Highlights
-
•
80 % of EVALI patients presented with respiratory symptoms.
-
•
60 % of the EVALI patients presented with gastrointestinal symptoms.
-
•
All of the EVALI patients had ground glass opacities on chest imaging.
-
•
Standardization of vaping history in both out-patient is highly recommended.
Keywords: e-cigarette, Vaping, EVALI, Lung, Injury
Abstract
Introduction
Recently, a rapidly increasing number of e-cigarette or vaping induced lung injury (EVALI) has been reported across the nation. Given the ongoing epidemic, it has been suggested that specific chemical substances used as additives in e-cigarettes could be highly related to EVALI. A history of vaping with positive radiographic changes and low suspicion for active infection are requirements for diagnosis but it still remains a diagnosis of exclusion. The course of the disease, mechanism of lung injury and the optimal management options need to be better understood. Here we aimed to discuss the clinical characteristics recognized in a case series of ten hospitalized EVALI patients with radiological findings of lung injury and provide an up today summary of the known literature of EVALI-induced lung injury.
Methods
A retrospective chart review was conducted on ten patients who presented to Saint Peter’s University Hospital in New Brunswick, NJ from July 2019 to February 2020, with a mean hospital stay of five days. According to the CDC recommended definition of the disease, our cases met the current working definition of confirmed or probable cases of EVALI.
Results
Ten patients, with mean age 30.8 years (50 % male) and average years of vaping 1.708 with 60 % endorsing a simultaneous history of cannabis-related products use, went under a retrospective review. 3/10 (30 %) had documented medically-managed pulmonary disease history, 8/10 (80 %) presented with the respiratory-related chief complaint, 6/10 (60 %) presented with gastrointestinal symptoms and 7/10 (70 %) had constitutional symptoms. All patients (100 %) were found to have bilateral ground-glass opacities on chest imaging. 9/10 were admitted, 6/10 (60 %) had an oxygen saturation of <95 % requiring oxygen supplementation with 4/10 managed in the intensive care unit.
Conclusion
EVALI patients with radiological findings of lung injury, although mainly present respiratory symptoms, may very often appear with constitutional and gastrointestinal symptoms. Based on the existing literature and our data it is argued that EVALI may be misdiagnosed and that closer monitoring is required to determine optimal diagnostic and therapeutic management of this condition. Our data and the existing literature suggest that laboratory and epidemiologic findings can be contributory for the diagnosis of the disease.
1. Introduction
The use of electronic-cigarettes (e-cigarettes) or vaping products has rapidly increased in the last few years, especially among adolescents and young adults [[1], [2], [3], [4]]. Although early studies have raised awareness about the harmful effect of e-cigarettes or vaping products [5], the healthcare industry remained largely neutral on the topic until convincing data started surfacing in 2019.
Electronic-cigarette or vaping induced lung injury (EVALI) was first reported in 2019 [2], and as January 14 of 2020, 2668 hospitalized cases of EVALI have been reported from 50 states of United States of America (USA), with 26 deaths [6]. The majority of patients were young, under the age of 35 years [2,6]. Although the mechanism of EVALI is still not well understood, it has been suggested that heating can transform the compounds and additives in vaping products into respiratory irritants and carcinogens [[7], [8], [9], [10]]. Blount et al. emphasized the effect of Vitamin E acetate, a substance used as a thickening agent in tetrahydrocannabinol (THC) containing products as a potential cause of respiratory endothelial injury, suggesting that it could be associated with transition of surfactant from gel to liquid crystalline form, leading to loss of surfactant’s ability to maintain surface tension [11].
Although there has been a lot of emerging data on EVALI, it still remains a diagnosis of exclusion with no confirmatory diagnostic test [1,2,6]. Significant overlap based on symptomatology can lead to misattribution and misdiagnosis [12]. Given the rapidly growing number of cases, a detailed report of clinical characteristics recognized in EVALI cases with radiological findings of lung injury will not only provide a better understanding of the natural history of the disease, but also create a strong foundation for the development of evidence-based guidelines for the diagnosis, prognosis, and treatment of this condition. Here we present the clinical characteristics of a series of hospitalized EVALI cases at our institution, and discuss the importance of our data based on the existing literature describing the unique characteristics of the disease that would be significant for the its proper diagnostic and therapeutic approach.
2. Methods
2.1. Patient selections
Ten patients, 16–66 years old, with symptomatology similar to the previous EVALI reported cases such as dyspnea, cough, fatigue, nausea, and vomiting were admitted to the Saint Peter's University Hospital in the period July 2019 – February 2020. The data were obtained according to our Institutional Review Board (IRB) approved protocol. The patients that were included in this study fulfilled the Centers for Disease Control (CDC) and prevention proposed criteria for the definition of confirmed or possible EVALI cases and were eventually discharged with a main diagnosis of EVALI [1,13].
