Abstract
Quantitative data on female external genital morphology are sporadic in the primate literature, and the intra-specific and inter-female variation is especially under investigated (e.g., external clitoris length). Since in most anthropoid primate species female external genitals are relatively small and often hidden, for those species whose external clitoris is described as hypertrophic, external genital resemblance may represent a source of confusion in distinguishing the sexes at a distance. This is the case of both captive and wild Sapajus apella infants. We provided data on external clitoral length and investigated differences in this trait at different ages in a captive female tufted capuchin population. Since likely allometric growth describes changes in relative dimensions of parts of the body that are correlated with changes in overall size, clitoris length has been analyzed by using body weight as a covariate. We measured clitoral length by adapting a technique developed for spotted hyenas (Crocuta crocuta). Our results suggest that the small body size may be only in part responsible of the perception of long clitoris in female infants, since the clitoris is actually longer in immature females compared to adult ones and its size is inversely related to body weight. While the cross-sectional nature of these data does not allow for conclusive interpretation of the results, we tentatively suggest this phenomenon as a transient male-mimicry by immature females. Our study contributed to the description of normative data in a clitoral trait, thus providing foundation for future studies about causal mechanisms and possible adaptive function(s).
Keywords: negative allometry, immature sexual mimicry, external clitoris
Graphical Abstract
INTRODUCTION
First described in early papers (e.g., Gerhardt, 1909; Pehrson, 1914), much of our understanding of non-human primate female external genital appearance has stemmed from research conducted primarily in the first half of 20th century. Early papers qualitatively characterized external genital variability in shape, size, length, and color in various primate species and provided detailed descriptions and illustrations, mostly emphasizing inter-sexual and interspecific differences (e.g., Pocock, 1918, 1920, 1925; Hill 1933, 1953, 1958, 1960, 1962; Harms, 1956; Hershkovitz, 1977). Quantitative data on female external genital morphology, however, are sporadic, and the intra-specific and inter-female variation is particularly under investigated (e.g., external clitoris size, but see qualitative observations in Ateles, Campbell and Gibson, 2008). The only quantitative data we are aware of are those by Drea and Weil (2008) who obtained a set of external genital measurements (external clitoris length, width, diameter and circumference; meatus length and distance; anogenital distance) in captive adult Lemur catta, and Goldschmidt and colleagues (2009) who measured external clitoris length in captive Macaca mulatta at different age classes.
The external clitoris of tufted capuchins (Sapajus apella) consists of a shaft that is ventrally grooved to the tip with the urethral opening at its base (Hill, 1960). The apex of the shaft slightly expands into a glans clitoridis, which is normally flattened and button-like shaped. When relaxed, the clitoris is enveloped in a preputial fold, which originates from the labia minora. However, when fully erect the clitoris becomes more visible. The base of the clitoris and the skin surrounding the vagina consist of darkly pigmented sparsely haired papillated tissue which corresponds to the development of the labioscrotal swelling into labia majora (Wislocki, 1936, Hill, 1960; Lima et al., 2015). Scientific literature describes the clitoris of tufted capuchins as resembling male penis (Cebus spp.: “…it is more penis-like” in Pocock, 1920; “The clitoris is shaped very much like the penis…” in Hill, 1960; C. apella: “…resembles the penis of male individuals” in Lima et al., 2015; “…the clitoris is developed like the penis” in Teixeira et al., 2015). In fact, external genital resemblance may, in some species and at different ages, represent a source of confusion in distinguishing the sexes at a distance (e.g., strepsirrhines Ankel-Simons, 1983; juvenile Alouatta palliata, Wislocki, 1936; Clarke, 1990, Clarke et al., 2007). This is what has been described in wild and captive tufted capuchins, whose infants have often been inaccurately sexed by human observers (Hill, 1960; Fragaszy et al., 2004; pers. obs.). In this species, however, inaccurate sexing is apparently limited to infancy, prompting us to question if the greater visibility of the clitoris in immature individuals (which is the cause for the reported confusion) might simply be due to different body proportions at different ages.
The insight that changes in proportion, as related to the growth of body size, are a major factor in evolution, has been especially significant for the studies on the relative growth of organs (Klingenberg, 1996). The growth of body parts at different rates, and the resulting variations in body proportions (i.e., allometry), can be considered with respect to either evolution (i.e., comparing adult individuals of related species) or development (i.e., comparing individuals of the same species and different age and size) (Huxley, 1932, Bertalanffy & Pirozynski, 1952, Gould, 1966). Three main types of allometry have been distinguished, such as static (size allometry, i.e., variation among same age individuals belonging to the same population), evolutionary (covariation of changes in different traits along phylogenetic branches), and ontogenetic allometry (Cock, 1966). The latter (growth allometry, Huxley & Tessier 1936), potentially interesting for the present study, deals with covariation among characters during growth. In this case, when longitudinal data on the same individuals along developmental stages cannot be obtained, data on different specimens at different ages (i. e., cross-sectional data) are used (Cock, 1966; Klingenberg, 1996). In fact, cross-sectional data are routinely used for estimating a population’s ontogenetic trajectory or average growth pattern (see Smith et al., 1994; Leigh, 2006; Mitteroecker et al., 2013).
