Abstract
The duration and lifetime pattern of hypertension is related to risk of stroke and dementia. In turn, cerebral small vessel disease (CSVD) is the most frequent form of cerebrovascular disease underlying dementia and stroke. Thus, study of the relation of mid to late life hypertension trends with CSVD late in life will help understand hypertension’s role, and inform preventive efforts of CSVD consequences. We studied 1686 Framingham Heart Study Offspring cohort participants free of stroke and dementia, who were examined in mid and late life, and had available brain MRI during late life. We related hypertension trends between mid and late life (normotension–normotension N-N, normotension-hypertension N-H, hypertension-hypertension H-H) to cerebral microbleeds (CMB) and covert brain infarcts (CBI), overall and stratified by brain topography. We used multivariable logistic regression analyses to calculate odds ratio (OR) and 95% confidence intervals (CI) for CSVD measures. The prevalence of CSVD in late life was 8% for CMBs and 13% for CBI, and increased with longer hypertension exposure across all brain regions. Compared to the trend pattern of N-N, both N-H and H-H trends had higher odds of mixed CMB (2.71 [1.08, 6.80], and 3.44 [1.39–8.60], respectively); H-H also had higher odds of any CMB or CBI (1.54 [1.12–2.20]), and any CBI (1.55 [1.08–2.20]). The burden of CSVD also increased with longer hypertension exposure. Our results highlight hypertension having a major role in subclinical CSVD, across subtypes and brain regions, and call attention to improve recognition and treatment of hypertension early in life.
Keywords: hypertension, magnetic resonance imaging, cerebral microbleeds, covert brain infarction, cerebral small vessel disease, epidemiology
Graphical Abstract
Introduction
Cerebral small vessel disease (CSVD) is the most frequent type of cerebrovascular disease in patients with stroke and dementia,1–3 contributing to approximately 20% and 45% of cases respectively,4 and detected in up to 55% and 40% of patients with confirmed established cerebrovascular disease and Alzheimer’s dementia (AD) respectively in autopsy studies.5 Cerebral microbleeds (CMBs) and covert brain infarcts (CBIs) represent CSVD markers of hemorrhage or ischemic vascular brain injury,3, 6 associated with increased risk of clinical stroke,7–9 cognitive impairment and dementia.6, 10 CMBs are present in 54 – 80% in association with intracerebral hemorrhage,11–13 18–65% with ischemic stroke,14–16 24% with AD and 58% with vascular dementia.17 Their topographic location in the brain has been suggested to represent the two most common types of CSVD: hypertensive angiopathy for lesions in deep brain regions, and cerebral amyloid angiopathy (CAA) for lesions in lobar regions,1 and mixed CMB likely representing the interplay of hypertensive and amyloid angiopathy and higher burden of CMB. Consequently, CSVD is not only common, but also closely linked to the pathophysiology of stroke and dementia. CSVD develops insidiously and progressively over long periods of time, emerging as a significant public health issue.
Hypertension (HTN) is the strongest, and one of the most treatable, risk factors for stroke and dementia. Hypertension was number one of the 5 leading factors for the burden of disease virtually worldwide in 201018 with a 56.1% rise in the actual number of deaths attributable to hypertension from 2007 to 2017.19 In clinical samples, hypertension is observed in 69 to 75% of patients with stroke20, 21 and 42 to 83% in patients with dementia with high burden of CSVD,22, 23 and 42 – 78% of individuals from population based studies with CMB and CBIs.24–26 Treatment of hypertension has resulted in a 30 to 40% RR reduction of stroke,27 and recent studies in FHS have shown decreasing trends of incident dementia coupled with improved vascular risk factor control, mainly hypertension.28 The duration of exposure to hypertension is a key factor in its relation to risk of stroke and dementia. In prior FHS reports, we observed that participants with longer exposure were at increased risk of clinical events: trends of HTN during midlife into late life were associated with a > 2-fold increase in dementia risk and stroke.29, 30 Investigators in the atherosclerosis risk in communities study (ARIC) also found that HTN trends from mid to late life identified individuals at highest risk of dementia.31 HTN is also related to subclinical CSVD. Although stronger relations have been described with lesions in deep brain regions,1 HTN is also relevant for CSVD in lobar regions, presumed to represent mostly CAA. For instance, in CAA patients, uncontrolled HTN is associated with a higher risk of hemorrhage.32 Thus, study of the relation of HTN with asymptomatic CSVD will allow us to increase our understanding of the early pathophysiology of vascular injury leading to stroke and dementia, presenting an opportunity to prevent both.
Framingham Heart Study participants have been followed prospectively for seven decades with accurate ascertainment of HTN throughout life. In the present study, we aimed to investigate the relation between HTN trends from mid to late life and prevalence of ischemic and hemorrhagic CSVD in our community cohort of neurologically healthy people.
Methods
Data and study materials will be made available to other researchers upon request following Framingham Heart Study procedures.
Sample
The Framingham Heart Study Offspring cohort was recruited in 1971 and has been followed prospectively for nearly five decades, with periodic examination approximately every 4 years. We included Framingham Offspring Cohort participants who attended a clinical examination during midlife (exam cycle 4 or 5, baseline) and in late life (exam cycle 7 or 8) for ascertainment of HTN status, and underwent brain MRI acquisition late in life (exam cycle 7 or 8). We excluded participants with prevalent stroke or dementia at baseline. Figure 1 shows our sample selection flow chart. Among participants attending a baseline exam, 1611 also attended exam cycle 7 and underwent brain MRI, and 559 participants attended exam cycle 8 and also had brain MRI. After excluding participants with prevalent stroke or dementia at baseline, and the hypertension-normal trend group due to the small number of participants in this subgroup for further analyses (n=38 participants), the final sample included 1686 Offspring Cohort participants. The Institutional Review Board of Boston University Medical Center approved the study protocol and informed consent was obtained from all subjects.
