Abstract
Background
Optimal management of chronic kidney disease (CKD) anaemia remains controversial and few studies have evaluated real-world management of anaemia in advanced CKD in the context of guideline recommendations.
Methods
We performed an observational study from the Swedish Renal Registry evaluating the epidemiology and treatment patterns of anaemia across Stages 3b–5 in non-dialysis (ND) and dialysis-dependent (DD) CKD patients during 2015. Logistic regression and Cox models explored the associations between anaemia treatments, inflammation, erythropoietin resistance index (ERI) and subsequent 1-year risk of major adverse cardiovascular events (MACEs).
Results
Data from 14 415 (ND, 11 370; DD, 3045) patients were included. Anaemia occurred in 60% of ND and 93% of DD patients. DD patients used more erythropoiesis-stimulating agents (ESAs; 82% versus 24%) and iron (62% versus 21%) than ND patients. All weekly ESA doses were converted to a weight-adjusted weekly epoetin equivalent dose. The prescribed ESA doses were low to moderate [median 48.2 IU/kg/week (ND), 78.6 IU/kg/week (DD)]. Among ESA-treated patients, 6–21% had haemoglobin (Hb) >13 g/dL and 2–6% had Hb <9 g/dL. Inflammation (C-reactive protein >5 mg/L) was highly prevalent and associated with ERI and higher ESA doses. Higher (>88 IU/kg/week) versus lower (<44 IU/kg/week) ESA doses were associated with a higher risk of MACEs [{ND hazard ratio [HR] 1.36 [95% confidence interval (CI) 1.00–1.86]; DD HR 1.60 [95% CI 1.24–2.06]}. There was no association between iron use and inflammation or MACEs.
Conclusions
Anaemia remains highly prevalent in advanced CKD. Patients with anaemia received moderate ESA doses with a relatively low prevalence of iron use. Higher doses of ESA were associated with inflammation and a higher risk of MACE.
Keywords: anaemia, chronic kidney disease, dialysis, epidemiology, ESA, haemoglobin, inflammation, iron
INTRODUCTION
Anaemia is a common complication of chronic kidney disease (CKD), occurring primarily as a result of impaired synthesis of erythropoietin from the failing kidneys, often in combination with absolute or functional iron deficiency and other contributing factors, including blood loss, inflammation and shortened red blood cell (RBC) lifespan [1]. Treatment of anaemia in advanced CKD includes iron replacement, either intravenous (IV) or oral, as initial therapy for iron-deficient patients and administration of erythropoiesis-stimulating agents (ESAs), with the primary aim of minimizing anaemia-related symptoms and avoiding RBC transfusions [2].
Optimal management of renal anaemia has yet to be fully defined for all patient subpopulations. While epidemiological studies have shown better outcomes associated with the attainment of higher haemoglobin (Hb) levels in patients with renal anaemia [3–9], the results of four key randomized clinical trials have not supported the hypothesis that full anaemia correction with ESAs is beneficial to all patients [10–13]. Collectively these clinical studies demonstrated that the strategy of targeting higher Hb levels (in the normal range) is associated with no benefit or with a potential risk of harm in at least some patients [i.e. increased mortality risk and/or cardiovascular (CV) side effects] [14]. Attempts to explain the apparent paradox between epidemiological and clinical data on Hb levels are grounded in the hypothesis that higher doses required in a subgroup of ESA hyporesponsive patients, precipitated by inflammation, may be the culprit rather than the Hb target per se [15]. Indeed, further post hoc analyses of both the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) study [16] and the Normal Haematocrit Trial [13] both demonstrated that higher ESA doses, albeit generally higher than those used in Europe at the time of this study, were associated with lower Hb levels and increased mortality in ESA hyporesponsive patients. However, there remains a lack of conclusive clinical evidence to indicate that high ESA doses are necessarily harmful. Current anaemia guidelines, issued between 2011 and 2013 after the pivotal results of the CHOIR [12], Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) [10] and Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) [11] trials, recommend individualizing the ESA dose based on patients’ clinical circumstances and using the lowest dose possible to reduce the risk of RBC transfusion (US Food and Drug Administration guidance) [17] and/or to maintain Hb <11.5 [2] or <12 g/dL [18]. Guidance also proposes broader use of iron to treat renal anaemia. We and others observed that these recommendations resulted in immediate changes in clinical anaemia practice, namely lower ESA use and lower Hb levels [19, 20]. However, few studies have evaluated the long-term and contemporary clinicians’ responses to these recommendations.
Using real-world Hb data from the Swedish national CKD registry, we explored the associations of anaemia treatments, erythropoietin resistance, inflammation and subsequent 1-year risk of major adverse CV events (MACEs) in a population of nephrology-referred patients within a broad spectrum of CKD severity.
