Abstract
As the second wave of coronavirus disease 2019 (COVID-19) is well under way around the world, the optimal therapeutic approach that addresses virus replication and hyperinflammation leading to tissue injury remains elusive. This issue of Clinical Kidney Journal provides further evidence of complement activation involvement in COVID-19. Taking advantage of the unique repeat access to chronic haemodialysis patients, the differential time course of C3 and C5 activation in relation to inflammation and severity of disease have been characterized. This further points to complement as a therapeutic target. Indeed, clinical trials targeting diverse components of complement are ongoing. However, a unique case of COVID-19 in a patient with pre-existent atypical haemolytic syndrome on chronic eculizumab therapy suggests that even early eculizumab may fail to prevent disease progression to a severe stage. Finally, preclinical studies in endotoxaemia, another hyperinflammation syndrome characterized by lung and kidney injury, suggest that cilastatin, an inexpensive drug already in clinical use, may provide tissue protection against hyperinflammation in COVID-19.
Keywords: acute kidney injury, cilastatin, complement, COVID-19, dialysis, tissue protection
As the second wave of coronavirus disease 2019 (COVID-19) is well under way in multiple countries around the world, the optimal therapeutic approach to the disease that addresses virus replication and hyperinflammation leading to tissue injury remains elusive. This issue of Clinical Kidney Journal (CKJ) contains several reports that shed light on the pathogenesis of tissue injury in COVID-19 and potential therapeutic approaches [1–3] that add to prior COVID-19 publications in the journal to enlighten the pathogenesis of tissue injury in COVID-19 [4].
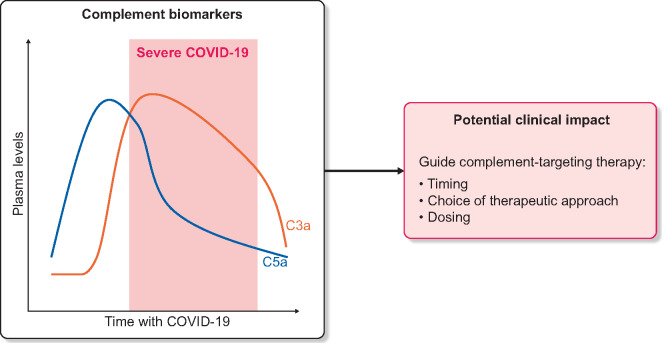
Two manuscripts provide insights into the role of complement activation and its potential as a therapeutic target [1, 2] in line with an increasing number of calls to target complement in COVID-19 [5–9]. While complement components are not usually assessed in routine care of COVID-19 patients, as illustrated by a recent detailed report of risk factors for severity of COVID-19 in chronic dialysis patients [10], Prendecki et al. [1] took advantage of the thrice-weekly venous access in haemodialysis patients to describe a characteristic time course of circulating complement activation products that may help to define the severity and stage of the disease. Plasma C3a and C5a were higher in haemodialysis patients with severe COVID-19 than in controls. Moreover, serial sampling identified a distinct temporal pattern in which C5a levels were elevated prior to clinical deterioration in patients who developed severe disease, while C3a more closely mirrored disease severity, increasing when disease became severe (Figure 1). Unfortunately there was a wide overlap in plasma C3a levels between patients with severe and non-severe disease. In any case, this report suggests that assessing complement peptides may eventually contribute to define clusters of COVID-19 patients, as has been done for C3 glomerulopathies/immune complex–mediated membranoproliferative glomerulonephritis [11, 12]. These clusters may eventually be used to guide the choice of therapy. In this regard, it would be interesting to assess some derivate parameters such as the C5a:C3a ratio to see whether they add information to the stage of the disease and the optimal timing of therapy initiation.
FIGURE 1.
Temporal pattern of circulating C5a and C3a levels in dialysis patients who developed severe COVID-19 [1] and potential clinical impact. Given the overlap between plasma C5a and C3a levels in non-severe and severe COVID-19, it is likely that additional parameters should be analysed to fulfil the aims presented in the figure.