Figure 1.
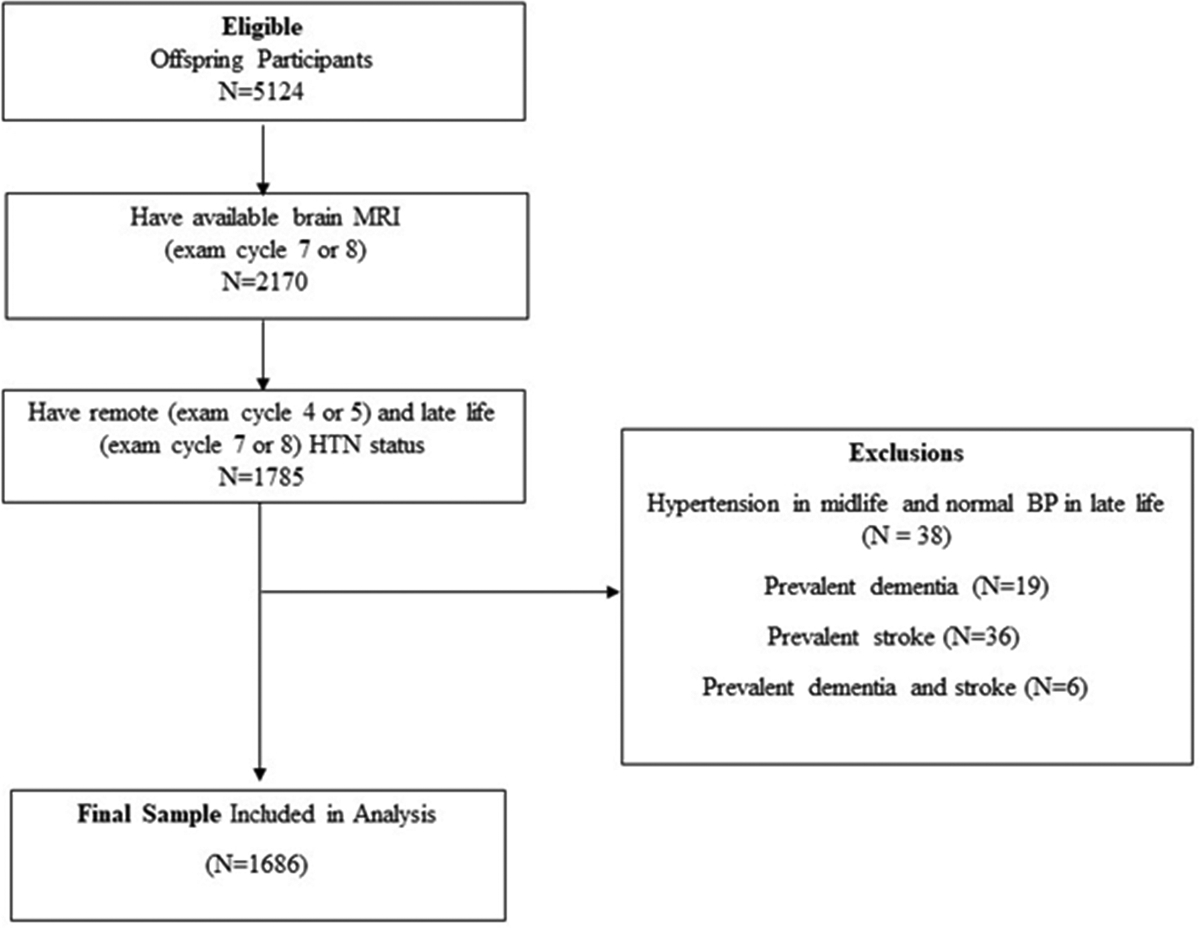
Sample selection flow chart
Exposure
All blood pressure measurements were taken in the left arm, in a seated position, using a mercury column sphygmomanometer and with an appropriate width-sized cuff. Readings were recorded to the nearest even number. Blood pressure was recorded by a physician at each examination cycle, and taken as the mean of two blood pressure measurements.29
Hypertension was defined as SBP ≥140 mmHg, DBP ≥ 90 mmHg and/or use of antihypertensive medications. Hypertension status was ascertained at mid (exam 4 or 5) and late life (exam 7 or 8). The study timeline is presented in figure 2. We categorized participants according to trends in HTN from mid to late life in the following categories: I. Normotensive-normotensive (N-N) category including participants who were normotensive at midlife exam and remained normotensive at late life exam; II. Normotensive-hypertensive (N-H) category including participants who were normotensive at midlife exam and hypertensive at late life exam; III. Hypertensive-hypertensive (H-H) category including participants who were hypertensive at midlife exam and remained hypertensive at late life exam.
Figure 2.
Study Timeline
Clinical and demographic characteristics
Clinical and demographic characteristics were measured at the baseline examination cycle. Prevalent diabetes mellitus was defined as a fasting blood glucose >126 mg/dL or use of oral hypoglycemic agents/ insulin. Current smoking was defined as self-reported smoking of at least one cigarette per day within the year preceding examination. Medication use was ascertained by self-report. Prevalent cardiovascular disease was defined as coronary heart disease, peripheral arterial disease and/or heart failure. APOE genotype was determined as previously described.33
Outcomes
MRI protocol
A 1.5-tesla MR machine (Siemens Magnetom) was used to obtain the following sequences: coronal T2-weighted 2470/20 to 80 (TR/ TE), echo train length 8, field of view 22 cm, acquisition matrix 192×256 interpolated to 256×256 with 1 excitation, 4-mm slice thickness from nasion to occiput, sagittal T1-weighted 11.4/4.4, 3D FLASH, 192 mm slab, 128 slices of 1.5-mm thickness, 12-degree flip angle and axial T2*gradient echo 656/26 (TR/TE), field of view 22cm, acquisition matrix 144×256, 30-degree flip angle, 19 slices of 5-mm thickness, and 2 mm gap.