MATERIALS AND METHODS
Study design
This was a retrospective, observational analysis conducted in the Swedish Renal Registry (SRR) to evaluate the prevalence, management and adverse clinical outcomes of renal anaemia in referred CKD patients in Sweden. The SRR comprises two interlinked nationwide registries of patients followed at nephrology clinics. The patients either have CKD Stages 3b–5 (SRR-CKD) or are dialysis-dependent (SRR-DD) [21]. Detailed information on the SRR is reported in the Supplementary data.
Patients included in this study were >18 years of age under a nephrologist's care in 2015. ND patients had an estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2 (CKD Stages 3b–5), had not yet started dialysis and had one or more visit recorded in 2015. Data for ND patients were extracted from the last recorded visit to an outpatient clinic during 2015. Data for DD patients were extracted from a randomly selected hospital visit from September to October 2015. CV events were assessed prospectively for 1 year following the recorded visit in 2015. The study was approved by the regional ethics committee in Stockholm.
Study covariates
Demographic data (i.e. age and sex), current medications and clinical data were obtained as entered into the SRR on the visit date by the local administrators. All weekly ESA doses for darbepoetin and methoxy polyethylene glycol-epoetin beta were converted to a weight-adjusted weekly epoetin equivalent dose derived from the allocated defined daily doses defined by the World Health Organization (WHO) Collaborating Centre [22]. Conversion factors of 1:222 and 1:250 were used for the conversion of epoetin:darbepoetin and epoetin:methoxy polyethylene glycol-epoetin beta, respectively. According to the SRR manual, a patient was considered as treated with IV iron for up to 6 months after the last administration of low-frequency, high-dose IV iron. Use of oral iron was identified from pharmacy dispensations as recorded in the Prescribed Drugs Registry up to 3 months before or 15 days after baseline. The laboratory parameters of interest [Hb (g/dL), eGFR (mL/min/1.73 m2), calculated using the CKD Epidemiology Collaboration equation [23] and high sensitivity C-reactive protein (hs-CRP; mg/L)] were also used as entered into the SRR on the visit date.
Records from SRR patients were cross-linked to the corresponding records in national healthcare registries. The National Prescribed Drug Registry was used to extract additional prescription data. The National Patient Registry was used to extract information on patient morbidity and records of transfusions within the 3 years prior to their nephrology visit in 2015. Comorbidities and medications considered in this study are detailed in Supplementary data, Table S1. Finally, we accessed the National Cause of Death Registry to obtain data on death and causes of death, which were used to evaluate the risk of MACEs associated with anaemia management strategies within 1 year of the date of record entry in the SRR. MACE was defined as the composite of hospitalization from non-fatal stroke, unstable angina, myocardial infarction or heart failure and death from all causes, whichever occurred first.
CKD stages were classified according to criteria of the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group [2]. Anaemia was defined according to the WHO definition (females, Hb <12 g/dL; males, <13 g/dL). Weight-standardized ESA doses were calculated and categorized into equivalent tertiles of distribution: lower (<44 IU/kg/week), middle (44–87.9 IU/kg/week) and upper (>88 IU/kg/week). The erythropoietin resistance index (ERI) [24] was calculated as epoetin dose (IU/week) per body weight (kg) divided by Hb level (g/dL) and was categorized into tertiles of distribution.
Statistical analysis
Descriptive statistics were used to describe demographics and clinical characteristics, including anaemia treatment, Hb levels, inflammation (hs-CRP >5 mg/L) and epidemiological endpoints. Logistic regression models were used to evaluate the relationship between inflammation and the use of iron (oral or IV) and separately to evaluate the relationship between inflammation and ESA dose. Cox proportional hazards models were used to estimate the hazard ratios (HRs) for the effect of iron, ESA dose or ERI on MACEs. Patients were followed from their recorded nephrology visit until an occurrence of a MACE, end of follow-up at 1 year after the recorded nephrology visit or administrative censoring on 31 December 2016, whichever occurred first. Further details are provided in the Supplementary data.
RESULTS
Patient characteristics
Of 15 130 individuals recorded in the SRR during 2015, 14 415 (ND, 11 370; DD, 3045) met the inclusion and exclusion criteria (Figure 1). The median age was similar in the ND and DD patient groups {ND, 73 years [interquartile range (IQR) 64–80]; DD, 70 years [IQR 58–78]} and more patients were male (ND, 63%; DD, 67%). Diabetes mellitus was common (ND, 29%; DD, 36%). Patients in both groups had established CV disease (heart failure, 12–13%; atrial fibrillation, 11–12%; cerebrovascular disease, 10–11%; previous myocardial infarction, 9–10%). The most commonly prescribed medications were β-blockers in ND patients (58%) and phosphate binders in DD patients (73%) (Table 1 and Supplementary data, Table S1).
FIGURE 1.
