Abstract
Chronic kidney disease (CKD) patients are at an increased risk of cardiovascular disease (CVD) and statins may not be protective in advanced CKD. The reasons for the limited efficacy of statins in advanced CKD are unclear, but statins may increase plasma levels of the highly atherogenic molecule lipoprotein(a), also termed Lp(a), as well as PCSK9 (protein convertase subtilisin/kexin type 9) levels. Lp(a) has also been linked to calcific aortic stenosis, which is common in CKD. Moreover, circulating Lp(a) levels increase in nephrotic syndrome with declining renal function and are highest in patients on peritoneal dialysis. Thus, the recent publication of the Phase 2 randomized controlled trial of pelacarsen [also termed AKCEA-APO(a)-LRx and TQJ230], a hepatocyte-directed antisense oligonucleotide targeting the LPA gene messenger RNA, in persons with CVD should be good news for nephrologists. Pelacarsen safely and dose-dependently decreased Lp(a) levels by 35–80% and a Phase 3 trial [Lp(a)HORIZON, NCT04023552] is planned to run from 2020 to 2024. Unfortunately, patients with estimated glomerular filtration rate <60 mL/min or urinary albumin:creatinine ratio >100 mg/g were excluded from Phase 2 trials and those with ‘significant kidney disease’ will be excluded from the Phase 3 trial. Optimized exclusion criteria for Lp(a)HORIZON would provide insights into the role of Lp(a) in CVD in CKD patients.
Keywords: cardiovascular disease, cardiovascular risk, chronic kidney disease, dyslipidaemia, lipoprotein(a), pelacarsen
CARDIOVASCULAR DISEASE IN CHRONIC KIDNEY DISEASE
Chronic kidney disease (CKD) is one of the fastest growing global causes of death and has been projected to be among the top five worldwide causes of death by 2040 and among the top two before the end of the century in long-lived countries [1, 2]. CKD is usually diagnosed by the presence of a decreased glomerular filtration rate (GFR, <60 mL/min/1.73 m2) for >3 months or an increased urinary albumin:creatinine ratio (UACR, >30 mg/g). These thresholds mark an increased risk of all-cause and cardiovascular death [3]. However, it is worrisome that this increased risk of cardiovascular death persists despite current therapeutic approaches, especially in advanced CKD [4]. This is in part related to an incomplete understanding of the drivers of cardiovascular disease (CVD) in CKD as well as to insufficient information about the optimal levels to be achieved for some therapeutic targets. Exclusion of CKD patients from pivotal randomized clinical trials (RCTs) as well as the small size of some CKD-specific trials or enrolment of a heterogenous mixture of dialysis and non-dialysis patients is not helpful [5]. Some relevant examples relate to well-established CVD risk factors, such as hypertension and dyslipidaemia. Regarding hypertension, the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease; the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults; and the 2018 European Society of Cardiology, European Society of Hypertension Guidelines for the Management of Arterial Hypertension refer to different CKD populations and propose different definitions of hypertension, different thresholds to initiate anti-hypertensive therapy in CKD patients and different blood pressure targets for drug-treated hypertension [6]. Consensus documents have tried to fill the void left by evidence-based medicine [7–9]. Management of hyperlipidaemia is also marred by an incomplete evidence base. Current guidelines recommend statins for non-dialysis patients with CKD aged ≥50 years as well as for younger CKD patients who already have CVD, are especially at high cardiovascular risk, are diabetic or carry a functioning kidney graft [10]. However, statins are not indicated for dialysis patients. The guidelines relied heavily on the results of the SHARP randomized controlled trial (RCT) of simvastatin/ezetimibe versus placebo, the largest trial to date of lipid-lowering therapy in CKD [11]. However, some recommendations in the guidelines defy evidence-based medicine principles and even apparent logical reasoning. Thus, most of the evidence was generated with simvastatin/ezetimibe but guidelines recommend a statin, not statin/ezetimibe. Additionally, since dialysis is frequently a transition stage towards a kidney transplant, it defies logic that if a statin was initiated pre-dialysis, it is maintained through dialysis, but statins should not be initiated de novo until the patient is transplanted. In any case, SHARP identified a high-risk population that despite major decreases in risk still had the highest residual risk while on statin/ezetimibe: CKD patients with baseline low-density lipoprotein (LDL)-cholesterol >100 mg/dL, UACR >30 mg/g and body mass index >28 kg/m2 [12]. These CKD patients may specially benefit from an add-on agent, such as PCSK9 (protein convertase subtilisin/kexin type 9) inhibitor [13]. In any case, even in CKD patients not on dialysis, statin/ezetimibe decreased events but not mortality. Thus, there is still margin for improvement of CVD outcomes in CKD patients by optimizing lipid-lowering therapy.
