Abstract
Liver cancer is a major contributor to the world’s cancer burden and incidence rates have increased in many countries in recent decades. As the principal histologic type of liver cancer, hepatocellular carcinoma (HCC) is responsible for the great majority of liver cancer diagnoses and deaths. Hepatitis B virus (HBV) and hepatitis C virus (HCV) remain, at present, the most important global risk factors for HCC, but it is likely their importance will decline in the coming years. The effect of HBV vaccination of newborns, already seen in young adults in some countries, will be more notable as vaccinated cohorts age. In addition, effective treatments for chronic infections with both HBV and HCV should contribute to declines in the rates of viral-associated HCC. Unfortunately, the prevalence of metabolic risk factors for HCC, including metabolic syndrome, obesity, type II diabetes and non-alcoholic fatty liver disease (NAFLD) are increasing and may jointly become the major cause of HCC globally. Excessive alcoholic consumption also remains an intractable risk factor, as does aflatoxin contamination of food crops in some parts of the world. While significant efforts in early diagnosis and better treatment are certainly needed for HCC, primary prevention efforts aimed at decreasing the prevalence and obesity and diabetes and controlling mycotoxin growth, and are just as urgently required.
International incidence, mortality, temporal trends
Primary liver cancer is the seventh most frequently occurring cancer in the world and the second most common cause of cancer mortality (1). The highest incidence rates in the world are found in Asia and Africa (Figure 1) (2). Mongolia has the highest incidence at 93.7 per 100,000, but China has the greatest number of cases, due to both an elevated rate (18.3 per 100,000) and the world’s largest population (1.4 billion persons) (1).
Figure 1.
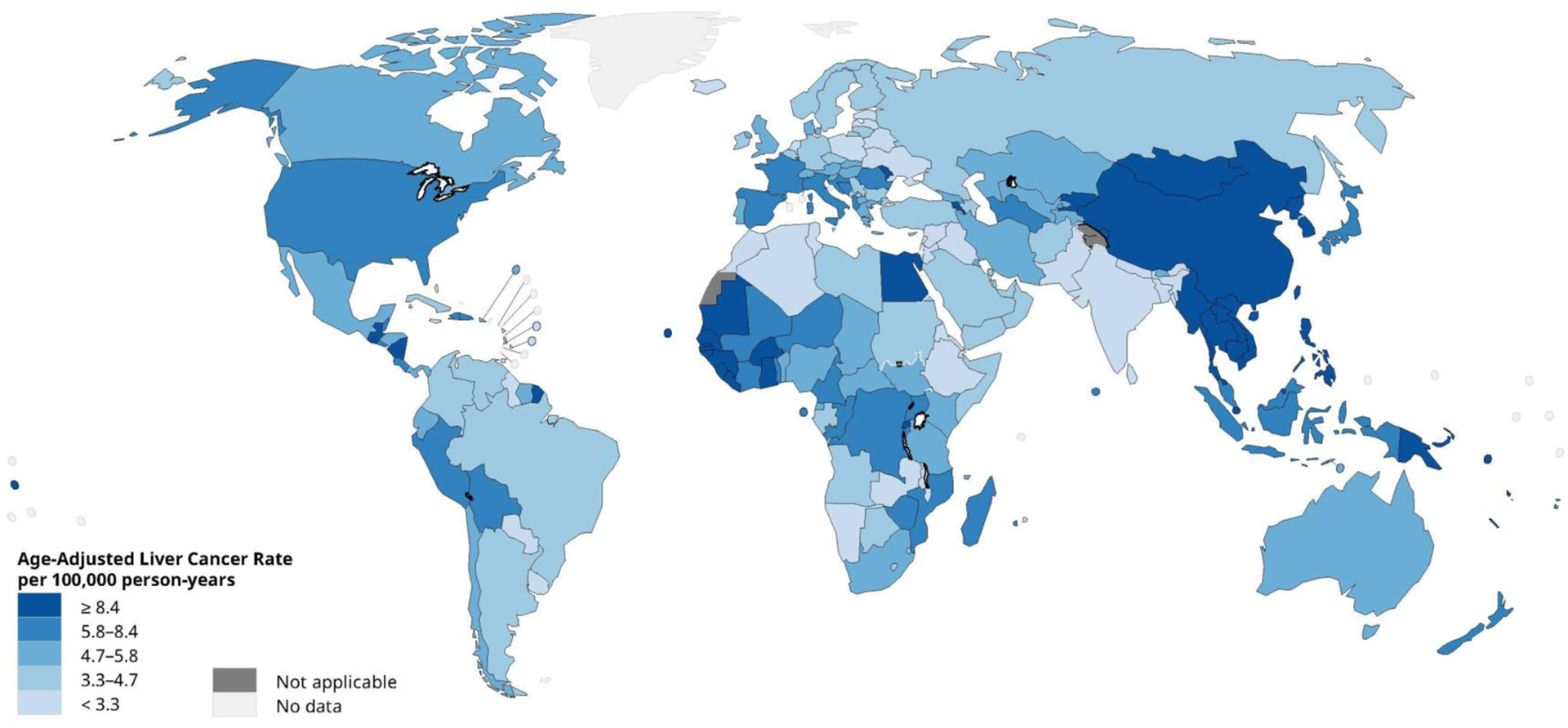
Global age-adjusted incidence rates of liver cancer, estimated for 2018. Data source: GLOBOCAN 2018. Graph production: IARC (http://gco.iarc.fr/today), World Health Organization.
