Abstract
Glomerulonephritis (GN) is the underlying cause of end-stage renal failure in 30–50% of kidney transplant recipients. It represents the primary cause of end-stage renal disease for 25% of the dialysis population and 45% of the transplant population. For patients with GN requiring renal replacement therapy, kidney transplantation is associated with superior outcomes compared with dialysis. Recurrent GN was previously considered to be a minor contributor to graft loss, but with the prolongation of graft survival, the effect of recurrent disease on graft outcome assumes increasing importance. Thus the extent of recurrence of original kidney disease after kidney transplantation has been underestimated for several reasons. This review aims to provide updated knowledge on one particular recurrent renal disease after kidney transplantation, immunoglobulin A nephropathy (IgAN). IgAN is one of the most common GNs worldwide. The pathogenesis of IgAN is complex and remains incompletely understood. Evidence to date is most supportive of a several hit hypothesis. Biopsy is mandatory not only to diagnose the disease in the native kidney, but also to identify and characterize graft recurrence of IgAN in the kidney graft. The optimal therapy for IgAN recurrence in the renal graft is unknown. Supportive therapy aiming to reduce proteinuria and control hypertension is the mainstream, with corticosteroids and immunosuppressive treatment tailored for certain subgroups of patients experiencing a rapidly progressive course of the disease with active lesions on renal biopsy and considering safety issues related to infectious complications.
Keywords: IgA nephropathy, immunosuppressive therapy, kidney transplant, pathology, recurrent glomerulonephritis
INTRODUCTION
Introduction of newer immunosuppressive agents has reduced graft loss directly by decreasing the incidence of acute rejection and indirectly through the consequent reduction of chronic allograft dysfunction [1, 2]. Glomerulonephritis (GN) is the underlying cause of end-stage renal failure in 30–50% of kidney transplant recipients [1]. It represents the primary cause of end-stage renal disease for 25% of the dialysis population [3] and 45% of the transplant population. For patients with GN requiring renal replacement therapy, kidney transplantation is associated with superior outcomes compared with dialysis [4]. Recurrent GN was previously considered to be a minor contributor to graft loss. With the prolongation of graft survival, the effect of recurrent disease on graft outcome assumes increasing importance. There is accumulating evidence that recurrent GN is an important cause of graft loss in the long-term follow-up of renal allograft recipients [1, 5–7]. Recurrence of GN is reported in 6–20% [8–12] of renal allograft recipients depending on the duration of follow-up [10], local protocol biopsy practice and type of primary GN [13]. Studies on recurrent disease are difficult since not all patients have undergone native kidney biopsy and most centres perform graft biopsies only when there are abnormal clinical or laboratory features. Briganti et al. [1] reported on 1505 patients with both native and graft biopsies that graft loss due to recurrent GN was the third most frequent cause of graft loss 10 years after kidney transplantation. The risk of graft loss from recurrence increased with the years of follow-up, from 0.6% at the first post-operative year to 8.4% at the 10th year. Moreover, a recent Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) publication focused on the recurrence rates of four representatives of original kidney diseases: membranoproliferative GN, immunoglobulin A nephropathy (IgAN), focal segmental glomerulosclerosis and membranous nephropathy [14]. It found that kidney allograft loss was more common in patients with recurrent disease than in others.
The recurrence rate, clinical course and impact on graft survival vary between different types of GN. This review aims to provide updated knowledge on one particular recurrent renal disease after kidney transplantation, IgAN. Many reports have described IgAN recurrence rates of 22–58%, with studies featuring scheduled protocol biopsies reporting shorter times to recurrence and higher recurrence rates [15–17]. The rate of graft loss attributable to IgAN recurrence was only 1.3–16% in retrospective cohort studies but up to 50% when protocol biopsies were scheduled [18]. Thus the risk of recurrence and its impact on outcomes are important questions for patients and clinicians in considering transplantation.
EPIDEMIOLOGY
IgAN in the general population
IgAN is now considered to be one of the most common GNs worldwide, affecting 1–3 patients/100 000 population/year [19]. IgAN may occur at any age, but there is a peak incidence in the second and third decades of life. It is predominant in males compared with females in North America, Western Europe and Australia [20, 21]. The frequency of IgAN, determined by renal histopathology, varies greatly and accounts for 50% of the histologic findings in Japan, Singapore, Australia, New Zealand and other countries in the Pacific Rim [22] . According to European studies, the incidence of the disease is lower in Europe, accounting for 20–30% of all primary glomerular diseases, whereas in North America, the contribution of IgAN appears to be ∼2–10%, with the exception of a 38% incidence in New Mexico [23]. In contrast to the geographic variations, IgAN is rare among blacks in all regions [24]. It is probable that the differences between various regions of the world reflect regional variations in referral practices and clinical indications of renal biopsy; furthermore, an inherited predisposition to IgAN is suggested by some researchers [25, 26]. On the other hand, there are also reports describing IgA deposition in other GNs, including thin basement membrane disease, minimal change disease, lupus nephritis and diabetic nephropathy, probably as a result of the frequent IgA deposition in the general population. These observations raise the point that there may be a large cohort of undiagnosed cases with IgAN in the general population.
IgAN recurrence in the graft
Patients with IgAN enjoy greater access to kidney transplantation as compared with those with many other forms of kidney disease. Rates of recurrence in the literature vary widely, primarily due to study differences in the indication for biopsy and length of post-transplant follow-up. This is likely because in the early stages of IgAN recurrence, positive biopsy findings are frequently not accompanied by clinical changes such as proteinuria, haematuria or graft dysfunction [17]. Recurrence of IgAN after transplantation appears to be a time-dependent phenomenon, with rates of recurrence increasing as time from transplant lengthens [16]. Initially, IgAN recurrence in the graft was considered to be a relatively benign phenomenon, but as several studies with longer observation periods have become available it appears that late in the course of follow-up, recurrence becomes a clinical problem, with ∼13–25% of patients exhibiting some recurrence-related graft dysfunction at 5 years, which may lead to graft loss in nearly 5–10% of cases. Protocol biopsies showed that IgAN recurs early in the graft, with 32% of 65 patients with IgAN as primary disease displaying one or more mesangial deposits of IgA in protocol biopsies performed before the second year after kidney transplant [17]. Interestingly, histological diagnosis of IgAN was not accompanied by abnormalities in the urinalysis in half of the patients [17].
