Abstract
Background:
Osimertinib is the treatment of choice for advanced EGFR-mutant non-small cell lung cancer (NSCLC). However, novel strategies to improve the duration of disease control are still urgently needed. Aspirin has been shown to decrease cancer incidence and improve outcomes in various malignancies. Therefore, we evaluated a cohort of patients who received osimertinib with or without concurrent use of aspirin to assess whether the addition of aspirin may lead to improved clinical outcomes.
Methods:
MD Anderson Cancer Center GEMINI database was retrospectively queried for EGFR-mutant NSCLC patients who received osimertinib with or without concurrent use of aspirin for progression-free survival (PFS) and overall survival (OS).
Results:
A total of 365 patients were identified including 77 which had concurrent use of aspirin. Patients in the aspirin-osimertinib group had significantly improved PFS (21.3 vs 11.6 months; HR, 0.52; 95% CI, 0.38 - 0.70) and OS (Not reached vs 32.3 months; HR, 0.56; 95% CI, 0.35 - 0.91) compared to osimertinib group. In subgroup analyses, the aspirin-associated PFS benefit was observed in patients with and without central nervous system (CNS) metastases, as well as in osimertinib first-line setting and in subsequent line setting. The median PFS in EGFR 19Del patients was longer than EGFR L858R patients with osimertinib, and when aspirin was added, the median PFS significantly improved in both groups regardless of lines of therapy. The benefit from aspirin was independent of age, gender, TP53 mutational status, or PD-L1 positivity.
Conclusion:
Concurrent aspirin use with osimertinib in EGFR-mutant NSCLC patients was associated with improved survival, regardless of lines of therapy, CNS metastatic status, EGFR mutation type, age, gender, TP53, and PD-L1 status.
Keywords: Non-small cell lung cancer, aspirin, osimertinib, EGFR, TP53, PD-L1
1. Introduction:
The treatment for advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations has evolved since the initial discovery of this oncogene-driver. [1-3] With the development of newer generations of tyrosine kinase inhibitors (TKIs), patients’ clinical benefit, measured by progression-free survival (PFS) and overall survival (OS), continues to improve. [4-6] Recently, osimertinib, a third-generation irreversible EGFR TKI, has demonstrated significant efficacy in metastatic EGFR-mutant NSCLC. In the AURA3 (NCT02151981) trial, osimertinib showed improved progression-free survival (PFS 10.1 vs 4.4 months) and objective response rate (ORR 71% vs 31%) when compared with platinum-pemetrexed in T790M-positive advanced NSCLC after first-line EGFR TKI.[7] Subsequently, the phase III FLAURA trial (NCT02296125) demonstrated a significant PFS (18.9 vs 10.2 months) and OS (38.6 vs 31.8 months) benefit when compared with erlotinib or gefitinib in the treatment-naïve patients.[8, 9] Currently in the United States, osimertinib is indicated both as the first-line treatment for metastatic EGFR-mutant NSCLC and as the subsequent treatment for patients with metastatic EGFR T790M-positive NSCLC after progression on other EGFR TKI therapy.
Despite the high rate of initial response, resistance to osimertinib inevitably emerges and patients ultimately succumb to the disease. [10-12] Therefore, therapeutic strategies that can further improve upon osimertinib in EGFR-mutant NSCLC patients are still urgently needed. Extensive efforts are being made on therapeutic combinations that might increase the long-term efficacy of osimertinib. For example, since the addition of anti-angiogenics have shown PFS benefit,[13, 14] ongoing trials are evaluating osimertinib with ramucirumab[15] or bevacizumab[16]. Also, the addition of chemotherapy with gefitinib demonstrated OS benefit compared to gefitinib alone,[17] so a large randomized phase 3 trial of osimertinib with chemotherapy to compare to osimertinib alone is now enrolling. [18] Although promising, these combinations come with a high risk of toxic effects and increased financial burden for patients. Identification of therapeutic strategies which are low-cost, have low toxicity, and improve osimertinib efficacy are beneficial to the patients and field.
There is evidence from observational studies that aspirin, a nonselective cyclooxygenase (COX) inhibitor, commonly used in patients with cardiovascular disease, is beneficial in reducing the incidence, metastasis, or the risk of death in various cancers.[19-22] In a recent study in patients with chronic hepatitis, the use of aspirin was associated with decreased incidence of liver cancer (4% vs 8.3%; HR 0.69; 95% CI, 0.62 - 0.76) in over 55, 000 patients.[23] In an analysis of over 10 million patients, aspirin use was associated with significantly reduced lung cancer risk (HR, 0.89; 95% CI, 0.84 - 0.94).[24] Furthermore, in a lung cancer preclinical study, Ogawa et al showed that cyclooxygenase (COX)-derived prostaglandin E2 (PGE2) is associated with poor prognosis by inducing tumor growth and metastasis, and aspirin use reduced the rate of metastasis to regional lymph nodes[25].
In EGFR-mutant NSCLC, a few lines of evidence indicate a potential benefit from aspirin usage to delay or overcome resistance from EGFR TKIs. Li et al. showed that aspirin use overcame acquired resistance to EGFR-TKI through inhibiting proliferation and promoting apoptosis of cancer cells using in vitro and in vivo models.[26] Han et al. reported that the combination of aspirin and osimertinib could induce strong antiproliferative and pro-apoptotic effects through inhibition of Akt/FoxO3a signaling component phosphorylation and increased Bim expression to promote apoptosis used in osimertinib-resistant NSCLC cell lines. Furthermore, they demonstrated that the use of aspirin was associated with improved median PFS (15.3 vs 9.3 months; p = 0.023) in a retrospective analysis of 45 patients with EGFR T790M mutant NSCLC.[27, 28] With aspirin being an inexpensive and relatively safe medication, it is of great interest to understand whether the concurrent use of aspirin enhances osimertinib’s benefit for EGFR-mutant NSCLC in a larger patient population. Here, we identified a cohort of 365 patients with advanced EGFR-mutant NSCLC treated with osimertinib and compared the clinical outcome of patients who had concurrent use of aspirin to the ones without. We further analyzed the impact of concurrent aspirin use in different clinical subgroups as well as different molecular subgroups.
