Abstract
Objective
To determine the feasibility and effectiveness of a quality improvement initiative (QI) to adopt universal screening for Lynch syndrome in uterine cancer patients at an institution that previously employed age-based screening.
Methods
Prior to the initiative, tumors of patients with uterine cancer diagnosed at age ≤ 60 years were screened for mismatch repair deficiency (MMR) and microsatellite instability (MSI). The QI process change model adopted universal testing of all uterine cancer specimens and implemented provider training, standardized documentation, and enhanced use of the electronic medical record (EMR). We compared screening rates, results of screening, follow up of abnormal results, and final diagnoses from the pre- and post-implementation periods.
Results
Pre- and post-implementation screening rates for women age ≤ 60 years at the time of diagnosis were 45/78 (57.7%) and 64/68 (94.5%), respectively. The screening rate for all patients with uterine cancer increased from 73/190 (38.4%) to 172/182 (94.5%). The rate of abnormal screening results increased from 15/190 (7.9%) to 44/182 (24.0%) cases. Genetics referral rates among screen positives increased from 3/15 (20.0%) to 16/44 (36.4%). Germline diagnoses increased from 2/190 (1.1%) with two Lynch syndrome diagnoses to 4/182 (2.2%) including three Lynch syndrome diagnoses and one BRCA1 germline diagnosis. The number of patients errantly not screened decreased from at least 32 patients to 3 patients after the intervention.
Conclusions
Adherence to screening guidelines significantly improved after interventions involving provider education, optimal use of the EMR, and simplification of screening indications. These interventions are feasible at other institutions and translatable to other screening indications.
Keywords: Endometrial cancer, Uterine cancer, Lynch syndrome, Genetic testing, Genetic screening, Quality improvement
Highlights
-
•
Compliance with Lynch syndrome screening guidelines can be dramatically improved with straightforward interventions.
-
•
Universal screening for Lynch syndrome in uterine cancer patients is feasible.
-
•
Universal screening for Lynch syndrome identifies substantially more patients eligible for targeted treatments.
1. Introduction
Lynch syndrome is an autosomal dominant inherited cancer susceptibility syndrome caused by defects in the DNA mismatch repair system [[1], [2], [3], [4]]. Lynch syndrome predisposes patients to cancers throughout the body, most commonly colorectal and uterine cancer [1,5]. The Society for Gynecology Oncology (SGO) recommends systematic clinical screening for Lynch Syndrome for all women diagnosed with uterine cancer through a review of personal and family history, tumor testing on all uterine tumors diagnosed before age 60 years, or universal tumor testing regardless of age [6]. Tumor testing uses immunohistochemistry (IHC) to detect for the presence or absence of protein expression for the mismatch repair genes MLH1, MSH2, PMS2, and MSH6 [4,7,8]. Microsatellite instability (MSI) is a cellular phenotype resulting from a mismatch repair (MMR) mutation. MSI is present in the majority of tumors from patients with Lynch syndrome but can also occur in sporadic uterine cancers [1,9]. Our institution's experience is that selectively testing patients based on age and family history created ample room for error in ordering the initial testing, requesting MLH1 promoter testing in patients with absent expression of MLH1 and/or PMS2, communicating the results to patients, and then placing a genetics referral when indicated [10]. The goal of our project was to determine the feasibility and effectiveness of adopting universal screening for Lynch syndrome in women with uterine cancer at a single academic institution. We hypothesized that universal testing of all uterine cancer patients would improve adherence to Lynch syndrome screening guidelines and minimize opportunities for human error in following up the screening. We secondarily sought to assess whether or not detection and genetic referral rates were different under a universal screening paradigm as compared to an age-based screening paradigm.
2. Materials and methods
This quality improvement initiative was implemented at a single, high volume, tertiary care, academic medical center in the United States. The institution sees approximately 200 new uterine cancer cases annually between eight gynecologic oncology faculty across four hospitals. The pathology department has four dedicated gynecologic pathologists and in-house capability to perform MSI testing and MMR immunohistochemistry (MMR/MSI). The institution employs oncologic genetic counselors who coordinate patient counseling as well as send-out testing. All of the above parties were involved in the design and implementation of the quality improvement initiative. The institution uses Epic as its Electronic Medical Record (EMR). The process change and related data gathering were approved under exempt status by the Duke Institutional Review Board given the quality improvement nature of this project (Protocol 00100854).
