Abstract
Imaging genetics offers the possibility of detecting associations between genotype and brain structure as well as function, with effect sizes potentially exceeding correlations between genotype and behavior. However, study results are often limited due to small sample sizes and methodological differences, thus reducing the reliability of findings. The IMAGEN cohort with 2000 young adolescents assessed from the age of 14 onwards tries to eliminate some of these limitations by offering a longitudinal approach and sufficient sample size for analyzing gene-environment interactions on brain structure and function. Here, we give a systematic review of IMAGEN publications since the start of the consortium. We then focus on the specific phenotype ‘drug use’ to illustrate the potential of the IMAGEN approach. We describe findings with respect to frontocortical, limbic and striatal brain volume, functional activation elicited by reward anticipation, behavioral inhibition, and affective faces, and their respective associations with drug intake. In addition to describing its strengths, we also discuss limitations of the IMAGEN study. Because of the longitudinal design and related attrition, analyses are underpowered for (epi-) genome-wide approaches due to the limited sample size. Estimating the generalizability of results requires replications in independent samples. However, such densely phenotyped longitudinal studies are still rare and alternative internal cross-validation methods (e.g., leave-one out, split-half) are also warranted. In conclusion, the IMAGEN cohort is a unique, very well characterized longitudinal sample, which helped to elucidate neurobiological mechanisms involved in complex behavior and offers the possibility to further disentangle genotype × phenotype interactions.
Subject terms: Neuroscience, Psychology, Psychology, Neuroscience
Introduction
Two decades ago, several groups started to associate genetic variants with measures of brain structure and function rather than clinically diagnosed disease categories [1–4]. For example, it was assumed that variance in the genetic constitution of monoamine transporters should have a stronger impact on in vivo transporter availability and, consequently, brain function than on subjective mood states [1, 2]. Likewise, genetic variants associated with the dopamine D2 receptor were associated with in vivo receptor availability [3, 5]. Shortly thereafter, genotype effects on MRI-derived functional brain activation rather than protein expression were studied, with a focus on working memory-dependent brain activation [6]. A meta-analysis of effect sizes reflecting the association of genetic variance with brain versus behavioral data confirmed that the assessed genetic variants displayed stronger associations with brain function than with cognition or clinical symptoms [7, 8]. However, many early candidate gene study findings failed to replicate and meta-analyses showed that the observed associations between genotype and functional activation were smaller than originally assumed [9]. In fact, most early studies were not sufficiently powered to reliably produce large effect sizes. Moreover, a variety of disease-related as well as comorbid factors including smoking or stress hormone activation can interact with genotype effects on brain correlates [10, 11], requiring adequate sample sizes to address complex interactions.
To address the issue of statistical power and to confirm whether brain structures and functions are directly associated with genetic variance or are a secondary consequence of the disorder (e.g., due to pharmacological drug effects), prospective long-term studies are required, which assess young participants before the manifestation of symptoms or clinical disorders. For this purpose, the IMAGEN consortium was established in 2010. It includes 8 European centers; each of them recruited at least 250 healthy adolescents aged 14, who have been followed up at ages 16, 19, and 22 [12].
Another important benefit of the shift towards large multidisciplinary collaborations such as IMAGEN and ABCD [13] is that it allows for data driven discovery science and out-of-sample prediction for in vivo imaging genetics researchers. The initial IMAGEN sample size (>2000) was large enough to assess the effect of previously identified SNPs or polygenic risk scores on functional activation and their interaction with additional factors including environmental measures [14–16] and comorbid factors such as smoking or stress hormone activation [10, 11]. Although not large enough for candidate gene or polygenic score construction [17], a phenotypically rich longitudinal dataset like IMAGEN is uniquely placed to identify how genes and behavior relate via psychological or neurobiological intermediate phenotypes.
Since its inception, the IMAGEN consortium has published a number of significant papers investigating how genetic and imaging findings contribute to specific traits, behaviors, symptoms, and disorders, for example with respect to impulsive decision making and drug consumption [18, 19]. Despite the 100+ IMAGEN publications in the last decade, no systematic review of IMAGEN findings has been published to date. Here we present all original IMAGEN imaging genetics papers, i.e., papers assessing effects of genetic variation on brain structure or functional brain activation during either reward anticipation, behavioral inhibition or processing of affective faces, and discuss their respective behavioral correlates with a focus on drugs of abuse including alcohol, tobacco, and cannabis.
Methods
Data sources
A systematic search for all IMAGEN publications was carried out by LMM and AR between October 1st, 2016, and December 31st, 2018. Relevant studies were identified using the list of published papers provided online by the IMAGEN consortium (https://imagen-europe.com/publications/) and by systematically searching the PUBMED database using the search terms “IMAGEN” and “consortium.” Study references of identified articles were additionally reviewed and taken into account. Altogether, we identified a total number of 110 papers from the IMAGEN consortium including manuscripts with IMAGEN as a contributor. Among these, 62 publications report interactions between genotype and functional or structural brain data.
Study selection
To provide a systematic account of all papers published by the IMAGEN consortium, we reviewed all identified papers. Abstracts were screened for relevance, and all identified articles were discussed by LMM, HW, AR, and AH. We excluded studies that were systematic literature reviews and animal studies that were not using the IMAGEN sample of participants (Fig. 1).
Fig. 1. Selection process.
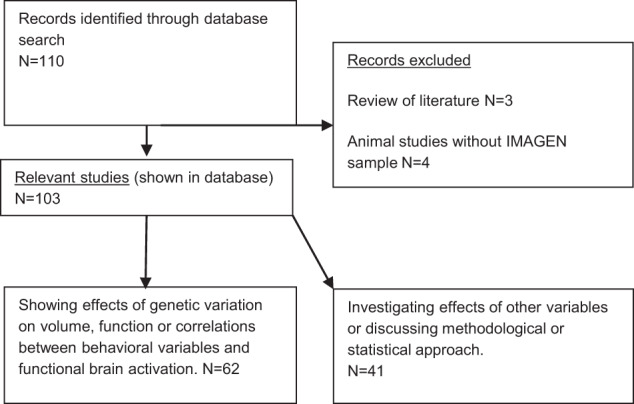
Flow-chart of selection process of identified publications.
We categorized papers from the IMAGEN consortium with respect to whether genetic variations were associated with (1) structural brain measures or (2) with one of the three tasks applied for functional imaging, i.e., the stop signal task (SST), the monetary incentive delay task (MID) and the Emotional faces (EF), or (3) intake of drugs of abuse. The variety of genetic variants addressed in IMAGEN studies was classified into four categories with respect to the main function of putatively associated genes: (1) neurodevelopment, (2) apoptosis and cell cycle, (3) neurotransmission, and (4) metabolic or endocrine function [20, 21].
To facilitate further use of the papers by the scientific community, we created three tables with key findings of all imaging genetics papers published by the IMAGEN consortium. The tables provide a brief overview of every paper, listing the title of the paper, authors, journal, year, the single nucleotide polymorphisms (SNPs) investigated and the key imaging variable (brain volume, structure, or functional task) addressed by the study. Genotype effects are discussed with respect to findings of other papers by the IMAGEN consortium and by independent groups.
From the 110 papers, 7 were reviews that did not contain original data or focused exclusively on animal data; these 7 papers are not further discussed in this review. The remaining 103 studies reviewed here include 41 papers that focused on methodological issues or behavioral features, while 62 manuscripts reported genotype associations with brain structure or function or correlations between behavioral variables and functional brain activation.
Results
Description of identified studies
We identified 103 relevant original papers including published abstracts. Among these papers, 12 manuscripts reported associations between genetic variations and brain volume [4, 14, 19, 22–30] (Table 1). Furthermore, 16 manuscripts reported associations of genetic variation on functional brain activation elicited by the MID task [15, 31–45]; 6 papers described functional brain activation elicited by the SST [16, 18, 46–49], and 4 papers showed functional brain activation elicited by the EF [50–53] (Table 2). We identified 24 papers that did not address imaging genetics but reported correlations between behavioral variables and functional brain activation elicited by (1) the MID task (n = 13) [54–66], (2) the SST (n = 3) [67–69], and (3) the FRT (n = 8) [70–77] (Table 3).
Table 1.
Influence of gene on brain structure volumes.
| Gene SNP minor allele frequency |
Title | Authors | Journal plus year | Number of participants | Mean age (years) | Main findings |
|---|---|---|---|---|---|---|
| Hippocampal findings | ||||||
|
Apo E Apolipoprotein E rs429358 T/C (0.1506a) rs7412 C/T (0.0751a) E4 rs7412 C, (rs429358 C) E3 rs7412 T, rs429358 C E2 (rs7412 T, rs429358 T) |
A multi-cohort study of ApoE 4 and amyloid- effects on the hippocampus in Alzheimer’s disease | Khan et al. | Journal of Alzheimer’s Disease, 2017 |
IMAGEN sample (N = 1387) Alzheimer’s disease (AD) and normal aging sample (N = 1781) |
IMAGEN e2 carriers(14.4, SD 0.4) e3 and e4 carriers (14.5, SD 0.4) |
AD and normal aging but not IMAGEN sample showed linear reduction in hippocampal volumes, with e4 carriers possessing the smallest volumes, e3 carriers possessing intermediate volumes, and e2 carriers possessing the largest volumes |
|
Apo E Apolipoprotein E rs429358 T/C (0.1506a) rs7412 C/T (0.0751a) E4 rs7412 C, (rs429358 C) E2 (rs7412 T, rs429358 T) |
No differences in hippocampal volume between carriers and noncarriers of the ApoE E4 and E2 alleles in young healthy adolescents | Khan et al. | Journal of Alzheimer Disease, 2014 | All participants N = 1412 |
APO E4, E2 noncarriers (14.45, SD 0.41) APOE4 carriers (14.44, SD 0.40) APO E2 carriers (14.45 ± 0.41) |
No hippocampal volume or asymmetry differences between carriers and noncarriers of the ApoE E4 or E2 alleles, no dose-dependent effects of either allele |
|
NR2F6 Nuclear receptor subfamily 2 group F member 6 rs4808611 C/T (0.102a) USHBP1 Usher syndrome type-1C protein-binding protein 1 rs35686037 C/T (0.088a) rs12982178 T/C (0.103a) BABAM1 BRISC and BRCA1 A Complex Member 1 rs10424178 C/T (0.173a) rs10406920 C/T (0.112a) rs8170 G/A (0.112a) TRPM8 Transient receptor potential cation channel rs11901004 G/T (0.113) rs17866592 C/T (0.135a) KIF26B Kinesin family member 26Brs9919234 G/T (0.404a) |
A genome-wide association study suggests novel loci associated with a Schizophrenia-related brain-based phenotype | Hass et al. | PLoS ONE, 2013 |
Consortia: MCICb (N = 241) ENIGMAc (N = 7795) IMAGEN (N = 1663) |
IMAGEN (~14, SD not reported) All other (~18, SD not reported) |
Six SNPs on chromosome 19, located within or in close proximity to the genes NR2F6, USHBP1, and BABAM1, as well as four SNPs in three other genomic regions (chromosome 1, 2 and 10) correlated with hippocampal volume with significant p-values between 6.75×10 − 6 and 8.3×10 − 7. Allelic differences in rs4808611 and rs8170 strongly associated with differential mRNA expression in the cis-acting region. Various top-ranking SNPs of MCIC association analysis hits replicated in IMAGEN sample, two KIF26BSNPs; three TRPM8 SNPs; 12 BABAM SNPS (e.g., rs2278897 with p = 5.1 × 10−4) |