The use of e-cigarettes within the last 90 days before the onset of symptoms and radiographic imaging of opacities or ground glass appearance are the main features of the suspected EVALI. Confirmed cases require the absence of signs and associated symptoms and laboratory findings indicating absence or low suspicion of ongoing infection. Probable cases are defined as having the symptomatology and imaging findings, however with laboratory studies indicating a possible ongoing infection. In both cases, no evidence of alternative diagnosis is required [1,13].
3. Results
3.1. Demographics
All of the patients included in this study presented in the emergency department of Saint Peter’s University Hospital during the period of July 2019 to February 2020 and were eventually discharged with a primary diagnosis of EVALI. The age range was 16–66 years with a mean of 30 years. Five of the patients were males (50 %) and five of them were females (50 %) (Table 1).
Table 1.
Demographics, past medical history and symptomatology of EVALI patients.
| Patient Demographics | |
|---|---|
| Sample size | N = 10 |
| Average age | 30.8 ± 21.7 |
| Female | 5/10 (50 %) |
| Past Medical History | |
| History of Asthma | 2/10 (20 %) |
| History of Anxiety | 3/10 (30 %) |
| Years of Vaping prior to admission | 1.7 ± 0.9 |
| Concomitant Marijuana Use | 10/10 (100 %) |
| Vaping of Cannabis-related products use | 6/10 (60 %) |
| Symptomatology | |
| Respiratory Symptoms on Presentation | 8/10 (80 %) |
| Gastrointestinal Symptoms on Presentation | 6/10 (60 %) |
| Constitutional Symptoms on Presentation | 6/10 (60 %) |
3.2. Vaping history and smoking history
All the patients reported marijuana use, however, only 60 % of the patients verified vaping history of cannabis-related products use [THC or Cannabichromene (CBC)] (Table 1). Urine toxicology was performed in all the patients with six of them (60 %) being positive. From the patients reported using cannabis-related vaping products three of them had positive THC urine test. The average years of vaping of the patients were 1.708 (range 1 month to 3 years) (Table 1). Only one patient reported a recent initiation of vaping (one month before the hospitalization). Only two out of ten patients (20 %) were actively using tobacco products, while three of them (30 %) were former tobacco users and five out of ten (50 %) never used tobacco before.
3.3. Symptomatology
Eight out of ten (80 %) had respiratory complaints such as cough, shortness of breath and pleuritic chest pain, six out of ten (60 %) presented with gastrointestinal symptoms such as nausea, vomiting, abdominal pain, seven out of ten (70 %) of the patient had constitutional symptoms of fever, fatigue and generalized weakness (Table 1).
3.4. Clinical course
All of the patients were hospitalized with a mean hospital stay of five days (variation 2–8.5 days) (Table 2). Two out of ten patients had a past medical history of asthma (Table 1) and one patient had a history of chronic obstructive lung disease. Five patients were evaluated as an outpatient for their complaints and treated with antibiotics without any significant improvement prompting them to come to the emergency department.
Table 2.
Management and hospitalization course of patents with EVALI.
| Management and hospital course | ||
|---|---|---|
| Antibiotics initiated in ED | 10/10 (100 %) | Absolute number (%) |
| IV Glucocorticoids initiated in ED | 4/10 (40 %) | Absolute number (%) |
| Admitted to hospital | 9/10 (90 %) | Absolute number (%) |
| Managed in ICU | 4/10 (40 %) | Absolute number (%) |
| Received NPPV* | 3/10 (30 %) | Absolute number (%) |
| Required intubation | 0/10 (0%) | Absolute number (%) |
| Deaths | 0/10 (0%) | Absolute number (%) |
| Readmission in 30 days | 0/10 (0%) | Absolute number (%) |
| Days of hospitalization | 5.2 ± 3.3 | Mean ± SD |
Abbreviations: NNPV: Non-invasive Positive Pressure Ventilation; ICU: Intensive Care Unit; IV intravenous; ED:emergency department.
Four out of ten patients were admitted to the Intensive Care Unit (ICU) of the hospital due to acute hypoxic respiratory failure (Table 2). Specifically, three patients were admitted directly to the ICU on the day of admission and one of the patients was initially managed on the medicine ward, and on the fifth day of hospitalization the patient’s respiratory status deteriorated and the patient was transferred to the ICU.
