Abstract
Purpose
This study aimed to present a single institutional experience with BRCA1/2 gene tests and the effects of pathogenic mutations in epithelial peritoneal, ovarian, and fallopian tube cancer (POFTC) on survival outcomes.
Materials and Methods
We identified patients with epithelial POFTCs who underwent BRCA1/2 gene testing by either germline or somatic methods between March 2007 and March 2020. Based on the BRCA1/2 test results, patients were divided into BRCA mutation and wild-type groups, followed by comparisons of clinicopathologic characteristics and survival outcomes after primary treatment.
Results
The annual number of POFTC patients who received BRCA1/2 gene tests increased gradually. In total, 511 patients were included and BRCA1/2 mutations were observed in 143 (28.0%). Among 57 patients who received both germline and somatic tests, three (5.3%) showed discordant results from the two tests. Overall, no differences in progression-free survival (PFS; p=0.467) and overall survival (p=0.641) were observed between the BRCA mutation and wild-type groups; however, multivariate analyses identified BRCA1/2 mutation as an independent favorable prognostic factor for PFS (adjusted hazard ratio [aHR], 0.765; 95% confidence interval [CI], 0.593 to 0.987; p=0.040). In 389 patients with International Federation of Gynecology and Obstetrics stage III-IV, different results were shown depending on primary treatment strategy: while BRCA1/2 mutation significantly improved PFS in the subgroup of neoadjuvant chemotherapy (aHR, 0.619; 95% CI, 0.385 to 0.995; p=0.048), it did not affect patient PFS in the subgroup of primary debulking surgery (aHR, 0.759; 95% CI, 0.530 to 1.089; p=0.135).
Conclusion
BRCA1/2 mutations are frequently observed in patients with epithelial POFTCs, and such patients showed better PFS than did those harboring wild-type BRCA1/2.
Keywords: Genital neoplasms, Female, Ovarian neoplasms, Germline test, Somatic test, BRCA1/2 mutation, Clinical outcome, Survival outcome
Introduction
Ovarian cancer is the most lethal gynecologic malignancy and is estimated to account for 295,000 new cases and 185,000 cancer deaths annually worldwide [1]. Recent studies view epithelial peritoneal, ovarian, and fallopian tube cancers (POFTCs) as a single disease group that shares a common pathogenesis, diagnosis, and treatment [2]. Epithelial POFTCs tend to be diagnosed at an advanced-stage and show high recurrence and mortality rates, despite the standard primary treatment. Approximately 15% to 20% of patients with epithelial POFTCs present genetic predisposition or hereditary factors, with BRCA1/2 identified as well-known causal genes [3,4].
Women harboring germline mutations in either BRCA1/2 are at an excessive risk of developing both breast cancer (BC) and ovarian cancer [5,6]. Offspring of a germline BRCA1/2-mutation carrier have a 50% chance of inheriting the pathogenic or likely pathogenic variant. Moreover, patients harboring germline or somatic BRCA1/2 mutations with primary or platinum-sensitive relapsed POFTC experience positive survival outcomes from poly(ADP-ribose) polymerase (PARP) inhibitors based on their synthetic lethality [7-12]. Therefore, current guidelines from the Korean Society of Gynecologic Oncology recommend that patients with epithelial POFTC patients undergo BRCA1/2 gene testing [13].
Previous studies have focused on the prognostic aspect of BRCA1/2 mutations, frequently reporting that BRCA1/2 mutations confer a survival advantage relative to wild-type BRCA1/2 due to better response to platinum-based chemotherapy [14]. However, further analysis revealed that the study populations and designs, as well as the specific results, differ among studies. Although overall survival (OS) was improved in patients carrying BRCA1/2 mutations [14,15], some studies identified advantages for only those harboring BRCA2 mutations [16,17]. In our previous study that included patients with advanced-stage ovarian high-grade serous carcinoma (HGSC), longer progression-free survival (PFS) but not OS was associated with germline BRCA1/2 mutations [18].
Therefore, additional scientific evidence concerning the effects of BRCA1/2 mutations on POFTC prognosis according to the primary treatment strategy is necessary, especially in patients of Korean ethnicity. In this study, we investigated the impact of BRCA1/2 mutational status on survival outcomes in patients with epithelial POFTC. Additionally, we present a single institutional experience with germline and somatic BRCA1/2 gene testing not limited by initial International Federation of Gynecology and Obstetrics (FIGO) stage or histologic type.
Materials and Methods
1. Study population
Since starting germline BRCA1/2 gene testing, our institution has conducted this test in patients with BC presenting a strong family history of BC or with family members harboring BRCA1/2 mutations. In March 2007, patients with epithelial POFTC also began to receive germline BRCA1/2 gene testing. In September 2017, our institutional hospital launched a targeted next-generation sequencing (NGS) cancer panel for clinical purposes, which enabled identification of somatic BRCA1/2 mutational status in patients with epithelial POFTC.
To include all possible cases meeting the study purpose, we established the following inclusion criteria: (1) patients pathologically diagnosed with and treated for epithelial POFTC; and (2) patients who received either germline BRCA1/2 gene testing or a somatic NGS cancer panel between March 2007 and March 2020, and thus whose germline or somatic BRCA1/2 mutational status was verified. By contrast, we excluded patients with insufficient clinicopathologic data or those lost to follow-up during primary treatment.
We identified 563 patients from the Ovarian Cancer Cohort of the institution who met these criteria. For fair comparisons, we further excluded 52 patients who were enrolled in past or current clinical trials, during their primary treatment, which could affect survival outcomes.
2. Germline and somatic BRCA1/2 gene test
Germline BRCA1/2 gene testing methods at the Seoul National University Hospital (SNUH) were described in our previous study [18]. As of February 2016, the method has been changed from direct sequencing (Sanger sequencing) to NGS of BRCA1/2 genes. Sequence variants found in NGS were confirmed by Sanger sequencing.