Considering the lack of normative data regarding external clitoris size in tufted capuchins, and based on the difficulty in sexing infants at a distance (see Fig. 1), this study firstly aims to establish normal standards for the external clitoris length, by using standardized measurements in a sample of captive S. apella at different ages. Secondly, by using cross-sectional data, we aim to determine if a negative (ontogenetic) allometry between body size and clitoris length can provide an explanation for the clitoris being less visible at later stages in life.
Figure 1.
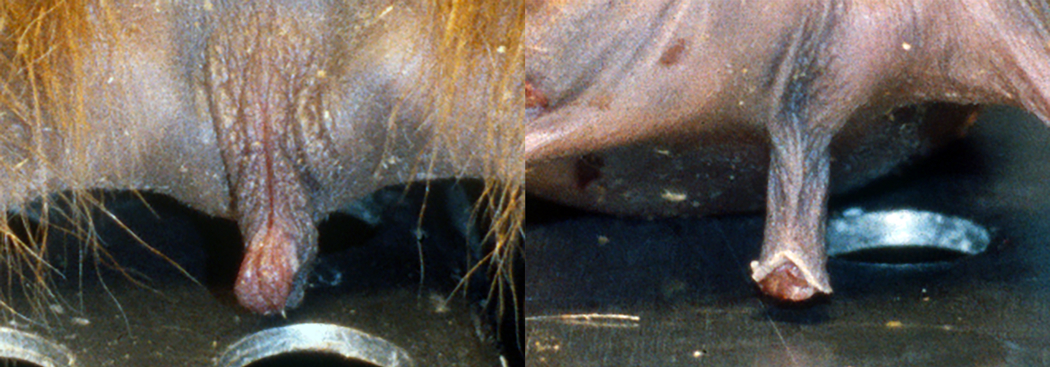
External genitals in newborn twins: a, female, and b, male
METHODS
Animal Subjects
The tufted capuchin females contributing to this study (N=24 ranging in age from 2 days to 38 years) were all housed at the National Institutes of Health (Animal Center, NICHD, Poolesville, MD) (Tab. 1). Animals were members of different social groups living in different housing conditions according to their group size (a large social group, N=26 individuals housed in an outdoor corncrib from May to October and in indoor runs from November to April; two smaller social groups, N=6 individuals, housed either in indoor runs from May to October and indoor cages from November to April, or in indoor cages year round). All animals were fed Purina New World Monkey chow twice a day, various types of fruits and nuts once a day, and had continuous access to water. Different subsets of the animal sample were used for the clitoral study.
Tab 1.
Female individuals, age at measurement and number of clitoral length measurements.
| Clitoris | |||
|---|---|---|---|
| Females | Age at measurement yrs. or mos. | No. measurements | |
| 1st | last | ||
| Lychee | 4 mos | 1.75 | 4 |
| Mara | 5 mos | 11 mos | 2 |
| Liv | 6 mos | 1.83 | 4 |
| Sam | 9 mos | 2.08 | 4 |
| Ivory | 1.08 | 2.5 | 4 |
| Lyla | 2.33 | 3.97 | 4 |
| Sagan | 2.92 | 4.25 | 4 |
| Claire | 3.17 | 4.5 | 4 |
| Spider | 3.25 | 4.25 | 3 |
| Shannon | 4.5 | 5.47 | 3 |
| Cathead | 5.25 | 5.66 | 4 |
| Magic | 5.58 | 6.97 | 4 |
| Lorena | 5.58 | 6.97 | 4 |
| Mocha | 6.83 | 8.25 | 4 |
| Lee | 8.25 | 9.58 | 4 |
| Lucy† | 13.0 | 15.0 | (1) |
| Suki | 13.58 | 14.97 | 4 |
| Jasmine | 15.0 | 16.0 | 5 |
| Shinade† | 15.2 | 16.6 | (1) |
| Carlina | 16.83 | 18.08 | 5 |
| Isabella | 18.0 | 20.0 | 5 |
| Coco | 36.0 | 38.0 | 3 |
| TOTAL | 80 | ||
= two females whose clitoral measurements could not be performed due to the extreme short clitoris, however contributing to analyses with a length set by default at 1mm
Clitoral length measurements.
During routine semi-annual TB testing at the Animal Center (NICHD, Poolesville, MD), animals (N=22, aged 4 months to 38 years, tested between December 1999- May 2001) were immobilized via intra-muscular injections of a combination of hydrochloride ketamine and acepromamzine (10 to 15 mg/kg depending on body weight). Following immobilization, we recorded body weight and clitoral length for each subject (see below). Each anesthetized monkey was placed in supine position, with the animal’s legs spread apart. Clitoral length was measured via a modified version of a technique developed for clitoral measurements in spotted hyenas (Crocuta crocuta) by Drea and collaborators (1998). A metal washer (4 cm in diameter, with an internal hole of 1 cm in diameter) was placed over the clitoris and the clitoris pulled through the hole in the washer. A “noose,” made of a loop of dental floss with a weight attached to one end, was tied under the glans clitoridis after a gentle extrusion from the prepuce and pulled taught. The washer was stabilized against the pubic bone, providing a standardized reference point for measurement. The floss was then hung over a PVC pipe (at about 50cm from the animal’s body) with the weight dangling freely, thereby pulling the clitoris with a standardized tension corresponding to the weight. After experimenting with various weights, we found that 36g provided the least amount of tension necessary to fully extend the clitoris for accurate measurement. The extended clitoris was then measured (from base to end of glans) by placing a ruler at a right angle on the washer and holding the floss parallel to the ruler (Fig. 2 a, b, c). Two measurements were obtained for each subject by two observers (MC and AU) and averages were later used for analyses. Each female subject was measured during a minimum of 2 to a maximum of 6 TB testing therefore contributing differently to the data set. For two adult females, whose clitoris was too short to fit the washer and could not be measured, clitoris length was set to a default of 1mm (Tab. 1, Fig. 2 d). Although clitoral measurements were obtained with standardized method and weight for all subjects, an uneven response to a given tension by different sized clitorises and/or different tissue elasticity at different ages might be expected however it was unquantifiable. Additionally, due to the tension applied, clitoral length data can neither reflect S. apella actual clitoris length nor be used for interspecific comparisons, but exclusively for comparisons in this study at the interindividual level.
