Abstract
Background:
Long-term engagement in opioid agonist therapy (OAT) has been consistently associated with reduced risk for morbidity and mortality in people with opioid use disorder (OUD). However, the dynamic nature of engagement/disengagement in OUD care for over time is poorly captured by traditional metrics. We characterized long-term longitudinal trajectories of engagement in OAT in Vancouver, Canada, between 2005 and 2018.
Methods:
Data were derived from two community-recruited prospective cohorts of people who use drugs. Retention in OAT was defined as self-reported enrolment in OAT for two consecutive follow-up periods (an approximately six-month retention interval). We used latent class growth analysis to identify OAT engagement trajectories during the first five years after OAT initiation and multivariable logistic regression to evaluate predictors of trajectory group membership.
Results:
We identified four OAT retention trajectories among 438 OAT initiators: “consistently high” (36%), “consistently low” (23%), “increasing” (23%), and “decreasing” (15%). Employment was a significant cross-cutting predictor of membership of all sub-optimal OAT engagement patterns compared to consistently high trajectories. We also found that initiating OAT after 2014 (when regulatory changes to the provincial OAT program were introduced) was associated with the “consistently low” engagement group relative to others.
Conclusions:
We identified four distinct OAT engagement trajectories in Vancouver, Canada, with employment being a common predictor of sub-optimal care trajectories, suggesting the need to explore alternative OAT models to address employment-related barriers. Care trajectory analysis could help inform tailored interventions to specific populations of people with OUD at specific time points to improve engagement in OAT, and decrease opioid-related morbidity and mortality.
Keywords: Latent class growth analysis, Opioid agonist therapy, Opioid use disorder, Performance measures, Quality metrics
INTRODUCTION
Settings throughout the United States (U.S.) and Canada continue to face an unprecedented and worsening opioid overdose crisis. In 2017, more than 47,000 people in the U.S. died from an opioid-related overdose (i.e., 14.9 per 100,000 people), a toll largely driven by exposure to synthetic opioids, including illicitly manufactured fentanyl and related analogues (Scholl, Seth, Kariisa, Wilson & Baldwin, 2018). Canada is experiencing similarly high fatal overdose rates, with some jurisdictions, like the province of British Columbia (BC) being particularly hard hit, with a rate of 31 overdose deaths per 100,000 people in 2018 (BC Coroners Service, 2019).
Despite the well-documented individual- and community-level benefits of opioid agonist therapy (OAT), including reductions in all-cause and overdose-specific rates of mortality (Blanco & Volkow, 2019; Sordo et al., 2017), access and retention in OAT programs is extremely low. It is estimated that in most settings less than 30% of individuals with OUD start OAT, and less than half of these remain in treatment for more than six months (Blanco & Volkow, 2019; Socias et al., 2018; Timko, Schultz, Cucciare, Vittorio & Garrison-Diehn, 2016; Williams et al., 2018). Barriers to access and retention in OAT are multifactorial and span throughout and beyond the health system. These include strict OAT programmatic requirements (e.g., frequent/daily visits to the pharmacy for witnessed ingestion of their medication, denial or revocation of take-home dosing privileges if positive urine drug tests), restricted coverage for OAT pharmacotherapies, limited number of trained addiction clinicians and geographic disparities in their availability, as well as a range of social-structural and attitudinal barriers stemming from the criminalized and stigmatized nature of drug use (Sharma et al., 2017).
A number of actions are being taken across the United States and Canada to curb the opioid crisis, including efforts to improve access to OAT (Murthy, 2016; Wood, 2018). There have also been increasing calls for the development and implementation of health system performance measures to monitor and evaluate the effectiveness of these initiatives (Socias et al., 2018; Socias, Volkow & Wood, 2016; Williams et al., 2018). While extremely valuable from a public health perspective, the majority of these metrics offer only a population-level snapshot of engagement in care at a given point in time. Consequently, they are limited in their ability to capture the multiple individual-level cycles of engagement in and disengagement from care common among individuals with OUD (Burns et al., 2015; Socias et al., 2018; Williams et al., 2018). Better understanding the longitudinal care trajectories of people with OUD and identification of socio-demographic, behavioural, social/structural and clinical predictors of sub-optimal care patterns could help to better target interventions to those most in need, and ultimately improve individual- and population-level outcomes. Therefore, the aim of the present study was to characterize long-term and distinctive patterns of engagement in care after OAT initiation in Vancouver, Canada, and to identify predictors of membership of each care trajectory.
