Abstract
Accompanying the aging of populations worldwide, and increased survival with chronic diseases, the incidence and prevalence of atrial fibrillation (AF) are rising, justifying the term global epidemic. This multifactorial arrhythmia is intertwined with common concomitant cardiovascular diseases, which share classical cardiovascular risk factors. Targeted prevention programs are largely missing. Prevention needs to start at an early age with primordial interventions at the population level. The public health dimension of AF motivates research in modifiable AF risk factors and improved precision in AF prediction and management. In this review, we summarize current knowledge in an attempt to untangle these multifaceted associations from an epidemiologic perspective. We discuss disease trends, preventive opportunities offered by underlying risk factors and concomitant disorders, current developments in diagnosis and risk prediction, and prognostic implications of AF and its complications. Finally, we review current technological (e.g. eHealth) and methodological (artificial intelligence) advances and their relevance for future prevention and disease management.
Keywords: atrial fibrillation, epidemiology, incidence, prevalence, risk factors, eHealth, artificial intelligence
Subject Terms: Electrophysiology
Introduction
With increased average global life expectancy and longer survival with chronic conditions, incidence and prevalence of atrial fibrillation (AF) has reached the dimension of a 21st century cardiovascular disease (CVD) epidemic.1–4 Despite multifaceted research efforts, the prevention of AF and its related complications remains challenging.5
Epidemiology.
The incidence and prevalence of AF are increasing globally. Based on data from the Framingham Heart Study (FHS), the prevalence of AF increased 3-fold over the last 50 years.1 The Global Burden of Disease project estimated a worldwide prevalence of AF around 46.3 million individuals in 2016.6 The lifetime risk of AF was estimated about one in four in white men and women older than 40 years in 2004,7 a decade later lifetime risk estimates reached about one in three in white individuals and one in five for African-Americans (AA) individuals (Figure 1).8
Figure 1. Challenges in AF epidemiology.
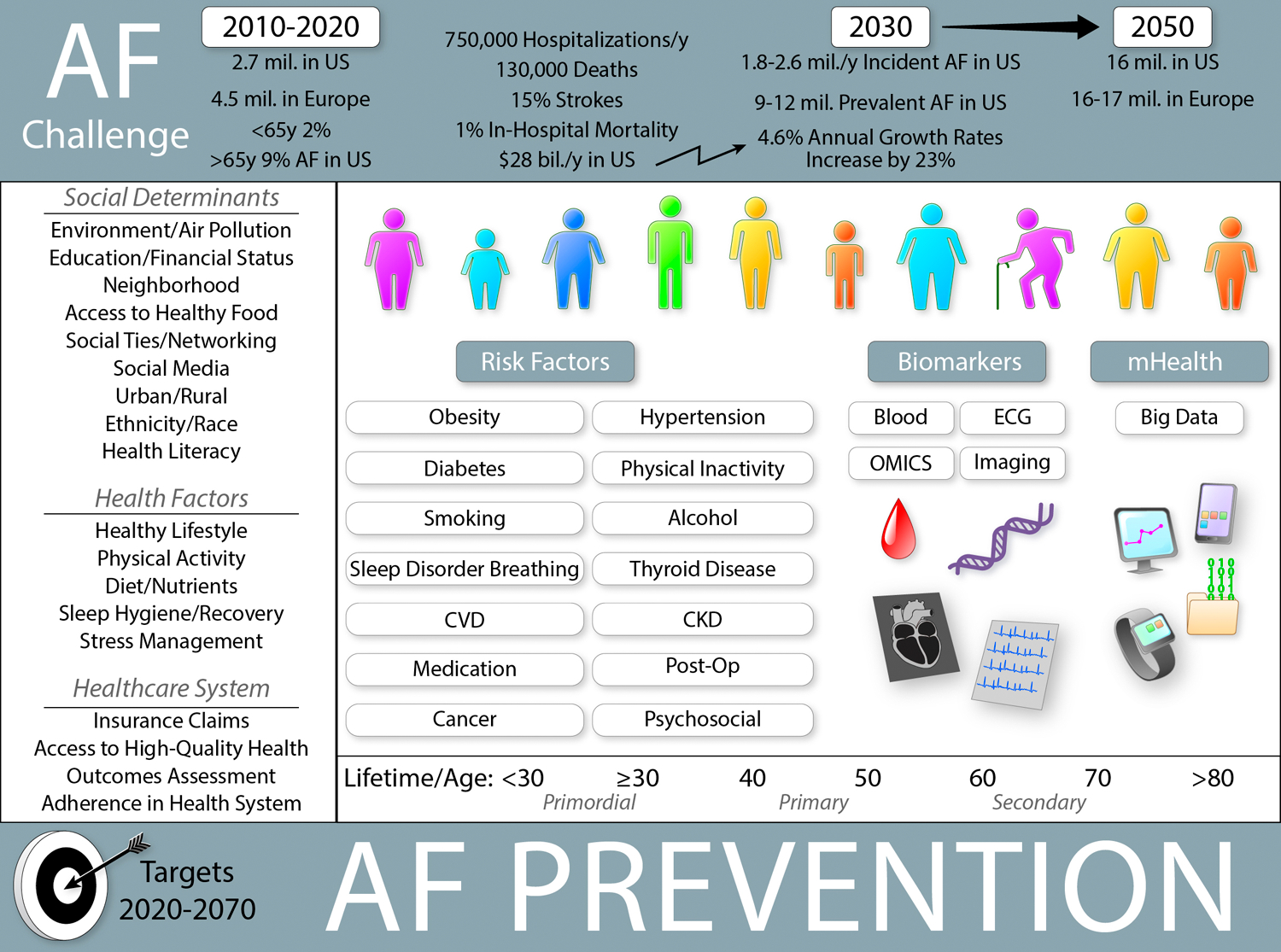
Prevention in AF becomes important because of epidemic character of disease development. While primary and secondary prevention play a crucial role in older individuals with cardiovascular- and non-cardiac diseases, primordial and primary prevention are fundamental in young adults and individuals without known co-morbidities. Personal, lifestyle, and social factors as well as societal and health system interventions remain essential prevention targets. (Illustration credit: Ben Smith)
In the United States (US) alone, at least 3–6 million people have AF, and the numbers are projected to reach ~6–16 million by 2050.9,10 In Europe, prevalent AF in 2010 was ~9 million among individuals older than 55 years and is expected to reach 14 million by 2060.11,12 It was estimated that by 2050 AF will be diagnosed at least in 72 million individuals in Asia, ~3 million with AF-related strokes.13
Awareness and enhanced detection of AF have improved over the past decade, which is important since about one third of the total AF population is asymptomatic.14 Therefore, the global AF burden is certainly underestimated (Figure 1). Facilitated and broadly applied rhythm monitoring by portable devices including smartphones and wearables initiated by consumers will further increase the prevalence of known AF.15 Precision medicine approaches are needed to identify individuals at higher risk for AF and its sequelae as well as to implement the most resource-efficient strategies to determine which subgroups of patients to screen, and to target for preventive and therapeutic management.
In this review, we summarize the role of common AF risk factors, biomarkers, and omics for prediction of incident AF, and their value for risk stratification of AF onset and perpetuation. Furthermore, we review comorbidities with important prognostic aspects and highlight future directions towards precision AF risk assessment and prevention using increasingly available artificial intelligence methods.
2. Primordial and primary prevention of AF
2.1. Non-modifiable factors
2.1.1. Advancing age
Age is the most important risk factor for AF. It is associated with increased AF burden, with a sharp incline after age 65 years. It is expected that the population of >65 year old adults will double from 12% in 2010 to 22% in 2040.16
In AF, many risk factors act over decades. For example, chronic subclinical inflammation, defined as continuous low-grade activation of the systemic immune response, is a hallmark of biological aging across multiple organ systems. Both AF and age are associated with elevated concentrations of reactive oxygen species. Further, inflammation is related to endothelial dysfunction, collagen catabolism, consequent increase of transforming growth factor (TGF)-ß1 activity, and changes in the extracellular matrix.17 Myocardial and vascular aging comprise changes at structural, functional, cellular, and molecular levels. Therefore, “healthy” aging could be considered as a goal in primordial and primary AF prevention. Controlling known AF risk factors would slow these degenerative processes and promote fit longevity.
2.1.2. Racial/ethnic differences in epidemiology of AF
The overall prevalence of AF in US is 1–2%.10 AF prevalence and incidence in Asians and AA are lower than in individuals with European ancestry, despite a higher burden of comorbidities in AA. Possible explanations comprise genetic, socioeconomic, and environmental determinants of health, which have not been completely evaluated.18,19 In the Multi-Ethnic Study of Atherosclerosis (MESA), the AF incidence was 46%–65% lower in Hispanics, Asians, and AA >65 years compared to non-Hispanic whites.19 In a study of over 600,000 Veteran Affairs patients, the age-adjusted prevalence of AF in Whites was almost two-fold higher than in other ethnicities.18
Although a lower AF incidence has been explained by under-detection caused by worse access to healthcare20 and more frequent paroxysmal AF,21 there is evidence that AA have up to 2 mm smaller atria on average compared to Whites.22 Genetic studies have shown a single-nucleotide polymorphism (SNP) mediating part of the increased risk of AF in European ancestry Americans compared to AA.23 Also, using ancestry informative markers, the Cardiovascular Health (CHS) and Atherosclerosis Risk in Communities Studies (ARIC) reported that European ancestry was associated with higher risk of AF.24
The same paradox has been found within the ethnic groups originating from India, Pakistan, Nepal, Sri Lanka, and Bangladesh, which represent about 20% of the world’s population.2,25 Similar to AA, lower AF incidence could be explained by smaller LA size indexed to body dimensions26 and ethnic variations in cardiac ion channels.27–29 However, systematic data on electrophysiological parameters in different ethnic groups remain an unmet need.
2.2. Modern lifestyle and modifiable risk factors for AF
Age, body mass index, height, hypertension, diabetes, obstructive sleep apnea, myocardial infarction, heart failure, smoking, and genetic predisposition are well-established risk factors for AF development and perpetuation.30 There is evidence that psychosocial and lifestyle factors are important modulators of AF occurrence, in particular at younger age.31 In this section, beside traditional risk factors such as hypertension and diabetes, we highlight the importance of lifestyle factors, e.g., smoking, alcohol, obesity, extreme sports, and psychological stress.
2.2.1. Hypertension
Up to one third of US adults have hypertension and its prevalence is expected to increase up to 46%.6, 32 The prevalence of hypertension reaches 80% in individuals >65 years and 26% in adults <45 years. Hypertension predisposes to cardiovascular complications, including coronary heart disease (CHD) and heart failure (HF), which contribute to AF initiation and mortality.33 Hypertension carries the largest population attributable risk for AF development worldwide. In the ARIC study, the hypothetical elimination of borderline or elevated risk factors was predicted to avoid more than half of diagnosed AF cases.34 Almost 25% of AF cases were attributed to elevated blood pressure.