Patients with IgAN as their primary disease receiving kidney transplant seem to have a better allograft survival in the first year after transplant compared with patients with other types of non-diabetic primary disease [15] and IgAN recurrence had negligible influence on 5- and 10-year graft survival [27]. This could be related to the lower immunological failure rate in IgAN. IgA anti-human luecocyte antigen (HLA) molecules found in pre-transplant sera of IgAN patients could play a role by blocking IgG anti-HLA or inhibiting cellular immune response [28].
The risk of recurrence is strongly influenced by the length of the follow-up time after kidney transplant. Odum et al. [16] initially reported that the only predictor identified for recurrence was length of time post-transplantation, with allograft tissue being studied at 45.9 ± 10.0 versus 15.3 ± 4.8 months post-transplantation in IgAN patients with and without recurrent deposits, respectively. More recently, a retrospective study from the ANZDATA registry showed that among a cohort of 2501 kidney transplant patients with biopsy-proven IgAN as the primary disease, ∼5.1, 10.1 and 15% of recipients experienced disease recurrence at 5, 10 and 15 years after transplant, respectively [14].
Up to 10 years after transplantation, neither patient nor graft survival differs between those patients with an underlying IgAN and patients with other types of non-diabetic primary renal disease. Data from registries showed that although IgAN recurrence was a significant cause of graft loss for those with GN, third only to chronic allograft nephropathy and death with a functioning graft, overall outcomes at 10 years were similar for those with IgAN as compared with those with all other causes of ESKD [1, 29].
The outcomes after 10 years have been insufficiently studied to draw firm conclusions, but a recent European Registry study and some long-term cohort studies suggest that progressive graft loss due to recurrence will continue to accrue beyond 10 years [15, 30, 31]. Choy et al. [30] were the first to report that at 12 years the graft survival became worse in IgAN patients than in controls. Aziz et al. [32] showed that patient survival was 92% at 10 years and graft survival was 53%; the clinicopathological recurrence was reported in 17.6%, leading to graft loss in 7% of cases.
More recently, Moroni et al. [15] extend these data by describing that the death-censored graft survival at 15 years was ∼10% lower in 190 IgAN patients when compared with 380 non-diabetic controls (63 versus 72%) with a median follow-up of almost 10 years. The latter appeared largely due to recurrent IgAN, as graft survival in non-recurrent patients was similar to that of controls, whereas it was only 51% in the recurrent patients at 15 years. In this study, IgAN recurrence in the graft was documented in 22.1% of the grafts.
We do not yet have a sufficient understanding of why some patients experience recurrence and others do not. Certain factors such as relatively fast prior recurrence of disease and loss of previous graft from recurrence, a rapidly progressive course of the original disease accompanied by extensive crescent formation [33] and the length of the follow-up time after kidney transplant [16] seem to play a role.
Two donor factors have emerged as possible contributors to IgAN recurrence: donor source and donor IgA deposits. The donor source has been studied as a possible risk factor. Given the genetic predisposition in at least a portion of patients with IgAN that could, at least in part, account for some of the demographic and clinical variability seen in IgAN, one might expect that patients with IgAN receiving a kidney from a related living donor might be at higher risk of recurrence. Andresdottir et al. [27] reported that a clinical recurrence of IgAN occurred in 4% of patients with a deceased-donor graft and in 20% of patients with a living-related donor graft. Later, another group [34] evaluated the long-term outcome, in terms of recurrence and graft survival, after a living related or unrelated donor kidney transplant, and showed an increase of recurrence to 44% with longer follow-up time, although this did not limit graft survival and recurrence was not affected by the type of living donor.
The other donor factor that has been considered is the presence of IgA deposits in the donor kidney, which was found to be one of the risk factors for recurrence of IgAN in a single-centre study [35]. Interestingly, when grafts with IgA deposits have been transplanted into non-IgA patients, the IgA deposits are rapidly cleared by the recipient, with follow-up biopsies indicating complete resolution [36].
Two registry studies have found that those with one or more HLA mismatches have a reduced rate of recurrence compared with those with zero-mismatch kidneys [37, 38].
Although heterogeneity in immunosuppressive protocols and disease pathogenesis across the spectrum of IgA disease is thought to be responsible, there have been no large, detailed, prospective, multicentre cohort studies to indicate the effects of a single immunosuppressive drug on the recurrence this disease. Although studies with longer follow-up periods have shown the negative impact of IgAN recurrence on graft survival, there is some evidence that recurrence of IgAN has diminished in recent decades [15], as well as graft loss due to recurrence [38, 39]. Moroni et al. [15] showed an overall reduction in recurrence rate from the 1980s to the first decade of 2000 in an Italian cohort of transplanted patients with IgAN as the primary disease. At the same time, in almost the same time period, Clayton et al. [39] found a reduction in graft loss due to recurrent IgAN when analysing ANZDATA registry data. One reason that could explain this change is the difference in the immunosuppressive protocols.
In the study by Moroni et al. [15], the year of transplant showed a direct correlation with the use of mycophenolate and with triple immunosuppressive therapy, while in the study by Clayton et al. [39], steroid use was found to be strongly associated with a reduced risk of recurrence and the proportion of patients receiving steroids was lowest in the late 1980s and higher in the first year of 2000. More recently, other registry data from the United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) database showed that steroid use was strongly associated with a reduced risk of recurrence after adjusting for other factors [40].
A group from France [41] analysed the impact of steroids as part of the maintenance therapy for kidney transplant and certain other immunosuppressive agents on the risk of IgAN recurrence post-transplant. They showed that the hazard risk of recurrence was significantly higher in patients managed with steroid-free and sirolimus-based regimens, without the use of antilymphocyte globulin for induction [41]. Other retrospective data from a single centre suggest that induction therapy with anti-thymocyte globulin prevents the development of IgAN recurrence [42].
However, the registry study from the UNOS/OPTN did not find any association between antithymocyte globulin induction therapy and IgAN recurrence [40]. Chandrakantan et al. [43] examined the impact of mycophenolate mofetil compared with azathioprine on the recurrence of IgAN and concluded that it did not lessen the time to IgAN recurrence or its clinical impact. Younger age at transplantation has also been found to be independently associated with the risk of recurrence of IgAN as well as for other common primary glomerular diseases [14].
