Abstract
Sodium–glucose co-transporter-2 (SGLT2) inhibitors decreased cardiovascular (CV) events and improved renal outcomes in CV safety studies in type 2 diabetes melitus (T2DM) patients at high CV risk. Canagliflozin also improved kidney outcomes in diabetic kidney disease in the Canagliflozin and Renal Events in Diabetes and Nephropathy Clinical Evaluationtrial. More recently, the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial showed that dapagliflozin improved CV outcomes in patients with HF with or without diabetes. Protection from HF in non-diabetics was confirmed for empagliflozin in the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced) trial. A meta-analysis of DAPA-HF and EMPEROR-Reduced confirmed reductions in all-cause and CV death and the combined risk of CV death or worsening HF, as well as in the composite renal endpoint {hazard ratio [HR] 0.62 [95% confidence interval (CI) 0.43–0.90]} without differences based on the presence of diabetes or baseline estimated glomerular filtration rate (eGFR). Moreover, the Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA-CKD) showed that dapagliflozin as an add-on over renin–angiotensin system blockade in patients with chronic kidney disease (CKD; with or without T2DM) reduced the HR for the primary endpoint (time to the first occurrence of ≥50% eGFR decline, end-stage kidney disease or renal or CV death) to 0.61 (95% CI 0.51–0.72) and for the secondary endpoints of worsening renal function or death from kidney failure [HR 0.56 (95% CI 0.45–0.68)], hospitalization for HF or CV death [HR 0.71 (95% CI 0.55–0.92)] and all-cause mortality [HR 0.69 (95% CI 0.53–0.88)]. These beneficial effects were consistent in patients with and without T2DM. In conclusion, SGLT2 inhibitors offer CV and kidney protection in both diabetic and non-diabetic CKD and, additionally, improve glycaemic control in T2DM, making them first-line therapy for CKD independent from diabetic status.
Keywords: chronic kidney disease, clinical trials, mortality, outcomes, SGLT2 inhibitor
Chronic kidney disease (CKD) is one of the fastest-growing global causes of death, projected to become the fifth leading global cause of death by 2040, while in countries with long life expectancy it may become the second leading cause of death before the end of the century [1, 2]. Diabetic kidney disease (DKD) is the most common cause of CKD and, together with ageing, hypertension and obesity, is thought to be a key contributor to the increasing prevalence and mortality from CKD. Indeed, hypertension is currently the second most common cause of CKD. Of note, >90% of patients with diabetes and almost all patients with diabetic CKD are also hypertensive. In this situation, drugs that improve glycaemic control and simultaneously decrease blood pressure and weight would be welcomed. Furthermore, since one of the key features of CKD is increased cardiovascular (CV) mortality [3], if such drugs also decrease CV events and mortality, they could go a long way towards decreasing the health impact of CKD. Finally, the current cornerstone of CKD therapy and one of the cornerstones of CV disease therapy is blockade of the renin–angiotensin system (RAS), but the associated trend towards hyperkalaemia limits their optimal use in everyday practice [4]. Thus it would be helpful if such drugs would help control hyperkalaemia. For several years now we have known that sodium–glucose co-transporter-2 (SGLT2) inhibitors possess many of these characteristics. Recent and accumulating evidence suggests that they may also decrease the significant residual CV and renal risk for diabetic and non-diabetic CKD patients treated by the current standard of RAS blockade.