For somatic BRCA1/2 gene testing, we used an NGS cancer panel named “SNUH FIRST-Cancer panel version 3.1” and performed DNA collection and profiling from archival formalin-fixed paraffin-embedded (FFPE) tumor tissues, as described previously [19]. Briefly, genomic DNA was extracted from FFPE tissues using the ReliaPrep FFPE gDNA miniprep system (Promega, Madison, WI), and a library was constructed using the SureSelectXT target enrichment protocol (Agilent Technologies, Carlsbad, CA) for Illumina paired-end sequencing (2×101 bp), which was performed on the Illumina Hiseq 2500 platform (Illumina, Carlsbad, CA). Details of the reporting algorithms used for single-nucleotide variants, copy number variants, and structural variants were also described previously [19]. The SNUH FIRST-Cancer panel version 3.1 provides information on all exons of 183 genes, specific introns of 23 fusion genes, the TERT promoter region, eight microsatellite-instability markers, and 45 drug-target lesions, covering a total length of approximately 1.949 Mbp. Of these, we focused on genomic alterations of BRCA1/2 genes.
We referenced the detected BRCA1/2 variants in two representative databases, the Breast Cancer Information Core (BIC) and the National Institutes of Health open-access database of clinically observed variants and their classification (ClinVar), and the literature. Sequence variants in BRCA1 and BRCA2 were classified into five categories according to the recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [20]. In the present study, we regarded patients with “pathogenic” and “likely pathogenic” variants as the BRCA mutation group (BRCAmut; study group) and the rest of the patients as the BRCA wild-type group (BRCAwt; control group).
3. Data collection
Review of medical records and pathologic reports allowed collection of the following clinicopathologic data: age at diagnosis, histologic type, FIGO stage, initial serum cancer antigen 125 levels, and primary treatment strategy. We considered optimal debulking to have occurred when the surgery resulted in the largest size of the residual tumor being < 1 cm. All patients received taxane- and platinum-based chemotherapy as part of their primary treatment unless they had low-grade IA/IB disease according to the 2014 FIGO staging system. Additionally, we retrieved personal and familial histories of cancer and the number of affected family members up to the second degree.
For survival analyses, PFS was defined as the time interval between the date of initial diagnosis and the date of disease progression confirmed by the Response Evaluation Criteria in Solid Tumours ver. 1.1 [21]. OS was defined as the time interval between the date of initial diagnosis to the date of cancer-related death or last visit.
4. Statistical analysis
Baseline clinicopathologic characteristics and survival outcomes were compared between the BRCAmut and BRCAwt groups. We used a Student’s t or Mann-Whitney U test for comparisons of continuous variables, and Pearson’s chi-squared or Fisher exact test for comparisons of categorical variables. For survival analyses, the Kaplan-Meier method with log-rank test and Cox proportional hazards regression models were used. We calculated the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for each variable. All statistical analyses were conducted by using SPSS software ver. 25.0 (IBM Corp., Armonk, NY), and a p < 0.05 was regarded as statistically significant.
5. Ethical statement
This retrospective cohort study was approved by the Institutional Review Board of SNUH (No. C-2005-042-1122) and performed in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived.
Results
1. BRCA1/2 gene test results
The annual number of POFTC patients who received BRCA1/2 gene tests increased gradually according to a series of sociomedical environment changes in Korea (Fig. 1). Of 511 patients who underwent BRCA1/2 gene tests (418, 36, and 57 for germline test only, somatic test only, and both tests, respectively), BRCA1/2 mutations were observed in 143 (28.0%), with 20.0% and 8.2% of patients harboring BRCA1 and BRCA2 mutations, respectively. One patient harbored mutations in both genes; however, germline testing identified only a BRCA2 mutation (c.9097dupA), whereas somatic testing identified an additional BRCA1 mutation (c.2206_2207delGA).
Fig. 1.
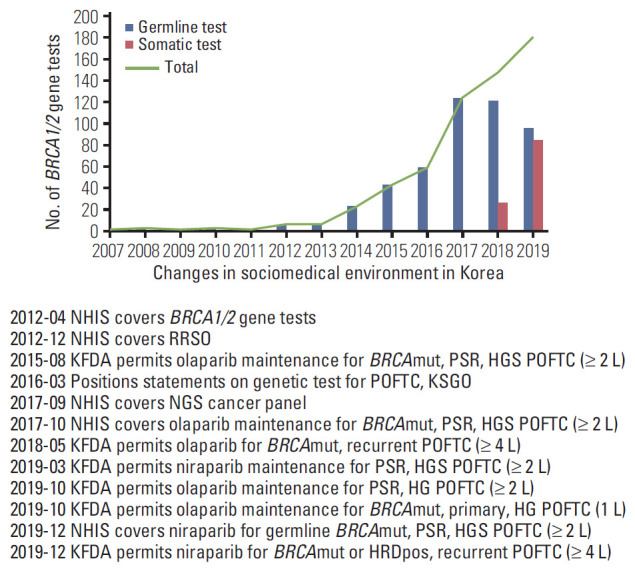
Annual number of BRCA1/2 gene tests among patients with peritoneal, ovarian, and fallopian tube cancers (POFTCs) and according to changes in sociomedical environment in Korea. NHIS, National Health Insurance Service; RRSO, risk reducing salpingo-oophorectomy; KFDA, Korea Food and Drug Administration; PSR, platinum-sensitive relapsed; HGS, high-grade serous; KSGO, Korean Society of Gynecologic Oncology; NGS, next-generation sequencing; HG, high-grade; HRDpos, homologous recombination deficiency-positive.
We observed differential BRCA1/2 mutational status in patients with POFTC according to the presence of BC and/or other cancers, such as colorectal and gastric cancers (S1 Fig.). Although the prevalence of BRCA1/2 mutations was lowest among patients presenting POFTC only (24.9%), it was highest among those presenting POFTC, BC, and another cancer (triple cancers; 75.0%). Of the 54 patients presenting both POFTC and BC, BRCA1/2 mutations were identified in 26 (48.1%).
Among 57 patients who received both germline and somatic tests, three (5.3%) showed discordant results in their classification into the BRCAmut and BRCAwt groups (Fig. 2). Specifically, one patient harboring germline BRCA1 mutation showed restoration of a wild-type BRCA1 sequence according to somatic testing (true reversion), and the other two with germline BRCA1/2 wild-type were identified as harboring somatic BRCA1 mutation (acquired mutation). Details of BRCA1/2 test results and clinical information of the 57 patients are presented in S2 Table.
Fig. 2.
