Summary of Problem
There exists no coherent national strategy for the early detection or prevention of gastric cancer in the United States (US), even among identified high-risk groups such as Asian Americans, African Americans, Hispanic Americans, and Alaska Native/American Indian peoples. As a result, patients with gastric cancer in the US are diagnosed at later stages and demonstrate worse overall survival compared to nations of East Asia with established screening programs (Table 1). The under-recognition of gastric cancer risk within minority communities is a significant unaddressed healthcare disparity.
Summit Framework
To address this disparity, the Division of Gastroenterology and Hepatology and the Center for Asian Health Research and Education at Stanford University hosted the inaugural Gastric Cancer Summit on March 5–6, 2020. This Summit brought together academic physicians, researchers, policy leaders, and patient advocates to identify strategies to combat gastric cancer in high-risk US populations through prevention and early detection. The first day of the Summit consisted of health policy- and advocacy-oriented presentations, and the second day consisted of scientific presentations on advances in early detection and prevention.
Day 1: Healthcare Policy and Advocacy
Gastric Cancer in the US
Dr. Joo Ha Hwang began the Summit by offering an overview of gastric cancer in the US, with a focus on the burden faced by Asian Americans. Dr. Hwang discussed that Asian Americans have a higher incidence (rate 15 cases per 100,000) of gastric cancer compared to non-Hispanic Whites (6 per 100,000). Certain Asian subgroups such as Koreans Americans (40 per 100,000) and Japanese Americans (30 per 100,000) face a particularly high risk.1 Dr. Dennis Deapen presented gastric cancer incidence data from Los Angeles County over the period 1976 to 2012, and showed that while Asian Americans have demonstrated secular decreases in incidence over time, certain groups such as Korean Americans remain at heightened risk. Dr. Eunjung Lee expanded on this using the California Cancer Registry, and showed that persistent disparities in gastric cancer incidence between Korean Americans and other groups warrant additional preventative strategies.2 Dr. Yanghee Woo discussed the advanced stage of diagnosis of most gastric cancers in the US, reasons for delays in diagnosis, and the curative role of surgery if early diagnosis can be achieved. Dr. Woo also reviewed the role of robotic gastrectomy.3 Using a decision analytic Markov model, Dr. Shailja Shah demonstrated that endoscopic noncardia gastric cancer screening for high-risk races and ethnicities could be cost effective in the US, with a cost of $71,451 / quality-adjusted life year for Asian Americans.4 Dr. Shah further showed that when disaggregated by subgroups, this strategy remained cost-effective for all Asian American populations. During the discussion panel, Dr. Woo and Dr. Shah discussed the need for additional risk factors (e.g. smoking status) as inputs for modeling studies.
Screening and Prevention in East Asia
South Korea, a nation of high Helicobacter pylori (Hp) prevalence and high gastric cancer incidence (34 per 100,000), initiated a biennial screening program for adults ≥40 years old in 2002. Since the initiation of the national screening program, the proportion of gastric cancers diagnosed at an early stage (defined as tumor with invasion limited to mucosa or submucosa) has increased from 39% in 2001 to 73% in 2016 (Figure 1). Dr. Il Ju Choi demonstrated in a nested case-control study that receipt of endoscopic screening was associated with a roughly 50% reduction in risk for gastric cancer death among 40–74 year olds (odds ratio 0.51, confidence interval [CI] 0.49–0.54).5 Dr. Choi also showed in a randomized controlled trial that testing and treating for Hp reduces gastric cancer incidence in first-degree relatives of gastric cancer patients, with a hazard ratio of 0.45 (CI 0.21–0.94) in the test-and-treat group.6 Dr. Hwoon-Yong Jung reported that Hp seropositivity in the South Korean population has fallen across all age groups, decreasing from 75% in 1992 to 51% in 2015. Dr. Jung further discussed that improvements in survival in South Korea are attributed in large part to earlier diagnosis, allowing for less-morbid endoscopic therapies such as endoscopic submucosal dissection.7 Japan (incidence rate 28 per 100,000) initiated a national radiography-based screening program for adults ≥40 years old in 1983, with endoscopy reserved for abnormal radiographic exams. In practice, participation in this program was low and many residents have sought direct outpatient endoscopy in private clinics. The national screening program was amended in 2016 to recommend either endoscopic or radiographic screening for adults ≥50 years old. Dr. Chisato Hamashima reviewed the evidence used by the Japanese Guideline Development Group to develop these guidelines,8 which included case-control studies (pooled odds ratio 0.77, CI 0.69–0.86) and a community cohort study (hazard ratio 0.58, CI 0.36–0.94) suggesting gastric cancer mortality benefits from having received endoscopic screening. Dr. Hamashima also reviewed testing characteristics including sensitivity and specificity of endoscopic screening,9 and discussed quality measures adopted in Japan such as the Double Check System of reviewing endoscopic images by a local committee. In the panel discussion, speakers discussed the lack of “gold standard” randomized controlled trial data for assessing the mortality benefit of screening, and the difficulties in conducting such a study.
