Abstract
Objectives
Mandated social distancing has been applied globally to reduce the spread of coronavirus disease 2019 (COVID-19). However, the beneficial effects of this community-based intervention have not been proven or quantified for the COVID-19 pandemic.
Study design
This is a regional population-level observational study.
Methods
Using publicly available data, we examined the effect of timing of mandated social distancing on the rate of COVID-19 cases in 119 geographic regions, derived from 41 states within the United States and 78 other countries. The highest number of new COVID-19 cases per day recorded within a geographic unit was the primary outcome. The total number of COVID-19 cases in regions where case numbers had reached the tail end of the outbreak was an exploratory outcome.
Results
We found that the highest number of new COVID-19 cases per day per million persons was significantly associated with the total number of COVID-19 cases per million persons on the day before mandated social distancing (β = 0.66, P < 0.0001). These findings suggest that if mandated social distancing is not initiated until the number of existing COVID-19 cases has doubled, the eventual peak would result in 58% more COVID-19 cases per day. Subgroup analysis on those regions where the highest number of new COVID-19 cases per day has peaked showed increase in β values to 0.85 (P < 0.0001). The total number of cases during the outbreak in a region was strongly predicted by the total number of COVID-19 cases on the day before mandated social distancing (β = 0.97, P < 0.0001).
Conclusions
Initiating mandated social distancing when the numbers of COVID-19 cases are low within a region significantly reduces the number of new daily COVID-19 cases and perhaps also reduces the total number of cases in the region.
Keywords: COVID-19, Mandated social distancing, Quarantine
Introduction
Quarantine and isolation are standard procedures to avoid transmission of infectious disease from infected to non-infected persons and have been used in numerous epidemics.1 Social distancing is another method for reducing frequency of contact between people to decrease the risk of disease transmission. Social distancing has been used in both influenza and coronavirus disease 2019 (COVID-19) pandemics (caused by severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]). Social distancing can be voluntary at the individual level or mandated at a community level by governing authorities.
Mandated social distancing comprises of a combination of travel restrictions, closure of non-essential group meeting venues (e.g., restaurants, schools, shops) and steps to avoid close contact at essential meeting venues (e.g., hospitals, food supply, pharmacies). Mandated social distancing is also referred to as ‘societal lockdown’ and will have a variable impact on the spread of disease depending on the mode of disease transmission and ability to identify and isolate persons infected with the disease.2 Critical analysis of mandated social distancing in 17 cities in the United States during the 1918 pandemic (caused by H1N1 influenza A virus) found that cities with mandated social distancing at an early phase of the epidemic had peak death rates 50% lower than in those cities that did not implement such early interventions.3 Although results from the 1918 pandemic, influenza pandemics and severe acute respiratory syndrome have been used to justify mandated social distancing in various parts of the world, limited analysis of the effect of mandated social distancing on the COVID-19 pandemic is available. The value of mandated social distancing requires a critical assessment for each pandemic because of inadvertent adverse psychological and health consequences on individuals4 , 5 and financial effects on society.6 We examined the effect of timing of mandated social distancing on the rate of COVID-19 cases in 119 geographic regions, derived from 41 states within the United States and 78 other countries.
Methods
Daily cumulative COVID-19 case numbers for individual regions (countries and individual states within the United States) from January 22, 2020, are publicly available.7 , 8 The start dates of mandated social distancing for different regions have been compiled and are also available.9 For this analysis, only regions that had data for both mandated social distancing start dates and daily cumulative case volumes for COVID-19 were included. For the United States, data were available for each state, thus allowing a detailed analysis. In countries other than the United States, we used national mandated social distancing start dates and national COVID-19 case volumes. For France, Denmark, the Netherlands and the United Kingdon, overseas regions were not included in the calculation of national case volumes.
New COVID-19 cases per day were calculated from cumulative daily case volumes up to April 25, 2020. The period of observation in this study was limited up to April 25, 2020, because after this date, relaxation of mandated social distancing occurred in various geographical units, thus confounding the results. We used 2019 population estimates for states in the United States and other countries to calculate daily new and cumulative total COVID-19 case volumes per million persons residing within the region.10 , 11 For further analysis, data were smoothed using a moving average to remove daily fluctuations in reported COVID-19 cases. Smoothed data were plotted over raw data for all geographical regions to ensure that they were representative of the raw data (see Appendix A in the supplementary material). China was excluded from the current analysis as the curve was visually different from other regions and the aforementioned methodology could not be reliably applied.