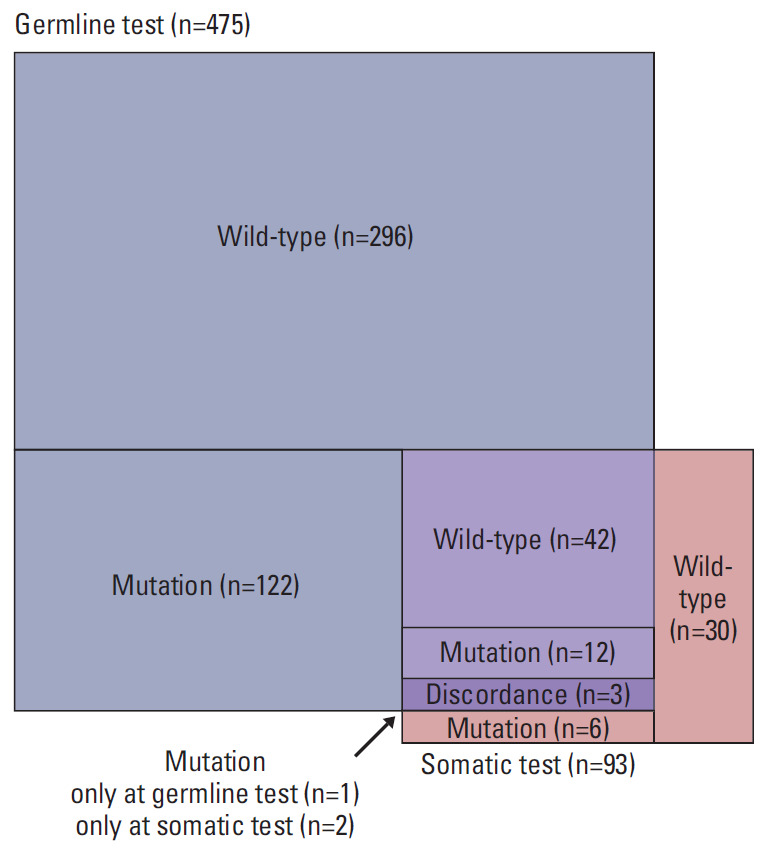
Aerial chart depicting proportion of patients who underwent germline and somatic BRCA1/2 gene tests along with the test results.
2. Characteristics of the study population
Patient characteristics are shown in Table 1. Age at diagnosis of POFTC was similar between the BRCAmut and BRCAwt groups. However, patients with BRCA mutations displayed significantly higher personal and family histories of BC and a higher family history of POFTC relative to those without BRCA mutations. Initial disease presentation also differed between groups, with the BRCAmut group showing more advanced disease and more frequent HGSC histology. In terms of primary treatment, there were no differences in the proportion of neoadjuvant chemotherapy (NAC) cases and residual tumor after debulking surgery between groups. In this study, 5.9% (30/511) of the study population received bevacizumab-containing chemotherapy during primary treatment, and the proportion of bevacizumab users was similar between the BRCAmut and BRCAwt groups. No patient received maintenance with a PARP inhibitor after primary treatment.
Table 1.
Clinicopathologic characteristics of the study population
| Characteristic | Total (n=511) | BRCA wild-type (n=368) | BRCA mutation (n=143) | p-value | |
|---|---|---|---|---|---|
| Age (yr) | 54.3±10.9 | 54.5±11.5 | 53.9±9.3 | 0.557 | |
| Parity | 1.9±1.3 | 1.9±1.3 | 1.9±1.0 | 0.394 | |
| Origin | |||||
| Ovary | 481 (94.1) | 345 (93.8) | 136 (95.1) | 0.705 | |
| Fallopian tube | 14 (2.7) | 10 (2.7) | 4 (2.8) | ||
| Peritoneum | 16 (3.1) | 13 (3.5) | 3 (2.1) | ||
| Hx of BC | 58 (11.4) | 29 (7.9) | 29 (20.3) | < 0.001 | |
| Hx of other cancers | 28 (5.5) | 18 (4.9) | 10 (7.0) | 0.349 | |
| Family Hx of POFTC | 27 (5.3) | 7 (1.9) | 20 (14.0) | < 0.001 | |
| No. of relatives | 0.1±0.2 | 0.0±0.1 | 0.2±0.4 | < 0.001 | |
| Family Hx of BC | 45 (8.8) | 16 (4.3) | 29 (20.3) | < 0.001 | |
| No. of relatives | 0.1±0.4 | 0.1±0.2 | 0.3±0.6 | < 0.001 | |
| Family Hx of other cancers | 111 (21.7) | 74 (20.1) | 37 (25.9) | 0.156 | |
| FIGO stage | |||||
| I | 72 (14.1) | 65 (17.7) | 7 (4.9) | 0.001 | |
| II | 50 (9.8) | 39 (10.6) | 11 (7.7) | ||
| III | 259 (50.7) | 177 (48.1) | 82 (57.3) | ||
| IV | 130 (25.4) | 87 (23.6) | 43 (30.1) | ||
| Histology | |||||
| High-grade serous | 368 (72.0) | 246 (66.8) | 122 (85.3) | 0.001 | |
| Low-grade serous | 11 (2.2) | 10 (2.7) | 1 (0.7) | ||
| Endometrioid | 43 (8.4) | 37 (10.1) | 6 (4.2) | ||
| Mucinous | 16 (3.1) | 13 (3.5) | 3 (2.1) | ||
| Clear cell | 42 (8.2) | 40 (10.9) | 2 (1.4) | ||
| Mixed | 14 (2.7) | 10 (2.7) | 4 (2.8) | ||
| Others | 8 (1.6) | 6 (1.6) | 2 (1.4) | ||
| Unknown | 9 (1.8) | 6 (1.6) | 3 (2.1) | ||
| Tumor grade | |||||
| 1 | 30 (5.9) | 27 (7.3) | 3 (2.1) | 0.027 | |
| 2 | 28 (5.5) | 23 (6.3) | 5 (3.5) | ||
| 3 | 438 (85.7) | 306 (83.2) | 132 (92.3) | ||
| Unknown | 15 (2.9) | 12 (3.3) | 3 (2.1) | ||
| CA-125 (IU/mL) | 695.5 (3.4-17,313) | 666.5 (3.4-15,700) | 767.0 (5.1-17,313) | 0.085 | |
| Primary treatment strategy | |||||
| PDS | 379 (74.2) | 278 (75.5) | 101 (70.6) | 0.255 | |
| NAC | 132 (25.8) | 90 (24.5) | 42 (29.4) | ||
| Residual tumor after PDS/IDSa) | |||||
| No gross | 374 (73.2) | 278 (75.5) | 96 (67.1) | 0.224 | |
| < 1 cm | 70 (13.7) | 48 (13.0) | 22 (15.4) | ||
| 1-2 cm | 23 (4.5) | 13 (3.5) | 10 (7.0) | ||
| ≥ 2 cm | 22 (4.3) | 17 (4.6) | 5 (3.5) | ||
| Unknown | 13 (2.5) | 6 (1.6) | 7 (4.9) | ||
| Chemotherapy at primary treatment | |||||
