Abstract
Objective:
This study aimed to do a genomewide transcriptomic profiling to develop a miRNA-based signature for the identification of peritoneal metastasis (PM) in patients with gastric cancer (GC).
Summary Background Data:
Even though PM in patients with GC has long been recognized to associate with poor survival, currently there is lack of availability of molecular biomarkers for its robust diagnosis.
Methods:
We performed a systematic biomarker discovery by analyzing miRNA expression profiles in primary tumors from GC patients with and without PM, and subsequently validated the expression of candidate miRNA biomarkers in three independent clinical cohorts of 354 patients with advanced GC.
Results:
Five miRNAs (miR-30a-5p, -134-5p, -337-3p, -659-3p, and -3917) were identified during the initial discovery phase; three of which (miR-30a-5p, -659-3p, and -3917) were significantly overexpressed in the primary tumors from PM-positive patients in the testing cohort (p=0.002, 0.04 and 0.007 respectively), and distinguished patients with vs. without peritoneal metastasis with the value of area under the curve (AUC) of 0.82. Furthermore, high expression of these miRNAs also associated with poor prognosis (HR=2.18, p=0.04). The efficacy of the combination miRNA-signature was subsequently validated in an independent validation cohort (AUC=0.74). Finally, our miRNA signature when combined together with the macroscopic Borrmann’s type score offered a much superior diagnostic in all three cohorts (AUC=0.87, 0.76, 0.79, respectively), and led us to establish a risk-prediction nomogram for the diagnosis of PM in GC patients.
Conclusions:
We have established a miRNA-based signature that have a potential to identify peritoneal metastasis in GC patients.
Keywords: biomarkers, gastric cancer, peritoneal metastasis, microRNAs, prognosis
MINI ABSTRACT
In this study, following a genomewide biomarker discovery and validation in multiple patient cohorts, we have identified a panel of miRNAs for the identification of peritoneal metastasis in patients with gastric cancer, and established a risk-probability model for its clinical application for a more personalized treatment approach for patients suffering from this malignancy.
INTRODUCTION
Gastric cancer (GC) is the fourth most common cause of cancer-related deaths worldwide [1]. In particular, GC patients with peritoneal metastasis (PM) have an exceptionally poor survival outcomes, which is further compounded by the lack of availability of effective treatments [2–5]. Currently, computed tomography (CT) or positron emission tomography (PET) are commonly used to diagnose presence of PM in GC patients, but the diagnostic sensitivity of these imaging modalities for identifying such metastatic lesions is quite inadequate [6, 7]
In this context, currently, staging laparoscopy is gaining increased clinical use for the detection of PM in GC patients, due to its improved diagnostic accuracy compared to CT or PET-CT [8–11]. In spite of its merits, the widespread implementation of staging laparoscopy as a routine modality in all GC patients has been hampered due to its invasive nature which requires general anesthesia, as well relatively higher costs [12]. Although several retrospective studies have shown the significance for the use of staging laparoscopy in detecting PM [13–16], there is still a lack of consensus agreement for its application in all GC patients with an advanced disease.
Considering that PM positive GC patients have a more aggressive disease, and it is well-recognized that its early detection can significantly improve patient outcomes; currently there are no molecular biomarkers used clinically to detect such metastases. Tumor markers, including CA125 and CA72-4 are frequently upregulated in patients with advanced GCs, and are also shown to be overexpressed in sera of patients with PM [17–19]. However, the challenge remains that individually or even as a combination, the diagnostic accuracy of these biomarkers for detecting PM remain inadequate [20]. Furthermore, if PM can be identified prior to the surgery, it can allow potentially more advanced treatment approaches including intra-peritoneal administration of paclitaxel [21, 22], or hyperthermic intraperitoneal chemotherapy (HIPEC) with cytoreductive surgery [23–26]. Although these treatments are yet not standardized and are only performed at limited institutions, accumulating evidence supports their significant therapeutic potential in improving outcomes in this subgroup of GC patients with PM [24, 27–31]. Therefore, availability of robust molecular biomarkers which could help facilitate identification of definitive PM in GC patients, could be clinically transformative as it will permit a timely intervention leading to reduction in the morbidity and mortality associated with this malignancy.
With the recent advances in next generation sequencing technologies, genomic as well as epigenomic profiling of various malignancies has allowed identification of previously unrecognized molecular biomarkers [32–34]. One such molecular substrates includes microRNAs (miRNAs), which are 18-25 nucleotides long single-stranded noncoding RNAs that act as post-transcriptional gene repressors [35, 36], and are frequently dysregulated in various human cancers including GC [37, 38]. More specifically, several miRNAs including miR-136, miR-203 and miR-3978, have been shown to be functionally involved in mediating PM in GC; however, their clinical significance as potential disease biomarkers for detecting such metastases has not been evaluated in large, adequately powered, independent patient cohorts [39–42].
Herein, we conducted a comprehensive miRNA expression profiling of primary GC tissues, with and without presence of PM. Subsequently, rigorous bioinformatic approaches were utilized to identify and prioritize key miRNAs as potential biomarkers for detecting PM, followed by their validation in multiple, independent cohorts of GC patients with advanced disease. Our efforts led to the identification and establishment of a novel miRNA-based signature that could be used to detect PM in GC patients.
MATERIALS AND METHODS
Study Design and patient specimens
The current study consisted of a systematic and comprehensive biomarker discovery and a validation phases. In the discovery phase, we generated microarray-based miRNA expression profiling results, followed by additional comparison in The Cancer Genome Atlas (TCGA) dataset, for the identification of candidate miRNAs that can detect PM in GC patients. Initially we identified differentially expressed miRNAs in the primary tumors from PM positive vs. negative patients (n=6, respectively), with a p value of <0.05 as an initial cut-off criteria in our miRNA-profiling data. Next, we analyzed TCGA dataset to identify miRNAs that were differentially expressed in the stage IV vs. other advanced GC patients. Subsequently, we selected miRNAs that were commonly dysregulated in both datasets of GC patients.
To evaluate the diagnostic accuracy of the miRNA biomarkers identified in the discovery phase, we examined their expression by TaqMan-based qRT-PCR assays in three independent cohorts of GC patients. The three clinical validation cohorts enrolled 354 gastric cancer patients, comprising of a testing cohort of 65 patients enrolled at the Mie University, a validation cohort of 85 patients from the Kumamoto University, and a performance evaluation cohort of 204 patients from the Nagoya University, Japan. The testing and validation cohorts included paraffin embedded tissues, while the performance evaluation cohort comprised of frozen tissues. Further information on patient demographics and clinicopathological characteristics are provided in the Table 1. A written informed consent was obtained from all patients, and the Institutional Review Boards of all participating institutions approved the study.