Figure 1:
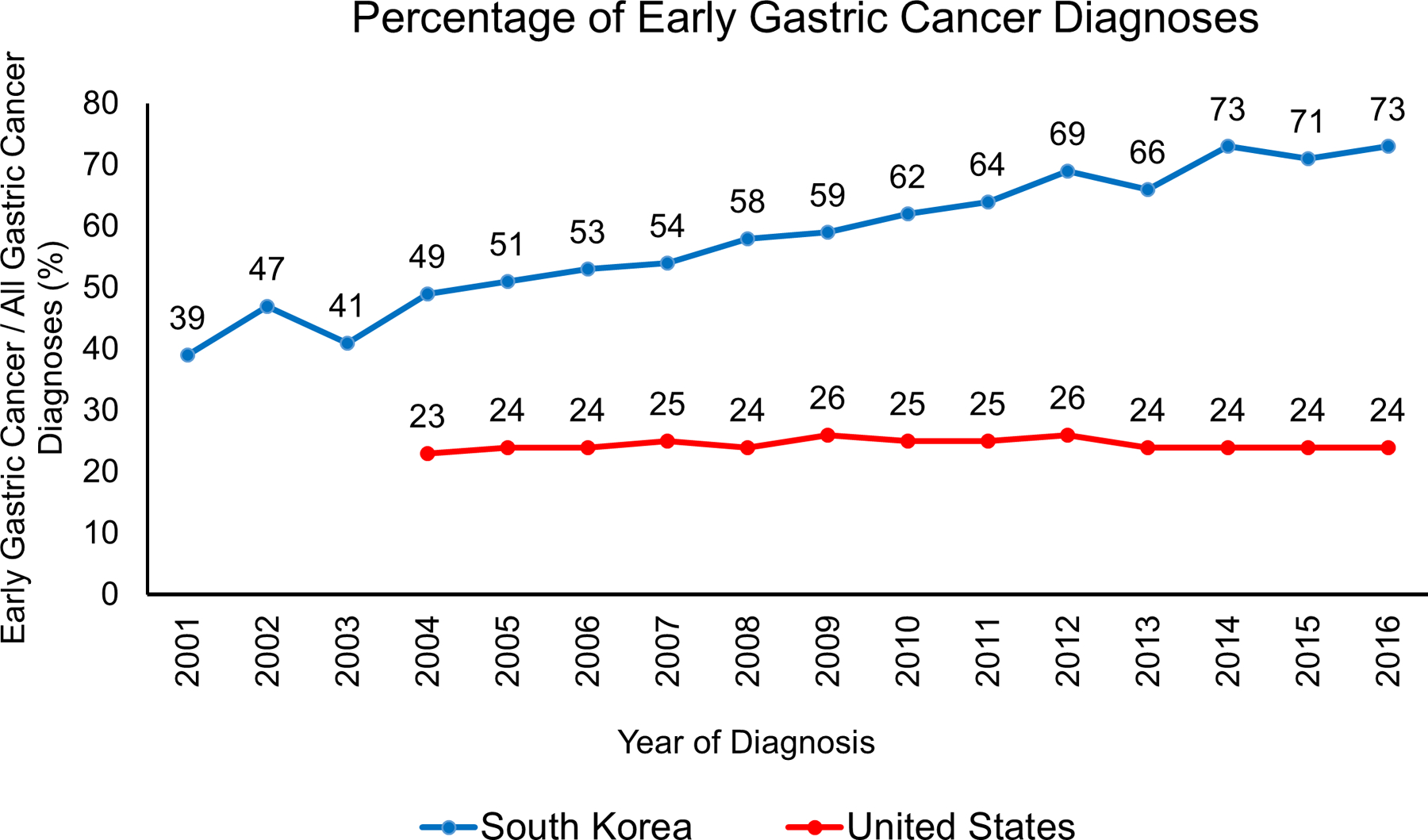
Comparison of the percentage of gastric cancers diagnosed at early stage (defined as tumor with invasion of no deeper than submucosa) between South Korea (South Korean National Cancer Center) and the United States (Surveillance, Epidemiology, and End Results Program). South Korea initiated a nationwide gastric cancer screening program in 2002. United States tumor staging data not available before 2004.
