Abstract
Hypertension and atherosclerosis, the predecessors of stroke and myocardial infarction, are chronic vascular inflammatory reactions. Tumor necrosis factor alpha (TNFα), the “master” proinflammatory cytokine, contributes to both the initiation and maintenance of vascular inflammation. TNFα induces reactive oxygen species (ROS) production which drives the redox reactions that constitute “ROS signaling.” However, these ROS may also cause oxidative stress which contributes to vascular dysfunction. Mice lacking TNFα or its receptors are protected against both acute and chronic cardiovascular injury. Humans suffering from TNFα-driven inflammatory conditions such as rheumatoid arthritis and psoriasis are at increased cardiovascular risk. When treated with highly specific biologic agents that target TNFα signaling (Etanercept, etc.) they display marked reductions in that risk. The ability of TNFα to induce endothelial dysfunction, often the first step in a progression toward serious vasculopathy, is well recognized and has been reviewed elsewhere. However, TNFα also has profound effects on vascular smooth muscle cells (VSMCs) including a fundamental change from a contractile to a secretory phenotype. This “phenotypic switching” promotes proliferation and production of extracellular matrix proteins which are associated with medial hypertrophy. Additionally, it promotes lipid storage and enhanced motility, changes that support the contribution of VSMCs to neointima and atherosclerotic plaque formation. This review focuses on the role of TNFα in driving the inflammatory changes in VSMC biology that contribute to cardiovascular disease. Special attention is given to the mechanisms by which TNFα promotes ROS production at specific subcellular locations, and the contribution of these ROS to TNFα signaling.
Keywords: atherosclerosis, blood pressure, hypertension, LRRC8A, Nox1, reactive oxygen signaling, TNFα, vascular smooth muscle
According to 2016 statistics from the World Health Organization, ischemic heart disease and stroke remain the top 2 causes of mortality worldwide, causing approximately the same number of deaths as the next 7 diagnoses combined.1 These “final common pathways” of mortality are promoted by chronic vascular inflammatory conditions such as hypertension, atherosclerosis, and diabetes. Understanding the risk factors that predispose to cardiovascular disease allows identification of individuals who may benefit from preventative therapy. However, vascular dysfunction progresses slowly and is asymptomatic until very late in the disease process. Effective prevention must be administered for many years, which necessitates a very favorable safety profile. This highlights the critical need to understand basic mechanisms of vascular inflammation. Therapies for hypertension that target blood pressure normalization may tangentially address the underlying vascular inflammation but do not treat it directly. Cholesterol lowering drugs like statins do address vascular inflammation by lowering serum levels of proinflammatory lipids, however, vascular disease still kills numerous individuals who have no abnormalities in their lipid profile. Primary treatment of vascular inflammation holds great appeal but has been elusive. To effectively target the inflammatory process, we must identify key steps in relevant signaling pathways and fully understand the mechanisms by which they proceed.
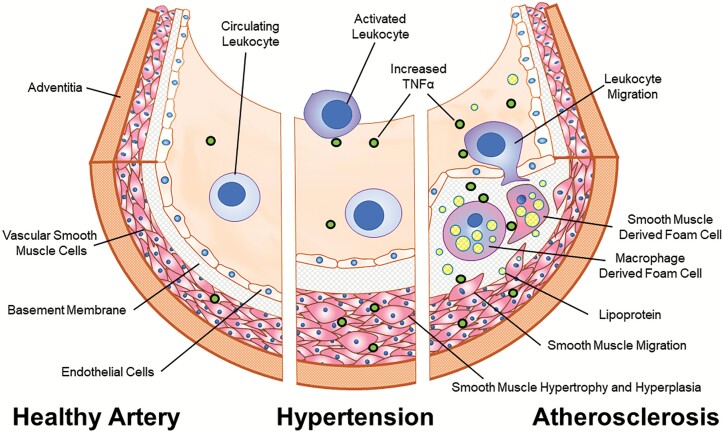
Blood vessels are composed of (i) an intimal layer of endothelial cells that cover a basement membrane, (ii) a media composed of layered, circumferentially oriented vascular smooth muscle cells (VSMCs) with interposed extracellular matrix, and (iii) an adventitia that includes extracellular matrix, fibroblasts, fat cells, nerve cells, and small arteries (vaso vasorum). All 3 layers are involved in the response to both acute and chronic inflammatory triggers. Tumor necrosis factor alpha (TNFα) is produced by inflammatory cells such as monocytes and neutrophils that invade the injured vascular wall, as well as by cells that are native to the tissue, particularly VSMCs. Circulating levels of TNFα are elevated and have been directly implicated in patients who develop cardiovascular disease including hypertension,2 atherosclerosis,3 and ischemic heart disease4 as well as in animal models of acute arterial injury.5 In addition, atherosclerotic plaques contain particularly high local levels of TNFα.6,7 An overview of the contributions of TNFα to the pathophysiology of vascular disease is provided in Figure 1.
Figure 1.
The role of TNFα in VSMC-related cardiovascular disease. In a healthy state, the primary functional components of the arterial wall; endothelial cells and VSMCs reside in a stable, interdependent and homeostatic state (left). In hypertension low grade inflammation is triggered by genetic, epigenetic, and/or environmental factors, and this is associated with increased serum and vascular levels of TNFα. This contributes to the development of altered endothelial and VSMC function, inducing both structural (hyperplasia and hypertrophy) and functional (contractility) changes (center). Atherosclerotic plaques begin as fatty streaks where endothelial injury results in local recruitment of macrophages and VSMCs to the intima. TNFα promotes phenotypic switching of VSMCs which is associated with proliferation, enhanced motility, and increased secretion of extracellular matrix proteins and lipid uptake (right). The lipid laden foam cells which constitute the bulk of cells within an atheroma are composed of approximately equal numbers of macrophages and cells of VSMC lineage which are indistinguishable histologically. Persistent high local levels of TNFα can lead to apoptosis and necrosis, destabilization of the neointima, and eventually plaque rupture with vessel occlusion. Abbreviations: TNFα, tumor necrosis factor alpha; VSMC, vascular smooth muscle cell.
A very proximal step of TNFα signaling in VSMCs is the production of extracellular superoxide anion (O2·−) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (Nox1).8,9 This signaling step is shared by several other signaling molecules that are also key drivers of vascular inflammation including platelet-derived growth factor, Angiotensin II (Ang II), and interleukin-1beta.9,10 A recurring challenge in understanding mechanisms of vascular inflammation is distinguishing the role of O2·− and its reactive oxygen species (ROS) metabolites in signaling from potentially independent deleterious effects of oxidants on cellular metabolism and survival. This review will focus on how the responses of VSMCs to TNFα contribute to vascular inflammation in hypertension and atherosclerotic disease. Current understanding of the mechanisms by which O2·− supports TNFα signaling and oxidative stress in VSMCs will be explored in detail.
TNFα AND VSMC IN VASCULAR DISEASE
Phenotypic switching
A fundamental mechanism by which inflammation promotes vascular disease is via a change in VSMC gene and protein expression from a pattern that promotes “normal” contractile function, to one supporting an increased capacity for migration and secretion of extracellular matrix proteins. This switch is associated with a decrement in the expression of proteins that support contractile function (myosin heavy chain, smooth muscle alpha actin, calponin, etc.) and increased expression of extracellular matrix proteins such as type I collagen and osteopontin.11 These phenotypic changes facilitate VSMC migration out of the media into the intimal layer. Once there, they can take on the appearance of fibroblasts or become indistinguishable from macrophage-derived foam cells and participate in formation of a neointima. Phenotypic switching is of fundamental importance to the atherosclerotic process. However, the roles of VSMCs are complex and stage-dependent. Recent consensus suggests that VSMC proliferation may be predominantly reparative and may not be the primary driver of plaque formation, while the role of migration remains controversial. In contrast, VSMC death and senescence clearly appear to promote atherogenesis and likely contribute to plaque instability.11
There was a longstanding controversy as to the primary source of the VSMCs that are responsible for the acute repair of injured blood vessels. While there may be some contribution from circulating VSMC progenitor cells, the primary cells responsible for neointima formation are now accepted to be resident VSMCs of the media that have undergone phenotypic modulation. These changes are widespread throughout the media and are thought to be reversible.11,12 A variety of signaling pathways promote phenotypic switching including growth factors such as platelet-derived growth factor,13 transforming growth factor β, vasoconstrictors such as endothelin-1 and Ang II,12 and cytokines such as TNFα and interleukin-1beta. Importantly, in addition to their proinflammatory signals, all these factors increase VSMC O2·− production which can reduce local nitric oxide concentrations and promote oxidative injury.