2. Materials and Methods
2.1. Study population
We queried the GEMINI database, a University of Texas MDACC Lung Cancer Moon Shot funded internal database, and identified patients with advanced EGFR-mutant lung cancers treated with osimertinib from March 2014 to July 2019. We collected patient demographics, clinical characteristics, reasons for aspirin use, survival data, and tumor molecular profiles (data cut-off was in October 2019, when the dataset was locked for the outcome analysis, Figure. 1). The reasons for aspirin use were divided into 4 categories which were defined as below: 1. Primary prevention: for the individuals with 10-year risk of cardiovascular disease ≥ 30%; 2. Secondary prevention: for the individuals with established cardiovascular disease (as well as those who had deep venous thrombosis or atrial fibrillation); 3. Mild pain; 4. Unclear reason. The written informed consent was collected from all of the patients and the studies were conducted following ethical guidelines including the Declaration of Helsinki and U.S. Common Rule. Eligibility criteria included histologic or cytologic confirmation of NSCLC, recurrent or metastatic disease, EGFR-mutant, and receipt of osimertinib. Patients with osimertinib as adjuvant therapy or induction therapy, lost to follow-up after osimertinib commenced, withdrawn from treatment because of toxicity, receipt of osimertinib < 1 cycle were excluded from the analysis. The study was approved by the Institutional Review Boards at MDACC.
Figure 1. Consort diagram for the retrospective cohort.
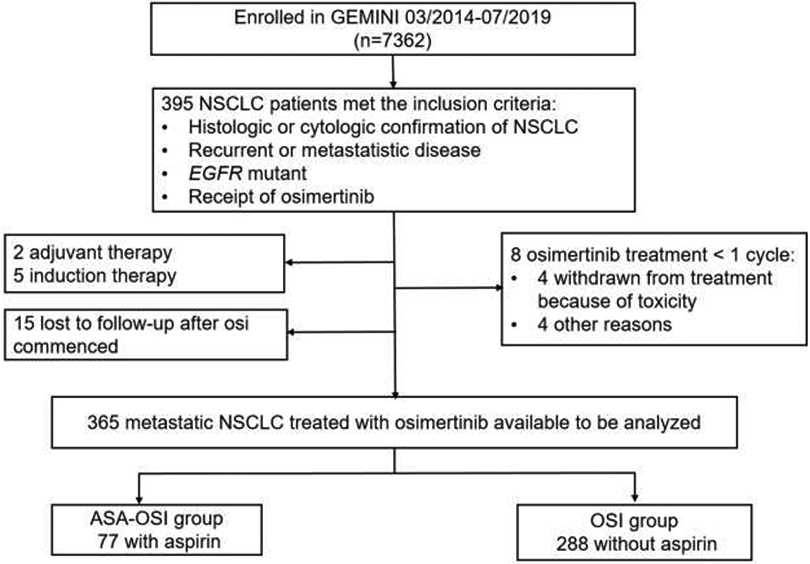
ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
2.2. Genomic profiling
The genomic profiling data were collected through molecular pathology reports. The cancer gene mutations including EGFR, TP53 were determined by next-generation sequencing panels of tumor tissue DNA (at MDACC Molecular Diagnostics Laboratory or FoundationOne - Foundation Medicine Inc.) or circulating tumor DNA (Guardant360 panel - Guardant Health). PD-L1 expression was assessed by immunohistochemistry using 22C3 pharmDx and quantified as a percentage of tumor cells expressing PD-L1 (TPS) at MDACC Molecular Diagnostics Laboratory.
2.2. Statistical analysis
Progression-free survival (PFS) was calculated from the date osimertinib treatment began to disease progression by the physician’s judgment, or death, whichever occurred first. Overall survival (OS) was defined as the time from the beginning of osimertinib treatment to death from any cause. The response was extracted from clinical notes, based on physician’s evaluation. Patients alive or the absence of disease progression at last follow-up were censored for analyses. The Kaplan–Meier method was used to estimate PFS and OS. The log-rank test was used to compare between-group differences. Fisher’s exact test was used to evaluate the impact of gender, smoking status, performance status, central nervous system (CNS) status, disease stage and previous lines of TKI therapy. The median age was determined by the Mann-Whitney test. All statistical analyses were performed on SPSS 24.0 statistical software package and p < 0.05 was considered statistically significant.
3. Results:
3.1. Patient characteristics
We identified a total of 395 patients with advanced EGFR-mutant NSCLC, whose tumor was not amenable to local treatment and had received osimertinib as systemic therapy (Figure. 1). Among them, 30 patients were excluded due to the following reasons: osimertinib used as adjuvant therapy or induction therapy (n = 7), lost to follow-up (n = 15), receipt of osimertinib < 1 cycle (28 days) (n = 8). In the total evaluable cohort of 365 patients, 77 patients received concurrent aspirin with osimertinib for at a minimum of one month (referred to as aspirin-osimertinib group hereafter). Among the 77 patients with aspirin use, 22 (29%) were treated for primary prevention, 38 (49%) for secondary prevention, 10 (13%) for treatment of mild pain, and remaining 7 (9%) for unclear reasons (Supplemental Table 1). There were no significant bleeding events in the aspirin-osimertinib group. Other common aspirin side effects (such as thrombocytopenia and gastrointestinal symptoms) are overlapping with osimertinib side effects, and therefore, they cannot be appropriately attributed. The remaining 288 patients received osimertinib without aspirin use (referred to as osimertinib alone group). The two groups were well-balanced in gender, histology, smoking status, disease stage, performance status and lines of TKI therapy (Table. 1). There were two differences between the two groups: (1) the incidence of CNS metastases prior to osimertinib treatment was lower in the aspirin-osimertinib group (27% vs 43%, p = 0.013), and (2) patients were older in the aspirin-osimertinib group (median age 69 vs 62 years; p < 0.001; Table 1). These differences are in line with clinical expectations as patients with brain metastases are less likely to use aspirin due to the concern of intracranial hemorrhage and older patients are more likely to use aspirin for treatment or prevention of cardiovascular diseases.
Table 1.