We used the total number of COVID-19 cases per million on the day before mandated social distancing was implemented as the independent variable and predictor for the analysis. The peak of the smoothed curve was used to determine the highest number of new COVID-19 cases per day (expressed in per million persons) and was used as the dependent variable. Owing to the skewness in both the dependent and independent variables, log transformation was applied. To determine if the number of daily new cases had plateaued or was still increasing, linear regression for the previous 13 days was used. The previous 13 days was selected after visually checking the trend for all geographic regions and repeating linear regression for various intervals, ranging from 5 to 13 days. The linear positive trend for the previous 13 days (April 12–25) correlated best with visual interpretation of an upward trend.
Log-transformed values of the highest number of new COVID-19 cases per day per million population and the total number of COVID-19 cases on the day before mandated social distancing were used for all regression analyses. Linear regression analysis was used to predict the highest number of new COVID-19 cases per day using the total number of COVID-19 cases on the day before mandated social distancing as the predictor (model A). Additional analysis of this association was performed after adjustment for the day mandated social distancing started in the course of the COVID-19 pandemic (calculated as the number of days since January 22, 2020), log-transformed population of the geographic region and proportion of persons living in urban areas (model B).12 , 13 We use adjusted R-squared (R2) to calculate how much of the correlation was determined by the addition of independent variables.
The analyses were repeated after classifying the geographic regions into those where the daily new COVID-19 case volume had plateaued and those where COVID-19 cases were still increasing.
Using Internet searches, individual elements of mandated social distancing were manually abstracted for each of the geographical regions included in the analyses (see Appendix B in the supplementary material), and additional analyses were performed after adjusting for these elements.
For regions where the average (over the last 5 days) daily new case volume had trended down to less than 20% of the peak daily new case volume (considered here as reaching the tail end of the epidemic), linear regression analysis was performed to predict the overall number of new COVID-19 cases per million from the total number of COVID-19 cases per million persons on the day before mandated social distancing after log transformation of both variables.
Results
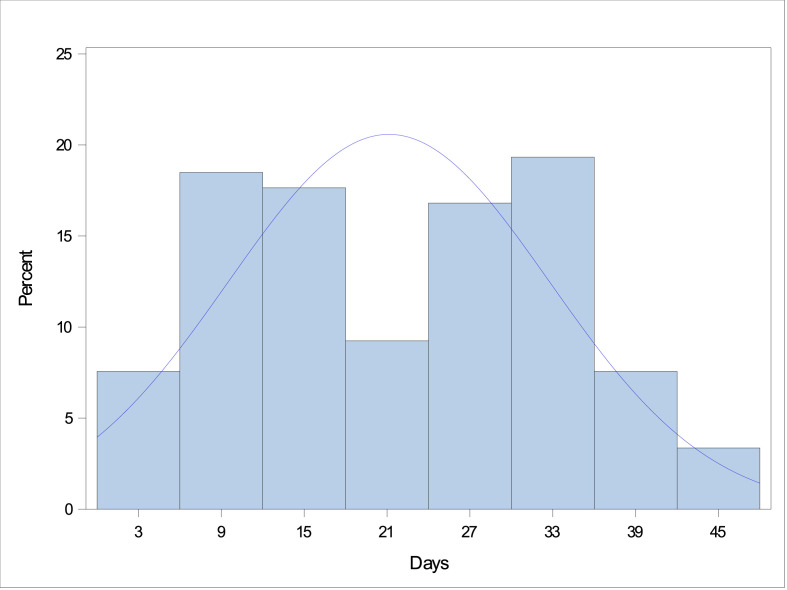
Initiation dates of mandated social distancing were available for 85 countries and 42 US states. Daily COVID-19 case volume data were available for 183 countries and all 52 US states. Both mandated social distancing starting dates and daily COVID-19 case data were available for 78 countries and 41 states. After excluding three regions where the date of the peak number of daily new cases was either before (Israel and Maine) or on the start day of mandated social distancing (Eritrea), the number of days from the start date of mandated social distancing to the peak in daily new COVID-19 cases ranged from 1 to 45 days (Fig. 1 ).
Fig. 1.
Interval (in days) between the date of mandated social distancing and reaching the highest number of new COVID-19 cases per day. COVID-19, coronavirus disease 2019.