SGLT2 INHIBITORS IMPROVE KIDNEY AND CV OUTCOMES IN T2DM
In CV safety trials, SGLT2 inhibitors improved CV outcomes in patients with type 2 diabetes mellitus (T2DM) [5]. Moreover, as antidiabetic drugs, they improved metabolic control. In addition, they decreased blood pressure and weight, likely because they promote the urinary excretion of sodium and calories from glucose since SGLT2 reabsorbs both from the glomerular ultrafiltrate. This iso-osmotic diuresis also leads to improved control of hyperkalaemia. On top of these advantages, CV safety trials suggested a nephroprotective effect of SGLT2 inhibitors in T2DM patients at high CV risk (reviewed in Górriz et al. [6]). Nephroprotection was clearly confirmed for T2DM patients with DKD in the Canagliflozin and Renal Events in Diabetes and Nephropathy Clinical Evaluation (CREDENCE) trial [6–8]. In patients with an estimated glomerular filtration rate (eGFR) of 30–<90 mL/min/1.73 m2 and a urinary albumin:creatinine ratio (UACR) of 300–5000 mg/g on RAS blockade, canagliflozin reduced by 30% the relative risk of a composite primary outcome of end-stage kidney disease (dialysis, transplantation or a sustained eGFR of <15 mL/min/1.73 m2), doubling of the serum creatinine level or death from renal or CV causes {hazard ratio [HR] 0.70 [95% confidence interval (CI) 0.59–0.82]}. This risk reduction was consistent for the different components of the primary endpoint as well as for CV death, myocardial infarction or stroke [HR 0.80 (95% CI 0.67–0.95), P = 0.01] and hospitalization for heart failure [HF; HR 0.61 (95% CI 0.47–0.80), P < 0.001], thus confirming in a CKD population the benefits observed in patients at high CV risk. Interestingly, while the antidiabetic effect of SGLT2 inhibitors decreases as eGFR decreases, since the total amount of glucose excreted decreases, the CV and kidney benefit is still observed at the lower end of the eGFR spectrum of patients enrolled in major clinical trials [9]. Furthermore, although there is discussion about the precise mechanisms responsible for kidney and heart protection by SGLT2 inhibitors, these drugs reduce intraglomerular pressure in individuals with and without diabetes, as glycosuria is also observed in non-diabetic individuals, since plasma glucose is continuously filtered by glomeruli and reabsorbed completely by proximal tubules independent of the presence of diabetes [10]. Besides decreasing single nephron GFR and promoting natriuresis, this mechanism decreases the proximal tubular workload, thus protecting tubules that may already be compromised by decreased oxygen availability because of the capillary rarefaction characteristics of CKD [11, 12]. Based on these results and considerations, more recent randomized controlled trials (RCTs) enrolled both diabetic and non-diabetic patients with either HF or CKD (Figure 1).
FIGURE 1.
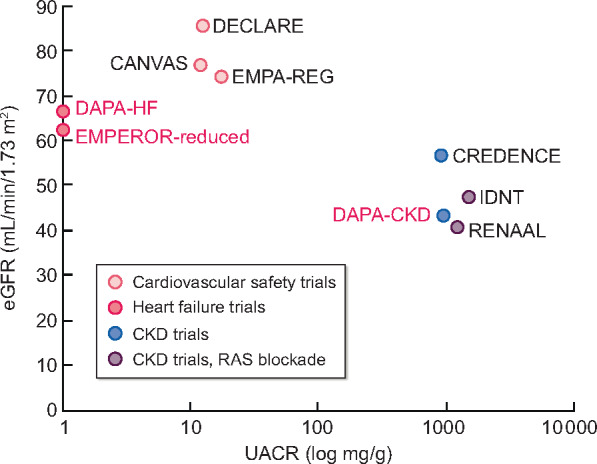
Baseline kidney characteristics for RCTs assessing RAS blockade in CKD as well as RCTs assessing different aspects of SGLT2 inhibitors: CV safety in T2DM, kidney protection in T2DM, HF outcomes in HF patients with and without T2DM and kidney protection in CKD patients with and without T2DM. RCTs assessing patients with and without T2DM are labeled in red. The following SGLT2 inhibitors were tested: canagliflozin (CANVAS and CREDENCE), dapagliflozin (DECLARE, DAPA-HF and DAPA-CKD) and empagliflozin (EMPA-REG and EMPEROR-reduced). No baseline albuminuria data were available for DAPA-HF and EMPEROR-reduced.