LIPOPROTEIN(a)
Lipoprotein(a) [Lp(a)] is a highly atherogenic, LDL-like lipoprotein containing Apolipoprotein-B (Apo B) linked by a disulphide bridge to Apolipoprotein(a) [Apo(a)] [14] (Figure 1). Apo(a) is encoded by the LPA gene and should not be confused with members of the Apolipoprotein A family, encoded by genes such as APOA1, APOA2 and others. The LPA gene is expressed mainly in the liver, with the kidney being the second-highest LPA messenger RNA (mRNA)-expressing organ according to normal tissue panel transcriptomics data, although in some of these data sets there are warnings regarding the reliability of the kidney LPA expression data [15–17]. Indeed, Protein Atlas identified kidney tubular staining for Lp(a) using two different antibodies [18]. The discussion on the potential kidney expression of LPA would be moot in the current state of knowledge if it were not for the adverse effect profile of Lp(a)-targeting approaches as discussed below. Lp(a) levels are determined mainly genetically, but Lp(a) is cleared by the kidney, among other clearance mechanisms, and may increase in CKD and in response to statins (Figure 2). In patients with large Apo(a) isoforms, plasma Lp(a) levels appear to increase early (from CKD category G2) in the course of CKD because of decreased clearance and they peak when GFR is <15 mL/min [19, 20]. Indeed, elevated Lp(a), >40 mg/dL, which is found in ∼25% of CKD patients, was independently associated with the risk of myocardial infarction and death in patients with CKD [21]. Patients with the highest Lp(a) values had lower GFR and higher UACR, and the impact of low estimated glomerular filtration rate (eGFR) was evidenced in patients with different genetic LPA backgrounds. Additionally, Lp(a) levels increase, independently of the size of Apo(a) isoforms, in patients on peritoneal dialysis and are increased up to 4-fold in patients with nephrotic syndrome (mean values 69 mg/dL, nearly 4-fold higher than the values in controls of 18 mg/dL) [22–24].
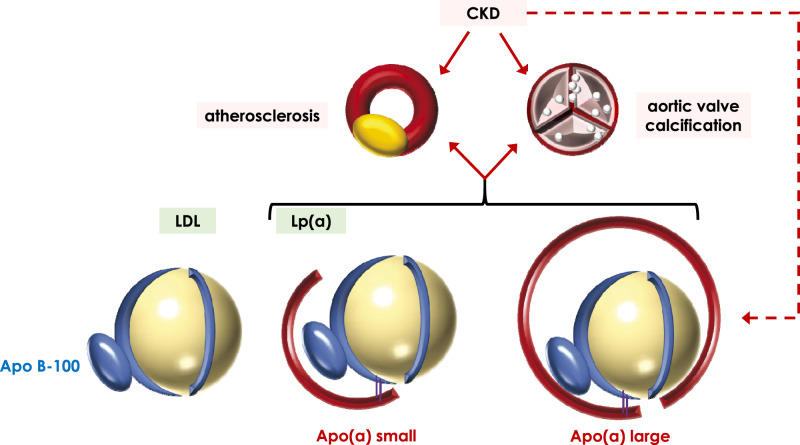
FIGURE 1.