Figure 2.
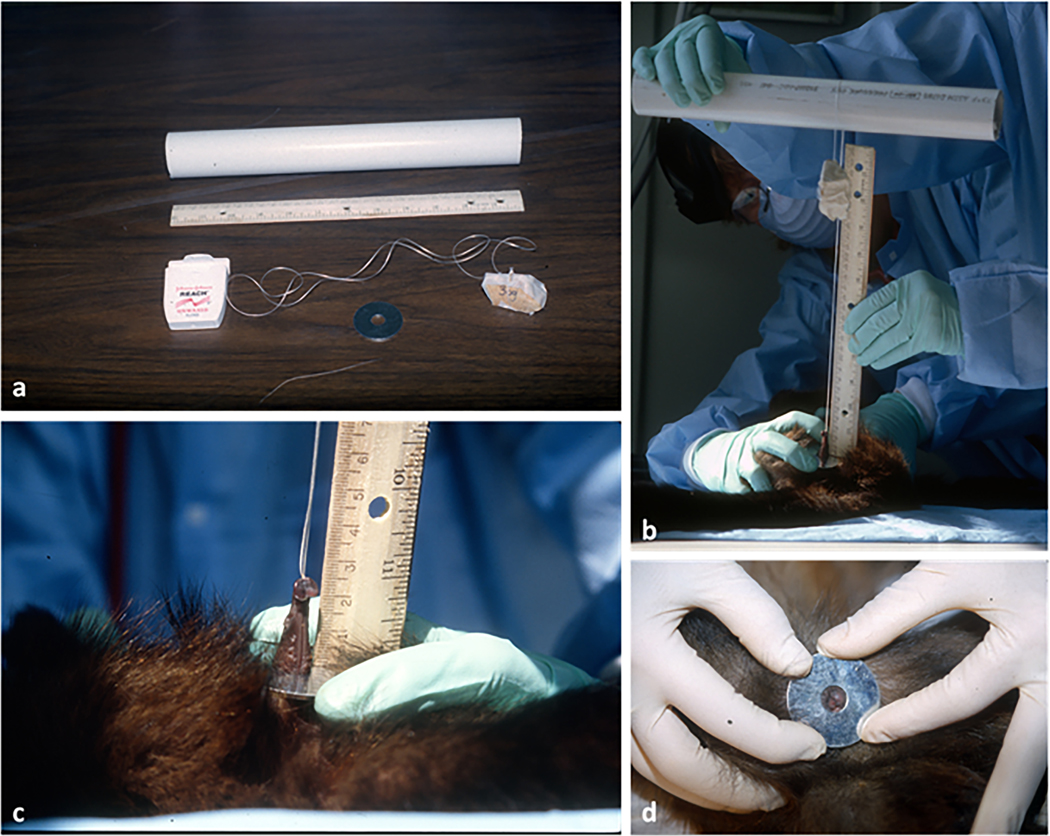
Method used for clitoral measurements (modified from technique developed by Drea et al., 1998 to measure clitoral length in spotted hyenas, Crocuta crocuta). a: materials used (PVC pipe, dental floss, 36g stone; washer); b: clitoral measurements (see text for details); c: close up of clitoral measurement (female Magic, 5 yrs. old); d: adult female with an extremely short clitoris which could not be measured by using this technique (female Lucy, DOB not known, not less than 13 yrs. old)
Ethical Note
Protocol and procedures employed in this study were ethically reviewed and approved and the study was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (approved Animal Study Proposal ASP 9#98 024). We also adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates.
Statistical analysis
We divided our sample in three age classes (infants [I], 0–1 year old; juveniles [JUV], 1.1–4 years old; adults [A], 4.1–38 years old). Transition from infancy to juvenile stage was set at 1 year (age at weaning, Fragaszy & Adam-Curtis, 1998) whereas transition to maturity was set at the age of first conception (4 years) based on youngest age at first delivery reported in this species (about 4.5 years) and duration of gestation (160 days, Fragaszy & Adam-Curtis, 1998). In order to verify the transition between the juvenile and the mature phase in our animal sample, female body weight data were plotted against age (x-axis). We investigated the relationships between clitoral length at age groups first by ANOVA (as done by Hawkins et al., 2002 and Goldschmidt et al., 2009) and second by ANCOVA, using body weight as a covariate. For significant ANOVA outputs, we performed planned contrast analyses. Finally, in order to analyze the variability of clitoral length at different body weights and ages, data were log-transformed and a regression analysis was carried out.