Globally, hepatocellular carcinoma (HCC) is the dominant type of liver cancer, accounting for approximately 75% of the total (2). Incidence rates of HCC have been decreasing in some high-rate areas but increasing in many low-rate areas (Figure 2) (3). In the interval between 1978 and 2012, HCC incidence declined in many Asian countries and Italy, but increased in India, the Americas, Oceania, and most European countries (3). In more recent years, however, the increase in some countries, such as the US, has abated, as rates in various subgroups have plateaued or declined (4, 5).
Figure 2.

Trends in hepatocellular carcinoma incidence rates by country, 1978–1982 through 2008–2012. Rates are per 100,000 person-years and age-adjusted to the world standard population.
Prognosis of HCC is poor in all regions of the world(6). As a result, incidence and mortality rates are roughly equivalent. In 2018, the estimated global incidence rate of liver cancer per 100,000 person-years was 9.3 while the corresponding mortality rate was 8.5 (1).
Demographic characteristics
Age.
In most populations, incidence rates of HCC and age are directly correlated until approximately 75 years of age (2). The median age at diagnosis, however, is generally somewhat younger. In the US, for example, the median age at diagnosis among men is between ages 60 and 64 years, while the median age among women is 65–69 years (7). By contrast, in Africa, there is a significant difference in median age at diagnosis between Egypt (58 years) and other African countries (46 years) (8).
Sex.
In most countries, incidence rates among men are two to four-fold higher than rates among women (2). For example, in the U.S., the 2016 age-adjusted incidence rate among men was 10.4 per 100,000, while the rate among women was 2.9 per 100,000. The greatest sex differences are seen in Europe, where rates among men can be greater than four-fold higher than rates among women (e.g., France M:F ratio = 5.0 and Malta M:F ratio = 4.8) (2). In some countries, however, the rates among men and women are much more similar. For example, Uganda (M:F ratio = 1.1), Costa Rica (M:F ratio = 1.6), Ecuador (M:F ratio = 1.0) and Colombia (M:F ratio = 1.6) report nearly equal rates (2).
Race/ethnicity.
In multi-ethnic societies such as the US, racial/ethnic disparities can be striking. In 2001, Asians/Pacific Islanders had the highest HCC rates in the US (11.3 per 100,000), but rates among Asians/Pacific Islanders began declining thereafter. As a result, in 2016, American Indians/Alaskan Natives had the highest incidence (11.4), followed by Hispanics (9.8), Asians/Pacific Islanders (9.1), non-Hispanic blacks (8.1), and non-Hispanic whites (4.6) (7).
The wide variability in incidence of HCC by geographic region, age, sex and race/ethnicity is largely, but not entirely, related to the prevalence, and age at acquisition, of major risk factors.
Risk factors
Hepatitis B Virus (HBV).
HBV is a DNA virus that induces chronic necroinflammatory disease that promotes mutations in liver cells and leads to HCC (9). When evaluating tumor tissue from HBV carriers, HBV DNA is commonly integrated into the genome (10).
