Abstract
Aims
Cognitive impairment may be greater in HIV-positive (HIV+) women than in HIV+ men. Whether sex-specific differences exist in brain microstructure of HIV+ individuals are unknown and were evaluated.
Method
39 HIV+ (21 men, 18 women) and 45 seronegative (SN, 20 men, 25 women) participants were assessed with brain diffusion tensor imaging and cognitive assessments (7 neuropsychological domains). Fractional anisotropy (FA) and mean diffusivity (MD) were measured with an automated atlas in selected brain regions. Group comparisons were assessed with linear mixed effects model, with sub-regions and hemisphere (left/right) as repeated factors for each region.
Results
HIV+ women, but not HIV+ men, were slower than sex-matched SN controls on sensorimotor function (Dominant-hand: interaction-p=0.007; Non-dominant hand: interaction-p=0.039). Similarly, only HIV+ women had lower FA in the globus pallidus (GP, interaction-p=0.011). Additionally, regardless of sex, the HIV+ group had poorer Fluency, Speed, and Attention than SN-controls (p=0.006–0.008), as well as lower FA and higher MD in multiple brain regions (p=<0.001–0.044). Across all participants, performance on Attention was predicted by uncinate-FA (p<0.001, r=0.5) and corpus callosum (CC)-FA (p=0.038, r=0.23), while the Speed of Information Processing was predicted by CC-FA (p=0.009, r=0.3). Furthermore, faster sensorimotor function correlated with higher CC-FA and uncinate-FA in men but not in women (Sex*DTI-interaction-p=0.03–0.06).
Conclusions
The relatively poorer sensorimotor function and abnormally lower GP_FA, suggesting lesser neuronal integrity, in HIV+ women demonstrate sex-specific effects from HIV-infection on these measures. These findings may be related to the greater immune activation and neuroinflammation in HIV+ women compared to HIV+ men.
Keywords: fractional anisotropy, mean diffusivity, sex, MRI, HIV, brain, sensorimotor
Introduction
Despite effective combined antiretroviral treatments (cART), up to half of the people living with HIV still suffer from HIV-associated neurocognitive disorders (HAND), which may involve deficits or impairments in executive function, learning, memory and motor function (Saylor et al., 2016). Furthermore, age-related decline on motor function was greater in HIV-infected (HIV+) men and women than that in the seronegative controls (Goodkin et al., 2017; Elicer I et al., 2018; Rubin et al., 2019), even when their HAND status were stable (Elicer I et al., 2018). Moreover, recent evidence suggests that HIV infection affects women more than men on sensorimotor function, processing speed and attention (Maki et al., 2018).
The greater sensorimotor deficits in HIV+ women than in HIV+ men may reflect even greater deficits since healthy women tend to have better manual dexterity than men (Ruff and Parker, 1993; Nicholson and Kimura, 1996; Junaid and Fellowes, 2006; Vasylenko et al., 2018). Comprehensive neuropsychological evaluations in many studies of HIV-infected individuals included the Grooved Pegboard test, an indicator of manual dexterity that gauges sensorimotor performance. Although the Grooved Pegboard test performance was poorer in HIV+ women than in HIV+ men, the causative mechanisms are unclear (Maki et al., 2018). Understanding the neural mechanisms responsible for this interaction may influence future treatment and targeted interventions.
Optimal sensorimotor performance requires engagement of multiple CNS regions, including the primary motor cortex, axons that contribute to the corticospinal tract, the basal ganglia, thalamic motor nuclei and the cerebellum (Papale and Hooks, 2018). In healthy participants, upper limb motor dexterity was associated with DTI metrics in the internal capsule, genu and splenium of the corpus callosum (Zahr et al., 2009). However, the sensorimotor dysfunction in HIV-infected individuals may be related to injury in other circuits that modulate the sensorimotor system circuits.
Prior studies found that HIV-associated cognitive deficits were associated with widespread abnormalities in white matter microstructure, including lower fractional anisotropy (FA) and higher mean diffusivity (MD), even in antiretroviral medication-treated cohorts (Pfefferbaum et al., 2009; Correa et al., 2016; Underwood et al., 2017). However, no study evaluated sex-specific effects on white matter microstructure in HIV+ individuals. Therefore, this study aimed to evaluate sex-specific effects in regional WM diffusivity and its contribution to the more pronounced sensorimotor, attention and psychomotor speed deficits in HIV+ women compared to HIV+ men. Because many cognitive tests are complex and may be dependent on WM integrity in multiple brain regions, we used a hierarchical approach evaluating regional and subregional effects of HIV and sex on WM microstructure. We hypothesized that alterations in WM diffusivity contribute to the more pronounced sensorimotor deficits in HIV infected women compared to HIV infected men.
Methods
Participants
A total of 84 participants, including 39 HIV+ (21 men and 18 women) and 45 HIV-seronegative participants (20 men and 25 women) were included in the current study. Participants were recruited from the community by flyers, online advertisement, word of mouth, and referrals from local health care providers. All participants were first screened by telephone, and potentially eligible participants were screened again in person with detailed medical history, physical and neurological examinations, screening blood and urine tests, and review of the medical records as needed to ensure eligibility. Inclusion criteria for all participants were age ≥ 18 years and ability to give written informed consent. The HIV-serostatus for all seronegative participants was confirmed onsite with the Clearview® COMPLETE HIV 1/2 test kits (Alere, altham, MA). Additional inclusion criteria for HIV+ participants were: 1) HIV seropositive (verified by medical records); 2) stable on a cART regimen for ≥ 6 months or medication naïve. Participants were excluded if they had: (1) a medical, neurologic or psychiatric disorders that might confound brain measurements; (2) active hepatitis C (verified by medical records or by QraQuick® HCV test); (3) abnormal screening laboratory tests (e.g. severe renal or hepatic dysfunction) or significantly abnormal electrocardiograms; (4) medications that would influence cognitive performance; (5) urine test verified pregnancy; (6) current or history of moderate or severe substance use disorder (other than tobacco smoking or medicinal cannabis use); (7) urine toxicology screen positive for cocaine, methamphetamine, opiates, and benzodiazepines; (8) contraindications for MR studies; (8) reading level less than 8th grade. All participants provided written informed consent approved by the Committee on Human Studies at our institution. The study was Health Insurance Portability and Accountability Act (HIPAA) compliant.
Clinical and Medical Assessments
All participants were evaluated by a study physician to ensure they fulfilled the study criteria. The medical history included HIV-related clinical measures, such as duration of HIV diagnosis, plasma viral load, nadir and most recent CD4 counts (typically within 6 months), their cART histories (number and % HIV patients taking cART), and detailed substance use histories including the usage of tobacco, alcohol and marijuana (see Table 1).
Table 1.