Abdominal aortic balloon injury in rabbits induces a subset of more proliferative VSMCs that also produce more TNFα. This suggests that VSMC-derived TNFα serves as a marker of a modulated smooth muscle cell phenotype after acute vascular injury.14 These local changes in TNFα abundance may also independently drive phenotypic changes in VSMCs. In pigs, chronic local exposure to TNFα-induced alterations in the smooth-muscle myosin heavy chain isoform expression that were consistent with VSMC dedifferentiation.15 In addition, the well-established ability of TNFα to promote either growth or apoptosis may be impacted by VSMC phenotype. Two stable subpopulations of VSMCs were isolated from human saphenous vein: spindle and epithelioid-shaped VSMCs. TNFα stimulated growth of spindle shaped cells but caused apoptosis of epithelioid ones which expressed higher levels of the type 1 TNFα receptor (TNFR1).16
Several signaling mechanisms contribute to TNFα-induced phenotypic switching. Proximal signaling involves phosphoinositide 3-kinase γ activation as demonstrated by inhibition or genetic knockdown of phosphoinositide 3-kinase γ in rat aortic aortic smooth muscle cells (SMCs), which inhibited TNFα-induced downregulation of VSMC contractile genes and increased proliferation and migration.17 A further downstream, but critical component of the response to TNFα is activation of the nuclear factor-kappaB (NF-κB) transcription factor, a master regulator of inflammation.18 Neointima formation was markedly reduced following carotid injury in VSMC-specific knockout mice that are unable to activate NF-κB (Ikappaβ null).19 Switching is also influenced by epigenetic mechanisms. Micro RNA-155 (miR-155) expression is increased in apolipoprotein E (ApoE) null mice on a high fat diet and in patients with atherosclerosis. TNFα-induced miR-155 expression in vessel segments and in cultured VSMCs which induced phenotypic switching in an NF-κB-dependent manner.20 Circular RNAs (circRNAs) are noncoding RNAs formed by back-splicing of exons to form a closed loop structure. This makes them highly stable in vivo compared with linear RNAs. Sirtuin 1 (Sirt1) is a histone deacetylase that can also deacetylate and inactivate the p65 subunit of NF-κB in response to TNFα, thereby mitigating the transcriptional response to the cytokine.21 A circRNA that arises from the Sirt1 gene (Circ-Sirt1) inhibits phenotypic switching of VSMCs in response to TNFα. This occurs via 2 mechanisms: (i) binding to and sequestration of NF-κB (p65) in the cytoplasm and (ii) binding to miR-132/212, which is known to degrade Sirt1 mRNA, thereby enhancing expression of Sirt1.22 TNFα also can induce phenotype changes via myocardin and Kruppel-like transcription factor 4 (KLF4)-regulated pathways. Targeting of KLF4 with small-interfering RNA (siRNA) blocked TNFα activation of inflammatory genes and suppression of contractile genes, and TNFα inhibition reversed pathologic vessel wall alterations in hypertension and under hemodynamic stress.23 Finally, atheromatous plaques have increased autophagy which is induced by TNFα and mediates protein and intracellular organelle degradation. The ability of TNFα to induce phenotypic switching in VSMCs is prevented by inhibition of autophagy.24
Hypertension
TNFα contributes to the vascular inflammation and remodeling25 which underlies the development of hypertension in humans.26 Ang II-induced hypertension was abrogated in TNFα knockout mice. Furthermore, administration of exogenous TNFα restored the increase in blood pressure induced by Ang II to levels similar to those observed in wild-type mice.27 Disruption of TNFα signaling using a biologic agent that binds up the free cytokine (Etanercept) also prevented Ang II-induced hypertension and aortic O2·− production in mice.28 Similarly, TNFR1 knockout mice were protected from ethanol-induced hypertension and displayed reduced O2·− in the aorta compared with wild-type mice.29 TNFα may also play an important role in the inflammatory response that drives pulmonary hypertension. In a rat model of monocrotaline-induced pulmonary hypertension, and in cultured pulmonary arterial VSMCs exposed to hypoxia, downregulation of miR-140-5p and upregulation of TNFα were observed. Furthermore, miR-140-5p directly targeted TNFα message for degradation and overexpression of this miRNA mitigated the rise in pulmonary blood pressure as well as proliferation, migration, and phenotypic variation of cultured pulmonary artery SMCs.30 Collectively, these reports suggest an important role for TNFα-induced inflammation in hypertension31, but they cannot discern the contributions of endothelial vs. VSMC inflammation or effects related to renal inflammation.32 Importantly, the response to TNFα differs remarkably between cultured endothelial cells and VSMCs. The predominant response of endothelial cells is cell death33,34 while VSMCs respond by increases in proliferation35–37 and migration.38 VSMCs produce hydrogen peroxide (H2O2) in response to TNFα 9 and this response has been linked to “hypertrophy” of individual VSMCs as reflected by the aggregate protein/DNA ratio of cultured cells.39
Human studies also support the association of TNFα with hypertension. While increased production of TNFα has been associated with essential hypertension and its various complications,40 it is challenging to isolate the pathophysiologic influence of TNFα in a complex environment of vascular inflammation. However, the more rare A allele at a polymorphic site in the promoter region of the TNFα gene (-308G/A) has consistently been associated with hypertension, including in a recent meta-analysis.41 The A allele has a significant positive effect on TNFα transcription in reporter gene assays.42 In addition to essential hypertension, TNFα also appears to play an important role in the inflammatory response associated with preeclamptic hypertension. Serum levels of TNFα are significantly higher in preeclamptic compared with normotensive pregnant women.43 This association is supported by animal data demonstrating that TNFα causes greater enhancement of phenylephrine-dependent contraction in aortae from pregnant compared with nonpregnant rats,44 and chronic infusion of TNFα increases mean arterial pressure in pregnant rats.45
As noted above in pregnant mice, TNFα can directly impact vascular contractility. In vivo TNFα infusion for 14 days increased in vitro aortic contractility compared with saline-treated controls.46 While a similar 14 day exposure to TNFα did not alter blood pressure in wild-type mice, it caused hypertension in interleukin 10 null animals and enhanced both aortic and mesenteric contractile responses to endothelin-1.47 VSMC-derived TNFα also augments myogenic tone in cerebral48 and skeletal muscle49 arterioles from humans and in murine mesenteric and olfactory resistance vessels.49 Furthermore, both inducible deletion of the TNFα gene selectively in smooth muscle cells, or blocking signaling with Etanercept, reduced total peripheral resistance and blood pressure in mice and were associated with a reduction in resistance artery myogenic responsiveness.50
Atherosclerosis
The leading cause of cardiovascular-associated mortality worldwide is atherosclerosis,51,52 the process through which vascular inflammation promotes fat deposition and immune cell infiltration into the vascular wall to form obstructive plaques. Vascular inflammation begins with endothelial cell dysfunction that can be triggered by a number of genetic and/or environmental factors31. This attracts and promotes the invasion of circulating monocytes/macrophages, which release TNFα in response to oxidized low-density lipoprotein.53 This mechanism is highlighted by the observation that rats injected with oxidized low-density lipoprotein display increased arterial TNFα expression within 24 hours.54 TNFα propagates the atherosclerotic process in part by reducing intracellular metabolism of lipids, allowing them to accumulate in specific macrophage and VSMC-derived foam cells55 that are virtually indistinguishable from each other. However, a large proportion of human neointimal and atherosclerotic lesions is composed of VSMC lineage cells.11,56 In addition to phenotypic VSMCs that are present in human atherosclerotic lesions, approximately half of the foam cells are derived from VSMCs that have undergone phenotypic switching.57,58 Similarly, lineage tracking in murine atheromas demonstrates that VSMC-derived cells account for approximately 70% of foam cells in ApoE null mice fed a Western diet for 6 or 12 weeks or a chow diet for longer periods.59 Importantly, the final common pathologic pathway of atherosclerotic lesions is cellular necrosis and apoptosis, which TNFα can trigger in VSMCs leading to plaque rupture and acute vessel occlusion.60
There has been some controversy regarding the role of TNFα in murine models of atherosclerosis. Perhaps the strongest evidence for the role of TNFα has been derived from mice that are both ApoE and TNFα deficient. Loss of ApoE decreases cholesterol release from foam cells which enhances inflammation and promotes atheroma development.61 When mice deficient in both ApoE and TNFα were fed a cholesterol-rich diet, they had a 50% reduction in the relative atherosclerosis lesion size after 10 weeks compared with ApoE null controls. Similar mice also demonstrated a reduced number of advanced atherosclerotic lesions (53.9% vs. 78.6%) as well as less necrosis and apoptosis within those lesions.62 Further, bone marrow transplantation of ApoE-deficient mice with dual ApoE/TNFα-deficient bone marrow resulted in a reduction of atherosclerotic lesion size of 83% compared with controls after 25 weeks of a cholesterol-rich diet.63 This suggests that TNFα from invading white blood cells is critical to the atherosclerotic process. TNFα null mice were also dramatically protected from neointima formation in response to carotid ligation,64 suggesting that TNFα can play an important role in atherogenesis independent of lipid status. In contrast to these findings, in low-density lipoprotein receptor knockout animals, use of a TNFα inhibitor had a mixed effect, reducing evidence of systemic inflammation but leading to increased plaque formation.65 The role of endothelial vs. VSMC responses to TNFα in these atherosclerosis models remains an important unknown.