Representation of Lp(a) and illustrative depiction of Lp(a) with large and small Apo(a) isoforms, as compared with LDL. LDL and Lp(a) share the Apo B100 apolipoprotein but only Lp(a) has an Apo(a) apolipoprotein of varying sizes. Lp(a) has been associated with increased risk of atherosclerosis and of calcific aortic stenosis, and CKD patients are also at increased risk of both diseases. Interestingly, CKD with either decreased GFR or severe albuminuria is associated with increased Lp(a) levels.
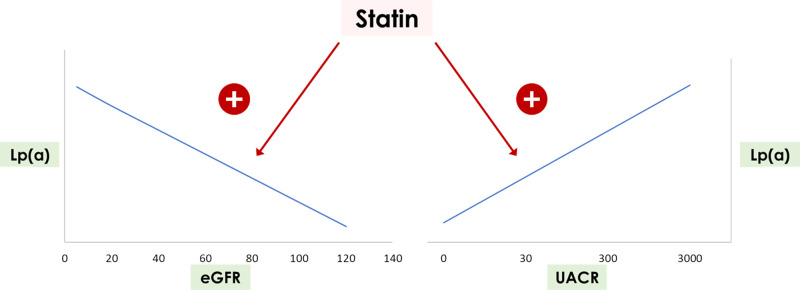
FIGURE 2.
Graphical representation of increased Lp(a) levels in association with CKD with either decreased GFR or severe albuminuria. Statins may increase Lp(a) levels, although the interaction between statins and CKD in the context of different GFR or albuminuria values or combinations thereof has not been adequately characterized. General trends are represented, not specific slopes or Lp(a) values.
Lp(a) has proinflammatory, proatherogenic and prothrombotic properties and has been linked to myocardial infarction, stroke and peripheral arterial disease by epidemiological studies, and meta-analyses, Mendelian randomization studies and genome-wide association studies [14]. The risk is regarded as significant by ESC guidelines when Lp(a) is >80th percentile (50 mg/dL) [22]. Additionally, it has been linked to calcific aortic valve stenosis by genetic and observational studies (Figure 1). It is thought that Lp(a) carries oxidized phospholipids and autotaxin to aortic valves, triggering calcification. Although there is little information on autotaxin and kidney disease, serum levels are increased in proteinuric diabetic patients [25]. In this regard, calcific aortic stenosis is particularly prevalent in CKD patients [26].
CURRENT THERAPY FOR HYPERCHOLESTEROLAEMIA AND Lp(a)
Currently, there are no approved medications to specifically lower Lp(a) and thus, it is not yet possible to precisely analyse the contribution of baseline Lp(a) or changes in Lp(a) levels to clinical CVD. Statins increase Lp(a) levels in the general population by 10–20%, although there is much less information in CKD patients, especially dialysis patients [27] (Figure 2). In this regard, statins are associated with increased PCSK9 levels in CKD patients and, as discussed below, PCSK9 targeting decreases Lp(a) [28]. Thus, it is currently unknown whether the lack of efficacy of statins in dialysis patients may be related to triggering of harmful compensatory responses, such as increased levels of PCSK9 and/or Lp(a). By contrast, niacin lowered Lp(a) levels by ∼20% and by up to 40% in patients with higher baseline Lp(a) levels, oestrogen/progestin lowered Lp(a) by 15–20% and mipomersen (antisense oligonucleotide targeting Apo B100 mRNA) and PCSK9 inhibitors by 20–30% [14]. Of these drugs, only PCSK9 inhibitors are widely used to decrease CVD risk and there is positive experience in CKD patients [12, 29, 30]. Indeed, and contrary to statins, the absolute reduction in CVD events on PCSK9 inhibitors was numerically greater in patients with more advanced CKD [29]. Apheresis can also reduce Lp(a) levels by 30–35%. However, the only drugs that decrease specifically Lp(a) and do so by >50% are antisense oligonucleotides [14].