Participant Demographics and Clinical Characteristics (Mean (SD))
| SN Women (n=25) | SN Men (n=20) | HIV+ Women (n=18) | HIV+ Men (n=21) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 47.0 (11.9) | 47.4 (12.7) | 47.3 (12.7) | 46.4 (11.3) | 0.99a |
| Education (years) | 14.1 (2.7) | 13.6 (2.1) | 13.4 (2.4) | 13.4 (2.1) | 0.70a |
| Race (White / Asian / Black / Native Hawaiian-Pacific Islander / American Indian or Alaska Native / Mixed) | 13 / 2/ 1 / 4 / 0 / 5 | 12 / 2 / 0 / 2 / 0 / 4 | 4 / 2 / 3 / 5 / 0 / 4 | 8 / 5 / 2 / 1 / 2 / 3 | 0.22d |
| CES-D scale score (0–60) | 10.6 (11.5) | 10.0 (10.8) | 11.1 (9.5) | 15.6 (9.8) | 0.29a |
| Wechsler Test of Adult Reading | 105 (10) | 106 (11.1) | 98.3 (8.0) | 106 (7.3) | 0.08 a |
| HIV Disease-Related | |||||
| Duration of diagnosis (months) | 139.7 (84.6) | 189.5 (95.8) | 0.10a | ||
| Detectable HIV RNA (>50 copies / mL, %) | 6/14 (42.9%) | 10/21 (47.6%) | 0.77e | ||
| Log Plasma HIV RNA (median, [IQR]) | 3.89 [3.89, 5.11] | 3.89 [3.89, 9.22] | 0.45b | ||
| Plasma HIV RNA (copies/mL) | Not Applicable | Not Applicable | 1102 (2548) | 13601 (27013) | 0.09b |
| # (%) maintained on cART | 16/18 (89%) | 17/19 (89%) | 1.00c | ||
| CD4 count (#/mm3) | 580 (416) | 385 (190) | 0.06a | ||
| Nadir CD4 count (#/mm3) | 200 [141, 398] | 160 [57, 325] | 0.19a | ||
| HIV Dementia Scale (0–16) | 12.7 (3.3) | 14.0 (2.5) | 0.16c | ||
| Karnofsky Score (0–100) | 91.2(9.9) | 89.8(10.3) | 0.67c | ||
| Tobacco usage, Median [IQR] | |||||
| # Lifetime Users (%) | 18/22 (81.8) | 11/20 (55) | 12/17 (70.6) | 13/20 (65) | 0.31d |
| Daily average use (# cigarettes) | 18.4 [9.2, 30.0.] | 15.0 [2.1, 16.7] | 20.3 [15.0, 30.0] | 30.0 [16.9, 36.5] | 0.10d |
| Total lifetime use (pack years) | 10.3 [3.1, 23.4] | 10.0 [1.8, 17.0] | 18.6 [5.0, 21.9] | 20.0 [10.0, 34.7] | 0.40b |
| Duration of tobacco use (years) | 20.9 [9.3, 33.7] | 20.0 [11.5, 28.1] | 16.3 [9.8, 32.9] | 23.1 [12.7, 33.7] | 0.84b |
| Marijuana usage, Median [IQR] | |||||
| # Lifetime marijuana users (%) | 13 (52) | 17 (85) | 12(66.7) | 19 (90.5) | 0.01d |
| # Users in the past month (%) | 7/16 (43.8) | 7/18 (38.9) | 4/14 (28.6) | 9/18 (50.0) | 0.67d |
| Average per use (g) ¶ | 0.30 [0.06, 0.75] | 0.75 [0.4, 0.75] | 0.75 [0.3, 1.8] | 0.75 [0.1, 1.1] | 0.40b |
| Total lifetime use (kg) ¶ | 0.15 [0.006, 1.0] | 0.27 [0.06, 0.7] | 2.8 [1.0, 9.9] | 0.26 [0.004, 1.6] | 0.14b |
| Duration of use (years) ¶ | 12.0 [3.5, 31.5] | 19.0 [5.2, 25.0] | 22.0 [10.9, 33.3] | 10.0 [4.3, 31.2] | 0.71b |
| Alcohol usage, Median [IQR] | |||||
| # Lifetime Alcohol users (%) | 23/24 (95.8) | 19/20 (95) | 15/16 (93.8) | 19/20 (95) | 1.00d |
| # Users in the past month (%) | 19/23 (82.6) | 16/20 (80) | 10/14 (71.4) | 15/19 (78.9) | 0.86d |
| Average per use (mL) ¶ | 35.4 [21.1,47.0] | 76.6 [37.3, 107.9] | 42.6 [31.9, 90.9] | 53.0 [30.2, 120.4] | 0.13b |
| Total lifetime use (Liter) ¶ | 20.1 [8.5, 81.4] | 62.2 [16.7,198.6] | 38.8 [3.6, 179.9] | 68.3 [27.0, 307.3] | 0.58b |
| Duration of use (years) | 24.9 [10.7, 33.8] | 20.7 [12.7, 37.2] | 19.0 [10.2, 30.1] | 21.0 [10.9, 34.5] | 0.91b |
p-values were derived from
= ANOVA
b = Kruskal-Wallis test
c = t test
d = Fisher exact test
CES-D = Center for Epidemiological Studies – Depression Scale;
cART = combined antiretroviral treatments
These variables were calculated only for the users who used these substances.
IQR = Interquartile range (middle 50% of the values from lowest to highest).
Neuropsychological Tests
To assess their neuropsychological performance, all except two participants completed a battery of tests that examined seven cognitive domains: Fluency (DKEFS or Ruff Figural Design Fluency, and Verbal Fluency with letters FAS), Executive Function (DKEFS Color Word Interference or Stroop Interference and Trail Making Test B), Speed of Information Processing (Symbol Digit, DKEFS Trail-making Number Sequencing or Trail Making Test A, DKEFS Color naming or Stroop Color Naming, and the Simple Reaction Time from the California Computerized Assessment Package), Attention / Working Memory (Arithmetic from Wechsler Adult Intelligence Scale-VI, Digit Span Backward, Letter-Number Sequencing, Arithmetic and Paced Auditory Serial Addition Test 1), Learning (Rey Auditory Verbal Learning Test Trial 5 and Rey-Osterreith Complex Figure Test-Immediate Recall), Memory (Rey Auditory Verbal Learning Test Delayed Recall (Trial 7) and Rey Complex Figure-Delayed Recall), and Sensorimotor skills with Grooved Pegboard in both Dominant and Non-dominant hands (Figure 1). Age and education adjusted Z-scores were computed using our normative dataset established in our laboratory from 507 healthy seronegative participants that were administered the same tests; higher Z-scores indicate better performance. HIV infected individuals were also evaluated with the HIV Dementia Scale (Power et al., 1995), Karnofsky Performance Scale Index (Karnofsky and Burchenal, 1949) and the Center for Epidemiologic Studies-Depression Scale (Radloff, 1977). The Wechsler Test of Adult Reading Test (WTAR) was also performed to estimate the verbal Intelligence Quotient, and to adjust for possible premorbid group differences on the cognitive performance.
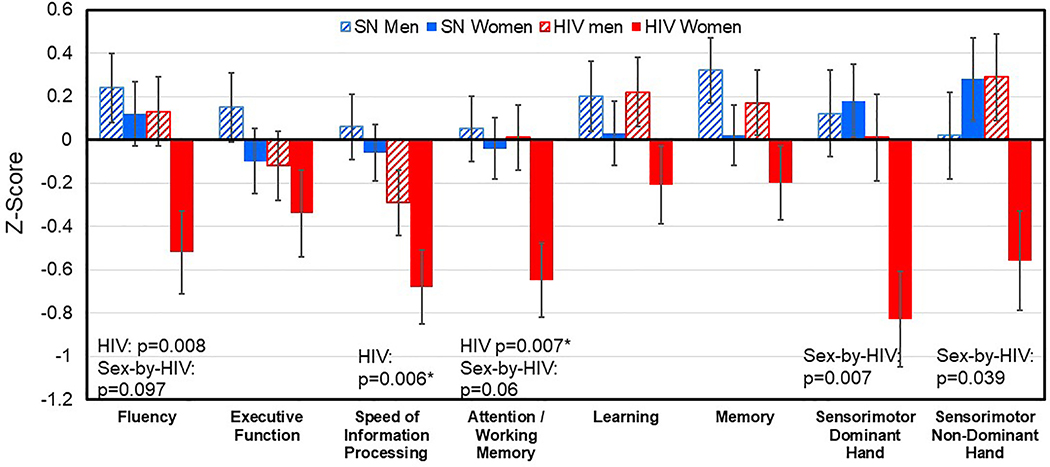
Fig. 1. Cognitive Domain Z-Scores in the four Study Participant Groups.