A variety of human studies support a role for TNFα in atherosclerosis. The same -308G/A polymorphism in the TNFα promoter that affects hypertension also impacts atheroma formation. Once again, the A allele confers an increased risk of coronary artery disease.66,67 Since the 1990s, TNFα inhibitors have been used clinically to treat inflammation associated with autoimmune diseases including rheumatoid arthritis, psoriasis, and inflammatory bowel disease.68 These patients are chronically inflamed and have a higher incidence of cardiovascular morbidity and mortality compared with the general population.69 The use of TNFα inhibitors in these disorders has been associated with a reduction in cardiovascular complications. This effect was well demonstrated in rheumatoid arthritis patients receiving anti-TNFα therapy who showed a marked reduction in the incidence of cardiovascular disease compared with controls.70 A recent meta-analysis showed that anti-TNFα therapy reduced the overall incidence of cardiovascular events in another cohort of patients with rheumatoid arthritis, though the relatively low sample size and heterogeneity of patients studies impaired the statistical significance.71 Patients with inflammatory bowel disease also have an increased risk of cardiovascular events compared with the general population72 and patients with inflammatory bowel disease who received anti-TNFα therapy showed a reduction in arterial stiffness compared with those who did not. Increased stiffness is associated with atherosclerosis and increased cardiovascular disease.73,74 However, not all inflammatory bowel disease patients receiving TNFα inhibitors have exhibited the same protection, suggesting a mixed picture that may be dependent upon disease severity.75
REACTIVE OXYGEN IN TNFα SIGNALING
A common pathophysiologic finding in hypertension and atherosclerosis is that both are associated with a more oxidized microenvironment in the vasculature and both endothelial cells and VSMCs are under “oxidative stress.” 76,77 There is a complex and relatively poorly understood interdependence between ROS generators (e.g., mitochondria and NADPH oxidases) and cellular antioxidant systems. Antioxidants are present diffusely within the cytoplasm (glutathione, vitamins C and E, etc.) and can also be more highly localized within subcellular compartments such as peroxisomes or in multiprotein complexes that incorporate antioxidant enzymes such as thioredoxins, peroxiredoxins, superoxide dismutase (SOD), or catalase. Relative deficiency or defective localization of antioxidant protection may theoretically be as damaging as overproduction of ROS. The need to better understand the localization of redox-dependent signaling events is highlighted by the fact that nonspecific antioxidant supplementation has thus far not proven to provide effective treatment of cardiovascular diseases.78,79
Many of the critical proinflammatory drivers of vascular inflammation (e.g., Ang II, endothelin-1, platelet-derived growth factor, interleukin-1beta, thrombin) share a critical commonality with TNFα signaling, that is a requirement for O2·− production by Nox enzymes. It has been proposed that oxidative stress can result from excessive activation of NADPH oxidases as part of these ROS-dependent signaling pathways. Given the large number of redox reactions within the cell, and the risks associated with off-target oxidation, it seems likely that effective redox-dependent signaling requires highly localized production of ROS. While we know relatively little about molecular colocalization of ROS generators with downstream targets, it seems likely that both tight local control of oxidant production and localization of antioxidant systems contribute to the creation of spatial and temporal constraints on “normal” signaling. Causes of oxidative stress may therefore include the disruption of local control of ROS production or scavenging. This might include failure of negative feedback or inappropriate activation of positive feedback influences on ROS signaling.80
An ideal therapeutic intervention to address oxidative stress will need to selectively target excessive ROS production without disrupting the relatively low levels of ROS production that are required for normal signaling and to support the many redox reactions that are part of normal biochemical homeostasis. These concepts present novel investigatory challenges and highlight the need to develop a detailed understanding of the topography and chronology of ROS generation. Using TNFα signaling in VSMCs as a model system, we will now focus on how localized ROS production and metabolism may confer specificity to subsequent signaling steps.
TNFα receptors
TNFα activates 2 receptor subtypes, both of which are expressed in VSMCs. TNFR1 and TNFα receptor type 2 (TNFR2) both share homology with the Fas death receptor, but only TNFR1 has a death domain that can activate caspase (Figure 2).81 This domain binds to the TNF receptor-associated death domain (TRADD) protein which can promote either apoptosis via association with the Fas-associated death domain (FADD) protein, or inflammation via TNF receptor-associated factor 2 (TRAF2) which promotes activation of NF-κB and leads to VSMC proliferation. TNFR2 signals only through the TRAF2-dependent pathway. Thus, TNFR1 activation appears to initiate most of the deleterious effects of TNFα, while TNFR2 receptors modify this response. Since the inflammatory response of cultured aortic VSMCs to TNFα was completely dependent on TNFR1,82 we will focus on this signaling pathway.
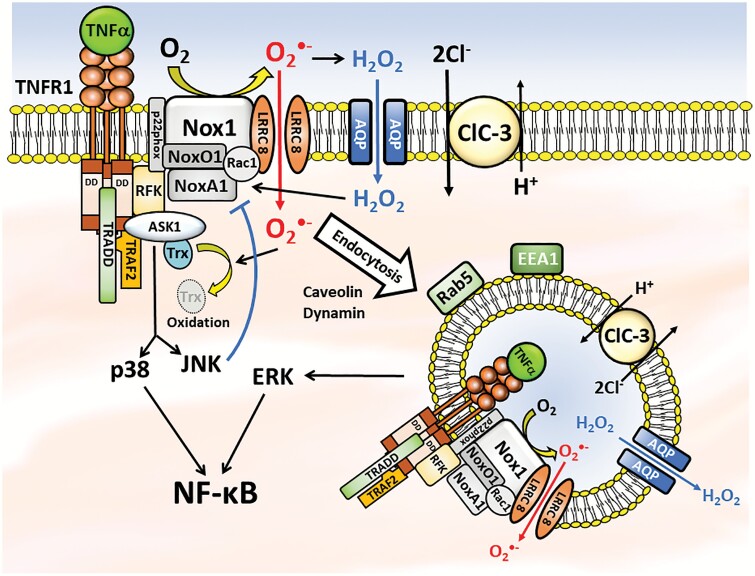
Figure 2.
Schematic representation of the complex interplay between the TNFR1, Nox1, and anion channels in oxidant-dependent signaling. The close association of these 3 proteins along with aquaporins may provide a mechanism by which highly localized changes in O2·− and H2O2 concentrations can affect intracellular signaling processes. Furthermore, endocytosis of redox-active vesicles that can by trafficked along the cytoskeleton may allow local delivery of oxidants throughout the cytoplasm. Collectively, this inflammatory signaling pathway influences NF-κB activation via redox-dependent activation of ASK1. Downstream activation of the p38 MAPK is a prime driver of NF-κB activation while JNK provides negative feedback regulation of Nox1. ERK also contributes to NF-κB activation but by an ASK1-independent mechanism that requires receptor endocytosis. LRRC8 anion channels support Nox1 activity at the plasma membrane while ClC-3 appears to be required for generation of endosomal O2·−. Understanding the details of O2·−-dependent TNFα signaling may identify novel opportunities to disrupt the process for therapeutic purposes. Abbreviations: ASK1, apoptosis signal-regulating kinase 1; ClC-3, Chloride Channel 3; ERK, extracellular signal-regulated kinase; H2O2, hydrogen peroxide; JNK, c-jun N-terminal kinase; LRRC8, leucine-rich repeat containing 8; NF-κB, nuclear factor-kappaB; Nox1, nicotinamide adenine dinucleotide phosphate oxidase 1; O2·−, superoxide; TNFα, tumor necrosis factor alpha; TNFR1, TNFα receptor type 1.