Patient demographics
| Overall | ASA-OSI group | OSI group | p Value | |
|---|---|---|---|---|
| No. of patient | 365 | 77 | 288 | NA |
| Median age, year (range) | 63 (27 - 87) | 69 (41 - 87) | 62 (27 - 82) | < 0.001 |
| Gender n (%) | ||||
| Male | 131 (35.9) | 33 (43.0) | 98 (35.0) | 0.181 |
| Female | 234 (64.1) | 44 (57.0) | 190 (65.0) | |
| Histology n (%) | ||||
| Adenocarcinoma | 352 (96.4) | 74 (96.1) | 278 (96.5) | 0.858 |
| Squamous cell carcinoma | 6 (1.6) | 1 (1.3) | 5 (1.7) | |
| Other | 7 (1.9) | 2 (2.6) | 5 (1.7) | |
| Smoking status n (%) | ||||
| Never | 259 (71.0) | 50 (65.0) | 209 (73.0) | 0.205 |
| Former/current | 106 (29.0) | 27 (35.0) | 79 (27.0) | |
| Performance status n (%) | ||||
| 0 - 1 | 236 (64.7) | 50 (64.9) | 186 (64.6) | 0.883 |
| 2 | 32 (8.8) | 6 (7.8) | 26 (9.0) | |
| 3 - 4 | 14 (3.8) | 2 (2.6) | 12 (4.2) | |
| Not known | 83 (22.7) | 19 (24.7) | 64 (22.2) | |
| CNS disease n (%) | ||||
| Yes | 145 (39.7) | 21 (27.0) | 124 (43.0) | 0.013 |
| No | 220 (60.3) | 56 (73.0) | 164 (57.0) | |
| Disease stage n (%) | ||||
| Recurrence | 78 (21.4) | 20 (26.0) | 58 (20.0) | 0.276 |
| Metastasis | 287 (78.6) | 57 (74.0) | 230 (80.0) | |
| Lines of TKI therapy n (%) | ||||
| 1 | 155 (42.5) | 38 (49.0) | 117 (41.0) | 0.195 |
| ≥ 2 | 210 (57.5) | 39 (51.0) | 171 (59.0) | |
| Previous lines of therapy n (%) | ||||
| 0 | 132 (36.2) | 32 (41.6) | 100 (34.7) | 0.272 |
| 1 | 139 (38.1) | 30 (39.0) | 109 (37.8) | |
| 2 | 53 (14.5) | 11 (14.3) | 42 (14.6) | |
| ≥ 3 | 41 (11.2) | 4 (5.1) | 37 (12.8) | |
Abbreviations: ASA-OSI - aspirin-osimertinib, OSI – osimertinib, CNS – central nervous system, TKI – tyrosine kinase inhibitor.
3.2. Concurrent use of aspirin with osimertinib was associated with improved survival
At the data cut-off on October 30, 2019, 64 (83% of 77) patients in the aspirin-osimertinib group and 207 (72% of 288) in the osimertinib alone group were still alive. The median follow-up was 14.3 months. The median PFS in the aspirin-osimertinib group (n = 77) was significantly longer than that in the osimertinib alone group (n = 288) (21.3 vs 11.6 months; HR, 0.52; 95% CI, 0.38 - 0.70; p < 0.001; Fig. 2A). Multivariable analysis showed that treatment with aspirin was independently associated with longer PFS (Table 2). The median OS in the aspirin-osimertinib group has not been reached after a follow up of 46.9 months, while the osimertinib alone group was 32.3 months, which also represents a significant difference (HR, 0.56; 95% CI, 0.35 - 0.91; p = 0.049; Fig. 2B).
Figure 2. Kaplan-Meier analyses of progression-free survival and overall survival.
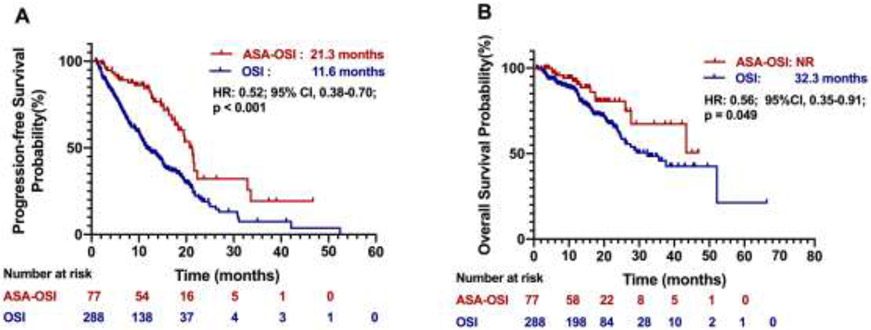
(A) Progression-free survival of patients in the ASA-OSI versus the OSI alone group, (B) Overall survival of patients in the ASA-OSI versus the OSI alone group. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Table 2.
Univariate and multivariate analyses for progression-free survival cohort
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Parameter | HR (95% CI) | p value | HR (95%CI) | p value |
| Aspirin use | ||||
| Yes vs. No | 0.48 (0.32 - 0.71) | < 0.001 | 0.45 (0.30 - 0.66) | < 0.001 |
| CNS | ||||
| No vs. Yes | 0.60 (0.45 - 0.81) | 0.001 | 0.58 (0.44 - 0.76) | < 0.001 |
| Age | ||||
| < 60 vs. ≥ 60 | 1.18 (0.88 - 1.56) | 0.260 | 0.73 (0.55 - 0.96) | 0.027 |
| Performance status | ||||
| 0 - 1 vs. ≥ 2 | 0.25 (0.16 - 0.38) | < 0.001 | 0.25 (0.17 - 0.28) | < 0.001 |
| Smoke status | ||||
| Never vs. Former/current | 0.98 (0.71 - 1.35) | 0.90 | 1.09 (0.8 - 1.47) | 0.570 |
| Gender | ||||
| Male vs. Female | 1.33 (0.99 - 1.78) | 0.062 | 1.22 (0.92 - 1.61) | 0.170 |
| Lines of TKI therapy | ||||
| Line 1 vs. Line ≥ 2 | 0.81 (0.59 - 1.11) | 0.190 | 0.68 (0.50 - 0.93) | 0.015 |
Abbreviations: CNS – central nervous system, TKI – tyrosine kinase inhibitor
As osimertinib was approved for both first- and subsequent-line treatment in EGFR-mutant NSCLC, we next performed subgroup analyses to evaluate aspirin’s benefit in different lines of osimertinib treatment. The median PFS of first-line osimertinib treatment in aspirin-osimertinib group (n = 38) has not been reached, while osimertinib alone group (n = 117) was 14.0 months (HR, 0.35; 95% CI, 0.20 - 0.62; p = 0.007; Fig. 3A). The median PFS of subsequent-line osimertinib treatment in aspirin-osimertinib group and osimertinib alone group was 19.6 months versus 10.9 months (HR, 0.50; 95% CI, 0.35 - 0.72; p = 0.002, Fig. 3B).
Figure 3. Kaplan–Meier estimates of progression-free survival for subgroups.