| Bevacizumab-containing regimen | 30 (5.9) | 19 (5.2) | 11 (7.7) | 0.362 | |
| Non-bevacizumab regimen | 464 (90.8) | 335 (91.0) | 129 (90.2) | ||
| No chemotherapy | 17 (3.3) | 14 (3.8) | 3 (2.1) | ||
| Recurrenceb) | 324 (63.4) | 231 (62.8) | 93 (65.0) | 0.634 | |
| PSRc) | 238 (46.6) | 156 (42.4) | 82 (57.3) | 0.001 | |
| PRR | 78 (15.3) | 67 (18.2) | 11 (7.7) | ||
| Genetic test methods | |||||
| Germline only | 418 (81.8) | 296 (80.4) | 122 (85.3) | 0.264 | |
| Somatic only | 36 (7.0) | 30 (8.2) | 6 (4.2) | ||
| Both | 57 (11.2) | 42 (11.4) | 15 (10.5) | ||
| BRCA1 mutational status | |||||
| Wild-type | 409 (80.0) | 368 (100) | 41 (28.7) | < 0.001 | |
| Mutation | 102 (20.0) | 0 | 102 (71.3) | ||
| BRCA2 mutational status | |||||
| Wild-type | 469 (91.8) | 368 (100) | 101 (70.6) | < 0.001 | |
| Mutation | 42 (8.2) | 0 | 42 (29.4) | ||
Values are presented as mean±SD, number (%), or median (range). Hx, history; BC, breast cancer; POFTC, peritoneal, ovarian, and fallopian tubal cancers; FIGO, International Federation of Gynecology and Obstetrics; CA-125, cancer antigen 125; PDS, primary debulking surgery; NAC, neoadjuvant chemotherapy; IDS, interval debulking surgery; PSR, platinum-sensitive recurrence; PRR, platinum-resistant recurrence; SD, standard deviation.
Nine patients did not receive debulking surgery,
Among the recurred, eight patients did not receive taxane- and platinum-based chemotherapy before,
PSR was defined as relapse ≥ 6 months after completion of taxane- and platinum-based chemotherapy, whereas PRR as relapse < 6 months.
BRCA1/2 mutations were observed in 33.2% of patients with HGSC (n=368), a higher percentage than in the whole study population. As shown in S3 Table, patient characteristics were similar between the BRCAmut and BRCAwt groups, except for patient age, personal history of BC, and family history of BC and POFTC. In patients who had histologic types other than HGSC (non-HGSC, n=134), incidence of BRCA1/2 mutation was 13.4%. As shown in S4 Table, patient characteristics, such as primary treatment strategy and residual tumor after debulking surgery, were similar between the two groups, whereas family history of BC and POFTC differed.
3. Clinical outcomes of all study populations
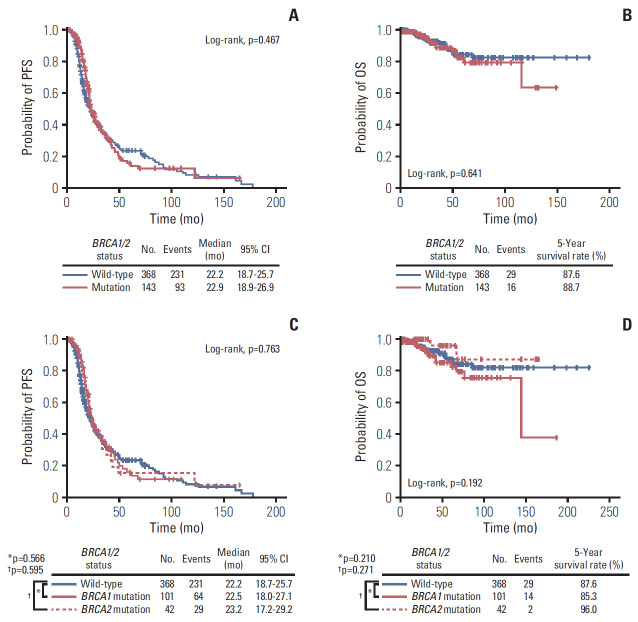
During the median observation period of 42.8 months, 93 patients (65.0%) in the BRCAmut group and 231 (62.8%) in the BRCAwt group experienced disease recurrence. Despite the higher proportion of platinum-sensitive recurrence in the BRCAmut group (p=0.001), which referred to recurrence within 6 months after completion of platinum-based primary treatment, the two groups showed similar PFS (median, 22.9 vs. 22.2 months; p=0.467) (Fig. 3A). However, multivariate analyses adjusting for age, FIGO stage, histologic type, primary treatment strategy, and residual tumor after debulking surgery revealed BRCA1/2 mutation as an independent favorable prognostic factor for PFS (aHR, 0.765; 95% CI, 0.593 to 0.987; p=0.040) (Table 2). Both BRCAmut and BRCAwt groups showed similar OS (5-year survival rate, 88.7% vs. 87.6%; p=0.641) (Fig. 3B), and multivariate analyses revealed that presence of BRCA1/2 mutations did not affect patient OS (Table 2). Use of bevacizumab in primary treatment did not improve patient PFS and OS in univariate and multivariate analyses.
Fig. 3.
Survival outcomes of the study population (A, B), and further comparisons according to the mutated BRCA gene (C, D). (A, C) Progression-free survival (PFS). (B, D) Overall survival (OS).
Table 2.
