] [PubMed] [Google Scholar]
- 25.Lomax P, Schonbaum E. Postmenopausal hot flushes and their management. Pharmacol Ther 1993;57:347–358. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, Tamura M, Hayashi M, et al. Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol 2000;278:R863–869. [DOI] [PubMed] [Google Scholar]
- 27.Opas EE, Gentile MA, Kimmel DB, Rodan GA, Schmidt A. Estrogenic control of thermoregulation in eralphako and erbetako mice. Maturitas 2006;53:210–216. [DOI] [PubMed] [Google Scholar]
- 28.Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev 2006;27:677–706. [DOI] [PubMed] [Google Scholar]
- 29.Brinton RD. Estrogens and alzheimer's disease In: Marwah J, Teitelbaum H, editors. Advances in neurodegenerative disorders vol2: Alzheimer's and aging. 1 ed. Scottsdale, Arizona: Prominent Press; 1998:99–130. [Google Scholar]
- 30.D'Hooge R, De Deyn PP. Applications of the morris water maze in the study of learning and memory. Brain Res Brain Res Rev 2001;36:60–90. [DOI] [PubMed] [Google Scholar]
- 31.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in alzheimer's disease and parkinson's disease. Prog Neurobiol 2001;63:71–124. [DOI] [PubMed] [Google Scholar]
- 32.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors 2004;22:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunha C, Brambilla R, Thomas KL. A simple role for bdnf in learning and memory? Front Mol Neurosci 2010;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bdnf Lu B. and activity-dependent synaptic modulation. Learn Mem 2003;10:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain bdnf function in normal aging and alzheimer disease. Brain Res Rev 2008;59:201–220. [DOI] [PubMed] [Google Scholar]
- 36.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 1995;92:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toran-Allerand CD, Miranda RC, Bentham WD, et al. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci U S A 1992;89:4668–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and bdnf induction in cultured hippocampal slices. Eur J Neurosci 2009;29:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang LC, Zhang QG, Zhou CF, et al. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One 2010;5:e9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (bdnf) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult cns. Front Neuroendocrinol 2006;27:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohrabji F, Lewis DK. Estrogen-bdnf interactions: Implications for neurodegenerative diseases. Front Neuroendocrinol 2006;27:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meltser I, Tahera Y, Simpson E, et al. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest 2008;118:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular ca(2+) dynamics leading to erk phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res 2007;1172:48–59. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased bcl-2 expression in primary hippocampal neurons. Brain Res 2004;1010:22–34. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L, Brinton RD. Estrogen receptor beta as a therapeutic target for promotion of neurogenesis and prevention of neurodegeneration. Drug Dev Res 2006;66:103–117. [Google Scholar]
- 46.Akaza H, Miyanaga N, Takashima N, et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in japanese, korean and american residents. Jpn J Clin Oncol 2004;34:86–89. [DOI] [PubMed] [Google Scholar]
- 47.Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol 2000;10:127–135. [DOI] [PubMed] [Google Scholar]
- 48.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–170. [DOI] [PubMed] [Google Scholar]
- 49.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 2002;132:3577–3584. [DOI] [PubMed] [Google Scholar]
- 50.Frankenfeld CL, McTiernan A, Thomas WK, et al. Postmenopausal bone mineral density in relation to soy isoflavone-metabolizing phenotypes. Maturitas 2006;53:315–324. Epub 2005 Jul 2012. [DOI] [PubMed] [Google Scholar]
- 51.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem 2007;18:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Oka J, Ezaki J, et al. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal japanese women: A double-blind, randomized, controlled trial. Menopause 2007. [DOI] [PubMed] [Google Scholar]