Advancing Health Policy
In the keynote address, Dr. Howard Koh discussed a framework to address healthcare disparities in the US. Dr. Koh reviewed his own experiences in government, first as the Commissioner of Public Health for Massachusetts and later as the US Assistant Secretary for Health under President Barack Obama. Dr. Koh emphasized that there is a striking degree of heterogeneity between Asian subgroups which must be accounted for in research studies and policy decisions. Dr. Koh also discussed his experience of promoting federal initiatives to increase preventative healthcare and screening for Asian Americans, such as for hepatitis B. Drawing example from modeling studies performed for esophageal adenocarcinoma, Dr. Chin Hur emphasized how combining multiple known gastric cancer risk factors (such as race, ethnicity, Hp infection status, diet, sex, and foreign born status) into a comprehensive multivariable risk model could allow for identification of high-risk sub-populations who could benefit from risk-attenuation programs.10 Dr. Hur further noted the need to develop better molecular and genetic markers for risk stratification. Pooling data from several US regional cohorts, Dr. Meira Epplein demonstrated that Hp prevalence has not decreased over time among African Americans. Dr. Epplein further reviewed data from eradication trials showing a 35–50% reduction in gastric cancer incidence with Hp eradication,11 highlighting the need to reduce disparities in Hp testing and treatment. Dr. Epplein also introduced an exciting community-based program to determine the prevalence of Hp and other gastric cancer risk factors in partnership with community leaders in Durham, NC (the Durham Initiative for Stomach Health). Dr. Asad Umar discussed ongoing National Cancer Institute (NCI) efforts at gastric cancer prevention. Dr. Umar highlighted primary prevention studies currently funded by the NCI, including international trials of the polyamine synthesis inhibitor difluoromethylornithine in patients with gastric precancerous lesions, curcumin as chemoprevention of carcinogenesis, and Epstein-Barr virus (EBV) vaccine development.
Advancing Advocacy
Dr. Samuel So described his experience in advocating for hepatitis B screening in high risk populations. Previously, there was no comprehensive national strategy to screen for hepatitis B in the US. Because of the efforts of Dr. So and others, a recommendation for hepatitis B screening in high-risk populations was adopted by the US Preventative Services Task Force (USPSTF) in 2014.12 Dr. John Inadomi reviewed the process of turning evidence into recommendations utilizing the Grading of Recommendations, Assessment, Development and Evaluations framework. This framework is used by the American Gastroenterological Association, of which Dr. Inadomi is the incoming president-elect. Dr. David Greenwald discussed the critical role of professional societies in addressing healthcare disparities through diverse avenues including symposia, white papers, and committees. Dr. Greenwald further discussed the national advocacy work of the American College of Gastroenterology, of which he is president-elect. Ms. Aki Smith delivered a deeply motivating and personal message about the need to improve awareness of gastric cancer among high-risk groups. In response to the gastric cancer diagnoses of both her father and close friend, Ms. Smith founded Hope for Stomach Cancer, a non-profit organization which promotes early detection of gastric cancer and provides critical resources to patients and families.