RESULTS
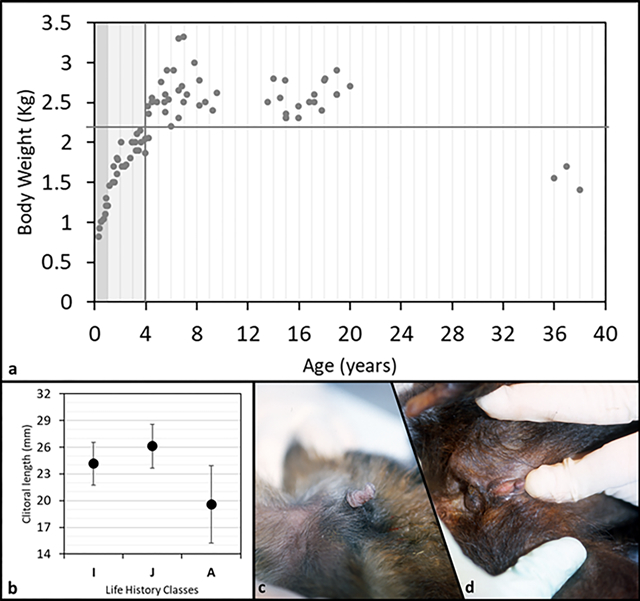
We plotted body weight versus age and set the age at maturity to four years (Fig. 3a, vertical line). We observed age related differences in body weight that suggested a continuous and rapid increase from birth to maturity, a plateau during early and mid-adulthood (body weight never below 2.2 kg, Fig. 3a, horizontal line), followed by a decrease in body weight in senescence (congruent pattern with longitudinal body weight and skeletal data, Jungers and Fleagle, 1980; Fragaszy and Adam-Curtis, 1998). In our sample clitoral length ranged from 1mm to 30mm (Tab 2). All female infants observed in the colony (as well as some not included in the sample measured) showed, without exception, a prominently long clitoris (N= 6). Clitoris length differed among three age classes (F(2,78)=21.7, p=0.001; Fig. 3b) with adult females having the smallest size, as shown in figure 3 by comparing infant’s (Fig. 3c) and adult’s (Fig. 3d) clitoris (contrast analyses: A vs. IM, F(1;75)=61.5, p=0.001; A vs. J, F(1;72)=37.4, p=0.001; A vs. I, F(1;57)=7.2, p=0.009). When the body weight was taken into account (ANCOVA) clitoris length differed among three age classes as well (F(2,77)=11.2, p=0.001). Regression revealed clitoral length as inversely correlated to both age (R=−0.6, p<0.001) and body weight (R=−0.37, p<0.001) (Fig. 4).
Figure 3.
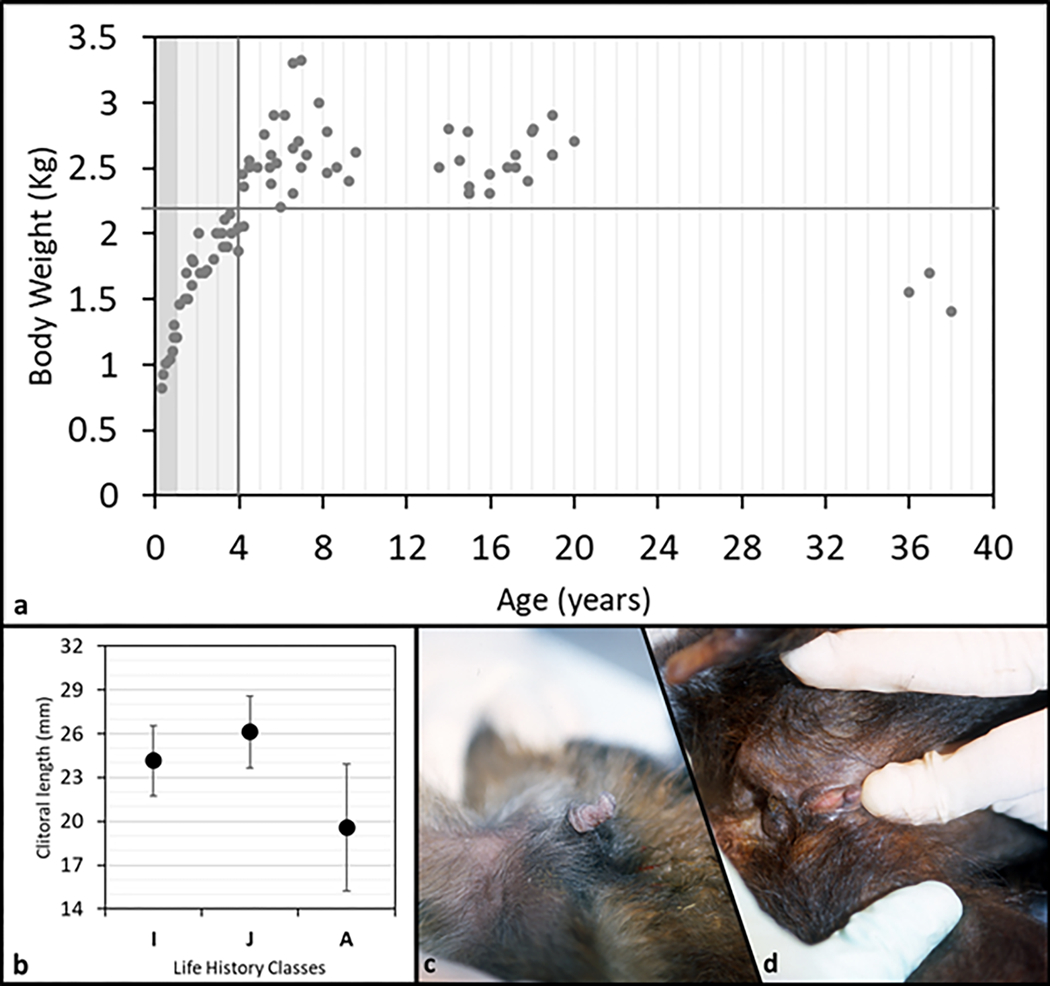
a: plot of body weight vs. age of 22 tufted capuchin (S. apella) females. Dark grey band stands for infants, I (0–1 year); light grey band stands for juveniles, J (1.1–4 years); white band stands for adults, A (4.1–38 years). Vertical grey line identifies the transition between immature and mature phase. b: clitoral mean length (SD bars) for each life history class. (I=infant; J=juvenile; A=adult). c: clitoris appearance in a representative infant female S. apella (6 mo. old). d: clitoris appearance in a representative old-adult female S. apella (37 yrs. old).