METHODS
Study setting
British Columbia (BC)’s OAT program was established in 1996 and has since evolved to include low-threshold models, including office-based care, dispensation of pharmacotherapies through community-based pharmacies, and free medical care and pharmacotherapies for low-income residents delivered through the province’s universal health care system (British Columbia Ministry of Health, 2020; Socias et al., 2018). Methadone was the only available OAT until 2010, when buprenorphine/naloxone was introduced to the provincial drug formulary (Socias et al., 2018). In 2014, regulatory changes were introduced to the BC OAT program, including a switch to a different methadone formulation and discontinuation of pharmacy deliveries, which resulted in a number of unintended consequences and dissatisfaction among many OAT clients (McNeil et al., 2015; Socias et al., 2017). In 2017, the provincial government endorsed the off-label use of alternative forms of OAT, including slow-release oral morphine and injectable diacetylmorphine or hydromorphone, as second- and third-line treatments, respectively (Wood, 2018).
Study design
Data for this study were drawn from two harmonized and ongoing prospective cohort studies of people who use illicit drugs in Vancouver, Canada: the Vancouver Injection Drug Users Study (VIDUS) and the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS). Eligibility criteria and study procedures for each cohort have been described in detail previously (Strathdee et al., 1998; Wood et al., 2008). In brief, VIDUS consists of HIV-negative adults (≥ 18 years) who injected drugs in the month prior to enrolment, and ACCESS of HIV-positive adults who used illicit drugs in the previous month. Participants are recruited through self-referral, word-of-mouth, and street outreach in Greater Vancouver.
After providing informed consent, at baseline and semi-annually thereafter, participants complete an interviewer-administered questionnaire that collects socio-demographic data, substance use patterns, health care access and utilization, including addiction care, as well as other relevant social-structural exposures. Additionally, at each visit, participants undergo HIV and HCV antibody testing, as well as HIV disease monitoring, as appropriate. Participants receive a CAD $40 honorarium at each study visit. The VIDUS and ACCESS studies have received approval by University of British Columbia/Providence Health Care Research Ethics Board.
Study sample
As in previous work investigating engagement in care for OUD (Socias et al., 2018), we restricted the analytic sample to participants enrolled between December 2005 and November 2018 who initiated OAT after recruitment into the cohorts. We further restricted the sample to participants who completed at least three study visits to have at least one year worth of data.
Outcome measure
Our main outcome of interest was retention in OAT, defined as a self-report of being on methadone-, buprenorphine/naloxone-, slow release oral morphine or injectable OAT-based treatment in the current and immediately previous study visit, an approximately six-month retention interval (Socias et al., 2018). In the event of missing information for a given interview, the last observation was carried forward.
Covariates
The following variables were assessed as potential predictors of membership of each trajectory. Socio-demographic characteristics and comorbidities examined included: age at enrolment (per year older), sex (male versus female), self-reported race (white versus others), HIV serostatus and history mental illness (e.g., depression, anxiety, post-traumatic stress disorder, psychosis, personality disorder). We also considered substance use patterns, including high intensity (i.e., ≥ daily versus < daily) non-medical opioid (e.g., heroin, street fentanyl, street oxycodone, street methadone), crack, cannabis use and high-risk drinking as per the U.S. National Institute on Alcohol Abuse and Alcoholism (NIAAA)’s definition (National Institute on Alcohol Abuse and, Alcoholism, 2020), and recent overdose; OAT characteristics, including year of OAT initiation, type of OAT; as well as social-structural exposures, such as and unmet health or social service needs, homelessness, employment and incarceration. Except for the fixed socio-demographic variables, and OAT related variables, all other variables refer to the six-month period prior to the interview where a participant first reported being on OAT. We decided to dichotomized the year of OAT initiation at </≥ 2014 to explore the impacts of the regulatory changes to BC OAT program (McNeil et al., 2015; Socias et al., 2017). Overall, the extent of missing values for covariates was extremely low (< 1.5%).
Statistical analyses
First, we used latent class growth analysis (LCGA) to identify patterns of engagement in OAT. Time zero was defined as the date of OAT initiation, and we included all interview data for each participant for five years after this date. To determine the optimal number and patterns of trajectory groups, we fitted a series of models, varying the number of groups from one to seven, and compared results based on the following criteria: the Bayesian Information Criterion, the average posterior probabilities of groups, examination of the shapes of the trajectories for similarities, and group membership probability (Muthen & Muthen, 2000; van de Schoot, Sijbrandij, Winter, Depaoli & Vermunt, 2017).