Day 2: Advances in Early Detection and Prevention
The Science of Interception
Dr. Andrew Chan began this session with a presentation describing the international team and proposal selected for the 2020 Stand Up To Cancer Gastric Cancer Interception Grant. Dr. Chan’s proposal will focus on improving early detection of both intestinal and diffuse gastric cancers using biomarkers such as circulating cell-free DNA. Dr. Khay Guan Yeoh focused his talk on molecular risk stratification of gastric precancerous lesions such as intestinal metaplasia (IM). Leveraging a large cohort of high-risk Singaporean patients with IM, Dr. Yeoh and colleagues identified several molecular markers for progression onto dysplasia or cancer, including telomere shortening and somatic copy number alterations.13 Dr. Alejandro Corvalan discussed ongoing gastric cancer endoscopic screening campaigns in high-risk areas of Chile (incidence rate 18 per 100,000). This campaign has resulted in over 3,700 patients being screened between 2016 and 2019, and included bio-specimen collection for use in future research studies.
Microbes and Cancer
Dr. Richard Peek demonstrated that Hp strains harvested from gerbils grown under iron-limited conditions exhibited enhanced virulence and inflammatory factors in a CagA-dependent manner.14 Moreover, iron depletion accelerated the development of Hp-induced premalignant and malignant lesions in gerbils. Translating these findings to humans from a high-incidence area of Colombia, Dr. Peek and colleagues discovered that serum ferritin concentrations decreased with increasing severity of gastric premalignant lesions.14 Dr. Manuel Amieva emphasized the importance of tissue location of Hp with regards to its virulence and ability to cause human pathology. Using quantitative confocal microscopy, Dr. Amieva’s group demonstrated that Hp grows as distinct micro-colonies deep in stomach glands, where they accelerate Lgr5(+) stem cell proliferation, and up-regulate expression of stem cell-related genes.15 Dr. Keith Wilson discussed the critical role of spermine oxidase in mediating CagA-induced oxidative DNA damage16 and pre-malignant changes.17 Dr. Wilson then overviewed an ongoing randomized chemoprevention trial of difluoromethylornithine (an inhibitor of ornithine decarboxylase upstream of spermine oxidase) in high-risk Honduran and Puerto Rican populations. Dr. Charles Rabkin discussed the epidemiology of EBV-associated gastric cancers including clinical features, risk factors, and immune profiling.18 Dr. Rabkin also reviewed the distinct hyper-methylation profile of EBV-associated gastric cancers derived from the Cancer Genome Atlas.
Gastric Cancer 2050, Shifting Burdens and Demographics
Using an Hp transmission model for Mexico which includes treatment resistance,19 Dr. Fernando Alarid-Escudero demonstrated a population-wide screen-and-treat strategy with concomitant antibiotic susceptibility testing was the most cost-effective strategy for gastric cancer prevention. Dr. Constanza Camargo discussed changes in the epidemiology of gastric cancer. Using cancer registration data covering ~80% of the US population, Dr. Camargo and colleagues reported an unexpected increase in incidence of noncardia gastric cancer among young (age <50 years) non-Hispanic whites.20 These data highlight the need for studies addressing novel factors related to major societal transitions that may provide clues to understanding re-emergence of gastric cancer. Dr. Christian Abnet demonstrated that Hp seropositivity is inversely associated with cardia cancers in Western populations, yet positively associated with cardia cancers in Asian populations.21 These dichotomous findings emphasize the need for personalized approaches to risk stratification across populations. Dr. Michael Bruce discussed steps he and colleagues at the Alaska Native Tribal Health Consortium have recently implemented to address the high burden of gastric cancer among Alaska Native/American Indian persons, including endoscopic screening and Hp testing for persons with a first-degree family history, working towards standardization of endoscopic biopsy protocols statewide, and plans to develop a clinical registry for high-risk individuals.22 The Alaskan approach provides a model by which to improve early detection and prevention in a targeted, high-risk population.