Chronic elevated blood pressure leads to LA and left ventricular (LV) structural remodeling and contributes to cardiac pro-fibrotic changes.35 The main contributor to remodeling in hypertension remains the renin-angiotensin axis (RAS) with upregulated TGF-ß1 expression, increased aldosterone production, activation of nicotinamide adenine dinucleotide phosphate oxidase, and apoptosis.35 Some post-hoc analyses suggested that inhibition of RAS could be considered an upstream therapy for AF prevention and management, but the observational or randomized data were inconsistent.36,37 RAS activation is present also in individuals with chronic kidney disease (CKD), which is closely related with hypertension and AF. Interestingly, all three diseases – AF, hypertension, CKD – share age and diabetes as the most important risk factors, and stroke – as a relevant complication.
2.2.2. Diabetes
Glucose intolerance and insulin resistance are main components in diabetes mellitus (DM) and are modulators in AF substrate development.38 Type 2 DM is increasingly diagnosed not only in older adults. During last decade the prevalence of type 2 DM increased by 30% in young, usually obese, individuals aged <20 years.39 DM has been associated with 1.6-fold increased risk of AF.40,41 A meta-analysis reported a 40% increased risk for AF development in adults with compared to individuals without diabetes.42 Human and animal studies revealed that oxidative stress and inflammation are central modulators for mitochondrial dysfunction and consequent DNA damage, generating a substrate for AF development in metabolically-stressed hearts.43,44 Also, there is evidence that TGF-β1, RhoA–Rho-associated protein kinase pathway, and advanced glycation end products and their receptor axis are activated in DM and contribute to AF initiation.45 However, the association of DM with AF is not as strong as with other CVD.
2.2.3. Smoking
In the US, up to 38 million people are current smokers.6 Compared to non-smokers, the risk for AF in current smokers was significantly higher in the CHARGE AF Consortium,46 although the association is not as strong as with other CVD. A meta-analysis of 29 prospective studies reported a dose-dependent association between smoking and increased AF risk.47
The main components in tobacco products are nicotine besides tar and carbon monoxide. Nicotine activates pro-fibrotic mechanisms and blocks potassium channels. Nicotine may thus be directly involved in the development of an electro-anatomical substrate for AF.48,49 Indirectly, smoking may increase systemic catecholamine release and promote coronary vasospasm leading to myocardial ischemia and, secondarily, to AF.50 Furthermore, smoking increases inflammation, oxidative stress, endothelial dysfunction, and pro-thrombotic conditions, which facilitate atherosclerotic changes and contribute to atrial ischemic processes.51 Similarly, it has been suggested that vaping leads to pro-inflammatory changes and endothelial dysfunction. Although data on e-cigarettes’ adverse cardiovascular effects are sparse, a recent retrospective study reported that e-cigarette use was associated with an almost 2-fold risk for myocardial infarction.52 Whether vaping is associated with AF risk needs to be examined.
2.2.4. Alcohol
Alcohol consumption is common in Western countries, with almost 50% of the American population regularly consuming alcohol. The American Heart Association recommends to limit alcoholic beverages to a maximum of 2 drinks daily for men and 1 drink for women, ideally consumed with meals.53 A meta-analysis found that low alcohol consumption defined as one drink/day was not associated with increased AF incidence.54
In US over 17% of adult drinkers (~37 million) are binge drinkers.55 A recent meta-analysis found almost 8% increased AF risk with each additional daily alcoholic drink suggesting a linear dose-response relation.56 The results of ARIC cohort indicate a duration- and dose-dependent association with a higher risk of developing AF.57 Lower AF incidence was associated with longer duration of alcohol abstinence among former heavy drinkers. Every decade of alcohol abstinence was associated with almost 20% decreased risk of incident AF (~2%/year).
Long-term alcohol consumption promotes supraventricular and ventricular arrhythmias, particularly after periods of heavy drinking. Chronic ethanol exposure is associated with longer HV interval, QRS duration, and atrial myocyte action potentials, explaining predisposition to arrhythmias in animal and human models.58 High alcohol consumption has direct toxic, inflammatory, and oxidative effects on LA myocardium. In the FHS, alcohol consumption predicted LA size enlargement and incident AF.59 Alcohol promotes LV remodeling and increases LV pressures facilitating diastolic dysfunction.60 A recent study demonstrated that abstinence or substantial reduction of alcohol intake was associated with fewer AF recurrences in regular drinkers.61 Besides the elimination of direct proarrhythmic effects, the results could be explained by weight reduction. Due to its high energy content (7 kcal/g), unrestricted alcohol intake may lead to weight gain and hypertension, contributing further to AF initiation.
Therefore, alcohol restriction or abstinence should be considered as one of the potentially effective strategies for AF prevention.
2.2.5. Obesity
The prevalence of overweight and obesity have increased significantly over the last decades worldwide with significant impact on public health with reduced quality of life and high medical costs.62 It is expected that by 2030 ~38% of the world’s adult population will be obese.63 However, not only measurements of weight at a single point in time, but also dynamic weight changes are associated with higher AF risk compared with stable body weight.64 Interestingly, body mass index (BMI) gain in later life posed higher AF risk than that during younger years.
A causal role of obesity for AF has been supported by a Mendelian randomization study, which demonstrated that a genetic risk score comprised of 39 polymorphisms associated with BMI were associated with AF.65 Sustained obesity is associated with hypertension, DM, metabolic syndrome, CHD, and obstructive sleep apnea, which provide the substrate for atrial remodeling and contribute to AF initiation and perpetuation (Figure 2).63 There are robust associations between weight gain and electro-anatomical remodeling,66 enhanced neurohormonal activation modulating LA enlargement and electrical instability.67 Furthermore, obesity is related to low-grade inflammation and greater epicardial fat thickness, which impair atrial electrophysiology.68 Some studies indicate that obesity may have a direct influence on myocardial structure via increased oxidative stress.69
Figure 2. Relationship between physical activity, obesity and lean body mass in AF.
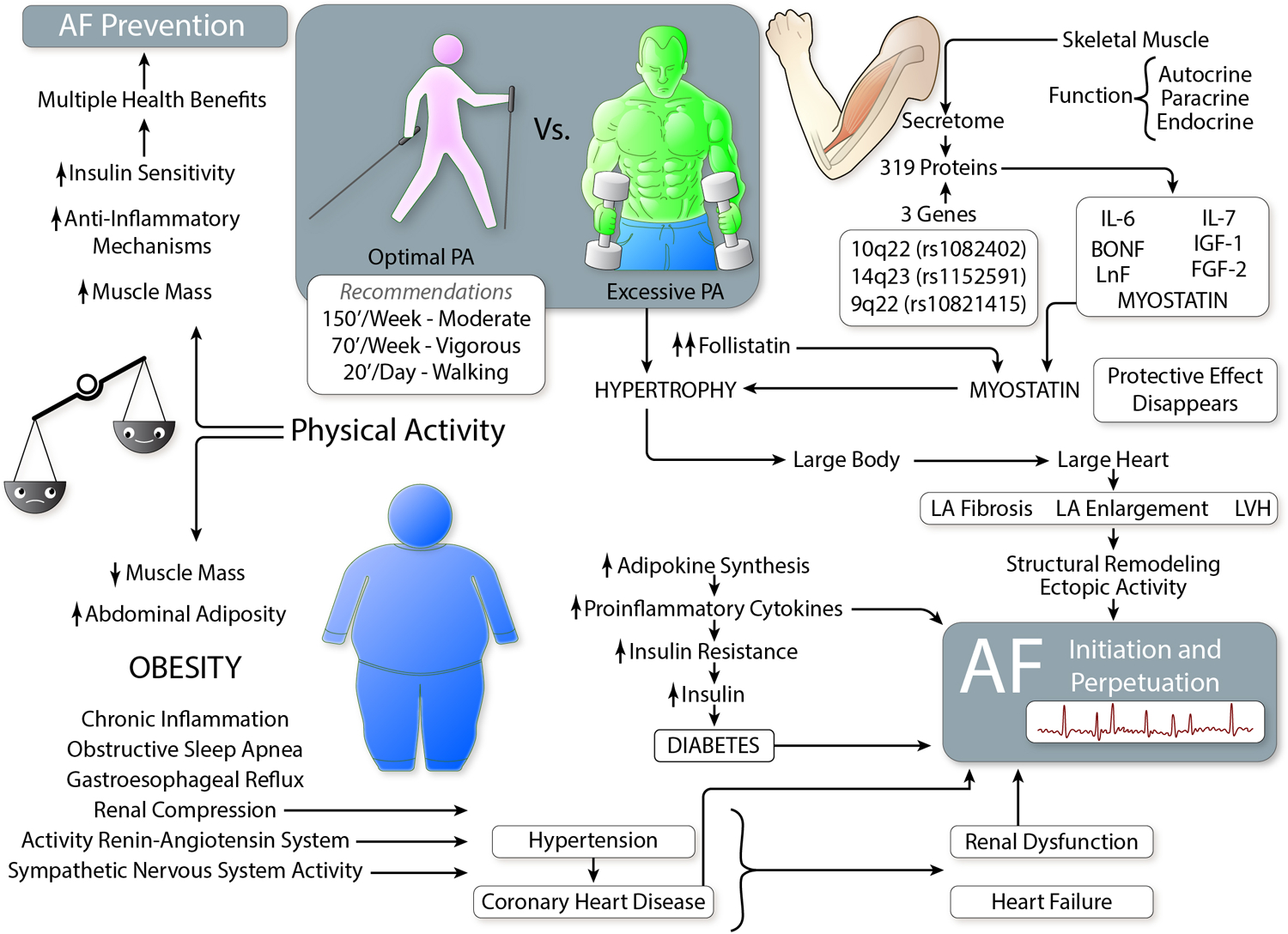
Moderate physical activity (PA) should be recommended for all AF prevention levels – primordial, primary, and secondary. Low and extreme PA predisposes to obesity (fat) or excessive muscle (lean body) mass, respectively. Through complex pathophysiological mechanisms, both are risk factors for AF. (Illustration credit: Ben Smith)
Weight variability >5% is another factor associated with an almost two-fold risk of AF recurrence.70 Importantly, weight regain during weight cycling leads to rapid adipose tissue growth and metabolic shifts facilitating lipid storage,71 which are associated with AF risk.