MRI data were analyzed using QUANTA 2 on a Linux operating system, blind to the subject’s demographic and clinical characteristics, and outcome ascertainment.
CSVD markers
CMBs and CBIs were considered categorical outcome measurements on brain MRI.
We defined CMBs following established guidelines,1 as small, round to ovoid areas of focal low signal on gradient-echo T2* weighted magnetic resonance images. We grouped CMB into 4 categories according to their brain location: any CMB, lobar only, deep only and deep + mixed (called deep/mixed). The intra-rater and inter-rater reliability for CMB ratings has been previously reported (kappa statistic 0.78).26
CBIs were defined using standard criteria,3 based on size (greater than 3 mm), location, and imaging characteristics of the lesion as previously described,34 clinically asymptomatic. We grouped them according to brain location in the same categories as CMB for analyses: any CBI, lobar only, deep only and deep + mixed (called deep/mixed). The intra-rater and inter-rater reliability for CBI ratings has been previously reported (kappa statistic 0.73 to 0.9).35
Statistical Analysis
Baseline characteristics of study participants were evaluated overall and by HTN trend category, presented in table 1. We used multivariable Firth-corrected logistic regression analysis to estimate odds ratios and 95% confidence intervals for prevalent CSVD (CMBs and/or CBIs), overall and stratified by brain region. The Firth correction, a penalized likelihood approach, improves estimation in logistic regression models by reducing bias when small event counts are present. We performed comparisons between each pair of hypertension trends groups, with respect to each of the following outcomes: presence of both CMB and CBI, compared to neither; presence of either CMB or CBI, compared to neither; presence of any CMB, lobar only CMB, deep or mixed CMB, deep only CMB, each compared to no CMB; and presence of any CBI, lobar only CMI, deep or mixed CMI, deep only CMI, each compared to no CMI. Model 1 was age and sex-adjusted; model 2, additionally adjusted for antiplatelet and anticoagulant use, for CMB outcomes only; model 3, additionally adjusted for diabetes mellitus and smoking for CBI outcomes only; and model 4, additionally adjusted for antiplatelet use, anticoagulant use, diabetes and smoking for the any CMBs and/or any CBIs outcomes. In exploratory analyses we examined a model additionally adjusted for APOE genotype (any ɛ4 alleles) and evaluated interaction with the APOE ɛ4 genotype in the relation of hypertension trend and any CMBs or CBIs outcomes. We evaluated CMB burden in the following categories: ≥1 CMBs, ≥2 CMB, ≥5 CMB, each compared to no CMB [referent]. We also evaluated separately the group of ≥ 2 lobar only CMB, considered to represent probable CAA by modified Boston criteria.36 Odds ratios in each case were based on comparisons to the group without CMB or CBI.
Table 1.
Baseline characteristics and covariates overall and by hypertension trend (at midlife, baseline exam)
| Hypertension Trend Category | ||||
|---|---|---|---|---|
| Characteristic | All | Normal-Normal (N-N) | Normal-Hypertensive (N-H) | Hypertensive-Hypertensive (H-H) |
| N (%) | 1686 (100%) | 770 (46%) | 505 (30%) | 411 (24%) |
| Age, mean (SD), y | 52 (9) | 49 (9) | 53 (8) | 56 (8) |
| Men, n (%) | 780 (46) | 306 (40) | 248 (49) | 226 (55) |
| Systolic Blood Pressure, mm Hg | 122.7 (17.5) | 111.5 (10.7) | 123.3 (9.5) | 142.9 (17.0) |
| Diastolic Blood Pressure, mm Hg | 74.4 (10.2) | 69.6 (8.0) | 74.8 (7.8) | 83.1 (10.6) |
| Diabetes mellitus | 78 (5) | 13 (2) | 20 (4) | 45 (11) |
| Cigarette smoking | 288 (17) | 144 (19) | 95 (19) | 49 (12) |
| Prevalent cardiovascular disease | 66 (4) | 14 (2) | 22 (4) | 30 (7) |
| Statin use | 38 (3) | 9 (1) | 13 (3) | 16 (5) |
| Atrial Fibrillation | 11 (1) | 2 (0) | 5 (1) | 4 (1) |
| Antihypertensive medication | 191 (11) | 0 (0) | 0 (0) | 191 (47) |
| ApoE4 (any allele) | 366 (22) | 164 (22) | 103 (21) | 99 (25) |
| Antiplatelet therapy | 2 (0) | 0 (0) | 0 (0) | 2 (1) |
| Anticoagulant therapy | 4 (0) | 0 (0) | 3 (1) | 1 (0) |
| At follow-up (late life) | ||||
| Any CMB, n (%) | 140 (8) | 44 (6) | 41 (8) | 55 (13) |
| Any CBI, n (%) | 221 (13) | 74 (10) | 66 (13) | 81 (20) |
Values are mean (SD) for continuous variables, and counts (%) for categorical variables.
CMB = cerebral microbleeds. CBI = covert brain infarcts.
Statistical analysis was performed using SAS version 10 (SAS Institute, Cary, NC).
Results
The mean age (SD) of the included participants at the midlife exam was 52 (9) years and 46% (n=780) were men. At baseline examination, our sample was considered healthy, with low prevalence of vascular risk factors overall (Table 1). Participants in the H-H trend group (i.e. hypertensive from midlife) were older, more likely to have diabetes, and had slightly higher proportion of APOE e4, but otherwise were similar to the other two groups (5% diabetes mellitus, 17% smokers, 5% cardiovascular disease, 1% atrial fibrillation).