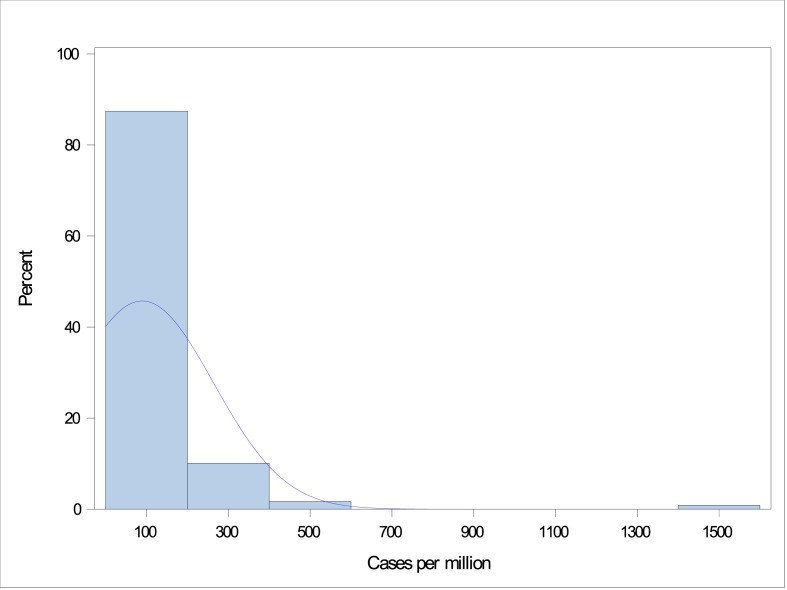
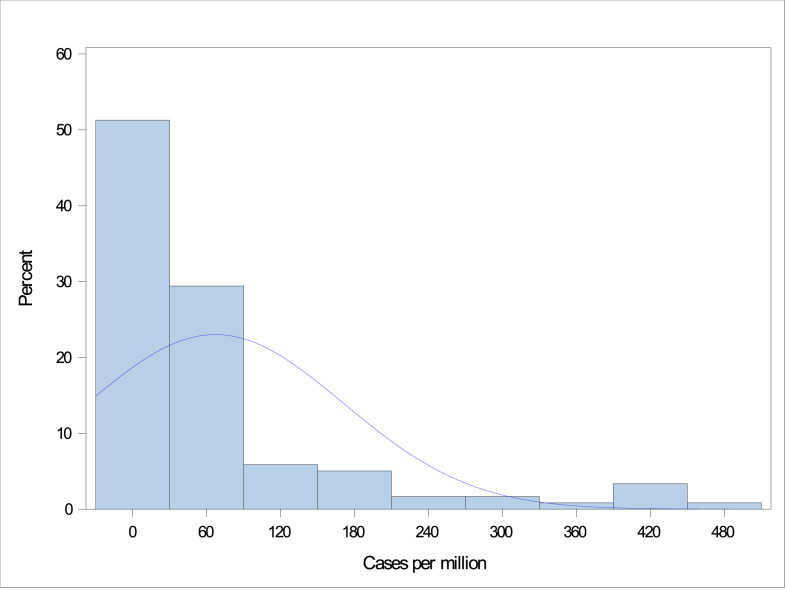
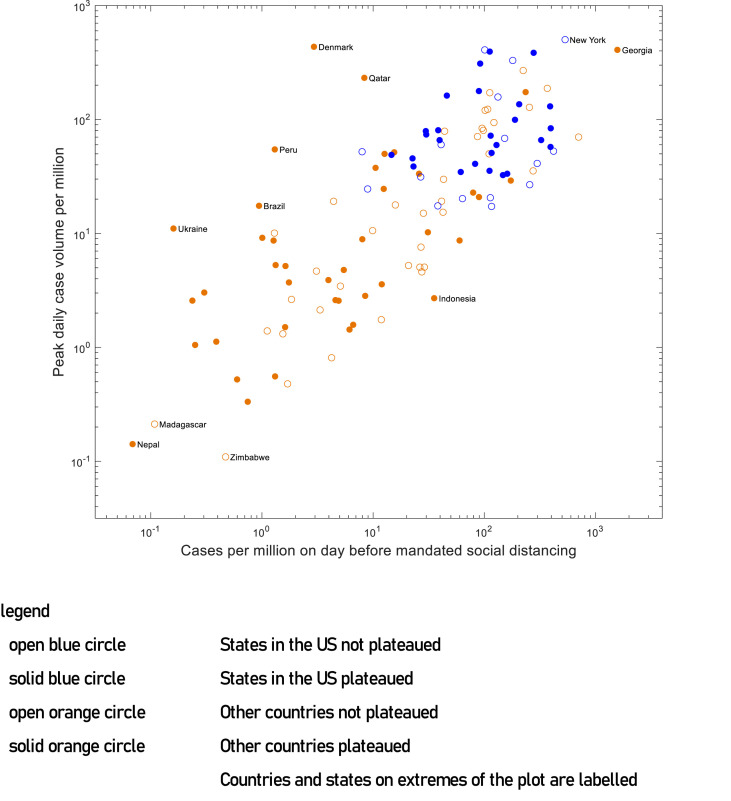
Mandated social distancing start dates within individual states of the United States ranged from March 17 to April 3, 2020, and for other countries ranged from March 9 to April 15, 2020. The total number of COVID-19 cases ranged from 0 to 1571 cases per million persons on the day before the start date of mandated social distancing (Fig. 2 ). The highest number of new COVID-19 cases per day ranged from 0.10 to 503 per million persons (Fig. 3 ). There was a clear trend towards the association between the total number of COVID-19 cases on the start date of mandated social distancing and the highest number of new COVID-19 cases per day when plotted on a logarithmic scale using a scatter plot (Fig. 4 ).