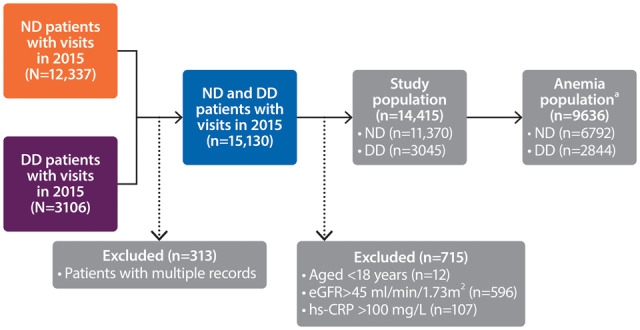
Flow chart of the study population. aIncluded patients with Hb levels <13 g/dL (males) or <12 g/dL (females) and those treated with ESAs.
Table 1.
Patient demographics and anaemia-related characteristics (full analysis set)
| Parameter | Non-DD patients | DD patients |
|---|---|---|
| (n = 11 370) | (n = 3045) | |
| Age (years), median (IQR) | 73 (64–80) | 70 (58–78) |
| Sex (male), n (%) | 7164 (63) | 2052 (67) |
| eGFR (mL/min/1.73 m2), median (IQR) | 25 (17–33) | – |
| CKD stage, n (%) | ||
| Stage 3b | 3722 (33) | – |
| Stage 4 | 5520 (49) | – |
| Stage 5 | 2128 (19) | – |
| Dialysis type, n (%) | ||
| PD | – | 708 (23) |
| HD | – | 2337 (77) |
| Dialysis vintage (years), median (IQR) | – | 2.3 (1.0–4.4) |
| Comorbidities, n (%) | ||
| Cancer (previous 3 years) | 1669 (15) | 463 (15) |
| Cerebrovascular disease | 1180 (10) | 337 (11) |
| Diabetes mellitus | 3309 (29) | 1087 (36) |
| Heart failure | 1345 (12) | 382 (13) |
| Myocardial infarction | 1006 (9) | 313 (10) |
| Peripheral vascular disease | 1306 (12) | 451 (15) |
| hs-CRP (mg/L), median (IQR) | 5 (2–9) | 5 (3–13) |
| >5 mg/L, n (%)a | 2905 (33) | 1441 (50) |
| Anaemia, n (%)b | 6792 (60) | 2844 (93) |
| Hb (g/dL), mean (SD) | 12.3 (1.63) | 11.4 (1.34) |
| ESA users, n (%) | 2692 (24) | 2491 (82) |
| Iron medication users, n (%) | 2359 (21) | 1880 (62) |
| Oral | 1662 (15) | 97 (3) |
| IV | 697 (6) | 1783 (59) |
| Concomitant medications, n (%) | ||
| ACEi/ARB | 6487 (57) | 1093 (36) |
| β-blockers | 6643 (58) | 1814 (60) |
| Statins | 5761 (51) | 1170 (38) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Percentage of patients with hs-CRP >5 mg/mL in those with a recorded level.
Included patients with Hb levels <13 g/dL (males) or <12 g/dL (females) and those treated with ESAs.
Prevalence of anaemia and anaemia treatment in CKD patients
The proportion of patients with anaemia was higher in DD (93%) than ND (60%) CKD patients. DD patients had higher use of ESAs (82% versus 24%) and iron (62% versus 21%) than ND patients. The prevalence of anaemia increased with CKD severity in ND patients (Stage 3b, 42.5%; Stage 5, 83.7%) and was numerically higher for haemodialysis (HD, 94.5%) than for peritoneal dialysis (PD, 89.7%) (Figure 2). A similar trend was also observed in males and females analysed separately when anaemia was defined as Hb <12.0 g/dL for both sexes. The definition of anaemia as Hb <12.0 g/dL resulted in a moderate reduction of anaemia prevalence, particularly in ND patients (Supplementary data, Figure S1). Although anaemia was frequently present, the proportion of patients with severe anaemia (<9 g/dL) was overall very low (ND, 1%; DD, 4%). The proportion of CKD patients receiving iron increased with CKD severity; the most frequent form of iron was oral in ND patients and IV in DD patients. More HD patients used iron/IV iron (70%/69%) than PD patients (iron 37%/IV 25%). ESA use also increased with CKD severity and was more common in HD (83%) than PD (77%) (Figure 2).
FIGURE 2.