The lifetime risk of developing HCC among HBV carriers ranges from 10–25% (10). In a US study, the annual HCC incidence was estimated to be 0.42% overall (11), but incidence can vary depending on whether the person has an active HBV infection and/or cirrhosis (12). Cofactors that also increase risk among HBV carriers include demographic characteristics (e.g., male sex, older age, Asian or African ancestry, family history of HCC), viral factors (e.g., high HBV replication levels, HBV genotype, infection duration, coinfection with HCV or HIV), and environmental exposures (e.g., aflatoxin, alcohol, tobacco, obesity, diabetes) (12). Some risk factors have been incorporated into scoring systems or surveillance recommendations, such as the one devised by the American Association for the Study of Liver Diseases based on cirrhosis, family history of HCC, age, and Asian or African American race/ethnicity. However, these recommendations may not be accurate for predicting HCC risk among HBV carriers who undergo antiviral therapy (13). Further, US data show that HCC risk is extremely low among individuals, including African Americans, who are less than 40 years of age.
Randomized controlled trials have shown that antiviral treatment of HBV infection can achieve sustained reductions in HBV-DNA levels and improve liver function and histology (14). The primary drugs used are nucleos(t)ide analogs (NAs) (15). Increasing evidence suggests that NA treatment can reduce, but not eliminate, the short and medium-term risk of HCC (16, 17). A meta-analysis found that HCC incidence was significantly lower among persons treated with the first NAs introduced, primarily lamivudine (17). Fewer studies have evaluated risk reduction with the newer NAs. Studies from Taiwan and Japan, however, have reported significantly lower risks of HCC in conjunction with entecavir therapy (18, 19). A US study investigating the effect of entecavir or tenofovir reported that the 5-year HCC risk was lower among persons with cirrhosis, but the overall risk was still higher than among persons without cirrhosis (20). Among patients on long-term NA therapy, older age, male sex, cirrhosis, low platelet count, and diabetes are likely HCC co-factors and have been incorporated in validated risk scores for HCC prediction (21).
HBV vaccination programs are a key HCC prevention strategy. The 30-year report on the neonatal HBV vaccination effort in Taiwan noted that HCC incidence declined 80% and mortality declined 92% in cohorts born after the vaccination program began (22). Many other countries that implemented programs in the 1980s, such as China, Singapore, and Spain are seeing reductions similar to those of Taiwan in the prevalence of HBV in vaccinated cohorts (10).
Hepatitis C Virus (HCV).
Chronic HCV infection is a firmly established risk factor for HCC, increasing risk by 10–20 fold (9). HCV is a RNA virus that does not integrate into the host’s genome and is, thus, unlikely to be the primary initiator of tumorigenesis. Rather, as approximately 90% of HCV-associated HCC cases are preceded by cirrhosis, HCV likely promotes tumorigenesis through repetitive damage, regeneration and fibrosis (23). The annual incidence of HCC in persons with HCV-related cirrhosis ranges from 0.5–10% (24).
A US model based on a population of HCV carriers estimated that the number of HCV-associated HCC cases increased by 130% between 1990–1999 and 2000–2009 (25). As the 1945–1965 birth cohort has a higher HCV prevalence than other birth cohorts, it has been estimated that the numbers of HCV-associated HCCs in the US will peak around 2020 (24, 26).
Among persons with active HCV infections, co-factors for HCC include male sex, Hispanic ethnicity, HCV genotype 3, longer duration of infection, coinfections with HBV or HIV, insulin resistance, obesity, diabetes, tobacco and alcohol (24, 27). The main factor that decreases HCC incidence is sustained virologic response (SVR) achieved via antiviral therapy (28). Several large studies of direct-acting antiviral (DAA) therapy, and meta-analyses of these studies (29), have demonstrated that HCC risk, while not eliminated, is reduced by 50–80% among persons who achieve SVR (30, 31). While HCC risk is reduced with SVR, the rates do not revert to baseline, especially among persons with cirrhosis. For example, it has been reported that the risk of HCC was reduced 76% in a cohort of patients who achieved SVR and the annual incidence of HCC was 0.9%, with the highest rate (1.0–2.2%) seen in conjunction with cirrhosis (32). Longer follow up of this cohort showed that cumulative 1, 2, and 3-year risks of HCC were 1.1%, 1.9% and 2.8%, respectively. These incidence rates are at, or below, the threshold for cost-effective HCC surveillance (32). Among patients who achieve SVR, HCC risk if higher in association with alcohol use, older age, infection with HCV genotype 3, and elevated markers of hepatic fibrosis (33).
Alcohol.