Finally, there are intriguing findings indicating that AF risk is primarily associated with high lean body mass (e.g. fat-free mass), and that fat tissue itself contributes remotely to AF development.72,73 (Figure 2) One possible explanation are skeletal muscles, which are a secretory organ distinguished by production and release of diverse cytokines and peptides with endocrine effects.74 Muscle activity promotes liver synthesis of follistatin. Follistatin is an inhibitor of myostatin, which is involved in metabolic homeostasis, influencing adipose tissue function and cardiac hypertrophy.74,75 Thus, inhibition of myostatin was associated with ventricular hypertrophy, atrial enlargement, atrial fibrosis, and spontaneous AF.75
2.2.6. Physical activity
Regular, moderate physical activity (PA) is a cornerstone of a healthy lifestyle (Figure 2). It is inversely and independently associated with clinical AF incidence and progression, and several studies indicate beneficial effects for AF prevention in individuals pursuing regular PA.76,77 Among the multifactorial beneficial effects of moderate PA are attenuation of many of the cardiovascular consequences related to obesity as insulin resistance, dyslipidemia, endothelial dysfunction, and reduced blood pressure.78 In overweight and obese individuals, moderate PA reduces systemic inflammation independently of weight loss, minimizing atrial arrhythmogenesis.79
Some investigators reported an association between moderate PA and decreased 80,81 AF risk, while vigorous PA increased AF risk.82 Although, a meta-analysis found that intermediate and high level PA was associated with lower risk for AF,83 there is a J-shaped relationship between exercise intensity and incident AF.84 In the Tromsø Study, compared with individuals without regular exercise history, individuals with moderate PA had a 28% lower AF risk.85
In contrast to moderate PA, a high volume of endurance exercise increases the risk of AF in elite athletes.86 The risk of AF development in athletes is 5-times higher than in referents.87 Some athletes may be more aware of their body and AF symptoms, possibly resulting in earlier diagnosis of AF. However, there are several underlying pathomechanisms explaining higher AF risk in athletes (Figure 3). Cardiac adaptation to vigorous exercise involves increased vagal tone, lower resting heart rate, and increased stroke volume, chamber dilatation, and hypertrophy, all of which may predispose to AF.88,89
Figure 3. Athletes’ heart and AF.
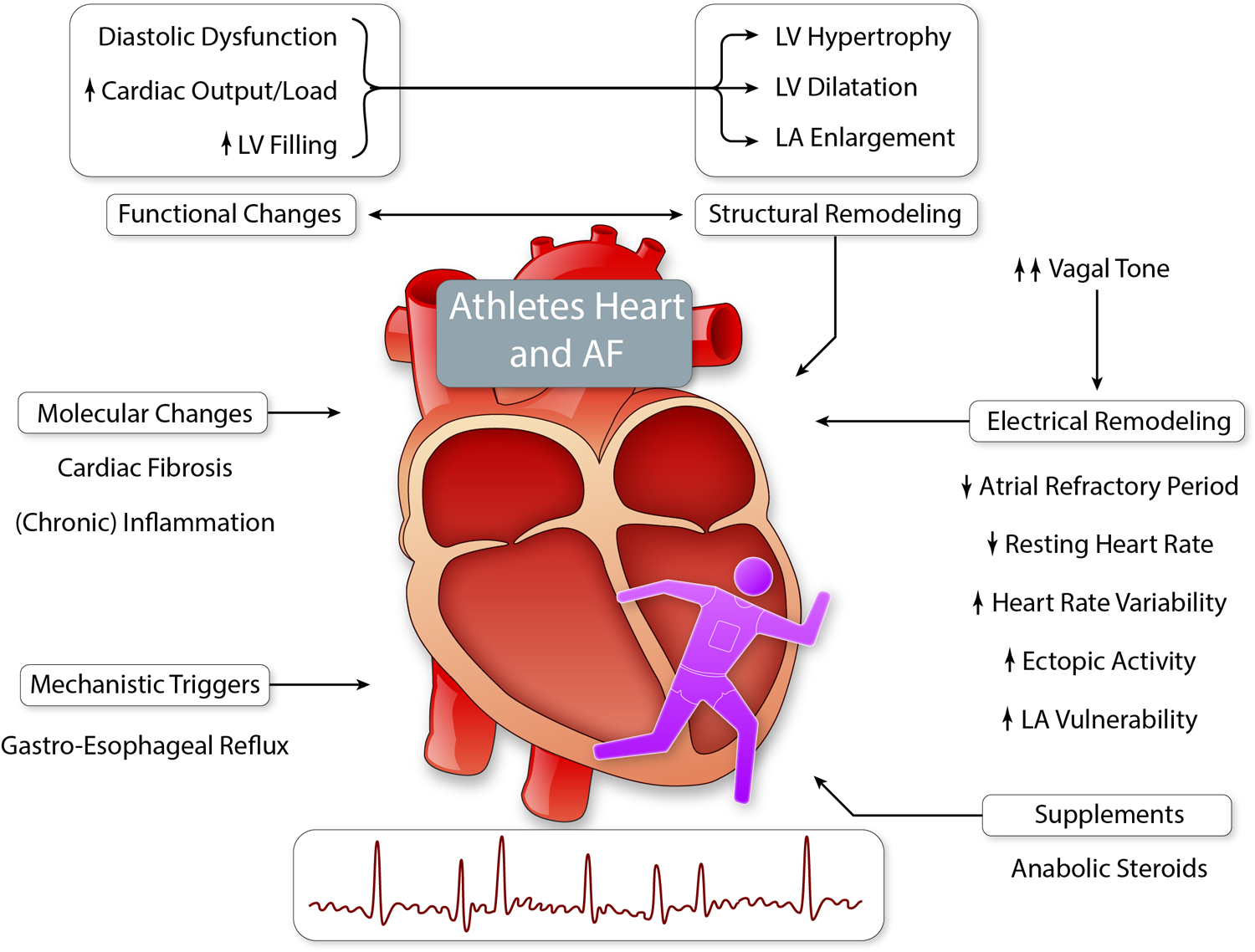
Cardiac adaptations to exercise are considered as beneficial, although vigorous physical activity and prolonged endurance exercise, may lead to cardiac ‘overadaptation’ or even ‘maladaptation’ and (patho)-physiological changes, which facilitate AF initiation and perpetuation. (Illustration credit: Ben Smith)
2.2.7. Psychological stress and psychosocial factors
The prevalence of chronic stress is ~8% in US, but in certain populations its exposure is ~40% (e.g., military deployment, sexual assault, natural disaster, gun violence).90 In a nationwide study with >1 million young veterans (median age 27 years), posttraumatic stress disorder (PTSD) was associated with 13% higher risk of incident AF.31 Pathophysiologicaly, psychosocial stress might lead to dysregulations in autonomic tone, hormonal imbalance, and catecholamine overload resulting in the alteration of LA electrophysiology91 and facilitating atrial fibrosis formation.92,93 Furthermore, there is evidence that chronic changes in autonomic tone impair atrial electrophysiological pattern and facilitate AF initiation.94 Chronic psychological stress including work-related stress as well as depression, anger, anxiety, and sleep deprivation are linked to the metabolic syndrome and its components,95–98 and associated with unhealthy behavior. The prevalence is higher in individuals with lower employment category or socioeconomic level, and in those with continuous stress at work.96,99 Also, psychological stress leads to sleep disorder including disturbances in sleep-wake cycle and sleep patterns with consequent, malfunction of the hypothalamic-pituitary-adrenal axis.100,101 Sleep deprivation impairs the physiological balance in circadian cortisol concentrations, which results in increased sympathetic nervous system activity102 and decreased vagal activity.91
The role of stress reduction for AF prevention has been incompletely studied, partly because of the potential for residual confounding. There is evidence that relaxation strategies including prayer, yoga, and meditation transiently modify indices of autonomic activation,103 and improve quality of life in AF patients.104 The role of stress reduction to prevent incident AF is less certain. Since stress is an exposure that may change over time, repeated measurements in longitudinal studies with extended follow-up would advance the field.
Future directions in primary AF prevention:
Overall there remain research needs to characterize optimal primordial AF prevention measures (PA, body composition, nutrition) and test implementation strategies targeted to specific populations.
For this aim improvement of AF study populations (e.g., inclusion of young individuals, large multi-ethnic/racial cohorts, long follow-up for lifetime risk analyses) is required to close the remaining, significant knowledge gaps.
Understudied external exposures with many unanswered questions are social determinants, geographic residential environment (rural vs urban), neighborhood and neighborhood-specific factors (as pollution, socioeconomic status). Also, the role of health literacy remains a challenging condition in vulnerable groups and should be addressed in future epidemiologic research.
3. Comorbidities of AF and their impact on prognosis
AF is associated with increased mortality. Patients generally do not die from the arrhythmia, but of accompanying comorbidities and complications, e.g., heart failure (HF), myocardial infarction (MI), chronic kidney disease (CKD), venous thromboembolism (VTE), stroke, dementia, and cancer. Beyond shared risk factors, there are multiple direct causal interactions between AF and its comorbidities,105 resulting in an interdependence in disease development (Figure 4).
Figure 4. Association between AF and system diseases.
