Abstract
Background
Coronavirus disease 2019 (COVID-19) is an ongoing global pandemic. The ability to predict cardiac injury and analyze lymphocyte immunity and inflammation of cardiac damage in patients with COVID-19 is limited. We aimed to determine the risk factors and predictive markers of cardiac injury in these patients.
Methods
Data from 124 consecutive hospitalized patients with confirmed COVID-19 were collected. We compared the proportion of cardiovascular disease history in moderate, severe, and critical cases. We obtained high-sensitivity cardiac troponin I (hs-cTn I) results from 68 patients. Patients were divided into two groups based on positive hs-cTn I result: those with cardiac injury (n = 19) and those without cardiac injury (n = 49).
Results
Compared with the group with moderate disease, hypertension, coronary heart disease, and smoking were more common in severe and critical cases. Diabetes mellitus was most common in the critical group. Age older than 65 years, presence of chronic kidney disease, and lower blood lymphocyte percentage were independent risk factors of cardiac injury. The total T- and B-lymphocyte counts and CD4+ and CD8+ T-cell counts were significantly lower in those with cardiac injury. A minimal lymphocyte percentage < 7.8% may predict cardiac injury. The interleukin (IL) 6 level in plasma was elevated in the group with cardiac injury.
Conclusions
The lymphocyte percentage in blood may become a predictive marker of cardiac injury in COVID-19 patients. The total T and B cells and CD4+ and CD8+ cell counts decreased and the IL-6 level increased in COVID-19 patients with cardiac injury.
Keywords: Coronavirus disease 2019, Cardiac injury, Lymphocyte immunity, Inflammation
Abbreviations: COVID-19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; hs-cTn I, high-sensitive cardiac troponin I; IQR, interquartile range; IL, interleukin; CI, confidence interval; TNF, tumor necrosis factor; ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II
1. Introduction
Coronavirus disease 2019 (COVID-19) is a worldwide pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and many patients have died across the globe. The outbreak was declared a Public Health Emergency of International Concern on January 30, 2020, by the World Health Organization (WHO). Although most patients with COVID-19 have respiratory symptoms, reports have suggested that coronavirus can also cause acute cardiac injury [[1], [2], [3]]. The demand for greater understanding of the clinical characteristics of cardiovascular injury in COVID-19 and its associated risk factors and mechanisms is increasing. The aim of this study was to compare the minimal levels of lymphocyte percentage during the disease to determine whether these values could predict cardiac injury.
2. Methods
We retrospectively collected data from 124 hospitalized patients with COVID-19 at Tongji Hospital of Tongji Medical College Hua Zhong University of Science & Technology—Zhongfa Division, China, from January 28, 2020 to March 28, 2020, including medical history, clinical manifestation, laboratory findings, complications, and outcomes data. All the patients in this study had confirmed diagnoses of COVID-19 according to the 7th edition guideline [4] issued by the National Health Commission of China. Patients who were suspected cases were excluded. Of the COVID-19 patients included, 68 had high-sensitivity cardiac troponin I (hs-cTn I) test results. We divided the patients into two groups based whether the hs-cTn I level was above 99th percentile upper limit of the normal reference (14 pg/mL). Moreover, 23 of the 68 cases had lymphocyte immunity analysis results. The severity types of COVID-2019 including mild, moderate, severe, and critical cases were defined according to the 7th edition guideline issued by the National Health Commission of China [4]. We did not include mild cases in our study because patients with mild disease were not admitted to hospital.
2.1. Data collection
We collected baseline characteristics (age and sex), chronic disease history, and clinical data (laboratory findings, complications, intensive care unit–associated treatment, and outcomes). Patients were divided into two groups based on the results of hs-cTn I.
2.2. Approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the First Hospital of Jilin University (2020−320). Informed consent was not required because of the retrospective design of the study.
2.3. Statistical analysis
Continuous variables were presented as mean (standard deviation) or median (interquartile range [IQR]) according to normal distribution. Categorical variables were expressed as proportions (%). The continuous variables of two groups were compared using t-test or Mann–Whitney U test according to the data. We used the Kruskal–Wallis test to compare the continuous variables of multiple groups. Categorical variables were compared using the chi-squared or Fisher's exact test. Logistic regression models were used to define the independent risk factors for cardiac injury of COVID-19 patients. The receiver operating characteristic curve was used to determine the sensitivity and specificity of lymphocyte percentage for estimating cardiac injury.
Statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY, USA). Statistical charts were generated using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA). For all data, p<0.05 was considered statistically significant.
3. Results
Of the 124 patients, 64 (52%) were men and 60 (48%) were women. The median age was 63 years (range, 30–86 years). Forty-four (35%), 62 (50%), and 18 (15%) patients were diagnosed as moderate, severe, and critical cases, respectively. Of the 68 confirmed COVID-19 patients with hs-cTn I results, 19 (28%) had cardiac injury and 49 (72%) did not have cardiac injury. There were 23 patients with peripheral blood immunological tests.
3.1. Cardiovascular disease–associated risk factors (hypertension, diabetes mellitus, coronary heart diseases, smoking) in 124 patients with COVID-19
The proportions of moderate, severe, and critical cases with hypertension were 13.6%, 43.5%, and 50%, respectively (p˂0.05). The proportions of patients with diabetes were 13.6%, 22.6%, and 50%, respectively (p˂0.05). The proportion of patients with coronary heart disease were 2.3%, 11.3%, and 11.1%, respectively (p˂0.05), and the proportion of those who smoked in each group were 2.3%, 19.4%, and 16.7%, respectively (p˂0.05). Thus, hypertension, coronary heart disease, and smoking were more common in severe and critical cases. Diabetes was more common in critical cases than in the other two groups (p˂0.05) (Supplementary Fig. 1).
3.2. Hs-cTn I results in the moderate, severe, and critical groups
We collected the hs-cTn I results of 68 patients, including 16 (24%) moderate cases, 36 (56%) severe cases, and 16 (24%) critical cases. The median peak levels of hs-cTn I of the three groups were 2.35 [IQR, 1.9–7.4], 4.45 [IQR, 1.9–9.9], and 56.6 [IQR, 24.1–362.9] pg/mL, respectively (p˂0.05). The peak hs-cTn I level was significantly higher in the critical group than that in the other two groups (Supplementary Fig. 2).
3.3. Baseline clinical characteristics and laboratory findings of 68 COVID-19 patients
The study included 68 hospitalized confirmed COVID-19 cases with hs-cTn I results. The hs-cTn I level higher than 14 pg/mL was considered positive. Patients were divided into two groups according to the hs-cTn I results: with cardiac injury (n = 19, 28%) and without cardiac injury (n = 49, 72%).
The proportion of patients older than 65 years was significantly higher in those with cardiac injury than in those without cardiac injury. Moreover, patients with cardiac injury were more likely to have diabetes and chronic kidney disease (CKD), but not other comorbidities, including smoking, hypertension, coronary heart disease, chronic liver disease, chronic respiratory disease, thyroid disease, cancer, and influenza. Regarding laboratory findings, lymphocyte percentage and minimal lymphocyte percentage were much lower in patients with cardiac injury than in those without cardiac injury. Elevated interleukin (IL) 6 and creatinine were higher in those with cardiac injury than in those without cardiac injury. In addition, the N-terminal pro-B-type natriuretic peptide level was much higher in those with cardiac injury than in those without cardiac injury (Table 1 ).
Table 1.
Baseline Clinical Characteristics and Laboratory Findings of 68 COVID-19 Patients.