Advancing Early Detection
Dr. Elena Martinez Stoffel discussed the management of patients with pathogenic or likely pathogenic CDH1 variants found on multi-gene panel testing. Dr. Stoffel and colleagues discovered that a high percentage of patients with no family history of gastric cancer but incidentally diagnosed pathogenic or likely pathogenic CDH1 variants harbored signet ring cell carcinomas.23 Dr. Jeremy Davis further demonstrated that 94% of patients who underwent prophylactic total gastrectomy for pathogenic variants of CDH1 demonstrated signet ring cell carcinomas in gastrectomy explants. These data suggest that pathogenic variants of CDH1 represent a highly-penetrant genetic defect. Dr. Hanlee Ji discussed his work characterizing the gastric cancer tumor microenvironment with single-cell genomic sequencing.24 Dr. Ji and colleagues revealed widespread immune reprogramming of the tumor microenvironment, data which allow for identification of novel targets for immunotherapy. Dr. Blanca Piazuelo provided an expert review of interpreting gastric biopsies using Operative Link scoring systems for IM and atrophy. Dr. Piazuelo further discussed the critical importance of distinguishing incomplete phenotypes of IM, as incomplete IM confers a much higher risk for subsequent gastric cancer.25
Summit Conclusions and Gaps in Knowledge
The 2020 Stanford Gastric Cancer Summit highlighted the high burden of gastric cancer among certain racial and ethnic groups, the evidence of a mortality benefit of prevention and screening strategies in East Asia, and the projections that gastric cancer will remain a disease of high burden into the 21st century. Based on these data, a broad consensus emerged among Summit participants that strategies of primary prevention (Hp testing and treatment) and secondary prevention (endoscopic screening) should be considered for adoption in the US for targeted, high-risk populations. The Summit participants also acknowledged remaining knowledge gaps, including:
A need for randomized trial data to show the mortality benefit of endoscopic screening in high-risk populations
An improved understanding of Hp prevalence and resistance patterns across ethnic and racial groups in the US
Clinical and biological markers to risk stratify patients with precancerous lesions (such as IM) for cancer progression across diverse US populations
A need for clinical trials to evaluate potential chemoprevention agents in high-risk individuals
Encouraged Actions and Future Directions
While acknowledging the aforementioned gaps in knowledge, the Summit participants felt there existed enough data to justify proposing adoption of preventative strategies (Hp testing and endoscopic screening) in high-risk groups (based on family history or race/ethnicity) as a national recommendation. The Summit participants are resolved to write a White Paper summarizing the Summit’s major findings, which will be submitted to the USPSTF and other professional societies for consideration. The Summit participants also felt a strong obligation to improve national awareness among both scientific and lay audiences through community outreach, partnership with non-profit organizations and professional societies, and social media.
Table 1:
Comparison of Gastric Cancer Stage of Diagnosis and Survival
| Country | South Korea | Japan | United States | |||
|---|---|---|---|---|---|---|
| Years | 2006–2010 | 2006–2008 | 2010–2014 | |||
| Screening | Biennial Radiography or Endoscopy | Biennial Radiography or Endoscopy | No screening program | |||
| Stage at diagnosis | Distribution (%) | 5-year Survival (%) | Distribution (%) | 5-year Survival (%) | Distribution (%) | 5-year Survival (%) |
| Localized | 92.4 | 95.9 | 70.3 | |||
| Regional | 55.7 | 50.0 | 32.0 | |||
| Distant | 5.5 | 5.7 | 5.8 | |||
| Unknown | 49.2 | - | 21.8 | |||
| All Stages | 67.0 | 64.6 | 32.1 | |||
South Korean data adapted from the Korea National Cancer Incidence Database. Japanese data derived from the Center from the National Cancer Center of Japan. United States data derived from Surveillance, Epidemiology, and End Results Program (SEER) of the National Cancer Institute. 5-year relative survival rates are presented. Summary stages defined by SEER criteria.
Funding:
ME is supported by R01 CA190428. LP is supported by K24 HL150476. MBP is supported by P01 CA028842. SCS is supported by IK2 CX002027. KTW is supported by P01 CA028842, R01 CA190612, and I01 CX002171.