SGLT2 INHIBITORS IMPROVE CV OUTCOMES ALSO IN NON-DIABETIC PATIENTS WITH HF
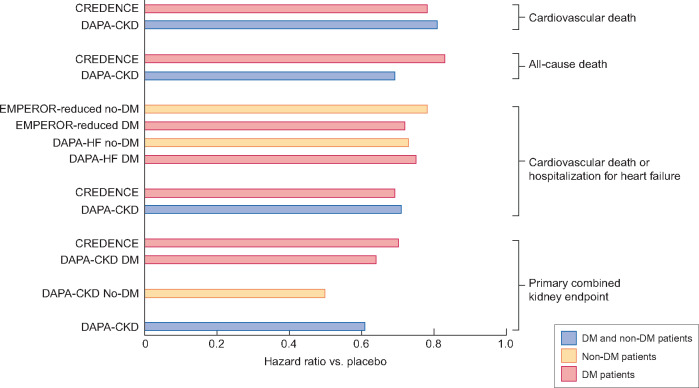
The first large Phase 3 trial of SGLT2 inhibitors enrolling non-diabetic patients in addition to diabetics was the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial [13]. DAPA-HF enrolled 4744 patients with HF and low ejection fraction with or without diabetes with an eGFR ≥30 mL/min/1.73 m2. Over a median of 18.2 months, the HR for the primary composite outcome of worsening HF or CV death was 0.74 (95% CI 0.65–0.85) (Figure 2). A reduction of worsening HF [HR 0.70 (95% CI 0.59–0.83)], death from CV causes [HR 0.82 (95% CI 0.69–0.98)] and all-cause death [HR 0.83 (95% CI 0.71–0.97)] was noted. Findings were similar for patients with or without diabetes (HR of primary outcome 0.75 and 0.73, respectively). However, serum creatinine increased more up to 8 months in patients on dapagliflozin (0.07 ± 0.24 versus 0.04 ± 0.25 mg/dL; P < 0.007). Given the short follow-up, this may be attributed to decreased glomerular hyperfiltration. Indeed, a post hoc analysis disclosed that the slope of the eGFR assessed from Day 14 after the introduction of dapagliflozin was −1.09 (95% CI −1.41 to −0.78) mL/min/1.73 m2/year for dapagliflozin versus −2.87 (95% CI −3.19 to −2.55) for placebo (P < 0.01), while the risk of doubling of serum creatinine was reduced with the SGLT2 inhibitor [HR 0.56 (95% CI 0.39–0.83)] and the difference between placebo and dapagliflozin increased with time [14]. Additionally, similar cardiac protection was observed for patients with eGFR <60 or ≥60 mL/min/1.73 m2; the HR for the primary outcome was 0.72 (0.59–0.86) and 0.76 (0.63–0.92), respectively [13].
FIGURE 2.
Key outcomes of RCTs assessing SGLT2 inhibitors in patients with HF or CKD. Results are presented for the full population, which in some RCTs (DAPA-HF, DAPA-CKD and EMPEROR-reduced) was comprised of patients with T2DM and non-diabetic individuals and separately for diabetics and non-diabetics. The primary combined kidney endpoint in CREDENCE and DAPA-CKD consisted of CKD progression, as assessed by a doubling of serum creatinine (CREDENCE) or ≥50% eGFR decline (DAPA-CKD), end-stage kidney disease, renal death or CV death. The endpoint of CV death or hospitalization for HF was the primary endpoint in HF trials (DAPA-HF and EMPEROR-reduced). The following SGLT2 inhibitors were tested: canagliflozin (CREDENCE), dapagliflozin (DAPA-HF and DAPA-CKD) and empagliflozin (EMPEROR-reduced).
SGLT2 INHIBITORS IMPROVE KIDNEY OUTCOMES IN NON-DIABETIC PATIENTS WITH HF
In August 2020, the results of two new trials testing SGLT2 inhibitors in diabetic and non-diabetic patients with HF or CKD were reported [15, 16]. The EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced) trial tested empagliflozin in patients with HF [15], while the Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA-CKD) tested dapagliflozin in patients with CKD [16]. In addition, a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials has been published [17], as well as a small crossover RCT of dapagliflozin in non-diabetic CKD (DIAMOND) [18].