Factors associated with survival outcomes
| Characteristic | Progression-free survival |
Overall survival |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
||||||||||
| HR | 95% CI | p-value | aHR | 95% CI | p-value | HR | 95% CI | p-value | aHR | 95% CI | p-value | ||
| Age (yr) | |||||||||||||
| < 55 | 1 | 1 | 1 | 1 | |||||||||
| ≥ 55 | 1.590 | 1.273-1.985 | < 0.001 | 1.325 | 1.043-1.683 | 0.021 | 2.237 | 1.218-4.107 | 0.009 | 1.704 | 0.904-3.211 | 0.099 | |
| FIGO stage | |||||||||||||
| I-II | 1 | 1 | 1 | 1 | |||||||||
| III | 2.435 | 1.746-3.397 | < 0.001 | 2.296 | 1.563-3.371 | < 0.001 | 1.502 | 0.613-3.678 | 0.374 | 1.078 | 0.378-3.079 | 0.888 | |
| IV | 3.568 | 2.478-5.139 | < 0.001 | 2.637 | 1.681-4.138 | < 0.001 | 2.768 | 1.068-7.176 | 0.036 | 1.490 | 0.465-4.775 | 0.502 | |
| Histology | |||||||||||||
| High-grade serous | 1 | 1 | 1 | 1 | |||||||||
| Non–high-grade serous | 0.620 | 0.472-0.813 | 0.001 | 1.085 | 0.795-1.483 | 0.607 | 0.803 | 0.396-1.625 | 0.541 | 1.219 | 0.545-2.724 | 0.630 | |
| Primary treatment strategy | |||||||||||||
| PDS | 1 | 1 | 1 | 1 | |||||||||
| NAC | 2.103 | 1.657-2.670 | < 0.001 | 1.511 | 1.131-2.019 | 0.005 | 2.071 | 1.117-3.837 | 0.021 | 2.003 | 0.969-4.142 | 0.061 | |
| Residual tumor after PDS/IDS | |||||||||||||
| < 1 cm | 1 | 1 | 1 | 1 | |||||||||
| ≥ 1 cm | 1.738 | 1.229-2.457 | 0.002 | 1.310 | 0.894-1.918 | 0.166 | 2.729 | 1.381-5.395 | 0.004 | 2.676 | 1.261-5.678 | 0.010 | |
| BRCA mutational status | |||||||||||||
| Wild-type | 1 | 1 | 1 | 1 | |||||||||
| Mutation | 0.914 | 0.718-1.164 | 0.467 | 0.765 | 0.593-0.987 | 0.040 | 1.156 | 0.627-2.131 | 0.642 | 1.163 | 0.623-2.171 | 0.636 | |
HR, hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio; FIGO, International Federation of Gynecology and Obstetrics; PDS, primary debulking surgery; NAC, neoadjuvant chemotherapy; IDS, interval debulking surgery.
Regarding the specific genes with mutations, we subdivided the BRCAmut group into the BRCA1mut (n=101) and BRCA2mut (n=42) groups. The one patient harboring mutations in both genes was placed into the BRCA2mut group for statistical purposes. The BRCA1mut and BRCA2mut groups showed similar PFS and OS relative to the BRCAwt group (Fig. 3C and D). In multivariate analyses, BRCA1 mutation rather than BRCA1/2 wild-type was not a prognostic factor for improved PFS (aHR, 0.773; 95% CI, 0.575 to 1.040; p=0.089) and OS (aHR, 1.689; 95% CI, 0.870 to 3.280; p=0.121). Additionally, BRCA2 mutation did not affect patient PFS (aHR, 0.780; 95% CI, 0.522 to 1.166; p=0.226) and OS (aHR, 0.403; 95% CI, 0.095 to 1.703; p=0.216), compared to BRCA1/2 wild-type.
4. Subgroup analysis according to histologic type
We performed subgroup analyses of patients in order to investigate the effect of BRCA1/2 mutations on survival outcomes according to the histologic type. Among patients with HGSC (n=368), no differences in PFS (p=0.576) and OS (p=0.980) were observed between the BRCAmut and BRCAwt groups (S5A and S5B Fig.). In multivariate analyses, BRCA1/2 mutation was not associated with patient PFS (aHR, 0.785; 95% CI, 0.586 to 1.051; p=0.104) (S6 Table).
Among patients with non-HGSC (n=134), the BRCAmut and BRCAwt groups showed similar PFS (p=0.321) and OS (p=0.450) (S5C and S5D Fig.). Multivariate analyses revealed that presence of BRCA1/2 mutations did not affect patient PFS (aHR, 0.530; 95% CI, 0.252 to 1.115; p=0.094) (S6 Table).
5. Subgroup analysis according to primary treatment strategy
We then performed subgroup analyses of only patients with stage III to IV disease (n=389) in order to determine differences in the effect of BRCA1/2 mutations on survival outcomes according to the primary treatment strategy. Overall, the BRCAmut and BRCAwt groups showed similar PFS (p=0.146) and OS (p=0.967) (S7A-S7C Fig.). However, multivariate analyses identified BRCA1/2 mutation as an independent favorable prognostic factor for PFS (aHR, 0.722; 95% CI, 0.546 to 0.956; p=0.023), although not for OS (aHR, 1.066; 95% CI, 0.547 to 2.078; p=0.851) (Table 3).
Table 3.