| Characteristics | All patients (n = 68) | With cardiac injury (n = 19) | Without cardiac injury (n = 49) | p Value |
|---|---|---|---|---|
| Age, years, median (range) | 67 (30–86) | 73 (57–86) | 64 (30–79) | |
| Age group, n (%) | ||||
| 20–49 years | 10 (15%) | 0 (0%) | 10 (20%) | 0.001 |
| 50–65 years | 19 (28%) | 2 (11%) | 17 (35%) | |
| ≥65 years | 39 (57%) | 17 (89%) | 22 (45%) | |
| Sex | ||||
| Male, n (%) | 34 (50%) | 6 (32%) | 28 (57%) | 0.052 |
| Female, n (%) | 34 (50%) | 13 (68%) | 21 (43%) | |
| Smokers, n (%) | 13 (19%) | 2 (11%) | 11 (22%) | 0.223 |
| Chronic disease, n (%) | ||||
| Hypertension | 29 (43%) | 11 (58%) | 18 (37%) | 0.095 |
| Diabetes | 16 (24%) | 8 (42%) | 8 (16%) | 0.03 |
| Coronary heart disease | 8 (18%) | 3 (16%) | 5 (10%) | 0.395 |
| Chronic kidney disease | 7 (10%) | 6 (32%) | 1 (2%) | 0.001 |
| Chronic liver disease | 4 (6%) | 2 (11%) | 2 (4%) | 0.317 |
| Chronic respiratory disease | 6 (8%) | 2 (11%) | 4 (8%) | 0.541 |
| Thyroid disease | 5 (7%) | 2 (11%) | 3 (6%) | 0.431 |
| Cancer | 4 (6%) | 1 (5%) | 3 (6%) | 0.69 |
| Influenza accompanied, n (%) | 24 (35%) | 7 (37%) | 17 (35%) | >0.05 |
| Heart rate, bpm, mean ± SD | 89 ± 16 | 91 ± 15 | 88 ± 16 | 0.357 |
| Arrhythmia, n (%) | 7 (10%) | 3 (16%) | 4 (8%) | >0.05 |
| Laboratory findings, median (IQR) | ||||
| Leukocytes, 109/L | 6.45 (5.22–7.96) | 6.48 (5.24–8.95) | 6.41 (5.24–7.70) | 0.217 |
| Lymphocyte percentage | 20.4 (11.1–27.4) | 11.2 (8.2–16.2) | 23.3 (14.7–29.9) | 0.004 |
| Minimal lymphocyte percentage | 16.2 (10.15–26.65) | 6.7 (3.2–16.2) | 20.3 (12.3–29.0) | 0.000 |
| Albumin, g/L | 36.4 (32.8–39.7) | 33.8 (32.1–37.7) | 37.9 (33.8–40.3) | 0.05 |
| Alanine aminotransferase, U/L | 29 (22–37.5) | 35 (28–57) | 25.5 (19–34) | >0.05 |
| Aspartate aminotransferase, U/L | 31 (20–49) | 30 (19–49) | 31 (19–49) | >0.05 |
| Creatinine, μmol/L | 73 (62–88) | 91 (66–108) | 69 (62–79) | 0.043 |
| C-reactive protein, mg/L | 7.5 (1.9–27.6) | 24.4 (2.5–100.2) | 6.5 (1.9–19.1) | 0.319 |
| Erythrocyte sedimentation rate, mm/H | 35 (17–68) | 38 (30–73) | 26 (14–62) | 0.102 |
| IL-6, pg/mL | 4.0 (2.7–3.5) (n = 47) | 8.2 (4.4–30.2) (n = 12) | 3.5 (2.7–6.5) (n = 35) | 0.048 |
| TNF-α, pg/mL | 7.5 (6.0–10.8) (n = 47) | 10.2 (6.2–12.2) (n = 12) | 7.3 (6.0–8.4) (n = 35) | 0.104 |
| D-dimer increased-No.,% | 44 (65%) | 15 (79%) | 29 (59%) | 0.163 |
| NT-proBNP, pg/mL | 127 (53–472) | 614 (295–1833) | 65 (36–195) | 0.000 |
| Severity type, n (%) | ||||
| Moderate | 16 (24%) | 1 (5%) | 15 (31%) | 0.000 |
| Severe | 36 (53%) | 4 (21%) | 32 (65%) | |
| Critical | 16 (23%) | 14 (74%) | 2 (4%) | |
| ICU associated treatment, n (%) | ||||
| Noninvasive ventilation | 7 (10%) | 6 (32%) | 1 (2%) | 0.001 |
| Invasive ventilation | 6 (9%) | 6 (32%) | 0 (0%) | 0.000 |
| Continuous renal replacement therapy | 2 (3%) | 2 (11%) | 0 (0%) | 0.075 |
| Complications, n (%) | ||||
| SIRS | 2 (3%) | 1 (5%) | 1 (2%) | 0.484 |
| MODS | 7 (10%) | 6 (32%) | 1 (2%) | 0.001 |
| Outcome, n (%) | ||||
| Discharged | 61 (90%) | 13 (68%) | 48 (98%) | 0.001 |
| Died | 7 (10%) | 6 (32%) | 1 (2%) |
Values are presented as median (interquartile range), mean ± standard deviation, or number (%).