Serum levels of “soluble” TNFR1 correlate directly with cardiovascular risk,2,83–86 particularly in women.83,86 These receptors were initially thought to represent only the extracellular TNFα-binding portion of the protein that had been proteolytically cleaved from the membrane by TNFα converting enzyme (TACE, ADAM 17).87 However, the predominant form of plasma TNFR1 is the full length membrane spanning protein expressed on exosomal vesicles.88 These particles are now recognized to mediate intercellular communication and act as important modulators of inflammation.89 The “outside out” orientation of TNFR1 allows them to bind circulating TNFα which has been theorized to act as a TNFα “sink,” but this orientation may also target these vesicles to cells expressing cell surface TNFα. The precise biologic role of exosomes is an exciting topic of current investigation.
The topography of ROS signaling
The critical requirement for NADPH oxidase activation in TNFα signaling in VSMCs was first established by the observation that p22phox, an essential membrane protein that is part of all Nox enzymes, was required.8 Nox1 was subsequently identified as the important isoform in VSMCs.9 NADPH oxidases localize to specific membrane regions including ruffles, lamellopodia, focal complexes, and endosomes.90 In resting VSMCs Nox1 was found in caveolae and lipid rafts.91 Neointimal VSMCs display enhanced Nox1 expression92 and heterologous Nox1 overexpression in VSMCs potentiates Ang II-induced hypertension and medial hypertrophy.93 Increased O2·− production by Nox1 impairs endothelium-dependent relaxation by reducing nitric oxide bioavailability via combined effects of oxidative endothelial nitric oxide synthase (eNOS) uncoupling and direct scavenging of nitric oxide by O2·−.94 Fully functional Nox1 requires not only membrane association with p22phox, but also recruitment of 3 cytoplasmic proteins: Nox Organizer 1 (NOXO1), Nox Activator 1 (NOXA1), and the Rac1 GTPase (Figure 2). TNFα causes Nox1 to be phosphorylated by the β1 subtype of protein kinase C at T429 and this event facilitates association between Nox1 and NOXA1. Thus, T429 phosphorylation is markedly increased by both acute and chronic vascular injury.95
The Nox1 complex is part of a larger multiprotein complex that supports TNFα signaling (Figure 2). TNFR1 is physically linked to p22phox through mutual binding to riboflavin kinase (RFK).96 This linkage is of functional importance because the product of RFK activity is flavin mononucleotide (FAD), an essential cofactor for all NADPH oxidases. Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen activated protein kinase kinase kinase (MAPKKK) that associates with the TNFR1 multiprotein complex via binding to TRAF2,97 and thus also coimmunoprecipitates with Nox1.98 Under resting conditions, ASK1 is inhibited by association with reduced thioredoxin (Trx). In response to oxidation, Trx dissociates from ASK1 causing activation of the kinase. TNFα induces Nox1-dependent activation of ASK1 in VSMCs.98 Targeting of ASK1 in VSMCs by pharmacologic inhibition or siRNA silencing reduced multiple downstream responses to TNFα including: c-jun N-terminal kinase (JNK) and p38 activation, phosphorylation of dynamin and caveolin, TNFα endocytosis and NF-κB activation.98 Thus, ASK1 appears to be an important redox-sensitive mediator of Nox1-dependent TNFα signaling.
Extracellular O2·− production by Nox1 is also required to initiate TNFR1 endocytosis. Uptake of labeled TNFα by VSMCs is disrupted by either siRNA knockdown of Nox1 or extracellular O2·− scavenging using exogenous extracellular SOD, but not by extracellular catalase.37 TNFα signaling continues in cytoplasmic vesicles following dynamin-dependent endocytosis of the occupied receptor.33 Nox1 generates superoxide into this compartment and endosomal ROS support specific aspects of the response of VSMCs to TNFα.9,99,100 The vesicles are early endosomes, coated by the early endosomal antigen 1 (EEA1) and the Rab5 GTPase.9 TNFα induces phosphorylation of both caveolin and dynamin via a process that is dependent upon ASK1 activation.98 Interference with the endocytic process (dominant negative dynamin) disrupts downstream extracellular signal-regulated kinase 1 and 2 (ERK1/2) and NF-κB activation, suggesting that ERK1/2 activation is driven by events that occur after receptor endocytosis.82 In contrast, activation of JNK was enhanced by inhibition of endocytosis, suggesting that TNFα activates this mitogen-activated protein kinase (MAPK) at the plasma membrane. The 2 MAPKs have very distinct downstream effects; JNK provides important feedback inhibition of Nox1 activity, while ERK1/2 promotes activation of NF-κB.82 Of note, TNFα-induced activation of p38, which also activates NF-κB and promotes inflammation, is indifferent to disruption of receptor endocytosis (Figure 2).
The mechanistic contribution of endosomal O2·− to TNFα signaling remains unknown. It may be important for local redox reactions or may be an important influence on endosomal pH, a critical parameter of endosomal function.101 In general, endocytic vesicles become progressively more acidic as they progress from early to late endosomes and then into lysosomes. However, Nox-derived O2·− has been shown to be a mechanism of phagosome alkalinization in dendritic cells,102 neutrophils, and macrophages.103 These effects are based on the ability of O2·− to consume protons during dismutation to H2O2. Therefore, phagosomal pH results from a balance of inward proton transport via the vacuolar ATPase (V-ATPase) and proton consumption by Nox-derived ROS. Alkaline conditions prolong the half-life of O2·− by approximately 10-fold for every 1 point rise in pH between pH 6 and 14.104 For this reason, the relative concentrations of O2·− and H2O2 within endosomes will be related to the pH of the compartment. Higher V-ATPase activity will create a compartment with a lower pH and a higher H2O2 content relative to O2·−. An alkaline endosome would still be likely to contain significant H2O2 (pKa 11.7) but would have a much higher concentration of O2·−. The redox biochemistry of endosomes has been discussed in detail elsewhere.105,106 We speculate that the presence of channels permeant to these oxidants could mediate pinpoint delivery to cytoplasmic targets based on trafficking of the vesicles via the cytoskeleton. While the molecular identity of an endosomal O2·− conductance remains unknown, one has been characterized in interleukin-1beta-induced Rab5 early endosomes from Michigan Cancer Foundation-7 (MCF-7) epithelial cells.107
Despite a growing recognition of the sequence of events associated with TNFα signaling in VSMC, it remains largely unclear how extracellular deposition of O2·− by Nox19,82 promotes specific cytoplasmic redox reactions that result in signaling. What are the critical extracellular oxidant species and what are their targets? For instance, by what mechanism does a cytoplasmic protein like thioredoxin bound to ASK1 become oxidized following deposition of O2·− into the extracellular space? Superoxide is rapidly and spontaneously converted into H2O2 in aqueous solution, and this reaction can be accelerated by SOD, 1 isoform of which (SOD type 3) is present in the extracellular space. H2O2 is much more stable than O2·− making it a superior paracrine signaling molecule, thus the contribution of H2O2 to TNFα signaling has received significant attention. Direct application of H2O2 to cultured rat aortic VSMCs activates ASK1 and induces hypertrophy.39 The site of action of H2O2 was found to be intracellular as these effects were blocked by siRNA targeting of aquaporin 1, the pathway through which H2O2 enters the cells. Importantly, H2O2 alone was not sufficient to activate ASK1, but surprisingly did activate Nox1, and this intermediate O2·− generating step initiated both ASK1 phosphorylation and subsequent VSMC hypertrophy. It is interesting to consider how extracellular O2·− might contribute to this process. It seems unlikely that formation of additional H2O2 is required in the presence of an already quite significant triggering concentration of H2O2. Alternatively, the Nox1-dependence of the response to H2O2 points to a critical role for O2·−, which is quite capable of oxidizing thiols.108 A key role for O2·− is consistent with the observation that exogenously applied and membrane impermeant SOD profoundly inhibited both TNFα endocytosis and JNK phosphorylation in response to TNFα, while extracellular catalase had no effect.37 This raises a critical question; how can a short-lived and charged molecule like O2·− directly influence an intracellular protein like thioredoxin? Might a pathway exist by which O2·− can directly cross membranes?