While excessive alcohol consumption is a well-established risk factor for liver cancer (34), the effect of lower levels of consumption has not been as thoroughly investigated. In a meta-analysis of prospective studies, heavy alcohol consumption (≥3 drinks/day) was associated with a 16% increased risk of HCC, but there was no association with lower levels of consumption (<3 drinks/day) (35). A US pooling project found, however, that lower levels of consumption (<3 drinks/day) were associated with a significantly decreased risk of HCC, even after excluding non-drinkers (36). The relationship varied by diabetes status however; there was no association with lower level consumption among persons with diabetes, while there was a 35% decreased risk among persons without diabetes (36). In addition, alcohol may have a stronger association with HCC risk among women than men. This could be due to differences in alcohol dehydrogenase activity (37), or due to a stronger association between alcohol and cirrhosis among women (38). In a meta-analysis examining heavy drinking (>4 drinks/day), alcohol was associated with an almost four-fold increased risk among women, but only a 59% increased risk among men (34). The trends of alcohol use and alcoholic liver disease vary among countries. In the US, several recent reports have reported a notable increase (39).
Metabolic Syndrome, Diabetes, and Obesity.
Increasing evidence suggests that metabolic syndrome, a collection of conditions including insulin resistance, abdominal obesity, atherogenic dyslipidemia and hypertension, increases risk of HCC. A 2014 meta-analysis estimated that metabolic syndrome was associated with an 81% increased risk (40). Treating one of the metabolic syndrome conditions, dyslipidemia, with statins, however, may ameliorate risk by 37–42% (41, 42).
Studies in diverse populations have reported that diabetes is associated with a 2 to 3-fold increased risk of HCC, with a significantly greater relative risk among men than women (43). Longer duration of diabetes may be also associated with an incremental increase in risk of HCC, but the relationship between diabetes severity or blood sugar control and HCC risk is unclear (44). The treatment of type 2 diabetes with metformin has been reported to decrease the risk of HCC, whereas treatment with insulin or sulfonylureas has been reported to increase risk (45–47). Metformin is a first-line therapy, however, while insulin and sulfonylureas are not, thus the correlation of medication with disease severity might lead to an overestimation of risk reduction by metformin (10).
It is well-established that excess adult adiposity increases the risk of liver cancer (48), but several studies also suggest that there is an effect of adiposity at earlier ages. A Danish study reported that a one-unit increase in body mass index (BMI) z-score at ages 7 or 13 years was associated with a 20–30% increased risk of liver cancer (49), while studies from the US and Sweden found that obesity in early adulthood was associated with 2 to 3-fold increased risk (50–52). BMI may not accurately capture important elements of obesity, thus recent studies have examined waist and hip circumference as measures of excess abdominal and gluteofemoral adiposity, respectively. Cohort studies in Europe and the US have reported that persons with the high waist circumference have a 2-fold increased HCC risk, which remains unchanged after adjustment for BMI or hip circumference (53–55). Further, excess abdominal size in one of the studies was associated with an increased HCC risk, even among individuals who had a BMI of 18.5–≤25 kg/m2, but excess gluteofemoral size alone conferred no increased risk (54).
Nonalcoholic Fatty Liver Disease (NAFLD).
The overall prevalence of NAFLD among US adults is 32.8%, but the prevalence varies by sex (men 34.7%; women 31.0%), and race/ethnicity (Mexican-Americans 41.2%; whites 32.5%; blacks 29.1%) (56). Among persons with NAFLD, 20–30% are estimated to progress to non-alcoholic steatohepatitis (NASH), which then progresses to cirrhosis in 10–20% of cases (57, 58). NAFLD is now a leading cause of cirrhosis and NASH is the second-leading cause of liver transplantation related to HCC in the US (59). The increasing number of HCCs due to NASH could offset the reductions in HCV-related HCC expected after 2020 (24). Factors related to HCC risk associated with NAFLD include clinical factors (cirrhosis, diabetes, obesity, hypertension), demographic characteristics (age, race/ethnicity), and genetic susceptibility (e.g., genetic variability in PNPLA3).
Between 70 and 80% of NAFLD-related HCCs develop in cirrhotic livers, but the HCC risk is lower than that associated with HCV-related cirrhosis. While 20–30% of NAFLD-related HCCs develop in the absence of cirrhosis (60), the determinants of this proportion are unclear. An analysis of studies investigating the link between NAFLD and HCC risk found that HCC risk varied from 0 to 38% after follow-up of 5–10 years. In comparison, persons with NAFLD-related cirrhosis had an HCC incidence ranging from 2.4 to 12.8%. A recent large cohort study reported an HCC incidence of 0.21 per 1000 person-years among persons with NAFLD, which was significantly higher than the risk among persons without NAFLD (60). There was also an increase in risk with each additional metabolic trait; for example, HCC risk was 2.6-fold higher in NAFLD patients with diabetes, obesity, dyslipidemia and hypertension compared to NAFLD patients without any of these traits (61). While weight loss can reduce NAFLD severity, no studies have shown weight loss to affect HCC risk.