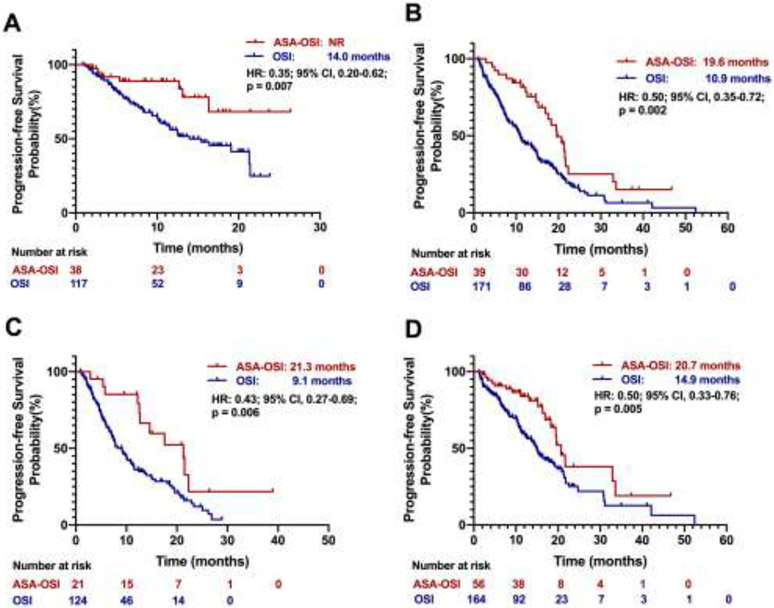
(A) Progression-free survival in osimertinib as the first-line treatment patients; (B) Progression-free survival in osimertinib as the subsequent-line treatment patients; (C) Progression-free survival in patients with baseline CNS metastases; (D) Progression-free survival in patients without baseline CNS metastases. CNS: central nervous system. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Next, we assessed whether the patients with CNS metastases also benefit from aspirin use. In our cohort of 365 patients, 145 (40%) had CNS metastasis at the time of osimertinib initiation. The PFS for patients who had baseline CNS metastasis was 21.3 months in the aspirin-osimertinib group (n = 21) compared to 9.1 months in the osimertinib alone group (n = 124) (HR, 0.43; 95% CI, 0.27 - 0.69; p = 0.006, Fig. 3C). For patients with no brain metastasis, the PFS was 20.7 versus 14.9 months in the aspirin-osimertinib (n = 56) and the osimertinib alone (n = 164) group respectively (HR, 0.50; 95% CI, 0.33 - 0.76; p = 0.005; Fig. 3D). For the 21 patients with CNS metastasis who had concurrent aspirin use with osimertinib, none of the patients had intracranial bleeding.
We also analyzed whether key clinical characteristics may impact the additional benefit from aspirin. The median PFS in females were 32.9 months versus 12.5 months (HR, 0.33; 95% CI, 0.22 - 0.50; p < 0.001; Supplemental Fig. 1A) in the aspirin-osimertinib (n = 44) and the osimertinib alone (n = 190) groups, respectively. In males (n = 33 vs 98), median PFS was 16.9 months versus 10.5 months (HR, 0.59; 95% CI, 0.37 - 0.95; p = 0.052; Supplemental Fig. 1B. In addition, we found that the PFS in the elderly group (age ≥ 60 years, n = 65 vs 175) were 21.3 versus 12.5 months (HR, 0.47; 95% CI, 0.33 - 0.68; p < 0.001; Supplemental Fig. 1C), while in the younger group (age < 60 years, n = 12 vs 113) were 20.7 versus 11.1 months (HR, 0.50; 95% CI, 0.25 - 0.98, p = 0.123; Supplemental Fig. 1D), respectively. No statistical significance in the younger group may be due to the limited sample size.
3.3. EGFR mutation type, TP53 and PDL1 status were not associated with the differential benefit of aspirin
It is known that lung cancers with EGFR exon 19 deletions (19Del) have a better prognosis than L858R mutations on exon 21.[29, 30] In our cohort, the 19Del patients (n = 212) had a better median PFS of 16.9 months compared to 13.0 months in L858R patients (n = 125, HR, 0.67; 95% CI, 0.50 - 0.91; p = 0.005; Fig. 4A), consistent with prior reports. When stratified by EGFR mutation type, we found that the median PFS of 19Del patients in the aspirin-osimertinib group (n = 50) was 20.7 compared to 14.2 months (HR, 0.49; 95% CI, 0.33 - 0.75; p = 0.006; Fig. 4B) in the osimertinib alone group (n = 162); whereas in L858R patients (n = 22 vs 103) the median PFS were 21.3 versus 11.5 months (HR, 0.44; 95% CI, 0.27 - 0.70; p = 0.009; Fig. 4B). We further analyzed the PFS for patients treated with osimertinib with or without aspirin in the first-line setting with regards to either 19Del or L858R. As shown in Supplemental Fig. 2, the median PFS was significantly longer in patients with 19Del in aspirin-osimertinib group (n = 22) compared to osimertinib alone group (n = 60) (Not reached vs 21.4 months; HR, 0.24; 95% CI, 0.10 - 0.60; p = 0.036). The same trend was observed in the L858R group (n = 13 vs 46), although the difference did not reach significance (16.3 vs 12.5 months; HR, 0.52; 95% CI, 0.22 - 1.77; p = 0.216). In patients with EGFR T790M mutation (n = 31 vs 114), the median PFS were 20.7 versus 13.5 months (HR, 0.44; 95% CI, 0.29 - 0.66; p = 0.001; Supplemental Fig. 3). These data suggest that the additional benefit from aspirin was not dependent on EGFR mutation type.
Figure 4. Kaplan–Meier estimates of progression-free survival based on gene status.