PELACARSEN
Pelacarsen is a hepatocyte-directed antisense oligonucleotide targeting the LPA gene mRNA (Figure 3). Pelacarsen has received multiple other names, such as those used in recent Phase 1 and 2 RCTs and the upcoming Phase 3 trial: ISIS 681257, IONIS APO(a)-LRx, AKCEA-APO(a)-LRx and TQJ230 [31–34]. In Phase 2 RCTs, a weekly or monthly subcutaneous administration of pelacarsen dose-dependently decreased Lp(a) by 35–80% [34]. Pelacarsen also decreased oxidized phospholipids by 30–90%, depending on dose and on the apolipoprotein examined, and LDL-cholesterol by ∼20% in patients already on lipid-lowering therapy: 90% on statins, 50% on ezetimibe and 25% on PCSK9 inhibitors. Unfortunately for nephrologists, most CKD patients were excluded from the RCT: those with GFR <60 mL/min (GFR categories G3–G5) and those with UACR >100 mg/g were excluded. Furthermore, the publication did not indicate how many participants had CKD G1/G2 based on the A2 albuminuria criterion or what results were obtained in these patients. Pelacarsen was found to be safe, with no excess nephrotoxicity among other safety parameters, and well tolerated. Of interest, the incidence of urinary tract infections was almost double in the pelacarsen compared with the placebo group (13% versus 6%). This adverse effect should be confirmed in larger trials. If confirmed, it may merit an in-depth look into potential Lp(a) expression in kidney cells and its function there, although pelacarsen theoretically only targets liver cells. In this regard, Lp(a) is present in urine, although it is thought to be derived from the circulation.
FIGURE 3.
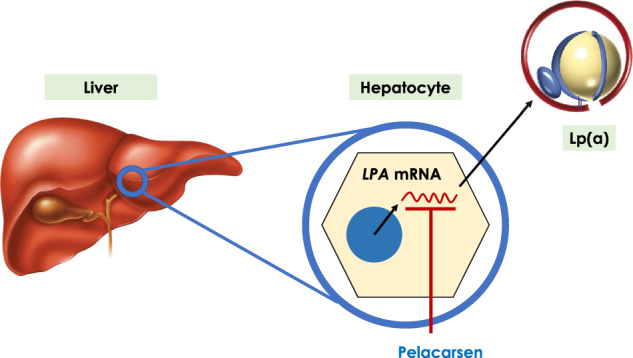
Mechanisms of action of pelacarsen, also known as AKCEA-APO(a)-LRx and TQJ230, among other names. Pelacarsen is a hepatocyte-directed antisense oligonucleotide targeting the mRNA transcribed from the LPA gene, resulting in decreased Apo(a) availability and lower Lp(a) levels.
A Phase 3 RCT [Lp(a)HORIZON, NCT04023552] with primary endpoint expanded major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or stroke and urgent coronary re-vascularization requiring hospitalization) in patients with Lp(a) ≥70 or ≥90 mg/dL is expected to start in 2020 and run through 2024 [32]. Key inclusion criteria are history of CVD and Lp(a) ≥70 mg/dL on optimal LDL-cholesterol-lowering treatment. Unfortunately, again, ‘significant kidney disease’ is an exclusion criterion, although, at the time of writing this piece, it is not publicly available what this entails. Let us hope the GFR threshold is lowered at least to 30 mL/min and the UACR threshold increased. In this regard, a Phase 1 RCT carried out in 2018 addressed the effects of pelacarsen in individuals with GFR 30–60 mL/min [31].
