Abstract
Pancreatitis is a fibro-inflammatory disorder of the pancreas that can occur acutely or chronically as a result of the activation of digestive enzymes that damage pancreatic cells, which promotes inflammation. Chronic pancreatitis with persistent fibro-inflammation of the pancreas progresses to pancreatic cancer, which is the fourth leading cause of cancer deaths across the globe. Pancreatic cancer involves cross-talk of inflammatory, proliferative, migratory, and fibrotic mechanisms. In this review, we discuss the role of cytokines in the inflammatory cell storm in pancreatitis and pancreatic cancer and their role in the activation of SDF1α/CXCR4, SOCS3, inflammasome, and NF-κB signaling. The aberrant immune reactions contribute to pathological damage of acinar and ductal cells, and the activation of pancreatic stellate cells to a myofibroblast-like phenotype. We summarize several aspects involved in the promotion of pancreatic cancer by inflammation and include a number of regulatory molecules that inhibit that process.
Keywords: Pancreatitis, pancreatic cancer, cytokines, chemokines, immune cell infiltration, inflammation signaling
1. INTRODUCTION
The pancreas produces a variety of digestive enzymes, including trypsin and chymotrypsin which digest proteins, amylase which digests carbohydrates, and lipase, which breaks down fats. Islet cells supply the endocrine component of pancreatic function by releasing insulin and glucagon to maintain sugar balance in the body.
Pancreatic cancer (PC) is the third leading cause of cancer deaths and poses a severe health burden globally. The median survival rate of PC is six months to a maximum of five years in less than 5% of patients. The histological futures of pancreatic cancer involve a progression from dysplasia to invasive carcinoma. The major genetic changes in carcinogenesis of the pancreas include the activation of KRAS and inactivation of TP53, CDKN2A, SMAD4. Less frequently, mutations in ARID1A, GL13, MLL3, DNAH11, SYNE1, HMCN1, LRP1B, MUC16, FLG, OBSCN, FAT3, DNAH14, ZNF559, WDFY4, ASTN1, RNF43, TGFBR2, ZNF568, MLL2, PXDN, PREX2, PCDH15, COL6A5 occur in pancreatic cancer [1]. Although prevention may be an effective strategy, early diagnosis is essential for successful therapy. Further research is required to design treatments to reduce the mortality rate of this devastating disease.
Cytokines are diverse group of molecules produced by nucleated cells that play essential roles in regulating cell growth, inflammation, and metastasis [2, 3]. Uncontrolled inflammation of the pancreas, chronic pancreatitis, is one of the risk factors for the development of various malignancies. Activation and recruitment of immune cells produce a cytokine- and chemokine-enriched environment, which promotes cancer development and impairs immune detection of the tumor. Chronic pancreatitis leads to changes in endocrine and exocrine functions of the pancreas and may lead to obesity, diabetes mellitus, calcification of the pancreatic parenchyma, dilatation, distortion, and stricturing of the pancreatic ducts [4–8]. The immune system has enormous potential to destroy tumors; however, immune dysregulation leads to the expansion of the tumor, metastasis, and poor survival of individuals [9].
In the present review, we discuss the role of cytokine-mediated immune cell infiltration and SDF-1α/CXCL12-CXCR4, SOCS, NLRP3, and NF-κB inflammation signaling that contributes to pancreatic acinar, stellate and ductal cell pathology in pancreatitis and progression to pancreatic cancer. Further, we discuss the role of cytokine inhibitors/inducers and chemokines, immune cells, and inflammation signaling inhibitors in combating pancreatitis and pancreatic cancer.
2. PANCREATITIS
Activation of digestive enzymes in the pancreas before release into the small intestine causes injures to pancreatic cells, leading to inflammation with abdominal pain. Acute or chronic pancreatitis may be related to autoimmunity or hyperlipidemia. A low-fat diet and avoiding alcohol consumption and smoking are key to controlling the progression of acute to chronic pancreatitis [10].
2.1. Acute Pancreatitis (AP)
Acute pancreatitis is the leading cause of hospitalization for gastrointestinal disorders accompanied by epigastric pain. The diagnosis of the disease involves the detection of serum amylase or lipase ≥3 times the upper limit of normal levels. Severe acute pancreatitis is accompanied by organ failure with peri-pancreatic fluid collection, pancreatic and peri-pancreatic necrosis, pseudocyst formation, and walled-off necrosis [11]. Acute pancreatitis can be diagnosed by computed tomography or magnetic resonance cholangiopancreatography identification of retained common bile duct stones. Endoscopic retrograde cholangiopancreatography for patients with suspected gallstone pancreatitis and magnetic resonance imaging helps in distinguishing walled-off necrosis from a pseudocyst. Endoscopic ultrasonography is a highly sensitive test for detecting cholelithiasis and chole-docholithiasis in acute pancreatitis [12]. Patients with acute pancreatitis can recover completely; however, risk factors like smoking, alcohol consumption, and pancreatic necrosis in those patients can lead to the development of recurrent or chronic pancreatitis after the first episode [13].
2.2. Chronic Pancreatitis (CP)
Chronic pancreatitis is a progressive fibro-inflammatory disease frequently related to excessive consumption of alcohol, cigarette smoking, exposure to industrial chemicals, or analgesics. Genetic mutations in a trypsin-controlling gene or the cystic fibrosis transmembrane conductance regulator account for hereditary forms of the disease [14]. Pancreatitis begins as pancreastasis or the prevention of apical exocytosis in pancreatic acinar cells. Consequently, newly synthesized and stored digestive enzymes are released via the basolateral membrane into lymphatics by way of the interstitium into the bloodstream, which causes inflammation [15–17].
2.3. Autoimmune Pancreatitis (AIP)
AIP is chronic inflammation due to the self-reactivity of the pancreas by the immune system, which leads to calcification and obstruction characteristic of chronic pancreatitis. Medication for AIP involves immune suppression by steroidal therapy. Type 1 AIP, also called lymphoplasmacytic sclerosing pancreatitis, is characterized by abundant infiltration with immunoglobulin G4 (IgG4)-positive plasma cells, whereas Type II AIP is characterized by granulocytic epithelial lesions in the pancreas without systemic involvement and is duct-centric [18]. The symptoms of AIP include dark urine, pale or floating stools, jaundice, pain in the upper abdomen, nausea, vomiting, weakness, loss of appetite, and weight loss. Pancreatic complications in AIP include pancreatic insufficiency/inability to make pancreatic enzymes, diabetes, and pancreatic calcifications.
2.4. Hyperlipidemia-Hypertriglyceridemia Pancreatitis (HTGP-AP)
Severe hypertriglyceridemia (HTG) is a common cause of acute pancreatitis. HTGP-AP occurs in approximately 15–20% of subjects referred to lipid clinics. Pathophysiology of HTGP-AP includes hydrolysis of triglycerides by pancreatic lipase and excessive formation of free fatty acids with inflammatory changes that promote capillary injury. Therapeutic measures in HTG-AP include dietary modifications, use of antihyperlipidemic agents, insulin, and heparin treatment [19]. Women with abnormal lipid metabolism are also at risk of developing hyperlipidemic gestational pancreatitis [20].
2.5. Obesity-Induced Pancreatitis (OIP)
Obesity, a risk factor for acute pancreatitis, aggravates the disease severity by damaging the intestinal mucosal barrier and changing the microbiota composition [21]. Adipose tissue produces adipokines, including adiponectin, leptin, visfatin, and resistin. In addition, adipose tissue-related MCP-1, TNF-α, and IL-6 enhance inflammation to worsen the severity of acute pancreatitis in diabetes patients [5]. Another comorbidity of chronic pancreatitis associated with obesity is an increased lifetime risk of developing pancreatic cancer. Upregulation of cytokines, chemokines, and other inflammatory mediators contributes to disease severity in pancreatitis and pancreatic cancer in obesity through activation of transcription factors such as NF-κB, AP-1, NFAT, STAT3 with immune suppression and a decrease in NK, i-NKT cells and immune surveillance function of CD8+ T cells [22].
2.6. Diabetes-Induced Pancreatitis (DIP)
There is a correlation between diabetes and pancreatitis and vice versa. Chronic pancreatitis is observed in type 1 diabetes patients with pancreatic ductal hyperplasia/dysplasia with a reduction in pancreas weight [23]. Animal studies showed that diabetes aggravates pancreatitis and suppresses regeneration of the pancreas [24]. Type 2 diabetes mellitus increased the risk of developing pancreatitis [6, 25]. Girman et al. [25] demonstrated that T2DM is a high-risk factor for acute pancreatitis compared with patients without diabetes. Chronic pancreatitis patients also develop Type 2 diabetes [26]. Diabetes mellitus secondary to chronic pancreatitis is accompanied by pancreatic exocrine dysfunction with deficient insulin secretion and classified as type 3c diabetes. In patients with chronic calcified or alcoholic pancreatitis, the incidence of retinopathy and neuropathy is high [27].
3. CHRONIC PANCREATITIS AND THE DEVELOPMENT OF PANCREATIC CANCER
Chronic pancreatitis is linked with an increased risk of pancreatic cancer. The incidence of pancreatic cancer is higher in chronic pancreatitis patients at an older age, and the prevalence increases with smoking and alcohol consumption. Diabetes, obesity, and an age >60 years also contribute to pancreatic cancer risk [28]. Metaplasia of pancreatic acinar cells is observed in chronic pancreatitis progression to pancreatic ductal adenocarcinoma. Oxido-nitrosative stress and fibro-inflammatory signals contribute to the development of pancreatitis and cooperate with oncogenic KRAS mutations and loss of tumor suppressor barriers p16/INK4A/CDKN2A, TP53 and SMAD4/DPC4 and subsequent progression to pancreatic intraepithelial neoplasias. The pathological progression increases from PanIN-1A, PanIN-1B, and PanIN 2/3 lesions and, ultimately, to invasive ductal adenocarcinoma [29].
4. CYTOKINES AND THEIR ROLE IN CHRONIC PANCREATITIS AND PANCREATIC CANCER
Cytokines are released in the systemic circulation in response to various stimuli to defend against attacks of antigens and pathogens in the biological system. The pro-inflammatory response is opposed by an anti-inflammatory response, and an imbalance between these two systems leads to localized tissue destruction and organ damage [30]. In pancreatitis, the excessive release of cytokines stimulates various inflammatory signals and cytokine release, which in turn induces accumulation of inflammatory cells and depletes T cell response. These events cause acinar cell injury accompanied by fibrosis with the activation of quiescent pancreatic stellate cells to activated myofibroblast-like phenotype and pancreatic damage [31] (Fig. 1). Likewise, in pancreatic cancer, the response to inflammatory cytokines leads to acinar, ductal, and stellate cell proliferation with epithelial to mesenchymal transition and progressive tumorigenesis. Treatment for pancreatic cancer requires a portion of the organ to be removed if detected early or complete removal of the pancreas if detected in later stages, and life expectancy reduces to less than five years in most cases [32]. Hence, understanding the role of cytokines in pancreatitis and its progression to pancreatic cancer will delineate new avenues for the discovery of anti-inflammatory and cytokine and chemokine inhibitory therapies in these disease states to reduce disease progression and to increase quality of life and survival. Cytokine inhibitors/inducers for pancreatitis and pancreatic cancer are listed in Table 1. Further, we describe some of the cytokines that play a crucial role in pancreatitis and pancreatic cancer.
Fig. (1).
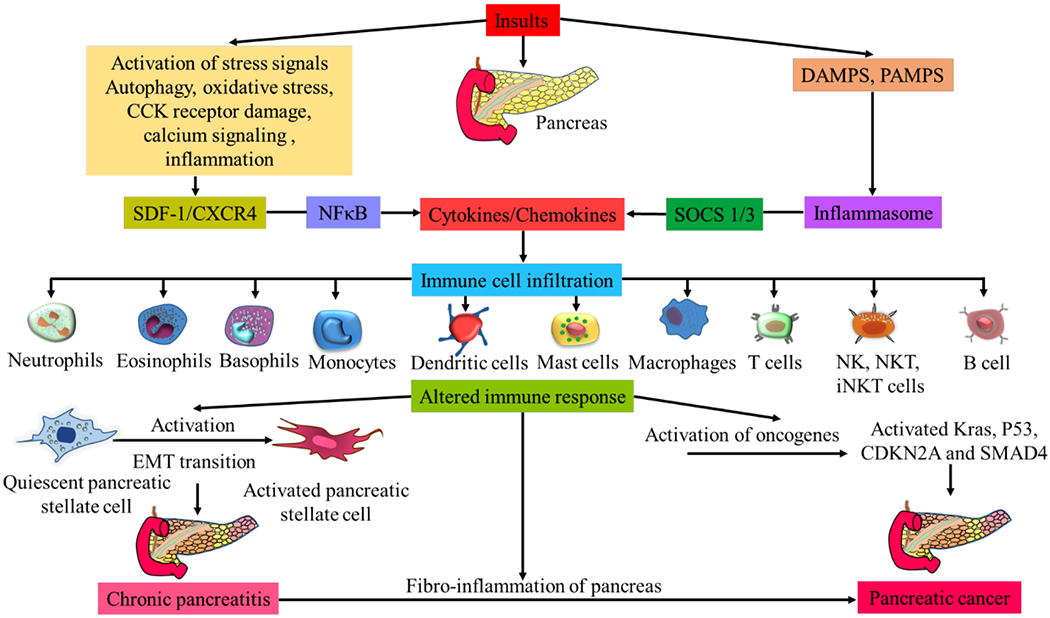
Activation of cytokines and chemokines, induction of inflammatory signaling mechanisms, immune cell infiltration, and fibro-inflammation in pancreatitis and progression to pancreatic cancer. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Cytokine inhibitors/ inducers for pancreatitis and pancreatic cancer.
| Cytokine Inhibition/Induction for Pancreatitis Therapy | ||||||
|---|---|---|---|---|---|---|
| S.NO | Cytokine | Cytokine Inhibitor/Inducer | Study Organism | Experimental Study | Biological Effects | References |
| 1. | IL-1β | Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethylketone (ICE) | Male Wistar rats | Pancreatitis by retrograde infusion of 0.1 ml/100 g body weight; 5% sodium taurocholate solution into the biliopancreatic duct | Myeloperoxidase inhibition, neutrophils, lymphocytes, and monocytes were attenuated | [33] |
| 2. | IL-4Rα | IL-4Rα signaling blockade peptide (a potent cyclic peptide ~1.5 kD) (50 μg per mouse, 100 μl daily for 5 days per week x 2 weeks) | C57BL/6 mice | Chronic pancreatitis by caerulein (50-μg kg−1 bodyweight) administration | The inhibitor (1μM) decreased mouse IL-4/IL-13-induced M2 polarization and CD206 expression, pancreas size, and α-SMA expression, pancreas fibrosis, blockade of alternative activation of pancreatic macrophages, and inhibited hPSC-mediated M2 polarization of human macrophages | [34] |
| 3. | IL-8 | Anti-IL 8 antibody (WS-4) | Rabbits | Pancreatitis by retrograde injection of 5% chenodeoxycholic acid into the pancreatic duct and duct ligation | Reduced the acute lung injury, via inhibition of circulating IL-8 and TNF-α, and CD11b/CD18 in lung tissue | [35] |
| 4. | IL-10 | Recombinant IL-10 | Mice | Acute pancreatitis by cerulein (50 μg/kg body weight) administration | reduction of TNF α and acinar cell necrosis | [36] |
| intraperitoneal IL-10 | Sprague-Dawley rats | Acute pancreatitis induced by intravenous cerulein (8.5 μg x kg(−1) administration | Reduction in serum amylase, TNF-α levels, and pancreatic edema | [37] | ||
| 5. | IL-11 | Recombinant human Interleukin-11 | Male BALB/c mice | cerulein (50 μg/kg) induced Acute necrotizing pancreatitis | Decreased amylase and lipase levels, serum and intrapancreatic TNF-α, pancreatic injury including edema, inflammatory cell infiltration, and hemorrhage | [38] |
| 6. | IL-15 | Recombinant IL-15 (5μg /mice) | Balb/c mice | Chronic pancreatitis by cerulein (50 μg/kg body weight) administration | Negotiated IFN-γ-responsive iNKT cells in the blood and tissue and protects cerulein-induced pancreatic fibro-inflammation in mice | [39] |
| 7. | IL-22 | Recombinant IL-22 or adenovirus IL-22 | C57BL/6 mice and IL-22 TG mice | Cerulein-induced acute and chronic pancreatitis | IL-22 treatment ameliorates cerulein-induced pancreatitis by inhibiting autophagy | [40] |
| 8. | TNF-α | CNI-1493 10mg/kg | NIH Swiss mice | Pancreatitis by cerulein (50 μg/kg body weight) administration | Inhibited serum amylase, lipase, and pancreatic necrosis | [41] |
| Cytokine Inhibitor/Inducer for Pancreatic Cancer Therapy | ||||||
| S.NO | Cytokine | Cytokine Inhibitor/Inducer | Study Organism | Experimental Study | Biological Effects | References |
| 1. | IL-2 | L19-Interleukin-2 (L19-IL2), consisting of the human singlechain Fv antibodyL19, highly specific for the extra-domain B (ED-B) of fibronectin, and the human cytokine IL-2 | Female NMRI nude mice and human pancreatic carcinoma tissues | Orthotropic mouse models for pancreatic cancer | Inhibited tumor growth, metastasis, long-term tumor control with the induction tumor necrosis and inhibition of tumor cell proliferation, increase of macrophages and NK cells in the tumor tissue | [42] |
| 2. | IL-6 | LLL12, a nonpeptide, cell-permeable small molecule | Pancreatic cancer cell lines | PANC-1 and ASPC-1 pancreatic cancer cell lines | Blocked exogenous IL-6-induced STAT3 phosphorylation and nuclear translocation in both PANC-1 and ASPC-1 pancreatic cancer cell lines | [43] |
| Anti-IL-6-receptor antibody tocilizumab; fusion protein sgp130Fc | An orthotopic model of human Colo357 cells in SCID/bg mice | Blocks IL-6 classical and trans-signaling | Reduced primary tumor weight metastases and tumor reoccurrence | [44] | ||
| 3. | IL-12 | IL-12 cDNA | Female BALB/c nu/nu and BALB/c scid/scid mice | Retrovirally transduced with IL-12 cDNA into nude mice | In IL-12-mediated antitumor effect, granulocytes are candidate cells and impaired tumorigenicity of human pancreatic cancer cells | [45] |
| 4. | IL-13 | Recombinant immunotoxin, a fusion of IL-13 and Pseudomonas exotoxin (IL-13-PE) and gemcitabine | Pancreatic cell lines and mouse model human pancreatic ductal cancer | Targeting IL-13Rα2 in pancreatic cell lines and an orthotopic mouse model of human PDA | Cytotoxicity to two pancreatic cancer cell lines, complete eradication of tumors and enhanced survival rate of mice | [46] |
| 5. | IL-15 | recombinant human IL-15 (10 ng/ml) | Pancreatic cancer cells (PCC) and pancreatic stellate cells (PSC) | NK cell-mediated killing of PCC and PSC cell lines | Pancreatic cancer cells and pancreatic stellate cells killing via upregulation of TIM-3 and NKG2D | [47] |
| 6. | IL-17 | Anti-IL-17RB antibody | NOD/SCIDγ mice, HPAF-II, BxPC3, Capan2, CFPAC-1, HPAC, SU.86.86, and MIA PaCa-2 human pancreatic cancer cells | Targeting IL-17B–IL-17RB signaling | Blocked tumor metastasis and promote survival in a mouse xenograft model | [48] |
| 7. | IL-21 | Cetuximab treatment in combination with mouse IL-21 adjuvant therapy | Human pancreatic cells and mouse tumor model | NK cells from normal donors or with pancreatic cancer, human pancreatic cancer cells, mouse subcutaneous and intraperitoneal model of pancreatic cancer | NK cell activation and inhibition of tumor growth | [49] |
| 8. | IL-23 | The murine IL-23 cDNA transfected Dendritic cell vaccine | Mouse | β-elemene combined with interleukin-23 gene-modified dendritic cells on murine pancreatic carcinoma | IL-23 increased the antigen-presenting ability of DCs specific Th1-type and CTL response against pancreatic carcinoma cells induced auto-immunity against pancreatic carcinoma | [50] |
| 9. | IL-24 | Adenovirus-mediated human IL-24 gene therapy | Human pancreatic carcinoma cell line, patu8988, nude mice bearing patu8988 tumors | Adenovirus (AdV)-mediated IL-24 gene therapy on human pancreatic carcinoma | Inhibited pancreatic carcinoma growth, tumor suppression by downregulating the vascular endothelial growth factor, CD34, and Bcl-2, and inhibiting tumor angiogenesis. | [51] |
| 10. | IL-27 | IL-27 (20 ng/ml) | Human pancreatic cell lines PANC-1, MiaPaCa-2, U937 cells, and M2 macrophages | IL-27 in the regulation of phenotypes and functions of tumor-associated macrophages | IL-27 decreased M2-polarized tumor-associated macrophages (TAM) and increased M1-polarized TAMs. IL-27 inhibits proliferation, migration, and invasion of pancreatic cancer cells | [52] |
| 11. | IFN-γ | Recombinant human IFN-γ | Human pancreatic carcinoma cells AsPc-1, Capan-1, and Capan-2, Dan-G cells | IFN-γ effects on growth and survival in human pancreatic cancer cells. | Inhibited pancreatic cancer cells growth, showed apoptosis by DNA fragmentation and PARP cleavage, and upregulation of procaspase-1 accompanied by proteolytic activation. | [53] |
4.1. IL-4
IL-4 is a Th2 cytokine that regulates cell proliferation and apoptosis and plays a role in inflammation [54, 55]. IL-4 levels are upregulated in cerulein-induced pancreatitis in mice, and IL-4 stimulates macrophages and activates pancreatic stellate cells. Blocking IL-4/IL-13 in the cerulein model using a peptide antagonist inhibits pancreatic damage and disease progression [34]. Pancreatic cancer cells and tissues express high levels of IL-4 and IL-4 receptors, and IL-4 acts as a growth factor in pancreatic cancer cells, facilitating pancreatic tumor growth and metastasis. Neutralizing IL-4 antibodies inhibits the growth of pancreatic cell lines [56]. IL-4 receptor-targeted cytotoxin is a potent target for pancreatic cancer therapy. Intra-tumoral injections of IL-4-Pseudomonas exotoxin exhibit antitumor activity against human pancreatic tumors implanted subcutaneously in immunodeficient animals [57].