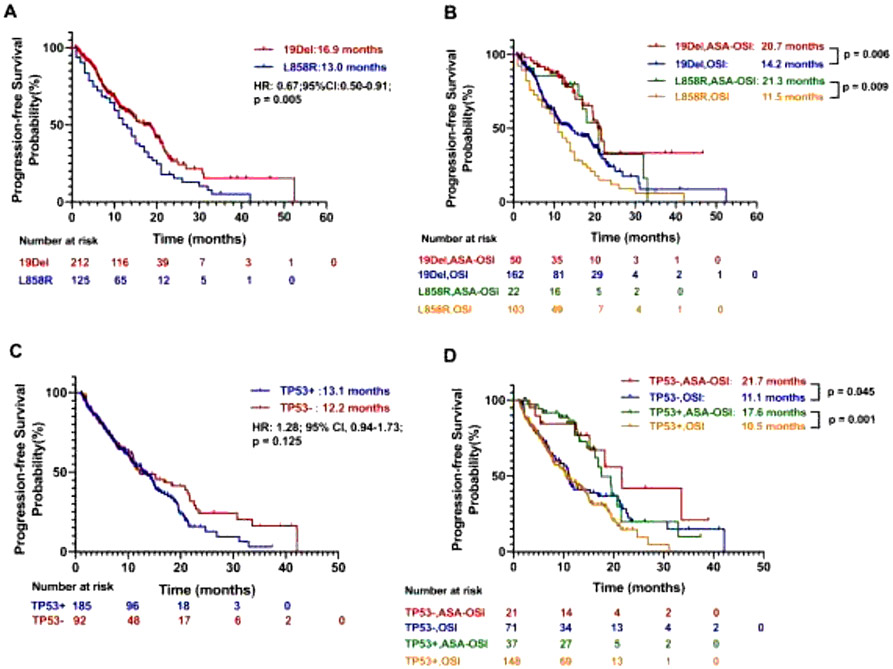
(A) Progression-free survival by EGFR 19Del versus L858R mutation; (B) Progression-free survival by EGFR 19Del versus L858R mutation and aspirin usage; (C) Progression-free survival by TP53 mutation status; (D) Progression-free survival by TP53 mutation status and aspirin usage. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Other than the EGFR mutation type, in some reports, co-occurring mutation of TP53 also impacts prognosis in EGFR-mutant NSCLC.[31, 32] In our cohort, we found no significant difference in median PFS between TP53 mutant patients (n = 185) and TP53 wild type (n = 92) with 13.1 versus 12.2 months (HR, 1.28; 95% CI, 0.94 - 1.73; p = 0.125; Fig. 4C). When analyzed by TP53 mutation status, the median PFS in TP53 wild type patients (n = 21 vs 71) were 21.7 versus 11.1 months (HR, 0.54; 95% CI, 0.29 - 0.99; p = 0.045, Fig. 4D), and the median PFS in TP53 mutant patients (n = 37 vs 148) were 17.6 versus 10.5 months (HR, 0.50; 95% CI, 0.33 - 0.76; p = 0.001; Fig. 4D) in aspirin-osimertinib versus osimertinib alone treated patients, respectively. These data suggest that the concurrent aspirin use confers benefit regardless of the TP53 mutational status.
Studies have shown that the clinical benefit with osimertinib was unaffected by PD-L1 expression status, [33] which was consistent with the results of our study (p = 0.293; Supplemental Fig. 4A), but aspirin might suppress cancer cell growth through PD-L1 related pathways.[34] We therefore evaluated whether PD-L1 status impacted the additional benefit of aspirin treatment in patients treated with osimertinib. Using equal or greater than 1% as the cut-off for being positive, the median PFS in PD-L1 positive patients (n = 27 vs 115) were 16.9 versus 10.2 months (HR, 0.61; 95% CI, 0.37 - 0.98; p = 0.071; Supplemental Fig. 4B). Similarly, the median PFS of PD-L1 negative patients in the aspirin-osimertinib group (n = 20) had not been reached yet, while the PFS in the osimertinib alone group (n = 73) was 11.1 months (HR, 0.42; 95% CI, 0.22 - 0.79; p = 0.036; Supplemental Fig. 4C), suggesting that the aspirin-osimertinib group had better median PFS regardless of PD-L1 status.
4. Discussion
In this study, we reviewed a cohort of patients with metastatic EGFR-mutated NSCLC treated with osimertinib with or without concurrent use of aspirin at MDACC. Our results demonstrated that the addition of aspirin to standard osimertinib therapy prolonged PFS and OS in EGFR-mutant NSCLC patients. Aspirin therapy with first-line osimertinib treatment (median PFS not yet reached) conferred the longest PFS in all subgroups analyzed. Furthermore, patients from different age groups, gender, EGFR mutation type, TP53 and PD-L1 status all benefited from the concurrent use of aspirin.
In this real-world dataset, it is worth noting that the PFS of first-line osimertinib alone group (14.0 months) was inferior to that from FLAURA trial [9] (18.9 months). This difference is likely due to different patient populations. Our real-world first-line osimertinib cohort (n = 117) included 17 patients (14.5%) with prior systemic chemotherapy or immunotherapy (anti-PD-1), 44 patients (37.6%) with CNS metastasis and 32 (30%) patients with performance status greater or equal to 2, all of which might be contributing factors to an inferior clinical outcome.
Due to the nature of the real-world retrospective study, this cohort of 365 patients had nearly 40% of patients having CNS metastasis at the time of osimertinib initiation. It could be related to physician’s choice due to the established efficacy of osimertinib in EGFR-mutant NSCLC patients with CNS metastases.[35-38] Our cohort demonstrated that the PFS in the osimertinib alone group was consistent with AURA3 in patients with measurable CNS metastasis (8.9 months).[39, 40] Interestingly, in the FLAURA study when osimertinib was used as first-line therapy, the median CNS PFS in patients with measurable and/or non-measurable CNS lesions treated with osimertinib was significantly better than that of the AURA3 trial, with CNS PFS not-reached (95% CI, 16.5 months to not reached). [41] We found when aspirin was added to the osimertinib treatment of CNS metastasis patients, the PFS emerged a substantial improvement to months from 9.1 months (p = 0.006). This significant improvement of PFS could be due to multiple factors. Our aspirin-osimertinib CNS group was small with only 21 patients. Furthermore, among them, 7 were treated as first line. 18 of the 21 patients received Gamma Knife or whole brain radiation therapy; 2 of the remaining 3 patients who did not have radiation achieved CR to osimertinib and 1 was asymptomatic with SD to osimertinib (Supplemental Table 2). Therefore, small sample size, first-line setting benefit and radiation therapy may all have contributed to the good outcome in this group of patients. Although our cohort was small at 21 patients with CNS metastasis and concurrent aspirin use, there was no intracranial bleeding, suggesting it is safe to use aspirin for EGFR-mutant NSCLC with controlled CNS metastasis to gain the potential PFS benefit.
It is known that patients with EGFR 19Del had better survival than EGFR L858R mutation, when treated with first-generation[42] and second-generation[43] EGFR TKI. One study showed that the significant OS benefit in EGFR 19Del group compared to EGFR L858R group (33.3 vs 26.4 months, p = 0.028) may be contributed by higher proportion of the EGFR T790M in the 19Del group compared to the L858R group (50.4% vs 36.5%, p = 0.043).[30] In this study, we also found that with the use of third-generation TKI (osimertinib), the median PFS in EGFR 19Del patients was significantly longer than EGFR L858R patients. Interestingly, when aspirin was added, the median PFS significantly improved in both groups to a similar duration (20.7 vs 21.3 months). When only first-line use of osimertinib was evaluated, removing the T790M rate as a confounding factor, the aspirin use continued to confer PFS benefit for both 19Del and L858R groups.