Fig. 2.
Distribution of the total number of COVID-19 cases (per million population) on the day before initiation of mandated social distancing. COVID-19, coronavirus disease 2019.
Fig. 3.
Distribution of the highest number of new COVID-19 cases per day (per million population). COVID-19, coronavirus disease 2019.
Fig. 4.
Relationship between the total number of COVID-19 cases on the day before mandated social distancing initiated and the highest number of new COVID-19 cases per day on the logarithmic scale. COVID-19, coronavirus disease 2019.
The results of the linear regression analyses with different models are reported in Table 1 . In model A, the highest number of new COVID-19 cases per day was significantly associated with the total number of COVID-19 cases on the day before mandated social distancing (β = 0.66, P < 0.0001). Model B showed improvements in the adjusted R2 values from 0.59 to 0.72, but no change was observed in terms of β values for the total number of COVID-19 cases on the day before mandated social distancing. Subgroup analyses on those regions where the daily new COVID-19 cases had already peaked showed increase in β values for the total number of COVID-19 cases on the day before mandated social distancing to 0.85 for both the unadjusted and adjusted models (P < 0.0001).
Table 1.
| Statistic | All regions | Plateaued | Not plateaued | |||
|---|---|---|---|---|---|---|
| Total | 119 | 51 | 68 | |||
| States within the United States | 41 | 15 | 26 | |||
| Other countries |
78 |
36 |
42 |
|||
| Model A |
Model B |
Model A |
Model B |
Model A |
Model B |
|
| F | 171.9 | 77.5 | 132.1 | 55.1 | 79.4 | 42.6 |
| Adjusted R2 | 0.59 | 0.72 | 0.72 | 0.81 | 0.54 | 0.71 |
| Constant | 10.1 (0.56) | 15.1 (1.65) | 11.8 (0.78) | 19.3 (2.15) | 9.6 (0.76) | 15.2 (2.56) |
| Log (cumulative case volume per million on the day before mandated social distancing) | 0.66** (0.05) | 0.66** (0.05) | 0.85** (0.07) | 0.85** (0.07) | 0.59** (0.07) | 0.61** (0.09) |
| Log (population of the region) | −0.06 (0.07) | −0.08 (0.08) | −0.12 (0.11) | |||
| Day of mandated social distancing (from January 22, 2020) | −0.09** (0.02) | −0.1** (0.02) | −0.08** (0.02) | |||
| Percentage of the urban population in the region | 0.02* (0.006) | 0.001* (0.008) | 0.02* (0.009) | |||
COVID-19, coronavirus disease 2019.
*P < 0.01, **P < 0.001.
Standard errors are reported in parentheses.
Model A = unadjusted; model B = adjusted for the day mandated social distancing started in the course of the COVID-19 pandemic (calculated as the number of days since January 22, 2020), for log-transformed population of geographic region and for proportion of persons living in urban areas.
Similar results from analyses for states within the United States are reported in Table 2 . There was a less clear association between the highest number of new COVID-19 cases per day and the total number of COVID-19 cases on the day before mandated social distancing (β = 0.3, P < 0.001) in the unadjusted model, but a stronger association was observed in the adjusted model (β = 0.72, P < 0.0001). In a model adjusted for only the day of mandated social distancing (not shown in the table), the association between the highest number of new COVID-19 cases per day and total number of COVID-19 cases on the day before mandated social distancing was strong (β = 0.78, P < 0.0001). Daily COVID-19 case volume plateaued in only 13 US states. Both the unadjusted (model A) and adjusted (model B) association between the highest number of new COVID-19 cases per day and the total number of COVID-19 cases on the day before mandated social distancing was stronger in US states where the number of new cases had plateaued compared with states where the number of new COVID-19 cases per day had not plateaued (Table 2).
Table 2.