Prior to the intervention, the policy at our facility was to perform Lynch syndrome screening with MMR/MSI for women who were 60 or younger at the time of uterine cancer diagnosis or who had a family history suspicious for an undiagnosed hereditary cancer syndrome. Testing could either be requested by the gynecologic oncologist based on family history or by the reading pathologist if the patient was under 60 years old. The QI initiative began with consensus agreement among leaders at our institution's gynecologic oncology division that the current system of age and family history-based testing was not optimal based on providers' subjective experience of the workflow and known screening heterogeneity among providers. Universal screening was an acceptable alternative to both gynecologic oncology and pathology providers to achieve a standardized process, and as above is one of the screening paradigms recommended by SGO.
The goal of the intervention was to achieve universal MMR/MSI testing on all patients with uterine cancer, regardless of age or family history, and to follow up abnormal results via additional tumor testing and genetics referrals as indicated. The intervention consisted of provider training, standardized pathology documentation, and optimization of EMR workflows.
2.1. Provider training
Provider training involved in-person and electronic communications with gynecologic oncology providers, pathologists, and clinical laboratories. Lynch syndrome epidemiology, pathophysiology, and prognosis were reviewed, as were SGO recommendations for screening. Discussion of current practice patterns again revealed significant heterogeneity in screening practices and many opportunities for human error. The insights reiterated were not part of the formal curriculum but served to increase enthusiasm for standardization.
2.2. Pathology documentation
To standardize pathology documentation, a non-optional field was added to the pathologists' uterine cancer pathology report template that prompted identification of a best block for subsequent molecular testing and staining. The pathology report for uterine cancer specimens could not be signed until this field was populated. Identification of a best block not only reminds the pathologist to submit the tumor for testing, but it also may increase the likelihood that the specimen to be tested contains characterizable-tumor.
2.3. Provider documentation
The note templates used in gynecologic oncology clinics were amended to include a mandatory section for “molecular testing and tumor genetics.” This section prompts the provider to manually review and document all testing that has been ordered, the results of those tests, whether or not a genetic counseling referral has been made, and the outcome of the genetic consultation (Fig. 1 ). Notes cannot be signed without completion of this field, unless it is deleted in its entirety.
Fig. 1.

Process change model to implement universal screening for Lynch syndrome in patients with uterine cancer. EMR, electronic health record; IHC, immunohistochemistry; MSI microsatellite instability.
The quality improvement initiative was launched on July 1, 2018. We retrospectively reviewed charts for all newly diagnosed uterine cancers during calendar year 2017. For all patients with a confirmed diagnosis of uterine cancer, data obtained included age, date of surgery, whether MMR/MSI testing was performed, results of MMR/MSI testing, whether MLH1 promoter hypermethylation testing was ordered (if indicated), date the provider communicated test results to the patient, date a genetic consult was placed, completion of a genetic counseling appointment, and results of genetic testing.
We prospectively collected the same data between July 1, 2018 and June 30, 2019. Data between January 1, 2018 and June 30, 2018 (“washout period”) was collected but not included in analysis as meetings had already been initiated about the upcoming interventions and practice patterns neither reflected the historical baseline or the post-intervention baseline. Patients were identified for inclusion based on automated review of billing and diagnostic codes in the EMR. Investigators then manually reviewed all patient records, excluded patients who did not have a new uterine cancer diagnosis, and collected the above data into a secure database.
To assess progress towards the goal of universal MMR/MSI testing for all uterine cancer patients, our primary outcomes were the percentage of newly diagnosed uterine cancer patients who had molecular Lynch syndrome screening, and the percentage of newly diagnosed uterine cancer patients under the age of 60 who had molecular Lynch syndrome screening. Additional metrics included the rates of positive screening results, referral rates to genetic counseling, and results of germline genetic testing.
The rates of MMR IHC and MSI testing were compiled for all patients with uterine cancer who underwent hysterectomy in the pre- and post-implementation periods. A non-parametric two-sided Fisher's exact test was used for the categorical variables. The Mann-Whitney test was used to compare continuous variables. All continuous variables are summarized with the median and range. Unadjusted p-values less than 0.05 were considered significant. GraphPad Prism 7 (Version 7.04) was used for data analysis.
Provider experience and the perceived effectiveness of the interventions was assessed qualitatively during regularly scheduled division meetings. Though not formally included in our analysis, feedback during these sessions failed to identify confounding variables such as new guidelines, new laboratory facilities, or new faculty that could explain changes in practice pattern. The institution's ethics review board was available throughout the study period, though there was no occasion that necessitated a consultation.