4.2. IL-5
IL-5 is a proinflammatory cytokine that plays a critical role in eosinophil initiation, development, migration, and recruitment to the tissues in allergy and inflammation [58]. GATA-1 and IL-5 deficiency inhibits the induction of eosinophil active chemokines and profibrotic cytokines, and protects mice from an experimental model of pancreatitis induced fibro-inflammatory pathology of the pancreas as described in Fig. (2) [59]. The role of IL-5 has been demonstrated in bladder cancer cells where it enhances the migration and invasion via activation of ERK1/2, MMP-9, NF-κB, AP-1, and p21WAF1 [60]. Eosinophils play an active role in tumor immune surveillance and kill methylcholanthrene-induced fibrosarcoma in IL-5 transgenic mice [61].
Fig. (2).
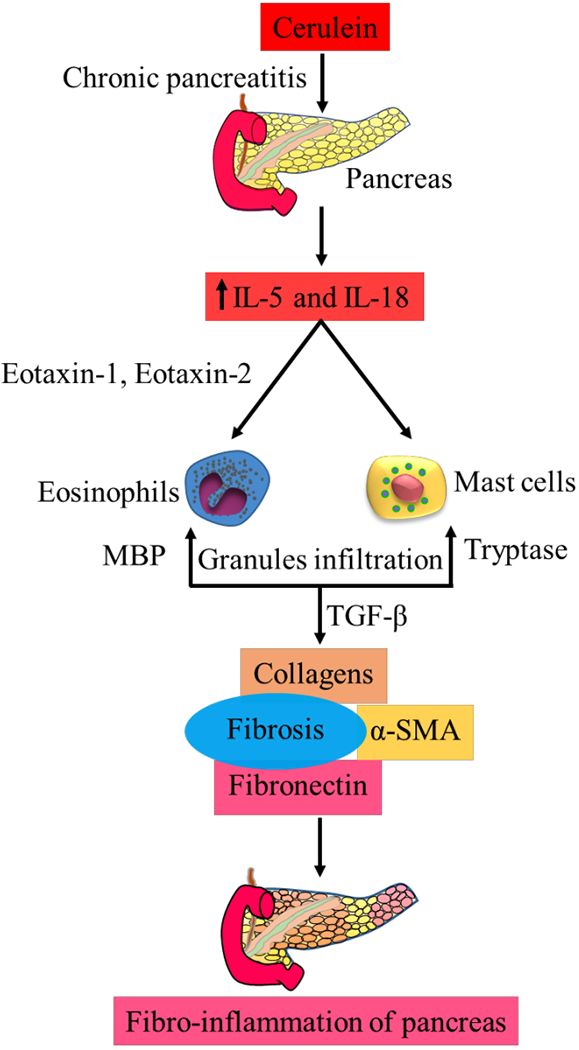
Role of eosinophils and mast cells in the initiation and progression of pancreatitis pathogenesis via induction of IL-5, IL-18 and fibrosis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.3. IL-6
IL-6 signaling plays a pivotal role in chronic inflammation, autoimmunity, and inflammation-associated cancer. IL-6 signaling controls the differentiation and activation of T lymphocytes via induction of the Jak/STAT-3 and Ras/Erk/C/EBP pathways and regulates the balance between Treg cells and Th17 cells [62]. Pancreatitis is associated with elevated IL-6 levels, which promotes acinar cell damage in mice. In humans, IL-6 levels are a prognostic indicator in acute pancreatitis [59]. Acute lung injury (ALI) is associated with severe acute pancreatitis. IL-6 KO mice had a lower death rate compared with wild-type mice with acute pancreatitis; however, mice challenged with IL-6 developed lethal ALI via phosphorylation of STAT3 and production of neutrophil attractant CXCL1. Therapeutic inhibition of IL-6 may prevent severe acute pancreatitis associated with acute lung injury [62]. IL-6 plays a role in tumor progression in pancreatic cancer, and its expression is localized to the stroma of tumors. IL-6 and PD-L1 blockade showed antitumor activity in mice bearing orthotopic KPC-luc tumors and inhibited tumor progression increased overall survival in KPC-Brca2 mice [63].
4.4. IL-13
IL-13 is produced by Th2 cells and plays a critical role in allergic diseases [64, 65]. Activation of macrophages is dependent on IL-4 and IL-13 signaling, and mice lacking IL4Ra and IL-4/IL-13 are less susceptible to cerulein-induced pancreatic fibrosis [34]. IL-13 is produced by PanIN and Tuft cells in the development of pancreatic cancer, and promotes macrophage polarization and contributes to PanIN cell proliferation and fibrosis. IL-13 neutralizing antibody decreases activated macrophages in ADM/PanIN lesions and reduces fibrosis and pancreatic lesion growth [66].
4.5. IL-15
IL-15 shares structural similarity with IL-2, and it activates NK cell proliferation, cytotoxicity, and cytokine production and regulates NK cell/macrophage interaction. IL-15 plays an anti-inflammatory role against asthma and eosinophil-mediated allergic diseases [65, 67]. IL-15 treatment produces an increase of interferon-γ-responsive invariant natural killer T (iNKT) cells in the blood. In the tissue, it protects against cerulein-induced pancreatic fibro-inflammation in mice, as illustrated in Fig. (3). [59]. IL-15 promotes NK cell-mediated cytotoxicity as a treatment for pancreatic cancer and stellate cells [47].
Fig. (3).
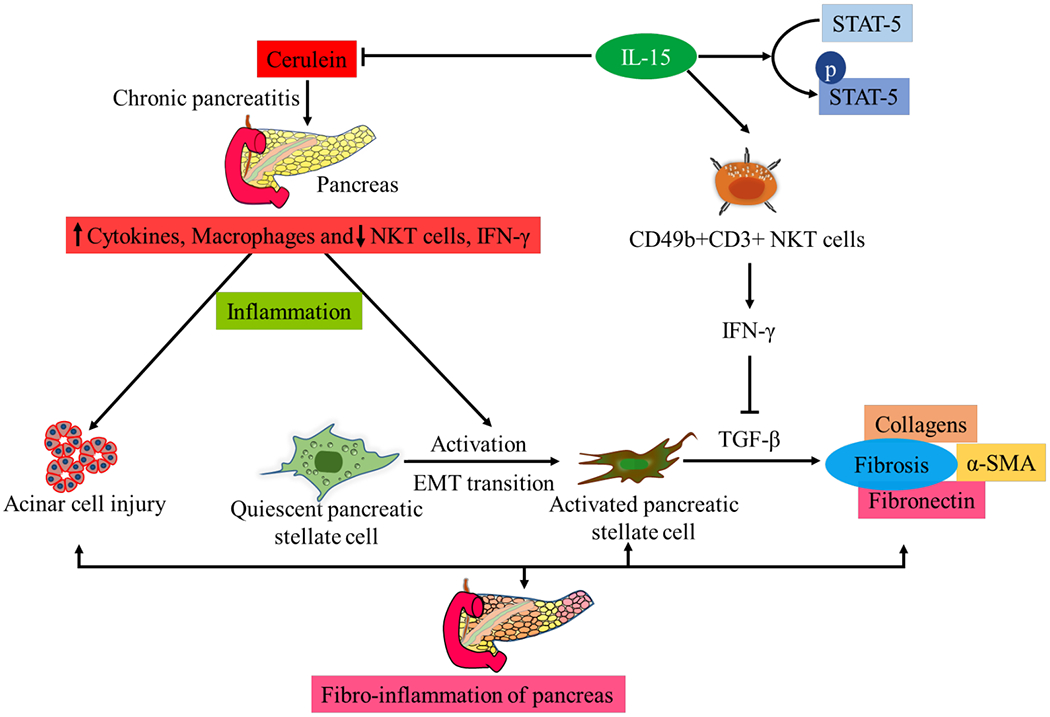
IL-15 treatment mitigates cerulein induced pancreatitis via induction of i-NKT cells, IFN-γ and inhibits pancreatic inflammation and fibrosis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.6. IL-17
IL-17 is a pro-inflammatory Th17 cytokine that plays a role in host defense, promoting inflammatory pathology and inducing eosinophil-mediated allergic diseases [68]. Stimulator of interferon genes (STING) activation worsens acute pancreatitis; however, it is protective in chronic pancreatitis and limits fibrosis. STING deficiency leads to IL-17 polarization, which is possibly inhibited by STING activation. IL-17A neutralization inhibits STING deficiency-mediated chronic pancreatitis [69]. Immune cell-derived IL-17 regulates the development of tuft cells and stem cell features of pancreatic cancer cells via increased expression of DCLK1, POU2F3, ALDH1A1, and IL-17RC and promotes pancreatic tumor growth and progression in mice and humans [70].
4.7. IL-18
IL-18, also called IFN-γ-inducing factor, is a proinflammatory cytokine that is converted to an active form by IL-1 p converting enzyme caspase-1. IL-18 plays a central role in inflammation and contributes to the pathogenesis and pathophysiology of inflammation and eosinophil-mediated allergic diseases [67, 71–73]. An increase in IL-18 levels was observed in chronic pancreatitis in mouse and human samples and served as a prognostic marker [39, 74]. IL-18 is increased in the blood and tissues of most cancer patients, including pancreatic cancer, and is associated with disease progression, metastatic recurrence risk, and reduced survival [75].
4.8. IFN-γ
IFN-γ plays a crucial role in host defense in innate and acquired immunity and exhibits both pro- and anti-tumorogenic roles [76]. Cerulein-induced pancreatitis is exacerbated in IFN-γ−/− mice with increased neutrophil recruitment. IFN-γ administration induced anti-inflammatory effects and attenuated cerulein-induced acute pancreatitis in both WT and IFN-γ−/− mice, with a reduction in NF-κB activation and COX-2 expression [77]. NKT cells are the source of IFN-γ, and recently we showed that cerulein induces a decrease in IFN-γ in a mouse chronic pancreatitis model. However, IL-15 administration induces IFN-γ and protects pancreatic pathology via NKT cell recruitment [59]. Interferon-γ released in the tumor microenvironment inhibits human pancreatic carcinoma cell growth via caspase-1 dependent induction of apoptosis [53].
4.9. TNF-α
TNF α is a proinflammatory cytokine that contributes to oxidative stress at sites of inflammation, participates in vasodilatation and edema formation, and plays a role in malignancy and inhibition of pancreatic cancer [78, 79]. TNFα directly induces premature protease activation and necrosis in pancreatic acinar cells via calcium and cathepsin-B activity. Genetic deletion of TNFα and neutralizing antibodies against TNFα prevented neutrophil- and macrophage-induced trypsin activity and necrosis in pancreatic acini treated with phorbol-12-myristate-13-acetate, cerulein, or TNFα, and prevented cerulein-induced experimental pancreatitis in mice [80]. Anti TNF-α reduces desmoplasia and the inflammatory microenvironment in pancreatic ductal adenocarcinoma, and anti-TNFα combined with chemotherapy killed tumor cells [81].
5. CHEMOKINES AND THEIR ROLE IN CHRONIC PANCREATITIS AND PANCREATIC CANCER
Chemokines are secondary pro-inflammatory mediators induced by cytokines that stimulate the recruitment of leukocytes. The major chemokine sub-families based upon the position of cysteine residues are CXC and CC chemokines [82]. Chemokines and their receptors are critical mediators of cell migration during immune surveillance. Chemokines promote the tumorigenesis, proliferation, metastasis, and angiogenesis of a variety of cancers [83, 84]. In an earlier study, Yubero et al. [85] reported that oxidant-mediated MAPK, NF-κB, and STAT3 activation triggers chemokine expression in pancreatic acinar cells in pancreatitis in rats [85]. Chemokine receptor antagonists are a favorable therapeutic approach for the treatment of inflammatory diseases and cancer. Chemokine inhibitors for pancreatitis and pancreatic cancer are listed in Table 2.
Table 2.
Chemokine inhibitors for pancreatitis and pancreatic cancer.
| Chemokine Inhibition for Pancreatitis Therapy | ||||||
|---|---|---|---|---|---|---|
| S.NO | Chemokine | Chemokine Inhibitor | Study Organism | Experimental Study | Biological Effects | References |
| 1. | CXCR2 | Antileukinate (52.63 mg/kg, s.c.) | Swiss mice | Caerulein (50 μg/kg/h) induced acute pancreatitis mediated pancreatic and lung injury | Reduced plasma amylase, pancreatic water content, pancreatic myeloperoxidase activity, pancreatic MIP-2 levels pancreas, and lung myeloperoxidase activity | [86] |
| 2. | CCL2/MCP-1 | Pepducin | BALB/c mice | Acute and chronic pancreatitis induced by intraperitoneal administration of (0.2 mg/kg body weight) caerulein | The decrease in pancreatitis induced neutrophils, macrophages, and acinar cell damage | [87] |
| Bindarit; inhibitor of MCP-1, | A rat model of severe acute pancreatitis (SAP) | SAP model was induced by retrograde infusion of (4%) sodium taurocholate into the biliopancreatic duct | Bindarit ameliorates SAP by inhibiting serum amylase MCP-1, levels, and pancreatic damage | [88] | ||
| Chemokine Inhibitors for Pancreatic Cancer Therapy | ||||||
| S.NO | Chemokine | Chemokine Inhibitor | Study Organism | Experimental Study | Biological Effects | References |
| 1. | CXCR2 | Anti-CXCR2 antibody | BxPC-3 cell line | Secreted CXC protein levels | Inhibition of neovascularization | [89] |
| CCR2 inhibitor PF-04136309 in combination with Folfirinox chemotherapy (oxaliplatin and irinote-can plus leucovorin and fluorouracil) | Human subjects with borderline resectable and locally advanced pancreatic cancer | Single-centre, open-label, dose-finding, non-randomised, phase 1b trial | Objective tumor response, with local tumor control in 32 (97%) patients. | [90] | ||
| AZ13381758 is a potent inhibitor of both murine and human CXCR2 | KPC mice | Immunotherapy by CXCR2 Inhibition in pancreatic ductal adenocarcinoma | Cxcr2 deficiency in KPC mice, Ly6G+ cell depletion, or CXCR2 inhibition suppresses metastasis in PDAC and enhance response to chemotherapeutics and immunotherapy to prolong survival. | [87] | ||
| CXCR2 inhibitor repertaxin and SB225002 | Mice bearing autochthonous PDAC. Ptf1acre/+; LSL-KrasG12D/+; Tgfbr2flox/flox | NF-κB inhibitor SC-514 (an IκB kinase-2 inhibitor) showed significant suppression of the CXCL1 and −5 expression | CXCR2 inhibitors repertaxin inhibited the PDAC–induced CTGF upregulation and tumor growth and exhibited antitumor effects in Ptf1acre/+; LSL-KrasG12D/+; Tgfbr2flox/flox PDAC mice | [91] | ||
| 2. | CCR5 | - | Human and mouse | Human pancreatic adenocarcinoma and a murine pancreatic tumor model (Pan02) | Reduction of T reg cells migration to tumors | [92] |
| 3. | CXCL12 | AMD3100, an inhibitor of chemokine (C-X-C motif) receptor 4, a CXCL12 receptor, | KPCD (LSL-KrasG12D/+; LSL-Tp53R172H/+; Pdx-1-Cre; FAP-DTR) mice, pancreatic cancer cell lines derived from tumors arising in KPC mice (TB32964, K8484), LL2 cell line expressing chicken OVA (LL2/OVA) | Targeting CXCL12 with anti–PD-L1 immunotherapy in pancreatic cancer | Revealed antitumor effects and greatly diminished cancer cells | [93] |
| 4. | CXCR4 | Zerumbone inhibitor of CXCR4 | PANC-1 (pancreatic duct cell carcinoma), PANC-28 (pancreatic carcinoma), MIA PaCa-2 (pancreatic carcinoma), AsPC-1 (pancreatic adenocarcinoma) | Zerumbone, a component of subtropical ginger (Zingiber zerumbet), as a regulator of CXCR4 expression leading to inhibition of CXCL12-induced invasion of pancreatic tumor Cells | Suppression of CXCR4 NF-κB, inhibition of CXCL12-induced invasion of pancreatic cancer cells | [94] |
| 5. | CCL21 | Recombinant murine CCL21 | C57BL/6 | CCL21 mediated anti-tumor cellular immunity | Intratumoral injection of CCL21 into pancreatic tumors reduced the growth of distant tumors and treated tumors, immune cell infiltration of the tumor mass, delayed growth of treated tumors, and generate a tumor-specific cellular immune response | [95] |
| 6. | CXCL2 | Triptonide | Patu8988 and Panc1 cancer cells and in vivo mouse model of matrigel assay | Pancreatic cancer cell-mediated vasculogenesis | Inhibition of CXCL2 via reduction of gene promoter activity and suppresses pancreatic cancer cell-mediated tumor vasculogenic mimicry by reducing tumor cell migration and invasion and inhibiting expression of VE-cadherin and CXCL2 | [96] |
5.1. The CC Family of Chemokines
CC chemokines influence allergic inflammation and progression of cancer [97, 98]. The following CC family chemokines are well reported in pancreatitis and pancreatic cancer and are reviewed in brief.
5.1.1. CCL2
Monocyte chemoattractant protein-1 (MCP-1/CCL2) regulates migration and infiltration of monocytes/macrophages [99]. MCP-1 upregulation is seen in acute and chronic pancreatitis in animal models and human tissues and contributes to the pathogenesis of mononuclear infiltration [100, 101]. Mutant MCP-1 inhibited intrapancreatic cytokine and chemokine expression and suppressed the development of pancreatic fibrosis in chronic pancreatitis induced by dibutyltin dichloride in rats [102]. Induction of pancreatitis by cerulein in mice involves the migration of CD11b high CD11c–Gr-1 low macrophages from the bone marrow mediated by CCL2 via CCR2 and SOCS-3 dependent activation. CCL2−/− mice exhibited less infiltration of CD11b high CD11c–Gr-1 low macrophages with less severe pancreatitis upon cerulein-induced pancreatitis [103]. Monocyte recruitment is critical to pancreatic cancer progression and increased monocyte prevalence in the peripheral blood, and its decrease in bone marrow correlates inversely with survival. Human pancreatic tumors express CCL2, and immunosuppressive CCR2+ macrophages infiltrate these tumors with low CD8 T-cells. CCR2 blockade depletes inflammatory monocytes and macrophages from cancer and exhibits antitumor immunity, decreased tumor growth, and reduced metastasis in mice [104].