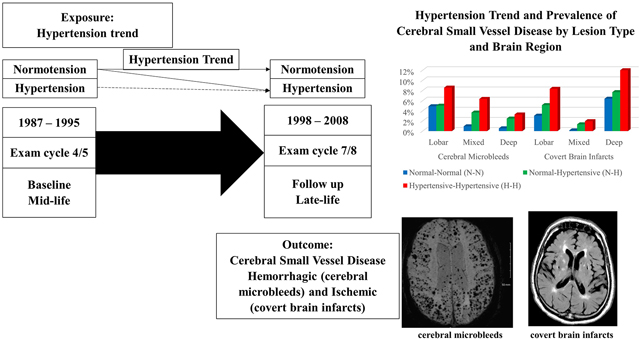
The prevalence of CMB and CBI among the 1686 participants included was 8% (n=140) and 13% (n=221) respectively, and 20.6% (n=361) had any CMB or any CBI. The frequency of both outcomes (CMB and CBI) increased with longer HTN exposure from N-N (6 and 10% respectively), to N-H (8 and 13%, respectively) to H-H (13 and 20%, respectively) groups, and the increase was observed across all brain locations for both CMB and CBI (Figure 3).
Figure 3.
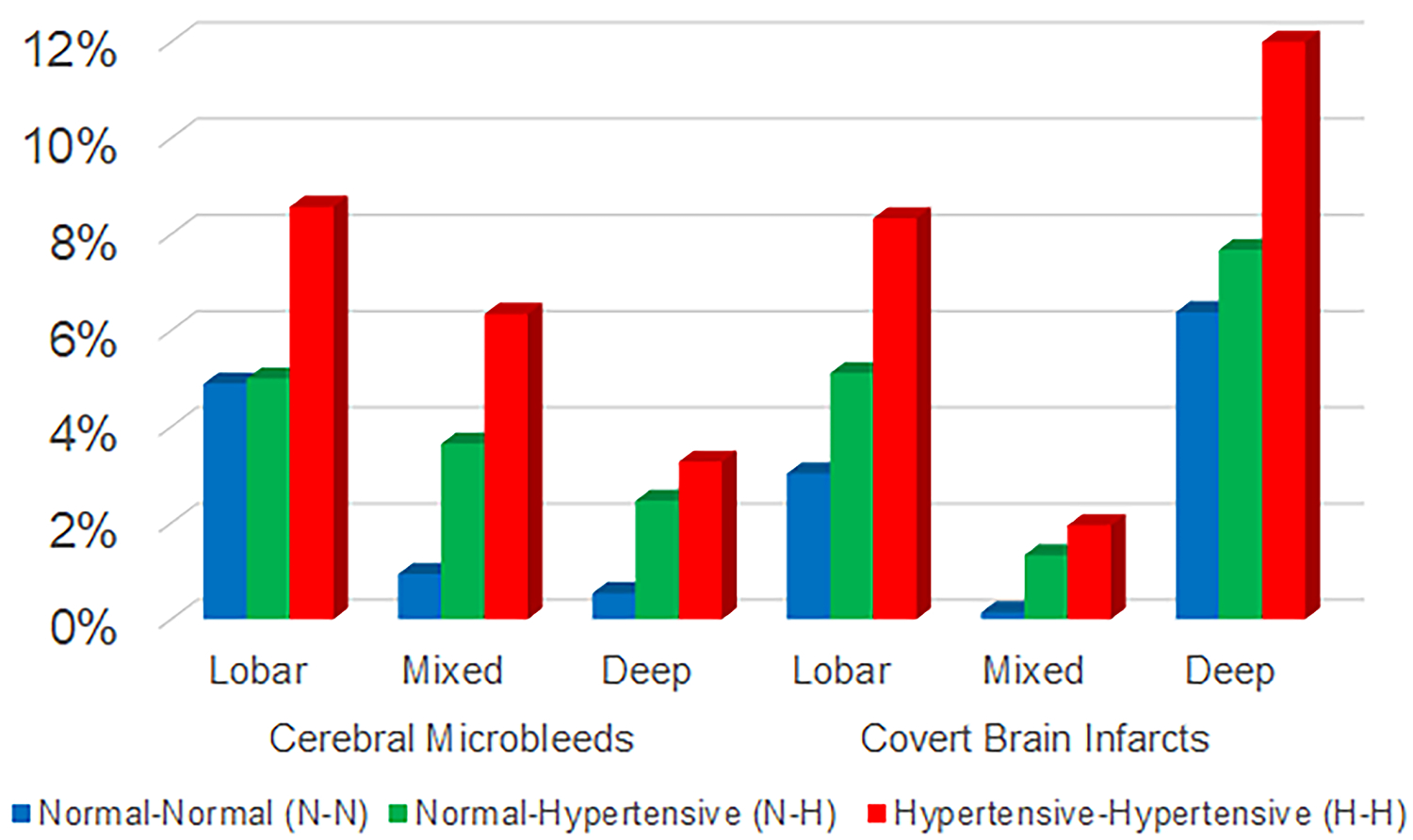
Prevalence of cerebral small vessel disease (CSVD) measures across brain regions and hypertension trend category
Multivariable logistic regression analysis of the association of hypertension trends with CMBs and CBI.
We present our primary analysis based on model 1, as no substantial differences were observed in additionally adjusted models, unless otherwise specified (Table 2). Comparisons with the N-N group showed that N-H had higher odds of mixed CMBs (OR [95% CI] 2.71 [1.08, 5.80], p= 0.033); H-H had higher odds of any CMB or CBI (1.54 [1.12–2.20], p = 0.007), mixed CMB (3.44 [1.39–8.60], p = 0.008), and any CBI (1.55 [1.08–2.20], p = 0.018). Comparison between the N-H and H-H trend groups showed higher odds of any CMB or CBI in those with H-H trend (1.49 [1.08–2.00], p = 0.014). Models with additional multivariable adjustment (models 2, 3 and 4) yielded similar results (Table 3). There was no significant interaction with APOE ɛ 4 genotype in the relation between HTN trend and any CMB or any CBI outcomes, and further adjustment for APOE ɛ 4 yielded similar results (data not shown).