Data from Prendecki et al. [1] build upon a growing body of evidence linking complement activation to tissue injury in COVID-19 [6–9]. Thus a recent report showed an increase in circulating soluble C5a levels proportional to COVID-19 severity and a high expression of its receptor C5aR1 in myeloid cells [13]. Indeed, anti-C5aR1 antibodies inhibited acute lung injury in human C5aR1 knock-in mice [13]. Additionally, increased complement receptor 3 (CR3) expression was found in granulocytes and monocytes from hypoxic COVID-19 patients, but not in those with less severe COVID-19 or in those without COVID-19 but ventilated for other reasons [14]. CR3 binds the C3d fragment of C3. Further recent reports support a potential pathogenic role of complement activation in severe COVID-19 and its relationship to the thrombotic diathesis. Increased plasma levels of neutrophil extracellular traps (NETs), tissue factor (TF) activity and soluble C5b-9 were detected in COVID-19 patients [15]. Thrombin or NETosis inhibition or C5aR1 blockade attenuated platelet-mediated NET-driven thrombogenicity. Furthermore, COVID-19 serum-induced complement activation in vitro and C3 inhibition with compstatin Cp40 disrupted TF expression in neutrophils, supporting a role of complement and NETs in COVID-19 immunothrombosis [15]. Indeed, both micro- and macrovascular thrombosis are features of COVID-19 that may contribute to kidney injury [16].
From a clinical point of view, a large study of >11 000 patients suspected of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified a history of macular degeneration (a proxy for complement-activation disorders) as an independent risk factor for severe COVID-19, and a genome-wide association study found an association with previously reported expression of quantitative trait loci for CD55 (a negative regulator of complement activation) and single-nucleotide polymorphisms in complement factor H and complement component 4 binding protein alpha [17]. Moreover, engagement of complement pathways was observed in transcriptional profiling of nasopharyngeal swabs, suggesting very early activation of complement during COVID-19, already locally at the site of virus entry [17]. Among other immune system abnormalities, non-survivors of COVID-19 had lower circulating C3 and C4 levels, suggesting complement pathway activation, and independent predictors of death included low C3, together with old age, co-morbidity of malignant tumour, neutrophilia, lymphocytopenia, low CD4+ T cells and low oxygen saturation [18].
The growing evidence that complement may contribute to tissue injury in COVID-19 has led to the compassionate use of complement targeting strategies in selected patients as well as to a flurry of ongoing randomized clinical trials (Table 1 and Figure 2). In non-controlled case series and case reports, relatively positive results have been reported for the anti-C5 monoclonal antibody eculizumab, for C3 inhibitor AMY-101, for the mannan-binding lectin-associated serine protease 2 blocker narsoplimab (OMS721), for aliskiren and for nafamostat mesylate, a US Food and Drug Administration–approved anticoagulant agent that has broad-spectrum serine protease inhibitory activity, including for C1 esterase [2, 19–26]. At least 22 patients have been treated with eculizumab and 1 with AMY-101 [2, 19–23]. As expected from the known bias of positive reporting, the limited published experience with complement targeting strategies has been overwhelmingly positive. Additionally, results are difficult to interpret given the use of other medications and the very variable severity and natural history of COVID-19. Of interest, in one report, 2 of 8 (25%) eculizumab-treated patients died [22]. The deaths included 2 of 3 (67%) patients on mechanical ventilation at the initiation of eculizumab. In a further report, 1 in 3 (33%) eculizumab-treated patients died [23].
Table 1.
Ongoing clinical trials, according to ClinicalTrials.gov (accessed 29 August 2020) and published experience with therapies targeting complement in COVID-19 (in bold, drugs or trials not commented on by Trimarchi et al. [ 2])
| Drug target | Drug | Phase | NCT number | Published experiencea |
|---|---|---|---|---|
| MASP2 | Narsoplimab | ND | ND | Yes |
| Broad-spectrum, synthetic serine protease (including C1 esterase) inhibitor | Nafamostat mesilate | 2/3 | Yes | |
| 3 | NCT04390594 | |||
| C1 esterase | Conestat alfa (Ruconest, human recombinant C1 esterase inhibitor) | 2 | NCT04414631 | Yes |
| NCT04530136 | ||||
| C3 | AMY-101 | 2 | NCT04395456 | Yes |
| APL-9 | 1|2 | NCT04402060 | ND | |
| C5 | Zilucoplan | 2 | NCT04382755 | ND |
| Eculizumab | 2 | NCT04346797 | Yes | |
| ND | NCT04355494 | |||
| NCT04288713 | ||||
| Ravulizumab | 4 | NCT04390464 | ND | |
| 3 | NCT04369469 | |||
| Renin (C3 activation) | Aliskirem | ND | ND | Yes |
ND: no data.
Case series and case reports.