The EMPEROR-Reduced trial enrolled 3730 patients with HF and an eGFR ≥20 mL/min/1.73 m2 who were followed for a median of 16 months [15]. It essentially reproduced the beneficial findings observed in DAPA-HF, with an HR for the primary endpoint of worsening HF or CV death of 0.75 (95% CI 0.65–0.86) (Figure 2), although the decrease in CV or all-cause mortality did not reach statistical significance. A careful analysis should address the reason for this discrepancy regarding the impact on mortality between the two HF trials of SGLT2 inhibitors. Again, there was no difference between diabetic and non-diabetic patients for the primary outcome (HR 0.72 and 0.78, respectively). With regards to renal outcomes, the mean slope of the eGFR was lower in patients on empagliflozin (–0.55 ± 0.23 versus –2.28 ± 0.23 mL/min/1.73 m2/year; P < 0.001) and in another prespecified analysis the HR for a composite renal outcome (dialysis or renal transplantation or a sustained reduction of eGFR defined in several ways according to baseline eGFR) was 0.50 (95% CI 0.32–0.77), although this outcome was uncommon, occurring in 3.1% of placebo patients. It is of interest that the EMPEROR-Reduced trial lowered the eGFR threshold at which SGLT2 inhibitors offer CV and kidney benefit to 20 mL/min/1.73 m2.
The meta-analysis of the EMPEROR-Reduced and DAPA-HF trials confirmed the CV and renal benefits [17]. Of interest, a reduction in the risk of worsening HF or CV death was also observed in patients with CKD and eGFR <60 mL/min/1.73 m2 [HR 0.77 (95% CI 0.68–0.88)] and in the elderly [HR for those ≥75 years of age 0.77 (95% CI 0.64–0.92)] and was similar for diabetic [HR 0.74 (95% CI 0.65–0.84)] and non-diabetic [HR 0.75 (95% CI 0.65–0.87)] individuals. However, the most interesting analysis was related to safety: the incidence of severe hypoglycaemia, fractures, ketoacidosis, lower limb amputation and Fournier’s gangrene was minimal (≤0.7% for every individual event except for fractures, which was ≤2.7%) and did not differ between SGLT2 inhibitors and placebo.
SGLT2 INHIBITORS ADDITIONALLY IMPROVE KIDNEY AND CV OUTCOMES IN NON-DIABETIC PATIENTS WITH CKD
The most interesting data relate to DAPA-CKD trial [16, 19, 20]. As in the case of the other large trial with a primary kidney endpoint (CREDENCE) [8], DAPA-CKD was stopped early for overwhelming efficacy. DAPA-CKD enrolled 4304 CKD patients with an eGFR of 25–75 mL/min/1.73 m2 and albuminuria of 200–5000 mg/g, 2906 (68%) of them with T2DM, with a mean age of 62 years and a median follow-up of 2.4 years [16, 20]. The mean baseline eGFR was 43.1 (standard deviation 12.4) mL/min/1.73 m2 and 14.5% of patients had an eGFR <30 mL/min/1.73 m2. The median baseline albuminuria was 949 mg/g and 90% of patients had albuminuria >300 mg/g. The cause of CKD was ischaemic/hypertensive nephropathy in 16%, immunoglobulin A (IgA) nephropathy in 6% and focal segmental glomerulosclerosis in 3% and it had been confirmed by biopsy in 20% of patients. An overwhelming majority (97%) were on RAS blockade. Dapagliflozin reduced the risk of the primary combined endpoint of >50% eGFR decline, onset of ESRD or renal or CV death [HR 0.61 (95% CI 0.51–0.72)] (Figure 2). The number needed to treat to avoid one primary event was low at 19. The benefit of dapagliflozin on the primary endpoint was consistent in patients with and without T2DM [HR 0.64 (95% CI 0.52–0.79) and 0.50 (0.35–0.72), respectively; P for interaction = 0.24]. It was also observed in patients with an eGFR <45 or >45 mL/min/1.73 m2 and with albuminuria <1000 or >1000 mg/g, i.e. in patients with different stages of CKD and different severity of albuminuria. Overall, the results were in the range found for canagliflozin in CREDENCE in DKD [8]. There were no statistically significant differences in DAPA-CKD in the primary endpoint between diabetics and non-diabetics, however, the HR was 22% lower for non-diabetics. Thus the kidney benefit afforded by dapagliflozin was at least as large in patients with non-DKD (including ischaemic/hypertensive nephropathy, IgA nephropathy and focal segmental glomerulosclerosis, among others) than in DKD. Additionally, dapagliflozin reduced all three secondary endpoints compared with placebo: worsening renal function or death from kidney failure [HR 0.56 (95% CI 0.45–0.68)], hospitalization for HF or CV death [HR 0.71 (95% CI 0.55–0.92)] and all-cause mortality [HR 0.69 (95% CI 0.53–0.88)]. Dapagliflozin was also found to be safe in patients with CKD, with no differences compared with placebo in the incidence of severe hypoglycaemia, fractures, ketoacidosis and lower limb amputation, which were all uncommon. Diabetic ketoacidosis was not reported in any patient randomized to dapagliflozin and occurred in two patients in the placebo group, while severe hypoglycaemia was not observed in patients without T2DM. Data on urinary tract infections were not presented. In this regard, data from major outcome trials suggest that SGLT2 inhibitors are not associated with an increased risk of urinary tract infections [21].