There is no high-level evidence to support or refute the value, method, or frequency of HCC surveillance among the NAFLD population. However, based on cost-effectiveness modelling, clinical practice guidelines recommend considering HCC surveillance among persons with cirrhosis when the expected annual HCC incidence is 1.5% or higher (62).
Aflatoxin B1.
Aflatoxins, mycotoxins produced by fungi of the Aspergillus species, contaminate a variety of foodstuffs, most notably, maize, ground nuts, and tree nuts. Of the four principal aflatoxins, B1, B2, G1, and G2, the most potent is aflatoxin B1 (AFB1) (63). AFB1 occurs in many locations around the world, especially in countries with warm, humid environments. The development of AFB1 biomarkers enabled the link between HCC and AFB1 to be definitely established and prompted IARC to classify AFB1 as a group 1 human carcinogen (63). AFB1 is particularly carcinogenic when it co-occurs with chronic HBV infection as the combination of factors has a synergistic effect on HCC risk. A 2012 meta-analysis estimated that AFB1 alone increased HCC risk by 6-fold, HBV alone by 11-fold, and the two factors together by 54-fold (64).
AFB1 contamination of crops is difficult to combat because contamination can occur both pre- and post-harvest (65). A very successful effort, however, was the replacement of maize with rice as the dietary staple in parts of China; an effort has been largely credited for the current decline in HCC rates (66, 67). However, the single most effective way to reduce HCC risk in regions where AFB1 and HBV co-occur is to vaccinate against HBV in order to eliminate the synergistic effect on risk.
Tobacco
In a review of 113 studies, the 2014 US Surgeon General’s report found that current cigarette smoking was associated with a 70% increased risk of liver cancer, while former smoking was associated with a 40% increased risk (68). A recent study, however, reported that years since smoking cessation was inversely associated with HCC risk, with individuals who stopped smoking >30 years ago having an HCC risk similar to that of never-smokers (36).
Dietary factors.
Coffee has been consistently associated with decreased risk of liver cancer (69). A 2017 meta-analysis of both cohort and case-control studies reported that an extra two cups of coffee per day was associated with a 35% reduced risk (70). Coffee has also been associated with lower liver enzyme levels, slower progression of fibrosis, and lower risk of diabetes. The mechanisms underlying a possible protective effect of coffee, however, are not clear. Experimental evidence suggests there may be beneficial effects of caffeine as well as many other coffee components (e.g., diterpenes) in reducing inflammation, fibrosis, insulin resistance and oncogenesis (71).
High iron intake has been long associated with increased risk (72, 73). The consumption of traditional iron-rich beer is associated with HCC in southern and central Africa (74). Further, a recent meta-analysis of prospective studies in Asia, Europe, and the US found that higher serum ferritin levels were associated with a 49% increased HCC risk, while higher serum iron levels were associated with a 2.5-fold greater risk (72).
Genetic susceptibility
Mutations in the genes for hemochromatosis (HFE), alpha 1-antitrypsin deficiency (SERPINA1), glycogen storage diseases (G6PC, SLC37A4), porphyrias (HMBS, UROD), tyrosinemia (FAH) and Wilson’s Disease (ATP7B) increase susceptibility to HCC. Polymorphisms, originally examined in candidate-locus studies, have also been related to risk. A 2011 meta-analysis found that polymorphisms in UGT1A7, MnSOD and IL-1B were all significantly associated with risk (75). More recently, genome-wide association studies (GWAS) conducted in Asian populations where HBV or HCV were factors reported increased risks in association with a number of loci, most commonly ones located in the HLA region (HLA-DP, HLA-DQ, HLA-DR, MICA) (76–85). Another reported association maps to chromosome 1p36.22, a region that may harbor a tumor suppressor gene for HCC (85). The KIF1B gene in this region has been reported to be associated with apoptosis, and its association with HCC was replicated in subsequent studies from other Asian populations. Associations with STAT4, GRIK1, EFCAB11, and EFCAB11 have also been found (77, 78, 80). In non-Asian populations, a polymorphism in the PNPLA3 gene has been demonstrated to be associated with HCC. The rs738409 SNP was first reported to be related to NAFLD (86), conferring a 4-fold increased risk among persons homozygous for the risk allele and an almost 2-fold increased risk among heterozygotes (87). Subsequent examinations of the polymorphism and risk of HCC also found associations. As reported by a recent meta-analysis, there is evidence of a significant association among white populations (OR=1.75), but no evidence of an association among Asian populations (88).