Table 2.
Clitoral length (mm) in a colony of Sapajus apella
| Age group (age range, yrs) | No. Females | No. Measurements | Average length | Standard deviation | Min | Max |
|---|---|---|---|---|---|---|
| Infant (0–1) | 4 | 7 | 24,1 | 2,39 | 20 | 26 |
| Juvenile (1.1–4) | 5 | 22 | 26,1 | 2,45 | 21 | 30b |
| Adult (> 4.1) | 13 | 51 | 18,8 | 5,60 | 1a | 29 |
| Total | 22 | 80 | ||||
= minimum length by two adult subjects (Shinade and Lucy; from 13 to 16 yrs old)
= maximum length by a young juvenile (Liv; 1.5 yrs old)
Figure 4.
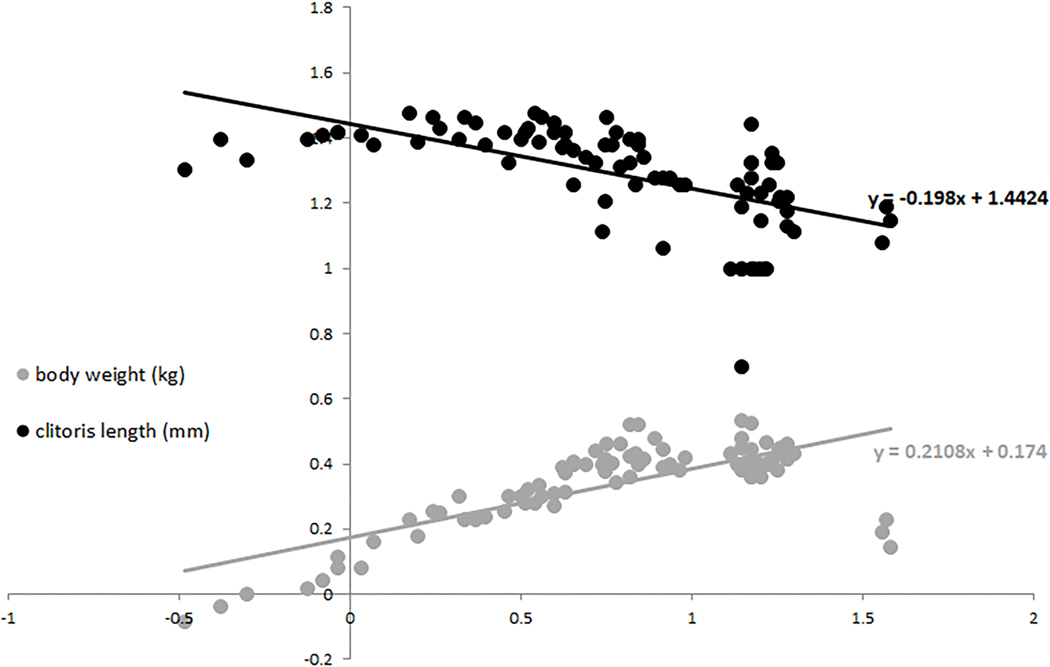
Scatter plot of body weight and clitoris length vs. age (all data were Log-transformed). Trend lines and associated equation are shown for each dataset.
DISCUSSION
Allometry designates the changes in relative dimensions of parts of the body that are correlated with changes in overall size. Specifically, ontogenetic allometry shows these changes in the context of growth. Results presented in this study are a first step in characterizing the clitoris in tufted capuchins by providing data on one of its traits (i.e., length) and investigating differences in this trait at different ages (from infant to adult individuals). Our data support the qualitative perception that immature female capuchins have longer clitorises than mature females. In fact, our results confirm that not only the naturally body growth may be in part responsible of a biased human perception, but that the clitoris is actually longer in immature females compared to adult ones.