Factors associated with survival outcomes in patients with FIGO stage III to IV disease
| Characteristic | Progression-free survival |
Overall survival |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
||||||||||
| HR | 95% CI | p-value | aHR | 95% CI | p-value | HR | 95% CI | p-value | aHR | 95% CI | p-value | ||
| Age (yr) | |||||||||||||
| < 55 | 1 | 1 | 1 | 1 | |||||||||
| ≥ 55 | 1.431 | 1.127-1.815 | 0.003 | 1.406 | 1.076-1.837 | 0.013 | 2.261 | 1.160-4.405 | 0.017 | 1.650 | 0.831-3.276 | 0.152 | |
| FIGO stage | |||||||||||||
| III | 1 | 1 | 1 | 1 | |||||||||
| V | 1.476 | 1.148-1.897 | 0.002 | 1.184 | 0.872-1.607 | 0.279 | 1.894 | 0.979-3.665 | 0.058 | 1.294 | 0.625-2.681 | 0.487 | |
| Histology | |||||||||||||
| High-grade serous | 1 | 1 | 1 | 1 | |||||||||
| Non–high-grade serous | 1.068 | 0.764-1.492 | 0.701 | 1.345 | 0.931-1.942 | 0.114 | 1.029 | 0.430-2.463 | 0.949 | 1.250 | 0.466-3.355 | 0.658 | |
| Initial serum CA-125 (IU/mL) | |||||||||||||
| < 900 | 1 | 1 | 1 | 1 | |||||||||
| ≥ 900 | 1.294 | 1.006-1.666 | 0.045 | 1.163 | 0.887-1.525 | 0.275 | 1.842 | 0.952-3.566 | 0.070 | 1.456 | 0.723-2.933 | 0.293 | |
| Primary treatment strategy | |||||||||||||
| PDS | 1 | 1 | 1 | 1 | |||||||||
| NAC | 1.683 | 1.315-2.153 | < 0.001 | 1.502 | 1.093-2.066 | 0.012 | 1.918 | 1.005-3.664 | 0.048 | 2.105 | 0.963-4.604 | 0.062 | |
| Residual tumor after PDS/IDS | |||||||||||||
| < 1 cm | 1 | 1 | 1 | 1 | |||||||||
| ≥ 1 cm | 1.435 | 1.010-2.038 | 0.044 | 1.596 | 1.078-2.364 | 0.020 | 2.438 | 1.210-4.913 | 0.013 | 2.769 | 1.258-6.095 | 0.011 | |
| BRCA mutational status | |||||||||||||
| Wild-type | 1 | 1 | 1 | 1 | |||||||||
| Mutation | 0.828 | 0.642-1.068 | 0.147 | 0.722 | 0.546-0.956 | 0.023 | 0.986 | 0.512-1.899 | 0.967 | 1.066 | 0.547-2.078 | 0.851 | |
FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio; CA-125, cancer antigen 125; PDS, primary debulking surgery; NAC, neoadjuvant chemotherapy; IDS, interval debulking surgery.
Among patients with stage III to IV disease who underwent primary debulking surgery (n=257), we observed no differences in PFS (p=0.705) or OS (p=0.768) between the BRCAmut and BRCAwt groups and no difference in PFS according to specific gene mutation (S7D-S7F Fig.). Multivariate analyses revealed that BRCA1/2 mutation did not affect patient PFS (aHR, 0.759; 95% CI, 0.530-1.089; p=0.135) (Table 4).
Table 4.
Factors associated with progression-free survival in patient with FIGO stage III to IV disease according to primary treatment strategy
| Characteristic | Primary debulking surgery |
Neoadjuvant chemotherapy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
||||||||||
| HR | 95% CI | p-value | aHR | 95% CI | p-value | HR | 95% CI | p-value | aHR | 95% CI | p-value | ||
| Age (yr) | |||||||||||||
| < 55 | 1 | 1 | 1 | 1 | |||||||||
| ≥ 55 | 1.286 | 0.952-1.737 | 0.101 | 1.491 | 1.060-2.097 | 0.022 | 1.543 | 1.020-2.333 | 0.040 | 1.508 | 0.948-2.401 | 0.083 | |
| FIGO stage | |||||||||||||
| III | 1 | 1 | 1 | 1 | |||||||||
| IV | 1.587 | 1.107-2.275 | 0.012 | 1.873 | 1.263-2.777 | 0.002 | 0.895 | 0.604-1.327 | 0.581 | 0.691 | 0.439-1.088 | 0.111 | |
| Histology | |||||||||||||
| High-grade serous | 1 | 1 | 1 | 1 | |||||||||
| Non–high-grade serous | 1.203 | 0.815-1.775 | 0.352 | 1.658 | 1.085-2.533 | 0.019 | 0.856 | 0.423-1.736 | 0.667 | 1.076 | 0.458-2.529 | 0.866 | |
| Initial serum CA-125 (IU/mL) | |||||||||||||
| < 900 | 1 | 1 | 1 | 1 | |||||||||
| ≥ 900 | 1.201 | 0.871-1.658 | 0.264 | 1.189 | 0.848-1.668 | 0.316 | 1.219 | 0.787-1.889 | 0.374 | 1.256 | 0.786-2.007 | 0.341 | |
| Residual tumor after PDS/IDS | |||||||||||||
| < 1 cm | 1 | 1 | 1 | 1 | |||||||||
| ≥ 1 cm | 1.471 | 0.975-2.218 | 0.066 | 1.340 | 0.857-2.096 | 0.200 | 1.887 | 0.943-3.775 | 0.073 | 3.200 | 1.357-7.544 | 0.008 | |
| BRCA mutational status | |||||||||||||
| Wild-type | 1 | 1 | 1 | 1 | |||||||||
| Mutation | 0.940 | 0.682-1.295 | 0.705 | 0.759 | 0.530-1.089 | 0.135 | 0.659 | 0.431-1.008 | 0.054 | 0.619 | 0.385-0.995 | 0.048 | |
FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio; CA-125, cancer antigen 125; PDS, primary debulking surgery; IDS, interval debulking surgery.
Among patients with stage III to IV disease who underwent NAC (n=132), the BRCAmut group showed better PFS with marginal significance than did the BRCAwt group (p=0.052), whereas a similar OS was observed between the two groups (p=0.619) (S7G-S7I Fig.). Additionally, multivariate analyses identified BRCA1/2 mutation as an independent favorable factor for improved PFS (aHR, 0.619; 95% CI, 0.385 to 0.995; p=0.048) (Table 4).
Discussion
In this single-institution, retrospective cohort study, we presented the BRCA1/2 mutational status of patients with epithelial POFTC and evaluated its effect on survival outcomes. We found a high incidence (28.0%) of BRCA1/2 mutation and that germline or somatic BRCA1/2 mutations were associated with better PFS than were wild-type BRCA genes.
Identification of patients with BRCA1/2 mutations and evaluation of their clinical outcomes are important issues in POFTC. Individuals with POFTC confirmed as harboring germline BRCA1/2 mutations have an opportunity to undergo treatment with PARP inhibitors. At the same time, they should undergo cancer surveillance for BC or other BRCA-related cancers. Additionally, their family members might benefit from BRCA1/2 gene testing in aspect of cancer prevention.