Results of the regression analysis predicting the highest number of new COVID-19 cases per day—states in the United States.a,b
| Statistic |
All regions |
Plateaued |
Not-plateaued |
|||
|---|---|---|---|---|---|---|
| States within the United States |
n=41 |
n=15 |
n=26 |
|||
| Model A | Model B | Model A | Model B | Model A | Model B | |
| F | 5.5 | 8.8 | 3.2 | 10.8 | 1.5 | 4.5 |
| Adjusted R2 | 0.1 | 0.44 | 0.13 | 0.74 | 0.02 | 0.36 |
| Constant | 7.1 (1.2) | 20 (4.01) | 7.9 (2.18) | 17.1 (5.15) | 6.1 (1.41) | 16.9 (5.85) |
| Log (cumulative case volume per million on the day before mandated social distancing) | 0.3∗∗ (0.13) | 0.72∗∗ (0.15) | 0.41∗∗ (0.23) | 0.7∗∗ (0.17) | 0.18∗∗ (0.15) | 0.52∗∗ (0.24) |
| Log (population of the region) | 0.01 (0.12) | 0.5 (0.19) | −0.26 (0.13) | |||
| Day of mandated social distancing (from January 22, 2020) | −0.15∗∗ (0.04) | −0.2∗∗ (0.04) | −0.08∗∗ (0.06) | |||
| Percentage of the urban population in the region | 0.009∗ (0.009) | −0.016∗ (0.013) | 0.023∗ (0.012) | |||
COVID-19, coronavirus disease 2019.
∗P < 0.05. ∗P < 0.01. ∗∗P < 0.001.
Standard errors are reported in parentheses.
Model A = unadjusted; model B = adjusted for the day mandated social distancing started in the course of the COVID-19 pandemic (calculated as the number of days since January 22, 2020), for log-transformed population of geographic region and for proportion of persons living in urban areas.
Internationally, there was a strong association between the highest number of new COVID-19 cases per day and the total number of COVID-19 cases on the day before mandated social distancing both in the unadjusted and adjusted models (Table 3 ). This association was stronger for countries where the number of new COVID-19 cases per day had already plateaued (β = 0.88, P < 0.0001).
Table 3.
Results of the regression analysis predicting the highest number of new COVID-19 cases per day—other countries.a,b
| Statistic |
All regions |
Plateaued |
Not-plateaued |
|||
|---|---|---|---|---|---|---|
| Total |
n=78 |
n=36 |
n=42 |
|||
| Model A | Model B | Model A | Model B | Model A | Model B | |
| F | 87.6 | 42.9 | 129.5 | 58.3 | 23.5 | 14.3 |
| Adjusted R2 | 0.53 | 0.69 | 0.79 | 0.87 | 0.35 | 0.57 |
| Constant | 9.6 (0.8) | 12.8 (2.05) | 12.1 (0.86) | 18.4 (2.16) | 8.3 (1.32) | 12.3 (3.68) |
| Log (cumulative case volume per million on the day before mandated social distancing) | 0.63∗∗ (0.07) | 0.6∗∗ (0.06) | 0.88∗∗ (0.08) | 0.83∗∗ (0.07) | 0.51∗∗ (0.1) | 0.55∗∗ (0.12) |
| Log (population of the region) | 0.02 (0.09) | −0.06 (0.08) | −0.02 (0.15) | |||
| Day of mandated social distancing (from January 22, 2020) | −0.09∗∗ (0.02) | −0.1∗∗ (0.02) | −0.08∗∗ (0.03) | |||
| Percentage of the urban population in region | 0.02∗ (0.008) | 0∗ (0.009) | 0.025∗ (0.011) | |||
COVID-19, coronavirus disease 2019.
∗P < 0.05. ∗P < 0.01. ∗∗∗P < 0.001.
Standard errors are reported in parentheses.
Model A = unadjusted; model B = adjusted for the day mandated social distancing started in the course of the COVID-19 pandemic (calculated as the number of days since 22 January, 2020), for log-transformed population of geographic region and for proportion of persons living in urban areas.
Addition of individual elements of mandated social distancing (e.g., closure of educational institutes, public transport, restaurants and other shops) did not affect the association between the highest number of new COVID-19 cases per day and the total number of COVID-19 cases on the day before mandated social distancing. Visually, Australia appeared to have plateaued; however, based on a positive trend over the last 13 days of regression, it was classified as not plateaued. The analysis of plateaued regions was repeated after manual addition of Australia, and no change in the aforementioned results was noticed.
For 17 regions (including three states within the United States), the daily new case volume reduced to less than 20% of the peak daily new case volume. The log-transformed total number of cases was strongly predicted by the total number of COVID-19 cases on the day before mandated social distancing (adjusted R2 = 0.87, F = 112, β = 0.97, P < 0.0001).