After determining the optimal number of trajectory groups that best fit the data, we used multinomial logistic regression models to determine baseline correlates of membership in each OAT engagement trajectory group, using as reference the “consistently high” engagement class. Given high prevalence (> 90%, and see Table 1 for more details) of opioid use and methadone-based OAT in the study sample at the time of OAT initiation, non-medical use of opioids and type of OAT were excluded from our regression models.
Table 1.
Baseline characteristics of OAT initiators, stratified by OAT engagement trajectory, Vancouver, British Columbia, 2005–2018.
| Characteristics | Total (N = 438) | OAT engagement trajectory groups, n (%) | |||
|---|---|---|---|---|---|
| Consistently low (n = 102) | Increasing (n = 114) | Decreasing (n = 66) | Consistently high (n = 156) | ||
| Socio-demographics | |||||
| Age (median, IQR) | 42 (35,48) | 42 (33,49) | 42 (35,49) | 43 (36,49) | 42 (36,48) |
| Male sex | 269 (61.4) | 64 (62.8) | 72 (63.2) | 42 (63.6) | 91 (58.3) |
| White race | 196 (44.8) | 40 (39.2) | 49 (43.0) | 33 (50.0) | 74 (47.4) |
| Comorbidities | |||||
| HIV-positive | 151 (34.5) | 31 (30.4) | 37 (32.5) | 25 (37.9) | 58 (37.2) |
| History of mental illness | 234 (53.4) | 60 (58.8) | 62 (54.4) | 38 (57.6) | 74 (47.4) |
| Substance use-related factorsa | |||||
| ≥ Daily non-medical opioid use | 426 (97.3) | 100 (98.0) | 109 (95.6) | 64 (97.0) | 153 (98.1) |
| High-risk drinking | 34 (7.8) | 8 (7.8) | 11 (9.7) | 6 (9.1) | 9 (5.8) |
| ≥ Daily crack use | 142 (32.4) | 30 (29.4) | 41 (36.0) | 20 (30.3) | 51 (32.7) |
| ≥ Daily cannabis use | 80 (18.3) | 16 (15.7) | 23 (20.2) | 10 (15.2) | 31 (19.9) |
| Recent non-fatal overdose | 32 (7.3) | 7 (6.9) | 11 (9.7) | 3 (4.6) | 11 (7.1) |
| OAT characteristics | |||||
| OAT initiation ≥ 2014 | 72 (16.4) | 25 (24.5) | 15 (13.2) | 7 (10.6) | 25 (16.0) |
| Type of OAT | |||||
| Methadone-based | 411 (93.8) | 96 (94.1) | 103 (90.4) | 64 (97.0) | 148 (94.9) |
| Buprenorphine-based | 11 (2.5) | 4 (3.9) | 3 (2.6) | 0 (0.0) | 4 (2.6) |
| Other (slow- release oral morphine, injectable diacetylmorphine) | 16 (3.7) | 2 (2.0) | 8 (7.0) | 2 (3.0) | 4 (2.6) |
| Other structural-level factorsa | |||||
| Unmet health or social needs | 95 (21.7) | 26 (25.5) | 21 (18.4) | 14 (21.2) | 34 (21.8) |
| Homelessness | 160 (36.5) | 31 (30.4) | 47 (41.2) | 24 (36.4) | 58 (37.2) |
| Employment | 83 (19.0) | 23 (22.6) | 24 (21.1) | 17 (25.8) | 19 (12.2) |
| Incarceration | 58 (13.2) | 17 (16.7) | 10 (8.8) | 7 (10.6) | 24 (15.4) |
IQR, interquartile range; OAT, opioid agonist therapy.
Refers to the six-month period prior to the baseline interview
Variables found to be associated with at least one trajectory group at P < 0.20 in bivariable analyses were considered for inclusion in the multivariable model selection process. As in previous research (Socias et al., 2018), the multivariable model was built using a backward selection approach. The Akaike information criterion (AIC) was used to identify the model with the best overall fit as indicated by the lowest AIC value.
In addition, to better inform targeting health system efforts to populations most in need, we conducted a sub-analysis, where we investigated unique factors associated with the “consistently low” group. We followed a similar approach as described above, but in this case, in the multivariable model, the “consistently low” group was compared with all other groups combined. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC), and all P values were two-sided.