ANION CHANNEL MODULATION OF TNFα SIGNALING
Anion channels regulate a variety of critical functions and contribute significantly to membrane depolarization of activated VSMCs.109 They can also regulate the Cl− concentration of the cytoplasm or of intracellular compartments and these changes may also act as signaling effectors.110,111 The efficacy of TNFα signaling in VSMCs has been linked to 2 anion conductances: (i) the leucine-rich repeat containing 8A (LRRC8A) subunit of volume-regulated anion channels (VRACs) associates with Nox1 and is required for extracellular superoxide production37 and (ii) the Chloride Channel 3 (ClC-3) 2Cl−/H+ antiporter which is required for Nox1 activity in endosomes.9
LRRC8A volume-regulated anion channels
Maintenance of proper volume is an essential function of all cells. Cells swell in response to hypotonic conditions and this activates VRACs.112 The subsequent efflux of anions and organic molecules (e.g., taurine) helps to return cell volume to normal. The proteins responsible for VRACs belong to the LRRC8 family (A through E). Hexameric VRACs with diverse biophysical properties result from combinations of 2 or even 3 subtypes of LRRC8 proteins, but all VRACs that function at the plasma membrane must contain the LRRC8A subunit.113 While VRACs composed of LRRC8A and D appear to mediate the efflux of osmolytes such as taurine and myo-inositol,114 the physiologic roles of LRRC8 subtypes are only beginning to be explored.
LRRC8A coimmunoprecipitates with both Nox137 and ASK1.98 Nox1 and LRRC8A also colocalize by immunostaining in murine VSMCs.37 Thus, LRRC8A is part of the TNFR1 signaling complex. Furthermore, channel activity is required for proper function of Nox1 because extracellular O2·− production in response to TNFα is markedly reduced when LRRC8A expression is either targeted by siRNA or inhibited pharmacologically.37 The nature of the functional relationship between VRAC channels and Nox1 remains unknown. We have considered the possibility that Cl− ion movement through LRRC8A VRACs provides charge compensation which is required to maintain electron flow through NADPH oxidases.115 Nox2 derives this support from a proton channel in neutrophils and macrophages. The observation that LRRC8 currents can be activated or inhibited by oxidation in a subtype-dependent manner116 raises the potential for tight local redox-dependent feedback regulation of Nox1 by the oxidants that it produces. This stresses the need to explore which VRAC subtypes interact with Nox1 in VSMCs and how this impacts TNFα signaling.
In view of the importance of extracellular O2·− for TNFα signaling it is worth considering a second role for LRRC8A VRACs; providing a pathway by which O2·− can enter the cell facilitated by the association of Nox1 with the channel. It is not possible to determine the local concentration of O2·− at the extracellular surface of the oxidase, but it would need to be high enough to drive inward O2·− movement against a negative membrane potential. However, it is also worth considering that this potential may be mitigated by local effects of the magnetic field imposed by electron flow through Nox1 in extreme proximity to the channel. O2·− has an anionic radius of 140 pm, between that of fluoride (119 pm) and chloride (167 pm), and has been indirectly demonstrated to be capable of moving through anion channels.107 Intracellular flux of O2·− through an LRRC8A channel could support extremely tight localization of redox signaling. Physical association of Nox1, LRRC8A, and ASK1 would allow very small amounts of O2·− to provide a redox signal capable of ASK1 activation. One appeal of such a system is reduction of off-target redox reactions which are clearly a higher risk if extracellular Nox-derived H2O2 is the primary signal. A second appeal is that following endocytosis of this multiprotein complex, trafficking of endosomes through the cytoplasm might allow highly localized delivery of either O2·− via LRRC8A, or H2O2 via an aquaporin to intracellular targets at a significant distance from the plasma membrane without exposing the entire cytoplasm to oxidative stress.
The ClC-3 2Cl−/H+ antiporter
ClC-3 is a member of the chloride channel (ClC) family of Cl− channels and Cl−/H+ antiporters117 that is expressed in virtually all cell types. Only a small fraction of the protein is expressed on the cell surface and the vast majority of ClC-3 protein localizes to intracellular vesicles.118 Assayed by patch-clamp recording in the plasma membrane, ClC-3 is a functionally a unidirectional transporter that is oriented such that it is only capable of carrying outward current (Cl− in, H+ out).119 Following endocytosis ClC-3 becomes oriented such that in response to negative voltage in the vesicular lumen, Cl− is transported out and H+ in. Immediately following endosome formation, negative cell surface charges may create such a negative vesicle lumen, allowing a ClC protein to contribute to the rapid fall in Cl− concentration and acidification that follows endosome formation.105,120 This orientation of ClC-3 makes it appear challenging for ClC-3 to provide charge compensation for proton pumping into these vesicles by the V-ATPase as initially proposed,117 but is consistent with an ability to provide charge compensation for endosomal Nox. This concept is supported by the observation that both Nox1 in VSMCs9 and Nox2 in neutrophils121,122 require the presence of ClC-3 in order for O2·− to be produced within endosomes. Of note, although the impact of the loss of ClC-3 on extracellular O2·− production in VSMCs has not been assessed, this function is completely unaffected in neutrophils.123 Taken together, these data are consistent with LRRC8A playing the key role supporting Nox1 at the plasma membrane while ClC-3 may become a critical partner for the oxidase in endosomes.
SUMMARY AND FUTURE DIRECTIONS
TNFα is an important driver of the inflammatory process that underlies the vascular pathology associated with both hypertension and atherosclerosis. A key response of VSMCs to TNFα is phenotypic switching which results in cells that are less contractile and more motile and proliferative. TNFα signaling is achieved via a multiprotein complex which incorporates key functionalities including: (i) generation of reactive oxygen by Nox1 which is required for multiple signaling steps including receptor endocytosis, (ii) charge compensation and/or superoxide conduction by LRRC8A anion channels, and (iii) redox signal sensing and transduction by thioredoxin/ASK1. The net response to TNFα is a complex mixture of signaling events occurring at both the plasma membrane and within early endosomes following receptor endocytosis.
The ultimate goal of understanding the molecular mechanisms of TNFα signaling is to identify novel ways to selectively interfere with the process. A survey of recent patent applications reveals that several companies are developing small molecule inhibitors for Nox1,124 and a mixed Nox1/Nox4 inhibitor (GTK137831) was shown to reduce atherosclerosis in mice.125 Monoclonal antibodies like Etanercept that selectively target TNFα signaling are first-line therapy for autoimmune inflammatory disease126 and also ameliorate the increased cardiovascular risk that is associated with these conditions.127–131 Unfortunately, due to their high target affinity, they completely block TNFα signaling, disrupting its adaptive roles, and increasing risk of infection and cancer. This precludes their use as primary preventative therapy in cardiovascular disease, even in patients at very high risk. An ideal agent for targeting the inflammation associated with cardiovascular disease would be titratable and capable of “normalizing” cytokine signaling while preserving the essential function of these important pathways. Ion channels are established targets of lower affinity ligands that allow titratable therapy of arrhythmias, hypertension and seizures. Selective Nox inhibitors or LRRC8 family channel blockers have the potential to downregulate TNFα signaling as well as multiple other proinflammatory signaling pathways simultaneously by targeting a shared mechanism. Finally, improved understanding of how oxidants support these signaling pathways may provide novel approaches to selective inhibition through the use of antioxidant agents that target-specific local reactions.