A LOOK INTO THE FUTURE
The increased CVD risk of CKD patients is not satisfactorily addressed by current therapeutic approaches based on statins. While they decrease events in non-dialysis CKD patients, they do not reduce mortality and are not effective in dialysis populations. The realization that statins may increase both Lp(a) and PCSK9, the beneficial effect of PCSK9 inhibitors in CKD patients, which appears to increase as GFR decreases (apparently contrary to observations for statins that are not protective in dialysis patients), the higher Lp(a) levels in CKD patients with low GFR or pathological proteinuria or in dialysis, and the frequent occurrence of calcific aortic stenosis, all point to a potential deleterious role of Lp(a) in CKD-associated vascular and valvular disease. At this point, it should be remembered that vascular calcification may even increase with statins or PCSK9 inhibitors [35]. In this regard, the successful and safe use of pelacarsen to lower Lp(a) levels and the Phase 3 RCT that will address its impact on CVD events should be good news for nephrologists. Unfortunately, CKD patients were grossly underrepresented in Phase 2 trial and we can only hope that the under-representation is not as gross in the Phase 3 RCT, so that some information can be obtained on the impact of pelacarsen in at least some CKD populations. The potential future availability of agents that target Lp(a) should spur research into the role and regulation of Lp(a) and its interaction with dialysis modalities, different lipid-lowering strategies and other therapies frequently prescribed in CKD patients. As an example, niacin, the oral drug with the greatest potential to decrease Lp(a), is under study for hyperphosphataemia [36].
FUNDING
Research by A.O. is supported by FIS PI16/02057, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009 Fondos FEDER, FRIAT, Sociedad Española de Nefrología, Comunidad de Madrid B2017/BMD-3686 CIFRA2-CM). M.V.P.-G. is supported by Rio Hortega programme ISCIII.
CONFLICT OF INTEREST STATEMENT
A.O. is a consultant for Sanofi Genzyme and has received speaker fees from Amgen.
REFERENCES
- 1. Foreman KJ, Marquez N, Dolgert A. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018;392:2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ortiz A, Sanchez-Niño MD, Crespo-Barrio M. et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019;39:29–34 [DOI] [PubMed] [Google Scholar]
- 3. Perez-Gomez MV, Bartsch L-A, Castillo-Rodriguez E. et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019;12:258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ortiz A, Covic A, Fliser D. et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014;383:1831–1843 [DOI] [PubMed] [Google Scholar]
- 5. Vanholder R, Van Laecke S, Glorieux G. et al. Deleting death and dialysis: conservative care of cardio-vascular risk and kidney function loss in chronic kidney disease (CKD). Toxins (Basel) 2018;10:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo-Rodriguez E, Fernandez-Fernandez B, Alegre-Bellassai R. et al. The chaos of hypertension guidelines for chronic kidney disease patients. Clin Kidney J 2019;12:771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarafidis PA, Persu A, Agarwal R. et al. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transplant 2017;32:620–640 [DOI] [PubMed] [Google Scholar]
- 8. Parati G, Ochoa JE, Bilo G. et al. Hypertension in chronic kidney disease Part 1: out-of-office blood pressure monitoring: methods, thresholds, and patterns. Hypertension 2016;67:1093–1101 [DOI] [PubMed] [Google Scholar]
- 9. Parati G, Ochoa JE, Bilo G. et al. Hypertension in chronic kidney disease Part 2: role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertension 2016;67:1102–1110 [DOI] [PubMed] [Google Scholar]
- 10. Tonelli M, Wanner C, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. Lipid management in chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2013 clinical practice guideline. Ann Intern Med 2014;160:182–189 [DOI] [PubMed] [Google Scholar]
- 11. Baigent C, Landray MJ, Reith C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng-Lin B, Ortiz A.. Lipid management in chronic kidney disease: systematic review of PCSK9 targeting. Drugs 2018;78:215–229 [DOI] [PubMed] [Google Scholar]