Table 1:
Clinicopathological characteristics of patients in each of the clinical cohorts
| Testing cohort | Validation cohort | Performance evaluation cohort | P Value | ||
|---|---|---|---|---|---|
|
| |||||
| Variable | number | number | number | ||
| Gender | Male | 40 | 62 | 158 | |
| Female | 25 | 23 | 46 | 0.04 | |
| Age at operation | median (range) | 69 (18-90) | 70 (25-92) | 69 (20-88) | 0.7 |
| Macroscopical type | type1, 2 | 31 | 43 | 100 | |
| type3,4 | 34 | 42 | 104 | 0.94 | |
| Tumor size (mm) | median (range) | 55 (12-170) | 55 (10-120) | 55 (15-180) | 0.21 |
| Histological type | intestinal | 27 | 48 | 76 | |
| Diffuse | 38 | 37 | 128 | 0.01 | |
| T factor | T2 | 12 | 20 | 40 | |
| T3 | 14 | 35 | 59 | ||
| T4 | 39 | 30 | 105 | 0.03 | |
| Lymph node metastasis | Negative | 21 | 29 | 59 | |
| Positive | 44 | 56 | 145 | 0.66 | |
| UICC Stage | 1B | 8 | 16 | 23 | |
| 2 | 15 | 26 | 47 | ||
| 3 | 18 | 17 | 69 | ||
| 4 | 24 | 26 | 65 | 0.2 | |
| Lymphatic invasion | Negative | 5 | 36 | 20 | |
| Positive | 60 | 49 | 184 | <0.0001 | |
| Venous invasion | Negative | 26 | 14 | 67 | |
| Positive | 39 | 71 | 137 | 0.004 | |
MiRNA microarray expression profiling
Custom miRNA microarray expression profiling was performed using the Agilent SurePrint G3 Human miRNA microarray 8X60K v3 (Agilent Technologies, Santa Clara, CA), in primary tumor tissue specimens from GC patients with PM (n = 6) and without PM (n = 6). Further details are provided in Supplementary Materials and Methods.
RNA isolation and quantitative real time PCR
The expression of each miRNAs was analyzed by TaqMan miRNA real-time qRT-PCR assays (Applied Biosystems, Foster City, CA) using the QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems). Further information are provided in Supplementary Materials and Methods.
Statistical analysis
A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using the Medcalc statistical software V.16.2.0 (Medcalc Software bvba, Ostend, Belgium), JMP software 10.0.2 (SAS Institute, Cary, NC), and GraphPad Prism V7.0 (GraphPad Software, San Diego, CA), and R (version 3.5.0, Vienna, Austria). Details are provided in Supplementary Materials and Methods.
RESULTS
Identification of candidate miRNAs overexpressed in gastric cancer patients with peritoneal metastasis
In order to identify miRNAs specifically dysregulated in GC patients with PM, we performed miRNA expression profiling in six patients each, with and without PM. We used a p-value of <0.05 as the initial criteria to identify differentially expressed miRNAs (Figure 1A). Subsequently, we identified 513 candidate miRNAs, of which 364 were upregulated in the primary tumors of GC patients with PM (Figure 1B). To further narrow down the list of miRNA candidates for developing a clinically relevant diagnostic signature, we next analyzed the TCGA dataset to identify miRNAs that were specifically differentially expressed in stage IV vs. other stages of GC patients. The rationale was to ensure that miRNAs identified in our microarray dataset were associated with metastatic GCs, and not dysregulated in other GC stages. A comparison of stage IV vs. stage IB-III tumors identified 104 differentially expressed miRNAs, of which 46 were upregulated and 58 were downregulated. We thereafter overlapped miRNAs that were dysregulated both in PM and stage IV GC patients, and identified eight miRNAs, of which five were upregulated (miR-30a-5p, miR-134-5p, miR-337-3p, miR-659-3p, and miR-3917) and three were downregulated (miR-718, miR-1281, and miR-3162). Since the read count of the three downregulated miRNAs were extremely low, we excluded these and were able to successfully validate the remaining five miRNAs in the TCGA dataset (Figure 1C).
Figure 1: Identification of candidate miRNAs in primary tumors from gastric cancer patients with peritoneal metastasis.
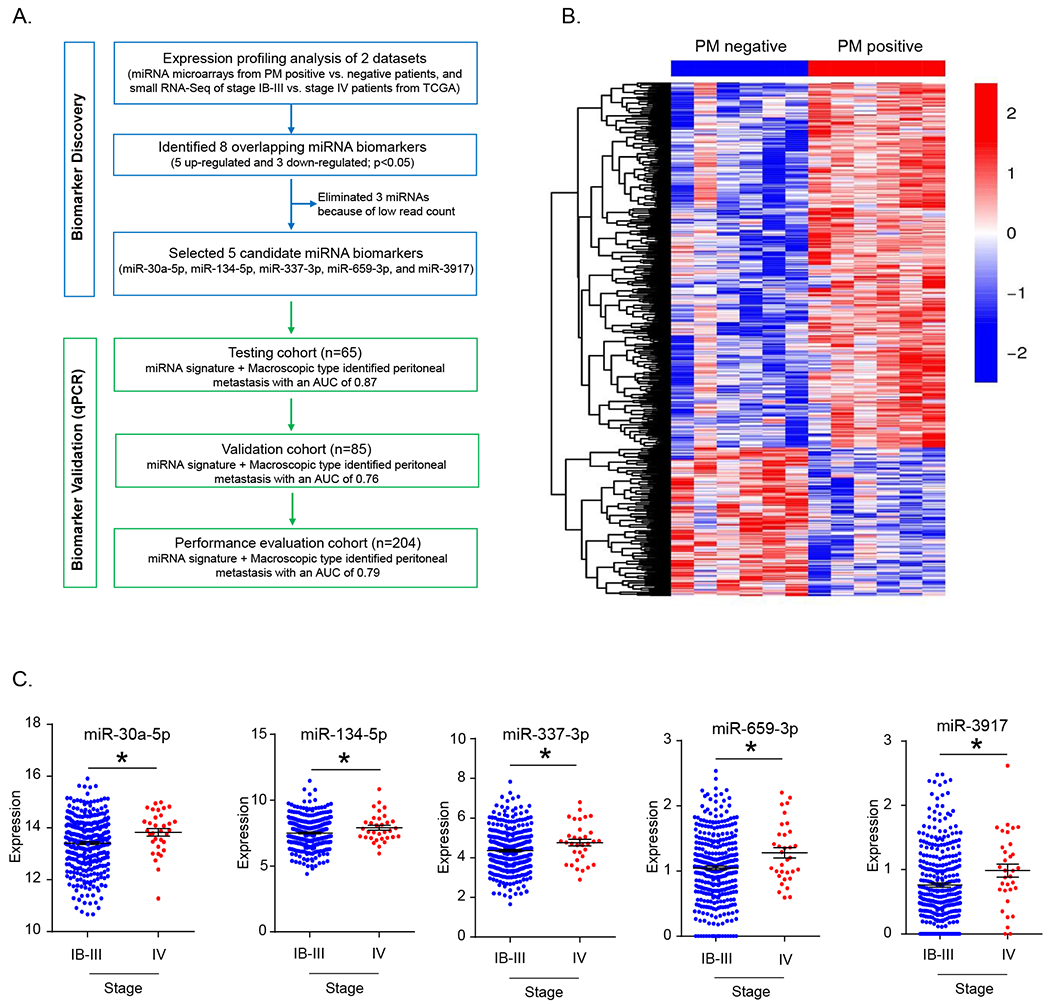
(A) Schematic of the study design for biomarker discovery and validation in various patient cohorts. (B) Heatmap illustrating differentially expressed miRNAs between peritoneal metastasis positive and negative samples identified from the miRNA-microarray dataset. (C) Differentially expressed candidate miRNAs between stage IV and stage IB-III GC patients in the TCGA dataset. Abbreviations; PM, peritoneal metastasis. *p<0.05, by Mann-Whitney U test.