3.5. Vital signs
At the time of presentation, 4 out of 10 patients (40 %) were febrile with a temperature of >100.4 F (Table 2). All patients were hemodynamically stable with a systolic blood pressure (BP) of more than 90 mmHg and half of the patients (50 %) were tachypneic with respiratory rate (RR) > 20 breaths per minute (Table 3). Six out of ten of the patients (60 %) were tachycardia with a heart rate of >100 beats per minute. Also, six out of ten (60 %) had an oxygen saturation of <95 % requiring oxygen supplementation via nasal cannula to maintain their oxygen saturation >95 % (Table 3).
Table 3.
Vitals and Investigations.
| Vital signs | ||
|---|---|---|
| Temperature >100.4 °F (>38 °C) | 4/10 (40 %) | Absolute number (%) |
| Systolic BP (mmHg) | 132 ± 27 | Mean ± SD |
| Diastolic BP (mmHg) | 77 ± 15 | Mean ± SD |
| RR >20 (breaths/min) | 5/10 (50 %) | Absolute number (%) |
| O2 on RA < 95 %, requiring supplemental O2 | 6/10 (60 %) | Absolute number (%) |
| Investigations | ||
| WBC >11,000 cells/mm3 | 10/10 (100 %) | Absolute number (%) |
| Na <135 | 8/10 (80 %) | Absolute number (%) |
| CXR Obtained | 10/10 (100 %) | Absolute number (%) |
| CT Chest Obtained | 9/10 (90 %) | Absolute number (%) |
| Bilateral, Ground Glass Opacities on Imaging | 10/10 (100 %) | Absolute number (%) |
Abbreviations: BP: Blood Pressure, RR: Respiratory Rate, RA: Room Air, WBC: White Blood Cells, Na+: Sodium, CXR: Chest x-ray, CT: Computed Tomography.
Four out of the ten patients demonstrated increase in oxygen demands required more aggressive oxygen therapy and admission to ICU for close monitoring. Although the oxygen saturation levels in two of these patients maintained good at presentation, an increase in oxygen demand and an increase in work of respiration, necessitated the use of non-invasive positive pressure ventilation (NPPV) or BiPAP (Table 4). Arterial Blood Gasses (ABG) that performed in these two patients before the initiation of NPPV showed significant hypoxia but not hypercapnia (Table 4). Specifically, in patient 1 the ABG showed pH 7.47, PCO2 38, PO2 50, HCO3 27 and O2 saturation of 86 % and in patient 2 pH 7.41, PCO2 39, PO2 77, HCO3 24 and 96 % (Table 4). Also, another patient, patient number 3, who was admitted to ICU, had an oxygen saturation 80 % at presentation, requiring immediately placement on Bilevel Positive Airway Pressure (BiPAP) (Table 4). ABG was performed after initiation of NPPV improvement of oxygen levels with pH 7.38, PCO2 34, PO2 94, HCO3 20 and O2 Sat 95 %. Another patient, patient number 4, that was directly admitted to ICU presented with oxygen saturation of 95 % on 3 L was placed on venti mask to maintain oxygen saturation levels (Table 4).
Table 4.
Oxygen saturation and Arterial Blood Gases in Patients with EVALI.
| Oxygen Saturation at admission | Oxygen Sat. Before ICU transition and ABGs | |
|---|---|---|
| Patient 1 | 90 % RA | 95 % on NC 5 L/min; ABG: pH 7.47, PCO2 38 mmHg, PO2 50 mmHg, HCO3 27 mEq/L and SaO2 86% |
| Patient 2 | 98% RA | 91 % NC 5 L/min; ABG: pH7.41, PCO2 39 mmHg, PO2 39, PO2 77 mmHg, HCO3 24 mEq/L and SaO2 96% |
| Patient 3 | 80 % RA | 81−80% RA followed by 94−99% BiPAP 35% O2; ABG while on BiPAP: pH 7.38, PCO2 34 mmHg, PO2 94 mmHg, HCO3 20 mEq/L and SaO2 95% |
| Patient 4 | 95 % RA | 95 % on NC 3 L/min followed by 99 % on Venti Mask 100 % O2 |
| Mean ± SD | 90 ± 9.0 | |
| Patient 5 | 97% RA | |
| Patient 6 | 95 % RA | |
| Patient 7 | 93% RA | |
| Patient 8 | 98% RA | |
| Patient 9 | 98% RA | |
| Patient 10 | 98% RA | |
| Mean ± SD | 96.2 ± 1.9 |
RA: Room Air; Sat: Oxygen Saturation; BiPAP: Bilevel Positive Airway Pressure, ICU: Intensive Care Unit; NC: Nasal Canula.