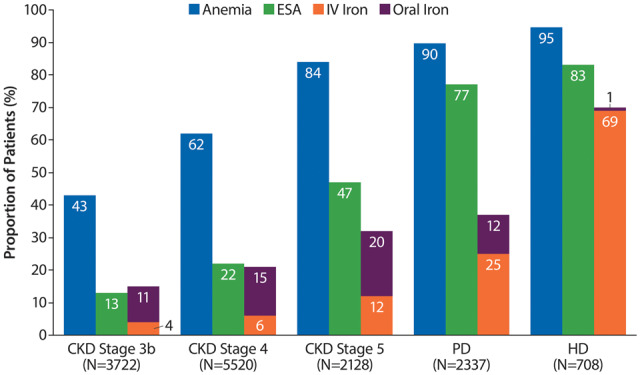
Prevalence of anaemia [included patients with Hb levels <13 g/dL (males) or <12 g/dL (females) and those treated with ESAs] and the proportion of patients receiving ESA and iron treatment by CKD Stages 3b–5 and on dialysis.
Iron use in ESA-treated patients
Approximately one-quarter of ESA-treated ND patients used concomitant oral iron, with a slight increase in the proportion of those taking IV versus oral iron in later CKD stages. Most ESA-treated HD patients used iron (~75%), while only 40% of ESA-treated PD patients received iron. The predominant form of iron in ESA-treated DD patients was IV (Figure 3).
FIGURE 3.
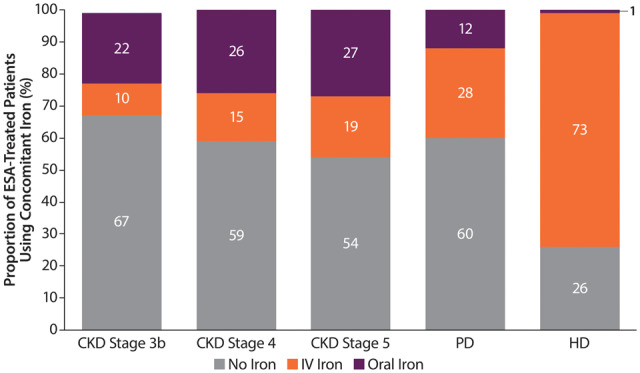
Prevalence of concomitant iron use in ESA-treated patients by CKD Stages 3b–5 (ND patients) or dialysis type (PD and HD patients). Percentages may not add up to 100 due to rounding.
ESA dose
The distribution of ESA doses was fairly consistent across ND CKD Stages 3b–5, with ~40% receiving doses in the lower tertile (<44 IU/kg/week). ESA doses in the upper tertile were more common in PD (35%) and HD (47%) patients than in ND (16–20%) patients (Figure 4). The median ESA dose was 48 (IQR 30–75) IU/kg/week for ND patients and 83 (IQR 46–138) IU/kg/week and 67 (IQR 36–110) IU/kg/week for HD and PD patients, respectively. The median dose of the upper tertile (>88 IU/kg/week) was 142 (IQR 110–192) for DD patients and 131 (IQR 104–177) for ND patients. The median ESA doses by tertile, proportions of patients using different ESA medications and number of transfusions are reported in Supplementary data, Tables S3–S5.
FIGURE 4.
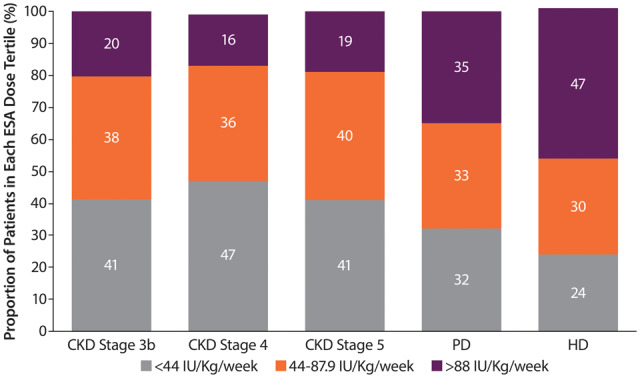
Dose of ESA by CKD stage (ND patients) or dialysis type (DD patients). Percentages may not add up to 100 due to rounding.
Hb levels in ESA-treated patients
The proportion of ESA-treated patients within the recommended European Hb target range of 10–12 g/dL was <60% across CKD Stages 3b–5 or in DD patients. There was a tendency for more DD patients to be in the target range than ND patients, where the proportion increased with increasing CKD severity. The proportion of ESA-treated patients with Hb levels <9 g/dL across the groups was small (2–6%), whereas 6–21% of patients had Hb levels >13 g/dL (Figure 5).
FIGURE 5.
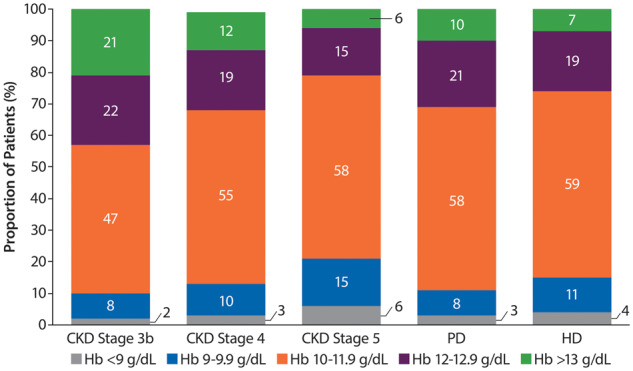
Distribution of Hb levels in ESA-treated patients. Percentages may not add up to 100 due to rounding.