|
Located between HRK Harakiri, BCL2 Interacting Protein and FBXW8 F-Box And WD Repeat domain containing 8 rs7294919 C/T (0.2119a) [influences expression of tescalcin gene] HMGA2 High mobility group AT-Hook 2 rs10784502C/T (0.3411a) DDR2 Discoidin domain receptor tyrosine kinase 2 rs10494373A/C (0.051a) |
Identification of common variants associated with human hippocampal and intracranial volumes | Stein et al. | Nature Genetics, 2012 |
Total (N = 7795) IMAGEN (N = 518) |
Total (39,9, SD9.24) IMAGEN (14.5, SD 0.4) |
rs7294919 associated with hippocampal volume (p = 6.70 × 10−16). C allele of rs10784502 associated with intracranial volume and general intelligence by approximately 1.29 IQ points per risk allele (P = 1.12 × 10 − 12). |
| Putamen findings | ||||||
|
DAT1/SLC6A3 Dopamine transporter gene rs40184 C/T (0.477a) |
Interaction between striatal volume and DAT1 polymorphism predicts working memory development during adolescence | Nemmi et al. | Developmental Cognitive Neuroscience, 2018 | All participants (N = 487) |
Baseline (~14, SD not reported) Follow-up (~19, SD not reported) |
TC heterozygotes with a larger putamen at age 14 showed greater WM improvement at age 19 (p = 0.0009) |
|
KTN1 Kinectin 1 rs945270 G/C (0.343a) DCC DCC Netrin 1 Receptor rs62097986 C/A (0.156a) BCL2L1 BCL2-Like 1 rs6087771 T/C (0.299a) DLG2 Disks large homolog 2 rs683250 A/G (0.417a) HRK Harakiri, BCL2 interacting protein rs77956314 T/C (0.05a) MSRB3 Methionine sulfoxide reductase B3 rs61921502 T/G (0.047a) FAT3 FAT Atypical Cadherin 3 rs1318862 T/C (0.462a) CRHR1 Corticotropin releasing hormone receptor 1 rs17689882 G/A (0.086a) HMGA2 High Mobility Group AT-Hook 2 rs10784502 T/C (0.341a) |
Common genetic variants influence human subcortical brain structures | Hibar et al. | Nature, 2015 |
Total: (N = 30,717) IMAGEN: (N = 1765) |
IMAGEN: (14.6, SD 0.4) |
Five genetic variants were associated with the volumes of the putamen and caudate nucleus. (rs945279, rs62097986, rs6087771, rs683250, rs1318862). Strongest effects for putamen was in rs945270 influencing the expression of the KTN1 gene (p = 1.08 × 10-33). Three loci replicated with previously established association with hippocampal and intracranial volume (rs77956314, rs6192150, rs 17689882) Rs10784502 did not survive genome-wide significance considering association with intracranial volume. |
| Cortex finding | ||||||
|
EFhd2 EF-Hand Domain Family Member D2 rs112146896 C/G (0.12a) |
EFhd2/Swiprosin-1 is a common genetic determinator for sensation-seeking/low anxiety and alcohol addiction | Mielenz et al. | Molecular Psychiatry, 2017 | All participants (N = 1980) | All participants (14.4, SD 0.41) | Minor C allele of rs112146896 shows a positive significant association with lifetime alcohol intake (EF = 0.099), a nominally positive association with binge drinking (EF = 0.055) and a negative association with anxiety. Negative association between lifetime alcohol intake and superior frontal gyrus volume. |
|
Polygenic risk sore ➢ 20.000 SNPs CNR1 Cannabinoid receptor 1 |
Early cannabis use, polygenic risk score for schizophrenia, and brain maturation in adolescence | French et al. | JAMA Psychiatry, 2015 |
Cohorts: SYSd (N = 949) ALSPACe (N = 295) IMAGEN (N = 333) |
All participants (16.4, SD 1.0) IMAGEN Baseline (14.5, SD 0.4) Follow-up (18.5, SD not reported) |
Negative association was between cannabis use in early adolescence and cortical thickness in male participants with a high polygenic risk score. Male participants showed an interactive effect of the high risk score and cannabis use on decreased cortical thickness from 14.5 to 18.5 years of age |
|
NPTN Neuroplastin rs7171755 G/A (C) (0.474a) |
Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents | Desrivières et al. | Molecular Psychiatry, 2013 | All participants (N = 1583) | All participants (14.4, SD 0.7) | Variant of rs7171755 (risk = minor allele A) is associated with smaller cortical thickness in frontal and temporal lobes, affecting left hemisphere (p = 1.12 × 10−7) more than right hemisphere and decreased verbal and non-verbal IQ |
| Other structural findings | ||||||
|
APOE Apolipoprotein E E4 rs7412 C, (rs429358 C) E3 (rs7412 T, rs429358 C) E2 (rs7412 T, rs429358 T) |
Tract based spatial statistic reveals no differences in white matter microstructural organization between carriers and noncarriers of the APOE ɛ4 and ɛ2 Alleles in Young Healthy Adolescents | Dell’Acqua et al. | Journal of Alzheimer’s Disease, 2015 | All participants (N = 575) | All participants(14.4, SD 0.5) | Microstructural properties of white matter are not associated with the APOE ɛ4 and ɛ2 alleles in adolescents |
| Set of 136 genes | Correlated gene expression supports synchronous activity in brain networks | Richiardi et al. | Science, 2015 | All participants (N = 259) | All participants(~14, SD not reported) | A set of 136 genes was significantly enriched for ion channels and polymorphisms in this gene set significantly affect resting-state functional connectivity, mostly within but also between functional networks |
|
KDM4C Lysine-specific demethylase 4C rs10511260 T/G (0.177a) rs10758821 G/T (0.465a) rs10975990 A/G (0.424a) PKNOX2 PBX/Knotted 1 Homeobox 2 rs10893366 C/T (0.285a) rs750338 A/G (0.358a); ADH1C Alcohol Dehydrogenase 1Crs1789891 C/A (0.095a) ANKS1A Ankyrin Repeat And Sterile Alpha Motif Domain Containing 1A rs2140418 C/T (0.432a) KIAA0040 Uncharacterized protein rs6701037 A/C (0.287a) KCNMA1 Potassium Calcium-Activated Channel Subfamily M Alpha 1 rs717207G/T (0.124a) RASGRF2 Ras Protein Specific Guanine Nucleotide Releasing Factor 2 rs26907 G/A (0) rs2369955 A/C (0.157a) nearMARK1 Microtubule Affinity Regulating Kinase 1 rs7530302 G/A (0.135a) PECR Peroxisomal Trans-2-Enoyl-CoA Reductase rs7590720 A/G (0.284a) EFNA5 Ephrin A5 rs770182 T/G (0.323a) |
Neuropsychosocial profiles of current and future adolescent alcohol misusers | Whelan et al. | Nature, 2014 |
IMAGEN Controls (N = 150) Current binge drinkers (N = 115) Future binge drinkers (N = 121) |
IMAGEN Controls (14.5, SD 0.4) Current binge drinkers (14.6, SD 0.4) Future binge drinkers (14.5, SD 0.4) |
Geneticvariantsrs2140418T, rs2369955C, rs7530302A, rs7590720G,and rs7916403T associated with current binge drinking; rs10758821T, rs10893366T, rs2140418T, and rs2369955C were predictive of future binge drinking |
aGlobal minor allele frequency based on 1000Genome phase 3 genotype data (1000 Genomes Project Consortium. (2015). A global reference for human genetic variation. Nature, 526(7571), 68–74.)
bMCIC The Mind Clinical Imaging Consortium.
cENIGMA Enhancing Neuro Imaging Genetics through Meta Analysis.
dSYS Saguenay Youth Study.
eALSPAC Avon Longitudinal Study of Parents and Children.
Table 2.
Genetic effects on functional brain activation elicited by MID, SST and EF task.
| SNP | Title | Authors | Journal plus year | Number of participants | Age | Main findings |
|---|---|---|---|---|---|---|
| MID | ||||||
|
DRD1 D1 dopamine receptor rs686, A/G (0.409a) PPP1R1B protein phosphatase 1 regulatory inhibitor subunit 1B rs8769 G/A (0.06a) DRD2 D2 dopamine receptor rs12364283 A/G (0.07a) ANKK1 Ankyrin repeat and kinase domain containing 1, rs1800497 G/A (0.188a) |
Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence | Baker et al. | Psychological Medicine, 2018 | All participants (N = 1840) | All participants (14.55, SD = 0.447) | Functional polymorphism rs686 of the D1 dopamine receptor (DRD1) (p = 0.01) gene and Taq1A of the ANKK1 gene (p = 0.002) influenced medial and lateral OFC activation during reward anticipation. rs686 of the DRD1gene was indirectly related to early onset of alcohol misuse through a medial orbitofrontal cortex × ventral striatum interaction. |
|
TTC12 tetratricopeptide repeat domain 12 ANKK1 ankyrin repeat and kinase domain containing 1 DRD2 dopamine receptor 2 — 33 SNPs, e.g., rs2236709 A/G (0.2588a) |
A neurobiological pathway to smoking in adolescence: TTC12-ANKK1-DRD2 variants and reward response | Macare et al. | European Neuropsychopharmacology, 2018 |
NFBC1966 (N = 4512), NFBC1986 (N = 4307) ALSPAC (N = 3674) IMAGEN (N = 1591) |
NFBC1966 (14, SD not reported) NFBC1986 (16, SD not reported) ALSPAC (15, SD not reported) IMAGEN (14, SD not reported) |
The minor G allele was linked to an increased ventral-striatal BOLD response during reward anticipation (p = 0.032) and with higher DRD2 gene expression in the striatum (p = 0.013). Same Allele was associated with self-reported smoking and higher plasma cotinine levels |
|
GABRB1 Gamma-aminobutyric acid receptor subunit Beta-1 rs2044081 C/T (0.148a) |
GABRB1 Single Nucleotide Polymorphism Associated with Altered Brain Responses (but not Performance) during Measures of Impulsivity and Reward Sensitivity in Human Adolescents | Duka et al. | Frontiers in Behavioral neuroscience, 2017 | All participants (N = 1299) | All participants (14, SD not reported) | Allele was not associated with an impulsive or reward-sensitivity phenotype as measured by SST and MID-Diff performance. Increased BOLD response in the right hemisphere inferior frontal gyrus, left hemisphere caudate/insula and left hemisphere inferior temporal gyrus during MID performance was higher in the minor (T) allelic group (p < 0.005). In contrast, during SST performance, the BOLD response found in the right hemisphere supramarginal gyrus, right hemisphere lingual and left hemisphere inferior parietal gyrus indicated reduced responses in the minor genotype (p < 0.005) |
|
KTN1 Kinectin 1 rs945270 C/G (0.3429a) |
Impact of a common genetic variation associated with putamen volume on neural mechanisms of attention deficit/hyperactivity disorder | Xu et al. | Journal of the American Academy of Child & Adolescent Psychiatry, 2017 | All participants (N = 1129) | All participants (14.4, SD 0.4) |
rs945270 C allele associated with lower ADHD symptoms. rs945270 C allele associated with higher putamen activation during reward anticipation in females and with lower putamen activation during successful response inhibition in males |
|
OPRL1 Opioid related nociceptin receptor 1 11 SNPs e.g., rs1335579 A/G (0.214a) |
Methylation of OPRL1 mediates the effect of psychosocial stress on binge drinking in adolescents | Ruggeri et al. | Journal of Child Psychology and Psychiatry, 2017 | All participants (N = 325) | All participants (14, SD not reported) | Methylation levels in intron 1 of OPRL1 are associated with higher psychosocial stress and higher frequency of binge drinking. In individuals with low methylation of OPRL1, frequency of binge drinking is associated with stronger BOLD response in the ventral striatum during reward anticipation. |
|
OPRM1 Opioid receptor Mu 1 rs1799971 A/G (0.223a) rs563649 C/T (0.1084a) |
Brain substrates of reward processing and the m-opioid receptor: a pathway into pain? | Nees et al. | Pain, 2017 |
All participants Baseline (N = 644) Follow-up (N = 44) |
All participants Baseline (14.58, SD 0.39) Follow-up (16.73, SD 0.21) |
Functional activation of the dorsal striatum during reward feedback predicted pain complaints independent of genetic variance. T allele of rs563649 had more pain complaints than CC-allele carriers. Relationship of pain complaints and activation in the periaqueductal gray and ventral striatum in carriers of theT-allele of rs563649 |
| Polygenic risk of psychosis and ventral-striatal activation during reward processing in healthy adolescents | Lancaster et al. | The American Journal of Psychiatry, 2016 | ||||
|
KALRN kalirin RhoGEF kinase rs6438839, G/A (0.17) rs4634050 (C/A) (0.22) |