The candidate miRNA biomarkers were overexpressed gastric cancer patients with peritoneal metastasis
To evaluate the clinical significance of the five miRNA candidates that we identified during the discovery phase, we examined their expression in tissue specimens from a clinical testing cohort of advanced GC patients (n=65; Table 1). Correlation between clinicopathological factors and expression of individual miRNAs (dichotomized into high and low groups) are shown in Supplementary Table 1. In brief, four of the miRNAs were significantly overexpressed in patients with Stage IV disease (Supplementary Table 1). Next, to directly address the clinical relevance of these candidate miRNAs for the detection of PM in GC patients, we interrogated their expression in patients with and without such metastases. Intriguingly, three of the five miRNAs, miR-30a-5p, miR-659-3p and miR-3917, were significantly overexpressed in the primary tumors of PM positive vs. negative patients (p=0.002, 0.04 and 0.007, respectively; Supplementary Figure 1A). Furthermore, these miRNAs were able to distinguish patients with vs. those without PM with corresponding AUC values for miR-30a-5p, miR-659-3p, and miR-3917 ;0.77, 0.68, and 0.74, respectively (Figure 2A). A sub-group analysis of stage IV patients revealed that the expression of these miRNAs in patients with PM tended to be higher compared to patients without such metastases (Supplementary Figure 1B). Next, we evaluated whether a combination signature of these three miRNA candidates might further improve the potential for PM detection; which was indeed the case as the risk scores and the AUC value of this signature significantly improved to 0.82 (sensitivity=85.7%, specificity=68.6%, positive predictive value (PPV) =42.9%, negative predictive value (NPV) =94.6%; Figure 2B and C) vs. individual markers in distinguishing PM positive GC patients. Collectively these data indicate that our miRNAs discovery phase from the expression profiling of PM patients was a success, and that these biomarkers were dysregulated and could detect GC patients harboring peritoneal metastases.
Figure 2: Candidate miRNAs were differentially expressed in peritoneal metastasis positive patients in the testing cohort.
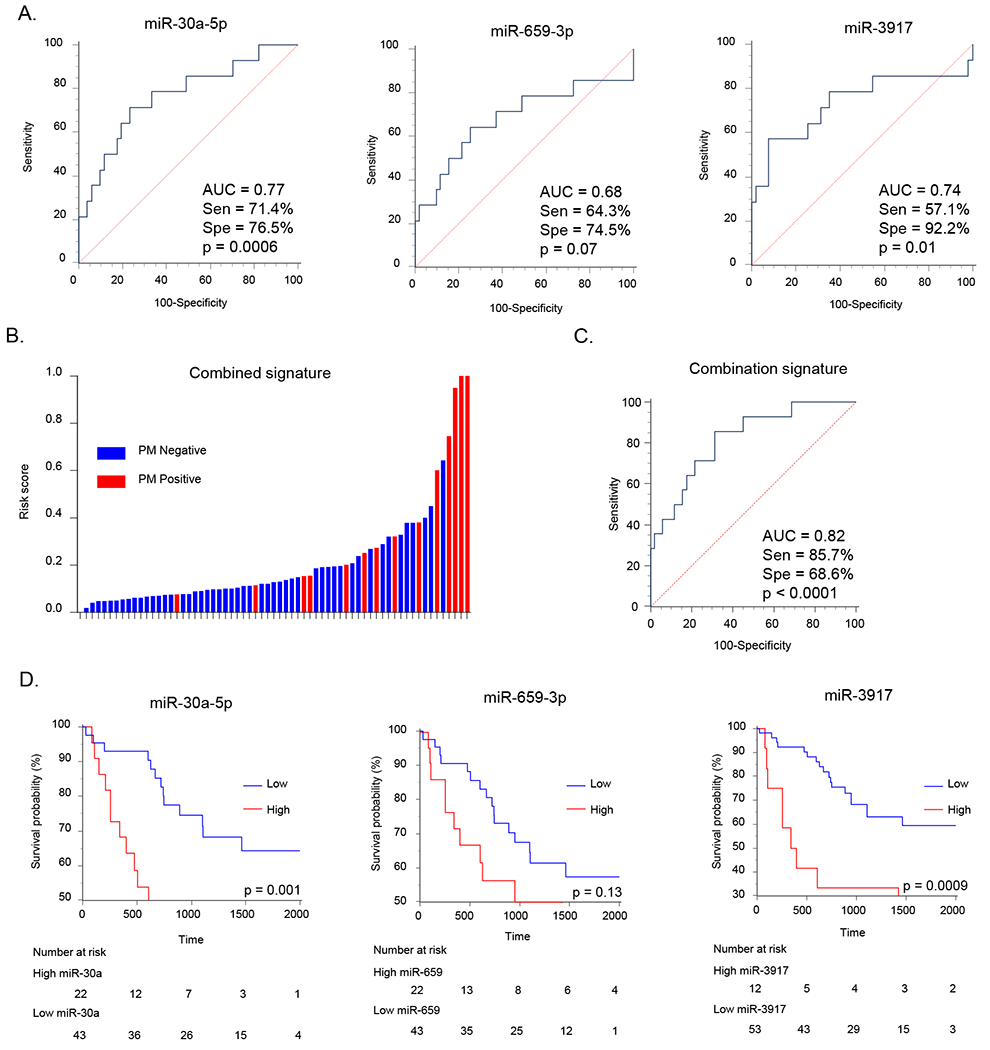
(A) Detection potential of miRNA candidates represented by receiver operating characteristic (ROC) curves. (B) The waterfall plot representing risk score of PM positive and negative patients based on the combined miRNAs signature. (C) ROC curve of the combined miRNA signature for the detection of PM. Abbreviations; Sen, sensitivity; Spe, specificity. (D) Kaplan-Meier analysis for overall survival (OS) between two groups dichotomized by Youden’s index for PM in individual overexpressing miRNAs.