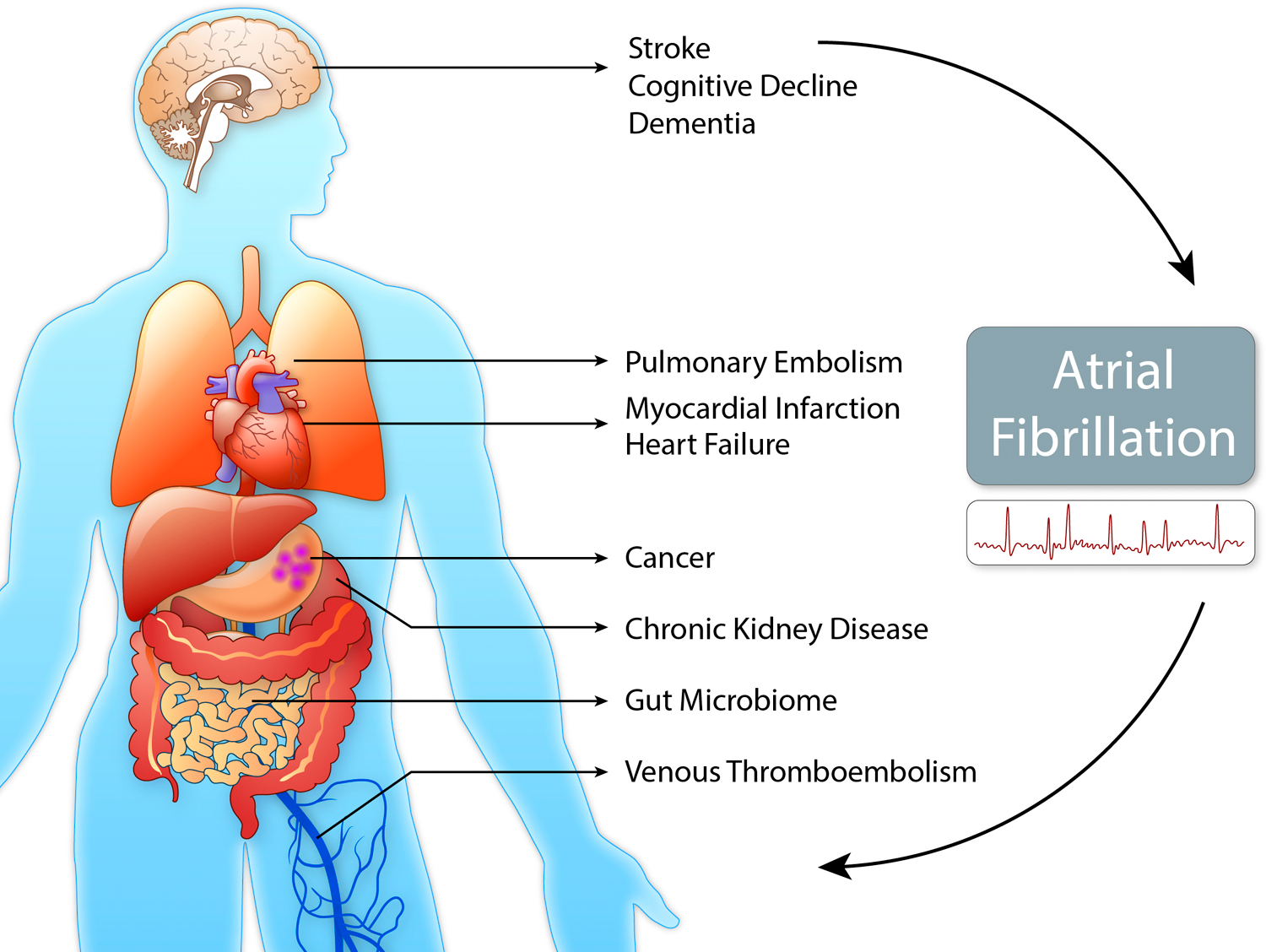
Atrial fibrillation (AF) is a multisystem disorder with complex relations to associated diseases, risk factors and the environment. An improved comprehension of this interplay may help improve risk assessment and management of AF and its comorbidities in the future. (Illustration credit: Ben Smith)
3.1. Heart failure
HF is closely related to AF and their coexistence is associated with substantially increased morbidity and mortality.106,107 Both AF and HF are associated with increased incidence of the other disease suggesting a bidirectional relationship. In a subset of FHS participants, who developed new AF, 37% had previously diagnosed HF. Vice versa, 57% of participants developing HF had prevalent AF.107 Incidence of both diseases increases steeply after age 60 years.16,108
In contrast to the general population, AF development is 4- to 6-times higher in HF patients.40 The most important underlying pathological feature seems to be LA vulnerability caused by structural and electrical remodeling as shown by electrophysiological mapping in HF patients.109 Also, the RAS, which is upregulated compensatorily in HF patients, contributes to the development of AF in HF patients. The prevalence of AF in HF patients is related to the severity of HF and rises from <10% in NYHA class I of >50% in NYHA class IV.108 Individuals with HF with preserved ejection fraction (HFpEF) are particularly at risk.107,110 Up to two thirds of patients with HFpEF develop AF simultaneously or close after the diagnosis, and in HFpEF AF is associated with a particularly adverse prognosis.110
HF becomes manifest in ~50% of AF patients.106 Modifiable cardiovascular risk factors strongly influence the risk of developing HF and their optimized management may decrease risk,111 but a strong evidence base to prevent HF in AF remains to be defined. Direct effects of AF such as irregular heart rate, shortened diastole, and loss of atrial contraction directly result in declines in cardiac output contributing to the development of HF.112 However, there are also long-term effects of AF, also referred to as AF-mediated cardiomyopathy.113 The resulting LV dysfunction is largely reversible after heart rate is controlled.114 However, an increased presence of diffuse fibrosis and cardiomyocyte apoptosis was described,115 which may explain the strong association between previous AF and HFpEF.116
3.2. Myocardial infarction
In AF patients, the risk of MI is ~2-fold increased.117 Conversely, MI is associated with increased incidence of AF, especially in the acute phase.118 Considering the frequent occurrence of asymptomatic AF, real incidence-rates may be much higher. The twelve-month incidence rate of AF in a post-MI trial monitoring patients with implantable cardiac devices was 32%.119
Not only common cardiovascular risk factors, but also several direct interactions explain the bidirectional relationship between both diseases. Tachyarrhythmic episodes may directly result in type-2 MI due to insufficient coronary artery perfusion.120 Furthermore, AF may induce MI by coronary thromboembolism, which accounts for ~3% of MI cases.121 Likewise, there are several mechanisms of how MI promotes the development of AF. In the acute phase after MI, risk factors for AF development include LV dysfunction, LV hypertrophy, and elevated heart rate.122 Atrial ischemia may cause early-onset AF after MI.123 Also, atrial stretching as a result of acute HF after MI may increase atrial excitability,124 and infarction-related pericarditis has been described as direct cause of AF.125 Further pathophysiologic links between AF and MI are systemic inflammation and endothelial dysfunction, which predispose both diseases. Otherwise, there is evidence that both AF and MI further aggravate cytokine release and systemic inflammation, eventually facilitating development of the other disease.126,127
The co-occurrence of MI and AF is associated with increased mortality of approximately 40%.128 Particularly at risk are patients with new-onset AF after MI, which is associated with a 87% higher mortality compared to permanent AF.129 An increased vulnerability for ventricular arrhythmias and an aggravation of ischemia by hemodynamic alterations have been discussed as possible explanations.130
3.3. Chronic kidney disease
Albuminuria, mild renal impairment, and declining renal function are associated with higher AF incidence.131,132 In a Japanese cohort, patients with glomerular filtration rate (GFR) of 30–59 ml/min had 32% higher risk AF compared to individuals with normal renal function. For those with GFR <30 ml/min the risk was 57% higher.132
AF and CKD share similar risk factors, but their association remains significant in individuals without hypertension or diabetes.132 A common pathophysiologic pathway is the activation of the RAS. In CKD patients, RAS activation eventually results in fibrogenesis, oxidative stress, and a downward spiral of kidney function. In AF, similar mechanisms have been described, leading to atrial fibrosis, increased atrial pressure, and modulation of ion channels.133 Likewise, an association with systemic inflammation has been described for both diseases.127 However, AF may also directly contribute to development of CKD by reduced cardiac output or thromboembolism.
The co-occurrence of AF and CKD is associated with an adverse prognosis. In patients with renal impairment, AF is associated with higher risk for HF, MI, and all-cause mortality.134 In a single-center study, patients with end-stage renal disease and AF had a 4-year mortality rate of 81%, compared to 29% in patients without AF.135
3.4. Venous thromboembolism
VTE and AF appear as distinct diseases, but are closely related to each other, often co-occur, and share multiple pathophysiological features. Both, incidence of AF after the diagnosis of VTE and incidence of VTE after the diagnosis of AF, are ≥70% higher compared to the general population. Within first six month after diagnosis of AF or VTE individuals are susceptible for the other disease.136
In patients with pulmonary embolism (PE) development of AF may partly be caused by increased right cardiac pressure and dilation resulting in atrial structural remodeling.137 In addition, neurohormonal mechanisms, like the release of 5-hydroxytryptamine by activated platelets, have been proposed. 138 More recently, hypercoagulability has been linked to induction of atrial fibrosis.139
As one possible cause of PE in AF patients, right-sided cardiac thrombus formation is assumed. This hypothesis is further supported by the fact, that mortality by PE is increased in patients with right heart thrombi.140 In addition, PE is more frequent in AF patients than deep vein thrombosis.141 AF itself has not only been associated with increased risk of pulmonary embolism but also of deep vein thrombosis,141 which implies more complex pathophysiological interactions of AF and VTE. The most important common denominators seem to be shared risk factors and comorbidities. Both, AF and VTE incidence are strongly age-dependent.142 As further risk factors of both diseases HF, obesity, sepsis, and autoimmune diseases have been identified.40,143–145 AF and VTE further have in common systemic inflammation with increased platelet activation, endothelial dysfunction and resulting in a prothrombotic state.
The co-occurrence of VTE and AF has serious prognostic implications and mortality is significantly elevated.146 This seems to account for both, prevalent AF and subsequent AF.147 However, it is unclear, whether AF is the cause of the increased mortality or only indicates a subset of patients with more comorbidities or more severe embolism.
3.5. Stroke
AF is associated with 4- to 5-fold increased risk of stroke, which also accounts for subclinical AF.36,148 Persistent forms of AF carry higher stroke risk compared to paroxysmal AF.149 Currently, the CHA2DS2-VASc score is the most widely used stroke risk score.150 However, there are several predictors, including obstructive sleep apnea151 and renal failure,152 which are not included in the score. Biomarkers such as high-sensitivity troponin T, N-terminal B-type natriuretic peptide, and growth differentiation factor-15 may improve performance of the current scoring systems.153
The underlying pathophysiological mechanisms of thrombus formation and stroke in AF include atrial fibrosis,154,155 atrial enlargement,156 and alterations of blood flow. Interestingly, new-onset AF is also increased after hemorrhagic stroke, which is not a direct result of AF.157 Pathophysiological mechanism may include dysregulation of autonomous nervous system and inflammation.158 Investigations on patients with implantable cardiac devices did not show a strong correlation between episodes of AF and onset of stroke, suggesting an association beyond thrombus formation.159 Both AF and stroke may indicate progressive CVD and represent risk factors for each other. A bidirectional temporal relationship of AF and stroke was confirmed in prospective community cohort studies.160
3.6. Dementia
Stroke in the setting of AF predisposes to dementia. Within five years, new-onset dementia was described in about one third of all stroke patients.161 In patients with AF, a meta-analysis revealed a 2.7-fold risk of dementia after first or recurrent stroke.162
However, there is an association between AF and dementia independent of stroke. Two meta-analyses revealed an approximately 30% increased risk of dementia in AF after adjustment for cerebrovascular events.162,163 Furthermore, AF is related to cognitive impairment or dementia in younger ages.164 In these studies, no brain imaging was performed to rule out clinically silent strokes as the underlying pathophysiology. In a case-referent study, that included MRI brain imaging, stroke-free individuals with AF showed difficulties in learning, memory, attention, and executive function compared to healthy referents.165
Non-ischemic mechanisms include cerebral hypoperfusion, vascular inflammation, and genetic factors. Cerebral hypoperfusion and hypoxia are mainly induced by AF-related HF, which appears in ~50% of AF patients.106 In the FHS, a relation between cardiac output and lower cognitive performance was described.166 In the Rotterdam Study, diastolic dysfunction was linked to stroke and dementia.167 Inflammatory markers such as C-reactive protein and interleukin-6 are elevated in AF and correlate with AF duration, success of cardioversion, and thrombogenesis.127,168 Inflammation itself is associated with cerebral microstructural changes and cerebral dysfunction and may be part of the common ground of atrial and cerebral disease.