Mouse and human genetic analyses associate kalirin with ventral striatal activation during impulsivity and with alcohol misuse | Peña-Oliver et al. | Frontiers in Genetics, 2016 | All participants (N = 1423) | All participants (14.43, SD = 0.41) | G major allele of the SNP rs6438839 in the KALRN gene was significantly associated with increased ventral striatum activation during reward anticipation (p = 5.9 × 10−3). A minor allele of SNP rs4634050, belonging to the same haplotype block, was associated with increased frequency of binge drinking (p = 4.87 × 10−2). |
|
VPS4A vacuolar protein sorting-associated protein 4A rs16958736 C/T (0.111) |
Neural basis of reward anticipation and its genetic determinants | Tianye et al. | Proceedings of the National Academy of Science USA, 2016 | All participants (N = 1423) | All participants (14.44, SD = 0.42) | The major C allele was associated with decreased activation in the striatal node during reward anticipation, although it did not reach the commonly used threshold for genome-wide significance. Lower activation in striatal node was associated with premature responding (p = 5.0 × 10 − 6). |
| EHD4 EH-domain containing 4 Haplotype blockconsisting ofrs 1648821 C/T(0.126a) | A translational systems biology approach in both animals and humans identifies a functionally related module of accumbal genes involved in the regulation of reward processing and binge drinking in males | Stacey et al. | Journal of Psychiatry and Neuroscience, 2016 | All participants (N = 907) | All participants (14.42, SD 0.41) | Functional activation of the right but not left ventral striatum during reward anticipation was significantlyassociatedwithhaplotype block 3 consisting ofrs 1648821 and 5 other SNPs in EHD4. M5 module, a functional gene-clusterassociated with mesolimbic dopamine signaling was linked to binge-drinking in both mice and male human adolescents |
|
BDNF Brain-derived neurotrophic factor rs6265 C/T (0.201a) |
BDNF Val66Met and reward-related brain function in adolescents: role for early alcohol consumption | Nees et al. | Alcohol, 2015 | All participants (N = 530) |
All participants Baseline (14.33, SD 0.98) Follow-up: (16.28, SD 0.88) |
Val/Val homozygotes versus Met -carriers had lower functional activation in putamen during reward anticipation. Low putamen activation during reward feedback in Met-carriers was associated with alcohol consumption at follow-up (EF = 0.024). Functional putamen activation during reward feedback in Met- but not Val/Val carriers at baseline predicted level of alcohol consumption 2 years later (EF = 0.011) |
| RSU1 Ras suppressor protein 1 rs7078011C/T (0.041) | Rsu1 regulates ethanol consumption in Drosophila and humans | Ojelade et al. | Proceedings of the National Academy of Science USA, 2015 | All participants (N = 1303) | All participants (14.4, SD 0.4) | Polymorphisms in RSU1 are associated with functional activation in the ventral striatum during reward anticipation alcohol consumption in adolescents |
| DRD2/ANKK1 Dopamine receptor D2/ Ankyrin repeat and kinase domain containing 1, rs1800497 G/A (0.326a) | DRD2/ANKK1 polymorphism modulates the effect of ventral striatal activation on working memory performance | Nymberg et al. | Neuropsychopharmacology, 2014 | All participants (N = 1080) | All participants (14.4, SD 0.4) |
Higher ventral striatum and caudate activation during reward feedback significantly associated with higher WM performance (EF = 0.010). This effect was only significant in carriers of the minor A allele (EF = 0.07) |
|
CHRNA5–CHRNA3–CHRNB4 Cholinergic receptor nicotinic alpha subunit (5–3–4) rs578776 G/A (0.445a) rs1051730 G/A (0.168a) |
Genetic risk for nicotine dependence in the cholinergic system and activation of the brain reward system in healthy adolescents | Nees et al. | Neuropsychopharmacology, 2013 | All participants (N = 999) | All participants (14.55, SD 0.25) | Carriers of the rs578776 GG allele versus A- carriers had significantly lower functional activation elicited by reward feedback in the right ventral and dorsal ACC(EF = 0.3157) |
|
MAOA Monoamine oxidase A rs12843268 A/G (0.470a) |
Neural mechanisms of attention deficit/hyperactivity disorder symptoms are stratified by MAOA genotype | Nymberg et al. | Biological Psychiatry, 2013 | All participants (N = 648) | All participants (14.4, SD 0 .4) | In male rs12843268 A hemizygotes, ADHD symptoms are associated with lower functional activation of the VS during reward anticipation and lower inferior frontal gyrus BOLD response during response inhibition (SST). In G hemizygotes, right inferior frontal gyrus activation during response inhibition 8ST) was positively correlated with ADHD symptoms in the presence of increased ventral striatal functional activation during reward anticipation (EF = 0.0053) |
|
TNM4 Teneurin transmembrane protein 4 rs12576775 A/G (0.104a) |
The risk variant in ODZ4 for bipolar disorder impacts on amygdala activation during reward processing. | Heinrich et al. | Bipolar Disorders, 2013 | All participants (N = 485) | All participants (14.26, SD 0.30) | rs12576775 G allele carriers had an increased functional activation in the amygdala during reward anticipation and feedback |
| Rasgrf2 Ras protein specific guanine nucleotide releasing factor 2 rs26907 G/A (0.157a) | RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. | Stacey et al. | PNAS, 2012 | All participants (N = 612) (in abstract n = 663 mentioned) | All participants (14.44, SD 0.40) |
Ethanol-induced dopamine release blunted in Rasgrf2 k.o.mice. A Rasgrf2 haplotype block containing rs26907, a SNP previously associated with alcohol intake, was significantly associated with ventral striatal functional activation during reward anticipation (EF = 0.0205) |
| SST | ||||||
|
PSD3 Pleckstrin and Sec7 domain containing 3 rs13265422 G/A (0.472a) |
The Arf6 activator Efa6/PSD3 confers regional specificity and modulates ethanol consumption in Drosophila and humans | Gonzalez et al. | Molecular Psychiatry, 2017 |
IMAGEN sample for association analyses (N = 1363) IMAGEN sample for fMRI task (N = 1771) SAGE sample (N = 2544) |
IMAGEN sample for association analysis (16.46,SD 0.51) IMAGEN sample for fMRI task (14.43, SD 0.42) (fMRI task) |
Haplotype containing rs13265422 and PSD3 Minor G allele of rs13265422 were significantly associated with increased frequency of alcohol consumption (EF = 0.2167) and binge drinking in the last 30 days (EF = 0.1125). Haplotype containing rs13265422 also modulates PFC activation during SST |
|
PPM1G Protein phosphatase, Mg2 + /Mn2+ Dependent 1G rs7602534 C/T (0.378a) rs11675428 A/C (0.050a) rs704791 T/C (0.454a) rs1260342 G/T (0.454a) rs2384629 G/A (0.0078a) |
Association of protein phosphatase PPM1G with alcohol use disorder and brain activity during behavioral control in a genome-wide methylation analysis | Ruggeri et al. | American Journal of Psychiatry, 2015 | IMAGEN (499 adolescents) 18 pair of twins |
IMAGEN (~14, SD not reported) Twins first follow-up (~16, SD not reported) |
Hypermethylation of PPM1G was positively associated with high daily alcohol intake drinking, impulsivity and functional activation of the right subthalamic nucleus during stop success in the SST task (η2 = 0.013). PPM1G genotype and methylation profile were not associated, thus indicating environmental causes |
|
COMT Catecholamine-0-methyl-transferase rs4680 G/A (0.369a) |
Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence | White et al. | Neuropsychopharmacology, 2014 | All participants (N = 1133) | All participants(14.45, SD 0.41) | Male but not female Val homozygotes displayed elevated functional activation in pre supplementary motor area (pre- SMA) during successful-inhibition trials and in both pre-SMA and inferior frontal cortex during failed-inhibition trials compared with other genotypes (EF = 0.2038) |
|
AMBRA1 Autophagy/beclin-1 regulator rs11819869 C/T (0.250a) |
From gene to brain to behavior: schizophrenia-associated variation in AMBRA1 alters impulsivity-related traits | Heinrich et al. | European Journal of Neuroscience, 2013 | All participants (N = 848) | All participants(14.44, SD 0.41) | T-risk allele carriers in the rs11819869 showed higher delay aversion (EF = 0.1634) and functional activation in an orbitofrontal target region during the SST(EF = 0.1651) |
|
SLC6A2 Solute carrier family 6 member 2 rs36024 G/A (0.463a) |
Adolescent impulsivity phenotypes characterized by distinct brain networks | Whelan et al. | Nature Neuroscience, 2012 | All participants (N = 1896) | All participants (14.55 ± 0.447) | Hypofunctioning of a specific orbitofrontal cortical network was associated with likelihood of initiating drug use in early adolescence. Right inferior frontal activity was related to the speed of the inhibition process and use of illegal substances and associated with genetic variation in a norepinephrine transporter gene |
|
ADRA2B Adrenoceptor Alpha 2B SNPs not reported |
A large-sample fMRI study (n = 1252) of individual differences in inhibitory control | Whelan et al. | Human brain mapping Quebec abstract book, 2011 | All participants (N = 1252) | All participants (14.51, SD 0.86) |
Participants with subclinical features of ADHD showed reduced functional activation during stop failure in bilateral putamen, pallidum,caudate,bilateral insula, ACC and IFG. ADRA2B was associated with activation during stop success in right lateralized IFG, insula and ACC |
| Emotional faces task | ||||||
| GWAS with 463,940 SNPs | Genetic risk for schizophrenia and autism, social impairment and developmental pathways to psychosis | Velthorst et al. | Translational Psychiatry, 2018 |
All participants (N = 2096) Subgroup used for structural equation modeling (SEM) (N = 643) |
All participants (14.46, SD 0.41) SEM (14.44, SD 0.43) 14.44 (0.43) |
Reduced brain activity to emotional stimuli (p = 0.009) as well as social impairments in late adolescence (p < 0.001) and high polygenic risk for schizophrenia (p = 0.014) independently contributed to the severity of psychotic experiences at age 18 |
|
CB1R Cannabinoid receptor 1 rs1049353 C/T (0.129a) rs806377 T/C (0.490a) |
The role of the cannabinoid receptor in adolescents′ processing of facial expressions | Ewald et al. | Cognitive Neuroscience, 2016 | All participants (N = 583) | All participants (14.38, SD 0.96) |
A-allele versus GG-carriers in rs1049353 displayed earlier recognition of facial expressions changing from anger to sadness (EF = 0.1735) or fear(EF = 0.175), increased functional activation elicited by angry but not neutral faces in the amygdala (EF = 0.1775) and insula (EF = 0.1981) No significant effects were observed for rs806377 |
|
OXTR Oxytocin receptor 23 SNPs, e.g., rs237915 T/C (0.159a) |
Oxytocin Receptor Genotype modulates ventral-striatal activity to social cues and response to stressful life events | Loth E et al. | Biological Psychiatry, 2014 | All participants (N = 1445) | All participants (14.4, SD 0.7) |
rs237915 CC-homozygoteshad significantly lower vs activation elicited by angry faces than T-allele carriers (left vs activity EF = 0.1779, right vs ES = 0.1672). In environments with low stressful life events, rs237915 CC homozygote girls had more emotional problems and boys had more peer problems. In high stressful environments, T-allele carriers had more clinical problems than CC homozygotes |
| GWAS with 511.089 SNPs | Global genetic variations predict brain response to faces | Dickie et al. | PLoS Genetics, 2014 | All participants (N = 1620) | All participants (~14, SD not reported) |
A significant proportion of the brain response to ambiguous but not angry facial expressions was predicted by common genetic variance in 9 out of 25 regions constituting a face network. The strength of the genotype-phenotype relationship varied according to the number of functional connections of each region, the identified 9 regions displayed the highest inter-individual variability in the number of connections with other network nodes |
aGlobal minor allele frequency based on 1000Genome phase 3 genotype data (1000 Genomes Project Consortium. (2015). A global reference for human genetic variation. Nature, 526(7571), 68–74).
Table 3.
Functional activation elicited by MID, SST, and EF task.