It is known that female patients often demonstrate better outcomes than males from EGFR TKI therapy.[44] Our data was generally in line with the prior report showing that the median PFS for females was significantly longer than that for males (32.9 vs 16.9 months, p = 0.036) when aspirin was added. To identify other potential confounding factors contributing to the long PFS in the female group, such as age, lines of therapy or mutation subtype, we further compared the female and male groups of patients. We found that 19Del was more common in females compared to males (75% vs 51%) within our cohort (Supplemental Table 3). What remains unclear is the magnitude of benefit that female patients derive from aspirin compared to the males. Although there was evidence that aspirin interacts with estrogen-related biological process [45-47] to reduce breast cancer risks for females, how the aspirin produces additional benefit in female patients receiving EGFR TKI still requires additional preclinical and clinical investigation.
The biological mechanisms underlying our observation that aspirin prolongs PFS for osimertinib are unclear, but could be from more than one process. Aspirin is a nonselective and irreversible inhibitor of COX-1 and COX-2 enzymes, and hence has many effects on human body. COX-2 enzymes are highly expressed in lung neoplasia[48] and shown as a potential contributor to EGFR TKI resistance,[49] possibly through epithelial-to-mesenchymal transition (EMT) that is related to prostaglandin E2 (PGE2) production downstream of COX-2.[50, 51] Previous studies have shown that aspirin inhibits the cyclooxygenase activity of the COX enzymes which leads to the decrease of PGE2 to suppress EMT. Decreased EMT potential has preventive or therapeutic effects in lung carcinogenesis[52] and overcomes TKI resistance in human EGFR-mutant NSCLC cell lines.[53] Other than suppressing PGE2 and MET, aspirin’s impact on apoptosis pathways could also be contributing to the additional cancer cell growth control. He et al. reported that the combination of aspirin and osimertinib significantly inhibited EGFR-mutant tumor cell growth through inhibition of Akt/FoxO3a signaling component phosphorylation and increased Bim expression to promote apoptosis.[27] Din et al. revealed that aspirin induced apoptosis through NFkB nuclear translocation, independent of p53 status. [54] In our study, we also found that aspirin’s benefit was independent of TP53 status.
Our study is the first to explore the clinical benefit of concurrent use of aspirin with osimertinib for patients with advanced NSCLC harboring EGFR mutations. In this real-world cohort of 365 patients, we found that EGFR-mutant NSCLC patients had prolonged PFS and OS from concurrent use of aspirin with osimertinib. Aspirin at low dose is relatively safe and inexpensive. An ongoing prospective randomized clinical trial (NCT04184921) will provide more direct evidence to advocate the use of aspirin with osimertinib in advanced EGFR-mutant lung cancer patients.
5. Conclusion
In summary, our study demonstrates that the addition of aspirin to osimertinib treatment in EGFR-mutated NSCLC patients significantly prolongs PFS and OS. The benefit remains regardless of lines of therapy, the presence of CNS metastasis, gender, age, EGFR mutation type, TP53 status or PD-L1 status.
Supplementary Material
Supplemental Figure 1. Kaplan–Meier estimates of progression-free survival for different gender and age groups. (A) Progression-free survival by aspirin usage in females; (B) Progress ion-free survival by aspirin usage in males; (C) Progression-free survival by aspirin usage in age ≥ 60 years patients; (D) Progression-free survival by aspirin usage in age < 60 years patients. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Supplemental Figure 2. Kaplan–Meier estimates of progression-free survival in 19Del and L858R mutation patients with osimertinib first line setting. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Supplemental Figure 3. Kaplan–Meier estimates of progression-free survival in T790M mutation patients. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Supplemental Figure 4. Kaplan–Meier estimates of progression-free survival regards to PD-L1 status. (A) Progression-free survival by PD-L1 status; (B) Progression-free survival by aspirin usage in PD-L1 positive patients; (C) Progression-free survival by aspirin usage in PD-L1 negative patients. PD-L1: programmed death ligand-1. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Highlights.
Concurrent aspirin use with osimertinib in EGFR-mutant NSCLC patients was associated with improved progression-free survival.
The benefit is independent of lines of therapy, CNS metastasis, EGFR mutation type, TP53 status, PD-L1 status, age or gender.
With aspirin use, the progression-free survival in L858R patients are similar to the EGFR 19Del patients.
Acknowledgement
This work is supported by the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Lung Moon Shot Program.
Funding support
Xiaoke Liu is supported by China Scholarship Council (CSC) under the Grant No. 201806240269. Jianjun Zhang is supported by MD Anderson Physician Scientist Award, NIH R01, AACR Johnson and Johnson Innovative Cancer Research Award, Khalifa Scholarship, and Conquer Cancer Foundation. Xiuning Le is supported by Calabresi Paul Award at MDACC (K12/NIH), Rexanna Foundation, Khalifa Scholarship, and Conquer Cancer Foundation. The funders are not involved in the design of the study, collection, analysis and interpretation of data, nor writing of this manuscript.
List of abbreviations:
- NSCLC
non-small cell lung cancer
- PFS
progression-free survival
- OS
overall survival
- NR
Not reached
- CNS
central nervous system
- TKIs
tyrosine kinase inhibitors
- COX
nonselective cyclooxygenase
- ORR
objective response rate
- EMT
epithelial-to-mesenchymal transition
- PGE2
prostaglandin E2.