MECHANISM OF DISEASE
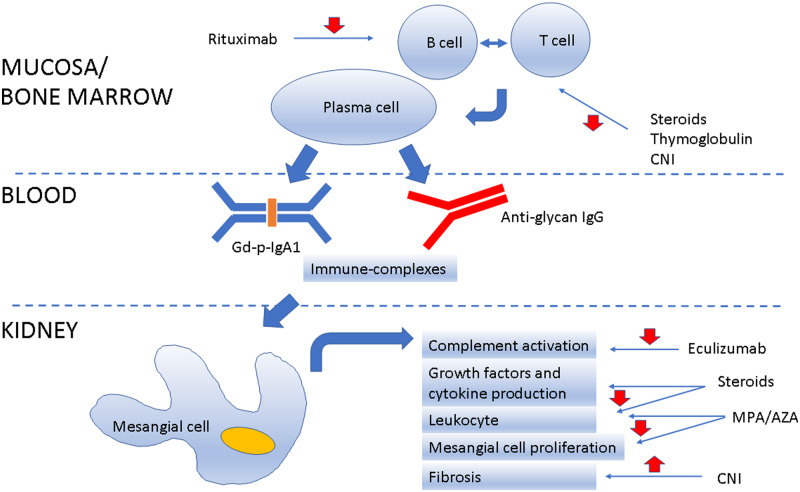
The pathogenesis of IgAN is complex and remains incompletely understood. There is marked variation in the clinical and pathological elements of IgAN, suggesting it is not one, but rather many different diseases. As such, it is unlikely that a single mechanism is either driving IgA deposition in the mesangium or generating inflammation and kidney injury from this misplaced IgA. There appears to be a genetic predisposition in at least a portion of those with IgAN, although how this manifests remains unclear. Areas of interest include specific HLA types, which have been found to be associated with IgAN in genetic association studies, and high serum IgA concentrations, which have been found in both patients as well as their non-affected family members [44, 45]. It is possible that differences in the underlying genetic defects may be partly responsible for some of the demographic and clinical variability seen in IgAN. Evidence to date is most supportive of a several hit hypothesis. IgAN occurs in an individual who is genetically predisposed to form atypical, poorly glycosylated IgA1, who then experiences a triggering respiratory or gastrointestinal illness, which in the setting of immune dysregulation leads to production of antiglycan antibodies of either IgG or IgA isotypes [44, 46]. Antiglycan antibodies bind poorly glycosylated IgA1 to form complexes that circulate in serum and are then deposited within the mesangium, leading to kidney injury [44, 46] (Figure 1). There may be a relationship between specific features of the multihit hypothesis and the likelihood of disease recurrence [47]. Berthelot et al. [48] suggest that high pre-transplant serum levels of poorly glycosylated IgA1 and antiglycan antibodies, as well as low levels of CD89 (a leucocyte cell surface receptor for IgA that has been shed), may be associated with more aggressive primary disease and a consequent increased risk of recurrence post-transplant.
FIGURE 1.
Schematic representation of IgAN recurrence in the graft and potential site of drug intervention.
A possible link between the gut mucosal immunity and IgAN has been hypothesized by three different groups: by genome-wide association studies, IgAN was linked with polymorphisms of genes involved in gut mucosal immunity [49]; an altered faecal microbiota has been recently described in patients with IgAN [50]; and treatment by corticosteroids targeting the gut mucosa could preserve renal function in IgAN patients [51]. Treatment with broad-spectrum antibiotics in a humanized mouse model of IgAN showed it prevented IgA1 mesangial deposits, glomerular inflammation and subsequent development of proteinuria [52]. Therefore, targeting gut microbiota represents a fascinating new target to treat this disease.
Another important key point in the pathogenesis of the recurrence of IgAN post-transplant is the involvement of the complement system. The complement system has been suggested to be involved in the pathogenesis of various graft pathologies, including ischaemia–reperfusion injury, antibody-mediated rejection and the recurrence of IgAN, with distinct mechanisms that remain to be elucidated [53–55]. Genetic genome-wide association studies have focused attention on the role of complement in IgAN, especially at the level of regulatory proteins [44, 56, 57].
Complement activation is a hallmark of IgAN pathogenesis, as evidenced by the C3 mesangial co-deposition observed in 90% of cases of IgAN [58]. Alternative and lectin pathways are two routes by which the complement is activated during IgAN [55]. IgAN is characterized by properdin or mannan-binding lectin (MBL)-associated serine proteases and co-deposition of IgA [59]. MBL deposition occurred in 20–25% of cases, but it was associated with significant proteinuria and increased renal injury [59]. Interestingly, C4d deposits can be used as biomarkers of lectin pathway activation [60, 61]; in agreement with previous data, patients who showed C4d deposits at the time of the first biopsy presented a significantly worse outcome in a 20-year follow-up [62].
During activation of the complement system, several products, such as C3a and C5a, have broad pro-inflammatory potential and are associated with the activity and severity of IgAN. C3a is also involved in inducing a mesangial cell secretory phenotype, which could explain the expansion of the mesangial matrix observed in human IgAN [63]. Their receptors could represent potential therapeutic targets in IgAN, as well as blocking complement via anti-C5 monoclonal antibodies. This could be an interesting therapeutic alternative considering that complement activation results in the generation of powerful inflammatory anaphylatoxins such as C5a. Indeed, anti-C5 antibody was used in a patient with crescentic IgAN, a rapidly progressive form of the disease, with encouraging results [64].
Interestingly, the expression patterns of CD46 and CD55, both complement protein regulators, were studied in the peripheral blood in patients with progressive IgAN [65]. The study showed that defective peripheral CD46 gene expression did not correlate with eGFR at sampling, but with a faster annual loss of GFR [65]. Similarly, Cernoch et al. [66], in a retrospective study of kidney-transplanted patients with IgAN recurrence, showed that there were no associations of eGFR with intrarenal CD46 complement transcripts, however they did describe the association of several intrarenal gene transcripts, including CD55, with CKD stage. In addition, new data coming from genetic and clinical studies are demonstrating a pivotal role of factor H and complement factor H–related proteins (CFHR) in IgAN development and progression [67, 68] Deletion of CFHR1–3 was demonstrated to be protective in IgAN and these molecules are competitors of factor H and can efficiently lead to uncontrolled complement activation [56, 69]. Therefore complement is considered to be an emerging new therapeutic target in IgAN that could be achieved by different pharmacological approaches [70].