An interesting piece of information is provided by the DIAMOND trial, although it had a small sample size and short follow-up [18]. In this crossover trial, 53 non-diabetic proteinuric (500–3500 mg/24 h) CKD patients with a mean eGFR of 58 ± 23 mL/min/1.73 m2 were randomly assigned to dapagliflozin–placebo or placebo–dapagliflozin. Although a transient reduction in eGFR of –6.6 mL/min/1.73 m2 (95% CI –9.0 to –4.2; P < 0.0001) was observed at Week 6 on dapagliflozin, there was no impact on proteinuria [difference in proteinuria change from baseline between dapagliflozin and placebo of 0.9% (95% CI –16.6–22.1; P = 0.93)], thus dissociating a haemodynamic impact on eGFR from an impact on proteinuria. Results on albuminuria and proteinuria for non-diabetic patients enrolled in DAPA-CKD are awaited, as this may provide clues as to the mechanism of nephroprotection afforded by SGLT2 inhibitors.
CRYSTAL BALL SGLT2-INOMICS
In conclusion, SGLT2 inhibitors appear to be primarily nephroprotective and cardioprotective agents that additionally improve glycaemic control in those T2DM patients who have better preserved kidney function. Large RCTs in diabetic and non-diabetic CKD patients on RAS blockade have shown that they slow kidney disease progression, decrease CV events and improve survival independent of the presence of diabetes. Thus the benefits appear to extend beyond diabetes to include patients with the second (hypertension/vascular nephropathy) and third (glomerulonephritis) most common causes of CKD, although subgroup analyses are awaited to confirm this. Additionally, and at least for HF, the benefits extend to old age, again pending detailed analysis of CKD trials. Finally, in more recent trials, SGLT2 inhibitors appear safer than in initial trials, likely because of better physician handling of SGLT2 inhibitors and concomitant drug prescription. In fact, they are as safe or even safer for certain adverse effects as a placebo. A further RCT of SGLT2 inhibitors in diabetics and non-diabetics with CKD is ongoing, testing empagliflozin. The Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY) will be a larger trial that will also enroll patients with Type 1 diabetes (T1DM) in addition to T2DM and non-diabetics and will have a lower eGFR entry criteria (20–90 mL/min/1.73 m2) than other CKD trials and will not require pathological albuminuria to enroll patients with low eGFR (20–<45 mL/min/1.73 m2), potentially expanding the population that benefits from SGLT2 inhibition if it meets the primary endpoint [9] (Figure 3). Of note, nephroprotection was also observed in HF trials in which pathological albuminuria was not an inclusion requirement. What is next?
FIGURE 3.