5.1.2. CCL5
CCL5, also known as RANTES, is expressed in various immune cells such as macrophages, dendritic cells, and memory T cells. The receptor for CCL5 is CCR5. CCL5 plays an essential role in inflammatory diseases and promotes carcinogenesis and stroma genesis [105]. In chronic pancreatitis, CCR5, CCL5, and MIP-1α mRNA levels were increased 12.9, 13.3, and 9.2-fold, respectively. Most CCR5-positive cells were also CD68-positive macrophages, and the study demonstrated that CCR5 is most likely involved in the attraction and activation of CD68-positive macrophages in chronic pancreatitis [106]. CCR5 and CCL5 interaction increased pancreatic cancer cell invasion through F-actin polymerization. Pancreatic cancer metastases showed elevated epithelial staining for CCR5 and CCL5. Pancreatic cancer cell lines (AsPc-1, BxPc-3, and MIA PaCa-2) showed higher expression levels of CCR5 and invasive potential. Treatment with the CCR5 inhibitor maraviroc appeared beneficial in preventing metastasis and may serve as a therapeutic strategy to control pancreatic cancer progression [107].
5.1.3. CCL18
CCL18, also called macrophage inflammatory protein 4, is expressed in monocytes, macrophages, and immature dendritic cells. The chemokine plays a crucial role in immune and inflammation responses and attracts lymphocytes and immature dendritic cells. CCL18 induces collagen deposition by fibroblasts and plays a role in the progression of malignant tumors [108]. Serum CCL18 levels were higher in patients with pancreatic ductal adenocarcinoma. Cancer epithelial cells and macrophages in pancreatic ductal adenocarcinoma tissues expressed CCL 18 that correlated with lymph node metastasis. Treatment with recombinant human CCL18 promoted the migration and invasion of pancreatic cancer cells and induced EMT by upregulation of SNAIL1 [109].
5.1.4. CCL20
CCL20, a direct target gene of RelA-containing NF-κB dimers, attracts immune cells to the site of the tumor and modulates the resistance of the cancer cells through their interaction with immune cells. The receptor for CCL20, CCR6, is expressed on a variety of immune cells, including macrophages, dendritic cells, and T-cells, as well as on different tumor cells. The TRAIL-RelA-CCL20 signaling pathway in pancreatic ductal adenocarcinoma cells leads to paracrine immune cell modulation and resistance towards TRAIL-induced apoptosis in pancreatic ductal adenocarcinoma cell lines. Dissection of the CCL20-CCR6 cancer-immune cell interaction is required for anti-tumor therapy [110]. CCL20 and CCR6 levels are increased in pancreatic carcinoma and play a role in the development and progression of the tumor. Inhibition of CCR6 signaling or neutralization of CCL20 or inhibition of its production and activity may be useful in preventing further progression of pancreatic carcinoma [111]. Aberrant expression of CCL20 is observed in tumor-associated macrophages of pancreatic cancer tissue. CCL20 secreted by IL-4-challenged M2 macrophages promotes the migration and epithelial-mesenchymal transition [112]. Expression of CCL20 was significantly higher in pancreatic cancer than in chronic pancreatitis and adjacent normal tissue, and may therefore be a new parameter for histological diagnosis and discrimination between pancreatic cancer and chronic pancreatitis [113].
5.1.5. CCL21
CCL21, an efficient chemoattractant for lymphocytes, is found on endothelial venules and within the T cell zones of both spleen and lymph nodes. It is selective in its recruitment of naive T cells and dendritic cells, and it influences integrin-mediated dendritic cell transmigration [114–117]. In the lymph nodes, CCL21 plays a role in the initiation of an immune response by colocalizing naive T cells with dendritic cells presenting antigens [118, 119]. CCR7 is a receptor for CCL21 that is expressed on all naive T cells, memory T cells, B cells, and mature dendritic cells. CCR7 plays a central role in lymphocyte infiltration and homing to lymph nodes [120, 121]. CCL21/CCR7 signaling is involved in regulating inflammation, development and progression of several types of cancer [122]. CCL21 expression is associated with microvessel density, while CCR7 expression is associated with microlymphatic vessel density. CCR7 and its ligand, CCL21, are critical in the progression of pancreatic cancer, and induction of angiogenesis and lymphangiogenesis by chemotactic interaction [123]. CCL21/CCR7 promotes migration and survival of CD133+ pancreatic cancer stem cells via activation of ERK/NF-κB signaling and promoting EMT and lymph node metastasis markers E-cadherin, N-cadherin, and LYVE-1 [124].
5.2. The CXC Family of Chemokines
CXC chemokines regulate tumor-associated angiogenesis, as well as cancer cell metastases [125]. Here we discuss the role of the following CXC chemokines in pancreatitis and pancreatic cancer.
5.2.1. CXCL1
CXCL1, also known as neutrophil-activating protein 3 and melanoma growth stimulating activity α, signals via CXCR2 on neutrophils, which mediates mammary tumor growth and lung metastasis [126, 127]. An experimental model of cerulein-induced pancreatitis and acute lung injury in C57BL/6 mice activated IL-6 and induced phosphorylation of STAT3, which elevated CXCL1 in the serum, BALF and pancreatic acinar cells [62]. RelA activation promotes oncogene-induced senescence via elevation of CXCL1, which activates CXCR2 during pancreatic carcinogenesis. In Kras mice, pancreas-specific inactivation of CXCR2 prevented oncogene-induced senescence, which correlated with increased tumor proliferation and decreased survival. In human tissues, reductions in CXCR2 levels were associated with advanced neoplastic lesions, which demonstrates that the RelA/CXCL1/CXCR2 axis is an essential mechanism of tumor surveillance in pancreatic ductal adenocarcinoma [128]. CXCL1 is highly expressed in mouse and human pancreatic ductal adenocarcinoma. High RIP3 (a critical regulator of programmed necrosis/necroptosis/inflammatory cell death) expression correlated with higher expression of CXCL1, and RIP3 deletion reduced in vivo and in vitro expression of CXCL1. Macrophage inducible Ca2+-dependent lectin receptor (Mincle) deletion also slowed the rate of oncogenesis. Targeting these networks represents a therapeutic approach for pancreatic ductal adenocarcinoma [129].
5.2.2. CXCL4
CXCL4, also called platelet factor 4, drives inflammation-mediated cancer aggravation and angiogenesis and promotes antitumor immunity [130]. CXCL4 secreted from platelets is a stimulator of neutrophil infiltration and subsequent pancreatic tissue damage via CXCL2 activation that also mediates cancer regrowth after chemotherapy [131]. Taurocholate infusion into the pancreatic duct or by intraperitoneal administration of L-arginine induced pancreatitis in C57BL/6 mice with increased plasma levels of CXCL4, whereas depletion of platelets markedly reduced CXCL4 plasma levels, demonstrating that circulating levels of CXCL4 are derived from platelets in acute pancreatitis. CXCL4 inhibition decreased taurocholate-induced neutrophil recruitment, IL-6 secretion, edema formation, amylase release, and tissue damage in the pancreas. CXCL4 reduction also diminished plasma and lung levels of CXCL2 and neutrophil infiltration and tissue damage in the inflamed pancreas [132]. CXCL4 levels are also elevated in mild and severe acute pancreatitis, which directs CXCL4 inhibition as a therapy for the treatment of pancreatitis. A paralog of CXCL4, CXCL4L1, hindered cell proliferation and migration in patient tumors, tumor cell lines, and murine xenografts and increased substantially in primary and metastatic pancreatic ductal adenocarcinoma. Myofibroblasts induce CXCL4L1 in tumor cells. Administration of a monoclonal antibody (mAb) against CXCL4L1 blocked the growth of tumors positive for CXCR3, a receptor for CXCL4, and inhibited pancreatic ductal adenocarcinoma development via the antiangiogenic function [130].
5.2.3. CXCL8
CXCL8, also called monocyte-derived neutrophil chemotactic factor or neutrophil-activating protein 1 or IL-8 in humans, is a pro-inflammatory chemokine. Leukocytes and tumor cells secrete CXCL8, which plays a role in immune surveillance, inflammation, and angiogenesis and modulates endothelial cell proliferation and migration. The cellular response to CXCL8 is affected by CXCR1 and CXCR2, which cross-link with CXCL8 and exert biological function. IL-8 levels increased in human subjects with pancreatitis, and Pooran et al. [133] recommend IL-8 as a marker for the evaluation of pancreatitis. Pancreatic cancer cell-derived CXCL8 and fibroblast-derived CXCL12 promote HUVEC proliferation, migration, and invasion. CXCL12 enhanced CXCL8 production by pancreatic cancer cells, and drugs targeting CXCR4 and CXCR2 block metastasis and angiogenesis in pancreatic cancer [134]. Chen et al. [135] found that IL-8 levels were increased in the serum of patients with pancreatic adenocarcinoma and recommend IL-8 as a serum marker for predicting the prognosis.
5.2.4. CXCL10
CXCL10, also called 10 KDa interferon gamma-induced protein, binds to its receptor CXCR3 to exert biological effects such as chemotaxis, induction of apoptosis, regulation of cell growth, and mediation of angiostatic effects. CXCL10 correlates with inflammation, immune dysfunction, tumor development, and metastasis [136]. In response to inflammation, immune cells like neutrophils, eosinophils, monocytes, and other cells such as epithelial cells, endothelial cells, and stromal cells secrete CXCL10 [137–139]. CXCL10 was elevated in human pancreatic ductal adenocarcinoma specimens and cocultures of pancreatic cancer cells with pancreatic stellate cells and correlated with high stroma content and decreased median survival in patients with pancreatic cancer. CXCL10 and its receptor CXCR3 are associated with the intratumoral presence of T reg cells, and CXCL10 stimulated the ex vivo recruitment of CXCR3+ effector T cells as well as CXCR3+ T reg cells leading to immunosuppressive and tumor-promoting effects [140].
5.2.5. CXCL16
CXCL16, a transmembrane and soluble chemokine, plays a role in inflammation, and its expression promotes tumor growth, proliferation, metastasis, NF-κB regulation, and angiogenesis [141, 142]. Increased levels of CXCL16 are observed in humans with severe pancreatitis and confirmed bacterial infection, and in mice challenged with sodium taurocholate and Escherichia coli-mediated necrotizing pancreatitis [143]. CXCL16 and CXCR6 are induced in chronic pancreatitis and pancreatic ductal adenocarcinoma tissues. Proinflammatory cytokines increase CXCL16 and silencing of ADAM10 inhibits CXCL16 [144]. sst2+/− mice showed PI3K/AKT activation, whereas KRASG12D sst2+/− mice showed premalignant lesions, tumors, and lymph node metastases and activation of PI3K signaling via AKT, NF-κB activation, and CXCL16 production, which prompted neoplastic lesions. Pancreatic ductal adenocarcinoma tissues and surrounding acini from mice and humans expressed higher CXCL16 and its receptor CXCR6 in pancreatic tissues. Neutralizing CXCL16 in KRASG12D mice reduced PI3K/AKT/NF-κB signaling and blocked carcinogenesis. sst2 is progressively lost in mouse lesions that expressed KRASG12D with PI3K activation that progressed to pancreatic ductal adenocarcinoma [145].
6. THE IMMUNE CELLS ACTIVATED IN CHRONIC PANCREATITIS AND PANCREATIC CANCER
Host immune cells defend against microbial and foreign substances in the tumor environment. Reports demonstrate that acute pancreatitis provoked after the consumption of food products like mustard, milk, egg, banana, fish, and kiwi fruits and food allergies are a possible cause for the initiation of pancreatitis associated with cytokine release and activation and release of immune cells [146]. In pancreatic cancer-immune cell infiltration like pan-macrophages, M2, Neu, or the ratio of T reg cells to CD4+T cells associated with shorter survival [147]. Proper activation of immune cells when required, and inhibition of aberrant infiltration of immune cells, are key to countering pancreatitis progression and development to pancreatic cancer. Immune cell inhibitors/inducers for pancreatitis and pancreatic cancer are listed in Table 3.
Table 3.
Immune cell inhibitors/ inducers for pancreatitis and pancreatic cancer.
| Immune cell inhibitors/inducers for pancreatitis therapy | ||||||
|---|---|---|---|---|---|---|
| S.NO | Immune Cells | Immune Cell Inhibitors | Study Organism | Experimental Study | Biological Effects | References |
| 1. | Neutrophils | Neutrophil–depleting anti–Ly6G antibody | BALB/c mice | Acute and chronic pancreatitis induced by intraperitoneal administration of caerulein (0.2 mg/kg body weight) | A decrease in pancreatitis induced neutrophils, macrophages, and acinar cell damage | [87] |
| Anti-mouse lymphocyte function antigen–1 antibody (5mg·g-1; i.p.) | C57BL/6 male mice | Pancreatitis was induced by retrograde infusion of sodium taurocholate into the pancreatic duct in mice | Reduced taurocholate-induced amylase levels, accumulation of neutrophils, production of CXC chemokines and tissue damage in the pancreas, leucocyte adhesion in postcapillary venules of the pancreas, attenuation of pulmonary infiltration of neutrophils | [148] | ||
| 2. | i-NKT cells | Recombinant IL-15 (5ug/mice) | Balb/c mice | Chronic pancreatitis by cerulein administration (50 μg/kg body weight) | Negotiated an increase of IFN-γ-responsive iNKT cells in the blood and tissue and protected cerulein-induced pancreatic fibro-inflammation in mice | [59] |
| Immune Cell Inhibitors for Pancreatic Cancer Therapy | ||||||
| S.NO | Immune Cells | Immune Cell Inhibitors | Study Organism | Experimental Study | Biological Effects | References |
| 1. | Dendritic cells | Dendritic cell vaccine modified with tumor lysate and IL-18 gene | BALB/C | Pancreatic carcinoma induced with Dimethylbenzanthracene | Induced tumor cell death and promoted NK and T cells to secrete IFN-γ and produced a specific and effective immune response against pancreatic carcinoma cells | [50] |
| 2. | Macrophages | Liposomal clodronate | LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre; CD11b-DTR (KPCD) mice | A genetic model of pancreatic cancer | Reduced CD11b-positive macrophages, tumor incidence, and growth slightly reduced, metastasis formation in the liver and lungs were significantly diminished after macrophage depletion, significantly impaired angiogenesis, reduced circulating vascular endothelial growth factor levels and circulating CD4+CD25+ T cells | [149] |
| 3. | T reg cells | Low-dose gemcitabine | Pancreatic carcinoma cells were injected orthotopically in C57Bl/6 mice | Orthotopic Panc02 model of pancreatic cancer | Selectively diminishes Treg prevalence at the tumor site and improves survival in pancreatic cancer | [150] |
6.1. Dendritic Cells
Dendritic cells protect against cell stress and are required for pancreatic viability in mice with acute pancreatitis. Major histocompatibility complex II+CD11c+ DCs are increased in pancreata of mice with acute pancreatitis along with increased IL-6 and TNF-α. With the depletion of dendritic cells, mice died upon challenge with cerulean or L-arginine and exhibited acinar cell death and neutrophil infiltration [151]. Blockade of the MyD88-independent TRIF pathway is protective against pancreatic cancer neoplastic transformation by augmenting the DC–Th2 axis, whereas blockade of the MyD88-dependent pathway exacerbates pancreatic inflammation and malignant progression. The protumorigenic and fibroinflammatory effects of MyD88 inhibition are mediated by dendritic cells (DCs), which induce pancreatic antigen-restricted Th2-deviated CD4+ T cells and promote the transition from pancreatitis to carcinoma [152].
6.2. Macrophages
Macrophages execute a critical role in disease progression in pancreatitis in mice and human tissues. M1 macrophages are predominant in acute pancreatitis. In contrast, macrophages are alternatively activated in chronic pancreatitis, promote proliferation and activation of PSCs and express high levels of TIMP2 and MMP9, which regulate ECM turnover. Alternatively, enabled macrophages are dependent on IL-4 and IL-13 signaling, and mice lacking IL-4Rα, and IL-4/IL-13 were less susceptible to pancreatic fibrosis. Pharmacologic inhibition of IL-4/IL-13 decreases alternatively activated macrophages and fibrosis in the pancreas [34]. Macrophages play an essential role in mediating tumor progression. M2-polarized tumor-associated macrophages induce epithelial-mesenchymal transition in the progression to metastasis. Activation of TLR4 on M2-polarized TAMs stimulates an increase in the cytokine IL-10 and increased EMT of pancreatic cancer cells [153]. PI3Kγ, an essential macrophage lipid kinase, regulates macrophage transcriptional programming, leading to stimulation of CD8+ T-cell–mediated tumor suppression, desmoplasia, tumor cell invasion, and metastasis in pancreatic adenocarcinoma. Genetic or pharmacologic inhibition of PI3Kγ restores antitumor immune responses and improves responsiveness to standard-of-care chemotherapy in animal models of pancreatic ductal adenocarcinoma. [154].
6.3. Mast Cells
Mast cell number and IgE-dependent mast cell activation are higher in chronic pancreatitis than in the healthy pancreas and localized in the fibrotic areas and the residual acinar parenchyma [155]. Perineural mast cells are enriched in pancreatic neuritis, a histopathological hallmark of pancreatic neuropathy in chronic pancreatitis and pancreatic adenocarcinoma [156]. Mast cell degranulation is observed in the pancreas with sodium taurodeoxycholate-induced pancreatitis in rats [157]. Mast cells are critical components of the tumor-stromal microenvironment in several solid and hematological malignancies. Mast cell infiltration increases in pancreatic cancer and plays a role in promoting angiogenesis and tumor growth [158]. In contrast to mast cells in acute pancreatitis, the mast cells in pancreatic ductal adenocarcinoma were found degranulated [159]. Mast cells contribute to the aggressiveness of pancreatic ductal adenocarcinoma, enhancing the expression of several pro-angiogenic factors such as VEGF, FGF-2, PDGF, and angiopoietin-1 as well as stimulating the pancreatic cancer cell proliferation by IL-13 and tryptase activity [160].
6.4. Neutrophils
Neutrophils are critical in mediating pancreatic and lung tissue damage in severe acute pancreatitis in taurocholate-induced pancreatitis in mice. Trypsinogen activation is dependent on neutrophil activation in the pancreas [161]. Neutrophils make use of histone citrullination, an epigenetic post-translational modification of histone arginine to citrulline by peptidyl arginine deiminase-4 (PADI4), upon contact with particulate agents to extrude decondensed chromatin as neutrophil extracellular traps (NETs) and form macroscopically visible aggregates. PADI4 is critical for intraductal aggregate formation, and PADI4-deficiency abrogates disease progression. Components of pancreatic juice, such as bicarbonate ions and calcium carbonate crystals, induce aggregated NET formation. Ductal occlusion by aggregated NETs emerges as a pathomechanism with relevance in a plethora of inflammatory conditions involving secretory ducts with IL-17A/cerulean challenge [162]. Neutrophil gelatinase-associated lipocalin secreted by neutrophils and other cell types is a prognostic marker in pancreatitis and pancreatic cancer [163, 164]. The transition of epithelial to mesenchymal phenotype in pancreatic cancers coincides with the polymorphonuclear infiltrate, a contribution of the inflammatory response in tumor progression [165].
6.5. Basophils
Basophils are proinflammatory granulocytes released in response to allergy and inflammation that infiltrate the tumor microenvironment [166]. Basophils activated by TLR2/TLR4 stimulation in type 1 AIP were significantly higher than those in healthy subjects and showed an essential role in the pathophysiology of type 1 AIP [167]. Mouse models of pancreatic cancer demonstrate the functional role of basophils during tumor progression. Basophils expressing IL-4 are enriched in tumor-draining lymph nodes of patients with pancreatic ductal adenocarcinoma. Basophils rely on the release of CCL7/MCP3 by “alternatively activated” monocytes, whereas basophil activation is induced by T-cell-derived IL-3. Basophils present in TDLNs correlate with the Th2/Th1 cell ratio in tumors [168].