The incidence of BRCA1/2 mutation in patients with POFTCs varies among different histologic types, with HGSC being the most common type and showing the highest mutation incidence (20%-25%) [22-24]. Consistently with previous studies, we found that the incidence of BRCA1/2 mutations was higher in patients with HGSC (33.2%) and lower in nonHGSC patients (13.4%) relative to the overall study population (28.0%). Specifically, incidences of BRCA1/2 mutations in endometrioid and clear cell carcinomas were 14.0% (6/43) and 4.8% (2/42), respectively. In Canadian and Australian populations, previous studies have reported that germline BRCA1/2 mutations were found in approximately 7% to 8% of patients with ovarian endometrioid and clear cell carcinoma [15,25]. Although our study included a substantial number of Korean patients with non-HGSC POFTC (n=134), the sample size for each histologic type was so small that proper comparisons were difficult between our study results and those from previous studies. Considering that ovarian clear cell carcinoma is more common in East Asian populations than in Western populations [26], BRCA1/2 test results from East Asians might differ from those from other regions. Therefore, an East Asian collaborative research is necessary to ascertain the exact incidences of BRCA1/2 mutations in specific histologic types of epithelial POFTC.
Regarding survival outcomes, we identified BRCA1/2 mutation as a favorable prognostic factor for PFS in the entire study population in consistence with previous studies reporting associations between BRCA1/2 mutation and improved PFS [14,15,18,27]. We also observed similar results in patients with stage III to IV disease, especially in those who underwent NAC. This improved PFS in patients with POFTC harboring BRCA1/2 mutations is likely due to a high response rate to platinum-based chemotherapy mediated by vulnerability to DNA double-strand breaks [28,29]. However, BRCA1/2 mutational status did not affect patient PFS in the subgroup of primary debulking surgery, which might be explained by our institution’s high optimal debulking rate (85.8%; 211/246), possibly offsetting BRCA-related favorable chemotherapy response.
Despite the elongated PFS in patients with BRCA1/2 mutations, we did not observe differences in patient OS according to BRCA1/2 mutational status, which differs from previous studies [15,30,31]. This deviation might originate from our study population not being limited by a specific stage or histologic type of epithelial POFTCs. In addition, as BRCA mutated tumor gains resistance through the sequential chemotherapy, it is likely that the initial high response to chemotherapy does not lead to improved OS. Although the mechanisms of acquired chemoresistance are heterogeneous, researchers have commonly reported secondary mutations in BRCA1/2 genes, or reversion mutations, that restores homologous recombination repair functions [32,33]. Sokolenko et al. [34] also reported rapid selection of pre-existing BRCA1-proficient tumor clones during chemotherapy in ovarian cancer patients who had germline BRCA1 mutations. Development of individualized, novel treatment strategies reflecting each patient’s specific mechanisms underlying chemoresistance are highly warranted to improve patient OS.
The advent of treatment strategies involving the two PARP inhibitors olaparib and niraparib for POFTC has increased the demand for BRCA1/2 gene testing in Korea. Based on the findings that tumors with somatically acquired BRCA1 or BRCA2 pathogenic mutations respond to PARP inhibitors [10-12], physicians at our institution are recommending somatic testing to patients harboring wild-type BRCA1/2 according to germline test results and vice versa in order to expand candidate options for PARP inhibitors. As a result, 57 patients from the study population received both germline and somatic tests. The results of both tests within the same patient can be inconsistent due to differences in both the methods and specimens used. In the present study, among 13 patients with both germline and somatic BRCA1/2 mutations, two (15.4%) showed different variations of BRCA1/2 mutations, which is similar to a previous study from another institution in Korea [35]. However, a difference in patient classification represents an important issue. Classification of patients into BRCAmut and BRCAwt groups resulted in a 5.3% (3/57) discordance rate. Of the 57 patients receiving both germline and somatic tests, solitary germline testing failed to identify two patients harboring somatic BRCA1/2 mutations (3.5%), and solitary somatic testing failed to identify one patient harboring germline BRCA1/2 mutations (1.8%). Therefore, this suggests an advantage to conducting both germline and somatic testing in order to identify single BRCA1/2 mutations. However, clinicians need to consider the accuracy of each test, as well as testing cost-effectiveness and available resources.
Although the Korea Food and Drug Administration (KFDA) recently permitted olaparib maintenance for newly diagnosed, high-grade POFTC involving BRCA1/2 mutation in October 2019, few patients at our institution have actually received olaparib in this setting due to its high price; in the current study, none of the patients received maintenance with olaparib after primary treatment. Additionally, the use of niraparib for first-line maintenance has not yet been permitted by the KFDA. Therefore, we could not observe the substantial survival benefit from PARP inhibitors reported in the phase 3 SOLO-1 [7] or PRIMA [8] trials in this study. It is expected that more patients will use PARP inhibitors in a primary setting if the price of the drugs is lowered or if changes in the sociomedical environment encourage the use of such drugs. However, as PARP inhibitors continue to increase in popularity, further investigation of the exclusive effect of BRCA1/2 mutations on survival outcomes will be increasingly difficult to conduct.
This study has several limitations. First, selection bias or survival bias might exist due to the retrospective study design. Especially, in terms of baseline characteristics, FIGO stage differed significantly between the BRCA mutation and wildtype groups. Second, initial tumor load and disease patterns were not examined. Third, despite collecting cases of BRCA1/2 gene tests over a considerable time period (e.g., > 10 years for the germline test), some might argue that the sample size was small, especially for further comparisons according to the mutated BRCA gene types. Fourth, we only investigated details of the primary treatment. Nevertheless, because very small portion (5.9%) of the study population received bevacizumab during primary treatment, we could not assess survival benefit from bevacizumab exactly in relation with the BRCA1/2 mutational status. Finally, although we recognize that somatic testing conducted using an NGS cancer panel reports variants of genes other than BRCA1/2, we only considered and collected BRCA1/2 results for study purposes. Currently, we are planning further studies to investigate associations between deficiency in homologous recombination repair genes other than BRCA1/2 and POFTC patient survival outcomes. Nevertheless, we attempted to organize the experiences of our institution regarding BRCA1/2 gene testing and present them with systematic survival analyses.