One in four patients treated with ESAs in the upper tertile (>88 IU/kg/week) had Hb levels <10 g/dL. Conversely, about a third of patients treated with ESA doses in the lower tertile (<44 IU/kg/week) had Hb levels >12 g/dL. A high proportion (20%) of patients treated with doses of ESAs in the upper tertile achieved Hb levels above the recommended level of 12 g/dL (Figure 6).
FIGURE 6.
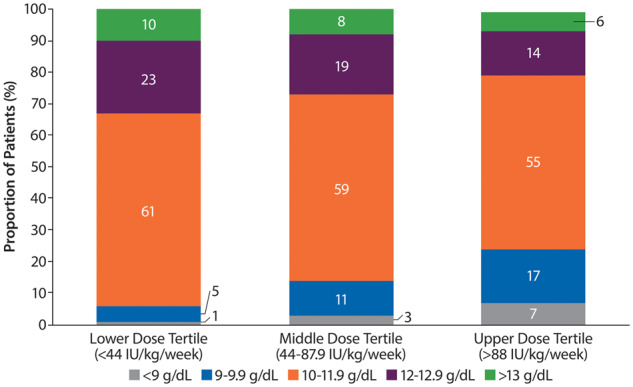
Distribution of Hb levels in patients with body weight–adjusted ESA doses in the upper, middle and lower equivalent tertiles. Percentages may not add up to 100 due to rounding.
Association between inflammation (hs-CRP >5 mg/L) and use of iron or ESA dose
Inflammation, defined as hs-CRP >5 mg/L, was common in patients receiving ESAs and was present in 43% of ND patients and 50% of DD patients (Supplementary data, Table S2). For both ND and DD patients, there was a clear relationship between increasing hs-CRP levels and higher ESA doses (Figure 7). The odds of receiving a higher ESA dose (>88 IU/kg/week) versus a lower ESA dose (<44 IU/kg/week) were ~2-fold in patients with hs-CRP >5 mg/L {fully adjusted odds ratio [OR] 1.85 [95% confidence interval (CI) 1.53–2.25]; Table 2}. The ERI was ~0.43 in ND patients and increased with CKD severity (PD, 0.58; HD, 0.74). These results are consistent with higher levels of inflammation in DD than in ND patients (Supplementary data, Table S2). The use of IV or oral iron was not associated with hs-CRP >5 mg/L [adjusted OR IV iron 1.15 (95% CI 0.98–1.35); oral iron, 0.82 (95% CI 0.65–1.03)] (Table 2).
FIGURE 7.
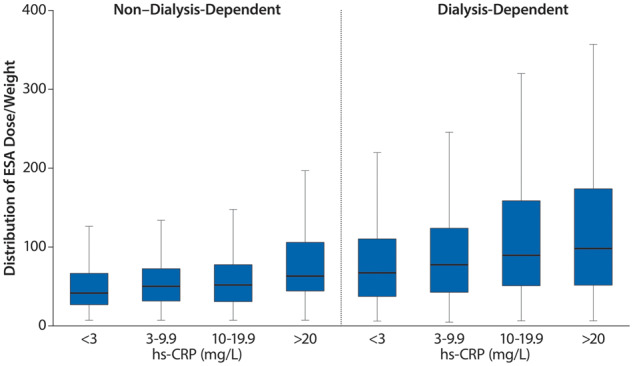
ESA dose per kilogram stratified by hs-CRP levels in ESA-treated ND and DD patients.
Table 2.
Association between inflammation (hs-CRP >5 mg/L) and use of iron (oral or IV) or ESA dose for ESA-treated patients
| Unadjusted OR | OR adjusted for age, sex and stage | OR further adjusted for comorbiditiesa | OR further adjusted for medicationb | Fully adjusted ORc | |
|---|---|---|---|---|---|
| Iron use (versus no iron) (n = 3770) | |||||
| IV | 1.24 (1.08–1.42) | 1.17 (1.01–1.37) | 1.14 (0.98–1.32) | 1.12 (0.96–1.31) | 1.15 (0.98–1.35) |
| Oral | 0.86 (0.69–1.05) | 0.89 (0.71–1.10) | 0.85 (0.69–1.06) | 0.87 (0.69–1.08) | 0.82 (0.65–1.03) |
| ESA (IU/kg/week) (categorized) (n = 3182) | |||||
| 44–87.9 versus <44 | 1.31 (1.09–1.56) | 1.30 (1.09–1.56) | 1.29 (1.08–1.55) | 1.30 (1.08–1.56) | 1.23 (1.02–1.48) |
| >88 versus <44 | 2.10 (1.77–2.50) | 2.14 (1.78–2.57) | 2.12 (1.76–2.55) | 2.12 (1.76–2.55) | 1.85 (1.53–2.25) |
Data are displayed as odds ratio (95% CI).