3. Results
There was no statistical difference in patient age between the pre- and post-implementation cohorts (years); 63 (31–84) versus 64.5 (25–89), p = 0.57 (Table 1 ). In the pre-implementation cohort, 190 patients underwent a hysterectomy for a diagnosis of uterine cancer. In the pre-implementation cohort a total of 73 (38.4%) of all patients received screening, and 45 (57.7%) of the 78 patients 60 years or younger received screening (Table 2 ). In the post-implementation cohort, 182 patients with uterine cancer underwent a hysterectomy and 172 (94.5%) of patients had MMR/MSI testing, including 64 (94.1%) of the 68 patients 60 years or younger.
Table 1.
Baseline demographics of pre- and post-implementation cohorts.
| Pre-implementation (n = 190) January 2017–December 2017 n (%) | Post-implementation (n = 182) July 2018–June 2019 n (%) | ||
|---|---|---|---|
| Age | NS | ||
| ≤40 | 5 (2.6) | 11 (6.0) | |
| 41–50 | 13 (6.8) | 19 (10.4) | |
| 51–60 | 60 (31.6) | 38 (20.9) | |
| ≥61 | 112 (58.9) | 114 (62.6) | |
| Race/Ethnicity | NS | ||
| African American | 46 (24.2) | 46 (25.3) | |
| Asian | 3 (1.6) | 2 (1.1) | |
| Caucasian | 120 (63.2) | 121 (66.5) | |
| Hispanic | 2 (1.1) | 2 (1.1) | |
| Native American or Alaskan Native | 5 (2.6) | 2 (1.1) | |
| Not reported | 14 (7.4) | 9 (4.9) |
NS = not significant.
Table 2.
Patients with uterine cancer eligible for screening for Lynch Syndrome.
| Pre-implementation (N = 190) January 2017–December 2017 |
Post-implementation (N = 182) July 2018–June 2019 |
p-Value | |||
|---|---|---|---|---|---|
| Evaluable patients | N (%) | Evaluable patients | N (%) | ||
| MMR/MSI testing (≤60 years old) | 78 | 45 (57.7) | 68 | 64 (94.1) | <0.0001 |
| MMR/MSI testing (all ages) | 190 | 73 (38.4) | 182 | 172 (94.5) | <0.0001 |
MMR = mismatch repair; MSI = microsatellite instability.
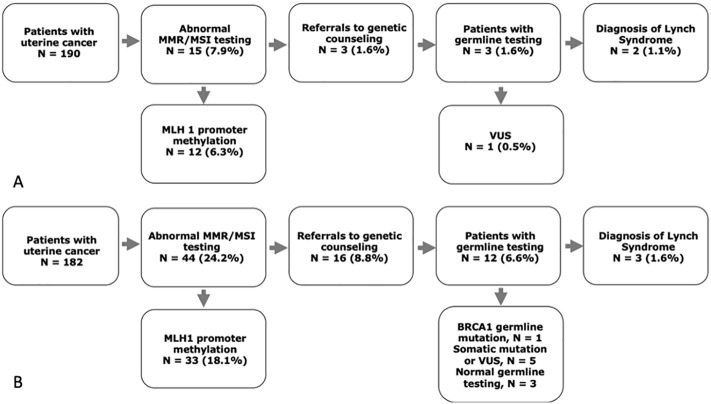
During pre-implementation, 15 (7.9%) patients undergoing staging surgery for uterine cancer had abnormal MMR testing, 12 (6.3%) of whom had loss of MLH1 and PMS2 protein expression as a result of MLH1 promoter hypermethylation. Three patients with absent MSH6 protein expression were referred for genetic counseling and all underwent germline testing. Two diagnoses of Lynch syndrome were confirmed (both pathogenic MSH6 mutations); the other patient had a variant of uncertain significance (VUS) in MSH6 (Fig. 2A). In the post-implementation cohort, 44 (24.2%) patients had abnormal MMR/MSI testing, of whom 33 (18.1%) were deemed sporadic in nature due to MLH1 promoter hypermethylation. Providers referred 16 (8.8%) patients to genetic counseling, which included 11 (6.0%) patients with abnormal MMR/MSI testing. One patient (0.5%) underwent commercially available sequencing of her tumor and a BRCA2 mutation was identified. She underwent germline testing and ultimately did not have a germline BRCA2 mutation. Four (2.2%) other patients with a significant family history or a history of multiple other cancers were referred. Twelve patients ultimately underwent germline testing. Of the four patients who were referred to genetic counseling but did not receive germline genetic testing, one declined referral, one declined testing after counseling, one deferred referral and testing until they return from an extended trip abroad, and one was lost to follow up despite multiple attempts at re-contacting them. Two of the four patients who did not receive germline genetic testing were referred because of abnormal MMR/MSI screening. Of the 12 patients who received germline genetic testing, 3 patients (1.6%) were identified as having Lynch syndrome with pathogenic MSH6 mutations, one of whom was over 60 years old a time of diagnosis. One patient was subsequently identified to have a germline BRCA1 mutation (she had a high grade serous endometrial adenocarcinoma and a personal history of breast cancer) (Fig. 2B). Five patients had either a somatic mutation or germline VUS.