| Title | Authors | Journal plus year | Number of participants | Age in years (mean) | fMRI task | Main finding |
|---|---|---|---|---|---|---|
| Examination of the neural basis of psychotic-like experiences in adolescence during reward processing | Papanastasiou et al. | Journal of American Psychiatry, 2018 | All participants (N = 298) |
Baseline (14.47, SD = 0.39) Follow-up (19.02, SD = 0.76) |
Monetary incentive delay task (MID) | Between baseline and follow-up, brain activation in two regions within the left and right middle frontal gyri increasedduring reward anticipation (p = 0.02; p = 0.03, respectively); there was no main group effect between high vs low psychotic-like experiences. |
| Epigenetic variance in dopamine D2 receptor: a marker of IQ malleability? | Kaminski et al. | Translational Psychiatry, 2018 |
All participants (N = 1475) |
All participants (14.43, SD = 0.45) | Functional striatal activation elicited by temporarily surprising reward-predicting cues (from MID task) as well as polygenic scores for intelligence and epigenetic modification of DRD2 gene and gray matter density in striatum were associated with general IQ | |
| Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents | Büchel et al. | Nature Communications, 2017 |
Healthy controls (N = 72) Problematic drug use (N = 72) |
Healthy controls (14.48, SD 0.40) Problematic drug use (14.38, SD 0.45) |
During reward anticipation, lower functional activation in dorsolateral PFC, ventral striatum and midbrain predict drug use at age 16 | |
| Ventral Striatum Connectivity During Reward Anticipation in Adolescent Smokers | Lee et al. | Developmental neuropsychology, 2016 | All (N = 206) | All Participants (~ 14, SD not reported) | Increased smoking frequency was associated with increased connectivity between ventral striatum and regions involved in saliency and valuation, including the orbitofrontal cortex during reward anticipation and with reduced connectivity with regions associated with inhibition and risk aversion, including the right inferior frontal gyrus | |
| Disentangling the autism−anxiety overlap: fMRI of reward processing in a community-based longitudinal study | Mikita et al. | Translational psychiatry, 2016 |
Reward anticipation (N = 1472) Negative feedback (N = 1601) Positive feedback (N = 1726) |
Baseline (14.4, SD not reported) Feedback (~16, SD not reported) |
Participants with autism spectrum disorder (ADS) traits had reduced BOLD responses in dorsal prefrontal regions during reward anticipation and negative feedback (p = 0.001). High anxiety symptoms were correlated with increased lateral prefrontal responses during reward anticipation and decreased responses to reward feedback (p < 0.05). Interaction between ASD and anxiety showed significantly lower activations compared to ASD alone. | |
| The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample | Stringaris et al. | The American Journal of Psychiatry, 2015 |
Healthy subjects Baseline (N = 123) Follow-up (N = 902) Subthreshold depression Baseline (N = 101) Follow-up (N = 68) Clinical depression Baseline (N = 22) Follow-up (N = 29) |
Healthy subjects Baseline (14.4, SD 0.4) Follow-up (16.4, SD 0.4) Subthreshold depression Baseline (14.5, SD 0.4) Follow-up (16.4, SD 0.4) Clinical depression Baseline (14.4, SD 0.3) Follow-up (16.5, SD 0.3–0.5) |
Bilaterally lower vs activation elicited by reward anticipation in groups with subthreshold and clinical depression compared to healthy group (p < 0.005). Low ventral left (EF = 0.050) and right (EF = 0.047) striatal activation during reward anticipation predicted transition to subthreshold or clinical depression in previously healthy adolescents at 2-year follow-up | |
| No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls | Müller et al. | Addiction Biology, 2014 |
Family history positive (FHD+) (N = 206) Family history negative (FHD−) (N = 206) FH+ (N = 77) FH− (N = 77) |
FHD+ (14.7, SD 0.4) FHD− (14.7, SD 0.3) |
Reward anticipation as well as reward feedback elicited activation in the ventral striatum in all participants, no significant differences between adolescents with versus without a positive family history for alcohol use disorders | |
| Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking | Müller et al. | JAMA Psychiatry, 2013 |
Participants exposed to intrauterine maternal smoking (N = 177) Nonexposed (N = 177) |
Exposed (14.7, SD 0.4) Nonexposed (14.6, SD 0.4) |
In adolescents prenatally exposed to cigarette smoke,reward anticipation but not feedback elicited a weaker functional activation of the right (EF = 0.04) and left (EF=) ventral striatum during reward anticipation | |
| A target sample of adolescents and reward processing: same neural and behavioral correlates engaged in common paradigms? | Nees et al. | Experimental Brain Research, 2012 | All participants (N = 54) | All Participants (~14, SD not reported) | Magnitude sensitive functional activation in VS response during reward anticipation (p = 0.036) and magnitude independent activation in the anterior cingulate cortex during feedback | |
| Risk taking and the adolescent reward system: a potential common link to substance abuse | Schneider et al. | American Journal of Psychiatry, 2012 | All participants (N = 266) | All participants (14.5, SD 0.4) | With increasing risk-taking bias, the ventral striatum showed decreased activation bilaterally during reward anticipation (EF = 0.57 and EF = 0.52) | |
| Maternal interpersonal affiliation is associated with adolescents’ brain structure and reward processing | Schneider et al. | Translational Psychiatry, 2012 | All participants (N = 63) | All participants (14.24, SD 0.25) | Maternal affiliation was significantly associated with ventral striatal (EF = 0.89) and caudate activation (EF = 1.1942) during reward feedback in female participants only | |
| Determinants of early alcohol use in healthy adolescents:the differential contribution of neuroimaging and psychological factors | Nees F et al. | Neuropsychopharmacology, 2012 | All participants (N = 324) | All participants (~14, SD not reported) | Reward-associated behavior, personality, and brain responses all contributed to alcohol intake with personality explaining a higher proportion of the variance (explained variance 16%) than behavior (explained variance 0.6%) and brain responses (explained variance 0.4%). | |
| Lower ventral striatal activation during reward anticipation in adolescent smokers | Peters et al | The American Journal of Psychiatry, 2011 | All participants (N = 86) | All participants (~14, SD not reported) | Neural responses in the ventral striatum during reward anticipation were significantly lower in the smokers than in the comparison subjects (p < 0.001), and in the smokers this response was correlated with smoking frequency. | |
| Distinct brain structure and behavior related to ADHD and conduct disorder traits | Bayard et al. | Molecular Psychiatry, 2018 | All participants (N = 1093) | All participants (14.47, SD 0.39) | Stop Signal Task (SST) | ADHD score correlated with SSRT (p = 0.021), while CD score did not (p = 0.740). This mirrored structural findings on prefrontal and anterior cingulate region. |
| Separate neural systems for behavioral change and for emotional responses to failure during behavioral inhibition | Deng et al. | Human Brain Mapping, 2017 | All participants (N = 1709) | All participants (~14, SD not reported) | Succesful inhibition was related to activation in the lateral orbitofrontal cortex, inferior frontal gyrus and the dorsolateral prefrontal cortex (DLPFC) (p < 0.05). Second, the anterior cingulate and anterior insula (AI) were activated more on failure trials (p < 0.05) | |
| Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence | Castellanos-Ryan et al. | The American Journal of Psychiatry, 2014 | All participants (N = 1778) |
Baseline (14.4, SD 0.35) Follow-up (~ 16, SD not reported) |
Impulsivity at age 14 significantly predicted the general externalizing factor at age 16, sensation-seeking at age 14 predicted substance misuse at age 16, and go/no-go commission errors as well as lower BOLD response in bilateral frontal cortex during failed inhibition at age 14 predicted ADHD/conduct disorder at age 16 | |
| Functional neuroimaging predictors of self-reportedpsychotic symptoms in adolescents | Bourque et al. | The American Journal of Psychiatry, 2017 |
Baseline (N = 300) Follow-up (N = 1196) |
Baseline: subjects with psychotic-like symptoms (14.4; SD 0.31) versus no symptoms (14.35; SD 0.38). Follow-up at age 16 |
Emotional faces SST MID |
Youths reporting psychotic-like experiencesshowed increased hippocampus/amygdala activity during processing of neutral faces (EF = 0.987). When controlling for baseline psychotic-like experiences and cannabis use, hyperactivation of the hippocampus/amygdala was the most prominent regional difference at age 16 in participants with mood fluctuation and psychotic symptoms versus subjects without such symptoms. |
| Psychosocial stress and brain function in adolescent psychopathology. | Quinlan et al. | American Journal of Psychiatry, 2017 | All participants (N = 1288) | All participants (14.4, SD 0.40) | Emotional faces task | Conduct or hyperactivity/inattention symptoms in combination with a higher number of stressful life events showed stronger right amygdala activation (EF = 0.1733) |
| Neural correlates of three types of negative life events during angry face processing in adolescents | Gollier-Briant et al. | Social Cognitive and Affective Neuroscience, 2016 |
Baseline (N = 685) Follow-up (N = 523) |
Baseline (14, SD not reported) Follow-up (16, SD not reported) |
Lifetime ‘distress’ positively correlated with orbitofrontal (p = 0.005) and temporal cortex activations (p = 0.007) during angry face processing. | |
| Cannabis use in early adolescence: evidence of amygdala hypersensitivity to signals of threat | Spechler et al. | Developmental Cognitive Neuroscience, 2015 | All participants (N = 140) | All participants (14 SD not reported) | Higher amygdala activation elicited by angry versus neutral faces in cannabis users only, potentially indicating hypersensitivity to stress | |
| Hormonal contraceptives, menstrual cycle and brain response to faces | Mareckova et al. | Social cognitive and affective neuroscience, 2014 | All participants (N = 110) | All participants (14.5, SD not reported) | Response in the left FFA elicited by emotional faces was higher in the group taking contraceptives versus freely cycling females and during mid-cycle versus menstruation (EF = 0.49) | |
| Do you see what i see? Sex differences in the discrimination of facial emotions during adolescence | Lee et al. | Emotion, 2013 | All participants (N = 1951) | All participants (14 SD not reported) | Female participants showed faster and more sensitive perception of facial emotions than boys. Both sexes overidentified happiness and anger | |
| Creating probabilistic maps of the face network in the adolescent brain: a multicenter functional MRI study | Tahmasebi et al. | Human Brain Mapping, 2012 | All participants (N = 1110) | All participants (14.5, SD 0.4) | Identification of 21 brain regions with high probability for responding to faces. Stronger neural response to ambiguous faces in the fusiform face area and further regions in female versus male adolescents, slightly stronger response to angry faces in the amygdala of male versus female adolescents | |
| Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents | Schneider et al. | Neuroimage, 2011 | Female (N = 235) Male (N = 235) |
Female(14.48, SD 0.4) Male (14.47, SD 0.4) |
Emotional faces elicit stronger right amygdale activation in males versus females (EF = 0.3279) |
Another 41 papers investigated methodological or statistical approaches or the associations of some other variables not listed above (e.g., maternal smoking and video gaming) on brain imaging parameters and behavioral variables and therefore were not included in the supplementary tables [12, 78–117].
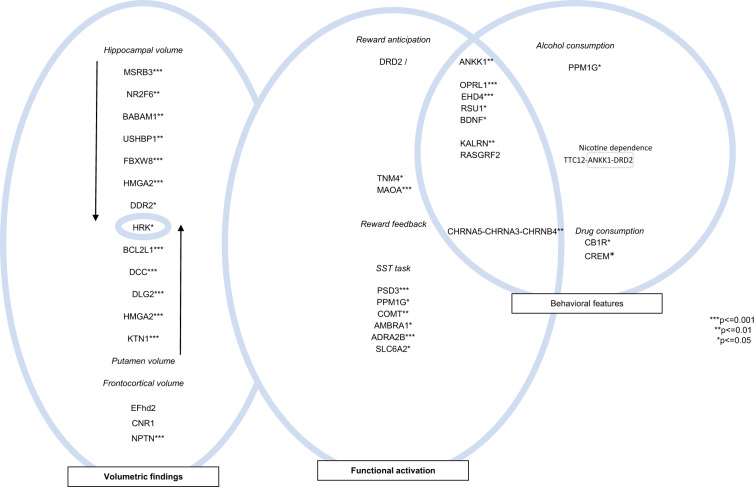
From the multitude of findings, we here focus on brain regions relevant for drug use, including the hippocampus, striatum and frontal cortex. Drug use frequently starts during adolescence and has been a focus of IMAGEN research [55–59]. Figure 2 illustrates the potential of a simultaneous assessment of the effect of genetic variations on brain structure, function and behavior, using as an example the effects of genetic variation on (1) the volume of frontal, hippocampal, amygdala and striatal brain areas, (2) functional activation during reward anticipation and feedback, behavioral inhibition, and (3) alcohol, tobacco, and cannabis consumption. We also indicate whether structure or function of the respective brain regions were themselves directly associated with drug consumption.
Fig. 2. Effect of genetic variations on brain structure, function and behavior.
Genes and epigenetic modifications associated with brain structure (hippocampus and putamen volume), functional activation (reward anticipation and feedback as elicited by the MID task; SST task) and behavior (alcohol consumption, nicotine and cannabis consumption).
Genotype effects on brain structure relevant for drug use
With respect to brain structure, frontocortical, hippocampal, and striatal (especially putamen) volumes were associated with variation in genes that contribute to metabolic and endocrine function, cell cycle and apoptosis, neurodevelopment and neurotransmission (Table 1). Notably, only variation in the HRK (“Between Harakiri, BCL2 Interacting Protein”) gene, which has been associated with cell cycle and apoptosis [20], contributed to both hippocampus and putamen volume [4, 25] (Fig. 2), indicating that the genetic contribution to brain volumes in adolescence may be rather site-specific. The other polymorphisms associated with brain volume at age 14 contribute to metabolic and endocrine function (APOE), cell cycle and apoptosis (BABAM1, FBXW8, HMGA2, DDR2, HRK, BCL2L1, HMGA2, and Efhd2), neurodevelopment (NR2F6, USHBP1, DCC, and FAT3), and neurotransmission (DLG2, CHR1, CNR1, and NPTN). The longitudinal design of the IMAGEN study will help to assess whether the effects of these genetic variations on brain volume are also age dependent [4, 14, 19, 22–28].
Genotype effects on functional brain activation relevant for drug use
With respect to reward anticipation and feedback (Table 2 and Fig. 2), functional activation elicited by the MID task was associated with polymorphisms in genes influencing neurodevelopment (TNM4 and BDNF) [15, 39] and neurotransmission (DRD2/ANKK1and MAOA) [36, 38]. Further, lower striatal activation during reward anticipation was associated with a high risk-taking bias (i.e., a strong tendency to engage in risky behavior) [59], while no significant difference was observed between adolescents with and without family history of alcohol use disorder [56].
With respect to the SST, distinct brain networks were associated with drug use versus attention deficit-hyperactivity symptoms; this study also reported that genetic variation in the norepinephrine transporter gene SLC6A2 was associated with the use of illegal substances but not functional activation elicited by behavioral inhibition [18]. The Arf6 activator Efa6/PSD3 was associated with ethanol-induced sedation and reduced tolerance development in drosophila and with altered prefrontal cortex activation during behavioral inhibition in the IMAGEN sample [46]. Moreover, epigenetic variation in the PPM1G gene locus was associated with increased functional activation of the right subthalamic nucleus during behavioral inhibition [16], and epigenetic modification of the OPRL1 gene mediated the effect of psychosocial stress and neutral striatal activation in the MID task on binge drinking in adolescence [31].
Regarding the EF paradigm, genetic variation in CB1R was associated with activation of the bilateral amygdala to angry faces, which in turn was correlated with higher drug intake [18].
Genotype and other associations with drug use
With respect to alcohol consumption, Fig. 2 shows genes associated with alcohol intake in the 30 days before study inclusion. The EHD4 gene, which has been associated with the cell cycle and apoptosis [21], showed a statistically significant association with alcohol consumption at age 14 [34]. Neuroimaging data additionally showed that low functional activation of the ventral striatum and of the putamen during reward anticipation at age 14 predicted high alcohol intake at age 16, with the association between functional putamen activation and alcohol intake being mediated by the BDNF Val66Met polymorphism [19]. The above mentioned epigenetic variation in the PPM1G gene was associated with high impulsiveness and early escalation of alcohol use [16]. Also, the human ortholog sof the Arf6 and Erf6 genes were associated with increased frequency of drinking and binge drinking episodes [46].