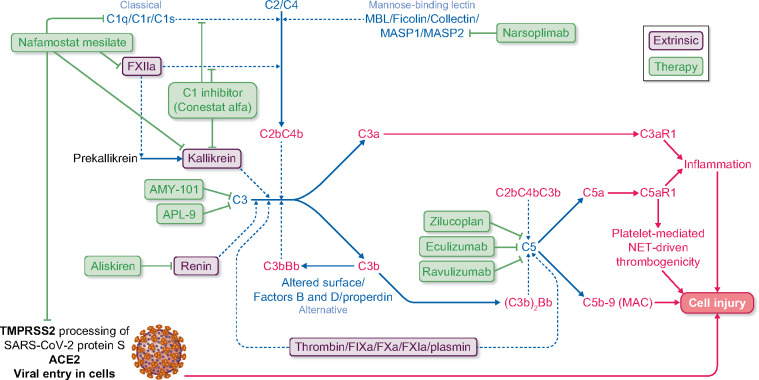
FIGURE 2.
Complement system and COVID-19. The complement system is represented as well as its contribution to cell and tissue injury in COVID-19 and therapeutic approaches that have been tested in isolated patients or which are undergoing clinical trials. The classical, alternative and mannose-binding lectin pathway for complement activation as well as extrinsic factors that may contribute to complement activation are displayed. C3aR1 and C5aR1 are cell surface receptors activated by C3a and C5a, respectively. Transmembrane serine protease 2 (TMPRSS2) is a cell surface enzyme that processes SARS-CoV-2 protein S, facilitating binding to ACE2, which functions as a cell surface receptor for SARS-CoV-2, allowing virus entry into cells. Purple proteins represent extrinsic pathways for complement activation, dark blue continuous lines represent protein processing or protein incorporation into protein complexes and green lines represent therapeutic agents inhibiting targets. Thin discontinuous lines represent enzymatic activity.
These results are open to several interpretations. One is that eculizumab was started too late in the course of the disease. The other is that it was not effective in severely affected patients. In this issue of CKJ, Trimarchi et al. [2] add another important piece of information related to the efficacy of very early initiation of eculizumab. They reported a patient presenting complex medical issues that complicated interpretation of the data, being a kidney transplant recipient with atypical haemolytic uraemic syndrome (aHUS). However, it is precisely this complexity that allowed the evaluation of early eculizumab therapy; shortly (3 days) after the diagnosis of COVID-19, the patient received the eculizumab dose, corresponding to chronic eculizumab therapy of aHUS. Despite eculizumab, the severity of COVID-19 increased over the next 6 days, with d-dimer values increasing 36-fold and total blood lymphocytes dropping from 470 to 213/μL. At this point, dexamethasone and convalescent plasma infusion was followed by improvement. Besides the question of co-morbidities, the question of appropriate dosing may be raised. However, this case report is a sobering reminder of the need of randomized controlled trials (RCTs) as reflected by Trimarchi et al. [2]. In fact, since their report was accepted for publication, the list of ongoing trials targeting complement listed on ClinicalTrials.gov has increased, although not all of them are controlled (Table 1).
There is anecdotal experience with other complement-targeting drugs. Narsoplimab was used in six patients with severe COVID-19 who survived, some of whom had low C3 and C4 levels [24]. Ongoing Phase 2 RCTs are testing narsoplimab for glomerulonephritis (NCT02682407). However, no narsoplimab trial for COVID-19 was listed on ClinicalTrials.gov as of 29 August 2020. Five patients with severe COVID-19 pneumonia received conestat alfa and also survived [25]. However, there was no uniform impact of conestat alfa on C3, C4 and C5a levels. Aliskiren also inhibits renin-mediated C3 activation and its capacity to inhibit complement activation in conjunction with eculizumab (dual complement inhibition concept) has been recently emphasized by case reports [27, 28]. There is a report on aliskiren use in four COVID-19 patients with hypertension who survived [29]. There is an additional preclinical report suggesting that aliskiren may reduce angiotensin-converting enzyme 2 (ACE2) expression, at least in the kidney [30]. However, no trials are further testing a potential role of aliskiren in COVID-19. Finally, nafamostat mesylate was also administered to three COVID-19 patients who survived [26]. In addition to its anticoagulant and C1 esterase inhibitory activity, nafamostat mesylate was recently shown to inhibit the cellular enzyme transmembrane protease serine 2 that processes SARS-CoV-2 protein S, allowing viral binding to cell surface ACE2 and entry into cells. In this regard, nafamostat mesylate inhibited SARS-CoV-2 protein S–mediated entry into host cells and blocked SARS-CoV-2 infection of human lung cells [31].