RESULTS
Between December 2005 and November 2018, 573 study participants reported initiating OAT after enrolment, of whom 438 (76%) had at least three study visits and were included in the analysis. Of these, just over half (n = 234, 53.4%) had a history of previous OAT. There were no significant differences between included and excluded participants in terms of age, sex or race. Selected baseline characteristics of the study sample are presented in Table 1. The median age at OAT initiation was 42 (IQR 35–48) years, 269 (61.4%) were male, 196 (44.8%) self-reported white race, around a third (n = 151, 34.5%) were living with HIV and over half reported a history of mental illness (n = 234, 53.4%). Most participants started OAT before 2014 (n = 235, 53,7% between 2006 and 2009, and n = 131, 29.9% between 2010 and 2013), and were enrolled in methadone-based OAT (n = 411, 93.8%). Of the 366 participants who initiated OAT before 2014, 285 (77.9%) had at least one report of enrolment in OAT after 2014. Additionally, at the time of OAT initiation, almost all of the study sample (n = 426, 97.3%) reported at least daily non-medical use of opioids.
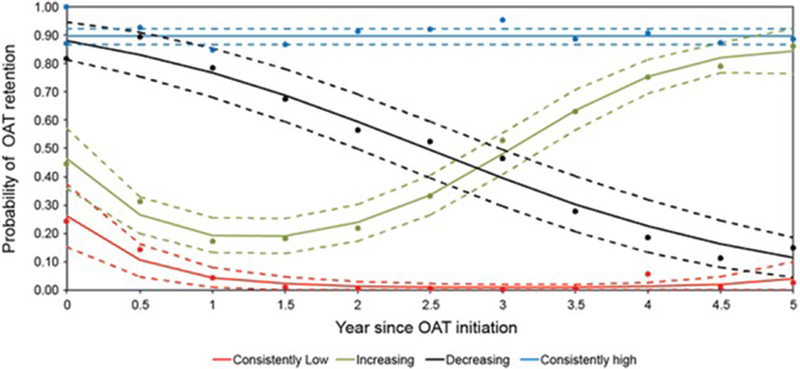
The median number of study visits per participant was 13 (IQR: 7–19), with a mean number of observations at each time point of 374 (range 290–438). On average, 54.0% of the sample were retained in OAT at any given study visit. Based on our LCGA model selection criteria, the optimal number of OAT engagement trajectories was four. The four-trajectory solution had average posterior probabilities ranging between 0.83 to 0.89, indicating good discrimination between groups (Mikolajczyk, Horn, Prins, Wiessing & Kretzschmar, 2014; Muthen & Muthen, 2000). Fig. 1 displays the patterns of OAT engagement over time based on the four-trajectory model. Specifically, 156 (35.6%) participants showed consistently high engagement in OAT (with an approximate 90% probability of being retained in OAT at each visit), 114 (26.0%) an initial low engagement with subsequent increase after the first year of OAT initiation (starting with < 50% probability, further decreasing to a low of < 20% at year 1, to steadily increase thereafter to > 80% at year 5), 66 (15.1%) a steadily decreasing engagement trend (starting at approximately 85% and decreasing to approximately 15% at year 5), and 102 (23.3%) a consistent low engagement pattern (< 15% retention probability).
Fig. 1.
Longitudinal trajectories of engagement in OAT among OAT initiators in Vancouver, Canada, 2005–2018.
Dots represent observed probability of retention in OAT in each 6-month interval. The average predicted probability of retention in OAT in each group, and corresponding 95% confidence interval are plotted with solid and dashed lines respectively.
Tables 2 and 3 present the bivariable and multivariable multinomial logistic regression analyses of factors associated with each trajectory group, when compared to the “consistently high” engagement pattern. In the adjusted model, employment was significantly and positively associated with the “consistently low” (Adjusted Odds Ratio [AOR] = 2.01, 95% Confidence Interval [CI]: 1.02, 3.93), “increasing” (AOR = 1.97, 95% CI: 1.02, 3.81) and “decreasing” (AOR = 1.97, 95% CI: 1.02, 3.81) engagement trajectories. No other predictors of membership of the sub-optimal trajectories were identified.
Table 2.