With respect to cannabis and other illegal drugs consumption, genetic variation in the norepinephrine transporter gene was associated with hypoactivation of the right inferior orbitofrontal network and speed of motor inhibition during SST, which was correlated with higher drug intake [18].
With respect to nicotine consumption, lower striatal activation during reward anticipation correlated with prenatal exposure to maternal smoking [57]. Risk variant rs578776, a variant in the CHRNA5–CHRNA3–CHRNB4 gene cluster, influences susceptibility to nicotine dependence by dampening the response of the anterior cingulate cortex to reward feedback, without recruiting the striatum or orbitofrontal cortex during feedback or anticipation [37].
Discussion
The IMAGEN study has identified several genetic polymorphisms that interact with adolescent brain function and behavior, thus helping to disentangle their general functional roles [16, 46, 50]. With 2000 adolescents aged 14 when included in the study, the IMAGEN cohort was the largest sample available to date for imaging genetics analyses, thus improving power compared with previous studies with smaller sample sizes [118]. Due to its longitudinal design, the IMAGEN study can help to reveal gene-environment interactions in the manifestation of mental disorders from adolescence to and young adulthood.
From the large body of IMAGEN publications, we focused on findings of genes interacting with (1) brain volume, (2) functional activation, and (3) drug intake, which tends to start during adolescence and has repeatedly been attributed to gene-environment interactions [18, 55]
To explore psychopathology-relevant brain functions, we selected tasks that probe key aspects of reinforcement-related behaviors and emotional processing implicated in frequent neuropsychiatric disorders: response inhibition, emotional reactivity, and reward sensitivity. The neuroimaging tasks were chosen because they reliably elicit strong activation in functional networks underlying inhibitory control (SST) [119], emotional reactivity to social stimuli (EF) [120] and reward anticipation/outcome (MID) [121]. Some limitations of using these three tasks include incomplete assessment of different aspects of behavioral constructs, e.g., SST measuring motor impulsivity but not delay discounting, which was assesed behaviorally; passive reception of socially salient stimuli (faces) outside of social context; lack of possibilities for computational modeling of reward-related decisions in the MID task.
The IMAGEN study has assessed a wide range of environmental factors including childhood trauma, bullying, stressful life events, family mental health, pre- and peri-natal events, and family conflict. For example, peer victimization was indirectly associated with increased anxiety via decrease in left putamen and caudate volume [114]. Moreover, stressful life events were associated with the interaction between functional amygdala activation elicited by the EF task in adolescents with conduct and hyperactivity symptoms [71]. We here describe genetic and environmental effects on brain structure, function, and behavior assessed in the IMAGEN study.
Regarding reward anticipation and feedback, the IMAGEN consortium focused on polymorphisms implicated in both impulsive and addictive behavior. Reduced ventral striatal activation during reward anticipation has repeatedly been observed in alcohol-dependent patients and may be attributed to dopamine dysfunction following detoxification [2, 122]. However, it was unclear whether reduced activation during reward anticipation is present prior to the development of alcohol use or alcohol use disorder. Here, Büchel et al. [55] observed that reduced functional activation of the ventral striatum, midbrain, and prefrontal cortex at age 14 predicts drug use at age 16. Environmental factors that may contribute to such a blunted ventral striatal activation include maternal smoking during pregnancy [57]. Blunted ventral striatal activation in adolescents has been associated with increased impulsivity [60], a finding that replicates a similar observation in adult alcohol-dependent patients and controls [123]. Moreover, impulsivity has been associated with early life stress, [2] and methylation of the OPRL1 gene was shown to mediate the effect of psychosocial stress on impulsive alcohol intake (binging) and the associated ventrostriatal activation in adolescence [31]. The OPRL1 genotype has repeatedly been replicated on alcohol use disorders [124, 125].
Beyond environmental factors, genetic variance contributes to reduced ventral striatal activation during reward anticipation elicited by cues that predict reward. In humans, Stacey et al. [40] observed an association between reduced functional activation of the ventral striatum and a haplotype block containing the Rasgrf2 polymorphism rs26907, which was associated with alcohol intake in a previous meta-analysis [126]. In animal experiments, Stacey et al. [40] observed that Rasgrf2 knockout mice displayed a significant reduction in ethanol intake relative to wild type controls, lower intake at the highest ethanol concentrations, and blunted diurnal drinking. Rasgrf2 knockout mice also showed a significant reduction in ethanol preference, which was measured by the percentage of ethanol relative to water intake [40]. In addition, Rasgrf knockout mice showed a diminished noradrenergic and serotonergic response in the ventral striatum/nucleus accumbens and a decrease in β1 adrenoreceptor gene expression [127]. Easton and colleagues have shown that Rasgrf2 mediates the presence of noradrenaline (NA) and serotonin (5-HT) in the synaptic cleft at both basal level and after acute alcohol exposure. After subchronic alcohol exposure, the NA system is modified, and Rasgrf2 regulates alterations in expression levels of adrenoceptor mRNA. Rasgrf2 may be a mechanism by which the NA system is prevented from adapting to subchronic alcohol intake, which could in turn influence vulnerability to the effects of repeated alcohol exposure [128]. Moreover, it was shown that Rasgrf2 controls dopaminergic signaling and adaptations to alcohol also in other brain regions, beyond the nucleus accumbens [127]. Another Rasgrf2 polymorphism (rs2369955) was associated with current and future binge drinking in the IMAGEN cohort [19]. Therefore, the IMAGEN findings regarding Rasgrf2 are neurobiologically plausible, however, replication in an independent imaging sample has yet to be done.
These IMAGEN findings can be explained by the fact that Rasgrf2 interacts with activation of the MAPK/ERK pathway, which is involved in neurotransmission through dopamine receptors and transporters and thus potentially associated with dopamine-dependent reward mechanisms in alcoholism [129, 130]. In mouse models, ethanol administration increased ERK activity in the nucleus accumbens, and inhibition of ERK activity influenced ethanol self-administration [19].
Further genetic variance impacting on ventral striatal activation was discovered by Nees et al. [15], who showed that BDNF Val homozygotes compared with Met carriers had lower putamen reactivity during reward anticipation. This is in partial accordance with results from Pecina et al., who also observed effects of BDNF ValMet genotype on functional activation during the anticipations of rewards and losses, albeit only significant during loss anticipation [131]. Lower striatal activation during reward anticipation may impact on personality traits reflecting the orientation towards positive reinforcers. Benzerouk et al. [132] indeed observed that BDNF Val carriers with a positive family history for alcohol use disorders displayed lower levels of reward dependence compared to probands without such a family history. ValMet genotype was also associated with risk of relapse in alcohol dependence, however, no functional brain imaging was performed in the study [133]. Furthermore, the BDNF Val68Met polymorphism regulates BDNF expression and was also implicated in rodent models of uncontrolled and excessive alcohol intake [134]. BDNF expression was lower in the nucleus accumbens [135] and in the central and medial nucleus of the amygdala of alcohol preferring versus non-preferring rats [136], and short versus long-term alcohol intake differentially regulate BDNF mRNA levels in the nucleus accumbens in rats actively versus passively consuming alcohol [137].
With respect to a dimensional approach towards mental disorders [138], the IMAGEN consortium also assessed whether blunted ventral striatal activation elicited by reward indicating cues is associated with psychotic or affective symptoms and disorders. Previous studies showed that low functional activation of the ventral striatum during reward anticipation in patients with depression and schizophrenia was related to the severity of negative mood states including depression and anhedonia [139, 140]. In the IMAGEN adolescents, Stringaris et al. [54] observed that low ventral striatum activation during reward anticipation at age 14 predicted transition to subthreshold or clinical depression in previously healthy adolescents at age 16. Vulser et al. [89] observed that adolescents with subthreshold depression also had smaller gray matter volumes in the ventromedial prefrontal and rostral anterior cingulate cortex as well as in the putamen [25]. In 2018, Vulser et al. showed that early fractional anisotropy variations in tracts projecting from the corpus callosum to the anterior cingulate cortex may denote a higher risk of transition to depression in adolescents [112]. Findings regarding the cingulate cortex volume are interesting in light of their association with major depression [141] and the role of this brain area in error detection and behavioral control [142], which may also play a role in recovery from affective disorders. Indeed, patients with sustained activation elicited by reward feedback across task runs in the anterior cingulate cortex were more responsive to behavioral activation therapy [143].
With respect to higher cognitive functions, Nymberg et al. [36] showed that higher ventral striatal and caudate activation during reward feedback was associated with higher working memory performance and that this interaction was limited to A allele carriers of the DRD2/ANKK1 polymorphism. Also Taq1A of the ANKK1 gene interacted with a lateral orbitofrontal activation during reward anticipation [45] and predicted alcohol drinking 2 years later [105]. These findings provide evidence for an interaction between reward processing and complex cognitive capacities [144, 145]. Differences in striatal dopaminergic neurotransmission have also been associated with differences in working memory performance [146, 147], in line with findings concerning dopaminergic markers and cognitive capacity [65].
Beyond reward anticipation and feedback, impulsivity as operationalized by behavioral inhibition has been implicated in the development and maintenance of substance use disorders [148]. The subthalamic nucleus modifies information processing in fronto-striatal networks relevant for impulse control [148, 149]. Increased functional activation of the subthalamic nucleus was associated with hypermethylation in the PPM1G gene, which also correlated with increased impulsiveness and alcohol use in adolescence [16]. Further brain areas implicated in behavioral inhibition are the prefrontal cortex [18], whose activation was associated with the human ortholog of Arf6 and orthologs of Efa6 (PSD1-4) [46].
Regarding the processing of affective faces, the study of Spechler et al. shows that stronger amygdala activation to signals of threat was associated with high cannabis intake. This observation points to another neurobiological dimension contributing both to negative mood states and drug intake [72]. Indeed, increased amygdala activation towards aversive stimuli has been shown to contribute to anxiety, feelings of being threatened and aggression, which in return predict excessive alcohol use [148]. Stronger amygdala activation during emotional processing was also observed in adolescents who experienced both a high number of stressful life events and strong symptoms of conduct disorder and hyperactivity [71], highlighting how experience can modulate brain-behavior relationships.
Overall, the IMAGEN consortium was able to detect a number of genetic polymorphisms with specific effects on brain structure and function and their association with symptoms of mental disorders in adolescents. IMAGEN’s study design has supported the elucidation of biological and environmental mechanisms of substance use-related behaviors, but we note limitations and ways to address them. The rather large-sample size overcomes some of the limitations of previous genetic imaging studies, however, independent replications are required. As studies like IMAGEN have their data repeatedly used by researchers, which—if systematic errors existed in the data would then be propagated into all research output, strict quality assurance and quality control measures are taken by the central database team that has overseen data management across all study time points. This allows for quick identification and resolution of errors that could occur before data are released for analyses (i.e., data entry and data upload/transfer).
The very nature of multivariate multimodal approaches bears a multiple comparison issue. Fortunately, clinical samples and other large phenotypically rich studies such as UK Biobank provide the opportunity to externally validate IMAGEN findings. Resampling methods such as bootstrapping and cross-validation (e.g., leave-one-out and split-half) are techniques we have used to internally validate model fit and estimate a model’s generalizability [41]. Moreover, the IMAGEN consortium asks for research proposals before data access is granted, and thus prevents unethical data dredging. Nonetheless, exploratory data analysis can be performed and has to be clearly described as such. Replication of such a cohort might be very costly, that is why rigorous statistics including the above mentioned internal cross-validation for estimation of predictive power and the reporting of confidence intervals in order to describe a range of plausible estimates should be promoted. With respect to intraindividual variance, reliability measures as well as developmental changes replications can be carried out within the IMAGEN sample, e.g., by assessing whether the same polymorphisms impact on subcortical brain volumes at age 14 and 16 or in young adulthood. Given the multisite design, variance from different acquisition sites were considered based on a central protocol.
The issue of participant attrition over time may limit some longitudinal multimodal statistical modeling and internal validation methods (i.e., due to reduced power and incomplete data), but with more than 1300 participants assessed at age 22, there is still great opportunity to understand the influence of biology and the environment on developmental trajectories of substance use. Relevant to identifying gene-environment interactions on brain and behavior, the relatively homogeneous socioeconomic background of the Western European participating families meant IMAGEN was not well placed to investigate particular environmental influences such as nutrition or hazardous exposures. Furthermore, no resources were available to independently confirm the reliability of the self-reported environmental data.
For the purpose of genetic analyses, the IMAGEN participants are of European ancestry—as a result the generalizability of findings to other cultures and stress/environmental exposures is unknown. Therefore, future replications should also be carried out outside of Europe, requiring careful harmonization of clinical tools and paradigms. As recently reported [150], the IMAGEN study has aligned itself with other global neuroimaging-genetics adolescent cohorts that will further enhance and clarify the work presented here.
Acknowlegements
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behavior in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAgingGEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant ‘c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the BundesministeriumfürBildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL 01EE1406A, 01EE1406B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940/2, TRR 265), the Medical Research Foundation and Medical research council (grant MR/R00465X/1). Further support was provided by grants from: ANR (project AF12-NEUR0008-01—WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la RechercheMédicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), USA (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence. Open access funding provided by Projekt DEAL.