Table 2.
Multivariable analysis of association of HTN trend (baseline midlife-later life) with CSVD (CMB and CBI)
| Model | Hypertension Trend Category | Reference group | CMB | CBI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both CMB and CBI | Any CMB or any CBI | Any CMB | Lobar only | Mixed and Deep | Deep only | Any CBI | Lobar only | Mixed and Deep | Deep only | |||
| OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | |||
| 1 | Normal-Hypertensive | Normal-Normal | 1.45 [0.56–3.80] 0.446 | 1.04 [0.76–1.40] 0.827 | 1.02 [0.65–1.60] 0.931 | 0.72 [0.42–1.20] 0.224 | 2.71 [1.08–6.80] 0.033 | ** | 1.11 [0.77–1.60] 0.589 | 1.40 [0.73–2.60] 0.310 | ** | 0.96 [0.60–1.60] 0.846 |
| Hypertensive-Hypertensive | Normal-Normal | 1.96 [0.78–5.00] 0.152 | 1.54 [1.12–2.20] 0.007 | 1.44 [0.92–2.20] 0.108 | 1.08 [0.65–1.80] 0.761 | 3.44 [1.39–8.60] 0.008 | ** | 1.55 [1.08–2.20] 0.018 | 2.03 [1.08–3.80] 0.029 | ** | 1.34 [0.85–2.20] 0.21 | |
| Hypertensive-Hypertensive | Normal-Hypertensive | 1.35 [0.60–3.00] 0.462 | 1.49 [1.08–2.00] 0.014 | 1.41 [0.91–2.20] 0.123 | 1.51 [0.88–2.60] 0.139 | 1.27 [0.66–2.40] 0.473 | ** | 1.41 [0.98–2.00] 0.064 | 1.45 [0.81–2.60] 0.213 | ** | 1.41 [0.88–2.20] 0.157 | |
| 2 | Normal-Hypertensive | Normal-Normal | NA | NA | 1.00 [0.63–1.60] 0.999 | 0.71 [0.42–1.20] 0.209 | 2.65 [1.06–6.60] 0.037 | ** | NA | NA | NA | NA |
| Hypertensive-Hypertensive | Normal-Normal | NA | NA | 1.44 [0.93–2.20] 0.105 | 1.08 [0.65–1.80] 0.761 | 3.43 [1.38–8.60] 0.008 | ** | NA | NA | NA | NA | |
| Hypertensive-Hypertensive | Normal-Hypertensive | NA | NA | 1.44 [0.93–2.20] 0.101 | 1.53 [0.88–2.60] 0.129 | 1.29 [0.67–2.40] 0.447 | ** | NA | NA | NA | NA | |
| 3 | Normal-Hypertensive | Normal-Normal | NA | NA | NA | NA | NA | NA | 1.10 [0.76–1.60] 0.621 | 1.27 [0.66–2.40] 0.469 | ** | 0.99 [0.62–1.60] 0.972 |
| Hypertensive-Hypertensive | Normal-Normal | NA | NA | NA | NA | NA | NA | 1.48 [1.02–2.20] 0.041 | 1.74 [0.91–3.40] 0.092 | ** | 1.38 [0.85–2.20] 0.191 | |
| Hypertensive-Hypertensive | Normal-Hypertensive | NA | NA | NA | NA | NA | NA | 1.35 [0.93–2.00] 0.115 | 1.37 [0.75–2.60] 0.31 | ** | 1.39 [0.86–2.20] 0.185 | |
| 4 | Normal-Hypertensive | Normal-Normal | 1.32 [0.50–3.40] 0.572 | 1.02 [0.74–1.40] 0.922 | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypertensive-Hypertensive | Normal-Normal | 1.96 [0.78–5.00] 0.149 | 1.49 [1.07–2.00] 0.017 | NA | NA | NA | NA | NA | NA | NA | NA | |
| Hypertensive-Hypertensive | Normal-Hypertensive | 1.49 [0.66–3.40] 0.34 | 1.46 [1.06–2.00] 0.022 | NA | NA | NA | NA | NA | NA | NA | NA | |
CMB = cerebral microbleeds. CBI = covert brain infarcts. CSVD= cerebral small vessel disease. HTN= hypertension.
Model 1 age and sex adjusted.
Model 2 additionally adjusted for antiplatelet and anticoagulant use (CMB outcomes).
Model 3 additionally adjusted for diabetes and smoking (CBI outcomes).
Model 4 additionally adjusted for antiplatelet or anticoagulant use, diabetes, and smoking. Too few events for meaningful statistical analyses (<5).
Hypertension definition uses blood pressure ≥140/90 mm Hg.
Table 3.