Population Attributable Risks
The extent to which individual risk factors contribute to the HCC burden can be estimated by calculation of population attributable fractions (PAFs), which are important measures for developing cancer control policies. PAFs are dependent on the strength of the risk factor-HCC association and the prevalence of the risk factor in the population, thus, PAFs vary widely by geographic location. For example, although it has been estimated that the global HBV PAF is 56%, the PAF for North America is estimated at just 7%, while the PAF for Eastern Asia is estimated at 69% (89). Similarly, the global HCV PAF is estimated at 20%, but the PAF for Eastern Asia is estimated at 11% while the PAF for Northern Africa is estimated at 79% (89). The global AFB1 PAF is estimated to be 17%, but the PAF ranges from 8% to 21% in populations where HBV is absent versus present, respectively (64). For alcohol, the global PAF is estimated at 26%, although the PAF in Eastern Europe is notably higher (39%) than the PAF in South Asia (13%). For obesity, the global PAF is estimated at 9%, with notably higher PAFs in North America (24%) than in Southeast Asia (4%) and sub-Saharan Africa (4%). For diabetes, the global PAF is estimated to be 7%, with higher PAFs in Oceania (12%) and the Middle East (12%) and lower PAFs in sub-Saharan Africa (4–5%).
Conclusions
HBV and HCV remain the most important global risk factors for HCC. However, the prevalence of both factors should decline in the coming years due to HBV vaccination of newborns, and more effective treatment of both HBV and HCV carriers. The prevalence of NAFLD/NASH is increasing and may soon overtake viral factors as the major cause of HCC globally. Excessive alcoholic consumption also remains an important risk factor. Due to climate change, so AFB1 could become a more dominant risk factor in the coming decades. These changing trends suggest that more effort needs to be focused on combating obesity and diabetes to decrease the incidence of NAFLD, and more effective strategies to control alcohol use and mycotoxin growth need to be implemented.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer 2019;E-pub (Oct 10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018;67:600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrick JL, Florio AA, Loomba R, McGlynn KA. Have incidence rates of liver cancer in the U.S. peaked? Cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, El-Serag HB, Thrift AP. Sex and Race Disparities in the Incidence of Hepatocellular Carcinoma in the United States Examined through Age-Period-Cohort Analysis. Cancer Epidemiol Biomarkers Prev 2020;29:88–94. [DOI] [PubMed] [Google Scholar]
- 6.Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore) 2017;96:e5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2018 Sub (1975–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. In; 2019.
- 8.Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, Abdelmaksoud AH, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol 2017;2:103–111. [DOI] [PubMed] [Google Scholar]
- 9.A review of human carcinogens: Biological agents. IARC Monogr Eval Carcinog Risk Hum 2012;100B:1–441. [PMC free article] [PubMed] [Google Scholar]
- 10.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, Spradling PR, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol 2014;12:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn J, Lim JK, Lee HM, Lok AS, Nguyen M, Pan CQ, Mannalithara A, et al. Lower Observed Hepatocellular Carcinoma Incidence in Chronic Hepatitis B Patients Treated With Entecavir: Results of the ENUMERATE Study. Am J Gastroenterol 2016;111:1297–1304. [DOI] [PubMed] [Google Scholar]
- 14.Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol 2015;13:2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. In: World Health Organization; Geneva, 2015. [PubMed] [Google Scholar]
- 16.Hsu YC, Wu CY, Lane HY, Chang CY, Tai CM, Tseng CH, Lo GH, et al. Determinants of hepatocellular carcinoma in cirrhotic patients treated with nucleos(t)ide analogues for chronic hepatitis B. J Antimicrob Chemother 2014;69:1920–1927. [DOI] [PubMed] [Google Scholar]
- 17.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol 2010;53:348–356. [DOI] [PubMed] [Google Scholar]
- 18.Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, Wu C, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology 2014;147:143–151. [DOI] [PubMed] [Google Scholar]
- 19.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013;58:98–107. [DOI] [PubMed] [Google Scholar]
- 20.Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 2017;66:1444–1453. [DOI] [PubMed] [Google Scholar]
- 21.Hsu YC, Yip TC, Ho HJ, Wong VW, Huang YT, El-Serag HB, Lee TY, et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol 2018;69:278–285. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013;310:974–976. [DOI] [PubMed] [Google Scholar]