IMAGEN consortium
Lisa Albrecht1, Chris Andrew2, Mercedes Arroyo19, Eric Artiges12, Semiha Aydin11, Christine Bach3, Tobias Banaschewski3, Alexis Barbot20, Gareth Barker2, Nathalie Boddaert12, Arun Bokde12, Zuleima Bricaud12, Uli Bromberg6, Ruediger Bruehl11, Christian Büchel6, Arnaud Cachia12, Anna Cattrell2, Patricia Conrod2, Patrick Constant21, Jeffrey Dalley19, Benjamin Decideur9, Sylvane Desrivieres2, Tahmine Fadai6, Herta Flor3, Vincent Frouin9, Jürgen Gallinat6, Hugh Garavan5, Fanny Gollier Briand12, Penny Gowland22, Bert Heinrichs23, Andreas Heinz1, Nadja Heym22, Thomas Hübner16, James Ireland24, Bernd Ittermann11, Tianye Jia2, Mark Lathrop25, Dirk Lanzerath23, Claire Lawrence22, Hervé Lemaitre12, Katharina Lüdemann1, Christine Macare2, Catherine Mallik2, Jean-François Mangin12, Karl Mann3, Jean-Luc Martinot12, Eva Mennigen16, Fabiana Mesquita de Carvahlo2, Xavier Mignon21, Ruben Miranda12, Kathrin Müller16, Frauke Nees3, Charlotte Nymberg2, Marie-Laure Paillere12, Tomas Paus14, Zdenka Pausova14, Jean-Baptiste Poline20, Luise Poustka15, Michael Rapp1, Gabriel Robert2, Jan Reuter1, Marcella Rietschel3, Stephan Ripke1, Trevor Robbins19, Sarah Rodehacke16, John Rogers24, Alexander Romanowski1, Barbara Ruggeri2, Christine Schmäl3, Dirk Schmidt16, Sophia Schneider6, MarkGunter Schumann2, Florian Schubert11, Yannick Schwartz20, Michael Smolka16, Wolfgang Sommer3, Rainer Spanagel3, Claudia Speiser26, Tade Spranger23, Alicia Stedman22, Sabina Steiner3, Dai Stephens27, Nicole Strache1, Andreas Ströhle1, Maren Struve17, Naresh Subramaniam19, Lauren Topper2, Henrik Walter1, Robert Whelan17, Steve Williams2, Juliana Yacubian6, Monica Zilbovicius12, C. Peng Wong2, Steven Lubbe2, Lourdes Martinez-Medina2, Alinda Fernandes2, Amir Tahmasebi14
Compliance with ethical standards
Conflict of interest
TB has served as an advisor or consultant to Bristol-Myers Squibb, DesitinArzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. GB has received honoraria from General Electric Healthcare for teaching on scanner programming courses and acts as a consultant for IXICO. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Members of the IMAGEN consortium are listed below Acknowledgements.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lea Mascarell Maričić, Henrik Walter, Gunter Schumann, Andreas Heinz
Contributor Information
Andreas Heinz, Email: andreas.heinz@charite.de.
IMAGEN consortium:
Lisa Albrecht, Chris Andrew, Mercedes Arroyo, Eric Artiges, Semiha Aydin, Christine Bach, Tobias Banaschewski, Alexis Barbot, Gareth Barker, Nathalie Boddaert, Arun Bokde, Zuleima Bricaud, Uli Bromberg, Ruediger Bruehl, Christian Büchel, Arnaud Cachia, Anna Cattrell, Patricia Conrod, Patrick Constant, Jeffrey Dalley, Benjamin Decideur, Sylvane Desrivieres, Tahmine Fadai, Herta Flor, Vincent Frouin, Jürgen Gallinat, Hugh Garavan, Fanny Gollier Briand, Penny Gowland, Bert Heinrichs, Andreas Heinz, Nadja Heym, Thomas Hübner, James Ireland, Bernd Ittermann, Tianye Jia, Mark Lathrop, Dirk Lanzerath, Claire Lawrence, Hervé Lemaitre, Katharina Lüdemann, Christine Macare, Catherine Mallik, Jean-François Mangin, Karl Mann, Jean-Luc Martinot, Eva Mennigen, Fabiana Mesquita de Carvahlo, Xavier Mignon, Ruben Miranda, Kathrin Müller, Frauke Nees, Charlotte Nymberg, Marie-Laure Paillere, Tomas Paus, Zdenka Pausova, Jean-Baptiste Poline, Luise Poustka, Michael Rapp, Gabriel Robert, Jan Reuter, Marcella Rietschel, Stephan Ripke, Trevor Robbins, Sarah Rodehacke, John Rogers, Alexander Romanowski, Barbara Ruggeri, Christine Schmäl, Dirk Schmidt, Sophia Schneider, MarkGunter Schumann, Florian Schubert, Yannick Schwartz, Michael Smolka, Wolfgang Sommer, Rainer Spanagel, Claudia Speiser, Tade Spranger, Alicia Stedman, Sabina Steiner, Dai Stephens, Nicole Strache, Andreas Ströhle, Maren Struve, Naresh Subramaniam, Lauren Topper, Henrik Walter, Robert Whelan, Steve Williams, Juliana Yacubian, Monica Zilbovicius, C. Peng Wong, Steven Lubbe, Lourdes Martinez-Medina, Alinda Fernandes, and Amir Tahmasebi
References
- 1.Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 2.Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, et al. In Vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. Am J Psychiatry. 1998;155:1023–8. doi: 10.1176/ajp.155.8.1023. [DOI] [PubMed] [Google Scholar]
- 3.Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvälahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 4.Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–61. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laruelle M, Gelernter J, Innis RB. D2 receptors binding potential is not affected by Taq1 polymorphism at the D2 receptor gene. Mol Psychiatry. 1998;3:261–5. doi: 10.1038/sj.mp.4000343. [DOI] [PubMed] [Google Scholar]
- 6.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose EJ, Donohoe G. Brain vs behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull. 2013;39:518–26. doi: 10.1093/schbul/sbs056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–27. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, et al. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol Psychiatry. 2013;18:512–20. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
- 10.Reimold M, Knobel A, Rapp MA, Batra A, Wiedemann K, Ströhle A, et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology. 2011;213:563–72. doi: 10.1007/s00213-010-1903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobiella A, Reimold M, Ulshöfer DE, Ikonomidou VN, Vollmert C, Vollstädt-Klein S, et al. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry. 2011;1:e37. doi: 10.1038/tp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–39. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 13.ABCD Study. 2019. https://abcdstudy.org/.
- 14.French L, Gray C, Leonard G, Perron M, Pike GB, Richer L, et al. Early Cannabis use, polygenic risk score for Schizophrenia, and brain maturation in adolescence. JAMA Psychiatry. 2015;72:1002–11. doi: 10.1001/jamapsychiatry.2015.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nees F, Witt SH, Dinu-Biringer R, Lourdusamy A, Tzschoppe J, Vollstädt-Klein S, et al. BDNF Val66Met and reward-related brain function in adolescents: role for early alcohol consumption. Alcohol. 2015;49:103–10. doi: 10.1016/j.alcohol.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Ruggeri B, Nymberg C, Vuoksimaa E, Lourdusamy A, Wong CP, Carvalho FM, et al. Association of protein phosphatase PPM1G with alcohol use disorder and brain activity during behavioral control in a genome-wide methylation analysis. Am J Psychiatry. 2015;172:543–52. doi: 10.1176/appi.ajp.2014.14030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–13. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 18.Whelan R, Conrod PJ, Poline J-B, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–5. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 19.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–9. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura M, Shimada K, Konishi N. The role of HRK gene in human cancer. Oncogene. 2009;27:S105. doi: 10.1038/onc.2009.48. [DOI] [PubMed] [Google Scholar]
- 21.George M, Rainey MA, Naramura M, Foster KW, Holzapfel MS, Willoughby LL, et al. Renal thrombotic microangiopathy in mice with combined deletion of endocytic recycling regulators EHD3 and EHD4. PLoS ONE. 2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3052385/. [DOI] [PMC free article] [PubMed]
- 22.Khan W, Giampietro V, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, et al. A multi-cohort study of ApoE ɛ4 and Amyloid-β effects on the hippocampus in Alzheimer’s disease. J Alzheimers Dis. 2017;56:1159–74. doi: 10.3233/JAD-161097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan W, Giampietro V, Ginestet C, Dell’Acqua F, Bouls D, Newhouse S, et al. No differences in Hippocampal volume between carriers and non-carriers of the ApoE ε4 and ε2 alleles in young healthy adolescents. J Alzheimers Dis. 2014;40(Jan):37–43. doi: 10.3233/JAD-131841. [DOI] [PubMed] [Google Scholar]
- 24.Hass J, Walton E, Kirsten H, Liu J, Priebe L, Wolf C, et al. A genome-wide association study suggests novel loci associated with a Schizophrenia-Related brain-based phenotype. PLoS ONE. 2013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3689744/. [DOI] [PMC free article] [PubMed]
- 25.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–9. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mielenz D, Reichel M, Jia T, Quinlan EB, Stöckl T, Mettang M, et al. EFhd2/Swiprosin-1 is a common genetic determinator for sensation-seeking/low anxiety and alcohol addiction. Mol Psychiatry. 2017. https://www.nature.com/mp/journal/vaop/ncurrent/full/mp201763a.html. [DOI] [PMC free article] [PubMed]
- 27.Desrivières S, Lourdusamy A, Tao C, Toro R, Jia T, Loth E, et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol Psychiatry. 2015;20:263–74. doi: 10.1038/mp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dell’Acqua F, Khan W, Gottlieb N, Giampietro V, Ginestet C, Bouls D, et al. Tract based spatial statistic reveals no differences in white matter microstructural organization between carriers and non-carriers of the APOE ɛ4 and ɛ2 alleles in young healthy adolescents. J Alzheimers Dis. 2015;47:977–84. doi: 10.3233/JAD-140519. [DOI] [PubMed] [Google Scholar]
- 29.Richiardi J, Altmann A, Milazzo A-C, Chang C, Chakravarty MM, Banaschewski T, et al. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–4. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemmi F, Nymberg C, Darki F, Banaschewski T, Bokde ALW, Büchel C, et al. Interaction between striatal volume and DAT1 polymorphism predicts working memory development during adolescence. Dev Cogn Neurosci. 2018;30:191–9. doi: 10.1016/j.dcn.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruggeri B, Macare C, Stopponi S, Jia T, Carvalho FM, Robert G, et al. Methylation of OPRL1 mediates the effect of psychosocial stress on binge drinking in adolescents. J Child Psychol Psychiatry. 2017;59:650–8. doi: 10.1111/jcpp.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B, Jia T, Macare C, Banaschewski T, Bokde ALW, Bromberg U, et al. Impact of a common genetic variation associated with putamen volume on neural mechanisms of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2017;56:436–444.e4. doi: 10.1016/j.jaac.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Nees F, Becker S, Millenet S, Banaschewski T, Poustka L, Bokde A, et al. Brain substrates of reward processing and the μ-opioid receptor: a pathway into pain? PAIN. 2017;158:212. doi: 10.1097/j.pain.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 34.Stacey D, Lourdusamy A, Ruggeri B, Maroteaux M, Jia T, Cattrell A, et al. A translational systems biology approach in both animals and humans identifies a functionally related module of accumbal genes involved in the regulation of reward processing and binge drinking in males. J Psychiatry Neurosci Jpn. 2016;41:192–202. doi: 10.1503/jpn.150138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci USA. 2015;112:E4085–93. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nymberg C, Banaschewski T, Bokde AL, Büchel C, Conrod P, Flor H, et al. DRD2/ANKK1 polymorphism modulates the effect of ventral striatal activation on working memory performance. Neuropsychopharmacology. 2014;39:2357–65. doi: 10.1038/npp.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nees F, Witt SH, Lourdusamy A, Vollstädt-Klein S, Steiner S, Poustka L, et al. Genetic risk for nicotine dependence in the cholinergic system and activation of the brain reward system in healthy adolescents. Neuropsychopharmacology. 2013;38:2081–9. doi: 10.1038/npp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nymberg C, Jia T, Lubbe S, Ruggeri B, Desrivieres S, Barker G, et al. Neural mechanisms of attention-deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biol Psychiatry. 2013;74:607–14. doi: 10.1016/j.biopsych.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich A, Lourdusamy A, Tzschoppe J, Vollstädt-Klein S, Bühler M, Steiner S, et al. The risk variant in ODZ4 for bipolar disorder impacts on amygdala activation during reward processing. Bipolar Disord. 2013;15:440–5. doi: 10.1111/bdi.12068. [DOI] [PubMed] [Google Scholar]
- 40.Stacey D, Bilbao A, Maroteaux M, Jia T, Easton AC, Longueville S, et al. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc Natl Acad Sci USA. 2012;109:21128–33. doi: 10.1073/pnas.1211844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia T, Macare C, Desrivières S, Gonzalez DA, Tao C, Ji X, et al. Neural basis of reward anticipation and its genetic determinants. Proc Natl Acad Sci USA. 2016;113:3879–84. doi: 10.1073/pnas.1503252113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peña-Oliver Y, Carvalho FM, Sanchez-Roige S, Quinlan EB, Jia T, Walker-Tilley T, et al. Mouse and human genetic analyses associate kalirin with ventral striatal activation during impulsivity and with alcohol misuse. Front Genet. 2016;7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4823271/. [DOI] [PMC free article] [PubMed]
- 43.Duka T, Nikolaou K, King SL, Banaschewski T, Bokde ALW, Büchel C, et al. GABRB1 single nucleotide polymorphism associated with altered brain responses (but not performance) during measures of impulsivity and reward sensitivity in human adolescents. Front Behav Neurosci. 2017;11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5309221/. [DOI] [PMC free article] [PubMed]
- 44.Macare C, Ducci F, Zhang Y, Ruggeri B, Jia T, Kaakinen M, et al. A neurobiological pathway to smoking in adolescence: TTC12-ANKK1-DRD2 variants and reward response. Eur Neuropsychopharmacol. 2018;28:1103–14. doi: 10.1016/j.euroneuro.2018.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker TE, Castellanos-Ryan N, Schumann G, Cattrell A, Flor H, Nees F, et al. Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence. Psychol Med. 2019;49:801–10. doi: 10.1017/S0033291718001459. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez DA, Jia T, Pinzón JH, Acevedo SF, Ojelade SA, Xu B, et al. The Arf6 activator Efa6/PSD3 confers regional specificity and modulates ethanol consumption in Drosophila and humans. Mol Psychiatry. 2017. https://www.nature.com/mp/journal/vaop/ncurrent/full/mp2017112a.html. [DOI] [PMC free article] [PubMed]
- 47.White TP, Loth E, Rubia K, Krabbendam L, Whelan R, Banaschewski T, et al. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology. 2014;39:2560–9. doi: 10.1038/npp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinrich A, Nees F, Lourdusamy A, Tzschoppe J, Meier S, Vollstädt-Klein S, et al. From gene to brain to behavior: schizophrenia-associated variation in AMBRA1 alters impulsivity-related traits. Eur J Neurosci. 2013;38:2941–5. doi: 10.1111/ejn.12201. [DOI] [PubMed] [Google Scholar]
- 49.Abstract_Whelan.pdf; 2017. http://91.206.61.6/media/Abstract_Whelan.pdf.