In tufted capuchin immature individuals, the prominent clitoris represents a source of confusion in sex determination (in addition to a sessile scrotum in immature males, Napier and Napier, 1967; Fragaszy et al, 2004). However, our species is not the only challenging case of visually equivocal external genitalia in immature animals. Both Wislocki (1936) and Hill (1962) reported difficulties in sexing immature individuals in mantled howlers (A. palliata). Clarke and colleagues (2007) stated that observational studies of free-ranging juvenile mantled howlers are impeded by visually undifferentiated genitalia. In contrast, the hypertrophic and pendulous clitoris in Ateles, instead of confusing sexual determination, is used as key for sexing both newborn and monomorphic adult individuals (Campbell and Gibson, 2008).
In the diverse landscape of size and morphology of primate species clitorises, Sapajus and Cebus are listed together with Ateles, Brachyteles and Lagothrix as the five platyrrhine genera characterized by clitoral hypertrophy (Dixson, 2012). As stated by Campbell (2017), the hypertrophy concept is usually applied when size goes beyond normal proportions, yet “normal proportions” of clitorises in the various primate species may greatly vary. Normative data regarding clitoral size are scanty in the primate literature. Goldschmidt and colleagues (2009) measured the clitoral lengths of a large sample of captive M. mulatta females (N=285) and, as in S. apella, found significant variability in clitoral length at different age classes (infant, juvenile, young adult and adult). However, they found an opposite trend if compared to our study species, with a positive correlation between clitoral length and age (also known in human females, see in Goldschmidt et al., 2009). Similarly, Drea and Weil (2008) reported quantitative data of female L. catta external genital morphology (e.g., anogenital distance, external clitoris length/diameter/circumference/width, meatus length/distance) and revealed a plateau in clitoral length at puberty. Differently, in Ateles genus, the distinctive and well known clitoral conspicuity has been reported as starting from birth and lasting throughout life (Hill, 1960). Nevertheless, inter-female variability in both clitoral length (Ateles spp.) and color (A. belzebuth belzebuth and A. b. chamek) has been reported as a useful trait for individual female and group membership identification (Campbell and Gibson, 2008). Unfortunately, no systematic data on clitoral length are available for these species. A genetic component to explain intraspecific variation in clitoral length has been hypothesized in both Ateles (differences between matrilines, Campbell and Gibson, 2008) and M. mulatta (inheritance effects for clitoris presence/absence, Goldschmidt et al., 2009).
Clarification of potential clitoral functions in non-human primates is needed (Dixson, 2012), and functional hypotheses explaining interspecific variation on clitoral appearance are limited to those species demonstrating potentially related behavioral patterns or traits. In Ateles, for example, the possible role of clitoris in chemical communication of the female’s reproductive state, is emphasized (Klein, 1971). In addition, observations of diverse clitoris-directed behaviors (Campbell, 2004; Pastor-Nieto, 2000) prompted researchers to hypothesize an array of functions for the extremely enlarged clitoris (the scent-marking and chemical signaling function, Klein, 1971, Pastor-Nieto, 2000; the sex distant identification function in a monomorphic species, Eisenberg and Kuehn, 1966; the “guide” facilitating male penetration function, Campbell, 2006; identification of adult females from a distance, Campbell and Gibson, 2008), all of which potentially correlate with clitoral peculiarities.
Since in most anthropoid primate species female external genitals are relatively small and often hidden (Ankel-Simons, 2010; Dixson, 2012), the prominently long external clitoris observed in immature capuchins might tentatively be interpreted as a form of male mimicry (male-like appearance of female external genitals, Hill, 1960; Fragaszy et al., 2004) and not related to the sexual-reproductive context. Virtually indistinguishable infants and juveniles have been described in howler monkeys as well. In this species, however, resemblance between sexes is based on a sort of undifferentiated morph which entails both external genitals and behavior (Clarke, 1990; Clarke et al., 2007). Juvenile monomorphism in this species has been hypothesized as related to the advantage juveniles would take in looking immature and nonthreatening thus delaying the expulsion from the natal group (Clarke & Zucker, 1989; Clarke et al., 2007). In a non primate mammal, the fossa (Cryptoprocta ferox), external genitals of immature females are characterized by peculiar specific masculine traits (including a prominent/elongated clitoris with spines), which progressively fade in adulthood. This “transient masculinization” (Hawkins et al., 2002) has been tentatively interpreted as an advantage for the young females, who during their juvenile dispersal phase, may benefit from mimicking males, decreasing the risk of both sexual harassment by males (annual estrus is brief, and females are dispersed) and attacks by territorial adult females. If further studies would support S. apella “transient male mimicry” what would the female advantage be? In social primates, the presence and appearance of external genitalia may affect how others interact with infants and juveniles, as for example in rhesus macaques whose male infants receive more genital inspection and less restriction than females (Dixson, 2012) In S. apella, a mothers’ biased behavior toward male infants, has never been described. During affiliative and playful interactions with both immature and adult individuals of both sexes, however, immature females have been observed erecting the clitoris, seemingly enhancing genital mimicry. Erection of the clitoris has never been observed in adult females (Fragaszy et al., 2004; MC, pers. obs.). Nevertheless, direct evidence for a social function remains lacking.
Quantitative descriptions of patterns of clitoral hypertrophy (elongation) in S. apella at different ages by mean of ontogenetic allometry, as well as comparisons with other species, revealed an interspecific variability potentially useful for both identifying different patterns of hypertrophy (e.g., Ateles spp, L. catta, M. mulatta) and hypothesizing diversified causal mechanisms, which, in turn, would possibly translate into different functional hypotheses. At the intra-specific level, future research should focus more on variability in clitoral size by collecting systematic quantitative measurements on a large sample in order to obtained normative data mandatory to disentangle likely functional hypotheses.