6.6. Monocytes
Monocytes can differentiate into macrophages and dendritic cells and play a role in host defense against microorganisms and dead cells. Monocyte infiltration in pancreatitis, and increased numbers of CD14+CD163- and CD14+CD163-MAC387+ monocytes were detected in mild acute pancreatitis patients [169]. An increase in monocytes in the blood and decrease in the bone marrow correlates with poor survival in pancreatic cancer. The chemokine CCL2 and CCR2+ macrophages infiltration with low CD8T cells are observed in pancreatic tumor patients. CCR2 blockade augments antitumor immunity, decreases tumor growth, and reduces metastasis with depletion of inflammatory monocytes and macrophages from the primary tumor and premetastatic liver in mice [104].
6.7. Eosinophils
Eosinophils are a type of leukocyte that are released in response to allergic stimuli and play a critical role in allergic disease. An increase in eosinophils is observed in mouse and human pancreatitis [39, 71]. Eosinophilic pancreatitis is a rare form of recurrent acute and/or chronic pancreatitis characterized by localized or diffuse periductal, acinar, and septal inflammatory eosinophilic infiltration of the pancreas and elevated serum immunoglobulin E levels [170, 171]. Eosinophil accumulation and degranulation were observed in human and mouse pancreatitis and may have a critical role in promoting pancreatitis pathogenesis and fibrosis [39]. Eosinophilia in pancreatic cancer is rare [172]. In a human study of pancreatic adenocarcinoma, eosinophilia was observed with infiltration of the duodenal wall characterized by multiple eosinophilic extracellular deposits consistent with non-calcified psammoma bodies [172].
6.8. T Cells
The pancreatic ductal adenocarcinoma microenvironment is predominantly infiltrated with immune suppressive cells. The checkpoint inhibitors such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death 1 (PD-1), and its ligand PD-L1 have failed to demonstrate responses given as single agents to PDA patients [173]. The combination of αCD40/chemotherapy plus αPD-1 and αCTLA-4 induced regression of subcutaneous tumors, and improved overall survival by priming T-cell response in pancreatic ductal adenocarcinoma [174]. PD-1 blockade increased effector CD8+ T lymphocytes and tumor-specific interferon-γ production of CD8+ T cells in the tumor microenvironment for pancreatic ductal adenocarcinoma [173].
6.8.1. CD4+T Cells
CD4+CD25high Tregs cells are observed in autoimmune pancreatitis patients and influence IgG4 production, and decreased T reg cells are involved in the pathogenesis of autoimmune pancreatitis [175]. The severity of cerulein-induced acute pancreatitis is also reduced by in vivo CD4+ (but not CD8+) T-cell depletion, and Fas ligand-targeted mutant mice display a decrease in histological lesions, serum hydroxylase and IFN-γ, IL-12, and FasL gene transcription [176].
6.8.2. CD8+T Cells
Tumor-derived GM-CSF is an essential regulator of inflammation and immune suppression within the tumor microenvironment. GM-CSF drives the development of Gr-1+ CD11b+ cells that suppress antigen-specific T cells in the KPC mouse model of spontaneous pancreatic ductal adenocarcinoma in which expression of oncogenic KrasG12D and mutant p53R172H are targeted to the pancreas. Abrogation of tumor-derived GM-CSF inhibited the recruitment of Gr-1+ CD11b+ cells to the tumor microenvironment and blocked tumor development dependent on CD8+ T cells to rescue the tumor growth [177].
6.8.3. NK Cells
Pancreatic tissue of chronic pancreatitis patients shows an increase in activated CD56+ NK cells, which mediate HLA-independent cytotoxicity [178]. NK cells constitutively express receptors for several cytokines, including IL-21, which promotes the maturation of NK cells. IL-21 enhances NK cell-mediated effector functions against cetuximab-coated pancreatic tumor cells irrespective of KRAS mutation status and reduced pancreatic tumor burden in vivo [49].
6.8.4. NKT Cells
The absence of NKT cells leads to aggressive development of pancreatic cancer, with an increase in pancreatic intraepithelial neoplasia lesions and 5-LOX and mPGES-1 expression in M2-type macrophages, and cancer stem-like cells in pancreatic tumors of CD1d−/− mice deficient in both invariant and variant NKT cells with the KrasG12D mice [179].
6.8.5. i-NKT Cells
Reduction in i-NKT cells is observed in human chronic pancreatitis and cerulean-induced chronic pancreatitis in mice, whereas IL-15 treatment mediates an increase in the interferon-γ-responsive invariant natural killer T cells in the blood and tissue and protects against cerulein-induced pancreatic pathology in mice [59].
6.8.6. T Regulatory Cells
Type 1 autoimmune pancreatitis patients show an increase in T reg cells and IgG4-sclerosing cholangitis (IgG4-SC) in the pancreas, and the numbers of infiltrated T reg cells correlate with IgG4-positive plasma cells. The increase in the inducible costimulatory molecule (ICOS)+ and IL-10+ T reg cells influence IgG4 production via IL-10 in Type 1 autoimmune pancreatitis [180]. T reg cell infiltration constitutes an immunosuppressive phenotype on tumor-associated CD11c+ DCs that then fail to activate a cytotoxic CD8+ T cell-mediated delay in tumor growth by suppressing the expression of costimulatory ligands. Targeting this interaction is a therapeutic strategy for the treatment of PDA and dependent on CD8+ T cell activation [181].
6.9. B Cells
Pancreatic neoplasms harboring oncogenic Kras are pointedly compromised in B-cell–deficient mice. B cells elicit a pro-tumorigenic effect via IL-35-mediated tumor cell proliferation released by CD1dhiCD5+ B cells in pancreatic cancer pathogenesis [182]. Hif1α deletion promotes pancreatic ductal adenocarcinoma initiation with increased intrapancreatic accumulation of B cells, B1b” B-cell subtype in KrasG12D-driven pancreatic neoplasia. B-cell depletion by αCD20 monoclonal antibodies suppresses pancreatic tumorigenesis [183]. CD19+CD24highCD27+ regulatory B cells are involved in the development of type 1 autoimmune pancreatitis and increase conquers the severity of disease activity [184]. IgG4-related autoimmune pancreatitis mimicking acute pancreatitis is observed in humans [185].
7. THE ROLE OF THE COMPLEMENT SYSTEM
The complement system is made of plasma proteins that defend the host by opsonizing pathogens and inducing inflammatory responses that help fight infections. Loss of complement component 5 (C5) or injection of a C5a-receptor antagonist reduces the level of fibrosis in cerulein-induced chronic pancreatitis in mice, and C5a induces activation of primary stellate cells delineating its antifibrotic effects in chronic pancreatitis [186]. The expression levels of complement C3, complement C4b1, and apoE were higher in pancreatic cancer. Complement C4b1 and apoE markedly correlated with tumor staging and lymph node metastasis. Complement C3 may be used as a marker for the diagnosis of early-stage pancreatic cancer, while C4b1 and apoE might be used as diagnostic markers of advanced pancreatic cancer [187].
8. INFLAMMATORY MECHANISMS IN CHRONIC PANCREATITIS AND PANCREATIC CANCER
Chronic pancreatitis increases the risk of pancreatic cancer by 10 to 20-fold, and inflammatory mediators play a crucial role in disease progression. Although the exact inflammatory mechanisms are not yet determined, disease initiation and progression is due to a combined effect of several inflammatory mechanisms in response to cytokine and chemokine infiltration in pancreatitis and pancreatic stroma (Fig. 4). Understanding these inflammatory pathways and development of inhibitors that inhibit aberrant activation of inflammatory signals is a key for pancreatitis and pancreatic cancer therapy [188]. Some of the inflammation signaling inhibitors for pancreatitis and pancreatic cancer are listed in Table 4.
Fig. (4).
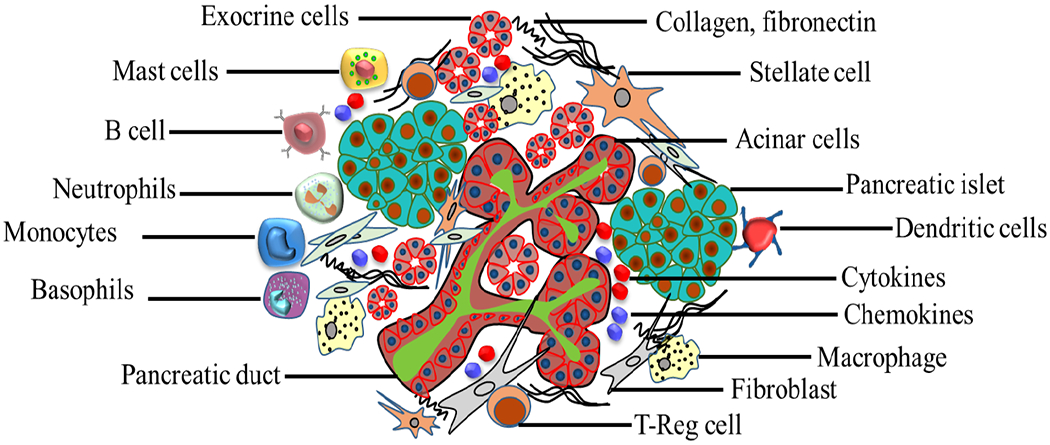
Pancreatic cancer microenvironment. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 4.
Inflammation signaling inhibitors for pancreatitis and pancreatic cancer.
| Inflammation Signaling Inhibitors for Pancreatitis | ||||||
|---|---|---|---|---|---|---|
| S.NO | Immune Cells | Inflammation Signaling Inhibitors | Study Organism | Experimental Study | Biological Effects | References |
| 1. | NLRP3 | INF-39 (50 mg/kg body weight) | C57BL/6 mice | Mouse model of severe acute pancreatitis by cerulean administration for 12 hours (50 μg), followed by one administration of LPS (10 mg/kg) | Reduce serum, amylase, IL-1β, TNFα, IL-6, and severity of severe acute pancreatitis | [189] |
| 2. | NF-κB | NF-κB essential modifier-binding domain peptide | Swiss Webster mice | Cerulein (50 μg/kg) induced acute pancreatitis | Decreased inflammation in the pancreas, hemorrhage in the lungs, and myeloperoxidase activity in pancreas and lungs. | [190] |
| Pyrrolidine derivative of dithiocarbamate (PDTC) | Rats | Taurocholate induced pancreatitis | Attenuated NF-κB activation and improved survival of the rats | [191] | ||
| Withaferin A, an inhibitor of NFκB | C57BL/6 mice | Acute and chronic pancreatitis by cerulein (50 μg/kg) administration | Blocks translocation of the p65 subunit and prevents caspase 3, IL-6, nitric oxide synthase 2, TNF-α; Inhibition of the ER stress pathway, PERK, ATF6, ATF4, ERN1, CHOP, Xbp1, HMGB1, Pycard and inflammasome signaling IL-18, IL-1β, NLRP3, | [192] | ||
| Inflammation Signaling Inhibitors for Pancreatic Cancer | ||||||
| S.NO | Immune Cells | Inflammation Signaling Inhibitors | Study Organism | Experimental Study | Biological Effects | References |
| 1. | SDF-1α/CXCL12-CXCR4 | CXCR4 antagonist, AMD3100 | Human pancreatic cancer cell lines Colo357, SW1990, AsPc1, BxPc3, CaPan1, HPAF II, CFPAC1, Panc1, MiaPaCa, Panc10. 05, Panc03.27, Panc02.03 | Rescue effect of activated CXCL12–CXCR4 signaling | Halted CXCL12-induced pancreatic cancer cell growth and drug resistance | [193] |
| CXCR4 antagonist, TN14003 | Human pancreatic cancer cell lines (CFPAC-1, Capan-2, AsPC-1, PANC-1, BxPC-3, and SUIT-2 | CXCR4 antagonist for SDF1 induced migration and invasion of human pancreatic cancer | Blocked SDF-1-induced migration and invasion of cancer cells via the alteration in phosphorylation of MAPK and reduction of actin polymerization. | [194] | ||
| Chloroquine | Tumor tissues resected from patients with PDAC before any neoadjuvant radiation or chemotherapy were implanted into immunocompromised mice, to establish low passage tissue xenografts, human pancreatic cancer cell lines Panc1, BxPC3, and 8988 T | Targeting pancreatic cancer stem cells via inhibition of CXCR4 | Inhibitory effect of chloroquine by inhibition of CXCL12/CXCR4 signaling, resulting in reduced phosphorylation of ERK and STAT3, reduction in sonic hedgehog-induced chemotaxis and down-regulation of downstream targets in highly tumorigenic and metastatic cancer stem cells | [195] | ||
| 2. | NLRP3 | CRID3 blocks ASC oligomerization in the NLRP3 inflammasome | WT, ASC−/−, and caspase-1−/− mice | Orthotopically implanted with KPC-derived tumor cells in mice | CRID3 offered synergistic efficacy when combined with TLR9 inhibition and reduced PANIN lesions in pancreatic ductal adenocarcinoma. | [196] |
| 3. | NF-κB | Pyrrolidine derivative of dithiocarbamate (PDTC), Tosylphe-chloromethyl ketone (TPCK) | Human pancreatic periacinar myofibroblasts | Assessed IL-8, MCP-1, RANTES, and MIP1α, activation of NF-κB and NF-IL-6 | Reduced the IL-1β– or TNFα– induced chemokine gene expression | [197] |
| Inhibitor of κB kinase (IKK) β | Pancreatic cancer cell lines, male athymic nu/nu mice | NDRG1/Cap43-induced reduction of tumor growth and angiogenesis | Reduced IKKμ expression in cells overexpressing NDRG1/Cap43, resulted in a reduction of both nuclear translocation of p65 and p50 and their binding to the NF-κB motif and reduced tumor growth and angiogenesis of pancreatic cancer | [198] | ||
8.1. Stromal-Derived Factor-1α/CXCL12-CXCR4 Signaling
SDF-1α/CXCR4 plays a role in the proliferation and maturation of human fetal pancreatic endocrine progenitor cells, where its increased expression is associated with inflammation [199]. Mice with experimental acute pancreatitis exhibit enhanced pancreatic SDF-1 α expression. The SDF-1α/CXCR4 axis promotes the migration of transplanted bone marrow mesenchymal stem cells towards the injured pancreas to facilitate a reparative process to combat the progression of acute pancreatitis [200]. CXCR4 upregulated on the surface of tumor cells of epithelial origin and CXCR4-positive tumor cells could migrate toward distant organs in response to an SDF-1α gradient [201]. SDF-1α /CXCR4 are expressed at higher levels in pancreatic cancer cells, and the expression level influences the clinical outcome of pancreatic ductal adenocarcinoma patients [202, 203]. Survival was lower in patients positive for CXCR4 expression than in patients negative for CXCR4 expression. Abrogation of SDF1α/CXCR4 influences the pancreatic cancer cell phenotype, including cell proliferation, colony formation, and cell invasion. Inhibition of Wnt targets genes and the mesenchymal markers vimentin and Slug, and may be a promising therapeutic target to delay pancreatic cancer progression [204].
8.2. SOCS Signaling
The suppressor of cytokine signaling (SOCS) proteins are inhibitors of activation of the JAK-STAT pathway, and studies demonstrated critical roles for SOCS1 and SOCS3 in inflammation and the development and progression of cancers [205]. Abnormal expression of SOCS1 and SOCS3 is associated with dysregulation of signals from cytokine receptors, Toll-like receptors (TLRs), and hormone receptors, resulting in inflammation and malignancies in cancer cells and human carcinomas including pancreatic cancer [206]. Inhibition of SOCs can exert protective effects against severe acute pancreatitis-associated acute lung injury, and this effect could be partially mediated by restraining mitochondrial-associated apoptosis of pulmonary microvascular endothelial cells [207]. SOCS3 suppresses the IL-6-mediated STAT3 activation that mediates the suppression of TLR/NF-κB signaling in macrophages, and the lack of the SOCS3 signaling pathway can accelerate STAT3 activation and amelioration of pancreatitis [103]. Activated IL-6/STAT3 signaling induces SOCS3 methylation via DNA methyltransferase 1 (DNMT1), which leads to pancreatic cancer growth and metastasis, whereas inhibitors of STAT3 or DNMT1 are therapeutics for treating pancreatic cancer [208].
8.3. NLRP3 Inflammasome Signaling
The db/db mice with diabetes are susceptible to acute pancreatitis, and the pancreatic tissues showed NLRP3 inflammasome activation [209]. NLRP3 signaling in macrophages drives the differentiation of CD4+ T cells into tumor-promoting Th2, Th17, and regulatory T cell populations and suppresses Th1 cell polarization and cytotoxic CD8+ T cell activation, while the transfer of PDA-entrained macrophages or T cells from NLRP3−/− mice was protective, and targeting NLRP3 is immunotherapy for PDA [196]. Caspase-1, ASC, and NLRP3 are required for inflammation in acute pancreatitis. TLR9 and P2X7 are important DAMP receptors upstream of inflammasome activation. Genetic deletion of TLR9 and pretreatment with the TLR9 antagonist IRS954 reduced pancreatic edema, inflammation, and pro–IL-1β expression in pancreatitis. IRS954 also decreased pancreatic necrosis and lung inflammation in taurolithocholic acid 3-sulfate-induced acute pancreatitis and is a therapeutic strategy for treating acute pancreatitis [210].
8.4. NF-κB Signaling
NF-κB is activated in the early stages of pancreatitis and regulates genes that control inflammation, survival, proliferation, and migration [211–212]. Huang et al. [213] demonstrated that acute pancreatitis by cerulein challenge to p65 transgenic mice showed higher levels of NF-κB activity in acinar cells and inflammation. Constitutive expression of IKK2 increased the activity of NF-κB in acinar cells and induced pancreatitis, and prolonged action of IKK2 for three months activated stellate cells, loss of acinar cells, and fibrosis with characteristic chronic pancreatitis. Co-expression of IKK2 and p65 also increased the inflammatory mediators and the severity of pancreatitis in mice. Activation of NF-κB is observed in pancreatic cancer, and its inhibition reduces pancreatic tumors. Blocking NF-κB by BAY11- 7082, an NF-κB pathway inhibitor, and recombinant IL-18 improved survival in a murine pancreatic cancer model [214].
9. PANCREATIC CELLS IN CHRONIC PANCREATITIS AND PANCREATIC CANCER
The pancreas, located in the upper left area of the abdomen behind the stomach near the duodenum, has endocrine and exocrine compartments. The exocrine compartment consists of acinar, ductal, and centroacinar cells that produce enzymes essential to digestion. The pancreatic juices and bile that release into the duodenum help digest fats, carbohydrates, and proteins. The endocrine compartment of the pancreas consists of islets of Langerhans cells α, β, δ, e, and pancreatic polypeptide cells that release hormones directly into the bloodstream. The primary pancreatic hormone insulin acts to lower blood sugar, and glucagon works to raise blood sugar, maintaining proper blood sugar level balance in the body for vital functioning of organs [215].
9.1. Pancreatic Acinar Cells
The pancreatic acinar cell is the functional unit for pancreas exocrine function. Premature activation of digestive enzymes in pancreatic acinar cells initiates pancreatitis [216]. In pancreatitis and pancreatic cancer, acinar cells may undergo redifferentiation into ductal cells and blockade of the ductal system [217]. Reducing Ca2+ influx and inhibition of amylase secretion reduced cellular damage in cerulean-induced pancreatitis in mice [218]. Defective lysosomal function, resulting in impaired autophagy, leads to pancreatitis. LAMP-2 deficient mice exhibit lysosomal/autophagic dysfunction, show a decrease in pancreatic digestive enzyme content, and inhibit cholecystokinin-induced amylase secretion by acinar cells and mimic the genetic model of human pancreatitis, explaining the homeostatic role of LAMP2 in pancreatic acinar cell health [219]. Cerulein-induced pancreatitis in mice revealed inflammation-associated death of acinar cells with pancreas-specific RelA/p65 truncation [220]. Kras activation itself is not enough to drive pancreatic carcinogenesis beyond the level of premalignancy. A secondary stimulus, such as inflammation-induced signaling, is required for tumor formation. Inflammatory transcription factor NFATc4 is highly induced and localizes to the nucleus in response to inflammation-induced EGFR signaling, and drives acinar-to-ductal conversion and pancreatic ductal adenocarcinoma initiation through direct transcriptional induction of Sox9 [221].