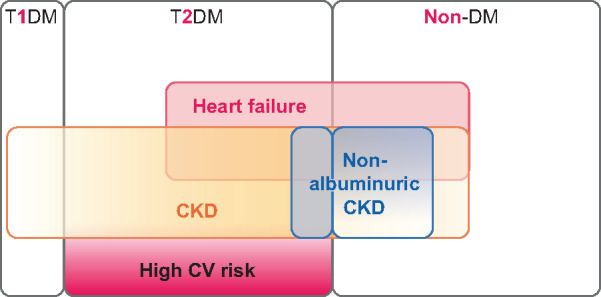
Current status and key future developments in the study of SGLT2 inhibitors and CV and kidney benefit. Coloured parts indicate populations in whom cardio- and nephroprotection have been demonstrated for SGLT2 inhibitors: dark red for high CV risk, light brown for CKD and light red for HF. Thus colours highlight high CV risk, HF and CKD patients and their overlaps within the T2DM and the non-diabetic populations. Additionally, non-albuminuric CKD is indicated in green when CV and kidney benefit has been demonstrated in large clinical trials, as for non-albuminuric CKD in T2DM patients within HF and high CV risk trials or non-diabetic patients with HF. However, no data are available regarding CV or kidney benefit in non-diabetic patients with CKD and an absence of HF, thus a white (non-coloured) spot is placed over patients with non-albuminuric CKD who are not diabetic and do not have HF. Additionally, there is no information on potential CV or renal benefits in T1DM patients. EMPA-REG will expand data from currently available trial results by exploring T1DM and non-albuminuric CKD in persons without diabetes. Of note, CV and kidney benefit has been observed up to now for populations with an eGFR ≥20 mL/min/1.73 m2 in the context of HF and EMPA-REG will also enroll CKD patients with an eGFR ≥20 mL/min/1.73 m2.
Guidelines should be updated to include SGLT2 inhibitor as first-line nephroprotective therapy on top of RAS blockade for any CKD, or at least for the causes of CKD tested in DAPA-CKD. Further RCTs focused on specific causes of CKD should confirm their efficacy in those, likely starting with IgA nephropathy and focal segmental glomerulosclerosis, which, although present in the DAPA-CKD trials, were clearly underrepresented. In this regard, the whole therapeutic approach to IgA nephropathy is being re-evaluated and draft Kidney Disease: Improving Global Outcomes (KDIGO) guidelines emphasize the need to enroll patients in clinical trials. Another issue would be how to integrate SGLT2 inhibitors with forthcoming broad nephroprotective drugs, such as finerenone, as kidney RCTs such asFIDELIO and FIGARO will be reporting later this year and next year [22, 23], sparsentan [24, 25] or bardoxolone [26], among others, which are undergoing RCTs in diverse nephropathies. For future trials, the question will be whether the addition of a new treatment to the standard therapy with RAS blockade and SGLT2 inhibitors is beneficial compared with such standard treatments in renal progression. In the meantime, if RCTs for novel drugs being compared with RAS blockade are positive, how would they be used in clinical practice? Added on top of RAS blockade plus SGLT2 inhibitors? Replace RAS blockade for sparsentan? Should novel RCTs address how they interact with SGLT2 inhibition? Finally, will all doomsday scenarios regarding the increasing contribution of CKD to global mortality need to be rewritten? This will also depend on the speed of the uptake of the drugs by physicians and a key issue will be a cost. Given the large size of the target population, it would be reasonable to lower the cost of SGLT2 inhibitors as far as possible to facilitate worldwide access to these lifesaving medications.
FUNDING
Funding was provided by FIS/Fondos FEDER (PI17/00257, PI18/01386, PI19/00588, PI19/00815), DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
CONFLICT OF INTEREST STATEMENT
A.O. is a consultant for Sanofi Genzyme and has received speaker fees or travel support from AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma and is director of the Catedra Mundipharma-UAM of DKD. J.F.N.G. has served as a consultant and has received speaker fees or travel support from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Esteve, Genzyme, Eli Lilly, Novartis, Servier, Shire and Vifor Fresenius Medical Care Renal Pharma. P.S. is an advisor/speaker for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Elpen Pharmaceuticals, Genesis Pharma, Menarini, Innovis Pharma and Winmedica and has received research support for an investigator-initiated study from AstraZeneca. J.L.G. has served as a consultant for Boehringer Ingelheim, Mundipharma, AstraZeneca, Novo Nordisk and has received speaker honoraria from Boehringer Ingelheim, Mundipharma, AstraZeneca, Novo Nordisk, Novartis and Eli Lilly. M.J.S. is a consultant for Novo Nordisk and received speaker fees from Janssen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, Mundipharma, Fresenius Medical Care and Esteve. B.F.F. reports speaker fees or travel support from AbbVie, AstraZeneca, Boehringer Ingelheim, Esteve, Menarini, Mundipharma, Novartis and Novo Nordisk, outside the submitted work.