he final report in this issue of CKJ on potential novel approaches to limit tissue injury in COVID-19 is derived from studies in experimental acute kidney injury (AKI). AKI is a serious problem in severely ill COVID-19 patients [32], to the point that the ability to provide renal replacement therapy may be compromised, as illustrated by attempts to replace classical haemodialysis or haemofiltration with acute peritoneal dialysis or by using home-dialysis machines instead of the more complex machines used in hospital or centre-based haemodialysis [33, 34]. González-Nicolás et al. [3] propose another approach to limit tissue (lung and kidney) injury due to hyperinflammation that may be tested in COVID-19: cilastatin, a small molecule inhibitor of renal dehydropeptidase I, used clinically in association with imipenem to prevent imipenem degradation and increase imipenem half-life. Cilastatin binds to dehydropeptidase I at the lipid rafts in the cell surface of proximal tubular cells and pulmonary cells, stabilizing lipid rafts and preventing the internalization of proteins associated to lipid rafts, such as death receptors. This provides cilastatin with anti-apoptotic activity against a wide range to tubular toxins [35–37]. González-Nicolás et al. [3] report on lung protection afforded by cilastatin in rat endotoxemia, an hyperinflammation syndrome characterized by lung and kidney injury, both key targets of hyperinflammation in COVID-19. This suggests that cilastatin may also protect from hyperinflammation-induced lung and kidney injury in COVID-19. As González-Nicolás et al. [3] emphasize, the fact that the SARS-CoV-2 cellular receptor ACE2 is expressed in lipid rafts may provide two mechanisms by which cilastatin may protect from severe COVID-19: (i) stabilizing ACE2 at the cell surface lipid rafts and preventing virus/ACE2 internalization and (ii) preventing hyperinflammation-induced tissue injury as observed in rat endotoxemia. Both hypotheses should be tested, the first initially in cell culture and eventually in RCTs. The fact that COVID-19 patients are frequently treated with antibiotics may facilitate the generation of early data by analysing large databases of conventionally treated COVID-19 patients and will also facilitate trials randomizing patients to receive either imipenem–cilastatin or an alternative antibiotic. However, definite proof of cilastatin tissue protection will require testing the compound by itself.
In conclusion, besides an emphasis on finding an effective vaccine and antiviral therapy, efforts at limiting tissue injury in severe COVID-19 should be expanded. Characterization of the C3a and C5a time course in patients with COVID-19 of diverse severity may help define a higher-risk population with enhanced probability of response to complement targeting therapy in which timing of this therapy is appropriate and dosing may be guided by assessing the impact on these complement components. On the other hand, the description of the tissue-protective effect of cilastatin against hyperinflammation and its potential, based on its known mechanism of action, to limit virus entry in target cells warrant detailed expanded studies into its role as a therapy for severe COVID-19.
FUNDING
Funding was provided by FIS/Fondos FEDER PI17/00257, PI18/01386, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
CONFLICT OF INTEREST STATEMENT
A.O. has received speaker fees from Alexion.