Multivariable analysis HTN trend (baseline midlife-later life) and CSVD burden (number of CMB and CBI)
| Model | Hypertension trend category | Reference group | CMB | CBI | |||||
|---|---|---|---|---|---|---|---|---|---|
| ≥ 1 | ≥ 2 | ≥ 5 | ≥ 2 lobar only | ≥ 1 | ≥ 2 | ≥ 5 | |||
| OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | |||
| 1 | Normal-Hypertensive | Normal-Normal | 1.02 [0.65–1.60] 0.931 | 1.08 [0.39–3.00] 0.876 | ** | ** | 1.11 [0.77–1.60] 0.589 | 1.64 [0.70–3.80] 0.254 | ** |
| Hypertensive-Hypertensive | Normal-Normal | 1.44 [0.92–2.20] 0.108 | 3.60 [1.52–8.60] 0.004 | ** | ** | 1.55 [1.08–2.20] 0.018 | 2.47 [1.08–5.60] 0.031 | ** | |
| Hypertensive-Hypertensive | Normal-Hypertensive | 1.41 [0.91–2.20] 0.123 | 3.32 [1.44–7.60] 0.005 | ** | ** | 1.41 [0.98–2.00] 0.064 | 1.51 [0.74–3.20] 0.261 | ** | |
| 2 | Normal-Hypertensive | Normal-Normal | 1.00 [0.63–1.60] 0.999 | 1.05 [0.37–3.00] 0.928 | ** | ** | NA | NA | NA |
| Hypertensive-Hypertensive | Normal-Normal | 1.44 [0.93–2.20] 0.105 | 3.58 [1.51–8.60] 0.004 | ** | ** | NA | NA | NA | |
| Hypertensive-Hypertensive | Normal-Hypertensive | 1.44 [0.93–2.20] 0.101 | 3.41 [1.47–8.00] 0.004 | ** | ** | NA | NA | NA | |
| 3 | Normal-Hypertensive | Normal-Normal | NA | NA | NA | NA | 1.10 [0.76–1.60] 0.621 | 1.81 [0.76–4.40] 0.179 | ** |
| Hypertensive-Hypertensive | Normal-Normal | NA | NA | NA | NA | 1.48 [1.02–2.20] 0.041 | 2.48 [1.04–6.00] 0.040 | ** | |
| Hypertensive-Hypertensive | Normal-Hypertensive | NA | NA | NA | NA | 1.35 [0.93–2.00] 0.115 | 1.37 [0.66–2.80] 0.401 | ** | |
CMB= cerebral microbleeds. CBIs= covert brain infarcts. CSVD= cerebral small vessel disease. HTN= hypertension.
Model 1 age and sex adjusted.
Model 2 additionally adjusted for antiplatelet and anticoagulant use (CMB outcomes).
Model 3 additionally adjusted for diabetes and smoking (CBI outcomes).
Too few events for meaningful statistical analyses (<10).
Hypertension definition uses blood pressure ≥140/90 mm Hg.
Analyses of the relation between hypertension trend and CSVD burden in some of the subgroups with CMB or CBI ≥ 1, ≥2 and ≥ 5, and the subgroup of CMBs ≥ 2 lobar only that represents probable CAA, were limited by small number of outcomes in subgroups of N-N and N-H trends (supplementary tables S1 and S2).
Nonetheless, two observations are noteworthy: we observed stronger associations with higher CSVD burden with longer hypertension exposure, and the strength of association increased with higher CSVD burden; participants with H-H trend had over three-fold higher odds of 2 or more CMB compared to N-H (3.32 [1.44–7.60], p = 0.005) and nearly four-fold higher odds of 2 or more CMB than those in N-N (3.60 [1.52–8.60], p= 0.004). Analyses in the probable CAA group (≥2 strictly lobar CMB) could not be performed to provide meaningful statistical results, but the highest proportion of participants in this group were from the H-H trend (3% vs 0.2% in the N-H group and 0.5% in the N-N group). Similarly, associations were stronger for CBI with higher burden: for ≥ 1 CBI OR = 1.55 (CI = 1.08–2.20, p = 0.018), and for ≥ 2 CBI OR = 2.47 (CI = 1.08–5.60, p = 0.031), comparing the H-H to N-N group.
In exploratory analyses, we evaluated the association of hypertension trends with CMB and CBI using two additional different hypertension definitions: use of antihypertensive medications or blood pressure equal or greater than 130/80, and separately, use of antihypertensive medications or blood pressure equal or greater than 150/90 mm Hg. We observed that the associations overall were stronger with higher blood pressure definitions, suggesting that higher hypertension values are related to higher risks (supplementary Table S3).
Discussion
In our study including a large community based sample of healthy individuals from the Framingham Heart Study we found that a pattern of long term exposure to hypertension is associated with higher prevalence and burden of cerebral small vessel disease. The presence of any CMB or any CBI was highest in the hypertension-hypertension category followed by normal-hypertension category. The highest number of both CMBs and CBIs were in the hypertension-hypertension category followed by normal-hypertension and normal-normal categories. The hypertension-hypertension category showed the strongest association with CMBs and CBIs in mixed/deep location when compared to the normal-normal blood pressure category. The associations observed were independent of other vascular risk factors, consistent with the knowledge that hypertension is one of the strongest, if not the strongest, predictor of cerebral small vessel disease. We did not observe a significant association of hypertensive trend groups with any or lobar CMBs only, but the proportion of participants with probable CAA (i.e. 2 or more strictly lobar CMB) was highest in the hypertension-hypertension trend group, suggesting the hypothesis that as HTN exposure duration increases, HTN may play a more prominent role, even for CAA related small vessel disease. Our analyses of CMB burden were limited by small number of participants in subgroups of HTN trend, but the results suggested an increasing effect with HTN exposure duration, both in strength of association and burden of CSVD.
Our results are novel as no other population based studies have reported the relation of long term hypertension trends with prevalence of CSVD. Given that CSVD is the most common underlying form of cerebrovascular disease in stroke and dementia, and the expected substantial increase in prevalence of both,37 our results are highly relevant for public health. Further, hypertension remains one of the major cardiovascular public health problems: the age-adjusted prevalence of hypertension in US adults ≥20 years was estimated to be 34.0% in NHANES in 2011 to 2014, equating an estimated 85.7 million adults, and is projected to increase to ≈41.4% of US adults by 2030. In addition, hypertension remains uncontrolled in most hypertensive adults: only 45.4% of adults in the US are estimated to have ideal BP control.19 Thus, there is a clear gap to be filled in public health, and improvement in recognition, treatment and control of HTN may result in substantial reductions of CSVD and their consequences.