Next, we evaluated the prognostic significance of these miRNAs by examining the overall survival (OS) using Kaplan-Meier analysis. While the patients with high expression of miR-659-3p did not demonstrate significant differences in survival, the ones with overexpression of miR-30a-5p and miR-3917 exhibited significantly inferior OS (p=0.001, and p=0.0009 respectively; Figure 2D). Patients with high risk score derived from the combination of three miRNA signature also revealed worse OS vis-à-vis those with low risk scores (p=0.04; Supplementary Figure 1C).
Validation of miRNA biomarkers for the identification of gastric cancer patients with peritoneal metastasis
Next, to further validate the efficacy of the three miRNAs identified in the testing cohort, we assessed their expression in an independent validation cohort (n=85; Table 1). The correlation between various clinicopathological factors and relative expression of the three miRNAs are shown in Supplementary Table 2. Consistent with the findings from our testing cohort, all three biomarkers demonstrated a significant upregulation in patients with stage IV disease (Supplementary Table 2). Furthermore, the expression of all three miRNAs, miR-30a-5p, miR-659-3p and miR-3917, was significantly upregulated in patients with PM vs. those without (p=0.02, 0.02, and 0.03 respectively; Supplementary Figure 2A). Furthermore, these three miRNAs certainly discriminated patients with vs. without PM as evidenced from the ROC curves and corresponding high AUC values (AUC for miR-30a-5p=0.69; miR-659-3p=0.73; and miR-3917=0.68; Supplementary Figure 2B). Next, we evaluated the efficacy of this combination signature in identifying patients with PM. The risk scores derived from the combination signature in patients with PM were significantly higher than those without PM (Figure 3A), and this signature was significantly superior vs. individual markers in discriminating GC patients with from those without PM (AUC=0.74, sensitivity=92.9%, specificity=52.1%, PPV=27.7%, NPV=97.4%; Figure 3B). Likewise, we successfully validated our findings from the testing cohort that patients with high expression of these three individual miRNAs, as well as the combination signature, resulted in significantly worse OS (miR-30a-5p, miR-659-3p, miR-3917, and combination signature: p=0.02, 0.0001, 0.006 and 0.02 respectively; Figure 3C and Supplementary Figure 2D).
Figure 3: Diagnostic potentialof peritoneal metastasis detection and prognostic significance of combined miRNA signature in validation cohort and performance evaluation cohort.
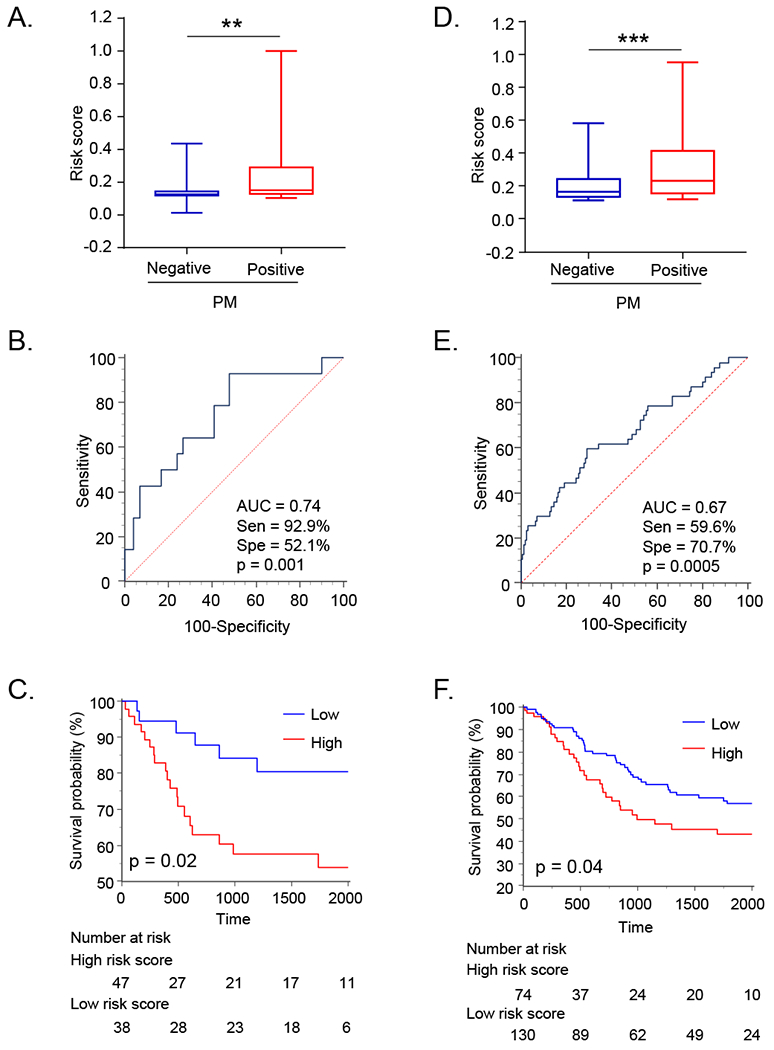
The box plot representing risk scores of PM positive and negative patients in the validation (A), and the performance evaluation cohort (D). ROC curves for the detection of PM in validation (B), and performance evaluation cohort (E). Kaplan-Meier analysis for OS between two groups dichotomized by Youden’s index for PM based on the combined miRNA signature in validation cohort (C), and the performance evaluation cohort (F). Abbreviations; PM, peritoneal metastasis; Sen, sensitivity; Spe, specificity. **p< 0.01, ***p< 0.01 by Mann-Whitney U test.