3.6. Investigations
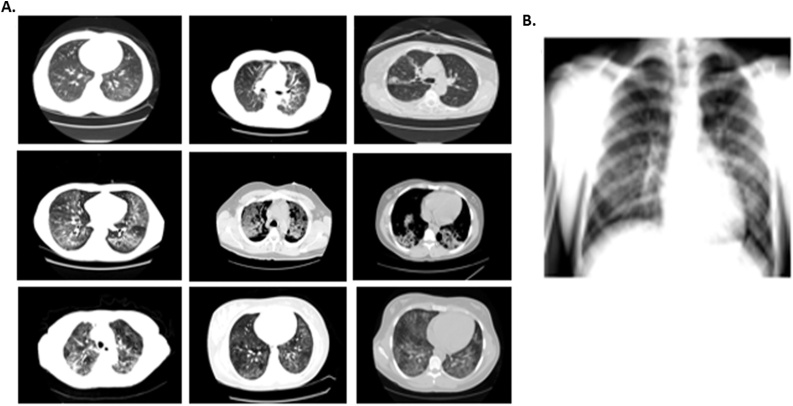
Imaging studies in all the patients were significant for radiological findings of lung disease. Computed Tomography (CT) was performed in nine of ten patients (90 %), showing opacities or infiltrates similar to previously reported EVALI cases (Table 3, Fig. 1). One of the patients had only a Chest X-ray showing characteristic peribronchial thickening and right and left sided opacities. One of the patients demonstrated emphysematous changes possibly related to chronic underlying lung disease. This patient is a former smoker with a reported history of 4-pack-years that quitted 30 years ago. Imaging on the presentation is shown below in Fig. 1.
Fig. 1.
Imaging findings of patients with probable or certain EVALI. A. Computer tomography (CT) images showing ground glass opacities. B. Chest-x-ray with right and left side opacities.
All the patients were found to have leukocytosis with a white blood cell (WBC) count of >11,000. Also, all the patients were found to have leukocytosis with lymphocyte count. Nine out of the ten patients that were tested for legionella urine antigen and all of them were found to be negative. More than half of the patients (60 %) were tested for pneumococcal urine antigen, and all of them found to be negative. The respiratory viral panel screening for influenza A (subtype H1 and H3), influenza B, respiratory syncytial virus A and B, adenovirus, human metapneumovirus, parainfluenza 1, 2, 3 and 4, and rhinovirus, was negative in seven patients; however, two cases were positive for rhinovirus. Although rhinovirus was positive in these patients, the clinical course and symptoms were not suggestive for rhinovirus infections. HIV test was offered and performed in seven out of ten patients (70 %) and was found to be non-reactive in all of them. Procalcitonin levels were assessed in most of the cases (80 %). In seven out of eight patients the procalcitonin was assessed was found to be normal (<0.5 ng/mL) with values in ng/mL: 0.9, 0.17, 0.36, < 0.05, 0.47, 0.29, 0.11, indicating a low probability for ongoing bacterial infection. One of the patient’s presented with elevated procalcitonin 1.22 ng/mL that decreased to the normal value 0.05 ng/mL within 3 days (<0.5 consider normal, ng/mL).
Additional laboratory investigations were performed in individual cases, such as C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR) and mononucleosis test, and quantiferon test. CRP was evaluated in two cases. In one of the cases the CRP was slightly elevated (31.27 mg/dl) while in the other case CRP was significantly increased (> 300 mg/dl). Normal value for CRP is considered < 10 mg/dl. ESR was assessed in two patients, and in both cases was found to be elevated (83 mg/dl and 88 mg/dl, respectively). Mononucleosis rapid test was performed in two cases and in both was negative. Also, the quantiferon TB gold test was performed in two cases and found to be negative. None of the reported patients underwent bronchoalveolar lavage.
3.7. Management
All the patients (100 %) were treated empirically with intravenous antibiotics for community-acquired and atypical pneumonia and 50 % were treated with a minimum of 5 days of systemic glucocorticoids, including intravenous steroid therapy followed by a tapering course of oral steroids (Table 2). All the ICU patients were treated with Hi-Flow Nasal Cannula for oxygen support, while two of them required further support with BiPAP (Table 2). None of the patients were intubated (Table 2). No deaths have been recorded. Follow up data are presented in Table 5, demonstrated that all the treated patients were not readmitted to the hospital. Laboratory findings for WBC counting were normal and imaging findings were found stable or improved.
Table 5.