Prior clinical trials of hypertension treatment are limited in their assessment of CSVD incidence. However, in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, including patients with stroke due to CSVD, although targeting systolic BP of< 130 mm Hg did not significantly reduce all stroke, there was a significant reduction in intracerebral hemorrhage (by 63%), which in turn is mainly caused by CSVD.20 In the Systolic Blood Pressure Intervention Trial (SPRINT) Trial, hypertensive adults targeting SBP < 120 mm Hg had significantly smaller increase in cerebral white matter lesion volume over the follow up period (3.1 years),38 and patients in the intensive BP lowering group tended to have reduced stroke recurrence, significant for ICH, which again is mainly due to CSVD.39 Despite the limited data from clinical trials, treatment of HTN is already recommended for primary and secondary cardiovascular disease prevention guidelines. Although our observations are limited by the epidemiological nature of the study design, our results emphasize the importance of implementing effectively those guidelines. We submit that hypertension remains a major target to minimize the public health impact of CSVD, but clinical trials with long follow up are needed to clarify blood pressure targets for prevention of CSVD progression and consequent stroke and dementia.
The mechanisms of injury by which HTN leads to increased prevalence of CSVD are likely multiple, and may also play synergistic roles, including vascular injury related to BP variability and fluctuating perfusion (hypo – hyper perfusion),40 ischemia,41 vascular inflammation42 and endothelial dysfunction.43 Importantly, most of these mechanisms are potential treatment targets.
Our study has several strengths including the large community-based sample, the accuracy of measurements, long term prospective follow up, the high reproducibility of the brain MRI measurements, both CMBs and CBIs. The limitations of our study come from the largely white race community cohort and cross-sectional design regarding outcome assessment, limiting the ability to evaluate the time relationship between the onset of hypertension and incident outcome events. Racial minorities have higher prevalence of untreated vascular risk factors, higher risk of subclinical CSVD and higher risk of stroke, which tends to occurs at younger age. Thus, it is likely that our findings underestimate the burden of CSVD in minorities, highlighting the pressing need for increased awareness and efforts to control hypertension early in life. However, further studies are needed including racial minorities to confirm this hypothesis. An additional limitation in our study is that at this time we are unable to evaluate the effect in specific subgroups of hypertension such as treatment resistant hypertension, or the effects of antihypertensive medication class, or number of treatments. Lastly, we are unable to assess mechanistic effects of sustained hypertension on CSVD such as blood brain barrier breakdown.
In conclusion, hypertension and duration of exposure to hypertension are strong influential risk factors for covert CSVD, both CMBs and CBIs in our study. Future research directions include additional studies in racial minorities to corroborate our findings, consideration of clinical trials of hypertension control with thorough evaluation of incident cerebral small vessel disease, and evaluation of impact in cognitive function.
Perspectives
HTN remains a clear target for prevention of cerebrovascular injury and its consequences, including dementia and stroke. Our results show that hypertension present since midlife and persistent at older age is strongly associated with measures of CSVD in late life. The effect of hypertension on clinically covert CSVD, CMBs and CBIs, is likely insidious as shown by our results comparing H-H to N-H, suggesting damaging effect of long-term exposure on the cerebral vasculature. The results on burden of CMBs and CBIs, while limited by small number of events, suggest that duration of exposure of hypertension is important for higher burden of CMBs. Further studies are needed to clarify optimal levels of blood pressure for prevention of CSVD prevention.
Supplementary Material
Novelty and significance.
What is New?
We studied the relation of hypertension trends from mid to late life in a large prospective community based cohort of individuals free of stroke and dementia, and found that the duration of hypertension exposure is related to late life presence and burden of cerebral small vessel disease (CSVD).
What is Relevant?
Cerebral small vessel disease is the main type of cerebrovascular disease detected in individuals with stroke and dementia. Hypertension is the main modifiable vascular risk factor for CSVD, and one of the strongest risk factors for clinical stroke and dementia. The public health impact of hypertension is expected to increase as the population ages: 1 in 3 Americans will develop hypertension throughout their lifetime, and a large proportion of the population remains untreated. Similarly, the prevalence of dementia and stroke is expected to increase dramatically over the next few decades.
Summary
Our findings highlight a tremendous opportunity to increase recognition and effective treatment of hypertension early in life, which could prevent both CSVD and its related consequences.
Sources of Funding
This work (design and conduct of the study, collection and management of the data) was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195; HHSN268201500001I, 75N92019D00031) and by grants from the National Institute of Neurological Disorders and Stroke (R01 NS017950), the National Institute on Aging (R01 AG008122; K23AG038444; R03 AG048180-01A1; AG054076, AG058589, AG049607, AG059421); NIH grant (1RO1 HL64753; R01 HL076784; 1 R01 AG028321, P30 AG010129, NS017950), and NHLBI grants (HL67288, 2K24HL04334 and T32 HL125232).