Footnotes
Conflict of interest statement
Dr. Roth reports consultant; scientific advisor; ownership interest; inventor on intellectual property licensed by Genprex; PI on Genprex sponsored research; and has received grants from Varian Medical Systems ClinicalTrials.gov (STARS: NCT00840749). Dr. Heymach receives advisory/consulting fees from Bristol-Myers Squibb, GlaxoSmithKline, Kairos Venture Investments, BrightPath Therapeutics, Hengrui Therapeutics, Eli Lilly, EMD Serono, and Foundation One Medicine, Spectrum, AstraZeneca, and Research Funding from NIH/NCI, American Cancer Society, and Checkmate Pharmaceuticals, and AstraZeneca, Spectrum; and Royalties and Patents from Spectrum. Dr. Zhang reports research funding from Merck and Johnson and Johnson, personal fees from AstraZeneca, Bristol-Myers Squibb, GenePlus-Beijing Institute, Innovent outside the submitted work. Dr. Le receives consultant and advisory fee from Eli Lilly, AstraZeneca, EMD Serono, and research funds from Eli Lilly, Boehringer Ingelheim, and Spectrum Pharmaceuticals. All outside of the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy, Science 304(5676) (2004) 1497–1500. [DOI] [PubMed] [Google Scholar]
- [2].Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib, Proceedings of the National Academy of Sciences 101(36) (2004) 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib, New England Journal of Medicine 350(21) (2004) 2129–2139. [DOI] [PubMed] [Google Scholar]
- [4].Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial, The lancet oncology 15(2) (2014) 213–222. [DOI] [PubMed] [Google Scholar]
- [5].Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial, The lancet oncology 13(3) (2012) 239–246. [DOI] [PubMed] [Google Scholar]
- [6].Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma, New England Journal of Medicine 361(10) (2009) 947–957. [DOI] [PubMed] [Google Scholar]
- [7].Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Osimertinib or platinum–pemetrexed in EGFR T790M-positive lung cancer, New England Journal of Medicine 376(7) (2017) 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC, New England Journal of Medicine 382(1) (2020) 41–50. [DOI] [PubMed] [Google Scholar]
- [9].Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, Investigators F, Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer, New England Journal of Medicine 378(2) (2018) 113–125. [DOI] [PubMed] [Google Scholar]
- [10].Schoenfeld AJ, Yu HA, The Evolving Landscape of Resistance to Osimertinib, J Thorac Oncol 15(1) (2020) 18–21. [DOI] [PubMed] [Google Scholar]
- [11].Passaro A, Malapelle U, Attili I, de Marinis F, Overcoming resistance to osimertinib in non-small cell lung cancer: Hopes, doubts, and in-between, Cancer 126(11) (2020) 2594–2596. [DOI] [PubMed] [Google Scholar]
- [12].Le X, Puri S, Negrao MV, Nilsson MB, Robichaux J, Boyle T, Hicks JK, Lovinger KL, Roarty E, Rinsurongkawong W, Tang M, Sun H, Elamin Y, Lacerda LC, Lewis J, Roth JA, Swisher SG, Lee JJ, William WN Jr., Glisson BS, Zhang J, Papadimitrakopoulou VA, Gray JE, Heymach JV, Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC, Clin Cancer Res 24(24) (2018) 6195–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study, The lancet oncology 15(11) (2014) 1236–1244. [DOI] [PubMed] [Google Scholar]
- [14].Nakagawa K, Garon E, Seto T, Nishio M, Aix SP, Chiu C-H, Park K, Novello S, Nadal E, Imamura F, Relay: a Multinational, Double-Blind, Randomized Phase 3 Study of Erlotinib (ERL) in Combination with Ramucirumab (RAM) or Placebo (PL) in Previously Untreated Patients with Epidermal Growth Factor Receptor Mutation-Positive (EGFRM) Metastatic Non-Small Cell Lung Cancer (NSCLC), ONCOLOGY RESEARCH AND TREATMENT, KARGER ALLSCHWILERSTRASSE 10, CH-4009 BASEL, SWITZERLAND, 2020, pp. 112–112. [Google Scholar]
- [15].Study of Osimertinib With and Without Ramucirumab in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC), https://ClinicalTrials.gov/show/NCT03909334.
- [16].AZD9291 (Osimertinib) With or Without Bevacizumab as Initial Treatment for Patients With EGFR-Mutant Lung Cancer, https://ClinicalTrials.gov/show/NCT04181060.
- [17].Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer, Journal of Clinical Oncology 38(2) (2020) 124–136. [DOI] [PubMed] [Google Scholar]
- [18].A Study of Osimertinib With or Without Chemotherapy as 1st Line Treatment in Patients With Mutated Epidermal Growth Factor Receptor Non-Small Cell Lung Cancer (FLAURA2), https://ClinicalTrials.gov/show/NCT04035486.
- [19].Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW, Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials, Lancet 379(9826) (2012) 1602–12. [DOI] [PubMed] [Google Scholar]
- [20].Algra AM, Rothwell PM, Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials, Lancet Oncol 13(5) (2012) 518–27. [DOI] [PubMed] [Google Scholar]
- [21].Dickson I, Aspirin associated with lower risk of liver cancer, Nat Rev Gastroenterol Hepatol 17(5) (2020) 260. [DOI] [PubMed] [Google Scholar]
- [22].Jackson SS, Pfeiffer RM, Liu Z, Anderson LA, Tsai HT, Gadalla SM, Koshiol J, Association Between Aspirin Use and Biliary Tract Cancer Survival, JAMA Oncol 5(12) (2019) 1802–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF, Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality, New England Journal of Medicine 382(11) (2020) 1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ye S, Lee M, Lee D, Ha EH, Chun EM, Association of Long-term Use of Low-Dose Aspirin as Chemoprevention With Risk of Lung Cancer, JAMA Netw Open 2(3) (2019) e190185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ogawa F, Amano H, Ito Y, Matsui Y, Hosono K, Kitasato H, Satoh Y, Majima M, Aspirin reduces lung cancer metastasis to regional lymph nodes, Biomed Pharmacother 68(1) (2014) 79–86. [DOI] [PubMed] [Google Scholar]
- [26].Li L, Hu M, Wang T, Chen H, Xu L, Repositioning Aspirin to Treat Lung and Breast Cancers and Overcome Acquired Resistance to Targeted Therapy, Front Oncol 9 (2019) 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yong H, Rui H, P1. 03-27 Aspirin Overcomes Acquired Resistance to Osimertinib in Human Lung Cancer Cells via Bim-Dependent Apoptosis Induction, Journal of Thoracic Oncology 14(10) (2019) S428–S429. [Google Scholar]
- [28].Han R, Hao S, Lu C, Zhang C, Lin C, Li L, Wang Y, Hu C, He Y, Aspirin sensitizes osimertinib-resistant NSCLC cells in vitro and in vivo via Bim-dependent apoptosis induction, Molecular Oncology 14(6) (2020) 1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou J, Ben S, Comparison of therapeutic effects of EGFR-tyrosine kinase inhibitors on 19Del and L858R mutations in advanced lung adenocarcinoma and effect on cellular immune function, Thorac Cancer 9(2) (2018) 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH, Zhang XC, Xu CR, Yang JJ, Tu HY, Yan HH, Zhang YC, Niu FY, Wu YL, A Higher Proportion of the EGFR T790M Mutation May Contribute to the Better Survival of Patients with Exon 19 Deletions Compared with Those with L858R, J Thorac Oncol 12(9) (2017) 1368–1375. [DOI] [PubMed] [Google Scholar]