Fig. 2.
(A) Results of testing in the pre-implementation and (B) post implementation cohorts. MMR= mismatch repair; MSI=microsatellite instability; VUS = variant of uncertain significance.
As with all process changes and quality improvement projects, a formalized review of the process must be undertaken to identify potential obstacles and missed opportunities. Ten patients with uterine cancer were not screened with MMR/MSI testing for Lynch syndrome in our post-implementation cohort. Six of these patients had neoadjuvant chemotherapy or pre-operative radiation therapy and had no residual tumor on the final specimen, which precluded testing. One patient had a known diagnosis of Lynch syndrome and was diagnosed with concurrent uterine and ovarian cancers during her risk reducing surgery. The three remaining patients represented true misses (Table 3 ). In the pre-implementation period 33 patients under the age of 60 were not screened, 32 of which were true misses by the screening paradigm in place at that time and one of which may potentially have been due to lack of residual tumor on her final pathology. It is possible that additional patients over 60 should have been screened based on their family history, though family history is not documented consistently enough to count true misses among those over 60 years of age in the pre-implementation cohort.
Table 3.
Missed opportunities for Lynch syndrome screening in patients with uterine cancer who met screening criteria during their respective time period.
| Reason | Pre-implementation, < 60 years old | Post-implementation, any age |
|---|---|---|
| No residual tumor on final pathology | 1 | 6 |
| Known diagnosis of Lynch syndrome | 0 | 1 |
| True misses | 32 | 3 |
4. Discussion
Most endometrial cancer patients in the pre-intervention period did not undergo screening for Lynch syndrome, including a substantial portion of patients under 60 years old at time of diagnosis. The post-intervention period demonstrated increased Lynch syndrome screening rates across all uterine cancer patients, but also among patients under 60 years old who should have received screening even under an age-based screening paradigm. Adherence to SGO Lynch syndrome screening guidelines was improved in the post-intervention period, with the rate of true misses decreasing at least 91%. Three patients were diagnosed with Lynch syndrome in the post-intervention period as compared to two patients in the pre-intervention period.
Our results are epidemiologically consistent with what is known about the incidence of Lynch syndrome among endometrial cancer patients. The incidence of Lynch Syndrome in our population was 1.1% in the pre and 2.2% in the post implementation periods (in addition to the three new Lynch Syndrome diagnoses, one patient in the post-implementation period was known prior to her hysterectomy to have Lynch Syndrome). The literature reports a Lynch Syndrome incidence of approximately 2–3% among endometrial cancer patients [7,8,11,12]. It is possible that additional Lynch Syndrome diagnoses were missed among the four patients who were referred to genetic counseling but did not receive genetic testing, among the three patients identified as “true-misses” who did not receive screening, or among the six patients who had no residual tumor to screen after neoadjuvant treatment with which to perform screening. There was one patient in both the pre and post-implementation periods with a VUS in MSH6 who may be reclassified as having Lynch Syndrome at a later date. In addition, we identified a patient with a germline BRCA1 mutation in the post-intervention period. She was diagnosed with a high grade serous endometrial adenocarcinoma, which has a known association with germline BRCA1 mutations [13].
Our pre-implementation results are consistent with prior literature that despite national recommendations for Lynch syndrome screening, these guidelines are often not well followed [14,15]. It has previously been demonstrated that in patients screened for hereditary cancer syndromes, referrals to genetic counseling are inconsistent and in some cases racially biased [16]. Our interventions addressed these shortcomings, with much improved adherence to guidelines in our post-intervention period. Near-perfect adherence to guidelines greatly reduces risk of racially or otherwise biased care. All patients received appropriate referrals to genetic counseling post-intervention, and 9 of 11 received germline genetic testing as a result of the referral. Importantly, 6 of these 11 patients were over 60 years old and would have been missed by age-based screening.