Footnotes
Disclosures
None
References
- 1.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM, Microbleed Study G. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das AS, Regenhardt RW, Vernooij MW, Blacker D, Charidimou A, Viswanathan A. Asymptomatic cerebral small vessel disease: Insights from population-based studies. J Stroke. 2019;21:121–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, nEuroimaging STfRVco. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in chinese vs white populations: A systematic review. Neurology. 2013;81:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the national alzheimer’s coordinating centre. Brain. 2013;136:2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37:550–555 [DOI] [PubMed] [Google Scholar]
- 8.Akoudad S, Portegies ML, Koudstaal PJ, Hofman A, van der Lugt A, Ikram MA, Vernooij MW. Cerebral microbleeds are associated with an increased risk of stroke: The rotterdam study. Circulation. 2015;132:509–516 [DOI] [PubMed] [Google Scholar]
- 9.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero JR, Beiser A, Himali JJ, Shoamanesh A, DeCarli C, Seshadri S. Cerebral microbleeds and risk of incident dementia: The framingham heart study. Neurobiol Aging. 2017;54:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koennecke HC. Cerebral microbleeds on mri: Prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171 [DOI] [PubMed] [Google Scholar]
- 12.Copenhaver BR, Hsia AW, Merino JG, Burgess RE, Fifi JT, Davis L, Warach S, Kidwell CS. Racial differences in microbleed prevalence in primary intracerebral hemorrhage. Neurology. 2008;71:1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke. 2000;31:2665–2669 [DOI] [PubMed] [Google Scholar]
- 14.Imaizumi T, Horita Y, Hashimoto Y, Niwa J. Dotlike hemosiderin spots on t2*-weighted magnetic resonance imaging as a predictor of stroke recurrence: A prospective study. J Neurosurg. 2004;101:915–920 [DOI] [PubMed] [Google Scholar]
- 15.Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on t2*-weighted gradient-echo mr images. AJNR Am J Neuroradiol. 2003;24:88–96 [PMC free article] [PubMed] [Google Scholar]
- 16.Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003;34:2459–2462 [DOI] [PubMed] [Google Scholar]
- 17.Sepehry AA, Lang D, Hsiung GY, Rauscher A. Prevalence of brain microbleeds in alzheimer disease: A systematic review and meta-analysis on the influence of neuroimaging techniques. AJNR Am J Neuroradiol. 2016;37:215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bromfield S, Muntner P. High blood pressure: The leading global burden of disease risk factor and the need for worldwide prevention programs. Curr Hypertens Rep. 2013;15:134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 20.Group SPSS, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: The sps3 randomised trial. Lancet. 2013;382:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY, Clinical Research Collaboration NETTN, the PI. Clopidogrel and aspirin in acute ischemic stroke and high-risk tia. N Engl J Med. 2018;379:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldholm RS, Persson K, Barca ML, Knapskog AB, Cavallin L, Engedal K, Selbaek G, Skovlund E, Saltvedt I. Association between vascular comorbidity and progression of alzheimer’s disease: A two-year observational study in norwegian memory clinics. BMC Geriatr. 2018;18:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Viswanathan A, Marshall GA, Alzheimer’s Disease Neuroimaging I. Vascular disease and risk factors are associated with cognitive decline in the alzheimer disease spectrum. Alzheimer Dis Assoc Disord. 2015;29:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: The rotterdam scan study. Neurology. 2008;70:1208–1214 [DOI] [PubMed] [Google Scholar]
- 25.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM, Rotterdam Scan S. Incidence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2003;34:392–396 [DOI] [PubMed] [Google Scholar]
- 26.Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the framingham heart study. Stroke. 2014;45:1492–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong KS. Blood pressure management for stroke prevention and in acute stroke. J Stroke. 2017;19:152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the framingham heart study. N Engl J Med. 2016;374:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seshadri S, Wolf PA, Beiser A, Vasan RS, Wilson PW, Kase CS, Kelly-Hayes M, Kannel WB, D’Agostino RB. Elevated midlife blood pressure increases stroke risk in elderly persons: The framingham study. Arch Intern Med. 2001;161:2343–2350 [DOI] [PubMed] [Google Scholar]
- 30.McGrath ER, Beiser AS, DeCarli C, Plourde KL, Vasan RS, Greenberg SM, Seshadri S. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89:2447–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, Knopman DS, Power MC, Rawlings AM, Mosley TH, Gottesman RF. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, Rosand J. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein e by gene amplification and cleavage with hhai. J Lipid Res. 1990;31:545–548 [PubMed] [Google Scholar]
- 34.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O’Donnell CJ, Yoshita M, D’Agostino RB Sr., DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the framingham offspring study. Stroke. 2008;39:2929–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510 [DOI] [PubMed] [Google Scholar]
- 36.Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: Evolution of the boston criteria. Stroke. 2018;49:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell SL. Advanced dementia. N Engl J Med. 2015;373:1276–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Group SMIftSR Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Doshi J, Erus G, Fine LJ, Gaussoin SA, Harris D, Johnson KC, Kimmel PL, Kurella Tamura M, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr., Wright CB, Williamson JD, Bryan RN. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitagawa K, Yamamoto Y, Arima H, Maeda T, Sunami N, Kanzawa T, Eguchi K, Kamiyama K, Minematsu K, Ueda S, Rakugi H, Ohya Y, Kohro T, Yonemoto K, Okada Y, Higaki J, Tanahashi N, Kimura G, Umemura S, Matsumoto M, Shimamoto K, Ito S, Saruta T, Shimada K, Recurrent Stroke Prevention Clinical Outcome Study G. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke: A randomized clinical trial and meta-analysis. JAMA Neurol. 2019. DOI: 10.1001/jamaneurol.2019.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, Papatheodorou S. Blood pressure variability and cerebral small vessel disease: A systematic review and meta-analysis of population-based cohorts. Stroke. 51:82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau KK, Li L, Simoni M, Mehta Z, Kuker W, Rothwell PM, Oxford Vascular S. Long-term premorbid blood pressure and cerebral small vessel disease burden on imaging in transient ischemic attack and ischemic stroke. Stroke. 2018;49:2053–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ihara M, Yamamoto Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke. 2016;47:554–560 [DOI] [PubMed] [Google Scholar]
- 43.Avolio A, Kim MO, Adji A, Gangoda S, Avadhanam B, Tan I, Butlin M. Cerebral haemodynamics: Effects of systemic arterial pulsatile function and hypertension. Curr Hypertens Rep. 2018;20:20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