Performance evaluation of miRNA biomarkers for the identification of peritoneal metastasis in gastric cancer patients
While we were able to identify and validate the biomarker potential of our miRNA biomarkers for the detection of PM in GC patients, we believe an ideal scenario for their clinical application would be evaluation in the pre-surgical biopsy specimens obtained during diagnostic upper gastro-endoscopy. Considering that such tiny biopsy specimens are generally preserved in a fresh frozen state, we next examined the performance of our miRNA biomarkers in fresh frozen specimens for the identification of PM in GC patients. The correlation between clinicopathological factors and relative expression of the three miRNAs is summarized in Supplementary Table 3. Interestingly, we observed that miR-30a-5p and miR-659-3p was significantly upregulated in patients with PM vs. those without (p=0.0007 and 0.004 respectively; Supplementary Figure 3A). Furthermore, these miRNAs were also able to distinguish GC patients with PM (AUC values for miR-30a-5p, miR-659-3p, and miR-3917 were 0.66, 0.64, and 0.58, respectively; Supplementary Figure 3B). As was the case with the testing and validation cohorts, in the sub-group analysis of stage IV patients, the expression of miR-30a-5p and miR-659-3p in patients with PM was significantly higher as well (Supplementary Figure 3C).
We next evaluated the performance of these miRNA biomarkers as a combination signature, and consistent with our results from the previous two cohorts, the risk scores derived from the combination signature in patients with PM were significantly higher than without PM (p<0.001; Figure 3D), and this signature significantly discriminated GC with PM (AUC=0.67, sensitivity=59.6%, specificity=70.7%, PPV=37.8%, NPV=85.4%; Figure 3E). In addition, we noted that the patients with high expression of miR-30a-5p showed poorer OS (p = 0.007, Supplementary Figure 3D), and patients with high risk scores derived from the combination signature showed significantly worse OS (p=0.04; Figure 3F).
Tumor macroscopic type together with the miRNA signature further improved the detection potential for identifying peritoneal metastasis in gastric cancer patients
Next, using a logistic regression model, we assessed the performance of various clinicopathological factors associated with PM, in conjunction with our miRNA signature, to determine whether our miRNA signature might serve as an independent factor for diagnosing PM in GC patients. We also aimed to identify if any of the clinical factor(s) could be used in conjunction with our miRNA signature in further improving its detection potential. We focused on important clinical factors that can be identified prior to surgery for a more optimal translation of this model in clinical settings. In the initial univariate analysis, tumor macroscopic Borrmann’s type III or IV (p=0.003), and the miRNA combination signature (p=0.0002) were associated with detection of PM in the testing cohort (Table 2). Subsequent multivariate analysis revealed that both tumor macroscopic Borrmann’s type III or IV (p=0.01), identification in GC patients. Similarly, tumor macroscopic Borrmann’s type III or IV, larger tumor size and high expression of the combination miRNA signature were significant in the validation cohort, both in univariate and multivariate analysis (macroscopic Borrmann’s type III or IV, p=0.03; larger tumor size, p=0.007; and high expression of combined miRNA signature, p=0.0007; Table 2). More importantly, even in the final performance evaluation cohort, the univariate analysis revealed that tumor macroscopic type III or IV (p<0.0001), larger tumor size (p=0.0006), diffused histology (p=0.02) and the miRNA combination signature (p=0.0002) were associated with detection of PM (Table 2), and majority of these features remained significant as independent factors for PM detection in GC patients in subsequent multivariate analysis (Table 2). Collectively, our risk-diagnosis model indicated that along with our miRNA signature, tumor macroscopic type appears to be an important factor for identifying GC patients with PM.
Table 2:
Multivariate logistic regression analysis for the diagnosis of peritoneal metastasis in gastric cancer patients in each cohort
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR | 95%CI | P-value | OR | 95%CI | P-value |
| Testing cohort | ||||||
| Age ≧ 69 | 1.87 | 0.57-6.82 | 0.31 | |||
| Male | 1.16 | 0.35-4.24 | 0.81 | |||
| Macroscopical type 3 or 4 | 7.91 | 1.91-54.27 | 0.003 | 6.83 | 1.45-50.61 | 0.01 |
| Tumor size >55mm | 2.19 | 0.66-7.99 | 0.2 | |||
| Poorly differentiated histology | 2.05 | 0.60-8.28 | 0.26 | |||
| MiRNA combined signature high risk | 13.12 | 3.12-90.96 | 0.0002 | 11.74 | 2.61-84.97 | 0.0008 |
|
| ||||||
| Validation cohort | ||||||
| Age ≧ 70 | 0.54 | 0.15-1.72 | 0.3 | |||
| Male | 1.44 | 0.40-6.84 | 0.6 | |||
| Macroscopical type 3 or 4 | 4.73 | 1.34-22.26 | 0.01 | 4.97 | 1.20-26.99 | 0.03 |
| Tumor size >55mm | 4.73 | 1.34-22.26 | 0.01 | 6.63 | 1.62-35.88 | 0.007 |
| Poorly differentiated histology | 2.76 | 0.86-9.80 | 0.09 | |||
| MiRNA combined signature high risk | 14.15 | 2.60-263.78 | 0.0007 | 17.52 | 2.89-343.90 | 0.0007 |
|
| ||||||
| Performance evaluation cohort | ||||||
| Age ≧ 69 | 1.48 | 0.77-2.88 | 0.24 | |||
| Male | 0.81 | 0.38-1.77 | 0.58 | |||
| Macroscopical type 3 or 4 | 5.82 | 2.74-13.59 | <0.0001 | 6.44 | 2.76-16.51 | <0.0001 |
| Tumor size >55mm | 3.25 | 1.64-6.72 | 0.0006 | 2.24 | 1.03-5.01 | 0.04 |
| Poorly differentiated histology | 2.31 | 1.13-5.07 | 0.02 | 1.73 | 0.76-4.17 | 0.19 |
| MiRNA combined signature high risk | 3.56 | 1.82-7.09 | 0.0002 | 5.22 | 2.42-11.80 | <0.0001 |
OR, Odds Ratio; CI, Confidence Interval. Bold indicates a statistically significant.
Subsequently, we assessed whether combining tumor macroscopic type with the miRNA signature might further improve the detection potential for PM in GC patients. The ROC curves revealed that indeed combination of miRNA signature and macroscopic type significantly improved the detection performance in all three clinical cohorts (Testing cohort; AUC=0.87, sensitivity=78.6%, specificity=84.3% PPV=57.9%, NPV=93.9%; Figure 4A, Validation cohort; AUC=0.76, sensitivity=71.4%, specificity=70.4%, PPV=32.3%, NPV=92.6%; Figure 4B, Performance evaluation cohort; AUC=0.79, sensitivity=68.1%, specificity=79%, PPV=49.2%, NPV-89.2%; Figure 4C). Collectively, these data suggest that our miRNA signature in combination with the tumor macroscopic type improved the overall detection potential of GC patients with peritoneal metastasis.