Our intervention resulted in a greater proportion of abnormal MMR/MSI results, but consistent with the rate of abnormal MMR/MSI results under other universal screening paradigms for endometrial cancer [17,18]. The majority of these abnormal results were due to MLH1 promoter methylation, a non-hereditary cause of cancer. The increase in the rate of positive screens is a notable departure from the colorectal Lynch syndrome literature, which suggests that universal screening increases the total number of diagnoses, but not the proportion of patients who screen positive [19].
Prior literature in both endometrial and colorectal cancer demonstrates that age-based screening fails to identify a substantial number of Lynch syndrome diagnoses, with strong data suggesting roughly 1 in 4 diagnoses missed [[20], [21], [22]]. In our post-intervention period, 1 of 3 new Lynch syndrome diagnoses would have been missed based on age-based screening. We are unable to ascertain how many Lynch syndrome diagnoses may have been missed in our pre-intervention period.
The primary implication of our study is that Lynch syndrome screening practices can be changed quickly and significantly using interventions widely available to most hospital systems. Provider education utilized freely available society guidelines, electronic communication, and simple PowerPoint presentations. Any health system in which note templates are used by pathologists and gynecologic oncologists, and fields in those note templates can be made mandatory before notes can be signed, would be able to replicate the documentation changes described in this intervention. Pathologists identified a best block for tumor profiling, but did not otherwise change specimen handling workflows. Though MSI and MMR testing was done on site, that is unlikely to explain the effectiveness of the intervention.
It is estimated that no more than 1.2% of all individuals with Lynch Syndrome are aware of their diagnosis [23]. This lack of knowledge provides oncologists with an opportunity to screen, diagnose, and educate patients with a new cancer diagnosis about a possible hereditary component to their tumor. Detection of hereditary cancer syndromes presents an opportunity for cancer prevention efforts in our patients as the lifetime risk of colorectal cancer in women with Lynch Syndrome is 25–50%. Additionally, testing is subsequently offered to other family members (“cascade testing”), which can identify other patients who could benefit from screening, surveillance, possibly risk reducing surgery, and targeted therapies should they develop cancer. Prior literature has demonstrated that each Lynch syndrome proband identified leads to an additional three diagnoses in family members, often occurring in a young patient when screening and prevention can be initiated to good effect [20]. Additionally, family members without the mutation can forgo additional screening, thereby realizing some cost savings and reduction in health-related anxiety.
In addition to identifying more patients and families with Lynch syndrome, our intervention increased both the absolute number and proportion of patients with abnormal MMR/MSI testing on initial screening. Pembrolizumab is approved as a treatment for patients with MMR-deficient endometrial cancer, so knowing the molecular phenotype has immediate treatment implications in the recurrent setting. In addition, knowing the MMR status at time of initial diagnosis can shorten the time to treatment in the recurrent setting, especially for those patients eligible for pembrolizumab.
Despite performing an additional 99 screening tests over a one-year period, all of which were within guidelines, only one additional case of Lynch Syndrome was diagnosed in the post-intervention period. Many benefits of hereditary cancer diagnoses are realized in the future, or even in the lives of family members, which makes the cost-effectiveness of universal screening unintuitive and therefore worthy of further exploration. Cost-effectiveness of Lynch syndrome screening strategies in endometrial cancer has not been extensively studied, but there is some suggestion that age-based screening is more costly and less effective than other strategies [24]. One analysis suggested that a screening strategy for patients <70 years old at time of diagnosis was more effective and cost-effective than a strategy for patients <50 years old, but that study did not evaluate universal screening as a method [25]. Prior literature in colorectal cancer is mixed about the cost-effectiveness of universal screening, with some studies suggesting cost effectiveness [26,27] while others suggest a substantially higher cost-per-proband diagnosed under a universal screening paradigm [19]. Whether universal screening is cost-effective appears to depend on accuracy of screening tests, cancer risk without surveillance, the number of relatives identified via cascade testing, and follow through with genetic testing [28].
While our intervention was focused on hereditary cancer screening, the tools we used, namely provider education and standardized EMR documentation, are readily available to all medical specialties. We believe that the behavior change demonstrated in our study motivates similar interventions in other medical fields where guidelines are imperfectly adhered to but standardization is possible.