3.7. Cancer
Even though cancer and AF frequently co-occur, their relation is understudied. The risk of newly diagnosed cancer in the first three months after new-onset AF is almost 3-fold increased. Similarly, newly diagnosed cancer is accompanied with a significantly increased risk of incident AF.169
Similar to the other AF comorbidities, shared risk factors may contribute to the co-occurrence of AF and cancer.170 The high incidence rate of cancer directly after diagnosis of AF may reflect improved detection of asymptomatic disease due to intensified medical examination. Furthermore, initiation of anticoagulation may trigger bleeding and consecutively lead to a cancer diagnosis.171
Conversely, there are multiple conceivable mechanisms how cancer might predispose to the development of AF. Thoracic cancer manifestations and surgery may induce AF by cardiac infiltration, inflammation, or mechanical disturbance.172 Likewise, radiotherapy, cytotoxic chemotherapy, targeted therapies, and high-dose corticosteroids have been associated with AF onset.173 The most notable chemotherapeutics associated with AF are alkylating agents, e.g. the incidence of AF with cisplatin is 15–32% and with anthracyclines is 10%. More recent developments in oncology like targeted therapies share similar problems. A prominent example is ibrutinib, a Bruton tyrosine kinase inhibitor, which is associated with LA remodeling. In patients treated with ibrutinib, AF incidence rates may reach 38%.174 Further pathophysiological links between cancer and AF include complications of cancer such as VTE, organ dysfunction, metabolic disorders, hypoxia, and systemic inflammation.93
The incidence of AF in cancer patients may predict unfavorable outcomes similar to the substantially increased risk of HF.175,176 However, the impact on overall mortality is uncertain. While in a large retrospective cohort of cancer patients with AF overall mortality was not increased,175 a smaller prospective cohort of lymphoma patients with new AF had nearly five-times higher mortality rate.176 In cancer patients with AF, disease management remains challenging and CVD is the primary cause of death not related to cancer.177 A predisposition to bleeding in cancer patients with AF has been reported.178
Future directions in secondary AF prevention:
A key research need in the understanding of AF and its frequent comorbidities is the disentangling of common and distinct pathophysiological pathways, their interactions and the identification of specific mechanisms that could be addressed for disease prevention in patients in whom comorbidities are prevalent.
4. Subclinical markers and risk prediction for incident AF
Many research groups have analyzed the prediction of incident AF using diverse clinical scores based on different combinations and weighting of classical risk factors (Online Table I). Biomarkers have been shown to increase accuracy of risk prediction. Biomarkers are objectively measurable and quantifiable markers of health and disease states. They include protein-based blood markers, cardiac imaging, or electrocardiographic markers, which can provide additional refinement to clinical risk stratification for identification of ‘high risk’ individuals for AF onset and complications. Electrocardiographic parameters such as PR interval and its indices represent atrial and atrioventricular conduction, therefore, association between pathological PR interval and AF seems to be appropriate.179 Multiple blood biomarkers – especially associated with cardiac damage and stress, cardiac pro-inflammatory and pro-fibrotic changes in particularly in the atria – have been analyzed in multiple observational and clinical studies to refine prediction of AF incidence and progression (Online Table II). Finally, recent developments and increasing availability of imaging tools including advanced echocardiography, computed tomography, and cardiac magnetic resonance imaging (cMRI) – have become a standard to assess risk profiles in AF, but may also contribute to risk prediction for imminent AF. The easily quantifiable echocardiographic antero-posterior LA diameter is a known indicator of AF progression,180 cMRI studies indicated the importance of anatomical and functional LA changes in AF.181,182
5. Next steps toward precision AF management and prevention
5.1. Omics
Recent advances in high-throughput technologies will accelerate our understanding of AF. Integrated approaches combining genomic, epigenomic, transcriptomic, proteomic, metabolomic, and microbiome data offer the opportunity to further define the molecular framework of AF and have already brought new insights.
Genetics and genomics have come closest to clinical practice. In familial or early-onset forms of AF several single rare genetic variants with large effect size in AF development have been reported over the last decades. These variants often encode ion channel or sarcomere protein components and provide a substrate for re-entry or early and delayed after-depolarization leading to AF.183,184 The reported mutations appear to be infrequent and rare variants appear to account for a relatively small proportion of genetically-determined AF in the general population. The genetic variants associated with AF in individuals of non-European ancestry also are largely unknown.
Over the last few years genome-wide association studies have revealed a polygenic basis with common genetic variations in over 100 loci associated with a modification of AF risk. 185,186 Polygenic scores based on common genetic variation may improve risk stratification beyond cardiovascular risk factors.187 The majority of these common genetic variants are located in regulatory, noncoding-regions of genes enriched within the transcriptional regulation, development and signaling pathways of electrophysiological, contractile and structural characteristics of cardiomyocytes. Many of these genes have previously been associated with other medical conditions, such as cardiovascular or musculoskeletal diseases. These massive genotyping efforts provide many candidate loci for AF and lay the ground for further mechanistic investigation and therapeutic targets.
Other ‘omics analyses also revealed new insights. Proteomic profiling recently identified eight proteins associated with incident AF, while their causal relation to AF development remains unclear.188 On the epigenetic level, a methylome-wide association study of AF revealed seven methylation signatures associated with prevalent or incident AF.189 By combining phenomic, metabolic, and genomic data, potassium, sodium ion, chitin, benzo[a]pyrene-7,8-dihydrodiol-9,10-oxide, and celebrex were found to be the five most important AF-related metabolites.190 Moreover, proton nuclear magnetic resonance spectroscopy revealed ketone body metabolism as a central step in persistent AF supported by proteomic analyses.191 In this study, metabolic profiles correctly predicted postoperative AF in >80% of patients undergoing cardiac surgery. Yet to be discovered is the role of the microbiome, its composition and quantity, in predicting AF. First evidence suggests an association of the gut microbiome in the AF development.192 Another aspect that has to be taken into account in a holistic approach is the so-called exposome including environmental influences, living circumstances, and biopsychological aspects.
Until now, most ‘omic studies have been small and remained without external validation. The step towards clinical implementation still must be taken. The hope is that better understanding of the triggers and mechanisms underlying AF may help define targets for prevention and treatment.
Future directions for translational research:
In the future, ‘omics-analyses may represent an important step towards AF precision medicine if ways for translational implementation of genetics and omics findings in clinical care with sufficient precision and justifiable costs can be defined.
5.2. eHealth and artificial intelligence
Health promotion and maintenance takes part largely outside the clinical setting. Significant advances are expected from technological developments known as electronic or mobile health (mHealth). A dynamic increase in the availability of mobile devices, wearable sensors, and software applications (apps) allow close health and disease monitoring.193 Following a general trend, mHealth has gained attention in cardiovascular prevention and diagnostics of AF.194,195 mHealth can be helpful to control and modify diverse risk factors associated with AF (e.g., PA, weight management, blood pressure, diabetes control, medication adherence). Also, it can help with AF detection (arrhythmic pulse, electrocardiograms). In patients with asymptomatic and/or paroxysmal AF with short episodes, mHealth gives the opportunity to improve early diagnosis of AF, with the potential to reduce future hospitalizations, morbidity, and mortality. However, overdiagnosis (and consequently overtreatment) is becoming a growing problem in contemporary medicine, partly due to rising influence of technical improvements. Detection of false-positive AF detection or ascertainment of very short self-limited AF may precipitate a testing and treatment cascade, which may significantly affect individuals’ lives.196 The risks of overdiagnosis are balanced against the thousands patients with undiagnosed cerebrovascular complications related to AF are associated with explosive health care costs.197
Alternatives for rhythm monitoring are smartphones and smartwatches, which have become popular across all population groups. These devices and wearable fitness trackers recognize arrhythmia pattern using AI systems.198 Deep learning algorithms monitor digital pulse waveforms and detect non-uniform and irregular heartbeats. Notifications permit timely medical consultation, further testing, and potential diagnosis of AF. Many evidence gaps remain, including whether high-risk individuals use such applications, whether wearable-detected AF carries a high enough risk to require anticoagulation, in particular, if only short episodes are present, whether anxiety and over-use of medical care are consequences of false positives, resulting in questions regarding whether the use of such devices for AF screening is efficient at the population level.
In the era of rapid advances of artificial intelligence (AI) in cardiovascular medicine, new methods of almost hypothesis-free interrogation of big data sets for the identification of AF predictors carry the potential to render AF screening and targeted treatment more efficient. AI structures large amounts of data, often instantaneously, and improves algorithms autonomously and may facilitate processes along the public health continuum from primordial to primary and secondary prevention of AF by increasing effectiveness and efficiency of diagnosis, screening, and treatment. Using automated learning algorithms machine learning (ML) represents an approach to generate unique prediction tools in primary care, to recognize high-risk individuals for AF, as well as guide physicians and patients managing AF.199
More comprehensive use of available information from multiple distinct sources, e.g., electronic health records, imaging, genetic, mHealth, etc. may promote understanding of disease patterns and permit the definition of clinically relevant AF subtypes. In initially healthy individuals in the deep-phenotyped MESA cohort, ML was able to improve predictive accuracy for AF.200 ML can select clinical and biomarker variables indicating prevalent AF.201 Recently, a ML data-driven approach assisted identification of multilevel interactions between clinical and ECG variables without pre-defined theoretical connections.202 ML was able to predict incident AF with high accuracy using ECG analysis with documented sinus rhythm. Nevertheless, external validation of these findings and demonstration of clinical relevance is necessary. Also, there is a risk of a significant amount of data with little or no clinical relevance. As recently reported, although ML diagnosed subclinical AF more accurately than clinicians, predicted AF was neither associated with prolonged hospital stay, nor with mortality.203 In fact, there is a risk of an unnecessary (over)-treatment and treatment-related complications.204 A further cautionary note about the use of ML in AF research and clinical care is awareness that ML may amplify biases inherent in previously collected data.205
Future directions for digital health:
Major research needs are the assessment of the effectiveness of mHealth, definition of the best-suited target population, and adaption of mHealth tools and requirements for AF screening, detection, monitoring and treatment.
Major knowledge gaps and research needs comprise the expansion of AI multilevel application to AF risk prediction, AF subtype classification, and management guidance, e.g. decision support tools, and demonstrate generalizability outside the derivation samples.
Conclusions
The significantly increasing incidence, prevalence, and high lifetime risk render AF a relevant disease in the population with high morbidity, mortality, and significant health care costs. Identification and targeting modifiable risk factors might be considered as the most relevant investment for AF risk modulation, number of lives saved, and healthcare resources freed. Primordial, primary, and secondary AF prevention include interventions at personal, healthcare and societal levels. Recent achievements in biomarker, omics, mHealth, and AI research will be essential to refine AF risk prediction and communication and will help modify AF prevention and management.
Supplementary Material
Acknowledgments
Funding
This project has received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 838259) for JK; German Center for Cardiovascular Research (DZHK e.V.) (81651/100) for CSB; NHLBI: R01HL128914; 2R01 HL092577; 1R01 HL141434 01A1; 2U54HL120163; American Heart Association, 18SFRN34110082; Robert Wood Johnson Grant 74624 for EJB; and European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 648131), from the European Union`s Horizon 2020 research and innovation programme under the grant agreement no.847770 (AFFECT-EU), and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103) for RBS.