9.2. Pancreatic Ductal Cells
In pancreatic injury due to KRAS hyperactivity and increased inflammatory signaling with the loss of cell-cell and cell-matrix contacts, loss of polarity can drive acinar cells to transdifferentiate to a duct-like phenotype with acinar-to-ductal metaplasia and initiate further progression to low-grade precancerous lesions [222]. Chronic stimulation and proliferation of the pancreatic duct gland in response to islet inflammation in type 2 diabetes mellitus (T2DM) is linked with increased risk for pancreatitis in T2DM [223]. Pancreatic ductal adenocarcinoma is a type of exocrine pancreatic cancer, and account for 95% of all pancreatic cancers. It is an aggressive malignancy, and surgical removal of the tumor is a possible cure. However, 90% of patients possess a high grade of the disease and are surgically incurable at the time of clinical presentation [224]. LCN2 is upregulated in patients with pancreatic ductal adenocarcinoma and obesity. Depletion of LCN2 diminished ECM deposition, immune cell infiltration, PanIN formation, tumor growth and increased survival in both obesity-driven and syngeneic orthotopic pancreatic ductal adenocarcinoma mouse models via modulation of proinflammatory cytokines secreted by pancreatic stellate cells [225]. TNF-α expression is elevated in the pancreatic ductal adenocarcinoma initiation process, and anti-TNF-α antibodies have shown promising effects in pancreatic ductal adenocarcinoma in preclinical models via killing tumor cells and diminishing desmoplasia and inflammation in the pancreatic ductal adenocarcinoma tumor stroma [81].
9.3. Pancreatic Stellate Cells (PSCs)
Pancreatic stellate cells, which comprise about 4-7% of the pancreas, are normally quiescent and play a role in standard tissue architecture by regulating extracellular matrix turnover [226]. PSCs can synthesize and secrete acetylcholine and play a role in mediating exocrine secretion from acinar cells [227]. PSCs transition to activated myofibroblast-like cells in response to inflammation. These cells play a vital role in ECM production, migration, and proliferation, and foster progression of chronic pancreatitis to pancreatic cancer [228]. PSCs respond to pro-inflammatory cytokines in acute pancreatitis and may exacerbate the disease to chronic pancreatitis with pancreatic injury and fibrosis [229]. TLR9 ligation induces pancreatic stellate cells to secrete chemokines and become fibrogenic and proliferative, and mediate pro-tumorogenic effects via CCL11 [230]. Understanding the biology of the pancreas and its cell types could provide avenues to treat pancreatic pathology in disease states.
CONCLUSION AND FUTURE THERAPEUTIC PERSPECTIVES
Understanding of the mechanisms of pancreatitis and progression of pancreatic cancer has advanced but much remains unclear. Inflammation and fibrosis with epithelial to mesenchymal transition are critical factors in pancreatic carcinogenesis, and hence novel anti-fibrotic agents in combination with those anti-inflammatory effects might be therapeutic in targeting acinar to ductal metaplasia and pancreatic ductal adenocarcinoma. A multitude of molecular, fibrotic, stress, and signal transduction pathways and factors that determine progression remains the main obstacle to combating this aggressive disease. Targeting multiple pathways may be effective in the treatment of pancreatitis and inhibiting its progression to pancreatic cancer.
Complementary and combinational therapeutics such as immunogenic, signal transduction targeted agents, chemotherapeutics, and therapies aimed against the tumor microenvironment will hopefully be a beneficial treatment. Targeted therapies towards the control of pro-inflammatory cytokines and chemokines, to inhibit immune cell infiltration and aberrant inflammatory signals and to enhance T cell activation that contributes to the disease pathology in pancreatitis and pancreatic cancer, might be helpful to inhibit pancreatitis progression to pancreatic cancer and metastasis.
ACKNOWLEDGEMENTS
Dr. Mishra is the Endowed Schlieder Chair; therefore, the authors thank Edward G. Schlieder Educational Foundation for the support. The authors are thankful to Dr. Gilbert Morris, Associate Professor, Dept. of Pathology and Laboratory Medicine, Tulane University School of Medicine, New Orleans, Louisiana, USA for the critical review of the manuscript. The authors are also thankful to Ms. Loula Burton, Editor for the Office of Research Proposal Development, Tulane University for the proofreading & editing of the manuscript.
FUNDING
The work is partially supported by the NIH Clinical Center, United States (NIH R01 AI08058l) grant funding (AM).
LIST OF ABBREVIATIONS
- ADM
Acinar-ductal metaplasia
- AIP
Autoimmune pancreatitis
- ALDH1A1
Aldehyde Dehydrogenase 1 Family Member A1
- ALI
acute lung injury
- AP-1
Activator protein 1
- ARID1A
AT-Rich Interaction Domain 1A
- AsPC-1
Human pancreatic adenocarcinoma cells
- ASTN1
Astrotactin 1
- BAY11-7082
irreversible inhibitor of IKK α and phosphorylation of cytokine-inducible IκBα
- Brca2
Breast Cancer Type 2 Susceptibility Protein
- BxPC-3
Human pancreas adenocarcinoma cell line
- C/EBP
CCAAT Enhancer Binding Protein Beta
- Cap43
N-Myc Downstream Regulated 1
- Capan-1
Human pancreatic ductal adenocarcinoma cell line
- Capan-2
Human pancreatic ductal adenocarcinoma cell line
- CC Chemokine
C-C motif chemokine
- CCL18
C-C Motif Chemokine Ligand 18
- CCL2
C-C Motif Chemokine Ligand 2
- CCL20
C-C Motif Chemokine Ligand 20
- CCL5
C-C Motif Chemokine Ligand 5
- CCR2
C-C Motif Chemokine Receptor 2
- CCR6
C-C Motif Chemokine Receptor 6
- CD11b
CD11 Antigen-Like Family Member B
- CD11c
CD11 Antigen-Like Family Member C
- CD18
Integrin beta chain-2
- CD206
Cluster of Differentiation 206
- CD4+T
Cluster of differentiation 4 T cells
- CD8+T
Cluster of differentiation 8 T cells
- CDKN2A
Cyclin Dependent Kinase Inhibitor 2A
- CFPAC-1
Pancreatic ductal adenocarcinoma cell line
- COL6A5
Collagen Type VI Alpha 5 Chain
- COX-2
Cyclooxygenase-2
- CXCL1
C-X-C Motif Chemokine Ligand 1
- CXCR2
C-X-C Motif Chemokine Receptor 2
- CXCR4
C-X-C Motif Chemokine Receptor 4
- DAMPS
Damage-associated molecular patterns
- Dan-G cells
Human Pancreas cancer cell line
- DCLK1
Doublecortin Like Kinase 1
- DNAH11
Dynein Axonemal Heavy Chain 11
- DNAH14
Dynein Axonemal Heavy Chain 11
- DNMT1
DNA methyltransferase 1
- DPC4
Deletion Target In Pancreatic Carcinoma 4
- ED-B
Extra-domain B
- EGFR
Epidermal Growth Factor Receptor
- EMT
Epithelial–mesenchymal transition
- ERK1/2
Extracellular signal-regulated kinases1/2
- FAT3
FAT Atypical Cadherin 3
- FLG
Filaggrin
- GATA-1
GATA Binding Protein 1
- GL13
Glioma-Associated Oncogene Family Zinc Finger 3
- Gr-1
Ly-6G/Ly-6C
- HMCN1
Hemicentin 1
- HPAC
Homo sapiens pancreas adenocarcinoma
- HPAF-II
Human pancreatic adenocarcinoma cell line
- HTGP-AP
Hyperlipidemia/ hypertriglyceridemia pancreatitis
- ICE
Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethylketone
- IFN-γ
Interferon Gamma
- IgG4
immunoglobulin G4
- IKK2
I-Kappa-B Kinase 2
- IKKβ
Inhibitor Of Nuclear Factor Kappa B Kinase Subunit Beta
- IL-10
Interleukin-10
- IL-11
Interleukin-11
- IL-13
Interleukin-13
- IL-13-PE
Pseudomonas exotoxin
- IL-15
Interleukin-15
- IL-17B
Interleukin-17B
- IL-17RB
Interleukin-17 receptor B
- IL-17RC
Interleukin 17 Receptor C
- IL-1β
Interleukin-1β
- IL-2
Interleukin-2
- IL-21
Interleukin-21
- IL-22
Interleukin-22
- IL-23
Interleukin-23
- IL-27
Interleukin-27
- IL-4
Interleukin-4
- IL-4Rα
Interleukin-4 receptor α
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- INK4A
Cyclin Dependent Kinase Inhibitor 2A
- i-NKT
Invariant natural killer T
- IRS954
TLR9 inhibitor
- JAK
Janus Kinase
- KPC mouse
PdxCretg/+ mouse
- KRAS
Kirsten Rat Sarcoma Viral Proto-Oncogene
- L19-IL2
L19-Interleukin-2
- LAMP2
Lysosomal Associated Membrane Protein 2
- LRP1B
Low-density lipoprotein receptor-related protein 1B
- Ly-6C
Lymphocyte antigen 6 complex locus C1
- Ly-6G
Lymphocyte antigen 6 complex locus G6D
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein-1
- MIA PaCa-2
pancreatic carcinoma
- MIP-1α
Macrophage Inflammatory Protein 1-Alpha
- MIP-2
Macrophage Inflammatory Protein 2-Alpha
- MLL2
Myeloid/Lymphoid Or Mixed-Lineage Leukemia 2
- MLL3
Myeloid/Lymphoid Or Mixed-Lineage Leukemia 3
- MMP-9
Matrix Metallopeptidase 9
- MUC16
Mucin 16 Cell Surface Associated
- NDRG1
N-Myc Downstream Regulated 1
- NFATc4
Nuclear Factor Of Activated T Cells 4
- NF-IL6
Nuclear Factor Of Interleukin 6
- NF-κB
Nuclear Factor Kappa B Subunit 1 Nuclear Factor Kappa B Subunit 1
- NK
Natural killer
- NLRP3
NLR Family Pyrin Domain Containing 3
- NOD
Nucleotide Binding Oligomerization Domain
- OBSCN
Obscurin
- p16
CDK4 Inhibitor P16-INK4
- p21WAF1
Wild-Type P53-Activated Fragment 1
- P2X7
Purinergic Receptor P2X 7
- p65
NF-Kappa-B Transcription Factor P65
- PAMPS
Pathogen-associated molecular patterns
- PANC-1
Human pancreas duct epithelioid carcinoma
- PANC-28
Human Pancreatic adenocarcinoma cell line
- PanIN-1A
Pancreatic Intraepithelial Neoplasia 1A
- PanIN-1B
Pancreatic Intraepithelial Neoplasia 1B
- PanIN-2/3
Pancreatic Intraepithelial Neoplasia 2/3
- PARP
Poly (ADP-Ribose) Polymerase 1
- PCC
Pancreatic cancer cells
- PCDH15
Protocadherin Related 15
- PDA/PDAC
Pancreatic Ductal Adenocarcinoma
- PD-L1
Programmed Cell Death 1 Ligand 1
- PDTC
Pyrrolidine derivative of dithiocarbamate
- POU2F3
POU Class 2 Homeobox 3
- PREX2
Phosphatidylinositol-3,4,5-Trisphosphate Dependent Rac Exchange Factor 2
- PSC
Pancreatic stellate cells
- PXDN
Peroxidasin
- RANTES
Regulated Upon Activation Normally T-Expressed And Presumably Secreted
- Ras
Rat Sarcoma Viral Oncogene Homolog
- RelA
Nuclear Factor NF-Kappa-B P65 Subunit
- RNF43
Ring Finger Protein 43
- SAP
Severe acute pancreatitis
- SCIDγ mice
Severe Combined Immunodeficiency
- SDF1α
Stromal Cell-Derived Factor 1
- SMAD4
SMAD Mothers Against DPP Homolog 4
- SNAIL-1
Snail Family Transcriptional Repressor 1
- SOCS
Suppressor of cytokine signaling
- SOX9
SRY-Box 9
- STAT3
Signal transducer and activator of transcription 3
- STING
Stimulator of interferon genes
- SU.86.86
Human pancreatic adenosarcoma cell line
- SYNE1
Spectrin Repeat Containing Nuclear Envelope Protein 1
- T Reg cell
Regulatory T cell
- T2DM
Type 2 diabetes
- TGFBR2
Transforming Growth Factor Beta Receptor 2
- Th1
Type 1 T helper
- TLRs
Toll-like receptors
- TNF-α
Tumor necrosis factor alpha
- TP53
Tumor Protein P53
- TPCK
Tosylphe-chloromethyl ketone
- TRAIL
TNF-Related Apoptosis Inducing Ligand
- U937
Human myeloid leukaemia cell line
- WDFY4
WD Repeat- And FYVE Domain-Containing Protein 4
- Wnt
Wingless/Integrated
- WS-4
Anti-IL 8 antibody
- ZNF559
Zinc Finger Protein 559
- α-SMA
α-smooth muscle actin
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Waddell N; Pajic M; Patch AM; Chang DK; Kassahn KS; Bailey P; Johns AL; Miller D; Nones K; Quek K; Quinn MC; Robertson AJ; Fadlullah MZ; Bruxner TJ; Christ AN; Harliwong I; Idrisoglu S; Manning S; Nourse C; Nourbakhsh E; Wani S; Wilson PJ; Markham E; Cloonan N; Anderson MJ; Fink JL; Holmes O; Kazakoff SH; Leonard C; Newell F; Poudel B; Song S; Taylor D; Waddell N; Wood S; Xu Q; Wu J; Pinese M; Cowley MJ; Lee HC; Jones MD; Nagrial AM; Humphris J; Chantrill LA; Chin V; Steinmann AM; Mawson A; Humphrey ES; Colvin EK; Chou A; Scarlett CJ; Pinho AV; Giry-Laterriere M; Rooman I; Samra JS; Kench JG; Pettitt JA; Merrett ND; Toon C; Epari K; Nguyen NQ; Barbour A; Zeps N; Jamieson NB; Graham JS; Niclou SP; Bjerkvig R; Grützmann R; Aust D; Hruban RH; Maitra A; Iacobuzio-Donahue CA; Wolfgang CL; Morgan RA; Lawlor RT; Corbo V; Bassi C; Falconi M; Zamboni G; Tortora G; Tempero MA; Gill AJ; Eshleman JR; Pilarsky C; Scarpa A; Musgrove EA; Pearson JV; Biankin AV; Grimmond SM Whole genomes redefine the mutational landscape of pancreatic cancer. Nature, 2015, 518(7540), 495–501. 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Łukaszewicz-Zając M; Gryko M; Mroczko B The role of selected chemokines and their specific receptors in pancreatic cancer. Int. J. Biol. Markers, 2018, 33(2), 141–147. 10.1177/1724600817753094 [DOI] [PubMed] [Google Scholar]
- [3].Szatmary P; Gukovsky I The role of cytokines and inflammation in the genesis of experimental pancreatitis. Pancreapedia; Exocrine Pancreas Knowledge Base, 2016. [Google Scholar]
- [4].Kleeff J; Whitcomb DC; Shimosegawa T; Esposito I; Lerch MM; Gress T; Mayerle J; Drewes AM; Rebours V; Akisik F; Muñoz JED; Neoptolemos JP Chronic pancreatitis. Nat. Rev. Dis. Primers, 2017, 3, 17060 10.1038/nrdp.2017.60 [DOI] [PubMed] [Google Scholar]
- [5].Evans AC; Papachristou GI; Whitcomb DC Obesity and the risk of severe acute pancreatitis. Minerva Gastroenterol. Dietol., 2010, 56(2), 169–179. PMID: 20485254 [PubMed] [Google Scholar]
- [6].Noel RA; Braun DK; Patterson RE; Bloomgren GL Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care, 2009, 32(5), 834–838. 10.2337/dc08-1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Papachristou GI; Papachristou DJ; Avula H; Slivka A; Whitcomb DC Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology, 2006, 6(4), 279–285. 10.1159/000092689 [DOI] [PubMed] [Google Scholar]
- [8].Taniguchi T; Seko S; Okamoto M; Hamasaki A; Ueno H; Inoue F; Nishida O; Miyake N; Mizumoto T Association of autoimmune pancreatitis and type 1 diabetes: autoimmune exocrinopathy and endocrinopathy of the pancreas. Diabetes Care, 2000, 23(10), 1592–1594. 10.2337/diacare.23.10.1592 [DOI] [PubMed] [Google Scholar]
- [9].Finn OJ Immuno-oncology: understanding the function and dysfunction of the immune system in cancer., Ann Oncol, 2012, 23(Suppl 8), viii6–9. 10.1093/annonc/mds256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].LaRusch J; Solomon S; Whitcomb DC Pancreatitis Overview GeneReviews; Adam MP; Ardinger HH; Pagon RA; Wallace SE; Bean LJH; Stephens K; Amemiya A, Eds.; Seattle, WA, 1993, Vol. R, . [PubMed] [Google Scholar]
- [11].Banks PA; Bollen TL; Dervenis C; Gooszen HG; Johnson CD; Sarr MG; Tsiotos GG; Vege SS Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut, 2013, 62(1), 102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- [12].Wu BU; Banks PA Clinical management of patients with acute pancreatitis. Gastroenterology, 2013, 144(6), 1272–1281. 10.1053/j.gastro.2013.01.075 [DOI] [PubMed] [Google Scholar]
- [13].Ahmed Ali U; Issa Y; Hagenaars JC; Bakker OJ; van Goor H; Nieuwenhuijs VB; Bollen TL; van Ramshorst B; Witteman BJ; Brink MA; Schaapherder AF; Dejong CH; Spanier BW; Heisterkamp J; van der Harst E; van Eijck CH; Besselink MG; Gooszen HG; van Santvoort HC; Boermeester MA Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin. Gastroenterol. Hepatol, 2016, 14(5), 738–746. 10.1016/j.cgh.2015.12.040 [DOI] [PubMed] [Google Scholar]
- [14].Braganza JM; Lee SH; McCloy RF; McMahon MJ Chronic pancreatitis. Lancet, 2011, 377(9772), 1184–1197. 10.1016/S0140-6736(10)61852-1 [DOI] [PubMed] [Google Scholar]
- [15].Braganza JM Evolution of pancreatitis Braganza JM In: The pathogenesis of pancreatitis. Manchester University Press, Manchester; , 1991; pp. 19–33. [Google Scholar]
- [16].Cook LJ; Musa OA; Case RM Intracellular transport of pancreatic enzymes. Scand. J. Gastroenterol Suppl, 1996, 219, 1–5. 10.3109/00365529609104990 [DOI] [PubMed] [Google Scholar]
- [17].Gaisano HY; Gorelick FS New insights into the mechanisms of pancreatitis. Gastroenterology, 2009, 136(7), 2040–2044. 10.1053/j.gastro.2009.04.023 [DOI] [PubMed] [Google Scholar]
- [18].Park DH; Kim MH; Chari ST Recent advances in autoimmune pancreatitis. Gut, 2009, 58(12), 1680–1689. 10.1136/gut.2008.155853 [DOI] [PubMed] [Google Scholar]
- [19].Valdivielso P; Ramíez-Bueno A; Ewald N Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med, 2014, 25(8), 689–694. 10.1016/j.ejim.2014.08.008 [DOI] [PubMed] [Google Scholar]
- [20].Crisan LS; Steidl ET; Rivera-Alsina ME Acute hyperlipidemic pancreatitis in pregnancy. Am. J. Obstet. Gynecol, 2008, 198(5), e57–e59. 10.1016/j.ajog.2008.01.003 [DOI] [PubMed] [Google Scholar]
- [21].Ye C; Liu L; Ma X; Tong H; Gao J; Tai Y; Huang L; Tang C; Wang R Obesity Aggravates Acute Pancreatitis via Damaging Intestinal Mucosal Barrier and Changing Microbiota Composition in Rats. Sci. Rep, 2019, 9(1), 69 10.1038/s41598-018-36266-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gukovsky I; Li N; Todoric J; Gukovskaya A; Karin M Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology, 2013, 144(6), 1199–209 e4 10.1053/j.gastro.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kobayashi T; Aida K; Fukui T; Jimbo E; Shimada A; Mori Y; Fujii T; Yagihashi S Pancreatic ductal hyperplasia/dysplasia with obstructive chronic pancreatitis: an association with reduced pancreatic weight in type 1 diabetes. Diabetologia, 2016, 59(4), 865–867. 10.1007/s00125-016-3867-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zechner D; Spitzner M; Bobrowski A; Knapp N; Kuhla A; Vollmar B Diabetes aggravates acute pancreatitis and inhibits pancreas regeneration in mice. Diabetologia, 2012, 55(5), 1526–1534. 10.1007/s00125-012-2479-3 [DOI] [PubMed] [Google Scholar]
- [25].Girman CJ; Kou TD; Cai B; Alexander CM; O’Neill EA; Williams-Herman DE; Katz L Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes. Metab, 2010, 12(9), 766–771. 10.1111/j.1463-1326.2010.01231.x [DOI] [PubMed] [Google Scholar]
- [26].Ewald N; Hardt PD Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J. Gastroenterol, 2013, 19(42), 7276–7281. 10.3748/wjg.v19.i42.7276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shiratori K Management of pancreatic diabetes secondary to chronic pancreatitis. An Integrated Textbook of Basic Science, Medicine, and Surgery, 2018, 495–502. 10.1002/9781119188421.ch62 [DOI] [Google Scholar]