Bivariable multinomial logistic regression analyses of factors associated with membership in suboptimal OAT engagement groups (versus membership in the “Consistently High” OAT engagement group) among OAT initiators, Vancouver, British Columbia, 2005–2018.
| OAT engagement trajectory groups | ||||||
|---|---|---|---|---|---|---|
| Consistently low | Increasing | Decreasing | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (per year older) | 0.99 | 0.96, 1.02 | 1.00 | 0.97, 1.03 | 1.02 | 0.99, 1.05 |
| Male sex | 1.20 | 0.72, 2.01 | 1.22 | 0.75, 2.01 | 1.25 | 0.69, 2.26 |
| White race | 0.72 | 0.43, 1.19c | 0.84 | 0.51, 1.36 | 1.11 | 0.62,1.97 |
| HIV-positive | 0.74 | 0.43, 1.26 | 0.81 | 0.49, 1.35 | 1.03 | 0.57, 1.87 |
| History of mental illness | 1.56 | 0.94, 2.59c | 1.31 | 0.80, 2.12 | 1.49 | 0.83, 2.66c |
| High-risk drinkinga | 1.41 | 0.52, 3.77 | 1.74 | 0.70, 4.36 | 1.63 | 0.56, 4.79 |
| ≥ Daily crack usea | 0.85 | 0.50, 1.47 | 1.13 | 0.68, 1.89 | 0.88 | 0.47, 1.64 |
| ≥ Daily cannabis usea | 0.75 | 0.39, 1.46 | 1.02 | 0.56, 1.86 | 0.72 | 0.33, 1.57 |
| Recent non-fatal overdosea | 0.98 | 0.37, 2.62 | 1.41 | 0.59, 3.37 | 0.63 | 0.17, 2.33 |
| OAT initiation ≥ 2014 | 1.70 | 0.91,3.17c | 0.79 | 0.40, 1.59 | 0.62 | 0.26, 1.52 |
| Unmet health or social needsa | 1.21 | 0.68,2.18 | 0.81 | 0.44, 1.48 | 0.94 | 0.47, 1.90 |
| Homelessnessa | 0.74 | 0.43,1.26 | 1.19 | 0.72, 1.94 | 0.97 | 0.53, 1.76 |
| Employment | 2.10 | 1.08, 4.09b | 1.92 | 1.00, 3.71c | 2.50 | 1.20, 5.20b |
| Incarcerationa | 1.10 | 0.56, 2.17 | 0.53 | 0.24, 1.16c | 0.65 | 0.27, 1.60 |
OAT, opioid agonist therapy; OR, odds ratio; CI, confidence interval.
Refers to the 6-month period prior to the baseline interview.
P < 0.05, and considered for inclusion in the multivariable model selection process.
P < 0.20, and considered for inclusion in the multivariable model selection process.
Table 3.
Multivariable multinomial logistic regression analyses of factors associated with membership in suboptimal OAT engagement groups (versus membership in the “Consistently High” OAT engagement group) among OAT initiators, Vancouver, British Columbia, 2005–2018.
| OAT engagement trajectory groupsb | ||||||
|---|---|---|---|---|---|---|
| Consistently low | Increasing | Decreasing | ||||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| OAT initiation ≥ 2014 | 1.61 | 0.86, 3.01 | 0.75 | 0.37, 1.51 | 0.57 | 0.23, 1.41 |
| Employmenta | 2.01 | 1.02, 3.93c | 1.97 | 1.02, 3.81c | 2.60 | 1.25, 5.43c |
OAT, opioid agonist therapy; AOR, adjusted odds ratio; CI, confidence interval.
Refers to the 6-month period prior to the baseline interview.
Only the variables included in the final multivariable model are presented.
P < 0.05.
Our sub-analysis found that initiation of OAT in years 2014–2018 (versus years 2006–2013) was associated with the “consistently low” pathway, when compared to all other groups combined (AOR = 2.00, 95% CI: 1.16, 3.45).
DISCUSSION
Our study identified four distinct trajectories of engagement in OAT among individuals starting OAT in Vancouver between 2005 and 2018: “consistently high”, “consistently low”, “increasing”, and “decreasing” engagement. Just over a third of the study population fell within the “consistently high” engagement group, under a quarter within the “consistently low” group, another quarter within the “increasing” group, and the remaining 15% within the “decreasing” group. Our analysis identified employment as a cross-cutting predictor of all sub-optimal OAT engagement trajectories when compared to the “consistently high” class. In addition, initiating OAT after 2014 was also found to predict membership in the “consistently low” engagement group relative to other groups.