Footnotes
Conflicts of interest
Starting January 2020, Dr. Benjamin serves as an uncompensated member for the MyHeartLab Steering Committee. The MyHeartLab Study is a PI-initiated study from the University of California San Francisco: PI, Jeffrey Olgin, MD, through a research grant to UCSF from Samsung.
References
- 1.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, Hiatt WR, Bristow MR, Packer M, Wasserman SM, Braunstein N, Pitt B, DeMets DL, Cooper-Arnold K, Armstrong PW, Berkowitz SD, et al. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol. 2015;65:1567–82. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 8.Mou L, Norby FL, Chen LY, O’Neal WT, Lewis TT, Loehr LR, Soliman EZ, Alonso A. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status: ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 11.Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M, Cattarinussi A, D’Alfonso MG, Gradia C, Sgherzi B, Pracucci G, Piccardi B, Polizzi B, Inzitari D, National Research Program: Progetto FAILFAiI. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace. 2019. [DOI] [PubMed]
- 13.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–97. [DOI] [PubMed] [Google Scholar]
- 14.Dilaveris PE, Kennedy HL. Silent atrial fibrillation: epidemiology, diagnosis, and clinical impact. Clin Cardiol. 2017;40:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. [DOI] [PubMed] [Google Scholar]
- 17.Shen MJ, Arora R, Jalife J. Atrial Myopathy. JACC Basic Transl Sci. 2019;4:640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–45. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr., Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–6, 76 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2015 National Healthcare Quality and Disparities Report and 5th Anniversary Update on the National Quality Strategy. Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- 21.Soliman EZ, Alonso A, Goff DC Jr. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–56. [DOI] [PubMed] [Google Scholar]
- 22.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JD, Hu D, Heckbert SR, Alonso A, Dewland TA, Vittinghoff E, Liu Y, Psaty BM, Olgin JE, Magnani JW, Huntsman S, Burchard EG, Arking DE, Bibbins-Domingo K, Harris TB, et al. Genetic Investigation Into the Differential Risk of Atrial Fibrillation Among Black and White Individuals. JAMA Cardiol. 2016;1:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway DS, Lip GY. Ethnicity in relation to atrial fibrillation and stroke (the West Birmingham Stroke Project). Am J Cardiol. 2003;92:1476–9. [DOI] [PubMed] [Google Scholar]
- 26.Ethnic-Specific Normative Reference Values for Echocardiographic LA and LV Size, LV Mass, and Systolic Function: The EchoNoRMAL Study. JACC Cardiovasc Imaging. 2015;8:656–65. [DOI] [PubMed] [Google Scholar]
- 27.Bezzina CR, Shimizu W, Yang P, Koopmann TT, Tanck MW, Miyamoto Y, Kamakura S, Roden DM, Wilde AA. Common sodium channel promoter haplotype in asian subjects underlies variability in cardiac conduction. Circulation. 2006;113:338–44. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman MJ, Splawski I, Makielski JC, Tester DJ, Will ML, Timothy KW, Keating MT, Jones G, Chadha M, Burrow CR, Stephens JC, Xu C, Judson R, Curran ME. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm. 2004;1:600–7. [DOI] [PubMed] [Google Scholar]
- 29.Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78:1479–87. [DOI] [PubMed] [Google Scholar]
- 30.Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res. 2017;120:1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosman L, Lampert R, Ramsey CM, Dziura J, Chui PW, Brandt C, Haskell S, Burg MM. Posttraumatic Stress Disorder and Risk for Early Incident Atrial Fibrillation: A Prospective Cohort Study of 1.1 Million Young Adults. J Am Heart Assoc. 2019;8:e013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–68. [DOI] [PubMed] [Google Scholar]
- 33.Lawler PR, Hiremath P, Cheng S. Cardiac target organ damage in hypertension: insights from epidemiology. Curr Hypertens Rep. 2014;16:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol. 2015;66:943–59. [DOI] [PubMed] [Google Scholar]
- 36.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 37.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 38.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–54. [DOI] [PubMed] [Google Scholar]
- 39.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020:CIR0000000000000757–CIR0000000000000757. [DOI] [PubMed]
- 40.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 41.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Zhang Z, Zhao Y, Jiang N, Qiu J, Yang Y, Li J, Liang X, Wang X, Tse G, Li G, Liu T. Alogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Alleviates Atrial Remodeling and Improves Mitochondrial Function and Biogenesis in Diabetic Rabbits. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odegaard AO, Jacobs DR Jr., Sanchez OA, Goff DC Jr., Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of atrial fibrillation: A systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol. 2018:2047487318780435. [DOI] [PubMed]
- 48.Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, Yang B. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83:465–72. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Shi H, Zhang L, Pourrier M, Yang B, Nattel S, Wang Z. Nicotine is a potent blocker of the cardiac A-type K(+) channels. Effects on cloned Kv4.3 channels and native transient outward current. Circulation. 2000;102:1165–71. [DOI] [PubMed] [Google Scholar]
- 50.DeFilippis EM, Singh A, Divakaran S, Gupta A, Collins BL, Biery D, Qamar A, Fatima A, Ramsis M, Pipilas D, Rajabi R, Eng M, Hainer J, Klein J, Januzzi JL, et al. Cocaine and Marijuana Use Among Young Adults With Myocardial Infarction. J Am Coll Cardiol. 2018;71:2540–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF Jr., Sutherland PA, Vasan A, Lipinska I, Evans JC, Benjamin EJ. Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis. 2008;201:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alzahrani T, Pena I, Temesgen N, Glantz SA. Association Between Electronic Cigarette Use and Myocardial Infarction. Am J Prev Med. 2018;55:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P. Alcohol and incident atrial fibrillation - A systematic review and meta-analysis. Int J Cardiol. 2017;246:46–52. [DOI] [PubMed] [Google Scholar]
- 55.Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual Total Binge Drinks Consumed by U.S. Adults, 2015. Am J Prev Med. 2018;54:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281–9. [DOI] [PubMed] [Google Scholar]
- 57.Dixit S, Alonso A, Vittinghoff E, Soliman EZ, Chen LY, Marcus GM. Past alcohol consumption and incident atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. PLoS One. 2017;12:e0185228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasek M, Bebarova M, Christe G, Simurdova M, Simurda J. Acute effects of ethanol on action potential and intracellular Ca(2+) transient in cardiac ventricular cells: a simulation study. Med Biol Eng Comput. 2016;54:753–62. [DOI] [PubMed] [Google Scholar]
- 59.McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, Benjamin EJ, Marcus GM. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and Atrial Fibrillation: A Sobering Review. J Am Coll Cardiol. 2016;68:2567–2576. [DOI] [PubMed] [Google Scholar]
- 61.Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, Wong G, Nalliah C, Sugumar H, Wong M, Kotschet E, et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl J Med. 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 62.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33:673–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol. 2014;7:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chatterjee NA, Giulianini F, Geelhoed B, Lunetta KL, Misialek JR, Niemeijer MN, Rienstra M, Rose LM, Smith AV, Arking DE, Ellinor PT, Heeringa J, Lin H, Lubitz SA, Soliman EZ, et al. Genetic Obesity and the Risk of Atrial Fibrillation: Causal Estimates from Mendelian Randomization. Circulation. 2017;135:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 67.Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, Dahlof B, Devereux RB. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for endpoint reduction in hypertension trial. Hypertension. 2007;49:311–6. [DOI] [PubMed] [Google Scholar]
- 68.Lin YK, Chen YC, Chang SL, Lin YJ, Chen JH, Yeh YH, Chen SA, Chen YJ. Heart failure epicardial fat increases atrial arrhythmogenesis. Int J Cardiol. 2013;167:1979–83. [DOI] [PubMed] [Google Scholar]
- 69.Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23:67–74. [DOI] [PubMed] [Google Scholar]
- 70.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 71.Strohacker K, Carpenter KC, McFarlin BK. Consequences of Weight Cycling: An Increase in Disease Risk? Int J Exerc Sci. 2009;2:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fenger-Gron M, Vinter N, Frost L. Body mass and atrial fibrillation risk: Status of the epidemiology concerning the influence of fat versus lean body mass. Trends Cardiovasc Med. 2019. [DOI] [PubMed]
- 73.Worm MS, Bager CL, Blair JPM, Secher NH, Riis BJ, Christiansen C, Nielsen HB. Atrial fibrillation is associated with lean body mass in postmenopausal women. Sci Rep. 2020;10:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. [DOI] [PubMed] [Google Scholar]
- 75.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–901. [DOI] [PubMed] [Google Scholar]
- 76.Blum S, Aeschbacher S, Meyre P, Zwimpfer L, Reichlin T, Beer JH, Ammann P, Auricchio A, Kobza R, Erne P, Moschovitis G, Di Valentino M, Shah D, Schlapfer J, Henz S, et al. Incidence and Predictors of Atrial Fibrillation Progression. J Am Heart Assoc. 2019;8:e012554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, Manson JE, Albert CM, LaMonte MJ, Larson JC, Li W, Martin LW, Nassir R, Garcia L, Assimes TL, Tharp KM, Hlatky MA, Perez MV. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol (1985). 2005;99:1193–204. [DOI] [PubMed] [Google Scholar]
- 79.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–61. [DOI] [PubMed] [Google Scholar]
- 80.Everett BM, Conen D, Buring JE, Moorthy MV, Lee IM, Albert CM. Physical activity and the risk of incident atrial fibrillation in women. Circ Cardiovasc Qual Outcomes. 2011;4:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu W, Shen Y, Zhou Q, Xu Z, Huang L, Chen Q, Hong K. Association of Physical Fitness With the Risk of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Clin Cardiol. 2016;39:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ricci C, Gervasi F, Gaeta M, Smuts CM, Schutte AE, Leitzmann MF. Physical activity volume in relation to risk of atrial fibrillation. A non-linear meta-regression analysis. Eur J Prev Cardiol. 2018;25:857–866. [DOI] [PubMed] [Google Scholar]
- 85.Morseth B, Graff-Iversen S, Jacobsen BK, Jorgensen L, Nyrnes A, Thelle DS, Vestergaard P, Lochen ML. Physical activity, resting heart rate, and atrial fibrillation: the Tromso Study. Eur Heart J. 2016;37:2307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, Rebato C, Elosua R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–23. [DOI] [PubMed] [Google Scholar]