Comorbidities included indicators for CV disease, diabetes, hypertension, inflammation, inflammatory bowel disease and chronic infections.
Medication data included indicators for angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statins, calcium channel blockers and β-blockers.
Further adjusted for laboratory data included continuous variables for albumin, calcium (albumin-adjusted) and phosphate.
Association between ESA dose, iron use, ERI and MACE
An association between oral iron treatment and increased CV risk compared with no iron was observed in DD patients [fully adjusted HR 1.59 (95% CI 1.02–2.46)]; the association was still present after further adjusting for dialysis modality and ESA dose [fully adjusted HR 1.21 (95% CI 0.97–1.52)]. This association was not seen with IV iron. Stratification based on the type of dialysis (PD, HD) was limited because of the small number of individuals treated with oral iron in the HD population, leading to low precision. Higher ESA doses (>88 IU/kg/week) compared with lower doses (<44 IU/kg/week) were also associated with increased CV risk, particularly in DD patients [HR 1.60 (95% CI 1.24–2.06)]. A higher ERI [0.88 (IU/week)/kg/(g/dL)] versus a lower ERI [<0.44 (IU/week)/kg/(g/dL)] was likewise associated with increased CV risk in both ND [HR 1.45 (95% CI 1.06–1.98)] and DD patients [HR 1.66 (95% CI 1.29–2.13)] (Table 3).
Table 3.
Association between the use of iron (oral or IV), ESA dose or ERI and MACEs a within 1-year follow-up for ESA-treated patients
| Unadjusted HR | Fully adjusted HRb | |
|---|---|---|
| Parameter | 1.30 (1.03–1.62) | 1.16 (0.92–1.46) |
| Iron use (versus no iron) | ||
| Non-DD | ||
| IV | 1.20 (0.92–1.56) | 1.05 (0.81–1.36) |
| DD | ||
| IV | 1.03 (0.85–1.24) | 0.97 (0.80–1.17) |
| Oral | 1.50 (0.97–2.33) | 1.59 (1.02–2.46) |
| ESA dose (IU/kg/week, categorized) | ||
| Non-DD | ||
| 44–87.9 versus <44 | 1.28 (0.98–1.66) | 1.02 (0.78–1.34) |
| >88 versus <44 | 1.90 (1.41–2.55) | 1.36 (1.00–1.86) |
| DD | ||
| 44–87.9 versus <44 | 1.27 (0.97–1.67) | 1.22 (0.93–1.60) |
| >88 versus <44 | 1.76 (1.38–2.24) | 1.60 (1.24–2.06) |
| ERI | ||
| Non-DD | ||
| 0.44–0.81 versus <0.44 | 1.34 (1.03–1.76) | 1.00 (0.75–1.33) |
| >0.81 versus <0.44 | 2.22 (1.67–2.96) | 1.45 (1.06–1.98) |
| DD | ||
| 0.44–0.81 versus <0.44 | 1.12 (0.86–1.47) | 1.12 (0.86–1.47) |
| >0.81 versus <0.44 | 1.79 (1.41–2.27) | 1.66 (1.29–2.13) |
Data are displayed as HR (95% CI).
MACEs were stroke, non-fatal myocardial infarction, non-fatal heart failure, hospitalization for unstable angina and death from all causes.
Adjusted for age, stage (for non-DD), sex, myocardial infarction, peripheral vascular disease, cerebrovascular disease, heart failure, prior diabetes, prior statin and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, Hb level and hs-CRP (categorized into <3, 3–10, 10–20 and >20).
DISCUSSION
In this nationwide contemporary study of anaemia prevalence and management in a Swedish nephrology-referred population across CKD Stages 3b–5, we confirmed that anaemia is a common complication of CKD, occurring in ~60% of ND and 90% of DD patients. These findings align broadly with results from other developed countries during 2007–10 [25–27]. Furthermore, we demonstrated that the association between ERI, inflammation and a higher risk of CV outcomes occurs in a real-world population of nephrology-referred patients. We observed that ~60% of ESA-treated patients had Hb levels within the range recommended in the international guidelines. Moreover, the proportion of HD and PD patients with Hb within the target range was similar to that reported in the UK [28].