In line with previous research (Socias et al., 2018; Timko et al., 2016; Williams et al., 2018), only approximately half of people initiating OAT in our study were considered retained in OAT in a given six-month period. Our study extends the previous literature, by providing a more fulsome picture of longitudinal trajectories in OAT engagement, including three distinct patterns of sub-optimal engagement in OUD care. For example, findings from this study suggest that low engagement in OAT in the first year of OAT initiation may improve (i.e., “increasing” group), but that if low engagement persists after the first year, this is unlikely to improve (i.e., “consistently low” group). Conversely, high engagement in the first year does not necessarily mean continued high engagement in the long-term, as suggested by the “decreasing” trajectory group. Given the well-known associations between OAT discontinuation and mortality, particularly in the four-week period immediately after treatment drop-out (Sordo et al., 2017), additional supports to OAT clients during the first year of OAT initiation, including early outreach triggered by missing medication refills, may help prevent OAT attrition. Taken together, results from this study suggest that better understanding the heterogeneities in engagement in OAT patterns could help inform targeted interventions to specific at-risk populations of individuals with OUD at specific time points to decrease the risk of OAT discontinuation, and associated mortality risk.
Another advantage of our analytical approach is that it allowed us to identify groups of individuals who might be more likely to experience barriers to OUD care. In our analysis of class membership, employment emerged as the only and common predictor of all sub-optimal OAT engagement trajectories. While this finding may seem counter-intuitive at first glance, prior research conducted in the same setting found similarly negative links between methadone-based OAT and employment (Richardson, Wood, Montaner & Kerr, 2012). Interactions between regulatory requirements of OAT programs and financial barriers to accessing OAT in BC may partially explain this finding. Specifically, in BC OAT dispensation (in particular methadone, the most common OAT during the study period) often requires frequent (i.e., daily) visits to the pharmacy for witnessed ingestion. These programmatic requirements may, in turn, interfere with employment schedules and responsibilities, which could result in persons with OUD who are employed forgoing OAT doses, or discontinuing OAT altogether (Borisova & Goodman, 2004; Lo et al., 2018; Richardson et al., 2012). Additionally, employed individuals may not be eligible for income assistance or other provincial drug plans providing free access to OAT medications to low-income individuals (BC Coroners Service, 2019). The lack of publicly-funded coverage for OAT medications for employed individuals with OUD without other forms of health insurance may further contribute to the strong association between employment and sub-optimal care patterns observed in this study. Given the critical role of employment in decreasing socio-economic marginalization and facilitating integration of people with OUD in the community, these findings highlight the need to address employment-related barriers to OAT access. These could range from universal coverage of OAT medications, to alternative OAT dispensation strategies that could help overcome the need for daily visits to the pharmacy (and associated dispensing fees), such as increasing take-home methadone doses for clients with demonstrated clinical and social stability, as well as to expand access to other safer forms of OAT (e.g., oral buprenorphine, extended-release injectable OAT).
Finally, we were also interested in investigating unique predictors of the “consistently low” engagement pattern given their continuously high mortality risk. The only factor associated with this pathway was initiation of OAT after 2014. We have previously documented a declining trend in linkage to and retention in OAT after 2014 in Vancouver (Socias et al., 2018), as well as immediate negative impacts following the regulatory changes introduced to the provincial OAT program in 2014, including disruptions in adherence to OAT and other co-dispensed medications (e.g., antiretroviral therapy), as well as increases in opioid injection, which have been partly attributed to “change intolerance” to the new methadone formulation (McNeil et al., 2015; Socias et al., 2017). The present analysis further suggests that this policy change, which was undertaken without consultation with the affected community, may have indeed been an important driver of attrition of OAT programs observed in BC after 2014, particularly among new OAT initiators. Alternatively, the association between OAT initiation after 2014 and the “consistently low” engagement trajectory may be partly explained by the introduction of illicitly manufactured fentanyl in BC, as demonstrated by a substantial increase in the proportion of fentanyl-related overdose deaths, from 2% in 2012 to 25% in 2014, to 87% in 2018 (BC Coroners Service, 2019). Limited evidence suggests that higher doses of OAT may be required to control withdrawal symptoms and cravings, given fentanyl’s higher potency compared to other opioids (Suzuki & El-Haddad, 2017), which in turn may have contributed to higher treatment attrition among people initiating OAT in more recent years.