Discussion
This study confirmed the benefit and provided a quantitative estimate of the value of mandated social distancing. The findings suggest that if mandated social distancing is not initiated until after the number of existing COVID-19 cases has doubled, there would be an eventual peak with 60% more COVID-19 cases per day. This investigation found that initiation of mandated social distancing when the number of existing COVID-19 cases had doubled would result in an eventual peak with 58% more COVID-19 cases (using β of 0. 66). If mandated social distancing is started when 100 persons are infected with COVID-19 and the subsequent highest number of cases is 1000 persons, initiating mandated social distancing when 200 persons are infected would increase the peak number of cases to 1580 persons. New York provides an example where mandated social distancing was initiated on day 61 when there were 10,356 cases. As per our analysis, if mandated social distancing was initiated on day 50 (142 cases), then the maximum number of cases per day would have been reduced by a factor of 16 (31 per million compared with 500 per million persons).
This study also identified what is considered a ‘spillover’ effect. There was a blunting of the quantitative value of mandated social distancing in states within the United States when mandated social distancing was initiated later in the course of the pandemic. It is suggested that this blunting of the effect was confounded by earlier mandated social distancing in the surrounding states, which resulted in mitigating the effect by reducing inflow of infected patients with COVID-19. This effect was not seen between countries, where boundaries between countries may serve to insulate by restricting travel into the country. There are no restrictions in movement between states in the United States, thus enhancing this spillover effect.
Ferguson et al.1 estimated that combining school and workplace closure with area quarantine and antiviral prophylaxis can result in 90% containment of infection (assuming the infection has a basic reproduction number [R0] = 1.9) and when containment was initiated with less than 200 detected cases. The model was based on the spread of H5N1, a highly pathogenic avian influenza in wild and domestic poultry in Southeast Asia. Longini et al.14 modelled the avian influenza A (subtype H5N1) outbreaks in Southeast Asia. They reported that the local household quarantine was effective in containing the epidemic if R0 ≤2.1, but is not as effective at an R0 value of 2.4. However, a combination of 80% antiviral prophylaxis plus quarantine was effective at an R0 as high as 2.4, and adding previous vaccination makes antiviral prophylaxis plus quarantine even more effective. Both analyses mentioned that one of the reasons limiting the beneficial effect of mandated social distancing is the continued contact between households and neighbourhoods during social distancing, which may offset the benefit with highly infectious agents. Ferguson et al.1 assumed in their model that household and random contact rates increase by 100% and 50%, respectively, for individuals no longer able to attend school or work. Previous models have been based on the H1N1 epidemiological experience. The R0 for H1N1 influenza has ranged between 1.25 in Canada,15 1.682 in China,16 1.96 in New Zealand,17 1.6 in Mexico,18 and 1.7 in the United States.19 One of the surprising findings is that the benefit of mandated social distancing in the COVID-19 pandemic has been seen, despite the high infectivity of SARS-CoV-2. The R0 of the SARS-CoV-2 infection was originally estimated between 2.2 and 2.7.20, 21, 22, 23, 24, 25 More recent data suggest that the R0 of SARS-CoV-2 infection may be as high at 5.7.20 The R0 of SARS-CoV-2 is higher than the threshold of 2.4 estimated by Longini et al.14 and 1.8 for new viral strains estimated by Ferguson et al.,1 meaning that a higher R0 will result in loss of benefit of mandated social distancing.
There may be other reasons to explain the beneficial effect of mandated social distancing in the COVID-19 pandemic. Ridenhour et al.26 indicated the importance of the role of transmission rate, recovery rate and size of the population in the overall speed of the epidemic, independent of R0. Tang et al.16 emphasised the role of asymptomatic patients and those who are in the prodromal period without symptoms in the spread of H1N1 influenza in the province of Shaanxi. The beneficial effect of mandated social distancing may also be related to a relatively long prodromal period and high proportion of asymptomatic SARS-CoV-2–infected patients. The time between transmission and symptoms ranges between 2 and 14 days for SARS-CoV-2.27 Data on 468 COVID-19 transmission events reported in mainland China outside of Hubei Province showed that 59 (12.6%) of the 468 patients developed symptoms before the potential source developed symptoms, suggesting that transmission occurred in the prodromal period.28
There have been small case studies highlighting that COVID-19 can be acquired from patients who are and will remain asymptomatic.29, 30, 31 The estimated proportion of asymptomatic COVID-19 was 17.9% based on screening of travellers on board a cruise ship32 and 30.8% from data of Japanese citizens evacuated from Wuhan.33 However, the viral loads in the upper respiratory specimens appeared to be similar in symptomatic and asymptomatic persons.34 It is possible that the beneficial effect of mandated social distancing may be related to reducing contact between asymptomatic individuals infected with SARS-CoV-2. Another unique aspect of SARS-CoV-2 is its ability to persist on various surfaces and thus be transmitted by indirect contact from high-touch surfaces.35 , 36 SARS-CoV-2 can persist on plastic, stainless steel, copper and cardboard, and viable virus has been detected for up to 72 h after application on these surfaces. The longest viability was on stainless steel and plastic; the estimated median half-life of SARS-CoV-2 is approximately 5.6 h on stainless steel and 6.8 h on plastic. Therefore, the mandated social distancing is likely to reduce contamination and transmission from high-touch surfaces within society.