Regardless of sex, HIV+ participants had significantly poorer performance in the Fluency (p=0.008), Processing Speed (p=0.006), Attention / Working Memory (p=0.007) domains than HIV-seronegative participants. In addition, across all participant groups, HIV+ women had the lowest Z-scores in all cognitive domains, especially the Sensorimotor (interaction-p=0.007 for dominant hand, p=0.039 for non-dominant hand), Fluency (interaction p=0.097) and Attention/Working Memory (interaction p=0.06) domains. Error bars=Standard Errors.
Image Acquisition
All participants were studied on a 3 Tesla TIM Trio MRI system (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. T1 magnetization-prepared rapid gradient-echo (MPRAGE: TR/TE/TI=2200/4.47/1000ms, flip angle=12°, FOV=256mm, 256*256 matrix, thickness=1mm) and T2 fluid-attenuated inversion recovery (FLAIR: TR/TE/TI=9100/84/2500 ms, flip angle=150°, FOV=230mm, 204*256 matrix, thickness=3 mm) sequences were used to assess structural alterations. For DTI, we used a spin-echo echo-planar imaging sequence (axial, TR/TE=3700/88mm, FOV=220mm, 128*128 matrix, thickness=4mm, four b=0 scans, 12 diffusion directions with b=1000s/mm2). After visual inspection of all MRI scans, two participants (1 seronegative female, 1 seropositive female) were removed from the analysis due to excessive head motion. The final DTI analyses included 82 participants.
DTI Processing
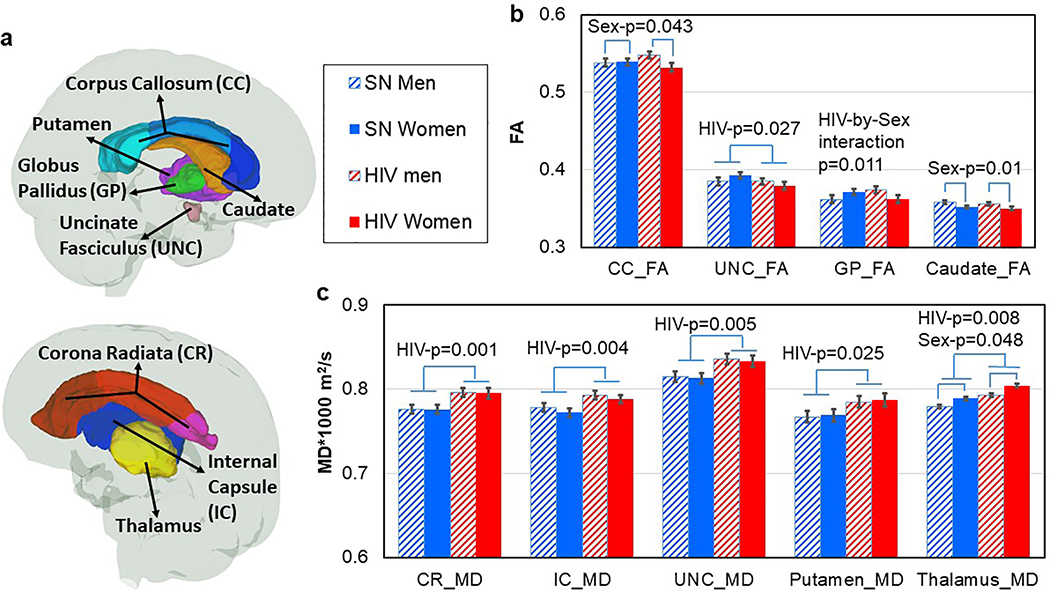
The raw diffusion-weighted images were co-registered to the b=0 image using a 12-parameter linear transformation. The tensor field was calculated using the DtiStudio software package (lbam.med.jhmi.edu or www.MriStudio.org) (Jiang et al., 2006). Three eigenvalues and eigenvectors were obtained from the tensor field, from which the fractional anisotropy (FA) and mean diffusivity (MD) maps were generated. DTI maps in the original space were transformed to the JHU-MNI atlas space using a 12-parameter linear transformation followed by dual-channel large deformation diffeomorphic metric mapping (LDDMM) (Ceritoglu et al., 2009). The inverse transformations generated with this process were applied to the JHU-MNI atlas Type II parcellation map to obtain 130 regions in native space, from which the FA and MD of each region was calculated (Oishi et al., 2009). Because AD and RD cannot be interpreted in many of the regions we assessed, with high percentage of crossing fibers, we did not examine these additional diffusivity measures. Final analyses were limited to regions that consistently show microstructural abnormalities in HIV-infected individuals and either directly or indirectly contribute to sensorimotor performance (Chang et al., 2008; Becker et al., 2011; Schulte et al., 2012; O’Connor et al., 2017; Sanford et al., 2018; O’Connor et al., 2019): the basal ganglia (BG), thalamus and uncinate fasiculi (UNC), as well as the sub-regions within the main regions of the corpus callosum (CC), corona radiata (CR), and internal capsule (IC) (Figure 2A).
Fig. 2. Group Differences in DTI Metrics.
a) Regions of interest for DTI measurements in the study. b) Regardless of HIV-serostatus, women had lower FA in the CC (p=0.043) and in caudate (p=0.01). Regardless of sex, HIV+ individuals had lower FA in the UNC (p=0.027) than SN participants. HIV-by-Sex interaction was found in the globus pallidus (GP), where HIV+women had lower FA than SN women, with no group difference in men (interaction-p=0.011). c) Regardless of sex, HIV+ had higher MD than SN participants in the Corona Radiata (CR), Internal Capsule (IC), Uncinate Fasciculus (UNC), putamen and thalamus (p=0.001–0.025). In addition, women had higher MD than men in the thalamus (p=0.048) regardless of HIV-serostatus.