IL-6, interleukin 6; TNF-α, tumor necrosis factor α; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ICU, intensive care unit; SIRS, systemic inflammatory response syndrome; MODS, multiple-organ dysfunction syndrome.
Patients with cardiac injury were more common in the critical group. Moreover, patients with cardiac injury required more non-invasive and invasive mechanical ventilations. More patients had multiple-organ dysfunction syndrome and died in the group with cardiac injury than in the group without cardiac injury (Table 1).
3.4. Multivariable logistic regression analysis of risk factors in cardiac injury patients
Risk factors that were significantly different in the two groups were analyzed by multivariable logistic regression, including age, diabetes, chronic kidney disease, lymphocyte percentage, minimal lymphocyte percentage, creatinine, and IL-6. Age ≥ 65 years, CKD, and minimal lymphocyte percentage were independent risk factors for cardiac injury (Supplementary Table 1).
3.5. Blood lymphocyte percentage in patients with cardiac injury
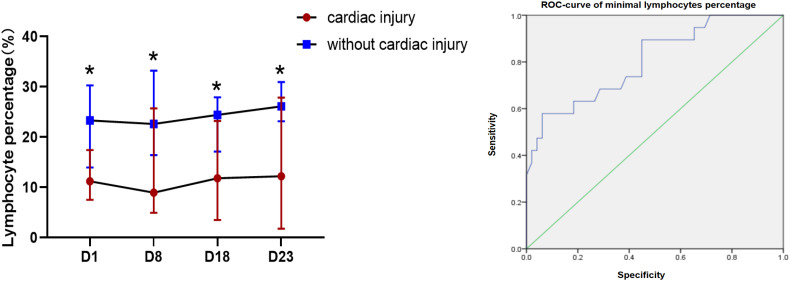
The blood lymphocyte percentages (%) were compared at four main time points (days 1, 8, 18, and 23, ±2 days) after admission (Fig. 1A). The lymphocyte percentage in the group with cardiac injury was significantly lower than that in the group without cardiac injury at four time points (median [IQR]: 11.2% [8.2–16.2] vs. 23.3% [14.7–29.9], p = 0.002; 9.0% [5.4–23.9] vs. 22.6% [16.5–33.1], p = 0.002; 11.8% [3.57–20.7] vs. 24.4% [18.2–27.8], p = 0.004; 12.2% [2.8–25.4] vs. 26.1% [23.3–30.3], p = 0.009, respectively). The minimal lymphocyte percentages during the disease were compared to predict cardiac injury. These values could predict cardiac injury with relative accuracy (p = 0.000, area under the curve = 0.803; Fig. 1B). The optimal cutoff for minimal lymphocyte percentage was 7.8%.
Fig. 1.
(A) Lymphocyte percentage with or without cardiac injury. (B) Receiver operating characteristic (ROC) curve of minimal lymphocyte percentage.
3.6. Lymphocyte immunological features of COVID-19 patients with and without cardiac injury
The lymphocyte immunological features in the peripheral blood of 23 patients with COVID-19 were analyzed, including 8 patients with cardiac injury and 15 patients without (Table 2 ). Compared with cases without cardiac injury, the absolute numbers of total T lymphocytes, total B lymphocytes, CD4+ T cells, and CD8+ T cells were significantly decreased in the group with cardiac injury. The percentages of cases with decreased total T lymphocytes, CD4+ T cells, and CD8+ T cells were higher in the group with cardiac injury than in the group without cardiac injury. The absolute numbers of natural killer (NK) cells showed no significant difference between the two groups.
Table 2.