Race and Follow-up data of Patients with EVALI.
| Age (years)/Sex | Race | Readmission | *Laboratory and imaging follow-up |
|---|---|---|---|
| 22/M | African American | NO | WBC: 11.9 > 10.6 |
| Absolute lymphocyte count: 0.83 | |||
| Imaging follow-up: N/A | |||
| 18/M | White | NO | WBC: 17.1 > 18.4 > 22.6 > 11.8 > 14.4 |
| Absolute lymphocyte count: 0.72 > 0.68 > 0.73 | |||
| Imaging follow-up: N/A | |||
| 76/F | White | NO | WBC: 11.6 > 12.3 > 11.4 |
| Absolute lymphocyte count: 0.46 > 0.86 > 0.46 | |||
| Imaging follow-up: N/A | |||
| 17/M | White | NO | WBC: 11.6 > 12.3 > 11.4 |
| Absolute lymphocyte count: 0.46 > 0.86 > 0.46 | |||
| Imaging follow-up: N/A | |||
| 32/M | White | NO | WBC: 12 > 12.9 > 15.4 > 13.6 > 13 > 20.1 > 12.3 > 11.2 > 11.4 > 12.5 |
| Absolute lymphocyte count: 1.40 > 1.23 > 0.52 > 1.60 | |||
| Imaging follow-up: CXR three and four days after admission showed an increase in opacities | |||
| 21/F | White | NO | WBC: 12.5 > 9.8 > 9.5 > 7.0 > 7.9 |
| Absolute lymphocyte count: 1.63 > 0.98 > 067 | |||
| Imaging follow-up: CXR two days after admission showed increased infiltrates, and six days after showed stable findings | |||
| 66/F | White | NO | WBC: 16.4 > 15.8 > 20.8 > 11.4 > 20.7 > 13.3 > 15.5 > 17.1 > 15.9 > 18.4 > 15.5 |
| Absolute lymphocyte count: 1.60 > 1.25 > 2.50 > 2.64 | |||
| Imaging follow-up: N/A | |||
| 20/F | Hispanic | NO | WBC 11.1 > 10.1 |
| Absolute lymphocyte count: 0.78 > 0.30 | |||
| Imaging follow-up: N/A | |||
| 20/F | White | NO | WBC: 11.5 > 10.7 > 8.4 > 6.5 |
| Absolute lymphocyte count: 0.35 > 0.54 > 0.76 > 0.70 | |||
| Imaging follow-up: CXR two days after admission showed stable opacities | |||
| 16/M | White | NO | WBC: 20 > 20.4 > 17.5 > 14.7 |
| Absolute lymphocyte count: 1.2 > 1.63 > 0.88 > 1.76 | |||
| Imaging follow-up: CXR four days after admission showed stable opacities |
White Blood Cells (WBC) trend (cells x 103/mm3)/Absolute lymphocyte count trend (cells x 103/mm3)/imaging follow-up; CXR: Chest x-ray.
4. Discussion
The increase in the prevalence of e-cigarette use in the last two years and the recent epidemic of EVALI necessitates a careful study of the characteristics and the pathophysiology of this syndrome [6,13,14]. Here, we present data from a series of ten cases that recognize clinical characteristics in hospitalized EVALI patients with radiological findings of lung injury.
The symptomatology of EVALI has been addressed by previous clinical studies, identifying both respiratory and non-respiratory symptoms related to the disease. The most common respiratory symptoms include dyspnea, cough, and chest pain, and in most cases accompanied by non-specific, constitutional symptoms, such as fever, chills, fatigue, and malaise [1,6,13,16]. In line with previous studies, the majority of our patients (80 %) presented with respiratory symptoms, such as cough and shortness of breath (Table 2). Gastrointestinal symptoms, such as vomiting, diarrhea, and abdominal pain, were also very common in patients included in this study. There is strong evidence that patients with EVALI can often present with gastrointestinal complaints [1,6,13,16], however the etiopathogenesis leading to these symptoms is not well understood. Also, fever and oxygen desaturation, may or may not be part of the initial presentation, and most of the auscultatory findings are considered non- specific [1,13]. Overall, given that symptoms are often non-specific, the diagnosis is usually challenging and a significant number of cases are misdiagnosed with pneumonia [1,13].
Our knowledge on the pathophysiology of EVALI is still growing. The most well-supported theory is that e-cigarette irritants lead to a native immunity reaction producing a sterile exogenous pneumonitis [15]. Recent histologic evaluation of series of EVALI patients showed non-specific inflammatory changes suggesting acute lung injury, such as fibrinous changes, diffuse damage of alveolar architecture, and bronchiolitis. A broad spectrum of substances found in e-cigarette products such as oils, additives, or even heavy metals, could potentially be involved in EVALI [9,11,[15], [16], [17]]. Some of them include substances that can be found in both nicotine and non-nicotine e-cigarettes, including additives such as glycerol [9,16] and propylene glycol or even compounds found in the metallic coil of the apparatus [17]. Although the toxicity of these substances might be minimal under normal conditions, rapid heating may induce their chemical transformation into toxic byproducts and promote their massive release in aerosol [9,16,17]. After vitamin E acetate was identified in bronchoalveolar lavage (BAL) fluid specimens of patients with EVALI, theoretical and experimental studies have focused on the exploration of the role of vitamin E in EVALI [11].