REFERENCES
- 1. Prendecki M, Clarke C, Medjeral-Thomas N. et al. Temporal changes in complement activation in haemodialysis patients with Covid-19 as a predictor of disease progression. Clin Kidney J 2020; R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trimarchi H, Gianserra R, Lampo M. et al. SARS-CoV-2 and atypical hemolytic uremic syndrome. Clin Kidney J 2020; R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. González-Nicolás MA, González-Guerrero C, Pérez-Fernández VA. et al. Cilastatin: a potential treatment strategy against COVID-19 that may decrease viral replication and protect from the cytokine storm. Clin Kidney J October issue 3. CKJ-00613 2020; R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carriazo S, Kanbay M, Ortiz A.. Kidney disease and electrolytes in COVID-19: more than meets the eye. Clin Kidney J 2020; 13: 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valga F, Vega-Díaz N, Macia M. et al. Targeting complement in severe coronavirus disease 2019 to address microthrombosis. Clin Kidney J 2020; 13: 477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noris M, Benigni A, Remuzzi G.. The case of complement activation in COVID-19 multiorgan impact. Kidney Int 2020; 98: 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song WC, FitzGerald GA.. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest 2020; 130: 3950–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lo MW, Kemper C, Woodruff TM.. COVID-19: complement, coagulation, and collateral damage . J Immunol 2020; 205: 1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polycarpou A, Howard M, Farrar CA. et al. Rationale for targeting complement in COVID-19. EMBO Mol Med 2020; 12: e12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lano G, Braconnier A, Bataille S. et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicenter French cohort. Clin Kidney J 2020; R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garam N, Prohászka Z, Szilágyi Á. et al. Validation of distinct pathogenic patterns in a cohort of membranoproliferative glomerulonephritis patients by cluster analysis. Clin Kidney J 2020; 13: 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iatropoulos P, Daina E, Curreri M. et al. Cluster analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex-mediated membranoproliferative GN. J Am Soc Nephrol 2018; 29: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carvelli J, Demaria O, Vély F. et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020; doi: 10.1038/s41586-020-2600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta R, Gant VA, Williams B. et al. Increased complement receptor-3 levels in monocytes and granulocytes distinguish COVID-19 patients with pneumonia from those with mild symptoms. Int J Infect Dis 2020; doi: 10.1016/j.ijid.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skendros P, Mitsios A, Chrysanthopoulou A. et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest 2020; doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Philipponnet C, Aniort J, Chabrot P. et al. Renal artery thrombosis induced by coronavirus disease 2019. Clin Kidney J 2020; 13: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramlall V, Thangaraj PM, Meydan C. et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat Med 2020; doi: 10.1038/s41591-020-1021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao Y, Nie HX, Hu K. et al. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect Dis Poverty 2020; 9: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mastaglio S, Ruggeri A, Risitano AM. et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol 2020; 215: 108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giudice V, Pagliano P, Vatrella A. et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol 2020; 11: 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diurno F, Numis FG, Porta G. et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci 2020; 24: 4040–4047 [DOI] [PubMed] [Google Scholar]
- 22.Peffault de Latour R, Bergeron A, Lengline E et al. Complement C5 inhibition in patients with COVID-19 – a promising target? Haematologica. 2020 doi: 10.3324/haematol.2020.260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laurence J, Mulvey JJ, Seshadri M. et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol 2020; 219: 108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rambaldi A, Gritti G, Micò MC. et al. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology 2020; doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urwyler P, Moser S, Charitos P. et al. Treatment of COVID-19 with conestat alfa, a regulator of the complement, contact activation and Kallikrein-Kinin system. Front Immunol 2020; 11:2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann M, Schroeder S, Kleine-Weber H. et al. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother 2020; 64: e00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez-Gomez MV, Ortiz A.. Aliskiren and the dual complement inhibition concept. Clin Kidney J 2020; 13: 35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plasse RA, Nee R, Olson SW.. Aliskiren as an adjunct therapy for atypical hemolytic uremic syndrome. Clin Kidney J 2020; 13: 39–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo Y, Zeng J, Li Q. et al. Preliminary clinical study of direct renin inhibitor aliskiren in the treatment of severe COVID-19 patients with hypertension. Zhonghua Nei Ke Za Zhi 2020; 59: E011. [DOI] [PubMed] [Google Scholar]
- 30. Ding W, Li X, Wu W. et al. Aliskiren inhibits angiotensin II/angiotensin 1-7(Ang II/Ang1-7) signal pathway in rats with diabetic nephropathy. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2018; 34: 891–895 [PubMed] [Google Scholar]
- 31. Jang S, Rhee JY.. Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int J Infect Dis 2020; 96: 500–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubin S, Orieux A, Prevel R. et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J 2020; 13: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mousseaux C, Mayet1 V, Poda A. et al. Home-dialysis machine use for emergency dialysis during the COVID-19 pandemic. Clin Kidney J October issue. CKJ-00606 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ponce D, Balbi AL, Durand JB. et al. Acute peritoneal dialysis in the treatment of COVID-19-related acute kidney injury. Clin Kidney J 2020; 13: 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Humanes B, Lazaro A, Camano S. et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int 2012; 82: 652–663 [DOI] [PubMed] [Google Scholar]
- 36. Humanes B, Jado JC, Camaño S. et al. Protective effects of cilastatin against vancomycin-induced nephrotoxicity. Biomed Res Int 2015; 2015: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pérez M, Castilla M, Torres AM. et al. Inhibition of brush border dipeptidase with cilastatin reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. Nephrol Dial Transplant 2004; 19: 2445–2455 [DOI] [PubMed] [Google Scholar]