In conclusion, we found that BRCA1/2 mutations were frequently observed in patients with epithelial POFTCs. This study demonstrated that patients harboring pathogenic BRCA1/2 mutations showed a better prognosis with longer PFS than did those harboring wild-type BRCA1/2. These findings might have important implications for real-world practice and clinical trial design.
Acknowledgments
This work was supported by grants from the Seoul National University Hospital Research Fund (No. 0320190260) and the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (No. 2020R1G1A1005711).
Footnotes
Conflicts of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
BRCA1/2 mutational status of the study population. POFT, peritoneal, ovarian, and fallopian tube.
Detected BRCA1/2 mutations and clinical information of patients who underwent both germline and somatic testing
Clinicopathologic characteristics of patients with high-grade serous carcinoma
Clinicopathologic characteristics of patients with non–high-grade serous carcinoma
Survival outcomes of patients according to histologic type; high-grade serous carcinoma (A, B) and non–high-grade serous carcinoma (C, D). (A, C) Progression-free survival (PFS). (B, D) Overall survival (OS).
Factors associated with progression-free survival according to histologic type
Survival outcomes of patients with International Federation of Gynecology and Obstetrics stage III to IV disease (A-C) and those who underwent primary debulking surgery (D-F) and neoadjuvant chemotherapy (G-I). (A, D, G) Progression-free survival (PFS). (B, E, H) PFS according to the mutated BRCA1/2 gene. (C, F, I) Overall survival (OS). CI, confidence interval.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine DA, Argenta PA, Yee CJ, Marshall DS, Olvera N, Bogomolniy F, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003;21:4222–7. doi: 10.1200/JCO.2003.04.131. [DOI] [PubMed] [Google Scholar]
- 4.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–90. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812–22. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 6.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation Carriers. JAMA. 2017;317:2402–16. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 7.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 9.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 10.Del Campo JM, Matulonis UA, Malander S, Provencher D, Mahner S, Follana P, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol. 2019;37:2968–73. doi: 10.1200/JCO.18.02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi MC, Lim MC, Suh DH, Song YJ, Kim TJ, Chang SJ, et al. Position statements on genetic test for peritoneal, ovarian, and fallopian tubal cancers: Korean Society of Gynecologic Oncology (KSGO) J Gynecol Oncol. 2016;27:e36. doi: 10.3802/jgo.2016.27.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–6. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 15.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyman DM, Zhou Q, Iasonos A, Grisham RN, Arnold AG, Phillips MF, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2012;118:3703–9. doi: 10.1002/cncr.26655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–65. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SI, Lee M, Kim HS, Chung HH, Kim JW, Park NH, et al. Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J Ovarian Res. 2019;12:40. doi: 10.1186/s13048-019-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park C, Kim M, Kim MJ, Kim H, Ock CY, Keam B, et al. Clinical application of next-generation sequencing-based panel to BRAF wild-type advanced melanoma identifies key oncogenic alterations and therapeutic strategies. Mol Cancer Ther. 2020;19:937–44. doi: 10.1158/1535-7163.MCT-19-0457. [DOI] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–75. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–6. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanley GE, McAlpine JN, Miller D, Huntsman D, Schrader KA, Blake Gilks C, et al. A population-based analysis of germline BRCA1 and BRCA2 testing among ovarian cancer patients in an era of histotype-specific approaches to ovarian cancer prevention. BMC Cancer. 2018;18:254. doi: 10.1186/s12885-018-4153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol. 2016;27:e5. doi: 10.3802/jgo.2016.27.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norquist BM, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner S, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: an NRG Oncology/Gynecologic Oncology Group study. Clin Cancer Res. 2018;24:777–83. doi: 10.1158/1078-0432.CCR-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, West SC. Distinct functions of BRCA1 and BRCA2 in double-strand break repair. Breast Cancer Res. 2002;4:9–13. doi: 10.1186/bcr417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett. 2015;369:363–7. doi: 10.1016/j.canlet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Dong F, Davineni PK, Howitt BE, Beck AH. A BRCA1/2 mutational signature and survival in ovarian high-grade serous carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1511–6. doi: 10.1158/1055-9965.EPI-16-0286. [DOI] [PubMed] [Google Scholar]
- 31.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–90. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lheureux S, Bruce JP, Burnier JV, Karakasis K, Shaw PA, Clarke BA, et al. Somatic BRCA1/2 recovery as a resistance mechanism after exceptional response to poly (ADP-ribose) polymerase inhibition. J Clin Oncol. 2017;35:1240–9. doi: 10.1200/JCO.2016.71.3677. [DOI] [PubMed] [Google Scholar]
- 33.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–15. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokolenko AP, Savonevich EL, Ivantsov AO, Raskin GA, Kuligina ES, Gorodnova TV, et al. Rapid selection of BRCA1-proficient tumor cells during neoadjuvant therapy for ovarian cancer in BRCA1 mutation carriers. Cancer Lett. 2017;397:127–32. doi: 10.1016/j.canlet.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Eoh KJ, Kim HM, Lee JY, Kim S, Kim SW, Kim YT, et al. Mutation landscape of germline and somatic BRCA1/2 in patients with high-grade serous ovarian cancer. BMC Cancer. 2020;20:204. doi: 10.1186/s12885-020-6693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BRCA1/2 mutational status of the study population. POFT, peritoneal, ovarian, and fallopian tube.
Detected BRCA1/2 mutations and clinical information of patients who underwent both germline and somatic testing
Clinicopathologic characteristics of patients with high-grade serous carcinoma
Clinicopathologic characteristics of patients with non–high-grade serous carcinoma
Survival outcomes of patients according to histologic type; high-grade serous carcinoma (A, B) and non–high-grade serous carcinoma (C, D). (A, C) Progression-free survival (PFS). (B, D) Overall survival (OS).
Factors associated with progression-free survival according to histologic type
Survival outcomes of patients with International Federation of Gynecology and Obstetrics stage III to IV disease (A-C) and those who underwent primary debulking surgery (D-F) and neoadjuvant chemotherapy (G-I). (A, D, G) Progression-free survival (PFS). (B, E, H) PFS according to the mutated BRCA1/2 gene. (C, F, I) Overall survival (OS). CI, confidence interval.