- [28].Raimondi S; Lowenfels AB; Morselli-Labate AM; Maisonneuve P; Pezzilli R Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol, 2010, 24(3), 349–358. 10.1016/j.bpg.2010.02.007 [DOI] [PubMed] [Google Scholar]
- [29].Pinho AV; Chantrill L; Rooman I Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett, 2014, 345(2), 203–209. 10.1016/j.canlet.2013.08.015 [DOI] [PubMed] [Google Scholar]
- [30].Verma AK; Kandikattu HK; Manohar M; Shukla A; Upparahalli Venkateshaiah S; Zhu X; Mishra A Intestinal overexpression of IL-18 promotes eosinophils-mediated allergic disorders. Immunology, 2019, 157(2), 110–121. 10.1111/imm.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xue R; Jia K; Wang J; Yang L; Wang Y; Gao L; Hao J A Rising Star in Pancreatic Diseases: Pancreatic Stellate Cells. Front. Physiol, 2018, 9, 754 10.3389/fphys.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Higuera O; Ghanem I; Nasimi R; Prieto I; Koren L; Feliu J Management of pancreatic cancer in the elderly. World J. Gastroenterol, 2016, 22(2), 764–775. 10.3748/wjg.v22.i2.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paszkowski AS; Rau B; Mayer JM; Möller P; Beger HG Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann. Surg, 2002, 235(1), 68–76. 10.1097/00000658-200201000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xue J; Sharma V; Hsieh MH; Chawla A; Murali R; Pandol SJ; Habtezion A Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat. Commun, 2015, 6, 7158 10.1038/ncomms8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Osman MO; Kristensen JU; Jacobsen NO; Lausten SB; Deleuran B; Deleuran M; Gesser B; Matsushima K; Larsen CG; Jensen SL A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut, 1998, 43(2), 232–239. 10.1136/gut.43.2.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Van Laethem JL; Marchant A; Delvaux A; Goldman M; Robberecht P; Velu T; Devière J Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology, 1995, 108(6), 1917–1922. 10.1016/0016-5085(95)90158-2 [DOI] [PubMed] [Google Scholar]
- [37].Rongione AJ; Kusske AM; Kwan K; Ashley SW; Reber HA; McFadden DW Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology, 1997, 112(3), 960–967. 10.1053/gast.1997.v112.pm9041259 [DOI] [PubMed] [Google Scholar]
- [38].Shimizu T; Shiratori K; Sawada T; Kobayashi M; Hayashi N; Saotome H; Keith JC Recombinant human interleukin-11 decreases severity of acute necrotizing pancreatitis in mice. Pancreas, 2000, 21(2), 134–140. 10.1097/00006676-200008000-00005 [DOI] [PubMed] [Google Scholar]
- [39].Manohar M; Verma AK; Venkateshaiah SU; Mishra A Mechanistic role of eosinophils in the initiation and progression of pancreatitis pathogenesis. J. Immunol, 2017, 198(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Feng D; Park O; Radaeva S; Wang H; Yin S; Kong X; Zheng M; Zakhari S; Kolls JK; Gao B Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int. J. Biol. Sci, 2012, 8(2), 249–257. 10.7150/ijbs.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Denham W; Fink G; Yang J; Ulrich P; Tracey K; Norman J Small molecule inhibition of tumor necrosis factor gene processing during acute pancreatitis prevents cytokine cascade progression and attenuates pancreatitis severity. Am. Surg, 1997, 63(12), 1045–1049. [PubMed] [Google Scholar]
- [42].Wagner K; Schulz P; Scholz A; Wiedenmann B; Menrad A The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin. Cancer Res, 2008, 14(15), 4951–4960. 10.1158/1078-0432.CCR-08-0157 [DOI] [PubMed] [Google Scholar]
- [43].Liu A; Liu Y; Li PK; Li C; Lin J LLL12 inhibits endogenous and exogenous interleukin-6-induced STAT3 phosphorylation in human pancreatic cancer cells. Anticancer Res, 2011, 31(6), 2029–2035. [PMC free article] [PubMed] [Google Scholar]
- [44].Goumas FA; Holmer R; Egberts JH; Gontarewicz A; Heneweer C; Geisen U; Hauser C; Mende MM; Legler K; Röcken C; Becker T; Waetzig GH; Rose-John S; Kalthoff H Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer, 2015, 137(5), 1035–1046. 10.1002/ijc.29445 [DOI] [PubMed] [Google Scholar]
- [45].Yoshida Y; Tasaki K; Miyauchi M; Narita M; Takenaga K; Yamamoto H; Yaaguchi T; Saisho H; Sakiyama S; Tagawa M Impaired tumorigenicity of human pancreatic cancer cells retrovirally transduced with interleukin-12 or interleukin-15 gene. Cancer Gene Ther, 2000, 7(2), 324–331. 10.1038/sj.cgt.7700118 [DOI] [PubMed] [Google Scholar]
- [46].Fujisawa T; Nakashima H; Nakajima A; Joshi BH; Puri RK Targeting IL-13Rα2 in human pancreatic ductal adenocarcinoma with combination therapy of IL-13-PE and gemcitabine. Int. J. Cancer, 2011, 128(5), 1221–1231. 10.1002/ijc.25437 [DOI] [PubMed] [Google Scholar]
- [47].Van Audenaerde JRM; De Waele J; Marcq E; Van Loenhout J; Lion E; Van den Bergh JMJ; Jesenofsky R; Masamune A; Roeyen G; Pauwels P; Lardon F; Peeters M; Smits ELJ Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget, 2017, 8(34), 56968–56979. 10.18632/oncotarget.18185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wu HH; Hwang-Verslues WW; Lee WH; Huang CK; Wei PC; Chen CL; Shew JY; Lee EY; Jeng YM; Tien YW; Ma C; Lee WH Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med, 2015, 212(3), 333–349. 10.1084/jem.20141702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McMichael EL; Jaime-Ramirez AC; Guenterberg KD; Luedke E; Atwal LS; Campbell AR; Hu Z; Tatum AS; Kondadasula SV; Mo X; Tridandapani S; Bloomston M; Ellison EC; Williams TM; Bekaii-Saab T; Carson WE III IL-21 Enhances Natural Killer Cell Response to Cetuximab-Coated Pancreatic Tumor Cells. Clin. Cancer Res, 2017, 23(2), 489–502. 10.1158/1078-0432.CCR-16-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tang ZH; Qiu WH; Wu GS; Yang XP; Zou SQ; Qiu FZ The immunotherapeutic effect of dendritic cells vaccine modified with interleukin-18 gene and tumor cell lysate on mice with pancreatic carcinoma. World J. Gastroenterol, 2002, 8(5), 908–912. 10.3748/wjg.v8.i5.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pan X; Sheng W; Zhu Q; Xie Y; Ye Z; Xiang J; Li D; Yang J Inhibition of pancreatic carcinoma growth by adenovirus-mediated human interleukin-24 expression in animal model. Cancer Biother. Radiopharm, 2008, 23(4), 425–434. 10.1089/cbr.2008.0461 [DOI] [PubMed] [Google Scholar]
- [52].Yao L; Wang M; Niu Z; Liu Q; Gao X; Zhou L; Liao Q; Zhao Y Interleukin-27 inhibits malignant behaviors of pancreatic cancer cells by targeting M2 polarized tumor associated macrophages. Cytokine, 2017, 89, 194–200. 10.1016/j.cyto.2015.12.003 [DOI] [PubMed] [Google Scholar]
- [53].Detjen KM; Farwig K; Welzel M; Wiedenmann B; Rosewicz S Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut, 2001, 49(2), 251–262. 10.1136/gut.49.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Amruta N; Kandikattu HK Apoptosis of inflammatory cells in Asthma. Int. J Cell Biol. Physiol, 2018, 1(1-2), 1–6. [Google Scholar]
- [55].Luzina IG; Keegan AD; Heller NM; Rook GA; Shea-Donohue T; Atamas SP Regulation of inflammation by interleukin-4: a review of “alternatives”. J. Leukoc. Biol, 2012, 92(4), 753–764. 10.1189/jlb.0412214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Prokopchuk O; Liu Y; Henne-Bruns D; Kornmann M Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br. J. Cancer, 2005, 92(5), 921–928. 10.1038/sj.bjc.6602416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kawakami K; Kawakami M; Husain SR; Puri RK Targeting interleukin-4 receptors for effective pancreatic cancer therapy. Cancer Res, 2002, 62(13), 3575–3580. [PubMed] [Google Scholar]
- [58].Mishra A; Wang M; Pemmaraju VR; Collins MH; Fulkerson PC; Abonia JP; Blanchard C; Putnam PE; Rothenberg ME Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology, 2008, 134(1), 204–214. 10.1053/j.gastro.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Manohar M; Kandikattu HK; Verma AK; Mishra A IL-15 regulates fibrosis and inflammation in a mouse model of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol, 2018, 135(6), G954–G965. 10.1152/ajpgi.00139.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee EJ; Lee SJ; Kim S; Cho SC; Choi YH; Kim WJ; Moon SK Interleukin-5 enhances the migration and invasion of bladder cancer cells via ERK1/2-mediated MMP-9/NF-κB/AP-1 pathway: involvement of the p21WAF1 expression. Cell. Signal, 2013, 25(10), 2025–2038. 10.1016/j.cellsig.2013.06.004 [DOI] [PubMed] [Google Scholar]
- [61].Simson L; Ellyard JI; Dent LA; Matthaei KI; Rothenberg ME; Foster PS; Smyth MJ; Parish CR Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J. Immunol, 2007, 178(7), 4222–4229. 10.4049/jimmunol.178.7.4222 [DOI] [PubMed] [Google Scholar]
- [62].Zhang H; Neuhöfer P; Song L; Rabe B; Lesina M; Kurkowski MU; Treiber M; Wartmann T; Regnér S; Thorlacius H; Saur D; Weirich G; Yoshimura A; Halangk W; Mizgerd JP; Schmid RM; Rose-John S; Algül H IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Invest, 2013, 123(3), 1019–1031. 10.1172/JCI64931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mace TA; Shakya R; Pitarresi JR; Swanson B; McQuinn CW; Loftus S; Nordquist E; Cruz-Monserrate Z; Yu L; Young G; Zhong X; Zimmers TA; Ostrowski MC; Ludwig T; Bloomston M; Bekaii-Saab T; Lesinski GB IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut, 2018, 67(2), 320–332. 10.1136/gutjnl-2016-311585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mishra A; Rothenberg ME Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology, 2003, 125(5), 1419–1427. 10.1016/j.gastro.2003.07.007 [DOI] [PubMed] [Google Scholar]
- [65].Upparahalli Venkateshaiah S; Niranjan R; Manohar M; Verma AK; Kandikattu HK; Lasky JA; Mishra A Attenuation of Allergen, IL-13- and TGF-alpha-Induced Lung Fibrosis Following the Treatment of IL-15 in Mice. Am. J. Respir. Cell Mol. Biol, 2019. 10.1165/rcmb.2018-0254OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liou GY; Bastea L; Fleming A; Döppler H; Edenfield BH; Dawson DW; Zhang L; Bardeesy N; Storz P The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis. Cell Rep, 2017, 19(7), 1322–1333. 10.1016/j.celrep.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Venkateshaiah SU; Manohar M; Verma AK; Blecker U; Mishra A Possible Noninvasive Biomarker of Eosinophilic Esophagitis: Clinical and Experimental Evidence. Case Rep. Gastroenterol, 2016, 10(3), 685–692. 10.1159/000452654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Murdock BJ; Falkowski NR; Shreiner AB; Sadighi Akha AA; McDonald RA; White ES; Toews GB; Huffnagle GB Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect. Immun, 2012, 80(4), 1424–1436. 10.1128/IAI.05529-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhao Q; Manohar M; Wei Y; Pandol SJ; Habtezion A STING signalling protects against chronic pancreatitis by modulating Th17 response. Gut, 2019, 68(10), 1827–1837. 10.1136/gutjnl-2018-317098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Y; Zoltan M; Riquelme E; Xu H; Sahin I; Castro-Pando S; Montiel MF; Chang K; Jiang Z; Ling J; Gupta S; Horne W; Pruski M; Wang H; Sun SC; Lozano G; Chiao P; Maitra A; Leach SD; Kolls JK; Vilar E; Wang TC; Bailey JM; McAllister F Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology, 2018, 155(1), 210–223 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kandikattu HK; Mishra A IL-18 overexpression promotes eosinophils-mediated peanut-induced intestinal allergy. J. Allergy Clin. Immunol, 2018, 143(2), AB254 10.1016/j.jaci.2018.12.776 [DOI] [Google Scholar]
- [72].Sandersa NL; Venkateshaiah SU; Manohar M; Verma AK; Kandikattu HK; Mishra A Interleukin-18 has an important role in differentiation and maturation of mucosal mast cells. Journal of mucosal immunology research, 2018, 2(1) [PMC free article] [PubMed] [Google Scholar]
- [73].Venkateshaiah SU; Mishra A; Manohar M; Verma AK; Rajavelu P; Niranjan R; Wild LG; Parada NA; Blecker U; Lasky JA; Mishra A A critical role for IL-18 in transformation and maturation of naive eosinophils to pathogenic eosinophils. J. Allergy Clin. Immunol, 2018, 142(1), 301–305. 10.1016/j.jaci.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Janiak A; Leśniowski B; Jasińska A; Pietruczuk M; Małecka-Panas E Interleukin 18 as an early marker or prognostic factor in acute pancreatitis. Prz. Gastroenterol, 2015, 10(4), 203–207. 10.5114/pg.2015.50993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vidal-Vanaclocha F; Mendoza L; Telleria N; Salado C; Valcárcel M; Gallot N; Carrascal T; Egilegor E; Beaskoetxea J; Dinarello CA Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev, 2006, 25(3), 417–434. 10.1007/s10555-006-9013-3 [DOI] [PubMed] [Google Scholar]
- [76].Zaidi MR; Merlino G The two faces of interferon-γ in cancer. Clin. Cancer Res, 2011, 17(19), 6118–6124. 10.1158/1078-0432.CCR-11-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hayashi T; Ishida Y; Kimura A; Iwakura Y; Mukaida N; Kondo T IFN-gamma protects cerulein-induced acute pancreatitis by repressing NF-kappa B activation. J. Immunol, 2007, 178(11), 7385–7394. 10.4049/jimmunol.178.11.7385 [DOI] [PubMed] [Google Scholar]
- [78].Kandikattu HK Oxido-nitrosative stress and antioxidants in asthma. J Basic Clin Immonol, 2018, 1, 9–12. [Google Scholar]
- [79].Zelová H; Hošek J TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res, 2013, 62(7), 641–651. 10.1007/s00011-013-0633-0 [DOI] [PubMed] [Google Scholar]
- [80].Sendler M; Dummer A; Weiss FU; Krüger B; Wartmann T; Scharffetter-Kochanek K; van Rooijen N; Malla SR; Aghdassi A; Halangk W; Lerch MM; Mayerle J Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut, 2013, 62(3), 430–439. 10.1136/gutjnl-2011-300771 [DOI] [PubMed] [Google Scholar]
- [81].Zhao X; Fan W; Xu Z; Chen H; He Y; Yang G; Yang G; Hu H; Tang S; Wang P; Zhang Z; Xu P; Yu M Inhibiting tumor necrosis factor-alpha diminishes desmoplasia and inflammation to overcome chemoresistance in pancreatic ductal adenocarcinoma. Oncotarget, 2016, 7(49), 81110–81122. 10.18632/oncotarget.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Turner MD; Nedjai B; Hurst T; Pennington DJ Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta, 2014, 1843(11), 2563–2582. 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- [83].O’Hayre M; Salanga CL; Handel TM; Allen SJ Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem. J, 2008, 409(3), 635–649. 10.1042/BJ20071493 [DOI] [PubMed] [Google Scholar]
- [84].Singh S; Sadanandam A; Singh RK Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev, 2007, 26(3-4), 453–467. 10.1007/s10555-007-9068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yubero S; Ramudo L; Manso MA; De Dios I The role of redox status on chemokine expression in acute pancreatitis. Biochim. Biophys. Acta, 2009, 1792(2), 148–154. 10.1016/j.bbadis.2008.12.002 [DOI] [PubMed] [Google Scholar]
- [86].Bhatia M; Hegde A Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul. Pept, 2007, 138(1), 40–48. 10.1016/j.regpep.2006.08.006 [DOI] [PubMed] [Google Scholar]
- [87].Steele CW; Karim SA; Foth M; Rishi L; Leach JD; Porter RJ; Nixon C; Jeffry Evans TR; Carter CR; Nibbs RJ; Sansom OJ; Morton JP CXCR2 inhibition suppresses acute and chronic pancreatic inflammation. J. Pathol, 2015, 237(1), 85–97. 10.1002/path.4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhou GX; Zhu XJ; Ding XL; Zhang H; Chen JP; Qiang H; Zhang HF; Wei Q Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis. HBPD INT, 2010, 9(2), 201–207. [PubMed] [Google Scholar]
- [89].Wente MN; Keane MP; Burdick MD; Friess H; Büchler MW; Ceyhan GO; Reber HA; Strieter RM; Hines OJ Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett, 2006, 241(2), 221–227. 10.1016/j.canlet.2005.10.041 [DOI] [PubMed] [Google Scholar]
- [90].Nywening TM; Wang-Gillam A; Sanford DE; Belt BA; Panni RZ; Cusworth BM; Toriola AT; Nieman RK; Worley LA; Yano M; Fowler KJ; Lockhart AC; Suresh R; Tan BR; Lim KH; Fields RC; Strasberg SM; Hawkins WG; DeNardo DG; Goedegebuure SP; Linehan DC Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol., 2016, 17(5), 651–662. 10.1016/S1470-2045(16)00078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ijichi H; Chytil A; Gorska AE; Aakre ME; Bierie B; Tada M; Mohri D; Miyabayashi K; Asaoka Y; Maeda S; Ikenoue T; Tateishi K; Wright CV; Koike K; Omata M; Moses HL Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J. Clin. Invest, 2011, 121(10), 4106–4117. 10.1172/JCI42754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tan MC; Goedegebuure PS; Belt BA; Flaherty B; Sankpal N; Gillanders WE; Eberlein TJ; Hsieh CS; Linehan DC Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol, 2009, 182(3), 1746–1755. 10.4049/jimmunol.182.3.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Feig C; Jones JO; Kraman M; Wells RJ; Deonarine A; Chan DS; Connell CM; Roberts EW; Zhao Q; Caballero OL; Teichmann SA; Janowitz T; Jodrell DI; Tuveson DA; Fearon DT Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA, 2013, 110(50), 20212–20217. 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sung B; Jhurani S; Ahn KS; Mastuo Y; Yi T; Guha S; Liu M; Aggarwal BB Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res, 2008, 68(21), 8938–8944. 10.1158/0008-5472.CAN-08-2155 [DOI] [PubMed] [Google Scholar]
- [95].Turnquist HR; Lin X; Ashour AE; Hollingsworth MA; Singh RK; Talmadge JE; Solheim JC CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int. J. Oncol, 2007, 30(3), 631–639. 10.3892/ijo.30.3.631 [DOI] [PubMed] [Google Scholar]
- [96].Han H; Du L; Cao Z; Zhang B; Zhou Q Triptonide potently suppresses pancreatic cancer cell-mediated vasculogenic mimicry by inhibiting expression of VE-cadherin and chemokine ligand 2 genes. Eur. J. Pharmacol, 2018, 818, 593–603. 10.1016/j.ejphar.2017.11.019 [DOI] [PubMed] [Google Scholar]