Abbreviations:
- US
United States
- Hp
Helicobacter pylori
- HR
CI, 95% confidence interval
- EBV
Epstein-Barr virus
- NCI
National Cancer Institute
- USPSTF
United States Preventative Services Task Force
- IM
intestinal metaplasia
Footnotes
Disclosures: No financial, professional, or personal conflicts of interest exist for any author. The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
REFERENCE
- 1.Huang RJ, Ende AR, Singla A, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc 2020;91:70–77 e1. [DOI] [PubMed] [Google Scholar]
- 2.Lee E, Liu L, Zhang J, et al. Stomach Cancer Disparity among Korean Americans by Tumor Characteristics: Comparison with Non-Hispanic Whites, Japanese Americans, South Koreans, and Japanese. Cancer Epidemiol Biomarkers Prev 2017;26:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 2011;146:1086–92. [DOI] [PubMed] [Google Scholar]
- 4.Saumoy M, Schneider Y, Shen N, et al. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018;155:648–660. [DOI] [PubMed] [Google Scholar]
- 5.Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017;152:1319–1328 e7. [DOI] [PubMed] [Google Scholar]
- 6.Choi IJ, Kim CG, Lee JY, et al. Family History of Gastric Cancer and Helicobacter pylori Treatment. N Engl J Med 2020;382:427–436. [DOI] [PubMed] [Google Scholar]
- 7.Ahn JY, Nam SH, Jung HY, et al. Endoscopic surveillance can increase the chance of resectability and endoscopic treatment in gastric cancer. Hepatogastroenterology 2014;61:1465–71. [PubMed] [Google Scholar]
- 8.Hamashima C Update version of the Japanese Guidelines for Gastric Cancer Screening. Japanese Journal of Clinical Oncology 2018;48:673–683. [DOI] [PubMed] [Google Scholar]
- 9.Hamashima C, Okamoto M, Shabana M, et al. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer 2013;133:653–9. [DOI] [PubMed] [Google Scholar]
- 10.Laszkowska M, Oh A, Hur C. Screening for Upper Gastrointestinal Malignancies in the United States-Which Immigrant Groups Should Be Considered High-Risk? Gastroenterology 2020;158:4–8. [DOI] [PubMed] [Google Scholar]
- 11.Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019;366:l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeFevre ML, Force USPST. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:58–66. [DOI] [PubMed] [Google Scholar]
- 13.Huang KK, Ramnarayanan K, Zhu F, et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 2018;33:137–150 e5. [DOI] [PubMed] [Google Scholar]
- 14.Noto JM, Gaddy JA, Lee JY, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest 2013;123:479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigal M, Rothenberg ME, Logan CY, et al. Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology 2015;148:1392–404 e21. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 2011;141:1696–708 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi R, de Sablet T, Asim M, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015;34:3429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camargo MC, Sivins A, Isajevs S, et al. Associations of Epstein-Barr Virus-Positive Gastric Adenocarcinoma with Circulating Mediators of Inflammation and Immune Response. Cancers (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alarid-Escudero F, Enns EA, MacLehose RF, et al. Force of infection of Helicobacter pylori in Mexico: evidence from a national survey using a hierarchical Bayesian model. Epidemiol Infect 2018;146:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamangar F, Qiao YL, Blaser MJ, et al. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer 2007;96:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolen LD, Vindigni SM, Parsonnet J, et al. Combating Gastric Cancer in Alaska Native People: An Expert and Community Symposium. Gastroenterology 2020;158:1197–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs MF, Dust H, Koeppe E, et al. Outcomes of Endoscopic Surveillance in Individuals With Genetic Predisposition to Hereditary Diffuse Gastric Cancer. Gastroenterology 2019;157:87–96. [DOI] [PubMed] [Google Scholar]
- 24.Sathe A, Grimes SM, Lau BT, et al. Single cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin Cancer Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah SC, Gawron AJ, Mustafa RA, et al. Histologic Subtyping of Gastric Intestinal Metaplasia: Overview and Considerations for Clinical Practice. Gastroenterology 2020;158:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]