B cells play a central role in the immunopathogenesis of GN, including IgAN. B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are proteins of the tumour necrosis factor superfamily that interact through three receptors [BAFF receptor (BAFF-R), transmembrane activator and cyclophilin ligand interactor), and to B-cell maturation antigen of B cells promoting survival and maturation of transitional B cells into mature B cells, contributing to their differentiation to memory B cells and plasma cells [71]. High expression of both proteins appears to be involved in the pathogenesis of IgAN as a stimulus for antiglycan antibody production [72, 73].
In a retrospective Spanish study, Martín-Penagos et al. [74] showed that higher levels at 6 months after transplant and mean values from 6 months to 3 years of APRIL but not BAFF were found to be related to the risk of IgAN recurrence.
Spleen tyrosine kinase (SYK) is an immunoreceptor-associated protein tyrosine kinase expressed in several cell types, although at the highest levels in B cells and myeloid cells, and has a role for intracellular signal transduction cascades upon engagement of immunoreceptors, including the B-cell receptor and activatory Fc receptors. Then it may have a role in disease pathogenesis via its activity in IgA- and IgG-producing B cells or plasma cells and/or in mediating the effects of the IgA1- and IgG-containing immune complexes when deposited in tissue, with possible beneficial effects of pharmacological inhibition [75].
PATHOLOGY
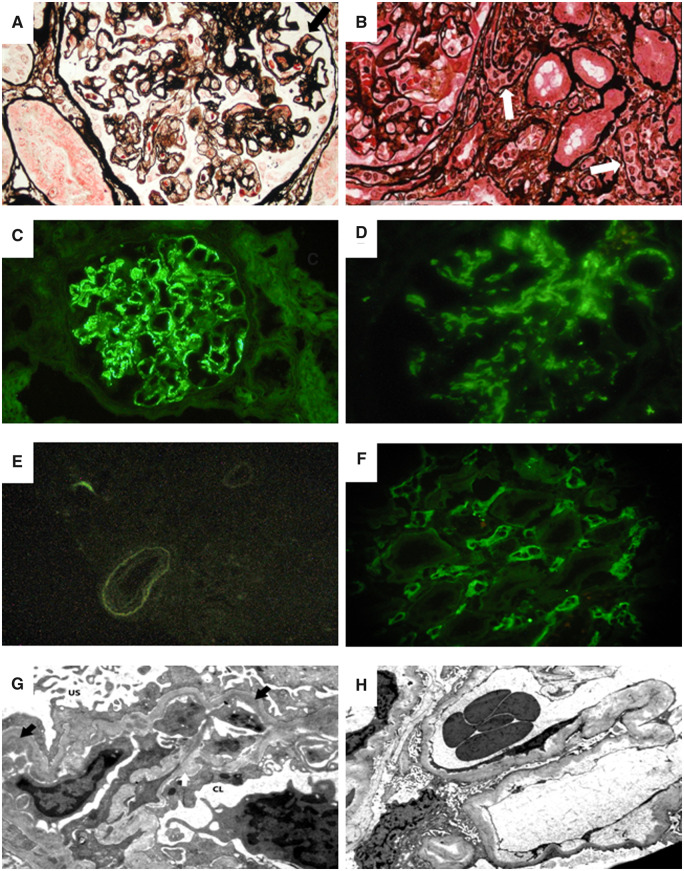
Biopsy is mandatory not only to diagnose disease in the native kidney, but also to identify and characterize graft recurrence of IgAN in the kidney graft. For this reason, the biopsy policies of different centres and the techniques of histological evaluation have an important role in the definition of the presence, frequency and type of recurrence. The pathology findings observed in graft biopsies of IgAN recurrent patients performed for clinical reasons are not different from those of the original disease. However, in this setting, interpretation of histology can be challenged by the simultaneous presence of other glomerular or vascular lesions secondary to acute or chronic graft rejections that can share a similar light microscopy appearance with IgAN. In these cases, not only light microscopy and immunofluorescence, but also electron microscopy may be needed to clearly define the possible association of two different diseases (Figure 2). The Oxford classification of IgAN in native kidneys found that semiquantitative scoring of five lesions (mesangial hypercellularity, endocapillary hypercellularity, segmental glomerulosclerosis, tubulointerstitial fibrosis and percentage of cellular and fibro-cellular crescent, known as the MEST-C score) has significant prognostic value [76].
FIGURE 2.
In a patient with recurrent IgAN, the light microscopy picture (A) shows a glomerulus with global membranoproliferative pattern of injury with several aspects of glomerular basement membrane double contours as well mesangial and capillary wall eosinophilic deposits (black arrow) (Jones silver stain). In this patient, immunofluorescence microscopy revealed mesangial and glomerular capillary wall deposition of IgA (C) with negative C4d in the peritubular capillaries (E). Electron microscopy in this case showed subendothelial electron dense deposits (black arrows) with newly formed lamina densa (i.e. double contours) and cellular interposition (white arrow) (G) (US, urinary space; CL, capillary lumen) [transmission electron microscopy (TEM), ×8900]. In a patient with recurrent IgAN and concurrent antibody-mediated rejection, a light microscopy image (B) shows a portion of a glomerulus (on the left) with segmental obliteration of CLs due to endothelial cells swelling and mononuclear inflammatory cells (i.e. glomerulitis) with inflammatory cells also in peritubular capillaries (white arrows) (Jones silver stain). In this patient, immunofluorescence microscopy revealed mesangial deposition of IgA (D) with diffuse strongly positive C4d in the peritubular capillaries (F). Electron microscopy of this case showed widening of the subendothelial spaces in the absence of subendothelial deposits (TEM, ×8900).
There is some evidence that MEST-C classification of IgAN could be of prognostic significance even in the setting of recurrence. Lim et al. [77] evaluated the prognostic value of MEST in a cohort of 125 renal allograft biopsies obtained from 114 patients diagnosed with IgAN, revealing that endocapillary hypercellularity (E), segmental sclerosis (S) and tubulointerstitial fibrosis (T) predicted graft survival. However, given the retrospective nature of the study, only 41 biopsies were tested for C4d and donor-specific antibody status was unknown, thus underestimating the possible role of concurrent acute or chronic antibody-mediated rejection on the pathogenesis of the lesions. Park et al. [78] recently published on the same topic, describing the prognostic importance of the MEST-C classification in a cohort of 333 patients with IgAN in their graft. They found that all the components of the updated IgAN classification system were significantly associated with prognosis. However, they also showed that E1 (i.e. endocapillary hypercellularity) was significantly positively correlated with antibody-mediated rejection and tubulointerstitial fibrosis was significantly positively correlated with both antibody-mediated rejection and acute T-cell-mediated rejection, underlying the complexity of pathogenesis in the lesions observed in the transplant setting that can recognize different and potentially synergistic aetiologies.