Results of hematologic tests may also vary and could be non-specific for diagnosis of EVALI. Similar to other studies, common findings in our patients included leukocytosis with neutrophilia. Previous studies also reported an increase in inflammatory markers such as ESR and CRP [1,13]. Inflammatory markers ESR and CRP were assessed in three of our patients, demonstrating relative elevations. However, given the absence of sufficient data and standardized guidelines, the diagnostic and prognostic utility of these markers is controversial.
Radiologic findings are closely associated with the severity and the progression of the disease [1,13,[18], [19], [20], [21]]. In most of the cases, chest x-ray demonstrates bilateral infiltrates and CT of the chest, often shows extensive bilateral ground-glass opacities [1,[18], [19], [20], [21]]. Pleural effusions, pneumomediastinum, and tree in bud opacities have also been described in previous case reports [13,13,18]. The imaging studies of our patients showed ground-glass opacities similar to those previously reported in other EVALI cases (Fig. 1). However, we should highlight that variation exists and multiple patterns may be seen.
Bronchoalveolar lavage (BAL) could have some applicability in cases where the suspicion of EVALI is high but clinical and imaging findings are not sufficient for diagnosis. In particular, bronchoscopy may be considered in cases of atypical radiologic findings suggesting alternative pathology, such as vasculitis, or in immunocompromised patients, but it is not strongly recommended for each patient with suspected EVALI [1,13]. BAL has been performed in previous cases, typically showing eosinophils and neutrophils or lipid-laden macrophages with oil red O stain, indicating lipoid pneumonitis and/or eosinophils, but findings may vary [2,3,6,10]. Although positive findings might have some specificity, and applicability, the necessity and the sensitivity are still questionable. Recent studies showed that vitamin E, a known component of many THC and CBC products, could be a marker for EVALI given the high prevalence in BAL fluids derived from patients with EVALI [11]. In the presented study, BAL was not performed given the sufficient exclusion of infectious causes and high suspicion for EVALI. We could speculate that the low positivity of urinary THC products in our cases may be due to the low frequency of use of cannabis-related-products or use of undetectable in urine toxicology CDC products.
Clinicians should be aware of the clinical features of EVALI and obtain detailed and proper e-cigarette/vaping history in all patients and especially the high-risk groups. Although significant elements of e-cigarette/vaping history have been suggested [2], incorporation of a routine screening for vaping in every inpatient and outpatient encounter should be performed. Until now there is no standardized approach to vaping history. Given the absence of specific inclusion criteria, EVALI remains a diagnosis of exclusion. The criteria as were previously described by Layden et al., require the use of an e-cigarette or dabbing in 90 days before onset followed by radiographic evidence of the disease such as opacities in the chest x-ray or ground-glass opacities on chest CT [1]. Confirmation of the case requires the exclusion of underlying infective causes after extensive workup. According to the same criteria, even if the infection is identified but the clinical picture and disease progression imply that this is not the sole cause of the respiratory disease, the case should be characterized as probable EVALI [1,13]. Absence also of other plausible diagnoses that could explain the clinical picture, such as cardiac, rheumatologic or neoplastic disease, is necessary for either confirmed or probable cases [1,13].
Although the nature of EVALI is a better understanding due to the knowledge to date, significant variations have been noticed in the course of the disease and several case reports have shown that the severity and the clinical picture may vary from patient to patient [1,2,13,22]. Despite the fact that, variations of the severity exist the average number of days of hospitalization of the current study is 5.2, similar to that reported by other case series and extended epidemiologic studies. Specifically, Layden et al. reported an average of 6 days, while Siegel reported an average of 6.7 days [1,2]. Layden et al., 32 % of patients were intubated at some point during hospitalization [1]. Siegel et al., in a larger patient sample of 338 patients showed that 22 % of them required intubation and mechanical ventilation. In our study, although a higher percentage of patients were found hypoxemic (60 %) compared to other studies (Layden et al., 31 % Siegel et al., 57 %), only three out of ten (30 %) of the presented patients required Non-invasive Positive Pressure Ventilation (NPPV) and none of them were intubated. Although limitations could apply due to the small number of patients in our study, the differences in requirements for aggressive airway management compared to other studies could indicate other compounding factors, such as coexisting comorbidities and age [2]. Specifically, in the present study none of the patients had reported cardiovascular disease and two out of the ten had asthma, one patient had chronic obstructive airway disease. This is in line with other studies in literature supporting that age and number of coexisting chronic cardiopulmonary conditions are highly associated with the severity of EVALI [2].