- 23.De Mitri MS, Poussin K, Baccarini P, Pontisso P, D’Errico A, Simon N, Grigioni W, et al. HCV-associated liver cancer without cirrhosis. Lancet 1995;345:413–415. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 2014;60:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010;138:513–521. [DOI] [PubMed] [Google Scholar]
- 26.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang KC, Wu YY, Hung CH, Lu SN, Lee CM, Chiu KW, Tsai MC, et al. Clinical-guide risk prediction of hepatocellular carcinoma development in chronic hepatitis C patients after interferon-based therapy. Br J Cancer 2013;109:2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015;162:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204–1212. [DOI] [PubMed] [Google Scholar]
- 30.Huang AC, Mehta N, Dodge JL, Yao FY, Terrault NA. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology 2018;68:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2018;68:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017;153:996–1005. [DOI] [PubMed] [Google Scholar]
- 33.Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology 2020;71:44–55. [DOI] [PubMed] [Google Scholar]
- 34.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, Corrao G, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol 2014;25:1526–1535. [DOI] [PubMed] [Google Scholar]
- 36.Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, Buring JE, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer 2018;118:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 1990;322:95–99. [DOI] [PubMed] [Google Scholar]
- 38.Corrao G, Arico S, Zambon A, Torchio P, Di Orio F. Female sex and the risk of liver cirrhosis. Collaborative Groups for the Study of Liver Diseases in Italy. Scand J Gastroenterol 1997;32:1174–1180. [DOI] [PubMed] [Google Scholar]
- 39.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol 2014;48:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradelli D, Soranna D, Scotti L, Zambon A, Catapano A, Mancia G, La Vecchia C, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev 2013;22:229–234. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013;144:323–332. [DOI] [PubMed] [Google Scholar]
- 43.Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia 2018;61:2140–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460–468. [DOI] [PubMed] [Google Scholar]
- 45.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One 2013;8:e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881–891. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol 2013;48:78–87. [DOI] [PubMed] [Google Scholar]
- 48.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working G. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berentzen TL, Gamborg M, Holst C, Sorensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol 2014;60:325–330. [DOI] [PubMed] [Google Scholar]
- 50.Hagstrom H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population-based cohort study in 1.2 million men. Gut 2018;67:1536–1542. [DOI] [PubMed] [Google Scholar]
- 51.Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, Hawk E, et al. Obesity Early in Adulthood Increases Risk but Does Not Affect Outcomes of Hepatocellular Carcinoma. Gastroenterology 2015;149:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang B, Petrick JL, Kelly SP, Graubard BI, Freedman ND, McGlynn KA. Adiposity across the adult life course and incidence of primary liver cancer: The NIH-AARP cohort. Int J Cancer 2017;141:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Beane Freeman LE, Buring JE, et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res 2016;76:6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Florio AA, Campbell PT, Zhang X, Zeleniuch-Jacquotte A, Wactawski-Wende J, Smith-Warner SA, Sinha R, et al. Abdominal and gluteofemoral size and risk of liver cancer: The liver cancer pooling project. Int J Cancer 2019;E-pub (Nov 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, Boffetta P, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer 2013;132:645–657. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez CS, Graubard BI, Thistle JE, Petrick JL, McGlynn KA. Attributable Fractions of NAFLD for Mortality in the United States: Results From NHANES III With 27 Years of Follow-up. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol 2015;13:2062–2070. [DOI] [PubMed] [Google Scholar]
- 58.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–285. [DOI] [PubMed] [Google Scholar]
- 59.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188–2195. [DOI] [PubMed] [Google Scholar]
- 60.Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018;155:1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanwal F, Kramer J, Li L, Dai J, Natarajan Y, Yu X, Asch SM, et al. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Non-alcoholic Fatty Liver Disease. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 62.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 63.Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl 1987;7:1–440. [PubMed] [Google Scholar]