Acknowledgements
A special thanks to the personnel of the Laboratory of Comparative Ethology (NICHD) for providing assistance and expertise during this study. We thank Drs. Mark Haines and Lynn Walker, veterinarians at the NIH Animal Center, provided technical support at the beginning of this study. The washer was added to the original technique used for hyenas thanks to the brilliant suggestion of Dr. Kathleen Rasmussen Miller. Melissa S. Gerald provided wise comments on an early draft of the manuscript, greatly improving the conceptual framework. This research was supported by funds from the Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
REFERENCES
- Ankel-Simons F. (1983). A survey of living primates and their anatomy. Macmillan Publishing Company. [Google Scholar]
- Ankel-Simons F. (2010). Primate anatomy: an introduction. Elsevier. [Google Scholar]
- Bertalanffy, von L, & Pirozynski W. (1952). Ontogenetic and evolutionary allometry. Evolution, 6, 387–392. DOI: 10.2307/2405701 [DOI] [Google Scholar]
- Campbell CJ (2004). Patterns of behavior across reproductive states of free‐ranging female black‐handed spider monkeys (Ateles geoffroyi). American Journal of Physical Anthropology, 124(2), 166–176. 10.1002/ajpa.10350 [DOI] [PubMed] [Google Scholar]
- Campbell CJ (2006). Copulation in free-ranging black-handed spider monkeys (Ateles geoffroyi). American Journal of Primatology, 68, 507–511. 10.1002/ajp.20246 [DOI] [PubMed] [Google Scholar]
- Campbell CJ (2017). Hypertrophied Clitoris. The International Encyclopedia of Primatology Vol II (p. 634). John Wiley & Sons; 10.1002/9781119179313.wbprim0463 [DOI] [Google Scholar]
- Campbell CJ, & Gibson KN (2008). Spider monkey reproduction and sexual behavior In Campbell Christina J. (Ed.) Spider Monkeys: Behavior, Ecology and Evolution of the Genus Ateles (pp. 266–287). Cambridge University Press; 10.1017/CBO9780511721915.010 [DOI] [Google Scholar]
- Clarke MR (1990). Behavioral development and socialization of infants in a free-ranging group of howling monkeys (Alouatta palliata). Folia Primatologica, 54, 1–15. 10.1159/000156422 [DOI] [PubMed] [Google Scholar]
- Clarke MR, & Zucker EL (1989). Social correlates of timing of sexual maturity in free-ranging howling monkeys (Alouatta palliata). American Journal of Primatology, 18 (2), 140. [Google Scholar]
- Clarke MR, Zucker EL, Ford RT, & Harrison RM (2007). Behavior and endocrine concentrations do not distinguish sex in monomorphic juvenile howlers (Alouatta palliata). American Journal of Primatology, 69, 477–484. 10.1002/ajp.20354 [DOI] [PubMed] [Google Scholar]
- Cock AG (1966). Genetical aspects of metrical growth and form in animals. Quarterly Review of Biology, 41, 131–190. 10.1086/404940 [DOI] [PubMed] [Google Scholar]
- Dixson AF (2012). Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Humans (2nd ed) Oxford University Press; DOI: 10.1093/acprof:osobl/9780199544646.001.0001 [DOI] [Google Scholar]
- Drea CM, & Weil A. (2008). External genital morphology of the ring‐tailed lemur (Lemur catta): Females are naturally “masculinized”. Journal of Morphology, 269(4), 451–463. 10.1002/jmor.10594| [DOI] [PubMed] [Google Scholar]
- Drea CM, Weldele ML, Forger NG, Coscia EM, Frank LG, Licht P & Glickman SE (1998). Androgens and masculinization of genitalia in the spotted hyena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. Journal of Reproduction and Fertility, 113, 117–127. 10.1530/jrf.0.1130117 [DOI] [PubMed] [Google Scholar]
- Eisenberg JF & Kuehn RE (1966). The behavior of Ateles geoffroyi and related species. Smithsonian Miscellaneous Collection, 151(8), 1–63. [Google Scholar]
- Fragaszy DM, & Adams‐Curtis LE (1998). Growth and reproduction in captive tufted capuchins (Cebus apella). American Journal of Primatology, 44(3), 197–203. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, & Fedigan LM (2004). The complete capuchin: the biology of the genus Cebus. Cambridge University Press; 10.5860/choice.42-4029 [DOI] [Google Scholar]
- Gerhardt U. (1909). Ueber das Vorkommen eines Penis-und Clitorisknochens bei Hylobatiden. Anatomischer Anzeiger, 35, 353–358 [Google Scholar]
- Goldschmidt B, Cabello PH, Kugelmeier T, Pereira BB, Lopes CA, Fasano DM, … & Marinho AM (2009). Variation in clitoral length in rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 48(5), 482–485. [PMC free article] [PubMed] [Google Scholar]
- Gould SJ (1966). Allometry and Size in Ontogeny and Phylogeny. Biological Reviews, 41, 587–640. 10.1111/j.1469-185X.1966.tb01624.x [DOI] [PubMed] [Google Scholar]