Statistical Analyses
Statistical analyses were conducted using R (version 3.5.2 https://www.R-project.org/) (R Core Team, 2019). For demographic and clinical variables, data distributions informed statistical tests to assess group differences (Table 1), with post-hoc analyses to further assess group differences as needed. Cognitive domain Z-scores were assessed with two-way ANOVA, since Z-scores were already adjusted for age and education, no additional covariates were used for the basic model. However, WTAR reading and CESD depression scores affect cognitive performance in women HIV+ patient (Maki et al., 2015; Sundermann et al., 2018), these two factors and the lifetime marijuana use were used as covariates for the additional two-way ANOVA models. Linear mixed models were used to analyze the HIV, sex and HV-by-sex effects on DTI measures, with hemisphere and sub-region as repeated factors, subject ID as a random factor and age and social-economic status as covariates. Separate models were run to examine FA and MD values in the CC, CR, IC, BG and thalamus. Since adding the depression scores and lifetime marijuana use as covariates did not change the findings on DTI data, they were removed from final models. Individual sub-regions were analyzed further when there was a significant HIV, sex or HIV-by-sex effect in a major region. The associations of WM microstructure with neuropsychological performance were explored for main regions that showed group differences, using general linear models, with cognitive task scores as dependent variable, DTI metrics, HIV and sex and all 2- and 3-way interactions thereof as independent variables, while covarying for age and scanner upgrade. We started with the full 3-way model and then removed non-significant interaction terms from the final model. To limit the number of contrasts, these models were primarily explored for main regions only, but followed up with post-hoc contrasts for sub-regions if a main region showed a significant effect. For this targeted exploratory study, statistical significance was defined as p≤0.05.
Results
Clinical Characteristics (Table 1)
The clinical characteristics of the four participant groups are summarized in Table 1.The groups were similar in age, race, and had similar years of education and depressive symptom scores. HIV-positive men and women were also similar in HIV disease severity, as assessed by duration of HIV diagnosis, current/nadir CD4 counts, current plasma HIV RNA copies/mL, the proportion of participants that were on stable regimens of cART, Karnofsky score and the HIV-dementia scale score. More men reported lifetime marijuana use than women, regardless of serostatus (p=0.01). Nevertheless, the four participant groups did not differ in their percentage of recent (past month) marijuana users, lifetime amounts of marijuana used, amounts per use and duration of marijuana use. The participant groups also had similar tobacco and alcohol usage, and none of the participants had severe alcohol use disorder.
Neuropsychological Performance (Figure 1)
Figure 1 shows mean and standard errors for the Z-scores of the seven cognitive domains for the four participant groups. Compared to seronegative controls, regardless of sex, HIV-infected individuals had poorer performance in Fluency (p=0.008), Speed of Information Processing (p=0.006), and Attention / Working Memory (p=0.007) domains. An HIV-by-sex interaction was observed for the Sensorimotor domain Z-scores (p=0.007 for dominant and p=0.039 for non-dominant hand). Specifically, HIV+ women performed significantly worse than SN women in the sensorimotor domain, while the HIV+ and SN men had similar performance. This interaction remained significant even after adjusting for the WTAR reading scores, the CES-depression scores and lifetime marijuana use. Similar trends for HIV-by-sex interactions were observed in the Fluency (P=0.097) and the Attention / Working Memory (P=0.061) domains, where HIV+ females had the poorest performance among the four groups; however, these trends were no longer present after adjusting for WTAR. In addition, Pearson correlations were explored between HIV related clinical features (Log transformed viral load, current and nadir CD4 cell counts and the duration of HIV diagnosis) and cognitive domains that showed HIV main effects (Speed, Fluency, Attention and Sensorimotor function); however, no significant correlations were found.
Effects of HIV and Sex on DTI Metrics (Table 2, Figure 2)
Table 2.
DTI Metrics of the Four Groups of Study Participants and the Effects from Linear Mixed Model
| Regional FA | SN Women n=24 | SN Men n=20 | HIV+ Women n=17 | HIV+ Men n=21 | Linear Mixed Model β (p-value) | ||
|---|---|---|---|---|---|---|---|
| HIV Effect | Sex Effect | HIV×Sex | |||||
| Corpus callosum (CC) | 0.539±0.005 | 0.538±0.005 | 0.532±0.006 | 0.548±0.005 | 0.0065 (0.380) | 0.016 (0.043) | −0.017(0.107) |
| CC Genu | 0.561±0.005 | 0.557±0.006 | 0.551±0.006 | 0.566±0.006 | 0.01 (0.209) | 0.015 (0.067) | −0.019 (0.092) |
| CC Body | 0.485±0.007 | 0.482±0.007 | 0.478±0.008 | 0.496±0.007 | 0.0068 (0.476) | 0.018 (0.072) | −0.02 (0.131) |
| CC Splenium | 0.571±0.005 | 0.574±0.006 | 0.568±0.006 | 0.582±0.006 | 0.0003 (0.592) | 0.0077 (0.165) | NS |
| Corona radiata (CR) | 0.414±0.003 | 0.415±0.003 | 0.412±0.004 | 0.413±0.003 | 0.0019 (0.574) | 0.0012 (0.724) | NS |
| Internal capsule (IC) | 0.495±0.004 | 0.500±0.004 | 0.489±0.005 | 0.494±0.004 | 0.0058 (0.135) | 0.0044 (0.263) | NS |
| Uncinate fasiculi | 0.393±0.004 | 0.385±0.005 | 0.379±0.005 | 0.385±0.004 | 0.0141 (0.027) | 0.0062 (0.192) | −.0142 (0.113) |
| Basal ganglia (BG) | 0.358±0.004 | 0.357±0.004 | 0.345±0.004 | 0.360±0.004 | 0.006 (0.041) | 0.0087 (0.618) | −0.1 (0.019) |
| Caudate | 0.352±0.002 | 0.358±0.002 | 0.350±0.002 | 0.356±0.002 | 0.0018 (0.456) | 0.0064 (0.010) | NS |