Figure 4: Diagnostic accuracy of peritoneal metastasis detection of combined miRNA signature and tumor macroscopic type in each clinical cohort.
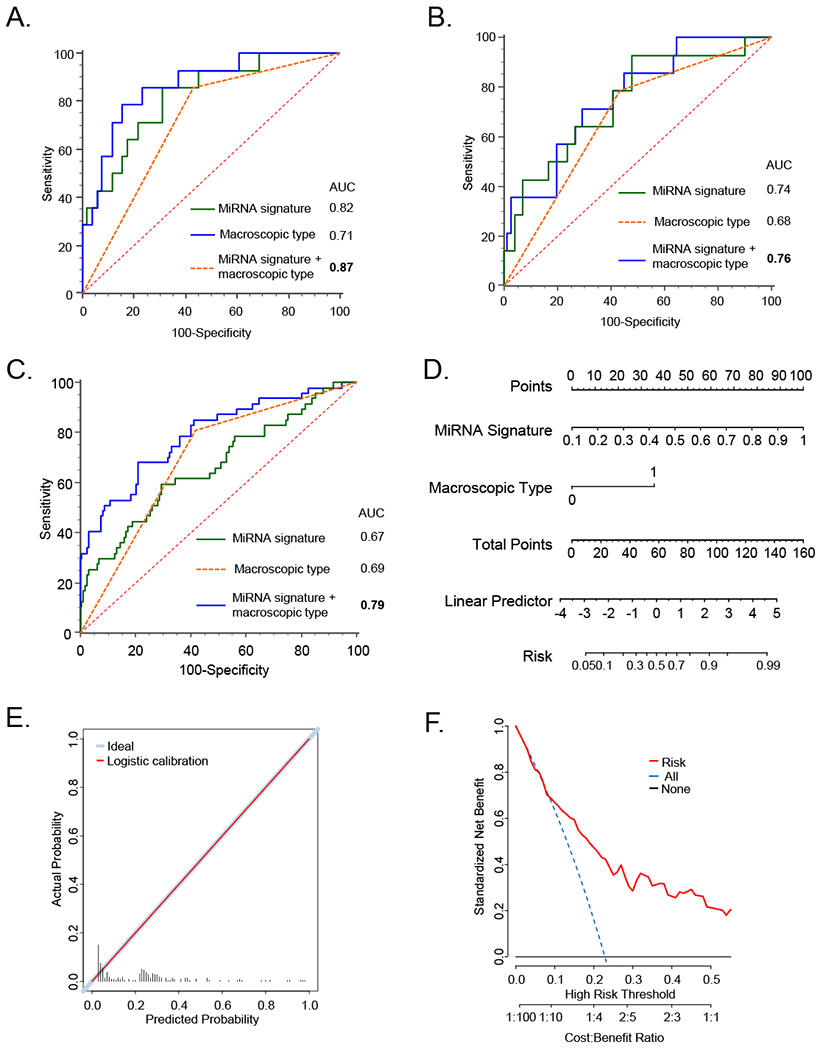
ROC curves derived from the combination miRNA signature, tumor macroscopic type, and their combination for detection of PM in the testing (A), validation (B), and performance evaluation cohort (C). Nomogram and their predicted probability plots in the performance evaluation cohort (Figure D, and E). Cost-benefit curves for PM detection in the performance evaluation cohort (Figure H).
Establishment of a prediction probability nomogram for the identification of high-risk gastric cancer patients with peritoneal metastasis
Subsequently, we established a nomogram for predicting the diagnostic probability for the presence of PM in GC patients using the statistically significant factors (miRNA signature and macroscopic type). We generated a nomogram using performance evaluation cohort which was composed of a large number of patients from whom fresh frozen specimens were available, which would also mimic biopsies in pre-surgical endoscopy settings. We demonstrated that our miRNA signature and the macroscopic type were significant predictors for the presence of PM in GC patients in this cohort (Figure 4D). Subsequent logistic regression analyses revealed that expected vs. observed predictability of our nomograms was significantly congruent, as evidenced from these plots in Figure 4E. Finally, we undertook cost-benefit analysis for the use of our biomarkers in detecting PM, and analysis revealed that our diagnostic model was significantly cost-effective as a diagnostic approach for identifying high-risk GC patients with peritoneal metastasis (Figure 4F). Collectively, these data suggest that our miRNA signature in combination with the tumor macroscopic type can provide risk-probability model for peritoneal metastasis in GC patients.
DISCUSSION
Currently, there is lack of availability of accurate biomarkers for the detection of peritoneal metastasis (PM) in gastric cancer (GC) patients. While computed tomography allows detection of larger foci with such metastasis, it lacks adequate diagnostic significance for microscopically smaller metastatic lesions. In this context, although staging laparoscopy has emerged as an approach with higher diagnostic accuracy for detecting PM in GC patients, its invasive nature and higher expense [12], provides a more appropriate rationale for its clinical application in not all, but selected subgroups of patients with advanced, high-risk disease. Therefore, in the current study we undertook a systematic effort to develop miRNA-based molecular biomarkers for the identification of high-risk patients with PM who can be more appropriate candidates for staging laparoscopy. We first performed computational analysis in two, genomewide expression profiling datasets to discover miRNAs dysregulated in GC patients with PM. We thereafter assessed the efficacy of a five miRNA panel in the two independent clinical cohorts, which led to the identification of three miRNAs which were consistently overexpressed in both cohorts (testing and validation), and the combination miRNA signature was significantly more accurate in discriminating GC patients with vs. without PM. In order to translate our findings into the clinical settings, we evaluated the potential of our miRNA biomarkers in a performance evaluation cohort of fresh frozen specimens that would mimic biopsies in pre-surgical endoscopy settings, and our miRNA signature performed well even in this cohort. Furthermore, high expression of all three miRNAs individually and their combination was associated with inferior overall survival. Finally, using linear regression analysis by combining risk scores from our miRNA signature together with the macroscopic Borrmann’s type, which were significantly superior for the identification of peritoneal metastasis in gastric cancer patients.
MiRNAs are short, single-stranded, noncoding RNAs which are frequently dysregulated in cancers. Previous studies have demonstrated that several miRNAs appear to have functional associations with PM in GC patients [39–41]. Using a peritoneal metastatic GC derived cell line, miR-136 was identified as a tumor-suppressive miRNA which attenuated metastatic potential of GC cells [39]. Similarly, a series of in vitro and in vivo experiments demonstrated miR-3978 as a potential tumor suppressor-miRNA, which inhibited legumain, a lysosomal cysteine endopeptidase [40, 41]. Collectively, these studies supported functional relevance of miRNAs in GC and suggested their potential clinical significance for the diagnosis of patients with PM.