- 50.Ewald A, Becker S, Heinrich A, Banaschewski T, Poustka L, Bokde A, et al. The role of the cannabinoid receptor in adolescents’ processing of facial expressions. Eur J Neurosci. 2016;43:98–105. doi: 10.1111/ejn.13118. [DOI] [PubMed] [Google Scholar]
- 51.Loth E, Poline J-B, Thyreau B, Jia T, Tao C, Lourdusamy A, et al. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol Psychiatry. 2014;76:367–76. doi: 10.1016/j.biopsych.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 52.Dickie EW, Tahmasebi A, French L, Kovacevic N, Banaschewski T, Barker GJ, et al. Global genetic variations predict brain response to faces. PLoS Genet. 2014 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4133042/. [DOI] [PMC free article] [PubMed]
- 53.Velthorst E, Froudist-Walsh S, Stahl E, Ruderfer D, Ivanov I, Buxbaum J, et al. Genetic risk for schizophrenia and autism, social impairment and developmental pathways to psychosis. Transl Psychiatry. 2018;8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6158250/. [DOI] [PMC free article] [PubMed]
- 54.Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry. 2015;172:1215–23. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Büchel C, Peters J, Banaschewski T, Bokde ALW, Bromberg U, Conrod PJ, et al. Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat Commun. 2017;8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5321762/. [DOI] [PMC free article] [PubMed]
- 56.Müller KU, Gan G, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, et al. No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addict Biol. 2015;20:534–45. doi: 10.1111/adb.12136. [DOI] [PubMed] [Google Scholar]
- 57.Müller KU, Mennigen E, Ripke S, Banaschewski T, Barker GJ, Büchel C, et al. Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking. JAMA Psychiatry. 2013;70:847–56. doi: 10.1001/jamapsychiatry.2013.44. [DOI] [PubMed] [Google Scholar]
- 58.Nees F, Vollstädt-Klein S, Fauth-Bühler M, Steiner S, Mann K, Poustka L, et al. A target sample of adolescents and reward processing: same neural and behavioral correlates engaged in common paradigms? Exp Brain Res. 2012;223:429–39. doi: 10.1007/s00221-012-3272-8. [DOI] [PubMed] [Google Scholar]
- 59.Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 60.Schneider S, Brassen S, Bromberg U, Banaschewski T, Conrod P, Flor H, et al. Maternal interpersonal affiliation is associated with adolescents’ brain structure and reward processing. Transl Psychiatry. 2012;2:e182. doi: 10.1038/tp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–9. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- 62.Nees F, Tzschoppe J, Patrick CJ, Vollstädt-Klein S, Steiner S, Poustka L, et al. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–95. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikita N, Simonoff E, Pine DS, Goodman R, Artiges E, Banaschewski T, et al. Disentangling the autism-anxiety overlap: fMRI of reward processing in a community-based longitudinal study. Transl Psychiatry. 2016;6:e845. doi: 10.1038/tp.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jollans L, Zhipeng C, Icke I, Greene C, Kelly C, Banaschewski T, et al. Ventral striatum connectivity during reward anticipation in adolescent smokers. Dev Neuropsychol. 2016;41:6–21. doi: 10.1080/87565641.2016.1164172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaminski JA, Schlagenhauf F, Rapp M, Awasthi S, Ruggeri B, Deserno L, et al. Epigenetic variance in dopamine D2 receptor: a marker of IQ malleability? Transl Psychiatry. 2018;8:1–11. doi: 10.1038/s41398-018-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papanastasiou E, Mouchlianitis E, Joyce DW, McGuire P, Banaschewski T, Bokde ALW, et al. Examination of the neural basis of psychoticlike experiences in adolescence during reward processing. JAMA Psychiatry. 2018;75:1043–51. doi: 10.1001/jamapsychiatry.2018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde ALW, et al. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry. 2014;171:1310–9. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- 68.Deng W, Rolls ET, Ji X, Robbins TW, Banaschewski T, Bokde ALW, et al. Separate neural systems for behavioral change and for emotional responses to failure during behavioral inhibition. Hum Brain Mapp. 2017;38:3527–37. doi: 10.1002/hbm.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayard F, Thunell CN, Abé C, Almeida R, Banaschewski T, Barker G, et al. Distinct brain structure and behavior related to ADHD and conduct disorder traits. Mol Psychiatry. 2018. 10.1038/s41380-018-0202-6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 70.Bourque J, Spechler PA, Potvin S, Whelan R, Banaschewski T, Bokde ALW, et al. Functional neuroimaging predictors of self-reported psychotic symptoms in adolescents. Am J Psychiatry. 2017;174:566–75. doi: 10.1176/appi.ajp.2017.16080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinlan EB, Cattrell A, Jia T, Artiges E, Banaschewski T, Barker G, et al. Psychosocial stress and brain function in adolescent psychopathology. Am J Psychiatry. 2017;174:785–94. doi: 10.1176/appi.ajp.2017.16040464. [DOI] [PubMed] [Google Scholar]
- 72.Spechler PA, Orr CA, Chaarani B, Kan K-J, Mackey S, Morton A, et al. Cannabis use in early adolescence: evidence of amygdala hypersensitivity to signals of threat. Dev Cogn Neurosci. 2015;16:63–70. doi: 10.1016/j.dcn.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee NC, Krabbendam L, White TP, Meeter M, Consortium I, Schumann G, et al. Do you see what I see? Sex differences in the discrimination of facial emotions during adolescence. Emotion. 2013;2013:1030–40. doi: 10.1037/a0033560. [DOI] [PubMed] [Google Scholar]
- 74.Tahmasebi AM, Artiges E, Banaschewski T, Barker GJ, Bruehl R, Büchel C, et al. Creating probabilistic maps of the face network in the adolescent brain: A multicentre functional MRI study. Hum Brain Mapp. 2012;33:938–57. doi: 10.1002/hbm.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, et al. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. NeuroImage. 2011;56:1847–53. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 76.Marečková K, Perrin JS, Nawaz Khan I, Lawrence C, Dickie E, McQuiggan DA, et al. Hormonal contraceptives, menstrual cycle and brain response to faces. Soc Cogn Affect Neurosci. 2014;9:191–200. doi: 10.1093/scan/nss128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gollier-Briant F, Paillère-Martinot M-L, Lemaitre H, Miranda R, Vulser H, Goodman R, et al. Neural correlates of three types of negative life events during angry face processing in adolescents. Soc Cogn Affect Neurosci. 2016;11:1961–9. doi: 10.1093/scan/nsw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fauth-Bühler M, de Rover M, Rubia K, Garavan H, Abbott S, Clark L, et al. Brain networks subserving fixed versus performance-adjusted delay stop trials in a stop signal task. Behav Brain Res. 2012;235:89–97. doi: 10.1016/j.bbr.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 79.Stringaris A, Castellanos-Ryan N, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, et al. Dimensions of manic symptoms in youth: psychosocial impairment and cognitive performance in the IMAGEN sample. J Child Psychol Psychiatry. 2014;55:1380–9. doi: 10.1111/jcpp.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thyreau B, Schwartz Y, Thirion B, Frouin V, Loth E, Vollstädt-Klein S, et al. Very large fMRI study using the IMAGEN database: sensitivity–specificity and population effect modeling in relation to the underlying anatomy. NeuroImage. 2012;61:295–303. doi: 10.1016/j.neuroimage.2012.02.083. [DOI] [PubMed] [Google Scholar]
- 81.Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, et al. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–38. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- 82.Fritsch V, Da Mota B, Loth E, Varoquaux G, Banaschewski T, Barker GJ, et al. Robust regression for large-scale neuroimaging studies. NeuroImage. 2015;111:431–41. doi: 10.1016/j.neuroimage.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 83.Schilling C, Kühn S, Romanowski A, Banaschewski T, Barbot A, Barker GJ, et al. Common structural correlates of trait impulsiveness and perceptual reasoning in adolescence. Hum Brain Mapp. 2013;34:374–83. doi: 10.1002/hbm.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schilling C, Kühn S, Paus T, Romanowski A, Banaschewski T, Barbot A, et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol Psychiatry. 2013;18:624–30. doi: 10.1038/mp.2012.56. [DOI] [PubMed] [Google Scholar]
- 85.Ng B, Baptiste Poline J, Thirion B, Greicius M. Bootstrapped permutation test for multiresponse inference on brain behavior associations. In: Ourselin S, Alexander DC, Westin C-F, Cardoso MJ, editors. Information processing in medical imaging. Sabhal Mor Ostaig, Isle of Skye, United Kingdom: Springer; 2015. p. 12. (Lecture Notes in Computer Science; vol. 9123). https://hal.inria.fr/hal-01185206. [DOI] [PubMed]
- 86.Ortuño-Sierra J, Fonseca-Pedrero E, Aritio-Solana R, Velasco AM, Luis EC, de, Schumann G, et al. New evidence of factor structure and measurement invariance of the SDQ across five European nations. Eur Child Adolesc Psychiatry. 2015;24:1523–34. doi: 10.1007/s00787-015-0729-x. [DOI] [PubMed] [Google Scholar]
- 87.Jurk S, Kuitunen-Paul S, Kroemer NB, Artiges E, Banaschewski T, Bokde ALW, et al. Personality and substance use: psychometric evaluation and validation of the substance use risk profile scale (SURPS) in English, Irish, French, and German Adolescents. Alcohol Clin Exp Res. 2015;39:2234–48. doi: 10.1111/acer.12886. [DOI] [PubMed] [Google Scholar]
- 88.Ducci F, Kaakinen M, Pouta A, Hartikainen A-L, Veijola J, Isohanni M, et al. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behaviour from adolescence to mid-adulthood. Biol Psychiatry. 2011;69:650–60. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vulser H, Lemaitre H, Artiges E, Miranda R, Penttilä J, Struve M, et al. Subthreshold depression and regional brain volumes in young community adolescents. J Am Acad Child Adolesc Psychiatry. 2015;54:832–40. doi: 10.1016/j.jaac.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galinowski A, Miranda R, Lemaitre H, Martinot M-LP, Artiges E, Vulser H, et al. Resilience and corpus callosum microstructure in adolescence. Psychol Med. 2015;45:2285–94. doi: 10.1017/S0033291715000239. [DOI] [PubMed] [Google Scholar]
- 91.Kühn S, Witt C, Banaschewski T, Barbot A, Barker GJ, Büchel C, et al. From mother to child: orbitofrontal cortex gyrification and changes of drinking behaviour during adolescence. Addict Biol. 2016;21:700–8. doi: 10.1111/adb.12240. [DOI] [PubMed] [Google Scholar]
- 92.O’Leary-Barrett M, Pihl RO, Artiges E, Banaschewski T, Bokde ALW, Büchel C, et al. Personality, attentional biases towards emotional faces and symptoms of mental disorders in an adolescent sample. PLoS ONE. 2015. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4457930/. [DOI] [PMC free article] [PubMed]
- 93.Paillère Martinot M-L, Lemaitre H, Artiges E, Miranda R, Goodman R, Penttilä J, et al. White-matter microstructure and gray-matter volumes in adolescents with subthreshold bipolar symptoms. Mol Psychiatry. 2014;19:462–70. doi: 10.1038/mp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Da Mota B, Fritsch V, Varoquaux G, Banaschewski T, Barker GJ, Bokde ALW, et al. Randomized parcellation based inference. NeuroImage. 2013;1:203–15. doi: 10.1016/j.neuroimage.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 95.Tzschoppe J, Nees F, Banaschewski T, Barker GJ, Büchel C, Conrod PJ, et al. Aversive learning in adolescents: modulation by amygdala–prefrontal and amygdala–hippocampal connectivity and neuroticism. Neuropsychopharmacology. 2014;39:875–84. [Google Scholar]
- 96.Kühn S, Lorenz R, Banaschewski T, Barker GJ, Büchel C, Conrod PJ, et al. Positive association of video game playing with left frontal cortical thickness in adolescents. PLoS ONE. 2014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3954649/. [DOI] [PMC free article] [PubMed]
- 97.Montigny C, Castellanos-Ryan N, Whelan R, Banaschewski T, Barker GJ, Büchel C, et al. A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS ONE. 2013 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3828212/. [DOI] [PMC free article] [PubMed]