- [97].Baggiolini M; Dahinden CA CC chemokines in allergic inflammation. Immunol. Today, 1994, 15(3), 127–133. 10.1016/0167-5699(94)90156-2 [DOI] [PubMed] [Google Scholar]
- [98].Matsuo Y; Raimondo M; Woodward TA; Wallace MB; Gill KR; Tong Z; Burdick MD; Yang Z; Strieter RM; Hoffman RM; Guha S CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int. J. Cancer, 2009, 125(5), 1027–1037. 10.1002/ijc.24383 [DOI] [PubMed] [Google Scholar]
- [99].Deshmane SL; Kremlev S; Amini S; Sawaya BE Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res, 2009, 29(6), 313–326. 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Grady T; Liang P; Ernst SA; Logsdon CD Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology, 1997, 113(6), 1966–1975. 10.1016/S0016-5085(97)70017-9 [DOI] [PubMed] [Google Scholar]
- [101].Saurer L; Reber P; Schaffner T; Buchler MW; Buri C; Kappeler A; Walz A; Friess H; Mueller C Differential expression of chemokines in normal pancreas and in chronic pancreatitis. Gastroenterology, 2000, 118(2), 356–367. 10.1016/S0016-5085(00)70218-6 [DOI] [PubMed] [Google Scholar]
- [102].Zhao HF; Ito T; Gibo J; Kawabe K; Oono T; Kaku T; Arita Y; Zhao QW; Usui M; Egashira K; Nawata H Antimonocyte chemoattractant protein 1 gene therapy attenuates experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Gut, 2005, 54(12), 1759–1767. 10.1136/gut.2004.049403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Saeki K; Kanai T; Nakano M; Nakamura Y; Miyata N; Sujino T; Yamagishi Y; Ebinuma H; Takaishi H; Ono Y; Takeda K; Hozawa S; Yoshimura A; Hibi T CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology, 2012, 142(4), 1010–1020 e9. 10.1053/j.gastro.2011.12.054 [DOI] [PubMed] [Google Scholar]
- [104].Sanford DE; Belt BA; Panni RZ; Mayer A; Deshpande AD; Carpenter D; Mitchem JB; Plambeck-Suess SM; Worley LA; Goetz BD; Wang-Gillam A; Eberlein TJ; Denardo DG; Goedegebuure SP; Linehan DC Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res, 2013, 19(13), 3404–3415. 10.1158/1078-0432.CCR-13-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Barmania F; Pepper MS C-C chemokine receptor type five (CCR5): An emerging target for the control of HIV infection. Appl. Transl. Genomics, 2013, 2, 3–16. 10.1016/j.atg.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Goecke H; Forssmann U; Uguccioni M; Friess H; Conejo-Garcia JR; Zimmermann A; Baggiolini M; Büchler MW Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery, 2000, 128(5), 806–814. 10.1067/msy.2000.108613 [DOI] [PubMed] [Google Scholar]
- [107].Singh SK; Mishra MK; Eltoum IA; Bae S; Lillard JW, Jr; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep, 2018, 8(1), 1323 10.1038/s41598-018-19643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schutyser E; Richmond A; Van Damme J Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J. Leukoc. Biol, 2005, 78(1), 14–26. 10.1189/jlb.1204712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Meng F; Li W; Li C; Gao Z; Guo K; Song S CCL18 promotes epithelial-mesenchymal transition, invasion and migration of pancreatic cancer cells in pancreatic ductal adenocarcinoma. Int. J. Oncol, 2015, 46(3), 1109–1120. 10.3892/ijo.2014.2794 [DOI] [PubMed] [Google Scholar]
- [110].Geismann C; Grohmann F; Dreher A; Häsler R; Rosenstiel P; Legler K; Hauser C; Egberts JH; Sipos B; Schreiber S; Linkermann A; Hassan Z; Schneider G; Schäfer H; Arlt A Role of CCL20 mediated immune cell recruitment in NF-kB mediated TRAIL resistance of pancreatic cancer. Biochim. Biophys. Acta Mol. Cell Res, 2017, 1864(5), 782–796. 10.1016/j.bbamcr.2017.02.005 [DOI] [PubMed] [Google Scholar]
- [111].Rubie C; Frick VO; Ghadjar P; Wagner M; Grimm H; Vicinus B; Justinger C; Graeber S; Schilling MK CCL20/CCR6 expression profile in pancreatic cancer. J. Transl. Med, 2010, 8, 45 10.1186/1479-5876-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Liu B; Jia Y; Ma J; Wu S; Jiang H; Cao Y; Sun X; Yin X; Yan S; Shang M; Mao A Tumor-associated macrophage-derived CCL20 enhances the growth and metastasis of pancreatic cancer. Acta Biochim. Biophys. Sin. (Shanghai), 2016, 48(12), 1067–1074. 10.1093/abbs/gmw101 [DOI] [PubMed] [Google Scholar]
- [113].Klemm C; Dommisch H; Göke F; Kreppel M; Jepsen S; Rolf F; Dommisch K; Perner S; Standop J Expression profiles for 14-3-3 zeta and CCL20 in pancreatic cancer and chronic pancreatitis. Pathol. Res. Pract, 2014, 210(6), 335–341. 10.1016/j.prp.2014.01.001 [DOI] [PubMed] [Google Scholar]
- [114].Campbell JJ; Bowman EP; Murphy K; Youngman KR; Siani MA; Thompson DA; Wu L; Zlotnik A; Butcher EC 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J. Cell Biol, 1998, 141(4), 1053–1059. 10.1083/jcb.141.4.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Manzo A; Bugatti S; Caporali R; Prevo R; Jackson DG; Uguccioni M; Buckley CD; Montecucco C; Pitzalis C CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am. J. Pathol, 2007, 171(5), 1549–1562. 10.2353/ajpath.2007.061275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Stein JV; Soriano SF; M’rini C; Nombela-Arrieta C; de Buitrago GG; Rodríguez-Frade JM; Mellado M; Girard JP; Martínez-A C CCR7-mediated physiological lymphocyte homing involves activation of a tyrosine kinase pathway. Blood, 2003, 101(1), 38–44. 10.1182/blood-2002-03-0841 [DOI] [PubMed] [Google Scholar]
- [117].Willimann K; Legler DF; Loetscher M; Roos RS; Delgado MB; Clark-Lewis I; Baggiolini M; Moser B The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol, 1998, 28(6), 2025–2034. [DOI] [PubMed] [Google Scholar]
- [118].Hedrick JA; Zlotnik A Identification and characterization of a novel beta chemokine containing six conserved cysteines. J. Immunol, 1997, 159(4), 1589–1593. [PubMed] [Google Scholar]
- [119].Nagira M; Imai T; Hieshima K; Kusuda J; Ridanpää M; Takagi S; Nishimura M; Kakizaki M; Nomiyama H; Yoshie O Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J. Biol. Chem, 1997, 272(31), 19518–19524. 10.1074/jbc.272.31.19518 [DOI] [PubMed] [Google Scholar]
- [120].Nagira M; Imai T; Yoshida R; Takagi S; Iwasaki M; Baba M; Tabira Y; Akagi J; Nomiyama H; Yoshie O A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol, 1998, 28(5), 1516–1523. [DOI] [PubMed] [Google Scholar]
- [121].Yoshida R; Nagira M; Kitaura M; Imagawa N; Imai T; Yoshie O Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem, 1998, 273(12), 7118–7122. 10.1074/jbc.273.12.7118 [DOI] [PubMed] [Google Scholar]
- [122].Raju R; Gadakh S; Gopal P; George B; Advani J; Soman S; Prasad TS; Girijadevi R Differential ligand-signaling network of CCL19/CCL21-CCR7 system. Database (Oxford), 2015, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhao B; Cui K; Wang CL; Wang AL; Zhang B; Zhou WY; Zhao WH; Li S The chemotactic interaction between CCL21 and its receptor, CCR7, facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Hepatobiliary Pancreat. Sci, 2011, 18(6), 821–828. 10.1007/s00534-011-0395-4 [DOI] [PubMed] [Google Scholar]
- [124].Zhang L; Wang D; Li Y; Liu Y; Xie X; Wu Y; Zhou Y; Ren J; Zhang J; Zhu H; Su Z CCL21/CCR7 Axis Contributed to CD133+ Pancreatic Cancer Stem-Like Cell Metastasis via EMT and Erk/NF-κB Pathway. PLoS One, 2016, 11(8)e0158529 10.1371/journal.pone.0158529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Keeley EC; Mehrad B; Strieter RM CXC chemokines in cancer angiogenesis and metastases. Adv. Cancer Res, 2010, 106, 91–111. 10.1016/S0065-230X(10)06003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Acharyya S; Oskarsson T; Vanharanta S; Malladi S; Kim J; Morris PG; Manova-Todorova K; Leversha M; Hogg N; Seshan VE; Norton L; Brogi E; Massagué J A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 2012, 150(1), 165–178. 10.1016/j.cell.2012.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sawant KV; Poluri KM; Dutta AK; Sepuru KM; Troshkina A; Garofalo RP; Rajarathnam K Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep, 2016, 6, 33123 10.1038/srep33123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lesina M; Wörmann SM; Morton J; Diakopoulos KN; Korneeva O; Wimmer M; Einwächter H; Sperveslage J; Demir IE; Kehl T; Saur D; Sipos B; Heikenwälder M; Steiner JM; Wang TC; Sansom OJ; Schmid RM; Algül H RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J. Clin. Invest, 2016, 126(8), 2919–2932. 10.1172/JCI86477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Seifert L; Werba G; Tiwari S; Giao Ly NN; Alothman S; Alqunaibit D; Avanzi A; Barilla R; Daley D; Greco SH; Torres-Hernandez A; Pergamo M; Ochi A; Zambirinis CP; Pansari M; Rendon M; Tippens D; Hundeyin M; Mani VR; Hajdu C; Engle D; Miller G The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature, 2016, 532(7598), 245–249. 10.1038/nature17403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Vandercappellen J; Van Damme J; Struyf S The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev, 2011, 22(1), 1–18. 10.1016/j.cytogfr.2010.10.011 [DOI] [PubMed] [Google Scholar]
- [131].Zhang Y; Gao J; Wang X; Deng S; Ye H; Guan W; Wu M; Zhu S; Yu Y; Han W CXCL4 mediates tumor regrowth after chemotherapy by suppression of antitumor immunity. Cancer Biol. Ther, 2015, 16(12), 1775–1783. 10.1080/15384047.2015.1095404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wetterholm E; Linders J; Merza M; Regner S; Thorlacius H Platelet-derived CXCL4 regulates neutrophil infiltration and tissue damage in severe acute pancreatitis. Transl. Res, 2016, 176, 105–118. 10.1016/j.trsl.2016.04.006 [DOI] [PubMed] [Google Scholar]
- [133].Pooran N; Indaram A; Singh P; Bank S Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J. Clin. Gastroenterol, 2003, 37(3), 263–266. 10.1097/00004836-200309000-00013 [DOI] [PubMed] [Google Scholar]
- [134].Matsuo Y; Ochi N; Sawai H; Yasuda A; Takahashi H; Funahashi H; Takeyama H; Tong Z; Guha S CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int. J. Cancer, 2009, 124(4), 853–861. 10.1002/ijc.24040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chen Y; Shi M; Yu GZ; Qin XR; Jin G; Chen P; Zhu MH Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J. Gastroenterol, 2012, 18(10), 1123–1129. 10.3748/wjg.v18.i10.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Liu M; Guo S; Stiles JK The emerging role of CXCL10 in cancer (Review). Oncol. Lett, 2011, 2(4), 583–589. [Review]. 10.3892/ol.2011.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Dyer KD; Percopo CM; Fischer ER; Gabryszewski SJ; Rosenberg HF Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood, 2009, 114(13), 2649–2656. 10.1182/blood-2009-01-199497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lo BK; Yu M; Zloty D; Cowan B; Shapiro J; McElwee KJ CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am. J. Pathol, 2010, 176(5), 2435–2446. 10.2353/ajpath.2010.081059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Luster AD; Ravetch JV Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med, 1987, 166(4), 1084–1097. 10.1084/jem.166.4.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lunardi S; Jamieson NB; Lim SY; Griffiths KL; Carvalho-Gaspar M; Al-Assar O; Yameen S; Carter RC; McKay CJ; Spoletini G; D’Ugo S; Silva MA; Sansom OJ; Janssen KP; Muschel RJ; Brunner TB IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget, 2014, 5(22), 11064–11080. 10.18632/oncotarget.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Deng L; Chen N; Li Y; Zheng H; Lei Q CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim. Biophys. Acta, 2010, 1806(1), 42–49. [DOI] [PubMed] [Google Scholar]
- [142].Liang K; Liu Y; Eer D; Liu J; Yang F; Hu K High CXC Chemokine Ligand 16 (CXCL16) Expression Promotes Proliferation and Metastasis of Lung Cancer via Regulating the NF-κB Pathway. Med. Sci. Monit, 2018, 24, 405–411. 10.12659/MSM.906230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wittel UA; Schmidt AI; Poxleitner PJ; Seifert GJ; Chikhladze S; Puolakkainen P; Hopt UT; Kylänpää L The chemokine ligand CXCL16 is an indicator of bacterial infection in necrotizing pancreatitis. Pancreatcology, 2015, 15(2), 124–130. 10.1016/j.pan.2015.01.004 [DOI] [PubMed] [Google Scholar]
- [144].Wente MN; Gaida MM; Mayer C; Michalski CW; Haag N; Giese T; Felix K; Bergmann F; Giese NA; Friess H Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. Int. J. Oncol, 2008, 33(2), 297–308. [PubMed] [Google Scholar]
- [145].Chalabi-Dchar M; Cassant-Sourdy S; Duluc C; Fanjul M; Lulka H; Samain R; Roche C; Breibach F; Delisle MB; Poupot M; Dufresne M; Shimaoka T; Yonehara S; Mathonnet M; Pyronnet S; Bousquet C Loss of Somatostatin Receptor Subtype 2 Promotes Growth of KRAS-Induced Pancreatic Tumors in Mice by Activating PI3K Signaling and Overexpression of CXCL16. Gastroenterology, 2015, 148(7), 1452–1465. 10.1053/j.gastro.2015.02.009 [DOI] [PubMed] [Google Scholar]
- [146].Manohar M; Verma AK; Upparahalli Venkateshaiah S; Goyal H; Mishra A Food-Induced Acute Pancreatitis. Dig. Dis. Sci, 2017, 62(12), 3287–3297. 10.1007/s10620-017-4817-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ino Y; Yamazaki-Itoh R; Shimada K; Iwasaki M; Kosuge T; Kanai Y; Hiraoka N Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer, 2013, 108(4), 914–923. 10.1038/bjc.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Awla D; Abdulla A; Zhang S; Roller J; Menger MD; Regnér S; Thorlacius H Lymphocyte function antigen-1 regulates neutrophil recruitment and tissue damage in acute pancreatitis. Br. J. Pharmacol, 2011, 163(2), 413–423. 10.1111/j.1476-5381.2011.01225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Griesmann H; Drexel C; Milosevic N; Sipos B; Rosendahl J; Gress TM; Michl P Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut, 2017, 66(7), 1278–1285. 10.1136/gutjnl-2015-310049 [DOI] [PubMed] [Google Scholar]
- [150].Shevchenko I; Karakhanova S; Soltek S; Link J; Bayry J; Werner J; Umansky V; Bazhin AV Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int. J. Cancer, 2013, 133(1), 98–107. 10.1002/ijc.27990 [DOI] [PubMed] [Google Scholar]
- [151].Bedrosian AS; Nguyen AH; Hackman M; Connolly MK; Malhotra A; Ibrahim J; Cieza-Rubio NE; Henning JR; Barilla R; Rehman A; Pachter HL; Medina-Zea MV; Cohen SM; Frey AB; Acehan D; Miller G Dendritic cells promote pancreatic viability in mice with acute pancreatitis. Gastroenterology, 2011, 141(5), 1915–26 e1–14. 10.1053/j.gastro.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ochi A; Nguyen AH; Bedrosian AS; Mushlin HM; Zarbakhsh S; Barilla R; Zambirinis CP; Fallon NC; Rehman A; Pylayeva-Gupta Y; Badar S; Hajdu CH; Frey AB; Bar-Sagi D; Miller G MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J. Exp. Med, 2012, 209(9), 1671–1687. 10.1084/jem.20111706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu CY; Xu JY; Shi XY; Huang W; Ruan TY; Xie P; Ding JL M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Invest, 2013, 93(7), 844–854. 10.1038/labinvest.2013.69 [DOI] [PubMed] [Google Scholar]
- [154].Kaneda MM; Cappello P; Nguyen AV; Ralainirina N; Hardamon CR; Foubert P; Schmid MC; Sun P; Mose E; Bouvet M; Lowy AM; Valasek MA; Sasik R; Novelli F; Hirsch E; Varner JA Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer Discov, 2016, 6(8), 870–885. 10.1158/2159-8290.CD-15-1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Esposito I; Friess H; Kappeler A; Shrikhande S; Kleeff J; Ramesh H; Zimmermann A; Büchler MW Mast cell distribution and activation in chronic pancreatitis. Hum. Pathol, 2001, 32(11), 1174–1183. 10.1053/hupa.2001.28947 [DOI] [PubMed] [Google Scholar]
- [156].Demir IE; Schorn S; Schremmer-Danninger E; Wang K; Kehl T; Giese NA; Algül H; Friess H; Ceyhan GO Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One, 2013, 8(3)e60529 10.1371/journal.pone.0060529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lopez-Font I; Gea-Sorlí S; de-Madaria E; Gutiérrez LM; Pérez-Mateo M; Closa D Pancreatic and pulmonary mast cells activation during experimental acute pancreatitis. World J. Gastroenterol, 2010, 16(27), 3411–3417. 10.3748/wjg.v16.i27.3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Strouch MJ; Cheon EC; Salabat MR; Krantz SB; Gounaris E; Melstrom LG; Dangi-Garimella S; Wang E; Munshi HG; Khazaie K; Bentrem DJ Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin. Cancer Res, 2010, 16(8), 2257–2265. 10.1158/1078-0432.CCR-09-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Karamitopoulou E; Shoni M; Theoharides TC Increased number of non-degranulated mast cells in pancreatic ductal adenocarcinoma but not in acute pancreatitis. Int. J. Immunopathol. Pharmacol, 2014, 27(2), 213–220. 10.1177/039463201402700208 [DOI] [PubMed] [Google Scholar]
- [160].Longo V; Tamma R; Brunetti O; Pisconti S; Argentiero A; Silvestris N; Ribatti D Mast cells and angiogenesis in pancreatic ductal adenocarcinoma. Clin. Exp. Med, 2018, 18(3), 319–323. 10.1007/s10238-018-0493-6 [DOI] [PubMed] [Google Scholar]
- [161].Abdulla A; Awla D; Thorlacius H; Regnér S Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J. Leukoc. Biol, 2011, 90(5), 975–982. 10.1189/jlb.0411195 [DOI] [PubMed] [Google Scholar]
- [162].Leppkes M; Maueröder C; Hirth S; Nowecki S; Günther C; Billmeier U; Paulus S; Biermann M; Munoz LE; Hoffmann M; Wildner D; Croxford AL; Waisman A; Mowen K; Jenne DE; Krenn V; Mayerle J; Lerch MM; Schett G; Wirtz S; Neurath MF; Herrmann M; Becker C Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat. Commun, 2016, 7, 10973 10.1038/ncomms10973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Chakraborty S; Kaur S; Muddana V; Sharma N; Wittel UA; Papachristou GI; Whitcomb D; Brand RE; Batra SK Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am. J. Gastroenterol, 2010, 105(9), 2050–2059. 10.1038/ajg.2010.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lippi G; Meschi T; Nouvenne A; Mattiuzzi C; Borghi L Neutrophil gelatinase-associated lipocalin in cancer. Adv. Clin. Chem, 2014, 64, 179–219, 179-219 10.1016/B978-0-12-800263-6.00004-5 [DOI] [PubMed] [Google Scholar]