Among the initially identified panel of miRNAs overexpressed in primary tumors of PM-positive patients, miR-30a, miR-659 and miR-3917 were consistently overexpressed and validated well in three independent patient cohorts. In pancreatic cancer, high expression of miR-30a was shown to promote migratory and invasive potential through upregulation of epithelial-to-mesenchymal-transition related genes [43]. Furthermore, overexpression of miR-30, including miR-30a, resulted in suppression of SOCS3, a key regulator of Jak/STAT3 pathway, and subsequently enhanced glioma stem cell growth [44]. Similarly, miR-30 is overexpressed in both GC tissues and its overexpression enhanced cellular proliferation and suppressed apoptosis through inhibition of p53 [45]. Collectively, these data indicate that miR-30a acts as an oncogene. In contrast, functional role of miR-659 and miR-3917 is unclear and remains to be elucidated.
Our current study demonstrates that a combination of our miRNA signature together with the tumor macroscopic type (Borrmann’s type III and IV) was significantly superior in identifying GC patients with PM. The Borrmann’s classification was developed in 1926, and is widely used to classify GCs based on endoscopic characteristics. Several studies have shown that Borrmann’s type correlates with other clinicopathological factors including depth of invasion, tumor stage, lymph node metastasis, distant metastasis [46, 47]. In addition, other studies have reported that macroscopic type III and IV Borrmann’s types are significant risk factors for PM in GC patients [13, 14, 16, 48, 49]; which was supported in our study, where we identified it to be one of the key factors for PM diagnosis in our multivariate analysis. Since, macroscopic stages for GC can be determined during endoscopy prior to the surgery, a combination of macroscopic type with the miRNA signature offers a potentially attractive choice for the identification of PM in gastric cancer patients.
Currently GC patients with PM are typically treated by chemotherapy or palliative surgery. However, there is no standardized treatment regimen for such patients, which can easily vary between institutions [50]. Nevertheless, recent studies have proposed various treatment strategies for these patients with PM. For example, intraperitoneal administration of paclitaxel significantly improved the outcome of GC patients with PM [29–31]. Although paclitaxel is a commonly used drug for ovarian, breast and lung cancers [51–53], it was recently recognized that its intraperitoneal administration was also effective for the treatment of PM in GC patients [21, 22, 54, 55]. Furthermore, recently the use of cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to be effective for GC patients with PM [23–26], and improving the overall outcome in these patients [24, 27, 56].
We would like to acknowledge some of the limitations of our present study. Although we used the clinical cohorts from multiple institutions, distribution of PM positive patients or other clinicopathological factors were different between cohorts, and both FFPE (testing cohort and validation cohort) and fresh-frozen specimens (performance evaluation cohort) were analyzed. Therefore, the expression profiles were different between cohorts and the risk scores (miRNA signature) were independently generated for each cohort. Second, although we conducted a comprehensive discovery of miRNA profiling using two datasets (microarray and TCGA), we selected candidate miRNAs commonly dysregulated in both datasets by Welch’s t-test in which we did not perform multiple comparison individually in each dataset. Third, as we showed in the supplementary data, there were a few outlier patients, which can be potential confounders for the final outcomes. Finally, due to the limited sample size in the present study, we were unable to evaluate whether our miRNA signature could identify patients with metachronous PM. Nonetheless, our study provides an important proof of principle concept, and these findings should be further confirmed in future, multi-institutional prospective studies before their clinical translation.
In conclusion, we have developed a novel miRNA-based signature for the detection of PM in GC patients, and validated its utility in multiple independent clinical cohorts. Furthermore, we have demonstrated that the combination of our miRNA signature and Bormann’s macroscopic type can offer a potentially attractive approach for detection of PM in gastric cancer patients, and offer an attractive tool for the selection of appropriate patients for staging laparoscopy for a more personalized treatment option.
Supplementary Material
Acknowledgements
We would like to acknowledge Xuan Wang for normalization of miRNA microarray data, acknowledge Jasjit Kaur Banwait for statistical support, acknowledge Fuminori Sonohara and Daisuke Izumi for technical advices, acknowledge Masaaki Iwatsuki and Kohei Yamashita for collecting clinical information, and acknowledge Lauren Patterson for her critical reading and valuable insights provided in improving the quality of this article.
Funding: This work was supported by CA181572, CA184792, CA187956 and CA214254 grants from the National Cancer Institute, National Institutes of Health (NCI/NIH); RP140784 from the Cancer Prevention Research Institute of Texas (CPRIT); grants from the Baylor Foundation and Baylor Scott & White Research Institute, Dallas, TX, USA.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Yoo CH, Noh SH, Shin DW et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87: 236–242. [DOI] [PubMed] [Google Scholar]
- 3.Ishizone S, Maruta F, Saito H et al. Efficacy of S-1 for patients with peritoneal metastasis of gastric cancer. Chemotherapy 2006; 52: 301–307. [DOI] [PubMed] [Google Scholar]
- 4.Koizumi W, Narahara H, Hara T et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008; 9: 215–221. [DOI] [PubMed] [Google Scholar]
- 5.Thomassen I, van Gestel YR, van Ramshorst B et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014; 134: 622–628. [DOI] [PubMed] [Google Scholar]
- 6.Kakroo SM, Rashid A, Wani AA et al. Staging Laparoscopy in Carcinoma of Stomach: A Comparison with CECT Staging. Int J Surg Oncol 2013; 2013: 674965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol 2013; 68: 251–255. [DOI] [PubMed] [Google Scholar]
- 8.Burke EC, Karpeh MS, Conlon KC, Brennan MF. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg 1997; 225: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer 2007; 10: 29–34. [DOI] [PubMed] [Google Scholar]
- 10.Karanicolas PJ, Elkin EB, Jacks LM et al. Staging laparoscopy in the management of gastric cancer: a population-based analysis. J Am Coll Surg 2011; 213: 644–651, 651 e641. [DOI] [PubMed] [Google Scholar]
- 11.Ajani JA, Bentrem DJ, Besh S et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013; 11: 531–546. [DOI] [PubMed] [Google Scholar]