IMMUNOSUPPRESSIVE THERAPY IN THE MANAGEMENT OF IGAN RECURRENCE ON THE GRAFT
The optimal therapy for IgAN recurrence in the renal graft is not known [42, 79–83]. However, required therapy is largely individualized and depends on the clinical syndrome of the disease at the time of histopathological diagnosis. Hence the clinical course of IgAN in the graft may differ substantially from that in the native kidneys, as the extent and intensity of glomerular lesions evolve and develop in an environment that is already immunosuppressed. In fact, one should keep in mind that the clinical course of the disease is most possibly modified by the fact that kidney transplant recipients are maintained with a triad of immunosuppressive agents.
Treatment aims to reduce proteinuria, optimize blood pressure and reduce the inflammatory state. Kidney Disease: Improving Global Outcomes provided clinical practice guidelines for native kidney IgAN that clarified recommended treatments and may reduce the variability seen in the management of these patients [84]. The standard of care for those with IgAN has been angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in those with proteinuria >0.5 g/day, tight blood pressure control to <130/80 mmHg in patients with protein <1 g/day and <125/75 in patients with protein >1 g/day and steroids for those with persistent proteinuria >1 g/day.
Another question is how we should prevent recurrence rather than symptom management once it has occurred. To this end, there is some evidence that immunosuppression matters. Steroid withdrawal, in particular, has been associated with increased rates of recurrence [39]. Retrospective data from a single centre suggest there may be a role for induction therapy, most notably the use of antithymocyte globulin, in prevention [42]. Furthermore, a reduction in the frequency of graft loss attributed to recurrence over successive eras in Italy and Australia suggests that the combination of mycophenolate and tacrolimus may also be protective [15, 39].
There is controversy on the use of steroids in IgAN in native kidneys. There are a few randomized controlled trials (RCTs) and a meta-analysis [85–88] that showed a benefit in terms of renal survival with steroid use on top of supportive therapy compared with supportive therapy alone for IgAN patients with proteinuria >1 g/day. However, more recently these benefits were questioned by the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) trial, which compared intensive supportive treatment to steroids alone or to steroids–immunosuppressive combination therapy [89]. The authors of this trial found that after 3 years of follow-up, a potential benefit from corticosteroid monotherapy was shown in the reduction of proteinuria but not in terms of renal function decline. This was not observed in the steroids–immunosuppressive combination therapy group. Although it is not possible to exclude that the higher rate of remission of proteinuria could translate into better long-term outcomes, a higher number of serious adverse events were reported in the STOP-IgAN trial in the steroid group and in the steroids–immunosuppressive combination therapy group. In another recent trial, the Therapeutic Evaluation of Steroids in IgAN Global Study, comparing steroids versus placebo in IgAN patients, recruitment was discontinued because of excess serious adverse events [90].
The use of steroids or other immunosuppressants in order to treat IgAN recurrence in the graft, although not well proved with clinical trials and prospective studies, may be required in selected patients [91]. Specifically, when biopsy-proven IgAN recurrence in the graft follows a rapidly progressive course, or nephrotic syndrome cannot be managed with conservative interventions, clinicians most often treat these patients according to the severity of the histopathology and the related recommendations for IgAN in the native kidneys [92–94]. Consequently, one approach for this group of patients could be therapy with high-dose glucocorticoids given orally or combining intravenous and oral administration for a few months, followed by a slow taper back to low doses, which are usually given to prevent rejection, carefully assessing individually the infection risk and adopting adequate prophylaxis strategies to counteract the possible metabolic and infectious side effects of steroids that may occur more frequently given the concomitant immunosuppressive therapy.
In patients with rapidly deteriorating renal function and histology showing features of crescentic GN, if the previous scheme has failed, cyclophosphamide could be used orally or intravenously for short period of time (up to 3 months) [95, 96]. In this case, the current anti-metabolite agent, which is used for transplant maintenance, should be discontinued as long as the patient remains on cyclophosphamide, and the patient should receive adequate prophylaxis for opportunistic infection and frequent evaluation to monitor serious adverse side effects.
B-cell-depleting agents (rituximab) have been successfully used in recurrent IgAN in a few case reports [97]. However, recently an RCT in native IgAN failed to show a beneficial effect of rituximab over supportive therapy [98].
Finally, the recent focus on the role of the gut–kidney axis in IgAN has led to the search for new drug formulations targeting the intestinal mucosal immune system (gut-associated lymphoid tissue). Interesting results in native IgAN were obtained by the NEFIGAN trial using a budesonide formulation, allowing selective drug delivery at intestinal gut-associated lymphoid tissue sites [51]. New treatment options for IgAN include drugs targeting BAFF and APRIL, B-cell factors crucial for IgA synthesis [99]. There are ongoing trials in native IgAN with humanized monoclonal antibodies against these factors: NCT02062684, Bright-sc, a Phase 2 trial on blisibimod, a selective antagonist of BAFF and NCT02808429, a Phase 2 trial on atacicept, an antagonist of both BAFF and APRIL. A SYK inhibitor, fostamatinib, is under evaluation in a Phase 2 trial on native IgAN (NCT02112838).
Relying on the potential involvement of complement disorders in the pathogenesis of IgAN, Rosenbland et al. [64] reported the case of an adolescent with rapidly progressive IgAN that led to renal failure despite immunosuppressive treatment. The patient was treated with an anti-C5 agent (eculizumab) in an attempt to rescue renal function. Treatment led to clinical improvement with stabilization of the glomerular filtration rate and reduced proteinuria, but discontinuation of treatment led to a rapid deterioration of renal function [64]. Finally, the concept of prevention of IgAN recurrence by using specific immunosuppressive agents for transplant maintenance has not concluded in certain regimens. Mulay et al. [100], after reviewing the US Renal Data System, concluded that in patients with a pre-transplant diagnosis of GN, the risk of graft loss due to recurrence was not associated with any specific immunosuppressive medication used for kidney transplant and therefore selection of immunosuppression for those recipients should not be made, with the goal of reducing graft failure due to recurrent GN. Most probably, the only strong association is that the use of steroids was strongly associated with a reduced risk of kidney graft loss from recurrent IgAN.