- 64.Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer 2012;48:2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Udomkun P, Wiredu AN, Nagle M, Muller J, Vanlauwe B, Bandyopadhyay R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application - A review. Food Control 2017;76:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen JG, Egner PA, Ng D, Jacobson LP, Munoz A, Zhu YR, Qian GS, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013;6:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Z, Chen T, Thorgeirsson SS, Zhan Q, Chen J, Park JH, Lu P, et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 2013;34:1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol 2014;179:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity and Liver Cancer. In: Continuous Update Project Report: Diet, Nutrition, Physical Activity and Liver Cancer; 2015. [Google Scholar]
- 70.Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open 2017;7:e013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torres DM, Harrison SA. Is it time to write a prescription for coffee? Coffee and liver disease. Gastroenterology 2013;144:670–672. [DOI] [PubMed] [Google Scholar]
- 72.Tran KT, Coleman HG, McCain RS, Cardwell CR. Serum Biomarkers of Iron Status and Risk of Primary Liver Cancer: A Systematic Review and Meta-Analysis. Nutr Cancer 2019;71:1365–1373. [DOI] [PubMed] [Google Scholar]
- 73.Gordeuk VR, McLaren CE, MacPhail AP, Deichsel G, Bothwell TH. Associations of iron overload in Africa with hepatocellular carcinoma and tuberculosis: Strachan’s 1929 thesis revisited. Blood 1996;87:3470–3476. [PubMed] [Google Scholar]
- 74.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 2014;3:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jin F, Xiong WJ, Jing JC, Feng Z, Qu LS, Shen XZ. Evaluation of the association studies of single nucleotide polymorphisms and hepatocellular carcinoma: a systematic review. J Cancer Res Clin Oncol 2011;137:1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568–574. [DOI] [PubMed] [Google Scholar]
- 77.Clifford RJ, Zhang J, Meerzaman DM, Lyu MS, Hu Y, Cultraro CM, Finney RP, et al. Genetic variations at loci involved in the immune response are risk factors for hepatocellular carcinoma. Hepatology 2010;52:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang DK, Sun J, Cao G, Liu Y, Lin D, Gao YZ, Ren WH, et al. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat Genet 2013;45:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet 2011;43:455–458. [DOI] [PubMed] [Google Scholar]
- 80.Lee MH, Huang YH, Chen HY, Khor SS, Chang YH, Lin YJ, Jen CL, et al. Human leukocyte antigen variants and risk of hepatocellular carcinoma modified by hepatitis C virus genotypes: A genome-wide association study. Hepatology 2018;67:651–661. [DOI] [PubMed] [Google Scholar]
- 81.Li S, Qian J, Yang Y, Zhao W, Dai J, Bei JX, Foo JN, et al. GWAS identifies novel susceptibility loci on 6p21.32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genet 2012;8:e1002791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin YY, Yu MW, Lin SM, Lee SD, Chen CL, Chen DS, Chen PJ. Genome-wide association analysis identifies a GLUL haplotype for familial hepatitis B virus-related hepatocellular carcinoma. Cancer 2017;123:3966–3976. [DOI] [PubMed] [Google Scholar]
- 83.Miki D, Ochi H, Hayes CN, Abe H, Yoshima T, Aikata H, Ikeda K, et al. Variation in the DEPDC5 locus is associated with progression to hepatocellular carcinoma in chronic hepatitis C virus carriers. Nat Genet 2011;43:797–800. [DOI] [PubMed] [Google Scholar]
- 84.Qu LS, Jin F, Guo YM, Liu TT, Xue RY, Huang XW, Xu M, et al. Nine susceptibility loci for hepatitis B virus-related hepatocellular carcinoma identified by a pilot two-stage genome-wide association study. Oncol Lett 2016;11:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H, Zhai Y, Hu Z, Wu C, Qian J, Jia W, Ma F, et al. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet 2010;42:755–758. [DOI] [PubMed] [Google Scholar]
- 86.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai G, Liu P, Li X, Zhou X, He S. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease (NAFLD) susceptibility and severity: A meta-analysis. Medicine (Baltimore) 2019;98:e14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Z, Guo X, Zhang G, Liang L, Nong B. Correlation between PNPLA3 rs738409 polymorphism and hepatocellular carcinoma: a meta-analysis of 10,330 subjects. Int J Biol Markers 2019;34:117–122. [DOI] [PubMed] [Google Scholar]
- 89.Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer 2018;142:2471–2477. [DOI] [PubMed] [Google Scholar]