FUNDING
This work was supported by National Health, Lung, Blood Institute (R01 HL128386, T32 HL 144446) and National Institute of General Medical Sciences (K08 GM 117367).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000 -2016. World Health Organization: Geneva, 2018. <https://www.who.int/healthinfo/global_burden_disease/estimates/en/> Accessed 10 June 2020. [Google Scholar]
- 2. Kim KI, Lee JH, Chang HJ, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Park KU, Kim CH. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J 2008; 72:293–298. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Park Y, Wu J, Chen Xp, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009; 116:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Battes LC, Cheng JM, Oemrawsingh RM, Boersma E, Garcia-Garcia HM, de Boer SP, Buljubasic N, Mieghem NA, Regar E, Geuns RJ, Serruys PW, Akkerhuis KM, Kardys I. Circulating cytokines in relation to the extent and composition of coronary atherosclerosis: results from the ATHEROREMO-IVUS study. Atherosclerosis 2014; 236:18–24. [DOI] [PubMed] [Google Scholar]
- 5. Lambert CM, Roy M, Meloche J, Robitaille GA, Agharazii M, Richard DE, Bonnet S. Tumor necrosis factor inhibitors as novel therapeutic tools for vascular remodeling diseases. Am J Physiol Heart Circ Physiol 2010; 299:H995–H1001. [DOI] [PubMed] [Google Scholar]
- 6. Niemann-Jönsson A, Söderberg I, Lindholm MW, Jovinge S, Nilsson J, Fredrikson GN. Medial expression of TNF-α and TNF receptors precedes the development of atherosclerotic lesions in apolipoprotein E/LDL receptor double knockout mice. Int J Biomed Sci 2007; 3:116–122. [PMC free article] [PubMed] [Google Scholar]
- 7. Orekhov AN, Nikiforov NG, Elizova NV, Korobov GA, Aladinskaya AV, Sobenin IA, Bobryshev YV. Tumor necrosis factor-α and C-C motif chemokine ligand 18 associate with atherosclerotic lipid accumulation in situ and in vitro. Curr Pharm Des 2018; 24:2883–2889. [DOI] [PubMed] [Google Scholar]
- 8. De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J 1998; 329(Pt 3):653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller FJ Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res 2007; 101:663–671. [DOI] [PubMed] [Google Scholar]
- 10. Lassègue B, Sorescu D, Szöcs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 2001; 88:888–894. [DOI] [PubMed] [Google Scholar]
- 11. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res 2016; 118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767–801. [DOI] [PubMed] [Google Scholar]
- 13. Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 1992; 71:1525–1532. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka H, Sukhova G, Schwartz D, Libby P. Proliferating arterial smooth muscle cells after balloon injury express TNF-alpha but not interleukin-1 or basic fibroblast growth factor. Arterioscler Thromb Vasc Biol 1996; 16:12–18. [DOI] [PubMed] [Google Scholar]
- 15. Fukumoto Y, Shimokawa H, Ito A, Kadokami T, Yonemitsu Y, Aikawa M, Owada MK, Egashira K, Sueishi K, Nagai R, Yazaki Y, Takeshita A. Inflammatory cytokines cause coronary arteriosclerosis-like changes and alterations in the smooth-muscle phenotypes in pigs. J Cardiovasc Pharmacol 1997; 29:222–231. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Rao PJ, Castresana MR, Newman WH. TNF-alpha induces proliferation or apoptosis in human saphenous vein smooth muscle cells depending on phenotype. Am J Physiol Heart Circ Physiol 2005; 288:H293–H301. [DOI] [PubMed] [Google Scholar]
- 17. Yu Q, Li W, Xie D, Zheng X, Huang T, Xue P, Guo B, Gao Y, Zhang C, Sun P, Li M, Wang G, Cheng X, Zheng Q, Song Z. PI3Kγ promotes vascular smooth muscle cell phenotypic modulation and transplant arteriosclerosis via a SOX9-dependent mechanism. EBioMedicine 2018; 36:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J, Petzelbauer P, Assinger A, Schmid JA. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol 2019; 10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshida T, Yamashita M, Horimai C, Hayashi M. Smooth muscle-selective inhibition of nuclear factor-κB attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. J Am Heart Assoc 2013; 2:e000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park M, Choi S, Kim S, Kim J, Lee DK, Park W, Kim T, Jung J, Hwang JY, Won MH, Ryoo S, Kang SG, Ha KS, Kwon YG, Kim YM. NF-κB-responsive miR-155 induces functional impairment of vascular smooth muscle cells by downregulating soluble guanylyl cyclase. Exp Mol Med 2019; 51:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004; 23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kong P, Yu Y, Wang L, Dou YQ, Zhang XH, Cui Y, Wang HY, Yong YT, Liu YB, Hu HJ, Cui W, Sun SG, Li BH, Zhang F, Han M. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res 2019; 47:3580–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH, Dumont AS. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab 2013; 33:1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. García-Miguel M, Riquelme JA, Norambuena-Soto I, Morales PE, Sanhueza-Olivares F, Nuñez-Soto C, Mondaca-Ruff D, Cancino-Arenas N, San Martín A, Chiong M. Autophagy mediates tumor necrosis factor-α-induced phenotype switching in vascular smooth muscle A7r5 cell line. PLoS One 2018; 13:e0197210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens 2013; 2013:808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virdis A, Dell’Agnello U, Taddei S. Impact of inflammation on vascular disease in hypertension. Maturitas 2014; 78:179–183. [DOI] [PubMed] [Google Scholar]
- 27. Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 2008; 51:1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simplicio JA, Gonzaga NA, Nakashima MA, De Martinis BS, Cunha TM, Tirapelli LF, Tirapelli CR. Tumor necrosis factor-α receptor 1 contributes to ethanol-induced vascular reactive oxygen species generation and hypertension. J Am Soc Hypertens 2017; 11:684–696.e3. [DOI] [PubMed] [Google Scholar]
- 30. Zhu TT, Zhang WF, Yin YL, Liu YH, Song P, Xu J, Zhang MX, Li P. MicroRNA-140-5p targeting tumor necrosis factor-α prevents pulmonary arterial hypertension. J Cell Physiol 2019; 234:9535–9550. [DOI] [PubMed] [Google Scholar]
- 31. Steyers CM 3rd, Miller FJ Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014; 15:11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen Y, Crowley SD. Renal effects of cytokines in hypertension. Adv Exp Med Biol 2019; 1165:443–454. [DOI] [PubMed] [Google Scholar]
- 33. Choi H, Nguyen HN, Lamb FS. Inhibition of endocytosis exacerbates TNF-α-induced endothelial dysfunction via enhanced JNK and p38 activation. Am J Physiol Heart Circ Physiol 2014; 306:H1154–H1163. [DOI] [PubMed] [Google Scholar]
- 34. Sato N, Goto T, Haranaka K, Satomi N, Nariuchi H, Mano-Hirano Y, Sawasaki Y. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst 1986; 76:1113–1121. [PubMed] [Google Scholar]
- 35. Rastogi S, Rizwani W, Joshi B, Kunigal S, Chellappan SP. TNF-α response of vascular endothelial and vascular smooth muscle cells involve differential utilization of ASK1 kinase and p73. Cell Death Differ 2012; 19:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davis R, Pillai S, Lawrence N, Sebti S, Chellappan SP. TNF-α-mediated proliferation of vascular smooth muscle cells involves Raf-1-mediated inactivation of Rb and transcription of E2F1-regulated genes. Cell Cycle 2012; 11:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi H, Ettinger N, Rohrbough J, Dikalova A, Nguyen HN, Lamb FS. LRRC8A channels support TNFα-induced superoxide production by Nox1 which is required for receptor endocytosis. Free Radic Biol Med 2016; 101:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jovinge S, Hultgårdh-Nilsson A, Regnström J, Nilsson J. Tumor necrosis factor-alpha activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler Thromb Vasc Biol 1997; 17:490–497. [DOI] [PubMed] [Google Scholar]
- 39. Al Ghouleh I, Frazziano G, Rodriguez AI, Csányi G, Maniar S, St Croix CM, Kelley EE, Egaña LA, Song GJ, Bisello A, Lee YJ, Pagano PJ. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res 2013; 97:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Navarro-González JF, Mora C, Muros M, Jarque A, Herrera H, García J. Association of tumor necrosis factor-alpha with early target organ damage in newly diagnosed patients with essential hypertension. J Hypertens 2008; 26:2168–2175. [DOI] [PubMed] [Google Scholar]