CONCLUSIONS
IgAN is a systemic disease mediated by circulating IgA-containing immune complexes. Despite kidney transplantation, the propensity to produce immune complex formation continues. Whether circulating immune complexes deposit in the mesangium and how immunosuppression modifies this and the subsequent responses of mesangial cells and recruited inflammatory cells likely determines whether IgAN recurs and the outcome of any recurrence.
The extent of recurrence of original kidney disease after kidney transplantation has been underestimated for several reasons. First, the duration of observation varies among studies. Second, the criteria used to schedule protocol and episode biopsies differ among institutions. And third, diagnostic modalities used for early detection of recurrent original kidney disease also vary. Thus rates of graft loss attributable to a recurrence of original kidney disease are often underestimated. However, the recurrence of original disease is often thought to be less important than chronic rejection followed by loss of a functioning allograft. It is important to note that recent data have shown that in patients with certain limited primary kidney diseases, as well IgAN, the predominant cause of graft loss is the recurrence of the original kidney disease. In addition, the rate of 5-year graft survival in patients with recurrent original kidney disease averages 45%. Thus understanding the cause of the clinical and histological variation in IgAN is a key to progressing our prevention and management of this disease. Better molecular characterization of IgAN may enable type-specific therapies. Thus research must address the recurrence of original kidney disease to accumulate more knowledge and obtain optimal management for our patients.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Briganti EM, Russ GR, McNeil JJ. et al. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 2002; 347: 103–109 [DOI] [PubMed] [Google Scholar]
- 2. Hariharan S, Johnson CP, Bresnahan BA. et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000; 342: 605–612 [DOI] [PubMed] [Google Scholar]
- 3. Schena FP. Epidemiology of end-stage renal disease: international comparisons of renal replacement therapy. Kidney Int 2000; 57(Suppl 74): S39–S45 [Google Scholar]
- 4. McDonald SP, Russ GR.. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant 2002; 17: 2212–2219 [DOI] [PubMed] [Google Scholar]
- 5. Hariharan S, Adams MB, Brennan DC. et al. Recurrent and de novo glomerular disease after renal transplantation: a report from renal allograft disease registry. Transplantation 1999; 68: 635–641 [DOI] [PubMed] [Google Scholar]
- 6. Briggs JD, Jones E.. Recurrence of glomerulonephritis following renal transplantation. Scientific Advisory Board of the ERA-EDTA Registry. European Renal Association-European Dialysis and Transplant Association. Nephrol Dial Transplant 1999; 14: 564–565 [DOI] [PubMed] [Google Scholar]
- 7. El-Zoghby ZM, Stegall MD, Lager DJ. et al. Identifying specific causes of kidney allograft loss. Am J Transplant 2009; 9: 527–535 [DOI] [PubMed] [Google Scholar]
- 8. Floege J. Recurrent glomerulonephritis following renal transplantation: an update. Nephrol Dial Transplant 2003; 18: 1260–1265 [DOI] [PubMed] [Google Scholar]
- 9. Hariharan S, Peddi VR, Savin V. et al. Recurrent and de novo renal diseases after renal transplantation: a report from the renal allograft disease registry. Am J Kidney Dis 1998; 31: 928–931 [DOI] [PubMed] [Google Scholar]
- 10. Yakupoglu U, Baranowska-Daca E, Rosen D. et al. Post-transplant nephrotic syndrome: a comprehensive clinicopathologic study. Kidney Int 2004; 65: 2360–2370 [DOI] [PubMed] [Google Scholar]
- 11. Freese PM, Svalander CT, Mölne J. et al. Renal allograft glomerulopathy and the value of immunohistochemistry. Clin Nephrol 2004; 62: 279–286 [DOI] [PubMed] [Google Scholar]
- 12. Chadban SJ. Glomerulonephritis recurrence in the renal graft. J Am Soc Nephrol 2001; 12: 394–402 [DOI] [PubMed] [Google Scholar]
- 13. Morozumi K, Takeda A, Otsuka Y. et al. Recurrent glomerular disease after kidney transplantation: an update of selected areas and the impact of protocol biopsy. Nephrology 2014; 19: 6–10 [DOI] [PubMed] [Google Scholar]
- 14. Allen PJ, Chadban SJ, Craig JC. et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int 2017; 92: 461–469 [DOI] [PubMed] [Google Scholar]
- 15. Moroni G, Longhi S, Quaglini S. et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant 2013; 28: 1305–1314 [DOI] [PubMed] [Google Scholar]
- 16. Odum J, Peh CA, Clarkson AR. et al. Recurrent mesangial IgA nephritis following renal transplantation. Nephrol Dial Transplant 1994; 9: 309–312 [PubMed] [Google Scholar]
- 17. Ortiz F, Gelpi R, Koskinen P. et al. IgA nephropathy recurs early in the graft when assessed by protocol biopsy. Nephrol Dial Transplant 2012; 27: 2553–2558 [DOI] [PubMed] [Google Scholar]
- 18. Cosio FG, Cattran DC.. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int 2017; 91: 304–314 [DOI] [PubMed] [Google Scholar]
- 19. D’Amico G. The commonest glomerulonephritis in the world. Q J Med 1987; 64: 709–727 [PubMed] [Google Scholar]
- 20. Taguchi T, Von Bassewitz DB, Takebayashi S. et al. A comparative study of IgA nephritis in Japan and Germany. An approach to its geopathology. Pathol Res Pract 1987; 182: 358–367 [DOI] [PubMed] [Google Scholar]
- 21. Galla JH. IgA nephropathy. Kidney Int 1995; 47: 377–387 [DOI] [PubMed] [Google Scholar]
- 22. Galla JH, Kohaut EC, Alexander RC. et al. Racial differences in the prevalence of IgA-associated nephropathies. Lancet 1984; 324: 522–529 [DOI] [PubMed] [Google Scholar]
- 23. Smith SM, Tung K.. Incidence of IgA-related nephritides in American Indians in New Mexico. Hum Pathol 1985; 16: 181–184 [DOI] [PubMed] [Google Scholar]
- 24. Jennette JC, Wall SD, Wilkman AS.. Low incidence of IgA nephropathy in blacks. Kidney Int 1985; 28: 944–950 [DOI] [PubMed] [Google Scholar]
- 25. Julian BA, Quiggins PA, Thompson JS. et al. Familial IgA nephropathy: evidence for an inherited mechanism of disease. N Engl J Med 1985; 312: 202–208 [DOI] [PubMed] [Google Scholar]
- 26. Schena FP, Scivittaro V, Ranieri E.. IgA nephropathy: pros and cons for familial disease. Contrib Nephrol 1993; 104: 36–45 [DOI] [PubMed] [Google Scholar]
- 27. Andresdottir MB, Hoitsma AJ, Assmann KJ. et al. Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol 2001; 56: 279–288 [PubMed] [Google Scholar]