| Putamen | 0.349±0.002 | 0.351±0.002 | 0.346±0.002 | 0.349±0.002 | 0.0027 (0.109) | 0.0024 (0.156) | NS |
| Pallidum | 0.371±0.004 | 0.362±0.005 | 0.362±0.005 | 0.374±0.004 | 0.011 (0.095) | 0.013 (0.122) | 0.023 (0.011) |
| Thalamus | 0.395±0.002 | 0.389±0.002 | 0.402±0.002 | 0.396±0.002 | −5.7e-04 (0.773) | −1.2e-03 (0.478) | NS |
| Region MD * 1000 m2/s | |||||||
| Corpus callosum (CC) | 0.901±0.006 | 0.905±0.007 | 0.916±0.008 | 0.910±0.007 | −1.0e-05 (0.109) | −3.7e-07 (0.954) | NS |
| CC Genu | 0.883±0.006 | 0.879±0.006 | 0.896±0.007 | 0.892±0.006 | −1.3e-05 (0.080) | −4.1e-06 (0.567) | NS |
| CC Body | 0.956±0.008 | 0.955±0.008 | 0.964±0.009 | 0.964±0.008 | −8.4e-06 (0.379) | −6.2e-07 (0.949) | NS |
| CC Splenium | 0.869±0.005 | 0.873±0.005 | 0.879±0.006 | 0.883±0.005 | −1.0e-05 (0.092) | 3.6e-06 (0.553) | NS |
| Corona radiata (CR) | 0.776±0.005 | 0.776±0.005 | 0.795±0.006 | 0.796±0.005 | −1.9e-05 (0.001) | 1.6e-07 (0.978) | NS |
| CR anterior | 0.800±0.006 | 0.791±0.006 | 0.820±0.007 | 0.810±0.006 | −1.8e-05 (0.009) | −9.7e-06 (0.184) | NS |
| CR posterior | 0.788±0.006 | 0.797±0.007 | 0.807±0.008 | 0.816±0.007 | −1.9e-05 (0.004) | 8.4e-06 (0.204) | NS |
| CR superior | 0.739±0.005 | 0.741±0.005 | 0.759±0.006 | 0.761±0.005 | −2.0e-05 (<0.001) | 1.8e-06 (0.763) | NS |
| Internal capsule (IC) | 0.772±0.005 | 0.778±0.005 | 0.788±0.005 | 0.793±0.005 | −1.4e-05 (0.004) | 7.3e-06 (0.332) | NS |
| IC anterior limb | 0.774±0.007 | 0.781±0.007 | 0.788±0.007 | 0.796±0.007 | −1.5e-05 (0.044) | 7.7e-06 (0.303) | NS |
| IC posterior limb | 0.804±0.005 | 0.807±0.005 | 0.823±0.005 | 0.827±0.005 | −1.9e-05 (<0.001) | 3.2e-06 (0.567) | NS |
| IC retrolenticular | 0.739±0.005 | 0.744±0.005 | 0.752±0.005 | 0.757±0.005 | −1.3e-05 (0.024) | 5.3e-06 (0.351) | NS |
| Uncinate fasiculi | 0.813±0.006 | 0.815±0.006 | 0.833±0.007 | 0.836±0.006 | −2.0e-05 (0.005) | 2.7e-06 (0.713) | NS |
| Basal ganglia (BG) | 0.791±0.006 | 0.799±0.006 | 0.803±0.007 | 0.811±0.006 | −1.1e-05 (0.116) | 8.0e-06 (0.265) | NS |
| Caudate | 0.815±0.008 | 0.828±0.008 | 0.819±0.009 | 0.831±0.008 | −3.7e-06 (0.674) | 1.3e-05 (0.157) | NS |
| Putamen | 0.769±0.007 | 0.767±0.007 | 0.787±0.008 | 0.785±0.007 | −1.8e-05 (0.025) | −1.7e-06 (0.839) | NS |
| Pallidum | 0.790±0.008 | 0.804±0.007 | 0.802±0.008 | 0.815±0.007 | −1.2e-05 (0.163) | 1.3e-05 (0.120) | NS |
| Thalamus | 0.789±0.002 | 0.779±0.002 | 0.804±0.002 | 0.793±0.002 | −4.5e-05 (0.008) | −1.1e05 (0.048) | NS |
All p-values shown are uncorrected. P-values in bold indicate significance (p<0.05) or a trend for significance (p<0.1). For the β values, HIV Effect: reference is HIV+; Sex Effect: reference is female; HIV×Sex: β is for SN men vs. HIV women. Corpus callosum (genu, body and splenium sub-regions are the repeated factors); Corona radiata (anterior, posterior and superior sub-regions are the repeat factors); Internal capsule (anterior limb, posterior limb and retrolenticular sub-regions are the repeated factors).
Independent of sex, HIV+ individuals had lower FA than SN controls in the uncinate fasciculi, and an apparently lower FA in the basal ganglia (which was due to the relatively lower FA, especially in the subregion of globus pallidus, in the HIV+ women only) (Table 2, Figure 2b). Additionally, independent of sex, HIV+ individuals had higher mean diffusivities than SN controls in the corona radiata (CR) and internal capsule (IC), including their sub-regions (p <0.001–0.044), as well as in the uncinate fasiculi, putamen and thalamus (Figure 2c). The corpus callosum and its sub-regions also showed trends for higher MD in HIV+ compared to SN subjects (Table 2).
Across the brain regions assessed, only three brain regions showed sex effects, with lower FA in the corpus callosum (p=0.043), lower FA in the caudate (p=0.01) and higher MD in the thalamus (p=0.048) in the women compared to men (Figure 2b & c).
Sex-by-HIV-serostatus interactions were observed for FA in the basal ganglia (p=0.019) and globus pallidus (p=0.011), where HIV+ women, but not HIV+ men, had lower FA than their SN counterparts (Table 2, Figure 2B). A trend for HIV+ women to have lower CC_FA and lower UNC_FA was also observed (Table 2, Figure 2B).
Associations between DTI Measures and Cognitive Performance (Figure 3)
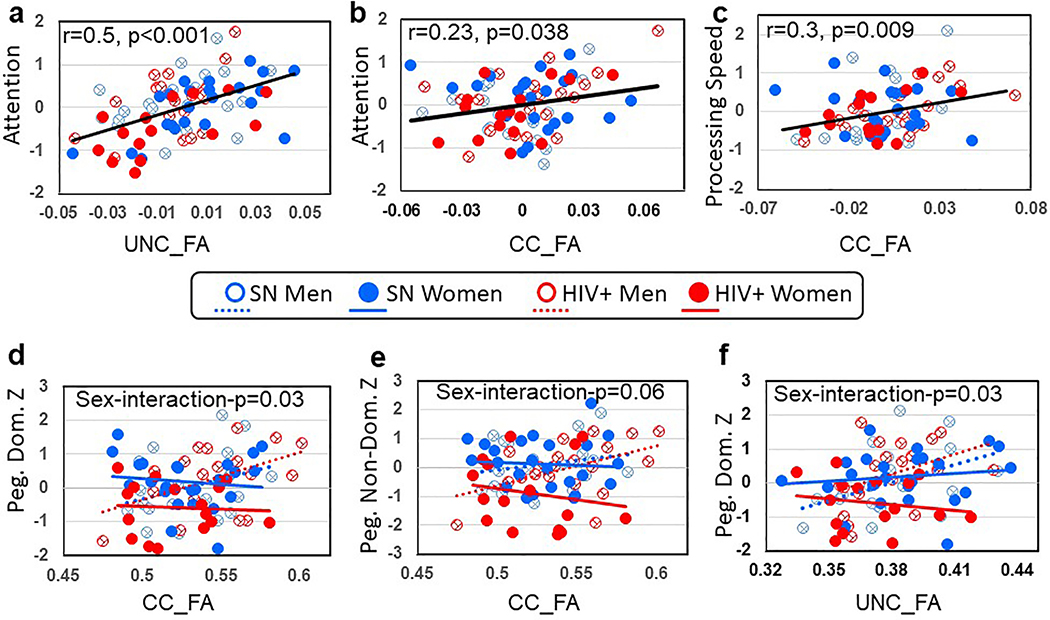
Fig. 3. Correlations between FA values in the Uncinate (UNC) or Corpus Callosum (CC) and Cognitive Performance Between Subject Groups or Across All Subjects.
a) UNC_FA predicted the Attention Z-score across all participants (Partial correlation, adjusted for age (r=0.5, p<0.001). b) CC_FA also predicted the Attention Z-score across all participants (adjusted for age, r=0.23, p=0.038). c) CC_FA predicted Processing Speed Z-score across all participants (adjusted for age, r=0.3, p=0.009). d) Higher CC_FA correlated with higher Pegboard-dominant hand performance in men but not in women (sex*FA-interaction-p=0.03). e Higher CC_FA correlated with higher Pegboard-Nondominant hand performance in men but not in women (trend for Sex*FA-interaction-p=0.06). (f) Higher UNC_FA correlated with higher Pegboard-Dominant hand performance in men but not in women (Sex*FA-interaction-p=0.03).
Since none of the 3-way interactions between HIV-serostatus, sex, and DTI measures on cognitive performance variables reached significance, all models were run with main effects and 2-way interaction terms only (HIV*Sex, HIV*DTI, Sex*DTI).
Regardless of HIV-serostatus or sex, better attention was predicted by higher UNC_FA (p<0.001; Figures 3a) and higher CC_FA (p=0.038, Figure 3b; and especially in the genu p=0.018, data not shown). Likewise, higher CC_FA across all groups predicted higher information processing speed (p=0.009, Figure 3C; again, especially in the genu p=0.004, data not shown).