Immunological Features of COVID-19 Patients.
| All patients (n = 23) | With cardiac injury (n = 8) | Without cardiac injury (n = 15) | p p Value | |
|---|---|---|---|---|
| Total T lymphocytes (%) | 73.23 (65.88–75.94) | 73.10 (63.21–74.04) | 73.23 (65.88–76.48) | 0.506 |
| Total T lymphocytes count,/ μL | 1022 (691–1298) | 598 (492–943) | 1233 (923–1406) | 0.003 |
| Decreased, n (%) | 11 (48%) | 7 (88%) | 4 (27%) | 0.009 |
| Total B lymphocytes (%) | 12.20 (9.14–17.73) | 12.42 (9.48–16.48) | 12.20 (9.35–17.36) | 0.825 |
| Increased, n (%) | 4 (17%) | 2 (25%) | 2 (13%) | 0.589 |
| Total B lymphocytes count, /μL | 151 (112–216) | 112 (67–162) | 170 (132–283) | 0.047 |
| Decreased, n (%) | 4 (17%) | 3 (37.5%) | 1 (6.7%) | 0.103 |
| CD4+ cells, (%) | 43.20 (37.22–48.08) | 41.46 (35.77–48.70) | 43.2 (37.64–46.31) | 0.776 |
| CD4+ cells count, /μL | 637 (364–779) | 351 (312–514) | 738 (561–868) | 0.004 |
| Decreased, n (%) | 10 (43%) | 6 (75%) | 4 (27%) | 0.039 |
| CD8+ cells, (%) | 24.54 (21.19–27.93) | 22.40 (13.61–27.77) | 24.86 (22.66–27.93) | 0.294 |
| CD8+ cells count, /μL | 357 (253–480) | 222 (126–300) | 452 (326–576) | 0.007 |
| Decreased, n (%) | 10 (43%) | 6 (75%) | 4 (27%) | 0.039 |
| NK cells (%) | 14.17 (10.78–23.28) | 16.48 (12.62–26.14) | 13.51 (9.91–20.49) | 0.357 |
| NK cells count, /μL | 211 (112–267) | 124 (86–185) | 229 (176–307) | 0.065 |
| decreased, n (%) | 9 (39%) | 5 (63%) | 4 (27%) | 0.179 |
| Th/Ts | 1.80 (1.40–2.34) | 1.98 (1.80–2.50) | 1.66 (1.31–2.02) | 0.357 |
Values are presented as median [interquartile range] or n (%).
COVID-19, coronavirus disease 2019; NK, natural killer cells; Th/Ts, helper T cells/suppressor T cells.
3.7. Inflammatory biomarkers in plasma of patients with and without cardiac injury
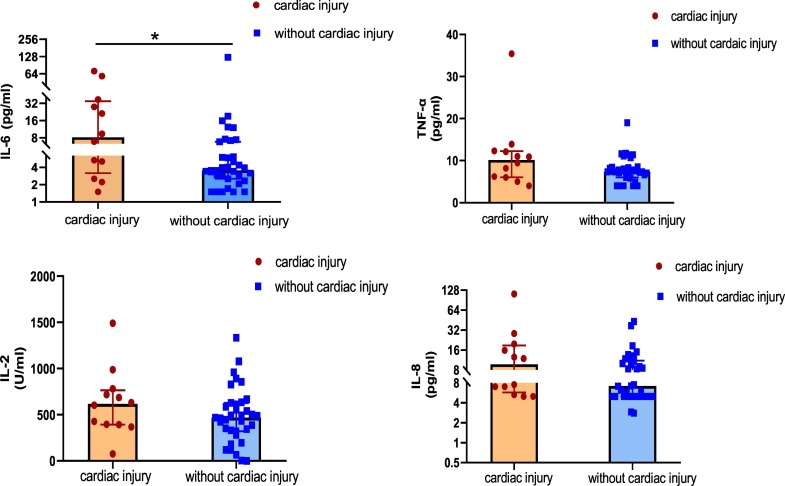
We collected plasma from 47 patients with COVID-19. The plasma levels of the inflammatory biomarker IL-6 was significantly elevated in the group with cardiac injury compared with the group without cardiac injury (median [IQR]: 8.2 [4.4–30.2] vs. 3.5 [2.7–6.5] pg/mL, p = 0.048). There was an uptrend of levels of tumor necrosis factor (TNF) α, IL-2, and IL-8 in the group with cardiac injury. However, the plasma levels of TNF-α, IL-2, and IL-8 did not differ between the two groups (Fig. 2 ).
Fig. 2.
Inflammatory biomarkers with or without cardiac injury.