- 28. Lim EC, Chia D, Gjertson DW. et al. In vitro studies to explain high renal allograft survival in IgA nephropathy. Transplantation 1993; 55: 996–999 [DOI] [PubMed] [Google Scholar]
- 29. Ponticelli C, Traversi L, Feliciani A. et al. Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int 2001; 60: 1948–1954 [DOI] [PubMed] [Google Scholar]
- 30. Choy BY, Chan TM, Lo SK. et al. Renal transplantation in patients with primary immunoglobulin A nephropathy. Nephrol Dial Transplant 2003; 18: 2399–2404 [DOI] [PubMed] [Google Scholar]
- 31. Pippias M, Stel VS, Aresté-Fosalba N. et al. Long-term kidney transplant outcomes in primary glomerulonephritis: analysis from the ERA-EDTA registry. Transplantation 2016; 100: 1955–1962 [DOI] [PubMed] [Google Scholar]
- 32. Aziz KA, Mousson C, Berthoux F. et al. Renal transplantation outcome in selected recipients with IgA nephropathy as native disease: a bicentric study. Ann Transplant 2012; 17: 45–51 [DOI] [PubMed] [Google Scholar]
- 33. Avasare RS, Rosenstiel PE, Zaky ZS. et al. Predicting post-transplant recurrence of IgA nephropathy: the importance of crescents. Am J Nephrol 2017; 45: 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YS, Moon JI, Jeong HJ. et al. Live donor renal allograft in endstage renal failure patients from immunoglobulin A nephropathy. Clin Transpl 2001; 71: 233–238 [DOI] [PubMed] [Google Scholar]
- 35. Moriyama T, Nitta K, Suzuki K. et al. Latent IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transplant 2005; 19: 41–48 [DOI] [PubMed] [Google Scholar]
- 36. Silva FG, Chander P, Pirani CL. et al. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation 1982; 33: 241–246 [PubMed] [Google Scholar]
- 37. Andresdottir MB, Haasnoot GW, Doxiadis II. et al. Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: a retrospective analysis of registry data. Transplantation 2005; 80: 1012–1018 [DOI] [PubMed] [Google Scholar]
- 38. McDonald SP, Russ GR.. Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation 2006; 82: 759–762 [DOI] [PubMed] [Google Scholar]
- 39. Clayton P, McDonald S, Chadban S.. Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant 2011; 11: 1645–1649 [DOI] [PubMed] [Google Scholar]
- 40. Leeaphorn N, Garg N, Khankin EV. et al. Recurrence of IgA nephropathy after kidney transplantation in steroid continuation versus early steroid‐withdrawal regimens: a retrospective analysis of the UNOS/OPTN database. Transpl Int 2018; 31: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Von Visger JR, Gunay Y, Andreoni KA. et al. The risk of recurrent IgA nephropathy in a steroid-free protocol and other modifying immunosuppression. Clin Transplant 2014; 28: 845–854 [DOI] [PubMed] [Google Scholar]
- 42. Berthoux F, El Deeb S, Mariat C. et al. Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation 2008; 85: 1505–1507 [DOI] [PubMed] [Google Scholar]
- 43. Chandrakantan A, Ratanapanichkich P, Said M. et al. Recurrent IgA nephropathy after renal transplantation despite immunosuppressive regimens with mycophenolate mofetil. Nephrol Dial Transplant 2005; 20: 1214–1221 [DOI] [PubMed] [Google Scholar]
- 44. Feehally J, Farrall M, Boland A. et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 2010; 21: 1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gharavi AG, Moldoveanu Z, Wyatt RJ. et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 2008; 19: 1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boyd JK, Cheung CK, Molyneux K. et al. An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 2012; 81: 833–843 [DOI] [PubMed] [Google Scholar]
- 47. Robert T, Berthelot L, Cambier A. et al. Molecular insights into the pathogenesis of IgA nephropathy. Trends Mol Med 2015; 21: 762–775 [DOI] [PubMed] [Google Scholar]
- 48. Berthelot L, Robert T, Vuiblet V. et al. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int 2015; 88: 815–822 [DOI] [PubMed] [Google Scholar]
- 49. Kiryluk K, Li Y, Scolari F. et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 2014; 46: 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Angelis M, Montemurno E, Piccolo M. et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 2014; 9: e99006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fellström BC, Barratt J, Cook H. et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017; 389: 2117–2127 [DOI] [PubMed] [Google Scholar]
- 52. Chemouny JM, Gleeson PJ, Abbad L. et al. Modulation of the microbiota by oral antibiotics treats immunoglobulin A nephropathy in humanized mice. Nephrol Dial Transplant 2019; 34: 1135–1144 [DOI] [PubMed] [Google Scholar]
- 53. Castellano G, Franzin R, Stasi A. et al. Complement activation during ischemia/reperfusion injury induces pericyte-to-myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front Immunol 2018; 23: 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simone S, Rascio F, Castellano G. et al. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic Biol Med 2014; 74: 263–273 [DOI] [PubMed] [Google Scholar]
- 55. Daha MR, van Kooten C.. Role of complement in IgA nephropathy. J Nephrol 2016; 29: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu XQ, Li M, Zhang H. et al. A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 2011; 25: 178–182 [DOI] [PubMed] [Google Scholar]
- 57. Gharavi AG, Kiryluk K, Choi M. et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 2011; 43: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol 2014; 10: 445–454 [DOI] [PubMed] [Google Scholar]
- 59. Roos A, Rastaldi MP, Calvaresi N. et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 2006; 17: 1724–1734 [DOI] [PubMed] [Google Scholar]
- 60. Maeng YI, Kim MK, Park JB. et al. Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria. Int J Clin Exp Pathol 2013; 15: 904–910 [PMC free article] [PubMed] [Google Scholar]
- 61. Faria B, Henriques C, Matos AC. et al. Combined C4d and CD3 immunostaining predicts immunoglobulin (Ig)A nephropathy progression. Clin Exp Immunol 2015; 179: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Espinosa M, Ortega R, Sánchez M. et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol 2014; 9: 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wan JX, Fukuda N, Endo M. et al. Complement 3 is involved in changing the phenotype of human glomerular mesangial cells. J Cell Physiol 2007; 213: 495–501 [DOI] [PubMed] [Google Scholar]