- 13. Vallejo-Vaz AJ, Ray KK, Ginsberg HN. et al. Associations between lower levels of low-density lipoprotein cholesterol and cardiovascular events in very high-risk patients: pooled analysis of nine ODYSSEY trials of alirocumab versus control. Atherosclerosis 2019;288:85–93 [DOI] [PubMed] [Google Scholar]
- 14. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017;69:692–711 [DOI] [PubMed] [Google Scholar]
- 15.GDS3113/186585. https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS3113:186585 (2 January 2020, date last accessed)
- 16.GDS181/31721_at. https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS181:31721_at (2 January 2020, date last accessed)
- 17.GDS422/31721_at. https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS422:31721_at (2 January 2020, date last accessed)
- 18.The Human Protein Atlas. Tissue expression of LPA—Staining in kidney. https://www.proteinatlas.org/ENSG00000198670-LPA/tissue/kidney (2 January 2020, date last accessed)
- 19. Hopewell JC, Haynes R, Baigent C.. The role of lipoprotein (a) in chronic kidney disease. J Lipid Res 2018;59:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bermudez-Lopez M, Forne C, Amigo N. et al. An in-depth analysis shows a hidden atherogenic lipoprotein profile in non-diabetic chronic kidney disease patients. Expert Opin Ther Targets 2019;23:619–630 [DOI] [PubMed] [Google Scholar]
- 21. Bajaj A, Damrauer SM, Anderson AH. et al. Lipoprotein(a) and risk of myocardial infarction and death in chronic kidney disease: findings from the CRIC study (Chronic Renal Insufficiency Cohort). Arterioscler Thromb Vasc Biol 2017;37:1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Catapano AL, Graham I, De Backer G. et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058 [DOI] [PubMed] [Google Scholar]
- 23. Agrawal S, Zaritsky JJ, Fornoni A. et al. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol 2018;14:57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wanner C, Rader D, Bartens W. et al. Elevated plasma lipoprotein(a) in patients with the nephrotic syndrome. Ann Intern Med 1993;119:263–269 [DOI] [PubMed] [Google Scholar]
- 25. Shimizu M, Furuichi K, Toyama T. et al. Serum autotaxin levels are associated with proteinuria and kidney lesions in Japanese Type 2 diabetic patients with biopsy-proven diabetic nephropathy. Intern Med 2016;55:215–221 [DOI] [PubMed] [Google Scholar]
- 26. Brandenburg VM, Schuh A, Kramann R.. Valvular calcification in chronic kidney disease. Adv Chronic Kidney Dis 2019;26:464–471 [DOI] [PubMed] [Google Scholar]
- 27. Tsimikas S, Gordts P, Nora C. et al. Statins and increases in Lp(a): an inconvenient truth that needs attention. Eur Heart J 2020;41:192–193 [DOI] [PubMed] [Google Scholar]
- 28. Elewa U, Fernández-Fernández B, Mahillo-Fernández I. et al. PCSK9 in diabetic kidney disease. Eur J Clin Invest 2016;46:779–786 [DOI] [PubMed] [Google Scholar]
- 29. Charytan DM, Sabatine MS, Pedersen TR. et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER Trial. J Am Coll Cardiol 2019;73:2961–2970 [DOI] [PubMed] [Google Scholar]
- 30. Ferro CJ, Mark PB, Kanbay M. et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 2018; 14: 727–749 [DOI] [PubMed] [Google Scholar]
- 31.Study of ISIS 681257 in patients with renal impairment compared to healthy patients - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03506854 (2 January 2020, date last accessed)
- 32.Assessing the impact of lipoprotein (a) lowering with TQJ230 on major cardiovascular events in patients with CVD—full text view—ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT04023552 (2 January 2020, date last accessed)
- 33. Viney NJ, van Capelleveen JC, Geary RS. et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–2253 [DOI] [PubMed] [Google Scholar]
- 34. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I. et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020; doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 35. Akers EJ, Nicholls SJ, Di Bartolo BA.. Plaque calcification: do lipoproteins have a role? Arterioscler Thromb Vasc Biol 2019;39:1902–1910 [DOI] [PubMed] [Google Scholar]
- 36. Malhotra R, Katz R, Hoofnagle A. et al. The effect of extended release niacin on markers of mineral metabolism in CKD. Clin J Am Soc Nephrol 2018;13:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]