- [31].Ohsaki Y, Tanno S, Fujita Y, Toyoshima E, Fujiuchi S, Nishigaki Y, Ishida S, Nagase A, Miyokawa N, Hirata S, Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression, Oncology reports 7(3) (2000) 603–610. [DOI] [PubMed] [Google Scholar]
- [32].VanderLaan PA, Rangachari D, Mockus SM, Spotlow V, Reddi HV, Malcolm J, Huberman MS, Joseph LJ, Kobayashi SS, Costa DB, Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: Correlation with clinical outcomes, Lung Cancer 106 (2017) 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brown H, Vansteenkiste J, Nakagawa K, Cobo M, John T, Barker C, Kohlmann A, Todd A, Saggese M, Chmielecki J, Markovets A, Scott M, Ramalingam SS, Programmed Cell Death Ligand 1 Expression in Untreated EGFR Mutated Advanced NSCLC and Response to Osimertinib Versus Comparator in FLAURA, J Thorac Oncol 15(1) (2020) 138–143. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Lv C, Dong Y, Yang Q, Aspirin-targeted PD-L1 in lung cancer growth inhibition, Thorac Cancer 11(6) (2020) 1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gourd E, Osimertinib for leptomeningeal metastases in NSCLC, Lancet Oncol 21(1) (2020) e17. [DOI] [PubMed] [Google Scholar]
- [36].Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, Okamoto I, Leighl N, Hodge R, McKeown A, Brown AP, Rukazenkov Y, Ramalingam SS, Vansteenkiste J, CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR- Mutated Advanced Non-Small-Cell Lung Cancer, J Clin Oncol (2018) JCO2018783118. [DOI] [PubMed] [Google Scholar]
- [37].Gourd E, CNS efficacy of osimertinib in EGFR-mutated advanced NSCLC, Lancet Oncol 19(10) (2018) e516. [DOI] [PubMed] [Google Scholar]
- [38].Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crino L, de Marinis F, Felip E, Morabito A, Hodge R, Cantarini M, Johnson M, Mitsudomi T, Janne PA, Yang JC, CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials, Ann Oncol 29(3) (2018) 687–693. [DOI] [PubMed] [Google Scholar]
- [39].Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, Investigators A, Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer, N Engl J Med 376(7) (2017) 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, Hodge R, Kaur P, Brown AP, Ghiorghiu D, Papadimitrakopoulou VA, Mok TSK, CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3), J Clin Oncol 36(26) (2018) 2702–2709. [DOI] [PubMed] [Google Scholar]
- [41].Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer, Journal of Clinical Oncology 36(33) (2018) 3290-+. [DOI] [PubMed] [Google Scholar]
- [42].Zhang Y, Sheng J, Kang S, Fang W, Yan Y, Hu Z, Hong S, Wu X, Qin T, Liang W, Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis, PloS one 9(9) (2014) e107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yang JC-H, Wu Y-L, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu C-P, O'Byrne K, Feng J, Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials, The lancet oncology 16(2) (2015) 141–151. [DOI] [PubMed] [Google Scholar]
- [44].Jiang H, Zhu M, Li Y, Li Q, Association between EGFR exon 19 or exon 21 mutations and survival rates after first-line EGFR- TKI treatment in patients with non - small cell lung cancer, Molecular and clinical oncology 11(3) (2019) 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI, Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk, Jama 291(20) (2004) 2433–2440. [DOI] [PubMed] [Google Scholar]
- [46].DuBois RN, Aspirin and breast cancer prevention: the estrogen connection, Jama 291(20) (2004) 2488–2489. [DOI] [PubMed] [Google Scholar]
- [47].Díaz-Cruz ES, Shapiro CL, Brueggemeier RW, Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells, The Journal of Clinical Endocrinology & Metabolism 90(5) (2005) 2563–2570. [DOI] [PubMed] [Google Scholar]
- [48].Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T, Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas, Cancer Res 58(17) (1998) 3761–4. [PubMed] [Google Scholar]
- [49].Krysan K, Riedl K, Sharma S, Dohadwala M, Dubinett SM, PGE2 activates MAPK/Erk pathway in non-small cell lung cancer cells in an EGF receptor-independent manner, American Association for Cancer Research, 2004. [Google Scholar]
- [50].Dohadwala M, Yang S-C, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E2 induces transcriptional repressors ZEB1 and Snail in non–small cell lung cancer, Cancer research 66(10) (2006) 5338–5345. [DOI] [PubMed] [Google Scholar]
- [51].Krysan K, Lee JM, Dohadwala M, Gardner BK, Reckamp KL, Garon E, John MS, Sharma S, Dubinett SM, Inflammation, epithelial to mesenchymal transition, and epidermal growth factor receptor tyrosine kinase inhibitor resistance, Journal of Thoracic Oncology 3(2) (2008) 107–110. [DOI] [PubMed] [Google Scholar]
- [52].Xu X-C, COX-2 inhibitors in cancer treatment and prevention, a recent development, Anti-cancer drugs 13(2) (2002) 127–137. [DOI] [PubMed] [Google Scholar]
- [53].Hu X, Wu LW, Weng X, Lin NM, Zhang C, Synergistic antitumor activity of aspirin and erlotinib: Inhibition of p38 enhanced aspirin plus erlotinib-induced suppression of metastasis and promoted cancer cell apoptosis, Oncol Lett 16(2) (2018) 2715–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Din F, Stark L, Dunlop M, Aspirin-induced nuclear translocation of NF κ B and apoptosis in colorectal cancer is independent of p53 status and DNA mismatch repair proficiency, British journal of cancer 92(6) (2005) 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan–Meier estimates of progression-free survival for different gender and age groups. (A) Progression-free survival by aspirin usage in females; (B) Progress ion-free survival by aspirin usage in males; (C) Progression-free survival by aspirin usage in age ≥ 60 years patients; (D) Progression-free survival by aspirin usage in age < 60 years patients. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Supplemental Figure 2. Kaplan–Meier estimates of progression-free survival in 19Del and L858R mutation patients with osimertinib first line setting. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Supplemental Figure 3. Kaplan–Meier estimates of progression-free survival in T790M mutation patients. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.
Supplemental Figure 4. Kaplan–Meier estimates of progression-free survival regards to PD-L1 status. (A) Progression-free survival by PD-L1 status; (B) Progression-free survival by aspirin usage in PD-L1 positive patients; (C) Progression-free survival by aspirin usage in PD-L1 negative patients. PD-L1: programmed death ligand-1. ASA-OSI: aspirin-osimertinib. OSI: osimertinib.