- 87.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–9. [DOI] [PubMed] [Google Scholar]
- 88.Wilhelm M Atrial fibrillation in endurance athletes. Eur J Prev Cardiol. 2014;21:1040–8. [DOI] [PubMed] [Google Scholar]
- 89.Guasch E, Benito B, Qi X, Cifelli C, Naud P, Shi Y, Mighiu A, Tardif JC, Tadevosyan A, Chen Y, Gillis MA, Iwasaki YK, Dobrev D, Mont L, Heximer S, et al. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. [DOI] [PubMed] [Google Scholar]
- 90.Sareen J Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taggart P, Boyett MR, Logantha S, Lambiase PD. Anger, emotion, and arrhythmias: from brain to heart. Front Physiol. 2011;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spragg D Left Atrial Fibrosis: Role in Atrial Fibrillation Pathophysiology and Treatment Outcomes. J Atr Fibrillation. 2013;5:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. [DOI] [PubMed] [Google Scholar]
- 94.Lampert R Behavioral influences on cardiac arrhythmias. Trends Cardiovasc Med. 2016;26:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–7. [DOI] [PubMed] [Google Scholar]
- 96.Golden SH, Williams JE, Ford DE, Yeh HC, Paton Sanford C, Nieto FJ, Brancati FL. Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2004;27:429–35. [DOI] [PubMed] [Google Scholar]
- 97.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26:986–9. [DOI] [PubMed] [Google Scholar]
- 98.Maes M Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. [DOI] [PubMed] [Google Scholar]
- 99.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Christensen MA, Dixit S, Dewland TA, Whitman IR, Nah G, Vittinghoff E, Mukamal KJ, Redline S, Robbins JA, Newman AB, Patel SR, Magnani JW, Psaty BM, Olgin JE, Pletcher MJ, et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marulanda-Londono E, Chaturvedi S. The Interplay between Obstructive Sleep Apnea and Atrial Fibrillation. Front Neurol. 2017;8:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. [DOI] [PubMed] [Google Scholar]
- 103.Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, Lagi A. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. BMJ. 2001;323:1446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko J, Vanga S, Dawn B. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol. 2013;61:1177–82. [DOI] [PubMed] [Google Scholar]
- 105.Bisbal F, Baranchuk A, Braunwald E, Bayes de Luna A, Bayes-Genis A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:222–232. [DOI] [PubMed] [Google Scholar]
- 106.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 107.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6a–13a. [DOI] [PubMed] [Google Scholar]
- 109.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–8. [DOI] [PubMed] [Google Scholar]
- 110.Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic PM, Maggioni AP, De Mora Martin M, Polonski L, Silva-Cardoso J, Amir O. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39:4277–4284. [DOI] [PubMed] [Google Scholar]
- 111.Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, Cook NR, Lee IM, Albert CM. Modifiable Risk Factors for Incident Heart Failure in Atrial Fibrillation. JACC Heart Fail. 2017;5:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viswanathan K, Daniak SM, Salomone K, Kiely T, Patel U, Converso K, Manning WJ, Silverman DI. Effect of cardioversion of atrial fibrillation on improvement in left ventricular performance. Am J Cardiol. 2001;88:439–41. [DOI] [PubMed] [Google Scholar]
- 113.Qin D, Mansour MC, Ruskin JN, Heist EK. Atrial Fibrillation-Mediated Cardiomyopathy. Circ Arrhythm Electrophysiol. 2019;12:e007809. [DOI] [PubMed] [Google Scholar]
- 114.Lee SH, Chen SA, Tai CT, Chiang CE, Wen ZC, Cheng JJ, Ding YA, Chang MS. Comparisons of quality of life and cardiac performance after complete atrioventricular junction ablation and atrioventricular junction modification in patients with medically refractory atrial fibrillation. J Am Coll Cardiol. 1998;31:637–44. [DOI] [PubMed] [Google Scholar]
- 115.Mueller KAL, Heinzmann D, Klingel K, Fallier-Becker P, Kandolf R, Kilias A, Walker-Allgaier B, Borst O, Kumbrink J, Kirchner T, Langer H, Geisler T, Schreieck J, Gramlich M, Gawaz M, et al. Histopathological and Immunological Characteristics of Tachycardia-Induced Cardiomyopathy. J Am Coll Cardiol. 2017;69:2160–2172. [DOI] [PubMed] [Google Scholar]
- 116.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
- 117.Soliman EZ, Lopez F, O’Neal WT, Chen LY, Bengtson L, Zhang ZM, Loehr L, Cushman M, Alonso A. Atrial Fibrillation and Risk of ST-Segment-Elevation Versus Non-ST-Segment-Elevation Myocardial Infarction: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;131:1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laurent G, Zeller M, Dentan G, Moreau D, Laurent Y, Beer JC, Makki H, Lhuillier I, Janin-Manificat L, Fraison M, Cottin Y. Prognostic impact of new onset atrial fibrillation in acute non-ST elevation myocardial infarction data from the RICO survey. Heart. 2005;91:369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jons C, Jacobsen UG, Joergensen RM, Olsen NT, Dixen U, Johannessen A, Huikuri H, Messier M, McNitt S, Thomsen PE. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm. 2011;8:342–8. [DOI] [PubMed] [Google Scholar]
- 120.Gupta S, Vaidya SR, Arora S, Bahekar A, Devarapally SR. Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta-analysis of observational studies. Cardiovasc Diagn Ther. 2017;7:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shibata T, Kawakami S, Noguchi T, Tanaka T, Asaumi Y, Kanaya T, Nagai T, Nakao K, Fujino M, Nagatsuka K, Ishibashi-Ueda H, Nishimura K, Miyamoto Y, Kusano K, Anzai T, et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation. 2015;132:241–50. [DOI] [PubMed] [Google Scholar]
- 122.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–74. [DOI] [PubMed] [Google Scholar]
- 123.Alasady M, Shipp NJ, Brooks AG, Lim HS, Lau DH, Barlow D, Kuklik P, Worthley MI, Roberts-Thomson KC, Saint DA, Abhayaratna W, Sanders P. Myocardial infarction and atrial fibrillation: importance of atrial ischemia. Circ Arrhythm Electrophysiol. 2013;6:738–45. [DOI] [PubMed] [Google Scholar]
- 124.Celik S, Erdol C, Baykan M, Kaplan S, Kasap H. Relation between paroxysmal atrial fibrillation and left ventricular diastolic function in patients with acute myocardial infarction. Am J Cardiol. 2001;88:160–2, a5. [DOI] [PubMed] [Google Scholar]
- 125.Nagahama Y, Sugiura T, Takehana K, Hatada K, Inada M, Iwasaka T. The role of infarction-associated pericarditis on the occurrence of atrial fibrillation. Eur Heart J. 1998;19:287–92. [DOI] [PubMed] [Google Scholar]
- 126.Parashar S, Kella D, Reid KJ, Spertus JA, Tang F, Langberg J, Vaccarino V, Kontos MC, Lopes RD, Lloyd MS. New-onset atrial fibrillation after acute myocardial infarction and its relation to admission biomarkers (from the TRIUMPH registry). Am J Cardiol. 2013;112:1390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–70. [DOI] [PubMed] [Google Scholar]
- 128.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Angeli F, Reboldi G, Garofoli M, Ramundo E, Poltronieri C, Mazzotta G, Ambrosio G, Verdecchia P. Atrial fibrillation and mortality in patients with acute myocardial infarction: a systematic overview and meta-analysis. Curr Cardiol Rep. 2012;14:601–10. [DOI] [PubMed] [Google Scholar]
- 130.Berton G, Cordiano R, Cucchini F, Cavuto F, Pellegrinet M, Palatini P. Atrial fibrillation during acute myocardial infarction: association with all-cause mortality and sudden death after 7-year of follow-up. Int J Clin Pract. 2009;63:712–21. [DOI] [PubMed] [Google Scholar]
- 131.Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R, Katz R, Kestenbaum B, Mathew J, Robinson-Cohen C, Sarnak MJ, Shlipak MG, Sotoodehnia N, Young B, Heckbert SR. eGFR and Albuminuria in Relation to Risk of Incident Atrial Fibrillation: A Meta-Analysis of the Jackson Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study. Clin J Am Soc Nephrol. 2017;12:1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158:629–36. [DOI] [PubMed] [Google Scholar]
- 133.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–8. [DOI] [PubMed] [Google Scholar]
- 134.Massicotte-Azarniouch D, Kuwornu JP, Carrero JJ, Lam NN, Molnar AO, Zimmerman D, McCallum MK, Garg AX, Sood MM. Incident Atrial Fibrillation and the Risk of Congestive Heart Failure, Myocardial Infarction, End-Stage Kidney Disease, and Mortality Among Patients With a Decreased Estimated GFR. Am J Kidney Dis. 2018;71:191–199. [DOI] [PubMed] [Google Scholar]
- 135.Vazquez E, Sanchez-Perales C, Lozano C, Garcia-Cortes MJ, Borrego F, Guzman M, Perez P, Pagola C, Borrego MJ, Perez V. Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. Am J Cardiol. 2003;92:868–71. [DOI] [PubMed] [Google Scholar]
- 136.Lutsey PL, Norby FL, Alonso A, Cushman M, Chen LY, Michos ED, Folsom AR. Atrial fibrillation and venous thromboembolism: evidence of bidirectionality in the Atherosclerosis Risk in Communities Study. J Thromb Haemost. 2018;16:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14:209–14. [PubMed] [Google Scholar]
- 139.Spronk HM, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, Rienstra M, van Hunnik A, Kuiper M, Lumeij S, Zeemering S, Linz D, Kamphuisen PW, Ten Cate H, Crijns HJ, et al. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J. 2017;38:38–50. [DOI] [PubMed] [Google Scholar]
- 140.Koc M, Kostrubiec M, Elikowski W, Meneveau N, Lankeit M, Grifoni S, Kuch-Wocial A, Petris A, Zaborska B, Stefanovic BS, Hugues T, Torbicki A, Konstantinides S, Pruszczyk P. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J. 2016;47:869–75. [DOI] [PubMed] [Google Scholar]
- 141.Enga KF, Rye-Holmboe I, Hald EM, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T, Braekkan SK, Hansen JB. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J Thromb Haemost. 2015;13:10–6. [DOI] [PubMed] [Google Scholar]
- 142.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. [DOI] [PubMed] [Google Scholar]
- 143.Stein PD, Matta F, Goldman J. Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb Res. 2011;128:518–23. [DOI] [PubMed] [Google Scholar]
- 144.Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014;18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Svendsen JH, Torp-Pedersen C, Hansen PR. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;344:e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barra SN, Paiva LV, Providencia R, Fernandes A, Leitao Marques A. Atrial fibrillation in acute pulmonary embolism: prognostic considerations. Emerg Med J. 2014;31:308–12. [DOI] [PubMed] [Google Scholar]
- 147.Ng AC, Adikari D, Yuan D, Lau JK, Yong AS, Chow V, Kritharides L. The Prevalence and Incidence of Atrial Fibrillation in Patients with Acute Pulmonary Embolism. PLoS One. 2016;11:e0150448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 149.Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M, Avezum A, Diaz R, Hohnloser SH, Lewis BS, Shestakovska O, Wang J, Connolly SJ. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36:281–7a. [DOI] [PubMed] [Google Scholar]