Some limitations should be considered when interpreting the findings of this study. First, our study sample was not randomly selected, and therefore, results from this analysis may not be generalizable to other individuals initiating OAT in Vancouver or other settings, particularly to jurisdictions with different health system arrangements. Likewise, given that almost all of the study sample initiated methadone-based OAT, results for other types of OAT should be interpreted with caution. As access to buprenorphine and alternative forms of OAT continue to expand, research should aim to tease out potential differences in engagement trajectories with different types of OAT. Second, our analytical approach only allowed for assessment of time-fixed variables at baseline as potential trajectory predictors. Given the chronic and dynamic nature of OUD, future analyses should aim to include time-varying predictors to identify potential changes in social-structural exposures and behaviours that could result in trajectories diverging at a specific time-points (e.g., “consistently high” and “decreasing” patterns). Third, our analysis relied mostly on self-reported data which may be subject to social-desirability or recall bias. That said, prior research has shown people who use drugs’ reports on substance use and addiction treatment to be reliable (De Irala, Bigelow, McCusker, Hindin & Zheng, 1996; Langendam, van Haastrecht & van Ameijden, 1999), and we have no reason to believe that there would be differential reporting of sensitive data among members of each OAT engagement trajectory. Fourth, membership in the “decreasing” group could, in some cases, reflect no need for continuous OAT. That said, among the 323 participants who had at least one observation characterized by no retention in OAT, 176 (54.6%) reported at least daily illicit opioid use and 226 (70.0%) any illicit opioid use at the first documentation of no retention in OAT. Importantly, current approaches to the treatment of OUD, typically recommend long-term OAT (possibly indefinite) given strong associations between OAT discontinuation, relapse to opioid use and mortality (Blanco & Volkow, 2019; Sordo et al., 2017).
In conclusion, we identified four longitudinal trajectories of OAT engagement in Vancouver, Canada, with employment emerging as a common predictor of sub-optimal patterns of care. This counter-intuitive finding points to the need to explore alternative OAT models and coverage that could address employment related barriers to OAT. As demonstrated by this study, the use of LCGA can complement more traditional cross-sectional measures of engagement in OUD care (Williams et al., 2018), and help inform targeted interventions to specific sub-populations of OAT clients at specific time-points to improve engagement in OAT, and maximize its clinical and population-level benefits. The key challenge for policy makers and researchers will be to use findings such as these to inform public health and clinical strategies to improve access and retention of persons with OUD in OAT.
Acknowledgments
The authors thank the study participants, as well as current and past researchers and staff.
Financial support
This work was supported by the US National Institute on Drug Abuse (NIDA) (U01-DA038886 and U01-DA021525). MES is supported by a Michael Smith Foundation for Health Research (MSFHR)/St Paul’s Foundation Scholar Award. EW is supported by the Canadian Institutes of Health Research (CIHR) Canada Research Chairs program. HD is supported by a CIHR doctoral award. KH is supported by a CIHR New Investigator and MSFHR Scholar Awards, and the St. Paul’s Foundation. LR is supported by CIHR New Investigator (MSH 217672) and MSFHR Scholar Awards, as well as a CIHR Foundation Grant (FDN 154320). M-JM is supported by NIDA (U01-DA021525), a CIHR New Investigator and MSFHR Scholar Awards. M-JM is the Canopy Growth professor of cannabis science at the University of British Columbia, a position created by unstructured gifts to the university from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. The University of British Columbia has also received unstructured funding from NG Biomed, Ltd. to support M-JM.
Footnotes
Conflict of Interest Statement
All authors declare no conflict of interests.
REFERENCES
- BC Coroners Service. (2019). Illicit drug overdose deaths in BC (January 1 2009–January 31, 2019).
- BC Coroners Service. (2019). Fentanyl-detected illicit drug overdose deaths (January 1, 2012 to March 31, 2019).
- Blanco C, & Volkow ND (2019). Management of opioid use disorder in the USA: Present status and future directions. Lancet, 393, 1760–1772. [DOI] [PubMed] [Google Scholar]
- Borisova NN, & Goodman AC (2004). The effects of time and money prices on treatment attendance for methadone maintenance clients. Journal of Substance Abuse Treatment, 26, 345–352. [DOI] [PubMed] [Google Scholar]
- British Columbia Ministry of Health.(2020) Coverage of drugs for the treatment of opioid use disorder. Retrieved June 11 2019, from https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/what-we-cover/drug-coverage/coverage-of-drugs-for-opioid-use-disorder.