- 98.Kühn S, Romanowski A, Schilling C, Banaschewski T, Barbot A, Barker GJ, et al. Manual dexterity correlating with right lobule VI volume in right-handed 14-year-olds. NeuroImage. 2012;59:1615–21. doi: 10.1016/j.neuroimage.2011.08.100. [DOI] [PubMed] [Google Scholar]
- 99.Mackey S, Chaarani B, Kan K-J, Spechler PA, Orr C, Banaschewski T, et al. Brain regions related to impulsivity mediate the effects of early adversity on antisocial behavior. Biol Psychiatry. 2017;82:275–82. doi: 10.1016/j.biopsych.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 100.Urrila AS, Artiges E, Massicotte J, Miranda R, Vulser H, Bézivin-Frere P, et al. Sleep habits, academic performance, and the adolescent brain structure. Sci Rep. 2017;7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5299428/. [DOI] [PMC free article] [PubMed]
- 101.Miller ML, Ren Y, Szutorisz H, Warren NA, Tessereau C, Egervári G, et al. Ventral striatal regulation of CREM mediates impulsive action and drug addiction vulnerability. Mol Psychiatry. 2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5656565/. [DOI] [PMC free article] [PubMed]
- 102.Toro R, Poline J-B, Huguet G, Loth E, Frouin V, Banaschewski T, et al. Genomic architecture of human neuroanatomical diversity. Mol Psychiatry. 2015;20:1011–6. doi: 10.1038/mp.2014.99. [DOI] [PubMed] [Google Scholar]
- 103.Da Mota B, Tudoran R, Costan A, Varoquaux G, Brasche G, Conrod P, et al. Machine learning patterns for neuroimaging-genetic studies in the cloud. Front Neuroinform. 2014;8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3986524/. [DOI] [PMC free article] [PubMed]
- 104.Shin J, Bourdon C, Bernard M, Wilson MD, Reischl E, Waldenberger M, et al. Layered genetic control of DNA methylation and gene expression: a locus of multiple sclerosis in healthy individuals. Hum Mol Genet. 2015;24:5733–45. doi: 10.1093/hmg/ddv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heinrich A, Müller KU, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, et al. Prediction of alcohol drinking in adolescents: personality-traits, behavior, brain responses, and genetic variations in the context of reward sensitivity. Biol Psychol. 2016;118:79–87. doi: 10.1016/j.biopsycho.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Lancaster TM, Linden DE, Tansey KE, Banaschewski T, Bokde ALW, Bromberg U, et al. Polygenic risk of psychosis and ventral striatal activation during reward processing in healthy adolescents. JAMA Psychiatry. 2016;73:852–61. doi: 10.1001/jamapsychiatry.2016.1135. [DOI] [PubMed] [Google Scholar]
- 107.Burt KB, Whelan R, Conrod PJ, Banaschewski T, Barker GJ, Bokde ALW, et al. Structural brain correlates of adolescent resilience. J Child Psychol Psychiatry. 2016;57:1287–96. doi: 10.1111/jcpp.12552. [DOI] [PubMed] [Google Scholar]
- 108.Castellanos-Ryan N, Brière FN, O’Leary-Barrett M, Banaschewski T, Bokde A, Bromberg U, et al. The structure of psychopathology in adolescence and its common personality and cognitive correlates. J Abnorm Psychol. 2016;125:1039–52. doi: 10.1037/abn0000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bartholdy S, Allen K, Hodsoll J, O’Daly OG, Campbell IC, Banaschewski T, et al. Identifying disordered eating behaviours in adolescents: how do parent and adolescent reports differ by sex and age? Eur Child Adolesc Psychiatry. 2017;26:691–701. doi: 10.1007/s00787-016-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sadaghiani S, Ng B, Altmann A, Poline J-B, Banaschewski T, Bokde ALW, et al. Overdominant effect of a CHRNA4 polymorphism on cingulo-opercular network activity and cognitive control. J Neurosci. 2017;37:9657–66. doi: 10.1523/JNEUROSCI.0991-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spechler PA, Allgaier N, Chaarani B, Whelan R, Watts R, Orr C, et al. The initiation of cannabis use in adolescence is predicted by sex-specific psychosocial and neurobiological features. Eur J Neurosci. 2019;50:2346–56. doi: 10.1111/ejn.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vulser H, Paillère Martinot M-L, Artiges E, Miranda R, Penttilä J, Grimmer Y, et al. Early variations in white matter microstructure and depression outcome in adolescents with subthreshold depression. Am J Psychiatry. 2018;175:1255–64. doi: 10.1176/appi.ajp.2018.17070825. [DOI] [PubMed] [Google Scholar]
- 113.Cury C, Glaunès JA, Toro R, Chupin M, Schumann G, Frouin V, et al. Statistical shape analysis of large datasets based on diffeomorphic iterative centroids. Front Neurosci. 2018;12. https://www.frontiersin.org/articles/10.3389/fnins.2018.00803/full. [DOI] [PMC free article] [PubMed]
- 114.Quinlan EB, Barker ED, Luo Q, Banaschewski T, Bokde ALW, Bromberg U, et al. Peer victimization and its impact on adolescent brain development and psychopathology. Mol Psychiatry. 2018. 10.1038/s41380-018-0297-9. [Epub ahead of print]. [DOI] [PubMed]
- 115.D’Alberto N, Chaarani B, Orr CA, Spechler PA, Albaugh MD, Allgaier N, et al. Individual differences in stop-related activity are inflated by the adaptive algorithm in the Stop Signal Task. Hum Brain Mapp. 2018;39:3263–76. doi: 10.1002/hbm.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huguet G, Schramm C, Douard E, Jiang L, Labbe A, Tihy F, et al. Measuring and estimating the effect sizes of copy number variants on general intelligence in community-based samples. JAMA Psychiatry. 2018;75:447–57. doi: 10.1001/jamapsychiatry.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brislin SJ, Patrick CJ, Flor H, Nees F, Heinrich A, Drislane LE, et al. Extending the construct network of trait disinhibition to the neuroimaging domain: validation of a bridging scale for use in the European IMAGEN Project. Assessment. 2019;26:567–81. doi: 10.1177/1073191118759748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bogdan R, Salmeron BJ, Carey CE, Agrawal A, Calhoun VD, Garavan H, et al. Imaging genetics and genomics in psychiatry: a critical review of progress and potential. Biol Psychiatry. 2017;82:165–75. doi: 10.1016/j.biopsych.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–77. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grosbras M-H, Paus T. Brain networks involved in viewing angry hands or faces. Cereb Cortex. 2006;16:1087–96. doi: 10.1093/cercor/bhj050. [DOI] [PubMed] [Google Scholar]
- 121.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 122.Charlet K, Beck A, Heinz A. The dopamine system in mediating alcohol effects in humans. In: Behavioral neurobiology of alcohol addiction. Berlin, Heidelberg: Springer; 2011. p. 461–88. (Current Topics in Behavioral Neurosciences). https://link.springer.com/chapter/10.1007/978-3-642-28720-6_130. [DOI] [PubMed]
- 123.Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–42. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 124.Xuei X, Flury‐Wetherill L, Almasy L, Bierut L, Tischfield J, Schuckit M, et al. HUMAN GENETIC STUDY: association analysis of genes encoding the nociceptin receptor (OPRL1) and its endogenous ligand (PNOC) with alcohol or illicit drug dependence. Addict Biol. 2008;13:80–7. doi: 10.1111/j.1369-1600.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- 125.Kuzmin A, Bazov I, Sheedy D, Garrick T, Harper C, Bakalkin G. Expression of pronociceptin and its receptor is downregulated in the brain of human alcoholics. Brain Res. 2009;1305(Suppl):S80–5. doi: 10.1016/j.brainres.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci USA. 2011;108:7119–24. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Easton AC, Rotter A, Lourdusamy A, Desrivières S, Fernández-Medarde A, Biermann T, et al. Rasgrf2 controls dopaminergic adaptations to alcohol in mice. Brain Res Bull. 2014;109:143–50. doi: 10.1016/j.brainresbull.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 128.Easton AC, Rotter A, Lourdusamy A, Desrivières S, Fernández-Medarde A, Biermann T, et al. Rasgrf2 controls noradrenergic involvement in the acute and subchronic effects of alcohol in the brain. Psychopharmacology. 2014;231:4199–209. doi: 10.1007/s00213-014-3562-x. [DOI] [PubMed] [Google Scholar]
- 129.Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia—psychopathological and behavioral correlates. Eur Psychiatry. 2002;17:9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- 130.Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74:387–98. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- 131.Peciña M, Martínez-Jauand M, Love T, Heffernan J, Montoya P, Hodgkinson C, et al. Valence-specific effects of BDNF Val66Met polymorphism on dopaminergic stress and reward processing in humans. J Neurosci. 2014;34:5874–81. doi: 10.1523/JNEUROSCI.2152-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benzerouk F, Gierski F, Raucher-chéné D, Ramoz N, Gorwood P, Kaladjian A, et al. Association study between reward dependence and a functional Bdnf polymorphism in adult women offspring of alcohol-dependent probands. Psychiatr Genet. 2015;25:208–11. doi: 10.1097/YPG.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 133.Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, et al. Association between Val66Met Brain-Derived Neurotrophic Factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pandey SC. A critical role of brain-derived neurotrophic factor in alcohol consumption. Biol Psychiatry. 2016;79:427–9. doi: 10.1016/j.biopsych.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan Q-S, Feng M-J, Yan S-E. Different expression of brain-derived neurotrophic factor in the nucleus accumbens of alcohol-preferring (P) and -nonpreferring (NP) rats. Brain Res. 2005;1035:215–8. doi: 10.1016/j.brainres.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 136.Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–50. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Orrù A, Caffino L, Moro F, Cassina C, Giannotti G, Di Clemente A, et al. Contingent and non-contingent recreational-like exposure to ethanol alters BDNF expression and signaling in the cortico-accumbal network differently. Psychopharmacology. 2016;233:3149–60. doi: 10.1007/s00213-016-4358-y. [DOI] [PubMed] [Google Scholar]
- 138.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 139.Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, et al. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol. 2015;6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4549553/. [DOI] [PMC free article] [PubMed]
- 140.Hägele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F, et al. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. 2015;232:331–41. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, et al. volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 143.Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord. 2016;203:204–12. doi: 10.1016/j.jad.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schlagenhauf F, Rapp MA, Huys QJM, Beck A, Wüstenberg T, Deserno L, et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 2013;34:1490–9. doi: 10.1002/hbm.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Friedel E, Schlagenhauf F, Beck A, Dolan RJ, Huys QJM, Rapp MA, et al. The effects of life stress and neural learning signals on fluid intelligence. Eur Arch Psychiatry Clin Neurosci. 2015;265:35–43. doi: 10.1007/s00406-014-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hill SY, Lichenstein S, Wang S, Carter H, McDermott M. Caudate volume in offspring at ultra high risk for alcohol dependence: COMT Val158Met, DRD2, externalizing disorders, and working memory. Adv. Mol Imaging. 2013;3:43–54. doi: 10.4236/ami.2013.34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Richter A, Barman A, Wüstenberg T, Soch J, Schanze D, Deibele A, et al. Behavioral and neural manifestations of reward memory in carriers of low-expressing versus high-expressing genetic variants of the Dopamine D2 receptor. Front Psychol. 2017;8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5410587/. [DOI] [PMC free article] [PubMed]
- 148.Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat Rev Neurosci. 2011;12:400–13. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- 149.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking Basal Ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 150.Quinlan EB, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Büchel C, et al. Identifying biological markers for improved precision medicine in psychiatry. Mol Psychiatry. 2020;25:243–53. doi: 10.1038/s41380-019-0555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]