- [165].Grosse-Steffen T; Giese T; Giese N; Longerich T; Schirmacher P; Hänsch GM; Gaida MM Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin. Dev. Immunol, 2012, 2012720768 10.1155/2012/720768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Rigoni A; Colombo MP; Pucillo C Mast cells, basophils and eosinophils: From allergy to cancer. Semin. Immunol, 2018, 35, 29–34. 10.1016/j.smim.2018.02.001 [DOI] [PubMed] [Google Scholar]
- [167].Yanagawa M; Uchida K; Ando Y; Tomiyama T; Yamaguchi T; Ikeura T; Fukui T; Nishio A; Uemura Y; Miyara T; Okamoto H; Satoi S; Okazaki K Basophils activated via TLR signaling may contribute to pathophysiology of type 1 autoimmune pancreatitis. J. Gastroenterol, 2018, 53(3), 449–460. 10.1007/s00535-017-1390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].De Monte L; Wörmann S; Brunetto E; Heltai S; Magliacane G; Reni M; Paganoni AM; Recalde H; Mondino A; Falconi M; Aleotti F; Balzano G; Algül H; Doglioni C; Protti MP Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res, 2016, 76(7), 1792–1803. 10.1158/0008-5472.CAN-15-1801-T [DOI] [PubMed] [Google Scholar]
- [169].Zhang ML; Jiang YF; Wang XR; Ding LL; Wang HJ; Meng QQ; Gao PJ Different phenotypes of monocytes in patients with new-onset mild acute pancreatitis. World J. Gastroenterol, 2017, 23(8), 1477–1488. 10.3748/wjg.v23.i8.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Reppucci J; Chang M; Hughes S; Liu X Eosinophilic Pancreatitis: A Rare Cause of Recurrent Acute Pancreatitis. Case Rep. Gastroenterol, 2017, 11(1), 120–126. 10.1159/000457788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tian L; Fu P; Dong X; Qi J; Zhu H Eosinophilic pancreatitis: Three case reports and literature review. Mol. Clin. Oncol, 2016, 4(4), 559–562. 10.3892/mco.2016.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ibrahim U; Asti D; Saqib A; Mudduluru BM; Ayaz S; Odaimi M Eosinophilia as the presenting sign in pancreatic cancer: an extremely rare occurrence. Postgrad. Med, 2017, 129(3), 399–401. 10.1080/00325481.2017.1246345 [DOI] [PubMed] [Google Scholar]
- [173].Soares KC; Rucki AA; Wu AA; Olino K; Xiao Q; Chai Y; Wamwea A; Bigelow E; Lutz E; Liu L; Yao S; Anders RA; Laheru D; Wolfgang CL; Edil BH; Schulick RD; Jaffee EM; Zheng L PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J. Immunother, 2015, 38(1), 1–11. 10.1097/CJI.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Winograd R; Byrne KT; Evans RA; Odorizzi PM; Meyer AR; Bajor DL; Clendenin C; Stanger BZ; Furth EE; Wherry EJ; Vonderheide RH Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol. Res, 2015, 3(4), 399–411. 10.1158/2326-6066.CIR-14-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Miyoshi H; Uchida K; Taniguchi T; Yazumi S; Matsushita M; Takaoka M; Okazaki K Circulating naïve and CD4+CD25high regulatory T cells in patients with autoimmune pancreatitis. Pancreas, 2008, 36(2), 133–140. 10.1097/MPA.0b013e3181577553 [DOI] [PubMed] [Google Scholar]
- [176].Demols A; Le Moine O; Desalle F; Quertinmont E; Van Laethem JL; Devière J CD4(+ )T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology, 2000, 118(3), 582–590. 10.1016/S0016-5085(00)70265-4 [DOI] [PubMed] [Google Scholar]
- [177].Bayne LJ; Beatty GL; Jhala N; Clark CE; Rhim AD; Stanger BZ; Vonderheide RH Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell, 2012, 21(6), 822–835. 10.1016/j.ccr.2012.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Hunger RE; Mueller C; Z’graggen K; Friess H; Büchler MW Cytotoxic cells are activated in cellular infiltrates of alcoholic chronic pancreatitis. Gastroenterology, 1997, 112(5), 1656–1663. 10.1016/S0016-5085(97)70048-9 [DOI] [PubMed] [Google Scholar]
- [179].Janakiram NB; Mohammed A; Bryant T; Ritchie R; Stratton N; Jackson L; Lightfoot S; Benbrook DM; Asch AS; Lang ML; Rao CV Loss of natural killer T cells promotes pancreatic cancer in LSL-KrasG12D/+ mice. Immunology, 2017, 152(1), 36–51. 10.1111/imm.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Uchida K; Kusuda T; Koyabu M; Miyoshi H; Fukata N; Sumimoto K; Fukui Y; Sakaguchi Y; Ikeura T; Shimatani M; Fukui T; Matsushita M; Takaoka M; Nishio A; Okazaki K Regulatory T cells in type 1 autoimmune pancreatitis. Int. J. Rheumatol, 2012, 2012795026 10.1155/2012/795026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Jang JE; Hajdu CH; Liot C; Miller G; Dustin ML; Bar-Sagi D Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep, 2017, 20(3), 558–571. 10.1016/j.celrep.2017.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Pylayeva-Gupta Y; Das S; Handler JS; Hajdu CH; Coffre M; Koralov SB; Bar-Sagi D IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov, 2016, 6(3), 247–255. 10.1158/2159-8290.CD-15-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Lee KE; Spata M; Bayne LJ; Buza EL; Durham AC; Allman D; Vonderheide RH; Simon MC Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov, 2016, 6(3), 256–269. 10.1158/2159-8290.CD-15-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Sumimoto K; Uchida K; Kusuda T; Mitsuyama T; Sakaguchi Y; Fukui T; Matsushita M; Takaoka M; Nishio A; Okazaki K The role of CD19+ CD24high CD38high and CD19+ CD24high CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis. Pancreatology, 2014, 14(3), 193–200. 10.1016/j.pan.2014.02.004 [DOI] [PubMed] [Google Scholar]
- [185].Chen X; Wang L; Zhang L; Zhao C IgG4-related Autoimmune Pancreatitis Mimicking Acute Pancreatitis: A Case Report. Chin. Med. Sci. J, 2017, 32(1), 65–68. 10.24920/J1001-9242.2007.009 [DOI] [PubMed] [Google Scholar]
- [186].Sendler M; Beyer G; Mahajan UM; Kauschke V; Maertin S; Schurmann C; Homuth G; Volker U; Volzke H; Halangk W; Wartmann T; Weiss FU; Hegyi P; Lerch MM; Mayerle J Complement Component 5 Mediates Development of Fibrosis, via Activation of Stellate Cells, in 2 Mouse Models of Chronic Pancreatitis. Gastroenterology, 2015, 149(3), 765–76 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chen J; Wu W; Zhen C; Zhou H; Yang R; Chen L; Hu L Expression and clinical significance of complement C3, complement C4b1 and apolipoprotein E in pancreatic cancer. Oncol. Lett, 2013, 6(1), 43–48. 10.3892/ol.2013.1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Farrow B; Sugiyama Y; Chen A; Uffort E; Nealon W; Mark Evers B Inflammatory mechanisms contributing to pancreatic cancer development. Ann. Surg, 2004, 239(6), 763–769. 10.1097/01.sla.0000128681.76786.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Fu Q; Zhai Z; Wang Y; Xu L; Jia P; Xia P; Liu C; Zhang X; Qin T; Zhang H NLRP3 Deficiency Alleviates Severe Acute Pancreatitis and Pancreatitis-Associated Lung Injury in a Mouse Model. BioMed Res. Int, 2018, 20181294951 10.1155/2018/1294951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Ethridge RT; Hashimoto K; Chung DH; Ehlers RA; Rajaraman S; Evers BM Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J. Am. Coll. Surg, 2002, 195(4), 497–505. 10.1016/S1072-7515(02)01222-X [DOI] [PubMed] [Google Scholar]
- [191].Satoh A; Shimosegawa T; Fujita M; Kimura K; Masamune A; Koizumi M; Toyota T Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut, 1999, 44(2), 253–258. 10.1136/gut.44.2.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Kanak MA; Shahbazov R; Yoshimatsu G; Levy MF; Lawrence MC; Naziruddin B A small molecule inhibitor of NFκB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J. Gastroenterol, 2017, 52(3), 352–365. 10.1007/s00535-016-1238-5 [DOI] [PubMed] [Google Scholar]
- [193].Singh S; Srivastava SK; Bhardwaj A; Owen LB; Singh AP CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br. J. Cancer, 2010, 103(11), 1671–1679. 10.1038/sj.bjc.6605968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Mori T; Doi R; Koizumi M; Toyoda E; Ito D; Kami K; Masui T; Fujimoto K; Tamamura H; Hiramatsu K; Fujii N; Imamura M CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol. Cancer Ther, 2004, 3(1), 29–37. 10.1186/1476-4598-3-29 [DOI] [PubMed] [Google Scholar]
- [195].Balic A; Sørensen MD; Trabulo SM; Sainz B, Jr; Cioffi M; Vieira CR; Miranda-Lorenzo I; Hidalgo M; Kleeff J; Erkan M; Heeschen C Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol. Cancer Ther, 2014, 13(7), 1758–1771. 10.1158/1535-7163.MCT-13-0948 [DOI] [PubMed] [Google Scholar]
- [196].Daley D; Mani VR; Mohan N; Akkad N; Pandian GSDB; Savadkar S; Lee KB; Torres-Hernandez A; Aykut B; Diskin B; Wang W; Farooq MS; Mahmud AI; Werba G; Morales EJ; Lall S; Wadowski BJ; Rubin AG; Berman ME; Narayanan R; Hundeyin M; Miller G NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J. Exp. Med, 2017, 214(6), 1711–1724. 10.1084/jem.20161707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Andoh A; Takaya H; Saotome T; Shimada M; Hata K; Araki Y; Nakamura F; Shintani Y; Fujiyama Y; Bamba T Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology, 2000, 119(1), 211–219. 10.1053/gast.2000.8538 [DOI] [PubMed] [Google Scholar]
- [198].Hosoi F; Izumi H; Kawahara A; Murakami Y; Kinoshita H; Kage M; Nishio K; Kohno K; Kuwano M; Ono M N-myc downstream regulated gene 1/Cap43 suppresses tumor growth and angiogenesis of pancreatic cancer through attenuation of inhibitor of kappaB kinase beta expression. Cancer Res, 2009, 69(12), 4983–4991. 10.1158/0008-5472.CAN-08-4882 [DOI] [PubMed] [Google Scholar]
- [199].Kayali AG; Lopez AD; Hao E; Hinton A; Hayek A; King CC The SDF-1α/CXCR4 axis is required for proliferation and maturation of human fetal pancreatic endocrine progenitor cells. PLoS One, 2012, 7(6)e38721 10.1371/journal.pone.0038721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Gong J; Meng HB; Hua J; Song ZS; He ZG; Zhou B; Qian MP The SDF-1/CXCR4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol. Med. Rep, 2014, 9(5), 1575–1582. 10.3892/mmr.2014.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Balkwill F The significance of cancer cell expression of the chemokine receptor CXCR4. Semin. Cancer Biol, 2004, 14(3), 171–179. 10.1016/j.semcancer.2003.10.003 [DOI] [PubMed] [Google Scholar]
- [202].Billadeau DD; Chatterjee S; Bramati P; Sreekumar R; Shah V; Hedin K; Urrutia R Characterization of the CXCR4 signaling in pancreatic cancer cells. Int. J. Gastrointest. Cancer, 2006, 37(4), 110–119. [DOI] [PubMed] [Google Scholar]
- [203].Wehler T; Wolfert F; Schimanski CC; Gockel I; Herr W; Biesterfeld S; Seifert JK; Adwan H; Berger MR; Junginger T; Galle PR; Moehler M Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol. Rep, 2006, 16(6), 1159–1164. 10.3892/or.16.6.1159 [DOI] [PubMed] [Google Scholar]
- [204].Wang Z; Ma Q; Liu Q; Yu H; Zhao L; Shen S; Yao J Blockade of SDF-1/CXCR4 signalling inhibits pancreatic cancer progression in vitro via inactivation of canonical Wnt pathway. Br. J. Cancer, 2008, 99(10), 1695–1703. 10.1038/sj.bjc.6604745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Tamiya T; Kashiwagi I; Takahashi R; Yasukawa H; Yoshimura A Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler. Thromb. Vasc. Biol, 2011, 31(5), 980–985. 10.1161/ATVBAHA.110.207464 [DOI] [PubMed] [Google Scholar]
- [206].Inagaki-Ohara K; Kondo T; Ito M; Yoshimura A SOCS, inflammation, and cancer. JAK-STAT, 2013, 2(3)e24053 10.4161/jkst.24053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wang L; Mehta S; Brock M; Gill SE Inhibition of Murine Pulmonary Microvascular Endothelial Cell Apoptosis Promotes Recovery of Barrier Function under Septic Conditions. Mediators Inflamm, 2017, 20173415380 10.1155/2017/3415380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Huang L; Hu B; Ni J; Wu J; Jiang W; Chen C; Yang L; Zeng Y; Wan R; Hu G; Wang X Transcriptional repression of SOCS3 mediated by IL-6/STAT3 signaling via DNMT1 promotes pancreatic cancer growth and metastasis. J. Exp. Clin. Cancer Res, 2016, 35, 27 10.1186/s13046-016-0301-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [209].Gao L; Lu GT; Lu YY; Xiao WM; Mao WJ; Tong ZH; Yang N; Li BQ; Yang Q; Ding YB; Li WQ Diabetes aggravates acute pancreatitis possibly via activation of NLRP3 inflammasome in db/db mice. Am. J. Transl. Res, 2018, 10(7), 2015–2025. [PMC free article] [PubMed] [Google Scholar]
- [210].Hoque R; Sohail M; Malik A; Sarwar S; Luo Y; Shah A; Barrat F; Flavell R; Gorelick F; Husain S; Mehal W TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology, 2011, 141(1), 358–369. 10.1053/j.gastro.2011.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kandikattu HK; Upparahalli Venkateshaiah S; Mishra A Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev, 2019, 47, 83–98. 10.1016/j.cytogfr.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kandikattu HK; Rachitha P; Jayashree GV; Krupashree K; Sukhith M; Majid A; Amruta N; Khanum F Antiinflammatory and anti-oxidant effects of Cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF-MS. Biomed. Pharmacother, 2017, 91, 191–201. 10.1016/j.biopha.2017.04.049 [DOI] [PubMed] [Google Scholar]
- [213].Huang H; Liu Y; Daniluk J; Gaiser S; Chu J; Wang H; Li ZS; Logsdon CD; Ji B Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology, 2013, 144(1), 202–210. 10.1053/j.gastro.2012.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Guo X; Zheng L; Jiang J; Zhao Y; Wang X; Shen M; Zhu F; Tian R; Shi C; Xu M; Li X; Peng F; Zhang H; Feng Y; Xie Y; Xu X; Jia W; He R; Xie C; Hu J; Ye D; Wang M; Qin R Blocking NF-κB Is Essential for the Immunotherapeutic Effect of Recombinant IL18 in Pancreatic Cancer. Clin. Cancer Res, 2016, 22(23), 5939–5950. 10.1158/1078-0432.CCR-15-1144 [DOI] [PubMed] [Google Scholar]
- [215].Röder PV; Wu B; Liu Y; Han W Pancreatic regulation of glucose homeostasis. Exp. Mol. Med, 2016, 48e219 10.1038/emm.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Leung PS; Ip SP Pancreatic acinar cell: its role in acute pancreatitis. Int. J. Biochem. Cell Biol, 2006, 38(7), 1024–1030. 10.1016/j.biocel.2005.12.001 [DOI] [PubMed] [Google Scholar]
- [217].Bockman DE Morphology of the exocrine pancreas related to pancreatitis.Microsc. Res. Tech, 1997, 37(5–6), 509–519. [DOI] [PubMed] [Google Scholar]
- [218].Williams JA Regulation of acinar cell function in the pancreas. Curr. Opin. Gastroenterol, 2010, 26(5), 478–483. 10.1097/MOG.0b013e32833d11c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Mareninova OA; Sendler M; Malla SR; Yakubov I; French SW; Tokhtaeva E; Vagin O; Oorschot V; Lüllmann-Rauch R; Blanz J; Dawson D; Klumperman J; Lerch MM; Mayerle J; Gukovsky I; Gukovskaya AS Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell. Mol. Gastroenterol. Hepatol, 2015, 1(6), 678–694. 10.1016/j.jcmgh.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Algül H; Treiber M; Lesina M; Nakhai H; Saur D; Geisler F; Pfeifer A; Paxian S; Schmid RM Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J. Clin. Invest, 2007, 117(6), 1490–1501. 10.1172/JCI29882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Hessmann E; Zhang JS; Chen NM; Hasselluhn M; Liou GY; Storz P; Ellenrieder V; Billadeau DD; Koenig A NFATc4 Regulates Sox9 Gene Expression in Acinar Cell Plasticity and Pancreatic Cancer Initiation. Stem Cells Int, 2016, 20165272498 10.1155/2016/5272498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Storz P Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol, 2017, 14(5), 296–304. 10.1038/nrgastro.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Schludi B; Moin ASM; Montemurro C; Gurlo T; Matveyenko AV; Kirakossian D; Dawson DW; Dry SM; Butler PC; Butler AE Islet inflammation and ductal proliferation may be linked to increased pancreatitis risk in type 2 diabetes. JCI Insight, 2017, 2(13), 92282 10.1172/jci.insight.92282 PMID: 28679961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Stark A; Eibl G Pancreatic Ductal Adenocarcinoma. Pancreapedia; Exocrine Pancreas Knowledge Base, 2015.
- [225].Gomez-Chou SB; Swidnicka-Siergiejko AK; Badi N; Chavez-Tomar M; Lesinski GB; Bekaii-Saab T; Farren MR; Mace TA; Schmidt C; Liu Y; Deng D; Hwang RF; Zhou L; Moore T; Chatterjee D; Wang H; Leng X; Arlinghaus RB; Logsdon CD; Cruz-Monserrate Z Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res, 2017, 77(10), 2647–2660. 10.1158/0008-5472.CAN-16-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Apte MV; Haber PS; Applegate TL; Norton ID; McCaughan GW; Korsten MA; Pirola RC; Wilson JS Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut, 1998, 43(1), 128–133. 10.1136/gut.43.1.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Phillips PA; Yang L; Shulkes A; Vonlaufen A; Poljak A; Bustamante S; Warren A; Xu Z; Guilhaus M; Pirola R; Apte MV; Wilson JS Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA, 2010, 107(40), 17397–17402. 10.1073/pnas.1000359107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Omary MB; Lugea A; Lowe AW; Pandol SJ The pancreatic stellate cell: a star on the rise in pancreatic diseases. J. Clin. Invest, 2007, 117(1), 50–59. 10.1172/JCI30082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Mews P; Phillips P; Fahmy R; Korsten M; Pirola R; Wilson J; Apte M Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut, 2002, 50(4), 535–541. 10.1136/gut.50.4.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Zambirinis CP; Levie E; Nguy S; Avanzi A; Barilla R; Xu Y; Seifert L; Daley D; Greco SH; Deutsch M; Jonnadula S; Torres-Hernandez A; Tippens D; Pushalkar S; Eisenthal A; Saxena D; Ahn J; Hajdu C; Engle DD; Tuveson D; Miller G TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med, 2015, 212(12), 2077–2094. 10.1084/jem.20142162 [DOI] [PMC free article] [PubMed] [Google Scholar]