Our study's greatest strength is its simplicity. None of our interventions were technologically or logistically prohibitive. Additionally, our outcomes of interest are easily ascertained and relatively unambiguous, specifically patients' age, whether they were screened, what the result of screening was, and whether positive results were followed up appropriately. Patient characteristics were largely similar pre- and post-intervention. No other confounding variables readily explain the degree of behavior change demonstrated pre- and post-intervention.
A notable limitation of the study is that, because the intervention was bundled, it is not possible to ascertain which of the components were most significant. Despite the interventions being designed with an eye towards generalizability, they were only trialed at a single large, academic institution. Further, this study was not powered to ascertain whether the increase in Lynch syndrome diagnoses from 1.1% of those tested to 1.6% represents a true change in detection rate. Finally, this study did not explore the cost effectiveness of the screening protocol pre- or post-intervention.
5. Conclusions
A bundled quality improvement intervention that targets provider education and optimizes use of the EMR dramatically improved adherence to Lynch syndrome screening guidelines.
Author contributions
Daniel Spinosa, MD: Chart review and data codification. Composition of the manuscript.
Tatiana Acosta, MD, MPH: Chart review and data codification. Composition of the manuscript.
Janice Wong: Chart review and data codification. Review of the manuscript.
Kelli Kurtovic, BS, MS: Project design. Workflow implementation. Data analysis. Review of the manuscript.
Jennifer Mewshaw, APN: Workflow implementation. Review of the manuscript.
Sarah Collins, APN: Workflow implementation. Review of the manuscript.
Noah Kauff, MD: Project design. Review of the manuscript.
Laura J. Havrilesky, MD, MHSc: Project design. Workflow implementation. Review of the manuscript.
Kyle Strickland, MD, PhD: Project design. Workflow implementation. Data analysis. Review of the manuscript.
Rebecca A. Previs, MD: Project design. Workflow implementation. Data analysis. Composition of the manuscript.
Disclosures
Daniel Spinosa has an equity interest in Invitae, his wife's employer. Noah Kauff has done consulting work for Astra Zeneca, Merck, and BGI. Laura Havrilesky has received grants from Astra Zeneca and Tesaro. Kyle Strickland is a consultant pathologist for Foundation Medicine Inc. and Almac Pharmaceuticals. Rebecca Previs has been on an advisory board for Myriad.
Precis
Provider education and optimal use of the electronic medical record significantly increased rates of screening for Lynch syndrome in patients with uterine cancer.
Presentations of this work
6th Annual Duke Cancer Institute Retreat, October 25th, 2019; Durham, NC.
Mid-Atlantic Gynecologic Oncology Society Annual Meeting, October 25th, 2019; Charlotte, NC.
Accepted for presentation at the Society of Gynecologic Oncology Annual Meeting, March 28th – 30th, 2020; Toronto, Canada (cancelled due to COVID-19).
Declaration of Competing Interest
Corresponding author (Daniel Spinosa) has an equity interest in Invitae, his wife's employer.
Acknowledgements
There was no funding obtained for this project. Dr. Previs is supported by grants from the AAOGF-GOG Foundation and the Emerson Collective. The authors acknowledge the providers of the Division of Gynecologic Oncology and the Department of Pathology for their assistance with this project.