One of the limitations of the current model is the variability in policies pertaining to mandated social distancing and compliance to the policies in various geographic regions. Mandated social distancing has several facets, which include special precautions on travel on public transit, ride-shares or taxis; only operating essential businesses, such as grocery stores, gas stations and banks; closure of non-essential businesses; using drive-thru, kerbside pickup or delivery services; prohibiting events and gatherings of more than 10 people; maintaining distance (approximately 6 feet or 2 m) from others when possible; avoid eating or drinking at restaurants, bars or food courts; closing of schools and non-essential factories and workplaces and limiting the number of patrons at retail shops. Compliance with mandated social distancing is an important factor in determining success of the intervention.1 There is also variability in exposure risk reduction within a given population as each individual does not have the same chance of coming in contact with others.26 There appears to be a difference exposure risk according to age of the individuals37 and population structure such as the number of households, workplaces, schools and community groups.38 Differences in age and population structure between geographic regions may also confound the results.
There is also a confounding effect of case identification and isolation, and robustness of testing for asymptomatic individuals, which may vary in different geographic units in the current analysis. The Centers for Disease Control and Prevention (CDC) concluded that the degree to which COVID-19 cases might go undetected or unreported varies in geographic regions because testing practices differ widely and might contribute significantly to the observed variations.39 , 40 For example, the state of New York (excluding New York City) reported administering 4.9 tests per 1000 population, which was higher than the national average of 1.6 (CDC, unpublished data, March 25, 2020). The confounding effect of contact tracing and isolation was not analysed in the present study. There was variability between geographic regions in implementation of contact tracing and isolation. Contact tracing and isolation was also affected by the number of COVID-19 cases within a geographic region and may not be possible if the number of new cases exceeds a certain threshold owing to limitations in resources. The socio-economic status and location (urban versus rural) also influence access to health care and thus case identification and may alter the differences between various geographic regions.
The variability in the highest number of new cases per day that was not explained in the statistical models of the present study is likely due to variability in mandating social distancing in different regions. Although most of the organisations were closed during mandated social distancing, certain businesses, such as meat- and poultry-processing facilities, were recognised as critical for infrastructure and permitted to continue work with precautions. Outbreaks in such places resulted in increasing numbers of new cases per day that are not explained by the current model.41 , 42 It is also noted that in some regions (excluded from the analysis), the highest number of new cases per day plateaued before mandated social distancing. This suggests that there may be other mechanisms that can reduce the number of new cases in certain regions.
There were certain analyses that could not be performed for all the regions included in the present study as the pandemic is ongoing, with changing numbers of COVID-19 cases. In subgroup analysis, it was clear that the relationship was strongest when the highest number of new cases per day had reached its peak. Some regions were still in the period wherein the number of new cases per day is continuing to increase. It is also important to note that the total number of COVID-19 cases in a region can only be determined after the pandemic subsides. In total, only 17 regions in the current analysis were thought to be at the tail end of the pandemic (i.e., where daily new cases had reached less than 20% of the highest number of new cases per day observed). There was a clear relationship between the total number of cases before the start date of mandated social distancing and overall total number of cases in the region, indicating that early mandated social distancing also reduced the total number of COVID-19–infected individuals over time.
Future studies should focus on identifying the effectiveness of individual components of mandated social distancing to determine the most effective model for prevention of COVID-19. Another issue is the re-emergence of COVID-19 (termed as the ‘second wave’) with relaxation of the mandated social distancing policy. Estimation of the impact of relaxation of the mandated social distancing policy is confounded by a staged and heterogenous set of policies, which make it difficult to identify a distinct effect. However, the differences in relaxation policies between regions may be correlated with regional re-emergence of COVID-19 to identify the most effective strategy for relaxation and termination of mandated social distancing.
Conclusions
The value of mandated social distancing in reducing the spread of COVID-19 has been questioned at multiple levels owing to widespread inadvertent effects on individuals' well-being and the financial consequences on society. This study demonstrates that initiating mandated social distancing when smaller numbers of COVID-19 cases are present will reduce the highest number of new cases per day and perhaps even the overall total number of COVID-19 cases in the region, highlighting the importance of this community-based intervention.
Author statements
Ethical approval
Not required.
Funding
None declared.