No significant interactions were found with Sex and HIV-serostatus on the correlations between DTI measures and cognitive performance. However, sex-interaction effects were found in several correlations between DTI metrics and fine sensorimotor performance (Figures 3d–f). Specifically, regardless of HIV-serostatus, higher CC_FA correlated with better sensorimotor function of the dominant hand in men but not in women (sex*FA interaction p=0.03, Figure 2d); this interaction was most pronounced in the body of CC (p=0.019; data not shown). The correlations between CC_FA and Pegboard-non-dominant hand Z-score showed a similar but borderline interaction with sex (p=0.06, Figure 2e). Similarly, higher FA_UNC correlated with better sensorimotor function of the dominant hand in men but not in women (interaction-p=0.03, Figure 3f). Finally, many of these 3-way models showed significant HIV*Sex interactions on the performance of the dominant and non-dominant hand, in general agreement with the findings from the simpler 2-way model described above (with only sex, HIV and their interaction as independent variables).
Discussion
The main findings of this study are: 1) Similar to prior cognitive studies, HIV+ participants had poorer performance on Executive Function, Speed of Information Processing, Working memory and Sensorimotor function compared to SN participants. However, in the current study, these HIV-serostatus effects were primarily driven by the poorer performance in the HIV+ women compared to SN women with little to no difference between HIV+ men and SN men, especially in the fine sensorimotor task. 2) Also similar to prior DTI studies, HIV+ participants, regardless of sex, had lower FA in the uncinate fasiculi and higher MD in the corpus callosum, corona radiata, internal capsule, uncinate fasiculi, basal ganglia and thalamus. We additionally demonstrated sex-specific effects in the current study; HIV+ women had significantly lower FA in the global pallidum and trends for lower FA in the corpus callosum and uncinate fasiculi than SN women while the two male participant groups showed similar or slightly higher measures in these regions. 3) We also found sex differences regardless of HIV serostatus, compared to men, women had lower FA in the CC and caudate and higher MD in the thalamus. 4) Across all participants, higher FA in the uncinate fasiculi correlated with better attention and working memory, while higher FA in the corpus callosum correlated with faster Speed of Information Processing and better attention / working memory. Lastly, regardless of HIV serostatus, only men showed that the higher FA in the uncinate fasiculi and corpus callosum correlated with faster Pegboard performance.
HIV and Sex-Specific Effects on Neuropsychological Function
Similar to prior studies, we demonstrated that HIV-infected individuals had relatively poorer performance in the domains of Fluency, Speed of Information Processing, Attention/Working Memory, and Sensorimotor function (Goodkin et al., 2017; Liang et al., 2018). Furthermore, we observed additional deficits in HIV+ women in Executive Function and Speed of Information Processing, as well as sex-specific (interactive) effects in some domains. Specifically, HIV+ women, but not HIV+ men, were slower than their same-sex SN controls on sensorimotor function, even after adjusting for reading score or depressive symptoms. Our findings are consistent with most, except one (Behrman-Lay et al., 2016), of the previous studies. For instance, earlier studies showed HIV+ women had greater cognitive impairment than HIV+ men, particularly in the psychomotor speed domain (Maki and Martin-Thormeyer, 2009). HIV+ women also showed greater than normal age-related decline on motor skills, despite undetectable viral loads (Rubin et al., 2017). Several studies also found sex-specific effects, with HIV+ women showing relatively poorer verbal learning and memory (Maki et al., 2015; Rubin et al., 2017), and processing speed (Manly et al., 2011; Maki et al., 2015). Although we only found significant sex-specific effects in the fine sensorimotor task in the current study, the HIV+ women in our study consistently showed the lowest performance amongst all participant groups in all cognitive domains, which is consistent with prior reports that found HIV-by-sex interactions in sensorimotor function (Martin et al., 2011; Burlacu et al., 2018; Maki et al., 2018), processing speed (Royal et al., 2016; Maki et al., 2018; Qiao et al., 2019), attention (Maki et al., 2018; Qiao et al., 2019) and verbal fluency (Royal et al., 2016).
Furthermore, our findings are consistent with prior reports despite the racial differences in our study, with more diverse race groups and very few Blacks, while the prior reports included 50–80% Blacks (Manly et al., 2011; Martin et al., 2011; Maki et al., 2015; Sundermann et al., 2015; Royal et al., 2016; Maki et al., 2018). One study conducted in Romania found that young women with HIV+ vertical transmission also performed relatively slower on a motor skill task than the SN women (Burlacu et al., 2018). Lastly, since education and reading levels were strong predictors of cognitive impairment in people living with HIV and could have confounded some earlier studies, two recent studies adjusted for these variables and found that sex-specific differences on cognitive impairment and motor skill were eliminated with these covariates (Maki et al., 2015; Sundermann et al., 2018). However, the current study adjusted for age, education, reading level with WTAR, as well as depressive symptom score, and the sex-specific effects in the sensorimotor domain remained. Therefore, our study provides additional evidence that HIV+ women may be even more vulnerable to cognitive deficits than HIV+ men, especially in the sensorimotor domain.
DTI Metrics and their Correlations with Neuropsychological Function across Groups
In addition to the poorer cognitive function, HIV+ participants, regardless sex, had lower FA in the basal ganglia and the uncinate fasiculi, and higher MD in multiple brain regions. Chronic neuroinflammation associated with ongoing HIV infection disrupts the microstructure in widespread brain regions which was shown consistently as lower FA and elevated diffusivity on DTI (Chang et al., 2008; O’Connor et al., 2017; Chang and Shukla, 2018; Liang et al., 2018). The relatively lower FA in HIV+ women in the CC, UNC and GP suggested that compared to men, women might have higher immune activation or neuroinflammation in these brain regions during chronic HIV infection. For instance, one study showed that dendritic cells (pDCs) derived from women produce significantly more interferon- α (IFN-α) in response to HIV-1-encoded Toll-like receptor (TLR7) ligands compared to those derived from men; furthermore, treatment naïve, chronically HIV-infected women also had significantly higher levels of CD8+ T cell activation compared to men who had the same level of HIV-1 viral load (Meier et al., 2009). Such enhanced immune activation in HIV+ women may indirectly contribute to the lower FA and higher brain mean diffusivity, indicating greater regional neuroinflammation, as observed in some brain regions in the current study.
Other reports also support the hypothesis that HIV+ women might have greater immune activation than HIV+ men. Prior to the cART era, HIV+ women progressed to AIDS at the same or even faster rate than men despite higher baseline CD4 count and lower plasma viral load (Farzadegan et al., 1998; Prins et al., 1999). Following cART, even with better clinical outcome than HIV+ men, HIV+ women consistently showed higher levels of plasma inflammatory markers, such as MCP-1, IL-10, sCD14, interferon γ, TNF-α and less decreased of C-reactive protein (Krebs et al., 2016; Mathad et al., 2016) or higher levels of inflammation burden (summarized from TNF-α, MCP-1 and sCD14) (Montoya et al., 2019). The mechanisms underlying the greater immune activation to HIV in women than in men remain unclear (Rubin et al., 2019). However, HIV+ women might have greater exposure to stressful life events (Machtinger et al., 2012) and therefore might have greater immune activation in response to the stress (do Prado et al., 2017). Other possible mechanisms involve female hormones, such as estrogen deficiency or dysfunction (Scherzer et al., 2015; Villa et al., 2016), gut microbiome-brain axis interactions and genetic factors (Scully, 2018; Rubin et al., 2019).