- 12.Ramos RF, Scalon FM, Scalon MM, Dias DI. Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur J Surg Oncol 2016; 42: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida K, Yoshikawa T, Tsuburaya A et al. Indications for staging laparoscopy in clinical T4M0 gastric cancer. World J Surg 2011; 35: 2703–2709. [DOI] [PubMed] [Google Scholar]
- 14.Miki Y, Tokunaga M, Tanizawa Y et al. Staging Laparoscopy for Patients with cM0, Type 4, and Large Type 3 Gastric Cancer. World J Surg 2015; 39: 2742–2747. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Li Z, Jia S et al. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis. Chin J Cancer Res 2017; 29: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosogi H, Shinohara H, Tsunoda S et al. Staging laparoscopy for advanced gastric cancer: significance of preoperative clinicopathological factors. Langenbecks Arch Surg 2017; 402: 33–39. [DOI] [PubMed] [Google Scholar]
- 17.Nakata B, Hirakawa YSCK, Kato Y et al. Serum CA 125 level as a predictor of peritoneal dissemination in patients with gastric carcinoma. Cancer 1998; 83: 2488–2492. [DOI] [PubMed] [Google Scholar]
- 18.Yamao T, Kai S, Kazami A et al. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn J Clin Oncol 1999; 29: 550–555. [DOI] [PubMed] [Google Scholar]
- 19.Byrne DJ, Browning MC, Cuschieri A. CA72-4: a new tumour marker for gastric cancer. Br J Surg 1990; 77: 1010–1013. [DOI] [PubMed] [Google Scholar]
- 20.Emoto S, Ishigami H, Yamashita H et al. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer 2012; 15: 154–161. [DOI] [PubMed] [Google Scholar]
- 21.Ishigami H, Kitayama J, Otani K et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology 2009; 76: 311–314. [DOI] [PubMed] [Google Scholar]
- 22.Ishigami H, Kitayama J, Kaisaki S et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010; 21: 67–70. [DOI] [PubMed] [Google Scholar]
- 23.Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer treatment reviews 2016; 48: 42–49. [DOI] [PubMed] [Google Scholar]
- 24.Yonemura Y, Elnemr A, Endou Y et al. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World journal of gastrointestinal oncology 2010; 2: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonemura Y, Canbay E, Li Y et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2016; 42: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 26.Ji ZH, Peng KW, Yu Y et al. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2017; 33: 562–570. [DOI] [PubMed] [Google Scholar]
- 27.Yonemura Y, Endou Y, Shinbo M et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol 2009; 100: 311–316. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara Y, Takiguchi S, Nakajima K et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol 2012; 105: 38–42. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi H, Kitayama J, Ishigami H et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer 2013; 119: 3354–3358. [DOI] [PubMed] [Google Scholar]
- 30.Ishigami H, Yamaguchi H, Yamashita H et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 2017; 20: 128–134. [DOI] [PubMed] [Google Scholar]
- 31.Ishigami H, Fujiwara Y, Fukushima R et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol 2018; 36: 1922–1929. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854. [DOI] [PubMed] [Google Scholar]
- 36.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993; 75: 855–862. [DOI] [PubMed] [Google Scholar]
- 37.Tsai MM, Wang CS, Tsai CY et al. Potential Diagnostic, Prognostic and Therapeutic Targets of MicroRNAs in Human Gastric Cancer. Int J Mol Sci 2016; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Guan DH, Bi RX et al. Prognostic value of microRNAs in gastric cancer: a meta-analysis. Oncotarget 2017; 8: 55489–55510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng J, Ge P, Liu X et al. MiR-136 inhibits gastric cancer-specific peritoneal metastasis by targeting HOXC10. Tumour Biol 2017; 39: 1010428317706207. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Wu YY, Jiang JN et al. MiRNA-3978 regulates peritoneal gastric cancer metastasis by targeting legumain. Oncotarget 2016; 7: 83223–83230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji FJ, Wu YY, An Z et al. Expression of both poly r(C) binding protein 1 (PCBP1) and miRNA-3978 is suppressed in peritoneal gastric cancer metastasis. Sci Rep 2017; 7: 15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imaoka H, Toiyama Y, Okigami M et al. Circulating microRNA-203 predicts metastases, early recurrence, and poor prognosis in human gastric cancer. Gastric Cancer 2016; 19: 744–753. [DOI] [PubMed] [Google Scholar]
- 43.Tsukasa K, Ding Q, Miyazaki Y et al. miR-30 family promotes migratory and invasive abilities in CD133(+) pancreatic cancer stem-like cells. Human cell 2016; 29: 130–137. [DOI] [PubMed] [Google Scholar]
- 44.Che S, Sun T, Wang J et al. miR-30 overexpression promotes glioma stem cells by regulating Jak/STAT3 signaling pathway. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2015; 36: 6805–6811. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Jiao Y, Cui L, Jiang L. miR-30 functions as an oncomiR in gastric cancer cells through regulation of P53-mediated mitochondrial apoptotic pathway. Bioscience, biotechnology, and biochemistry 2017; 81: 119–126. [DOI] [PubMed] [Google Scholar]
- 46.An JY, Kang TH, Choi MG et al. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2008; 12: 1364–1369. [DOI] [PubMed] [Google Scholar]
- 47.Li C, Oh SJ, Kim S et al. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology 2009; 77: 197–204. [DOI] [PubMed] [Google Scholar]
- 48.Huang B, Sun Z, Wang Z et al. Factors associated with peritoneal metastasis in non-serosa-invasive gastric cancer: a retrospective study of a prospectively-collected database. BMC cancer 2013; 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu YF, Deng ZW, Liu H et al. Staging laparoscopy improves treatment decision-making for advanced gastric cancer. World J Gastroenterol 2016; 22: 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Japanese Gastric Cancer A Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wani MC, Taylor HL, Wall ME et al. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971; 93: 2325–2327. [DOI] [PubMed] [Google Scholar]
- 52.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979; 277: 665–667. [DOI] [PubMed] [Google Scholar]
- 53.Guchelaar HJ, ten Napel CH, de Vries EG, Mulder NH. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol (R Coll Radiol) 1994; 6: 40–48. [DOI] [PubMed] [Google Scholar]
- 54.Kodera Y, Takahashi N, Yoshikawa T et al. Feasibility of weekly intraperitoneal versus intravenous paclitaxel therapy delivered from the day of radical surgery for gastric cancer: a preliminary safety analysis of the INPACT study, a randomized controlled trial. Gastric Cancer 2017; 20: 190–199. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer 2017; 20: 111–121. [DOI] [PubMed] [Google Scholar]
- 56.Yonemura Y, Ishibashi H, Hirano M et al. Effects of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy and Neoadjuvant Intraperitoneal/Systemic Chemotherapy on Peritoneal Metastases from Gastric Cancer. Annals of surgical oncology 2017; 24: 478–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