- 150.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 151.Yaranov DM, Smyrlis A, Usatii N, Butler A, Petrini JR, Mendez J, Warshofsky MK. Effect of obstructive sleep apnea on frequency of stroke in patients with atrial fibrillation. Am J Cardiol. 2015;115:461–5. [DOI] [PubMed] [Google Scholar]
- 152.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–32. [DOI] [PubMed] [Google Scholar]
- 153.Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA, Sritara P, Mercuri MF, Kamphuisen PW, Antman EM, Braunwald E, Giugliano RP. Efficacy and Safety of Edoxaban in Patients With Active Malignancy and Atrial Fibrillation: Analysis of the ENGAGE AF - TIMI 48 Trial. J Am Heart Assoc. 2018;7:e008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kottkamp H Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012;23:797–9. [DOI] [PubMed] [Google Scholar]
- 155.Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–41. [DOI] [PubMed] [Google Scholar]
- 157.van Bree MD, Roos YB, van der Bilt IA, Wilde AA, Sprengers ME, de Gans K, Vergouwen MD. Prevalence and characterization of ECG abnormalities after intracerebral hemorrhage. Neurocrit Care. 2010;12:50–5. [DOI] [PubMed] [Google Scholar]
- 158.Wang Y, Qian Y, Smerin D, Zhang S, Zhao Q, Xiong X. Newly Detected Atrial Fibrillation after Acute Stroke: A Narrative Review of Causes and Implications. Cardiology. 2019:1–10. [DOI] [PubMed]
- 159.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, Fain E, Nakamya J, Mairesse GH, Halytska M, Deng WQ, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 160.Camen S, Ojeda FM, Niiranen T, Gianfagna F, Vishram-Nielsen JK, Costanzo S, Soderberg S, Vartiainen E, Donati MB, Lochen ML, Pasterkamp G, Magnussen C, Kee F, Jousilahti P, Hughes M, et al. Temporal relations between atrial fibrillation and ischaemic stroke and their prognostic impact on mortality. Europace. 2019. [DOI] [PubMed]
- 161.Poggesi A, Inzitari D, Pantoni L. Atrial Fibrillation and Cognition: Epidemiological Data and Possible Mechanisms. Stroke. 2015;46:3316–21. [DOI] [PubMed] [Google Scholar]
- 162.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saglietto A, Matta M, Gaita F, Jacobs V, Bunch TJ, Anselmino M. Stroke-independent contribution of atrial fibrillation to dementia: a meta-analysis. Open Heart. 2019;6:e000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park H, Hildreth A, Thomson R, O’Connell J. Non-valvular atrial fibrillation and cognitive decline: a longitudinal cohort study. Age Ageing. 2007;36:157–63. [DOI] [PubMed] [Google Scholar]
- 165.Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, Heindel W, Breithardt G, Berger K, Ringelstein EB, Kirchhof P, Wersching H. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29:2125–32. [DOI] [PubMed] [Google Scholar]
- 166.Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am J Cardiol. 2011;108:1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Bruijn RF, Portegies ML, Leening MJ, Bos MJ, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW, Franco OH, Koudstaal PJ, Ikram MA. Subclinical cardiac dysfunction increases the risk of stroke and dementia: the Rotterdam Study. Neurology. 2015;84:833–40. [DOI] [PubMed] [Google Scholar]
- 168.Phillips KP. Role of Inflammation in Initiation and Perpetuation of Atrial Fibrillation: A Systematic Review of the Published Data. J Atr Fibrillation. 2013;6:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM. Risk of Malignant Cancer Among Women With New-Onset Atrial Fibrillation. JAMA Cardiol. 2016;1:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding--could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med. 2014;46:672–8. [DOI] [PubMed] [Google Scholar]
- 172.Beck-Nielsen J, Sorensen HR, Alstrup P. Atrial fibrillation following thoracotomy for non-cardiac diseases, in particular cancer of the lung. Acta Med Scand. 1973;193:425–9. [DOI] [PubMed] [Google Scholar]
- 173.Tamargo J, Caballero R, Delpon E. Drug-induced atrial fibrillation. Expert Opin Drug Saf. 2012;11:615–34. [DOI] [PubMed] [Google Scholar]
- 174.Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, Escudier M, Ammar C, Gaubert M, Dolladille C, Barraud J, Peyrol M, Cohen A, Paganelli F, Alexandre J, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6:e001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu YF, Liu CJ, Chang PM, Tsao HM, Lin YJ, Chang SL, Lo LW, Tuan TC, Li CH, Chao TF, Chung FP, Liao JN, Chen TJ, Chen SA. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–7. [DOI] [PubMed] [Google Scholar]
- 176.Amioka M, Sairaku A, Ochi T, Okada T, Asaoku H, Kyo T, Kihara Y. Prognostic Significance of New-Onset Atrial Fibrillation in Patients With Non-Hodgkin’s Lymphoma Treated With Anthracyclines. Am J Cardiol. 2016;118:1386–1389. [DOI] [PubMed] [Google Scholar]
- 177.Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, Meyer JE. Causes of death among cancer patients. Ann Oncol. 2017;28:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Melloni C, Shrader P, Carver J, Piccini JP, Thomas L, Fonarow GC, Ansell J, Gersh B, Go AS, Hylek E, Herling IM, Mahaffey KW, Yu AF, Peterson ED, Kowey PR, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes. 2017;3:192–197. [DOI] [PubMed] [Google Scholar]
- 179.Schumacher K, Dagres N, Hindricks G, Husser D, Bollmann A, Kornej J. Characteristics of PR interval as predictor for atrial fibrillation: association with biomarkers and outcomes. Clin Res Cardiol. 2017;106:767–775. [DOI] [PubMed] [Google Scholar]
- 180.D’Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, Natale A, Hunter RJ, Schilling RJ, Miyazaki S, Tada H, Aonuma K, Yenn-Jiang L, Tao H, Ma C, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. 2013;167:1984–9. [DOI] [PubMed] [Google Scholar]
- 181.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 182.Seewoster T, Spampinato RA, Sommer P, Lindemann F, Jahnke C, Paetsch I, Hindricks G, Kornej J. Left atrial size and total atrial emptying fraction in atrial fibrillation progression. Heart Rhythm. 2019. [DOI] [PubMed]
- 183.Christophersen IE, Ellinor PT. Genetics of atrial fibrillation: from families to genomes. J Hum Genet. 2016;61:61–70. [DOI] [PubMed] [Google Scholar]
- 184.Choi SH, Weng LC, Roselli C, Lin H, Haggerty CM, Shoemaker MB, Barnard J, Arking DE, Chasman DI, Albert CM, Chaffin M, Tucker NR, Smith JD, Gupta N, Gabriel S, et al. Association Between Titin Loss-of-Function Variants and Early-Onset Atrial Fibrillation. JAMA. 2018;320:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, Mathis MR, Yamazaki M, Crawford RD, Gabrielsen ME, et al. Genome-wide association study of 1 million people identifies 111 loci for atrial fibrillation. bioRxiv. 2018:242149.
- 186.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, Arking DE, Barnard J, Bartz TM, Benjamin EJ, Bihlmeyer NA, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weng LC, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B, Trinquart L, McManus DD, Staerk L, Lin H, Lunetta KL, Ellinor PT, Benjamin EJ, Lubitz SA. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation. 2018;137:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ko D, Benson MD, Ngo D, Yang Q, Larson MG, Wang TJ, Trinquart L, McManus DD, Lubitz SA, Ellinor PT, Vasan RS, Gerszten RE, Benjamin EJ, Lin H. Proteomics Profiling and Risk of New-Onset Atrial Fibrillation: Framingham Heart Study. J Am Heart Assoc. 2019;8:e010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lin H, Yin X, Xie Z, Lunetta KL, Lubitz SA, Larson MG, Ko D, Magnani JW, Mendelson MM, Liu C, McManus DD, Levy D, Ellinor PT, Benjamin EJ. Methylome-wide Association Study of Atrial Fibrillation in Framingham Heart Study. Sci Rep. 2017;7:40377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yan ZT, Huang JM, Luo WL, Liu JW, Zhou K. Combined metabolic, phenomic and genomic data to prioritize atrial fibrillation-related metabolites. Exp Ther Med. 2019;17:3929–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, Ladroue C, Madhu B, Roberts N, De Souza A, Fredericks S, Stubbs M, Griffiths JR, Jahangiri M, Xu Q, et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–94. [DOI] [PubMed] [Google Scholar]
- 192.Yu L, Meng G, Huang B, Zhou X, Stavrakis S, Wang M, Li X, Zhou L, Wang Y, Wang M, Wang Z, Deng J, Po SS, Jiang H. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92–98. [DOI] [PubMed] [Google Scholar]
- 193.Eapen ZJ, Turakhia MP, McConnell MV, Graham G, Dunn P, Tiner C, Rich C, Harrington RA, Peterson ED, Wayte P. Defining a Mobile Health Roadmap for Cardiovascular Health and Disease. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Milani RV, Lavie CJ, Bober RM, Milani AR, Ventura HO. Improving Hypertension Control and Patient Engagement Using Digital Tools. Am J Med. 2017;130:14–20. [DOI] [PubMed] [Google Scholar]
- 195.Turakhia MP, Kaiser DW. Transforming the care of atrial fibrillation with mobile health. J Interv Card Electrophysiol. 2016;47:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, Nagpal S, Saini V, Srivastava D, Chalmers K, Korenstein D. Evidence for overuse of medical services around the world. Lancet. 2017;390:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Turakhia MP, Shafrin J, Bognar K, Goldman DP, Mendys PM, Abdulsattar Y, Wiederkehr D, Trocio J. Economic Burden of Undiagnosed Nonvalvular Atrial Fibrillation in the United States. Am J Cardiol. 2015;116:733–9. [DOI] [PubMed] [Google Scholar]
- 198.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, Venugopalan S, Widner K, Madams T, Cuadros J, Kim R, Raman R, Nelson PC, Mega JL, Webster DR. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316:2402–2410. [DOI] [PubMed] [Google Scholar]
- 199.Hill NR, Ayoubkhani D, McEwan P, Sugrue DM, Farooqui U, Lister S, Lumley M, Bakhai A, Cohen AT, O’Neill M, Clifton D, Gordon J. Predicting atrial fibrillation in primary care using machine learning. PLoS One. 2019;14:e0224582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ambale-Venkatesh B, Yang X, Wu CO, Liu K, Hundley WG, McClelland R, Gomes AS, Folsom AR, Shea S, Guallar E, Bluemke DA, Lima JAC. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ Res. 2017;121:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, Fabritz L. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J. 2019;40:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S, Friedman PA. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019. [DOI] [PubMed]
- 203.Moss TJ, Calland JF, Enfield KB, Gomez-Manjarres DC, Ruminski C, DiMarco JP, Lake DE, Moorman JR. New-Onset Atrial Fibrillation in the Critically Ill. Crit Care Med. 2017;45:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Marcus PM, Prorok PC, Miller AB, DeVoto EJ, Kramer BS. Conceptualizing overdiagnosis in cancer screening. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed]
- 205.Parikh RB, Teeple S, Navathe AS. Addressing Bias in Artificial Intelligence in Health Care. JAMA. 2019. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