Given the prevalence of anaemia and the current recommendations, we found that the use of iron was surprisingly low in the overall ND population and in ESA-treated ND patients. Although we acknowledge the lack of information on iron status parameters in these patients, iron use has been shown to increase Hb levels even in the presence of adequate iron stores [29] and to be ESA dose sparing. Even though current guidelines recommend initiating treatment of anaemia with iron, and using iron as an adjuvant to sustain or enhance the erythropoietic response to ESAs, previous studies [25, 26] have also found low iron use. Iron utilization patterns may be the result of perceived tolerability issues with oral iron [30], concerns about the general safety of IV iron [31] and poor convenience of IV iron administration in the outpatient setting. However, in our study, the use of IV iron was not associated with the presence of inflammation or with an increased risk of MACEs.
In our study, the proportion of patients treated with ESA across a spectrum of CKD severity was always less than the proportion of patients with anaemia, particularly at earlier CKD stages. This probably reflects guideline recommendations [2, 18] to initiate ESA therapy only after Hb levels fall to <10 g/dL and to aim for partial correction of anaemia only. ESA doses administered across the anaemic CKD population were moderate, with ~80% of ND and about half of DD patients receiving <88 IU/kg/week, which can be considered the upper dose interval of ESA initiation, but still considerably lower than the highest recommended dose according to the KDIGO guidelines (twice the initiation dose) [2]. The moderate use of ESA may be due to the fact that high ESA doses have been linked to excess mortality. Indeed, we found an increased risk of MACEs in patients treated with ESA doses in the upper tertile (>88 IU/kg/week) compared with those in the lower tertiles, even after correction for potential confounding factors. We also observed that higher ERI and ESA doses, even within the recommended dose interval, were associated with worse CV outcomes.
The presence of inflammation as measured here (hs-CRP >5 mg/L) was a common finding across the spectrum of CKD severity and was associated with the need for higher ESA does. Inflammation is one of the key factors linking ESA resistance and outcomes. Inflammation upregulates the iron-regulating hormone hepcidin, which traps iron inside the cell, making it unavailable for erythropoiesis. Inflammation is a key factor promoting ESA resistance, high ferritin levels and functional iron deficiency. However, several other factors interplay with hepcidin regulation and erythropoiesis in inflamed CKD patients [32], and measuring hepcidin levels has not been established as a reliable biomarker of functional iron deficiency in CKD [33].
Another noteworthy finding in our study was the relatively high proportion of ESA-treated patients above the target Hb range of 10–12 g/dL. Other studies have found similar results [25]. In comparison with data from the US Renal Data System, the proportion of HD patients within the target range of 10–12 g/dL is similar, but fewer dialysis patients in the USA have Hb >12 g/dL (14.5% versus 26%) [34]. This observation may reflect that there are different interpretations of the evidence from observational studies and secondary analyses of randomized clinical trials [16] and that it is generally acknowledged in Sweden that patients with good response to ESAs—who may easily achieve higher Hb levels—have improved survival. Furthermore, because of the Hb variability during treatment with ESAs, maintaining Hb levels in a relatively narrow range (10–12 g/dL) with ESAs is a universal challenge. Indeed, in our study, more patients with doses in the lower two tertiles were above target than those given doses in the upper tertile.
Our study has several strengths, one being the complete coverage of nephrology-referred patients across a broad CKD spectrum in a nationwide registry with detailed information about diagnosis, laboratory data and treatments. Additionally, the possibility of linking the records to other healthcare resources allowed us to obtain extensive data on comorbidities, other medications and outcomes. Information on ESA use and ESA doses is mandatorily reported in the registry, and there was virtually no missing information concerning Hb. One weakness is missing data for some variables (e.g. hs-CRP, ferritin and transferrin) and a lack of information on iron doses. Furthermore, the study was performed in a point-prevalence design in Sweden, so the results on anaemia management, in particular, may not reflect those in other countries. Nevertheless, the key findings that iron use was low and that there is a high proportion of ESA-treated patients with Hb levels above the 10–12 g/dL target have been seen in studies conducted in other countries [25, 26].
To conclude, anaemia is a common complication of Stages 3b–5 CKD. The management of anaemia in the CKD population remains challenging and, given the proportion of ESA-treated patients with Hb >12 g/dL, maintaining Hb levels within the recommended target appears difficult. Iron use may be suboptimal in this population, especially in ND CKD. ESA doses and ESA resistance are affected by the high prevalence of inflammation in this population, and while ESA doses are generally kept within recommended intervals, ERI is also associated with CV outcomes in this dose interval.
FUNDING
This study was funded with a grant from Astellas Pharma to the Karolinska Institutet. Medical writing/editorial support was provided by Rosalba Satta, PhD and Elizabeth Hermans, PhD, from OPEN Health Medical Communications, Chicago, IL, USA and funded by the study sponsor.