As it was expected, the oxygen saturation levels at the admission were lower in the patients that were finally admitted to ICU compared to the patients that treated in the medical ward (Table 4). Further investigation of the association between oxygen saturation or arterial PO2 levels at the admission and need for immediately NPPV and ICU transfer could be useful in the development of an evidence-based approach for the management of patients with EVALI.
Even though rehospitalization of patients previously treated for EVALI have been reported, in our study none of the patients was readmitted to the hospital in a period of 3 months after hospitalization. According to recent epidemiologic studies coexistence of multiple chronic medical conditions may significantly increase the risk for rehospitalization of patients with EVALI [22]. As in the previous report, only a small percentage of patients in our sample group had chronic cardiopulmonary disease (20 %). In general, our patients appear to have relatively mild disease. We may assume that because our patients are young (<30 years old) the disease was mild. Investigation of the effect of age on severity of EVALI requires a larger sample that includes a wide range of ages.
There is a rapid increase in the number of deaths related to EVALI. Specifically, Layden et al. showed that 2 % of the patients did not completely recover and died due to EVALI [1].However, the existing data show that the mortality rates of EVALI is relatively low [1,2].
The therapeutic approach and management of EVALI is not clear yet. Since the knowledge is limited regarding the optimal treatment in the early and late stages of the disease, in most of the cases, antibiotic therapy is administered empirically, until underlying pneumonia is excluded. The use of steroids for treatment of EVALI remains controversial and although previous studies recommend the use of high doses of steroids [1,7,23], there is not enough evidence to clarify if there is a dose-dependent steroid effect. Also, based on the recent data the extent of the steroid treatment varies and could be related to the severity of the disease. In our study, half of the patients (50 %) received steroid treatment. All of the patients that presented with higher severity requiring transfer to ICU and NIPPV were treated with steroids. Generally, no clear guidelines exist for the management of EVALI, but considering that the pathophysiology of the disease includes immune reaction to e-cigarette irritants and the development of inflammation, steroid use is a reasonable approach.
Given the ongoing SARS-Cov-2 epidemic, a disease with clinical symptomatology that presents similarities with EVALI, an expected question is what are the unique clinical and laboratory findings that would help us differentiate the two diseases. According to previous reports, leukocytosis, favor the diagnosis of EVALI given lymphopenia is a common finding in COVID-19. Vaping history remains key in making the diagnosis. [24,25] Also, patients with EVALI were found to improve within corticosteroids compared to COVID-19 [26].
4.1. Limitations of the study and further investigations
We acknowledge that a number of limitations apply to our study due to the small sample size. Further investigations including a larger number of patients could increase the power of the study and provide stronger evidence for our conclusions and suggestions. Given the majority of the patients defined themselves as non-Hispanic whites (Table 5), investigation of the race effect on EVALI presentation, progression and mortality requires a larger and more variable sample.
5. Conclusion
Our ten-case series data recognized that clinical characteristics in hospitalized EVALI patients with radiological findings of lung injury, may include both respiratory and gastrointestinal or constitutional symptoms and based on the current literature may be misdiagnosed. In an era of new diseases affecting the respiratory system such as COVID-19, the clinician should be aware of the unique imaging and laboratory findings associated with EVALI. The reinforcement and standardization of the importance of e-cigarette and vaping history in both outpatient and inpatient settings are highly recommended, especially in high-risk groups like young adults. Based on our data and the existing literature, the presence of lymphocytosis and the epidemiologic data, such as the age of the patient could be contributory in the diagnosis of the disease. Future studies focusing on the comparison of clinical, laboratory and imaging findings of EVALI patients with patients presenting with similar respiratory conditions, with similar clinical manifestations, such as COVID-19, will contribute significantly in the development of standardized approach for EVALI diagnosis and treatment.