- 41. Yao YS, Chang WW, Jin YL. Association between TNF-a promoter -308G/A polymorphism and essential hypertension in the Asian population: a meta-analysis. J Renin Angiotensin Aldosterone Syst 2017; 18:1470320317741066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A 1997; 94:3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vitoratos N, Economou E, Iavazzo C, Panoulis K, Creatsas G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators Inflamm 2010; 2010:908649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giardina JB, Green GM, Cockrell KL, Granger JP, Khalil RA. TNF-alpha enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol 2002; 283:R130–R143. [DOI] [PubMed] [Google Scholar]
- 45. Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 2002; 15:170–175. [DOI] [PubMed] [Google Scholar]
- 46. Zemse SM, Chiao CW, Hilgers RH, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am J Physiol Heart Circ Physiol 2010; 299:H1160–H1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giachini FR, Zemse SM, Carneiro FS, Lima VV, Carneiro ZN, Callera GE, Ergul A, Webb RC, Tostes RC. Interleukin-10 attenuates vascular responses to endothelin-1 via effects on ERK1/2-dependent pathway. Am J Physiol Heart Circ Physiol 2009; 296:H489–H496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, Backx PH, Wier WG, Strieth S, Wangemann P, Voigtlaender-Bolz J, Lidington D, Bolz SS. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke 2010; 41:2618–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sauvé M, Hui SK, Dinh DD, Foltz WD, Momen A, Nedospasov SA, Offermanns S, Husain M, Kroetsch JT, Lidington D, Bolz SS. Tumor necrosis factor/sphingosine-1-phosphate signaling augments resistance artery myogenic tone in diabetes. Diabetes 2016; 65:1916–1928. [DOI] [PubMed] [Google Scholar]
- 50. Kroetsch JT, Levy AS, Zhang H, Aschar-Sobbi R, Lidington D, Offermanns S, Nedospasov SA, Backx PH, Heximer SP, Bolz SS. Constitutive smooth muscle tumour necrosis factor regulates microvascular myogenic responsiveness and systemic blood pressure. Nat Commun 2017; 8:14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 2016; 118:535–546. [DOI] [PubMed] [Google Scholar]
- 52. Mortality GBD; Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jovinge S, Ares MP, Kallin B, Nilsson J. Human monocytes/macrophages release TNF-alpha in response to Ox-LDL. Arterioscler Thromb Vasc Biol 1996; 16:1573–1579. [DOI] [PubMed] [Google Scholar]
- 54. Niemann-Jönsson A, Dimayuga P, Jovinge S, Calara F, Ares MP, Fredrikson GN, Nilsson J. Accumulation of LDL in rat arteries is associated with activation of tumor necrosis factor-alpha expression. Arterioscler Thromb Vasc Biol 2000; 20:2205–2211. [DOI] [PubMed] [Google Scholar]
- 55. Persson J, Nilsson J, Lindholm MW. Interleukin-1beta and tumour necrosis factor-alpha impede neutral lipid turnover in macrophage-derived foam cells. BMC Immunol 2008; 9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Albarrán-Juárez J, Kaur H, Grimm M, Offermanns S, Wettschureck N. Lineage tracing of cells involved in atherosclerosis. Atherosclerosis 2016; 251:445–453. [DOI] [PubMed] [Google Scholar]
- 57. Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014; 129:1551–1559. [DOI] [PubMed] [Google Scholar]
- 58. Katsuda S, Boyd HC, Fligner C, Ross R, Gown AM. Human atherosclerosis. III. Immunocytochemical analysis of the cell composition of lesions of young adults. Am J Pathol 1992; 140:907–914. [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, Jaw JE, Sin DD, Seidman MA, Leeper NJ, Francis GA. Smooth muscle cells contribute the majority of foam cells in ApoE (Apolipoprotein E)-deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol 2019; 39:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Niemann-Jönsson A, Ares MP, Yan ZQ, Bu DX, Fredrikson GN, Brånén L, Pörn-Ares I, Nilsson AH, Nilsson J. Increased rate of apoptosis in intimal arterial smooth muscle cells through endogenous activation of TNF receptors. Arterioscler Thromb Vasc Biol 2001; 21:1909–1914. [DOI] [PubMed] [Google Scholar]
- 61. Greenow K, Pearce NJ, Ramji DP. The key role of apolipoprotein E in atherosclerosis. J Mol Med (Berl) 2005; 83:329–342. [DOI] [PubMed] [Google Scholar]
- 62. Boesten LS, Zadelaar AS, van Nieuwkoop A, Gijbels MJ, de Winther MP, Havekes LM, van Vlijmen BJ. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc Res 2005; 66:179–185. [DOI] [PubMed] [Google Scholar]
- 63. Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2004; 24:2137–2142. [DOI] [PubMed] [Google Scholar]
- 64. Rectenwald JE, Moldawer LL, Huber TS, Seeger JM, Ozaki CK. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation 2000; 102:1697–1702. [DOI] [PubMed] [Google Scholar]
- 65. Oberoi R, Vlacil AK, Schuett J, Schösser F, Schuett H, Tietge UJF, Schieffer B, Grote K. Anti-tumor necrosis factor-α therapy increases plaque burden in a mouse model of experimental atherosclerosis. Atherosclerosis 2018; 277:80–89. [DOI] [PubMed] [Google Scholar]
- 66. Sbarsi I, Falcone C, Boiocchi C, Campo I, Zorzetto M, De Silvestri A, Cuccia M. Inflammation and atherosclerosis: the role of TNF and TNF receptors polymorphisms in coronary artery disease. Int J Immunopathol Pharmacol 2007; 20:145–154. [DOI] [PubMed] [Google Scholar]
- 67. Zhang P, Wu X, Li G, He Q, Dai H, Ai C, Shi J. Tumor necrosis factor-alpha gene polymorphisms and susceptibility to ischemic heart disease: a systematic review and meta-analysis. Medicine (Baltimore) 2017; 96:e6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Willrich MA, Murray DL, Snyder MR. Tumor necrosis factor inhibitors: clinical utility in autoimmune diseases. Transl Res 2015; 165:270–282. [DOI] [PubMed] [Google Scholar]
- 69. Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol 1997; 24:445–451. [PubMed] [Google Scholar]
- 70. Jacobsson LT, Turesson C, Gülfe A, Kapetanovic MC, Petersson IF, Saxne T, Geborek P. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1213–1218. [PubMed] [Google Scholar]
- 71. Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011; 63:522–529. [DOI] [PubMed] [Google Scholar]
- 72. Wu P, Jia F, Zhang B, Zhang P. Risk of cardiovascular disease in inflammatory bowel disease. Exp Ther Med 2017; 13:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zanoli L, Rastelli S, Inserra G, Lentini P, Valvo E, Calcagno E, Boutouyrie P, Laurent S, Castellino P. Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis 2014; 234:346–351. [DOI] [PubMed] [Google Scholar]
- 74. Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 2016; 77:1–7. [DOI] [PubMed] [Google Scholar]
- 75. Thapa SD, Hadid H, Schairer J, Imam W, Jafri SM. Effect of inflammatory bowel disease-related characteristics and treatment interventions on cardiovascular disease incidence. Am J Med Sci 2015; 350:175–180. [DOI] [PubMed] [Google Scholar]
- 76. Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol 2018; 175:1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Small HY, Migliarino S, Czesnikiewicz-Guzik M, Guzik TJ. Hypertension: focus on autoimmunity and oxidative stress. Free Radic Biol Med 2018; 125:104–115. [DOI] [PubMed] [Google Scholar]
- 78. Buglak NE, Batrakova EV, Mota R, Bahnson ESM. Insights on localized and systemic delivery of redox-based therapeutics. Oxid Med Cell Longev 2018; 2018:2468457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 2015; 71:40–56. [DOI] [PubMed] [Google Scholar]
- 80. Ward JPT. From physiological redox signalling to oxidant stress. Adv Exp Med Biol 2017; 967:335–342. [DOI] [PubMed] [Google Scholar]
- 81. Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 1999; 17:331–367. [DOI] [PubMed] [Google Scholar]
- 82. Choi H, Dikalova A, Stark RJ, Lamb FS. c-Jun N-terminal kinase attenuates TNFα signaling by reducing Nox1-dependent endosomal ROS production in vascular smooth muscle cells. Free Radic Biol Med 2015; 86:219–227. [DOI] [PubMed] [Google Scholar]
- 83. Carlsson AC, Jansson JH, Söderberg S, Ruge T, Larsson A, Ärnlöv J. Levels of soluble tumor necrosis factor receptor 1 and 2, gender, and risk of myocardial infarction in Northern Sweden. Atherosclerosis 2018; 272:41–46. [DOI] [PubMed] [Google Scholar]