REFERENCES
- 1. Foreman KJ, Marquez N, Dolgert A. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ortiz A, Sanchez-Niño MD, Crespo-Barrio M. et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019; 39: 29–34 [DOI] [PubMed] [Google Scholar]
- 3. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E. et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreira JP, Butler J, Rossignol P. et al. Abnormalities of potassium in heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75: 2836–2850 [DOI] [PubMed] [Google Scholar]
- 5. Sarafidis P, Ferro CJ, Morales E. et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019; 34: 208–230 [DOI] [PubMed] [Google Scholar]
- 6. Górriz JL, Navarro-González JF, Ortiz A. et al. Sodium-glucose cotransporter 2 inhibition: towards an indication to treat diabetic kidney disease. Nephrol Dial Transplant 2020; 35: i13–i23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez-Fernandez B, Fernandez-Prado R, Górriz JL. et al. Canagliflozin and renal events in diabetes with established nephropathy clinical evaluation and study of diabetic nephropathy with Atrasentan: what was learned about the treatment of diabetic kidney disease with canagliflozin and atrasentan? Clin Kidney J 2019; 12: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 9. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrannini E, Baldi S, Frascerra S. et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016; 65: 1190–1195 [DOI] [PubMed] [Google Scholar]
- 11. Satirapoj B, Korkiatpitak P, Supasyndh O.. Effect of sodium-glucose cotransporter 2 inhibitor on proximal tubular function and injury in patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J 2019; 12: 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Afsar B, Afsar RE, Dagel T. et al. Capillary rarefaction from the kidney point of view. Clin Kidney J 2018; 11: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMurray JJV, Solomon SD, Inzucchi SE. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008 [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJV. Presented at the European Society of Cardiology Congress, August 29, 2020; https://esc2020.escardio.org/detail/video/ref: S31328?_ga=2.107325186.1440733159.1598629931-849659886.1598629931 (30 August 2020, date last accessed)
- 15. Packer M, Anker SD, Butler J. et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 16. Heerspink HL. Dapagliflozin in patients with chronic kidney disease DAPA-CKD. Presented at the European Society of Cardiology Meeting 2020. https://esc2020.escardio.org/detail/video/ref: S31328?_ga=2.77892628.1440733159.1598629931-849659886.1598629931 (20 August 2020, date last accessed) [Google Scholar]
- 17. Zannad F, Ferreira JP, Pocock SJ. et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trial. Lancet 2020; doi: 10.1016/S0140-6736(20)31824-9 [DOI] [PubMed] [Google Scholar]
- 18. Cherney DZI, Dekkers CCJ, Barbour SJ. et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 2020; 8: 582–593 [DOI] [PubMed] [Google Scholar]
- 19. Heerspink HJL, Stefansson BV, Chertow GM. et al. ; for the DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020; 35: 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wheeler DC, Stefansson BV, Batiushin M. et al. The Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020; 10.1093/ndt/gfaa234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarafidis PA, Ortiz A.. The risk for urinary tract infections with sodium-glucose cotransporter 2 inhibitors: no longer a cause of concern? Clin Kidney J 2019; 13: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bakris GL, Agarwal R, Anker SD. et al. Design and baseline characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease trial. Am J Nephrol 2019; 50: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruilope LM, Agarwal R, Anker SD. et al. Design and baseline characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial. Am J Nephrol 2019; 50: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trachtman H, Nelson P, Adler S. et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol 2018; 29: 2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Komers R, Diva U, Inrig JK. et al. Study design of the phase 3 sparsentan versus irbesartan (DUPLEX) study in patients with focal segmental glomerulosclerosis. Kidney Int Rep 2020; 5: 494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nangaku M, Kanda H, Takama H. et al. Randomized clinical trial on the effect of bardoxolone methyl on GFR in diabetic kidney disease patients (TSUBAKI study). Kidney Int Rep 2020; 5: 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]