In our study, higher FA in the CC and UNC correlated with better cognitive function across all subjects; however, the correlation between CC-FA and UNC-FA and sensorimotor function was observed only in men but not in women regardless of HIV serostatus. Sensorimotor function is known to be correlated with CC-FA (Gooijers et al., 2013); the lack of such correlation in women might be due to other factors that contribute to the sensorimotor performance, such as finger size and learning strategy (Bryden and Roy, 2005; Grohs et al., 2018). A study in preschool children, however, showed that CC-FA correlated with better sensorimotor function in girls but not in boys (Grohs et al., 2018). Future studies with larger sample sizes across different age groups are needed to delineate these sex-differences in the relationship between DTI metrics and sensorimotor function.
HIV+ women in this study had lowest sensorimotor function and lowest globus pallidus_FA, suggestive of lesser neuronal integrity, amongst all participants. Although globus palidus is involved in complicate motor function including motor initiation and adjustment (Obeso et al., 2000; Kuoppamaki et al., 2005), GP-FA did not correlated with sensorimotor function in our participants. Similarly, lower GP_FA also did not correlate with Groove Pegboard test performance, but it did correlate with a multi-digit synergy metric, in a group of asymptomatic welders (Lewis et al., 2016). Therefore, the Groove Pegboard test may not be sensitive for detecting globus palidus dysfunction. Since HIV+ women had relatively lower GP_FA, indicating more impaired neuronal integrity in the GP, compared with HIV+ men, HIV+ women may need to be assessed with more specific sensorimotor function tasks to monitor their HIV treatment outcome.
In this study, women had lower FA in the CC and caudate, and higher MD in the thalamus, than men, regardless of HIV serostatus, which is consistent with prior findings in healthy volunteers (Pizzini et al., 2009; Liu et al., 2010; Menzler et al., 2011). FA is indeed more sensitive for detecting sexual dimorphism in the white matter microstructure. For example, compared to women, men had less dense but thicker fibers in the CC, and therefore had higher FA (Pizzini et al., 2009; Liu et al., 2010; Menzler et al., 2011), but similar MD (Pizzini et al., 2009; Liu et al., 2010). In addition, lower FA and higher radial diffusivity in the thalamus were also reported in healthy women than in men (Menzler et al., 2011).
Limitations
Our study has several limitations. First, due to the lower prevalence of HIV infection in women, the current study had a relatively small sample size for assessing the sex-specific effects on cognition and DTI measures. Second, possibly also due to the small sample size, HIV-by-sex interaction on FA was observed only in the globus pallidus, with trends for significance in the CC and UNC. Therefore, future study with a larger sample size may clarify these possible sex-specific regional effects on FA. Third, our DTI data were measured using an automated atlas with relatively large regions of interest, which might have lower sensitivity to detect WM microstructural abnormalities in specific fiber tracts; tractography may be more sensitive for detecting smaller regional effects. Finally, while severe substance use disorders (for example, methamphetamine, cocaine, hallucinogen and opioids) were exclusionary in our study, the use of; mild and moderate amounts of alcohol, tobacco and marijuana were allowed in the study, and these substances are known to have effects on white matter microstructure (Pfefferbaum et al., 2007; Orr et al., 2016; Liang et al., 2018). Although our four participant groups did not differ in daily and lifetime usage patterns for these substances, the variance due to substance use might be larger than, and could confound, the outcome measures. Future studies with larger sample size may allow further delineation of co-morbid substance use on sex-specific effects in HIV-positive individuals.
Conclusion
Our study confirms prior findings of greater cognitive impairment in HIV+ women compared to HIV+ men, and extends these sex-specific abnormalities to microstructural (DTI) measures. The abnormally lower FA in GP suggests lesser neuronal integrity in this brain region in HIV+ women, but not in HIV+ men. Likewise, the abnormally slower sensorimotor function in HIV+ women, but not in HIV+ men, relative to same-sex controls also suggests sex-specific effect on the sensorimotor network. These findings may be related to the greater immune activation, and its effects on neuroinflammation, found in HIV+ women than in HIV+ men.
Acknowledgments
This study was supported by NIH grants: National Institute on Mental Health (R01-MH61427); National Institute on Drug Abuse (2K24-DA16170); National Institute of Neurological Disorders and Stroke (U54-NS56883); National Institute on Minority Health and Health Disparities (G12-MD007601); National Institute on Aging (R01AG034852-09). We are grateful to our research participants, the referral physicians and community providers, in Honolulu, Hawaiì. We also appreciate the meticulous and hard work from the multiple clinical and technical research staff members who assisted in the data collection, and David Greenstein, B.A., for DTI image processing.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM, Multicenter ACS (2011) Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman-Lay AM, Paul RH, Heaps-Woodruff J, Baker LM, Usher C, Ances BM (2016) Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. J Neurovirol 22:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden PJ, Roy EA (2005) A new method of administering the Grooved Pegboard Test: performance as a function of handedness and sex. Brain Cogn 58:258–268. [DOI] [PubMed] [Google Scholar]
- Burlacu R, Umlauf A, Luca A, Gianella S, Radoi R, Ruta SM, Marcotte TD, Ene L, Achim CL (2018) Sex-based differences in neurocognitive functioning in HIV-infected young adults. AIDS 32:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceritoglu C, Oishi K, Li X, Chou MC, Younes L, Albert M, Lyketsos C, van Zijl PC, Miller MI, Mori S (2009) Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. Neuroimage 47:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Shukla D (2018) Imaging Studies of the HIV-Infected Brain. In: The Neurology of HIV Infection for Handbook of Clinical Neurology, 3 Edition (Brew BJ, ed), pp 229–264: Elsevier. [DOI] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T (2008) Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol 3:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DG, Zimmermann N, Tukamoto G, Doring T, Ventura N, Leite SC, Cabral RF, Fonseca RP, Bahia PR, Gasparetto EL (2016) Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging 44:1262–1269. [DOI] [PubMed] [Google Scholar]
- do Prado CH, Grassi-Oliveira R, Daruy-Filho L, Wieck A, Bauer ME (2017) Evidence for Immune Activation and Resistance to Glucocorticoids Following Childhood Maltreatment in Adolescents Without Psychopathology. Neuropsychopharmacology 42:2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elicer I M, Byrd D, Clark US, Morgello S, Robinson-Papp J (2018) Motor function declines over time in human immunodeficiency virus and is associated with cerebrovascular disease, while HIV-associated neurocognitive disorder remains stable. J Neurovirol 24:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D (1998) Sex differences in HIV-1 viral load and progression to AIDS. Lancet 352:1510–1514. [DOI] [PubMed] [Google Scholar]
- Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, Selnes OA, Ostrow DG, Sacktor NC, Multicenter ACS (2017) Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV 4:e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooijers J, Caeyenberghs K, Sisti HM, Geurts M, Heitger MH, Leemans A, Swinnen SP (2013) Diffusion tensor imaging metrics of the corpus callosum in relation to bimanual coordination: effect of task complexity and sensory feedback. Hum Brain Mapp 34:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohs MN, Reynolds JE, Dewey D, Lebel C (2018) Corpus callosum microstructure is associated with motor function in preschool children. Neuroimage 183:828–835. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116. [DOI] [PubMed] [Google Scholar]