Author statement
The manuscript is original and it has not been published or accepted for publication, either in whole or in part, in any form. This manuscript is not under consideration elsewhere. The manuscript has been read and approved by all authors. The authors whose names are listed in this article certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
CRediT authorship contribution statement
Sotirios G. Doukas: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Leena Kavali: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing - original draft, Writing - review & editing. Rohan S. Menon: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing - original draft, Writing - review & editing. Boris N. Izotov: Conceptualization, Validation, Writing - review & editing. Amar Bukhari: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Layden J.E., Ghinai I., Pray I. Pulmonary illness related to E-Cigarette use in Illinois and Wisconsin- final report. N. Engl. J. Med. 2020;382:903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 2.Siegel D.A. Update: interim guidance for health care providers evaluating and caring for patients with suspected E-cigarette, or vaping, product use associated lung injury-united states, October 2019. MMWR morb. Morb. Mortal. Rep. Surveill. Summ. 2019;68 doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirbolouk M. Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Ann. Intern. Med. 2018;169:429. doi: 10.7326/M17-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miech R., Johnston L., O’Malley P.M., Bachman J.G., Patrick M.E. Trends in adolescent vaping, 2017–2019. N. Engl. J. Med. 2019;381:1490–1491. doi: 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flouris A.D. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal. Toxicol. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 6.Krishnasamy V.P., Hallowell B.D., Ko J.Y. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury-United States, August 2019–January 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:90–94. doi: 10.15585/mmwr.mm6903e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson K. Outbreak of electronic-cigarette–associated acute lipoid pneumonia — north Carolina, July–august 2019. MMWR morb. Morb. Mortal. Rep. Surveill. Summ. 2019;68 doi: 10.15585/mmwr.mm6836e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillman I.G., Kistler K.A., Stewart E.W., Paolantonio A.R. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol. 2016;75:58–65. doi: 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 9.National Academies of Sciences, E . National Academies Press (US); 2018. Toxicology of E-Cigarette Constituents. [Google Scholar]
- 10.Zhao P., Liu G., Cui Y., Sun X. Propylene glycol alginate sodium sulphate attenuates LPS-induced acute lung injury in a mouse model. Innate Immun. 2019;25:513–521. doi: 10.1177/1753425919874491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blount B.C., Karwowski M.P., Shields P.G. Vitamin e acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Works K., Stack L. E-cigarette or vaping product‐use‐associated lung injury (EVALI): A case report of a pneumonia mimic with severe leukocytosis and weight loss. JACEP Open. 2020;1:46–48. doi: 10.1002/emp2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schier J.G. Severe pulmonary disease associated with electronic-cigarette-Product use - interim guidance. MMWR Morb. Mortal. Wkly. Rep. 2019;68:787–790. doi: 10.15585/mmwr.mm6836e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King B.A., Jones C.M., Baldwin G.T., Briss P.A. The EVALI and youth vaping epidemics - implications for public health. N. Engl. J. Med. 2020;382:689–691. doi: 10.1056/NEJMp1916171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chand H.S., Muthumalage T., Maziak W., Rahman I. Pulmonary toxicity and the pathophysiology of electronic cigarette, or vaping product, use associated lung injury. Front. Pharmacol. 2020;10 doi: 10.3389/fphar.2019.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaumont M. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316:L705–L719. doi: 10.1152/ajplung.00492.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olmedo P. Metal concentrations in e-Cigarette liquid and aerosol samples: the contribution of metallic coils. Environ. Health Perspect. 2018;126:027010. doi: 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He T., Oks M., Esposito M., Steinberg H., Makaryus M. Tree-in-Bloom”: severe acute lung injury induced by vaping Cannabis oil. Ann. Am. Thorac. Soc. 2017;14:468–470. doi: 10.1513/AnnalsATS.201612-974LE. [DOI] [PubMed] [Google Scholar]
- 19.Itoh M., Aoshiba K., Herai Y., Nakamura H., Takemura T. Lung injury associated with electronic cigarettes inhalation diagnosed by transbronchial lung biopsy. Respirol. Case Rep. 2018;6:e00282. doi: 10.1002/rcr2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry T.S., Kanne J.P., Kligerman S.J. Imaging of vaping-associated lung disease. N. Engl. J. Med. 2019;381:1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 21.Agustin, M., Yamamoto, M., Cabrera, F. & Eusebio, R. Diffuse Alveolar Hemorrhage Induced by Vaping. Case Reports in Pulmonology https://www.hindawi.com/journals/cripu/2018/9724530/ (2018) doi:10.1155/2018/9724530. [DOI] [PMC free article] [PubMed]
- 22.Mikosz C.A. Characteristics of Patients Experiencing Rehospitalization or Death After Hospital Discharge in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury — United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020;68:1183–1188. doi: 10.15585/mmwr.mm685152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddock S.D. Pulmonary lipid-laden macrophages and vaping. N. Engl. J. Med. 2019;381:1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 24.Callahan S.J., Harris D., Collingridge D.S., Guidry D.W., Dean N.C., Lanspa M.J., Blagev D.P. Diagnosing EVALI in the time of COVID-19. Chest. 2020;S0012-3692(20) doi: 10.1016/j.chest.2020.06.029. 31818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Lancet Respiratory Medicine The EVALI outbreak and vaping in the COVID-19 era. Lancet Respir. Med. 2020;8:831. doi: 10.1016/S2213-2600(20)30360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansari-Gilani K., Petraszko A.M., Gilkeson R.C. COVID-19 pneumonia versus EVALI, distinguishing the overlapping CT features in the COVID-19 era. Heart Lung. 2020;S0147-9563(20) doi: 10.1016/j.hrtlng.2020.06.008. 30268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]