CONFLICT OF INTEREST STATEMENT
M.E. reports speaker or advisory board fees from Astellas Pharma, AstraZeneca and Vifor Pharma. E.C. is employed by Astellas Pharma. S.H.J. received personal fees from Astellas Pharma, Amgen, AstraZeneca, Vifor Pharma and Baxter outside the submitted work. J.J.C. reports funding from AstraZeneca, Novartis, Merck Sharp & Dohme and Vifor Pharma outside the submitted work and speaker or advisory board fees from Baxter, AstraZeneca and Astellas Pharma. P.B. and H.B. have no conflicts of interest to report.
DATA SHARING STATEMENT
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Supplementary Material
REFERENCES
- 1. Babitt JL, Lin HY.. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 3. Collins AJ. Influence of target hemoglobin in dialysis patients on morbidity and mortality. Kidney Int Suppl 2002; 80: 44–48 [DOI] [PubMed] [Google Scholar]
- 4. Ofsthun N, Labrecque J, Lacson E. et al. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63: 1908–1914 [DOI] [PubMed] [Google Scholar]
- 5. Rigatto C, Parfrey P, Foley R. et al. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 2002; 13: 1084–1090 [DOI] [PubMed] [Google Scholar]
- 6. Harnett JD, Foley RN, Kent GM. et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int 1995; 47: 884–890 [DOI] [PubMed] [Google Scholar]
- 7. Foley RN, Parfrey PS, Harnett JD, Kent GM, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 1996; 28: 53–61 [DOI] [PubMed] [Google Scholar]
- 8. Levin A, Thompson CR, Ethier J. et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34: 125–134 [DOI] [PubMed] [Google Scholar]
- 9. Regidor DL, Kopple JD, Kovesdy CP. et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 2006; 17: 1181–1191 [DOI] [PubMed] [Google Scholar]
- 10. Drueke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 11. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 12. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 13. Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 14. Szczech L. Anemia trials: lessons for clinicians, politicians, and third-party payers. Kidney Int 2010; 77: 479–480 [DOI] [PubMed] [Google Scholar]
- 15. Singh AK. What is causing the mortality in treating the anemia of chronic kidney disease: erythropoietin dose or hemoglobin level? Curr Opin Nephrol Hypertens 2010; 19: 420–424 [DOI] [PubMed] [Google Scholar]
- 16. Szczech LA, Barnhart HX, Inrig JK. et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin Alfa (Marketed as Procrit E, Darbepoetin Alfa (Marketed as Aranesp).2017. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-erythropoiesis-stimulating-agents-esa-epoetin-alfa-marketed-procrit-epogen-darbepoetin (28 August 2019, date last accessed)
- 18. Locatelli F, Barany P, Covic A. et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 2013; 28: 1346–1359 [DOI] [PubMed] [Google Scholar]
- 19. Evans M, Suttorp MM, Bellocco R. et al. Trends in haemoglobin, erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant 2016; 31: 628–635 [DOI] [PubMed] [Google Scholar]
- 20. Freburger JK, Ng LJ, Bradbury BD. et al. Changing patterns of anemia management in US hemodialysis patients. Am J Med 2012; 125: 906–914.e9 [DOI] [PubMed] [Google Scholar]
- 21.Swedish Renal Registry. https://www.medscinet.net/snr/ (20 August 2019, date last accessed)
- 22.World Health Organization. Collaborating Center for Drug Statistics Methodology: Guidelines for ATC Classification and DDD assignment 2019.https://www.whocc.no/ (10 July 2019, date last accessed)
- 23. Levey AS, Stevens LA.. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010; 55: 622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panichi V, Rosati A, Bigazzi R. et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant 2011; 26: 2641–2648 [DOI] [PubMed] [Google Scholar]
- 25. Cases-Amenos A, Martinez-Castelao A, Fort-Ros J. et al. Prevalence of anaemia and its clinical management in patients with stages 3–5 chronic kidney disease not on dialysis in Catalonia: MICENAS I study. Nefrologia 2014; 34: 189–198 [DOI] [PubMed] [Google Scholar]
- 26. Ryu SR, Park SK, Jung JY. et al. The prevalence and management of anemia in chronic kidney disease patients: result from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci 2017; 32: 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stauffer ME, Fan T.. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014; 9: e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UK Renal Registry. 20th annual report of the renal association. Nephron 2018; 139(Suppl 1): 165-190 [DOI] [PubMed] [Google Scholar]
- 29. Coyne DW, Kapoian T, Suki W. et al. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 2007; 18: 975–984 [DOI] [PubMed] [Google Scholar]
- 30. Kalra PA, Bhandari S, Saxena S. et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant 2016; 31: 646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macdougall IC. Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin Kidney J 2017; 10: i16–i24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ganz T, Nemeth E.. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol 2016; 36: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ueda N, Takasawa K.. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients 2018; 10: 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Renal Data System. Annual Data Report. 2018. https://www.usrds.org/ (28 August 2019, date last accessed)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