3.8. Duration of infection and of symptoms with or without cardiac injury as well as severity
The durations of viral shedding and symptoms did not statistically differ between the group with cardiac injury and the group without cardiac injury. In addition, the durations of viral shedding and symptoms were longer in the critical group than in the severe and moderate groups. The mean duration of viral shedding in the critical, severe, and moderate groups were 35 [IQR, 29–39], 25 [IQR, 19–31], and 20 [IQR, 16–24] days, respectively (p˂0.05), and the mean duration of symptoms were 35 [IQR, 25–46], 21 [IQR, 16–27], and 22 [IQR, 16–30] days, respectively (p˂0.05) (Supplementary Fig. 3).
4. Discussion
This study demonstrates that cardiovascular disease–associated risk factors were more likely to be present in severe and critical cases of COVID-19. In addition, the degree of cardiac injury was strongly associated with COVID-19 severity. Patients with cardiac injury had more complications and a worse prognosis. This is consistent with the findings of previous report [3]. Therefore, we summarized the risk factors of cardiac injury in COVID-19 patients and found that immunity and inflammation factors were involved in cardiac injury: (1) Our results suggest that cardiac injury was more likely in older patients with diabetes and CKD. (2) Lymphopenia was an independent risk factor of cardiac injury, and the lymphocyte percentage in peripheral blood could predict cardiac injury. (3) Immunological analysis suggested that cardiac injury was mainly associated with the decrease in T and B lymphocytes. (4) The level of the inflammatory biomarker IL-6 was elevated in the group with cardiac injury.
Reports have suggested that SARS-CoV-2 can cause cardiac injury, which aggravates the disease and leads to a worse prognosis [[5], [6], [7]]. An autopsy report of a patient with COVID-19 showed degeneration and necrosis of myocardial cells as well as infiltration of a few monocytes, lymphocytes, and/or neutrophils in the interstitium [8]. The underlying mechanisms of cardiac injury are still not very clearly understood. We summarized the possible mechanisms as follows: (1) The most likely mechanism is angiotensin-converting enzyme 2 (ACE2)–associated signaling involved in cardiac injury [5]. ACE2 is involved in heart function, diabetes, and hypertension and plays a potential protective role for the heart by negatively regulating the renin–angiotensin system. SARS-CoV-2 enters human cells by glycoprotein binding to ACE2 receptors of the host cell surface. (2) The virus causes an immune system aberration and cytokine storm [9]. (3) Severe and critical cases always have hypoxemia, which leads to acidosis, an increase in intracellular free radicals, and calcium overload. This may be another mechanism of cardiac injury.
In our study, the results showed that diabetes and hypertension were more often found in severe or critical cases. This might be explained by a direct endocrine link among diabetes, hypertension, and ACE2. ACE2 plays a role in maintaining cardiovascular homeostasis [10]. ACE2 could degrade angiotensin II (Ang II) into angiotensin 1–7. When ACE2 activity is inhibited, Ang II plays a pro-inflammatory role and increases blood pressure. On the other hand, angiotensin 1–7 exerts anti-inflammatory and anti-fibrosis function. Patients with severe and critical COVID-19 were more likely to have diabetes and hypertension, probably because of an imbalance in the ACE2-associated pathways [11].
Our results suggest that severe and critical cases were more likely to have coronary heart disease. This may be associated with an increase in ACE2 secretion in these patients [5]. The high level of ACE2 made individuals particularly susceptible to SARS-CoV-2. Besides the lung and heart, ACE2 is also expressed in the kidney [12]; hence, people with CKD might be more vulnerable to infection. We showed that a history of diabetes was related to cardiac injury, which can be explained by ACE2 activity levels being elevated in the pancreas and diabetes inducing ACE2 expression in other tissues, such as the heart and lung [13]. Therefore, diabetes can contribute to damage of other organs in COVID-19 patients, including the heart [11]. However, after infection, SARS-CoV-2 reduces ACE2 activity and consumes the receptors [14]; hence, the ACE2/Ang II balance is disturbed and the heart may not be protected anymore, resulting in cardiac injury. This might be the reason why patients with underlying cardiovascular disease were more prone to infection and exhibited severe and critical disease, especially cardiac injury due to ACE2.