- Harms JW (1956). Fortpflanzungsbiologie In Hofer H, Schultz AH & Starck D, (Eds.). Primatologia. Handbuch der Primatenkunde, Vol. 1: Systematik, Phylogenie, Ontogenie. (pp. 561–660). Basel, Karger. [Google Scholar]
- Hawkins C, Dallas J, Fowler P Woodroffe R, & Racey P. (2002). Transient masculinization in the fossa, Cryptoprocta ferox (Carnivora, Viverridae). Biology of Reproduction, 66, 610–615. 10.1095/biolreprod66.3.610 [DOI] [PubMed] [Google Scholar]
- Hershkovitz P. (1977). Living new world monkeys (Platyrrhini). University of Chicago Press. [Google Scholar]
- Hill WCO (1933). A monograph on the genus Loris. Ceylon Journal of Science (B), 18, 89–129. [Google Scholar]
- Hill WCO (1953). Primates: Comparative anatomy and taxonomy, Volume I, Strepsirhini. Edinburgh, Edinburgh University Press. [Google Scholar]
- Hill WCO (1958). External genitalia In Hofer H, Schultz AH & Starck D (Eds.). Primatologia: Handbuch der Primatenkunde, Vol. 3 (pp. 630–704). Basel, Karger. [Google Scholar]
- Hill WCO (1960). Primates: Comparative anatomy and taxonomy, Volume IV, Cebidae, Part A. Edinburgh University Press, Edinburgh. [Google Scholar]
- Hill WCO (1962). Primates: Comparative anatomy and taxonomy, Volume V, Cebidae, Part B. Edinburgh University Press, Edinburgh. [Google Scholar]
- Huxley JS (1932). Problems of relative growth. Methuen: London. Reprinted 1972, Dover Publications, New York. [Google Scholar]
- Huxley JS& Tessier G. (1936) Terminology of relative growth. Nature 137, 780–781. 10.1038/137780b0 [DOI] [Google Scholar]
- Jungers WL, & Fleagle JG (1980). Postnatal growth allometry of the extremities in Cebus albifrons and Cebus apella: A longitudinal and comparative study. American Journal of Physical Anthropology, 53, 471–478. 10.1002/ajpa.1330530403 [DOI] [PubMed] [Google Scholar]
- Klein LL (1971). Observations on copulation and seasonal reproduction of two species of spider monkeys, Ateles belzebuth and A. geoffroyi. Folia Primatologica, 15, 233–248. 10.1159/000155382 [DOI] [PubMed] [Google Scholar]
- Klingenberg CP (1996). Multivariate Allometry In Leslie FM et al. (Eds.), Advances in Morphometrics (pp 23–49). Boston, MA: Springer; 10.1007/978-1-4757-9083-2_3 [DOI] [Google Scholar]
- Leigh SR (2006). Cranial ontogeny of Papio baboons (Papio hamadryas). American Journal of Physical Anthropology, 130: 71–84. 10.1002/ajpa.20319 [DOI] [PubMed] [Google Scholar]
- Lima AR, Guimarães SB, Branco É, Giese EG, Muniz JAPC, Pereira WLA, … & Miglino MA (2015). Morphological and morphometric description of female reproductive tract of Sapajus apella (Capuchin monkey). Anatomia, histologia, embryologia, 44(4), 262–268. 10.1111/ahe.12134| [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Windhager S, & Schaffer K. (2013). A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix, the Italian Journal of Mammalogy, 24(1), 59–66, doi: 10.4404/hystrix-24.1-6369. [DOI] [Google Scholar]
- Napier JR, & Napier PH (1967). Handbook of living primates. New York, USA: Academic Press [Google Scholar]
- Pastor-Nieto R. (2000). Female reproductive advertisement and social factors affecting the sexual behavior of captive spider monkeys. Laboratory primate newsletter, 39(3), 5–10. [Google Scholar]
- Pehrson T 1914. The external female genitalia in monkeys, lemurs and insectivores. Anatomischer Anzeiger 46(7–8), 161–179. [Google Scholar]
- Pocock RI (1918). On the external characters of the lemurs and of Tarsius. In Proceedings of the Zoological Society of London 88(1‐2), 19–53. 10.1111/j.1096-3642.1918.tb02076.x| [DOI] [Google Scholar]
- Pocock RI (1920). On the external characters of the South American monkeys. Proceedings of the Zoological Society of London, 90(1–2), 91–113. [Google Scholar]
- Pocock RI (1925). The External Characters of the Catarrhine Monkeys and Apes. Proceedings of the Zoological Society of London, 95(4), 1479–1579. 10.1111/j.1469-7998.1925.tb07446.x [DOI] [Google Scholar]
- Smith BH, Crummet TL, & Brandt KL (1994). Ages of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Yearbook of Physical Anthropology, 37, 177–231. 10.1002/ajpa.1330370608 [DOI] [Google Scholar]
- Teixeira DG, Hamlett WC, Guimarães MADBV, Morini AC, Araújo KPC, Cury FS, … & Miglino MA (2015). Morphological tools for describing the male external genitalia of Sapajus apella. Zoological Science, 32(1), 97–104. 10.2108/zs140175 [DOI] [PubMed] [Google Scholar]
- Wislocki GB (1936). The external genitalia of the simian primates. Human Biology, 8(3), 309–347. [Google Scholar]