References
- 1.Lynch H.T., Snyder C.L., Shaw T.G., Heinen C.D., Hitchins M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer. 2015;15:181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 2.Lynch H.T., Lynch P.M., Lanspa S.J., Snyder C., Lynch J., Boland C. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin. Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H.K., Broaddus R.R. Gynecologic cancers in Lynch syndrome/HNPCC. Familial Cancer. 2005;4:249–254. doi: 10.1007/s10689-005-1838-3. [DOI] [PubMed] [Google Scholar]
- 4.Umar A., Boland C.R., Terdiman J.P., Syngal S., Adl Chapelle, Rüschoff J. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu K.H., Dinh M., Kohlmann W., Watson P., Green J., Syngal S. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet. Gynecol. 2005;105:569–574. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 6.SGO Clinical Practice Statement: Screening for Lynch Syndrome in Endometrial Cancer. 2014. [Google Scholar]
- 7.Hampel H., Frankel W.L., Martin E., Arnold M., Khanduja K., Kuebler P. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N. Engl. J. Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H., Frankel W., Panescu J., Lockman J., Sotamaa K., Fix D. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 9.ACOG practice bulletin No. 147: Lynch syndromeObstetrics and gynecology. 2014;124:1042–1054. doi: 10.1097/01.AOG.0000456325.50739.72. [DOI] [PubMed] [Google Scholar]
- 10.Uyar D., Neary J., Monroe A., Nugent M., Simpson P., Geurts J.L. Implementation of a quality improvement project for universal genetic testing in women with ovarian cancer. Gynecol. Oncol. 2018;149:565–569. doi: 10.1016/j.ygyno.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 11.Meyer L.A., Broaddus R.R., Lu K.H. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control. 2009;16:14–22. doi: 10.1177/107327480901600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ollikainen M., Abdel-Rahman W.M., Moisio A.-L., Lindroos A., Kariola R., Järvelä I. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J. Clin. Oncol. 2005;23:4609–4616. doi: 10.1200/JCO.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 13.Shu C.A., Pike M.C., Jotwani A.R., Friebel T.M., Soslow R.A., Levine D.A. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434–1440. doi: 10.1001/jamaoncol.2016.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beamer L.C., Grant M.L., Espenschied C.R., Blazer K.R., Hampel H.L., Weitzel J.N. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J. Clin. Oncol. 2012;30:1058. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller C., Matthews L., Kupfer S.S., Weiss J.M. Effective identification of Lynch syndrome in gastroenterology practice. Curr. Treat Options Gastroenterol. 2019;17:666–680. doi: 10.1007/s11938-019-00261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller C., Lee S.M., Barge W., Siddique S.M., Berera S., Wideroff G. Low referral rate for genetic testing in racially and ethnically diverse patients despite universal colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2018;16 doi: 10.1016/j.cgh.2018.08.038. (1911–8. e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon J.L., Gonzalez J.L., DeMars L., Bloch K.J., Tafe L.J. Universal screening for Lynch syndrome in endometrial cancers: frequency of germline mutations and identification of patients with Lynch-like syndrome. Hum. Pathol. 2017;70:121–128. doi: 10.1016/j.humpath.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Egoavil C., Alenda C., Castillejo A., Paya A., Peiro G., Sánchez-Heras A.-B. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erten M.Z., Fernandez L.P., Ng H.K., McKinnon W.C., Heald B., Koliba C.J. Universal versus targeted screening for Lynch syndrome: comparing ascertainment and costs based on clinical experience. Dig. Dis. Sci. 2016;61:2887–2895. doi: 10.1007/s10620-016-4218-y. [DOI] [PubMed] [Google Scholar]
- 20.Hampel H., Frankel W.L., Martin E., Arnold M., Khanduja K., Kuebler P. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J. Clin. Oncol. 2008;26:5783. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najdawi F., Crook A., Maidens J., McEvoy C., Fellowes A., Pickett J. Lessons learnt from implementation of a Lynch syndrome screening program for patients with gynaecological malignancy. Pathology. 2017;49:457–464. doi: 10.1016/j.pathol.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Goodfellow P.J., Billingsley C.C., Lankes H.A., Ali S., Cohn D.E., Broaddus R.J. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group study. J. Clin. Oncol. 2015;33:4301. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampel H., de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev. Res. 2011;4:1–5. doi: 10.1158/1940-6207.CAPR-10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick K., Straughn J.M., Backes F., Hampel H., Matthews K.S., Cohn D.E. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstet. Gynecol. 2009;114:530–536. doi: 10.1097/AOG.0b013e3181b11ecc. [DOI] [PubMed] [Google Scholar]
- 25.Goverde A., Spaander M.C., van Doorn H.C., Dubbink H.J., van den Ouweland A.M., Tops C.M. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70 years of age. Gynecol. Oncol. 2016;143:453–459. doi: 10.1016/j.ygyno.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y.-E., Kao S.-S., Chung R.-H. Cost-effectiveness analysis of different genetic testing strategies for Lynch syndrome in Taiwan. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladabaum U., Wang G., Terdiman J., Blanco A., Kuppermann M., Boland C.R. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann. Intern. Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snowsill T., Coelho H., Huxley N., Jones-Hughes T., Briscoe S., Frayling I.M. 2017. Molecular Testing for Lynch Syndrome in People with Colorectal Cancer: Systematic Reviews and Economic Evaluation. [DOI] [PMC free article] [PubMed] [Google Scholar]