Competing interests
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2020.10.015.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ferguson N.M., Cummings D.A.T., Cauchemez S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 2.Ishola D.A., Phin N. Could influenza transmission be reduced by restricting mass gatherings? Towards an evidence-based policy framework. J Epidemiol Glob Health. 2011;1:33–60. doi: 10.1016/j.jegh.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatchett R.J., Mecher C.E., Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar A., McMunn A., Demakakos P., Hamer M., Steptoe A. Social isolation and loneliness: prospective associations with functional status in older adults. Health Psychol. 2017;36:179–187. doi: 10.1037/hea0000437. [DOI] [PubMed] [Google Scholar]
- 5.Shankar A., McMunn A., Banks J., Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30:377–385. doi: 10.1037/a0022826. [DOI] [PubMed] [Google Scholar]
- 6.Correia S., Luck S., Verner El. 2020. Pandemics depress the economy, public health interventions do not: evidence from the 1918 flu.https://ssrn.com/abstract=3561560 Available at: SSRN: or. [DOI] [Google Scholar]
- 7.Novel coronavirus (COVID-19) cases data. https://data.humdata.org/dataset/novel-coronavirus-2019-ncov-cases (last accessed 25 April 2020).
- 8.Coronavirus source data. https://ourworldindata.org/coronavirus-source-data (last accessed 25 April 2020).
- 9.Global Covid-19 lockdown tracker. https://auravision.ai/covid19-lockdown-tracker/ (last accessed 20 April 2020).
- 10.State population totals and components of change: 2010-2019. https://www.census.gov/data/tables/time-series/demo/popest/2010s-state-total.html (last accessed 20 April 2020).
- 11.Countries in the world by population. https://www.worldometers.info/world-population/population-by-country/ (last accessed 20 April 2020).
- 12.Census urban and rural classification and urban area criteria. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html (last accessed 20 April 2020).
- 13.Urban and rural populations. https://population.un.org/wup/Download/ (last assessed 22 April 2020).
- 14.Longini I.M., Nizam A., Xu S. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 15.Tuite A.R., Greer A.L., Whelan M. Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. CMAJ (Can Med Assoc J) 2010;182:131–136. doi: 10.1503/cmaj.091807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang S., Xiao Y., Yang Y., Zhou Y., Wu J., Ma Z. Community-based measures for mitigating the 2009 H1N1 pandemic in China. PloS One. 2010;5 doi: 10.1371/journal.pone.0010911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiura H., Wilson N., Baker M.G. Estimating the reproduction number of the novel influenza A virus (H1N1) in a Southern Hemisphere setting: preliminary estimate in New Zealand. N Z Med J. 2009;122:73–77. [PubMed] [Google Scholar]
- 18.Fraser C., Donnelly C.A., Cauchemez S. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White L.F., Wallinga J., Finelli L. Estimation of the reproductive number and the serial interval in early phase of the 2009 influenza A/H1N1 pandemic in the USA. Influenza Other Respir Viruses. 2009;3:267–276. doi: 10.1111/j.1750-2659.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Z., Wang L., Cauchemez S. Risk for transportation of coronavirus disease from Wuhan to other cities in China. Emerg Infect Dis. 2020;26:1049–1052. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.4.2000058. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J., Li M., Lv G., Lu Z.K. Monitoring transmissibility and mortality of COVID-19 in Europe. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.200282. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridenhour B., Kowalik J.M., Shay D.K. Unraveling R0: considerations for public health applications. Am J Public Health. 2014;104:e32–e41. doi: 10.2105/AJPH.2013.301704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C., Ji F., Wang L. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.200718. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020 doi: 10.7326/M20-0504. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Z., Xu X., Wu Y., Wang L., Cowling B.J., Meyers L.A. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2606.200357. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2565. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan J.F.-W., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiura H., Kobayashi T., Suzuki A. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santarpia J., Rivera D., Herrera V., Morwitzer M., Creager H., Santarpia G. 2020. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. pre print. [DOI] [Google Scholar]
- 36.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nokes D.J., Anderson R.M. The use of mathematical models in the epidemiological study of infectious diseases and in the design of mass immunization programmes. Epidemiol Infect. 1988;101:1–20. doi: 10.1017/s0950268800029186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellis L., Ferguson N.M., Fraser C. Threshold parameters for a model of epidemic spread among households and workplaces. J R Soc Interface. 2009;6:979–987. doi: 10.1098/rsif.2008.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CDC COVID-19 Response Team Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:465–471. doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30243-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meat and poultry processing workers and EmployersInterim guidance from CDC and the occupational safety and health administration (OSHA). https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/meat-poultry-processing-workers-employers.html (last assessed 3 May 2020).
- 42.COVID-19 among workers in meat and poultry processing facilities? 19 states. 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6918e3.htm?s_cid=mm6918e3_w#T1_down [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.