Lymphocytes play an important role in immune homeostasis and inflammatory response. Our results showed that the lymphocyte percentage decreased significantly with cardiac injury. A minimal lymphocyte percentage in peripheral blood <7.8% could predict a high risk of cardiac injury in patients with COVID-19. This will be helpful for physicians to judge the heart condition of patients. The probable mechanism of lymphopenia is the presence of ACE2 receptors in lymphocytes, which can be a target for SARS-CoV-2. The virus may also destroy immune organs. An inflammatory response leads to the release of pro-inflammatory cytokines, which in turn reduces the lymphocyte count. Furthermore, patients with cardiac injury were mostly severe and critical cases, with accompanying hyperlacticacidemia, which could inhibit the proliferation of lymphocytes [15].
Data from other reports have suggested that older adults were more susceptible to COVID-19, and aging is strongly associated with infection fatality in COVID-19 [16]. Our results showed that age ≥ 65 years was a risk factor for cardiac injury. A possible mechanism of cardiac injury is through immune function disruption. The immune system of older patients undergoes many age-related changes, known as immune senescence [17]. Lymph nodes, which produce naive T and B cells, become less able to maintain naive T cells and differentiation in the final third of life. B cells also undergo a similar reduction but much more slowly than T cells [18]. This is consistent with the immunological analysis results in this study, which suggested that the numbers of T and B lymphocytes were significantly decreased in the group with cardiac injury, but the decreased proportion of B lymphocytes did not differ. Another problem in the immune system is that T cells cannot move quickly to “fight” due to dysregulation of chemokines, which guide T-cell migration. Therefore, aging is associated with cardiac injury, probably due to immune senescence.
SARS-CoV-2 infection can cause inflammatory cytokine storms in the immune system. In this study, we compared several inflammatory biomarkers between the groups with and without cardiac injury. The IL-6 level was elevated in the group with cardiac injury, but this was not the case with other biomarkers. The reason might be because cardiac damage was related to aging and immune dysregulation. Thus, the inflammatory response was not as strong in older people as it was in younger people. However, the inflammatory response was still excessive and active at an early stage. Our results showed that more patients had CKD and elevated levels of creatinine in the blood in the group with cardiac injury than in the group without cardiac injury. Therefore, we considered that cytokine overproduction was involved in kidney–heart bidirectional damage in COVID-19 patients. Previous reports suggested that COVID-19 patients with diabetes are affected by a low-grade inflammation that facilitates the cytokine storm, and IL-6 was more elevated in COVID-19 patients with diabetes than in those without diabetes [19]. Therefore, we thought that diabetes resulted in metabolic inflammation and cytokine storms that damaged the heart further. In addition, the imbalance and loss of ACE2 might aggravate the inflammatory cytokine storm [20].
There are some limitations to our study. First, it was a single-center study and the number of patients was not very large. Second, we lacked some echocardiographic information and so did not include echocardiography results. Third, the prognosis in hospital is limited, and we did not follow-up.
In conclusion, the risk factors for cardiac injury in COVID-19 patients include not only aging but also chronic diseases such as diabetes and CKD. These factors affect the immune system and cause an inflammatory response, which damages the heart further. Lymphopenia is an effective marker that might be able to predict cardiac injury in COVID-19 patients and help physicians estimate disease conditions.
Funding
None.
Declaration of Competing Interest
None.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2020.10.049.
Appendix A. Supplementary data
Supplementary Fig. 1
Supplementary Fig. 2
Supplementary Fig. 3
Supplementary Table 1
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;39:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo G., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Health Commission of China . Chinese Society of Cardiology; 2020. National Administration of Traditional Chinese Medicine, Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment (7th edition)http://kjfy.meetingchina.org/msite/news/show/cn/3337.html [Google Scholar]
- 5.Zheng Z.Z., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200225-00123. E008. [DOI] [PubMed] [Google Scholar]
- 7.He X.W., Lai J.S., Cheng J., Wang M.W., Liu Y.J., Xiao Z.C., Xu C., Li S.S., Zeng H.S. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200228-00137. E011. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z., Fei G., Ren L., Zhou Y.W., Liu L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhmerov A., Marban E. COVID-19 and the heart. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020 doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunuba Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 18.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020 doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3321. e33213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1
Supplementary Fig. 2
Supplementary Fig. 3
Supplementary Table 1