- 84. Carlsson AC, Östgren CJ, Nystrom FH, Länne T, Jennersjö P, Larsson A, Ärnlöv J. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in type 2 diabetes. Cardiovasc Diabetol 2016; 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carlsson AC, Ruge T, Kjoller E, Hilden J, Kolmos HJ, Sajadieh A, Kastrup J, Jensen GB, Larsson A, Nowak C, Jakobsen JC, Winkel P, Gluud C, Arnlov J. 10-Year associations between tumor necrosis factor receptors 1 and 2 and cardiovascular events in patients with stable coronary heart disease: a CLARICOR (Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease) trial substudy. J Am Heart Assoc 2018; 7(9):e008299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cortez-Cooper M, Meaders E, Stallings J, Haddow S, Kraj B, Sloan G, McCully KK, Cannon JG. Soluble TNF and IL-6 receptors: indicators of vascular health in women without cardiovascular disease. Vasc Med 2013; 18:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem 2000; 275:14608–14614. [DOI] [PubMed] [Google Scholar]
- 88. Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A 2004; 101:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Benedikter BJ, Weseler AR, Wouters EFM, Savelkoul PHM, Rohde GGU, Stassen FRM. Redox-dependent thiol modifications: implications for the release of extracellular vesicles. Cell Mol Life Sci 2018; 75:2321–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE 2006; 2006:re8. [DOI] [PubMed] [Google Scholar]
- 91. Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2004; 24:677–683. [DOI] [PubMed] [Google Scholar]
- 92. Xu S, Shriver AS, Jagadeesha DK, Chamseddine AH, Szőcs K, Weintraub NL, Griendling KK, Bhalla RC, Miller FJ Jr. Increased expression of Nox1 in neointimal smooth muscle cells promotes activation of matrix metalloproteinase-9. J Vasc Res 2012; 49:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005; 112:2668–2676. [DOI] [PubMed] [Google Scholar]
- 94. Dikalova AE, Góngora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 2010; 299:H673–H679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Streeter J, Schickling BM, Jiang S, Stanic B, Thiel WH, Gakhar L, Houtman JC, Miller FJ Jr. Phosphorylation of Nox1 regulates association with NoxA1 activation domain. Circ Res 2014; 115:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermöhlen O, Brüning JC, Schütze S, Krönke M. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 2009; 460:1159–1163. [DOI] [PubMed] [Google Scholar]
- 97. Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem 2005; 280:37033–37040. [DOI] [PubMed] [Google Scholar]
- 98. Choi H, Stark RJ, Raja BS, Dikalova A, Lamb FS. Apoptosis signal-regulating kinase 1 activation by Nox1-derived oxidants is required for TNFα receptor endocytosis. Am J Physiol Heart Circ Physiol 2019; 316:H1528–H1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Matsuda JJ, Filali MS, Moreland JG, Miller FJ, Lamb FS. Activation of swelling-activated chloride current by tumor necrosis factor-alpha requires ClC-3-dependent endosomal reactive oxygen production. J Biol Chem 2010; 285:22864–22873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Miller FJ Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal 2010; 12:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 2008; 20:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Duménil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006; 126:205–218. [DOI] [PubMed] [Google Scholar]
- 103. El Chemaly A, Nunes P, Jimaja W, Castelbou C, Demaurex N. Hv1 proton channels differentially regulate the pH of neutrophil and macrophage phagosomes by sustaining the production of phagosomal ROS that inhibit the delivery of vacuolar ATPases. J Leukoc Biol 2014; 95:827–839. [DOI] [PubMed] [Google Scholar]
- 104. Valentine JS, Curtis AB. A convenient preparation of solutions of superoxide anion and the reaction of superoxide anion with a copper (II) complex. J Am Chem Soc 1975; 97:224–226. [DOI] [PubMed] [Google Scholar]
- 105. Lamb FS, Moreland JG, Miller FJ Jr. Electrophysiology of reactive oxygen production in signaling endosomes. Antioxid Redox Signal 2009; 11:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 2009; 11:1313–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol 2008; 28:3700–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med 1999; 27:322–328. [DOI] [PubMed] [Google Scholar]
- 109. Matchkov VV, Secher Dam V, Bødtkjer DM, Aalkjær C. Transport and function of chloride in vascular smooth muscles. J Vasc Res 2013; 50:69–87. [DOI] [PubMed] [Google Scholar]
- 110. Valdivieso ÁG, Santa-Coloma TA. The chloride anion as a signalling effector. Biol Rev Camb Philos Soc 2019; 94:1839–1856. [DOI] [PubMed] [Google Scholar]
- 111. Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol 2013; 75:453–477. [DOI] [PubMed] [Google Scholar]
- 112. Strange K, Yamada T, Denton JS. A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J Gen Physiol 2019; 151:100–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. König B, Stauber T. Biophysics and structure-function relationships of LRRC8-formed volume-regulated anion channels. Biophys J 2019; 116:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lutter D, Ullrich F, Lueck JC, Kempa S, Jentsch TJ. Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J Cell Sci 2017; 130:1122–1133. [DOI] [PubMed] [Google Scholar]
- 115. Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta 2006; 1757:996–1011. [DOI] [PubMed] [Google Scholar]
- 116. Gradogna A, Gavazzo P, Boccaccio A, Pusch M. Subunit-dependent oxidative stress sensitivity of LRRC8 volume-regulated anion channels. J Physiol 2017; 595:6719–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev 2018; 98:1493–1590. [DOI] [PubMed] [Google Scholar]
- 118. Zhao Z, Li X, Hao J, Winston JH, Weinman SA. The ClC-3 chloride transport protein traffics through the plasma membrane via interaction of an N-terminal dileucine cluster with clathrin. J Biol Chem 2007; 282:29022–29031. [DOI] [PubMed] [Google Scholar]
- 119. Rohrbough J, Nguyen HN, Lamb FS. Modulation of ClC-3 gating and proton/anion exchange by internal and external protons and the anion selectivity filter. J Physiol 2018; 596:4091–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Smith AJ, Lippiat JD. Direct endosomal acidification by the outwardly rectifying CLC-5 Cl−/H+ exchanger. J Physiol 2010; 588(Pt 12):2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lamb FS, Hook JS, Hilkin BM, Huber JN, Volk AP, Moreland JG. Endotoxin priming of neutrophils requires endocytosis and NADPH oxidase-dependent endosomal reactive oxygen species. J Biol Chem 2012; 287:12395–12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Moreland JG, Davis AP, Matsuda JJ, Hook JS, Bailey G, Nauseef WM, Lamb FS. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter ClC-3. J Biol Chem 2007; 282:33958–33967. [DOI] [PubMed] [Google Scholar]
- 123. Moreland JG, Davis AP, Bailey G, Nauseef WM, Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem 2006; 281:12277–12288. [DOI] [PubMed] [Google Scholar]
- 124. Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 2011; 21:1147–1158. [DOI] [PubMed] [Google Scholar]
- 125. Di Marco E, Gray SP, Chew P, Koulis C, Ziegler A, Szyndralewiez C, Touyz RM, Schmidt HH, Cooper ME, Slattery R, Jandeleit-Dahm KA. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe(−/−) mice. Diabetologia 2014; 57:633–642. [DOI] [PubMed] [Google Scholar]
- 126. Hoffman HM. Therapy of autoinflammatory syndromes. J Allergy Clin Immunol 2009; 124:1129–1138; quiz 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Brezinski EA, Follansbee MR, Armstrong EJ, Armstrong AW. Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 2014; 20:513–528. [DOI] [PubMed] [Google Scholar]
- 128. Tam LS, Kitas GD, Gonzalez-Gay MA. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology 2014; 53:1108–1119. [DOI] [PubMed] [Google Scholar]
- 129. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, Joshi FR, Ostör AJ, Hall FC, Rudd JH, Wilkinson IB. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 2012; 126:2473–2480. [DOI] [PubMed] [Google Scholar]
- 130. Sen D, González-Mayda M, Brasington RD Jr. Cardiovascular disease in rheumatoid arthritis. Rheum Dis Clin North Am 2014; 40:27–49. [DOI] [PubMed] [Google Scholar]
- 131. Reich K. The concept of psoriasis as a systemic inflammation: implications for disease management. J Eur Acad Dermatol Venereol 2012; 26(Suppl 2):3–11. [DOI] [PubMed] [Google Scholar]