- Burns L, Gisev N, Larney S, Dobbins T, Gibson A, Kimber J, et al. (2015). A longitudinal comparison of retention in buprenorphine and methadone treatment for opioid dependence in New South Wales, Australia. Addiction, 110, 646–655. [DOI] [PubMed] [Google Scholar]
- De Irala J, Bigelow C, McCusker J, Hindin R, & Zheng L (1996). Reliability of self- reported human immunodeficiency virus risk behaviors in a residential drug treat- ment population. American Journal of Epidemiology, 143, 725–732. [DOI] [PubMed] [Google Scholar]
- Langendam MW, van Haastrecht HJ, & van Ameijden EJ (1999). The validity of drug users’ self-reports in a non-treatment setting: Prevalence and predictors of in- correct reporting methadone treatment modalities. International Journal of Epidemiology, 28, 514–520. [DOI] [PubMed] [Google Scholar]
- Lo A, Kerr T, Hayashi K, Milloy MJ, Nosova E, Liu Y, et al. (2018). Factors associated with methadone maintenance therapy discontinuation among people who inject drugs. Journal of Substance Abuse Treatment, 94, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil R, Kerr T, Anderson S, Maher L, Keewatin C, Milloy MJ, et al. (2015). Negotiating structural vulnerability following regulatory changes to a provincial methadone program in Vancouver, Canada: A qualitative study . Social Science & Medicine, 133, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk RT, Horn J, Prins M, Wiessing L, & Kretzschmar M (2014). Trajectories of injecting behavior in the Amsterdam Cohort Study among drug users. Drug and Alcohol Dependence, 144, 141–147. [DOI] [PubMed] [Google Scholar]
- Murthy VH (2016). Ending the opioid epidemic—A call to action. The New England Journal of Medicine, 375, 2413–2415. [DOI] [PubMed] [Google Scholar]
- Muthen B, & Muthen LK (2000). Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and Experimental Research, 24, 882–891. [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (December 2019). Alcohol Facts and Statistics. British Columbia Ministry of Heatlh. Coverage of drugs for the treatment of opioid use disorder. Retrieved June 11 2019 from https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/what-we-cover/drug-coverage/drug-coverage-opioid-use-disorder.
- Richardson L, Wood E, Montaner J, & Kerr T (2012). Addiction treatment-related employment barriers: The impact of methadone maintenance. Journal of Substance Abuse Treatment, 43, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2018). Drug and opioid-in- volved overdose deaths - United States, 2013–2017. Morbidity and Mortality Weekly Report, 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, & Schwartz RP (2017). Update on barriers to pharmacotherapy for opioid use disorders. Current Psychiatry Reports, 19, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socias ME, Volkow N, & Wood E (2016). Adopting the ‘cascade of care’ framework: An opportunity to close the implementation gap in addiction care? Addiction, 111, 2079–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socias ME, Wood E, Kerr T, Nolan S, Hayashi K, Nosova E, et al. (2018a). Trends in engagement in the cascade of care for opioid use disorder, Vancouver, Canada, 2006–2016. Drug and Alcohol Dependence, 189, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socias ME, Wood E, Lake S, Nolan S, Fairbairn N, Hayashi K, et al. (2018b). High- intensity cannabis use is associated with retention in opioid agonist treatment: A longitudinal analysis. Addiction, 113, 2250–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socias ME, Wood E, McNeil R, Kerr T, Dong H, Shoveller J, et al. (2017). Unintended impacts of regulatory changes to British Columbia methadone main- tenance program on addiction and HIV-related outcomes: An interrupted time series analysis. International Journal of Drug Policy, 45, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ, 357 j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, et al. (1998). Barriers to use of free antiretroviral therapy in injection drug users. JAMA, 280, 547–549. [DOI] [PubMed] [Google Scholar]
- Suzuki J, & El-Haddad S (2017). A review: Fentanyl and non-pharmaceutical fentanyls. Drug and Alcohol Dependence, 171, 107–116. [DOI] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic re- view. Journal of Addictive Diseases, 35, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Schoot R, Sijbrandij M, Winter SD, Depaoli S, & Vermunt JK (2017). The GRoLTS-Checklist: Guidelines for reporting on latent trajectory studies. Structural Equation Modeling: A Multidisciplinary Journal, 24, 451–467. [Google Scholar]
- Williams AR, Nunes EV, Bisaga A, Pincus HA, Johnson KA, Campbell AN, et al. (2018). Developing an opioid use disorder treatment cascade: A review of quality measures. Journal of Substance Abuse Treatment, 91, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E (2018). Strategies for reducing opioid-overdose deaths—Lessons from Canada. The New England Journal of Medicine, 378, 1565–1567. [DOI] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, et al. (2008). Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA, 300, 550–554. [DOI] [PubMed] [Google Scholar]