- Junaid KA, Fellowes S (2006) Gender differences in the attainment of motor skills on the Movement Assessment Battery for Children. Physical & occupational therapy in pediatrics 26:5–11. [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: Evaluation of chemotherapeutic agents. (MacLeod CM, ed), pp 191–205. New York: Columbia University Press. [Google Scholar]
- Krebs SJ, Slike BM, Sithinamsuwan P, Allen IE, Chalermchai T, Tipsuk S, Phanuphak N, Jagodzinski L, Kim JH, Ananworanich J, Marovich MA, Valcour VG, team Ss (2016) Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS 30:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuoppamaki M, Rothwell JC, Brown RG, Quinn N, Bhatia KP, Jahanshahi M (2005) Parkinsonism following bilateral lesions of the globus pallidus: performance on a variety of motor tasks shows similarities with Parkinson’s disease. J Neurol Neurosurg Psychiatry 76:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MM, Lee EY, Jo HJ, Du G, Park J, Flynn MR, Kong L, Latash ML, Huang X (2016) Synergy as a new and sensitive marker of basal ganglia dysfunction: A study of asymptomatic welders. Neurotoxicology 56:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Chang L, Chen R, Oishi K, Ernst T (2018) Independent and Combined Effects of Chronic HIV-Infection and Tobacco Smoking on Brain Microstructure. J Neuroimmune Pharmacol 13:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Vidarsson L, Winter JD, Tran H, Kassner A (2010) Sex differences in the human corpus callosum microstructure: a combined T2 myelin-water and diffusion tensor magnetic resonance imaging study. Brain Res 1343:37–45. [DOI] [PubMed] [Google Scholar]
- Machtinger EL, Wilson TC, Haberer JE, Weiss DS (2012) Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS Behav 16:2091–2100. [DOI] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E (2009) HIV, cognition and women. Neuropsychol Rev 19:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K (2015) Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, Valcour V, Young MA, Becker JT, Martin EM, Neuropsychology Working Groups of the Women’s Interagency HIVS, the Multicenter ACS (2018) Differences in Cognitive Function Between Women and Men With HIV. J Acquir Immune Defic Syndr 79:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Robison E, Martin EM, Young M (2011) Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV- women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol 33:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P (2011) HIV+ men and women show different performance patterns on procedural learning tasks. J Clin Exp Neuropsychol 33:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathad JS, Bhosale R, Balasubramanian U, Kanade S, Mave V, Suryavanshi N, Gupte N, Joshi S, Chandanwale A, Dupnik KM, Kulkarni V, Deshpande P, Fitzgerald DW, Gupta A (2016) Quantitative IFN-gamma and IL-2 Response Associated with Latent Tuberculosis Test Discordance in HIV-infected Pregnant Women. Am J Respir Crit Care Med 193:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M (2009) Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, Hamer HM, Oertel WH, Rosenow F, Knake S (2011) Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 54:2557–2562. [DOI] [PubMed] [Google Scholar]
- Montoya JL, Campbell LM, Paolillo EW, Ellis RJ, Letendre SL, Jeste DV, Moore DJ (2019) Inflammation Relates to Poorer Complex Motor Performance Among Adults Living With HIV on Suppressive Antiretroviral Therapy. J Acquir Immune Defic Syndr 80:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Kimura D (1996) Sex differences for speech and manual skill. Percept Mot Skills 82:3–13. [DOI] [PubMed] [Google Scholar]
- O’Connor EE, Jaillard A, Renard F, Zeffiro TA (2017) Reliability of White Matter Microstructural Changes in HIV Infection: Meta-Analysis and Confirmation. AJNR Am J Neuroradiol 38:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EE, Zeffiro T, Lopez OL, Becker JT, Zeffiro T (2019) HIV infection and age effects on striatal structure are additive. J Neurovirol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW (2000) Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 23:S8–19. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S (2009) Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage 46:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Paschall CJ, Banich MT (2016) Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin 12:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale AE, Hooks BM (2018) Circuit changes in motor cortex during motor skill learning. Neuroscience 368:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130:48–64. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV (2009) Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS 23:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzini FB, Tassinari G, Zoccatelli G, Magon S, Alessandrini F, Rizzo P, Beltramello A (2009) Review of corpus callosum topography, analysis of diffusion values for the different callosal fibers and sex differences. Neuroradiol J 21:745–754. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC (1995) HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol 8:273–278. [DOI] [PubMed] [Google Scholar]
- Prins M, Robertson JR, Brettle RP, Aguado IH, Broers B, Boufassa F, Goldberg DJ, Zangerle R, Coutinho RA, van den Hoek A (1999) Do gender differences in CD4 cell counts matter? AIDS 13:2361–2364. [DOI] [PubMed] [Google Scholar]
- Qiao X, Lin H, Chen X, Ning C, Wang K, Shen W, Xu X, Xu X, Liu X, He N, Ding Y (2019) Sex differences in neurocognitive screening among adults living with HIV in China. J Neurovirol 25:363–371. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: A language and environment for statistical computing In. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Radloff LS (1977) The CES-D Scale:A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement 1:385–401. [Google Scholar]
- Royal W 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA (2016) Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PLoS One 11:e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Neigh GN, Sundermann EE, Xu Y, Scully EP, Maki PM (2019) Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr Psychiatry Rep 21:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, Villacres MC, Jiang X, Adimora AA, Waldrop-Valverde D, Vance DE, Bolivar H, Alden C, Martin EM, Valcour VG, Women’s Interagency HIVS (2017) Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 89:1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, Parker SB (1993) Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills 76:1219–1230. [DOI] [PubMed] [Google Scholar]
- Sanford R, Fellows LK, Ances BM, Collins DL (2018) Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol 75:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer R, Bacchetti P, Messerlian G, Goderre J, Maki PM, Seifer DB, Anastos K, Karim R, Greenblatt RM (2015) Impact of CD4+ lymphocytes and HIV infection on Anti-Mullerian Hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol 73:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A (2012) White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia 50:2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully EP (2018) Sex Differences in HIV Infection. Curr HIV/AIDS Rep 15:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Bishop JR, Rubin LH, Little DM, Meyer VJ, Martin E, Weber K, Cohen M, Maki PM (2015) Genetic predictor of working memory and prefrontal function in women with HIV. J Neurovirol 21:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Heaton RK, Pasipanodya E, Moore RC, Paolillo EW, Rubin LH, Ellis R, Moore DJ, Group H (2018) Sex differences in HIV-associated cognitive impairment. AIDS 32:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J, Cole JH, Caan M, De Francesco D, Leech R, van Zoest RA, Su T, Geurtsen GJ, Schmand BA, Portegies P, Prins M, Wit F, Sabin CA, Majoie C, Reiss P, Winston A, Sharp DJ, Comorbidity in Relation to AC (2017) Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin Infect Dis 65:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasylenko O, Gorecka MM, Rodriguez-Aranda C (2018) Manual dexterity in young and healthy older adults. 1. Age- and gender-related differences in unimanual and bimanual performance. Dev Psychobiol 60:407–427. [DOI] [PubMed] [Google Scholar]
- Villa A, Vegeto E, Poletti A, Maggi A (2016) Estrogens, Neuroinflammation, and Neurodegeneration. Endocr Rev 37:372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV (2009) Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage 44:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]