- 64. Rosenblad T, Rebetz J, Johansson M. et al. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr Nephrol 2014; 29: 2225–2228 [DOI] [PubMed] [Google Scholar]
- 65. Coppo R, Peruzzi L, Loiacono E. et al. Defective gene expression of the membrane complement inhibitor CD46 in patients with progressive immunoglobulin A nephropathy. Nephrol Dial Transplant 2019; 34: 587–596 [DOI] [PubMed] [Google Scholar]
- 66. Cernoch M, Hruba P, Kollar M. et al. Intrarenal complement system transcripts in chronic antibody-mediated rejection and recurrent IgA nephropathy in kidney transplantation. Front Immunol 2018; 9: 2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H. et al. Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int 2017; 92: 942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tortajada A, Gutiérrez E, Goicoechea de Jorge E. et al. Elevated factor H-related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int 2017; 92: 953–963 [DOI] [PubMed] [Google Scholar]
- 69. Kiryluk K, Novak J.. The genetics and immunobiology of IgA nephropathy. J Clin Invest 2014; 124: 2325–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rizk DV, Maillard N, Julian BA. et al. The emerging role of complement proteins as a target for therapy of IgA nephropathy. Front Immunol 2019; 19: 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoffman W, Lakkis FG, Chalasani G.. B cells, antibodies, and more. Clin J Am Soc Nephrol 2016; 11: 137–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Castigli E, Scott S, Dedeoglu F. et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci USA 2004; 101: 3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCarthy DD, Kujawa J, Wilson C. et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 2011; 121: 3991–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martín-Penagos L, Benito-Hernàndez A, San Segindo D. et al. A proliferation-inducing ligand increase precedes IgA nephropathy recurrence in kidney transplant recipients. Clin Transplant 2019; 33: e13502. [DOI] [PubMed] [Google Scholar]
- 75. McAdoo S, Tam F.. Role of the spleen tyrosine kinase pathway in driving inflammation in IgA nephropathy. Semin Nephrol 2018; 38: 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Trimarchi H, Barratt J, Cattran DC. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 77. Lim BJ, Joo DJ, Kim MS. et al. Usefulness of Oxford classification in assessing immunoglobulin A nephropathy after transplantation. Transplantation 2013; 95: 1491–1497 [DOI] [PubMed] [Google Scholar]
- 78. Park S, Go H, Baek CH. et al. Clinical importance of the updated Oxford classification in allograft IgA nephropathy. Am J Transplant 2019; 19: 2855–2864 [DOI] [PubMed] [Google Scholar]
- 79. Maes BD, Oyen R, Claes K. et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int 2004; 65: 1842–1849 [DOI] [PubMed] [Google Scholar]
- 80. Bumgardner GL, Amend WC, Ascher NL. et al. Single-center long-term results of renal transplantation for IgA nephropathy. Transplantation 1998; 65: 1053–1060 [DOI] [PubMed] [Google Scholar]
- 81. Ohmacht C, Kliem V, Burg M. et al. Recurrent immunoglobulin A nephropathy after renal transplantation: a significant contributor to graft loss. Transplantation 1997; 64: 1493–1496 [DOI] [PubMed] [Google Scholar]
- 82. Frohnert PP, Donadio JV Jr, Velosa JA. et al. The fate of renal transplants in patients with IgA nephropathy. Clin Transpl 1997; 11: 127–133. [PubMed] [Google Scholar]
- 83. Bachman U, Biava C, Amend W. et al. The clinical course of IgA-nephropathy and Henoch-Schonlein purpura following renal transplantation. Transplantation 1986; 42: 511–515 [DOI] [PubMed] [Google Scholar]
- 84. Radhakrishnan J, Cattran DC.. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines–application to the individual patient. Kidney Int 2012; 82: 840–856 [DOI] [PubMed] [Google Scholar]
- 85. Pozzi C, Bolasco PG, Fogazzi GB. et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 1999; 353: 883–887 [DOI] [PubMed] [Google Scholar]
- 86. Manno C, Torres DD, Rossini M. et al. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 2009; 24: 3694–3701 [DOI] [PubMed] [Google Scholar]
- 87. Lv J, Zhang H, Chen Y. et al. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 2009; 53: 26–32 [DOI] [PubMed] [Google Scholar]
- 88. Natale P, Palmer SC, Ruospo M. et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 2020; 3: CD003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rauen T, Eitner F, Fitzner C. et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225–2236 [DOI] [PubMed] [Google Scholar]
- 90. Lv J, Zhang H, Wong MG. et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 2017; 318: 432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9: S1–S55 [DOI] [PubMed] [Google Scholar]
- 92. Messina M, di Vico MC, Ariaudo C. et al. Treatment protocol with pulse and oral steroids for IgA Nephropathy after kidney transplantation. J Nephrol 2016; 29: 575–583 [DOI] [PubMed] [Google Scholar]
- 93. Matsukuma Y, Masutan K, Tsuchimoto A. et al. Effect of steroid pulse therapy on post-transplant immunoglobulin A nephropathy. Nephrology 2018; 23: 10–16 [DOI] [PubMed] [Google Scholar]
- 94. Cattran DC, Coppo R, Cook HT. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 95. Ballardie FW, Roberts IS.. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 2002; 13: 142–148 [DOI] [PubMed] [Google Scholar]
- 96. Walker RG, Yu SH, Owen JE. et al. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin: a two-year prospective trial. Clin Nephrol 1990; 34: 103–107 [PubMed] [Google Scholar]
- 97. Chancharoenthana W, Townamchai N, Leelahavanichkul A. et al. Rituximab for recurrent IgA nephropathy in kidney transplantation: a report of three cases and proposed mechanisms. Nephrology 2017; 22: 65–71 [DOI] [PubMed] [Google Scholar]
- 98. Lafayette RA, Canetta PA, Rovin BH. et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 2017; 28: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Coppo R. Treatment of IgA nephropathy: recent advances and prospects. Nephrol Ther 2018; 14(Suppl 1): S13–S21 [DOI] [PubMed] [Google Scholar]
- 100. Mulay AV, van Walraven C, Knoll GA.. Impact of immunosuppressive medication on the risk of renal allograft failure due to recurrent glomerulonephritis. Am J Transplant 2009; 9(4): 804–811 [DOI] [PubMed] [Google Scholar]