The cardiovascular disease (CVD) risk trajectory arguably begins in utero.1 Following birth, CVD risk is influenced by genetic and environmental factors.2 Considering that prenatal care is the sole access to health care for most women, obstetrics and gynecology (OBGYN) clinics present an important opportunity to track CVD risk trajectories of offspring from birth. To accomplish this goal, we need a measure of CVD risk suitable for clinical practice.
CVD risk can be estimated via arterial stiffness.3 As a biological marker of vascular aging, arterial stiffness occurs when an artery becomes less compliant and its ability to adequately expand and recoil is compromised.4 Following systole, forward-traveling pulse waves transmit through conduit vessels and partly reflect along the vasculature at sites of impedance mismatch. With stiffening, the reflected wave prematurely returns to the heart late in systole rather than diastole,4 increasing myocardial burden and contributing to CVD. Arterial stiffening can occur along the arterial tree, particularly along the aorto-iliac pathway, and can be noninvasively estimated via pulse wave velocity (PWV).4 PWV is calculated by measuring the transit time (TT) for the arterial waveform to pass between two points of a measured distance apart.4 Carotid-femoral PWV (cfPWV) predicts future CVD events,5 and international reference norms have been established for population, age, and risk factor strata.6
Recently, our group conducted a meta-regression analysis to determine the reported trajectory of cfPWV in children.3 We identified 9 articles (3 longitudinal and 6 cross-sectional) that included reference data for children. For the longitudinal studies, the age of the participants ranged from 14.5 to 15.1 years, with a 2–5-year follow-up. The age range across the cross-sectional studies was 6–18 y. From the meta-regression, the increase in cfPWV per year (age) was 0.12 (95% Confidence Interval (CI): 0.07, 0.16) m/s. The cfPWV intercept (0 years) was 3.61 (95% CI: 3.07, 4.16) m/s. These findings compliment those of a recent review from our group that reported that, in adults, cfPWV increases at a rate of 0.2 to 0.7 m/s every 5 years.7
While cfPWV may be suitable for assessments of arterial stiffness in children, it has not been rigorously studied in neonates. For neonates, the carotid and brachial waveforms have similar contours and likely provide comparable information.8 Thus, we measured brachial-femoral PWV (bfPWV), a less obtrusive measurement that can be conducted using oscillometry rather than tonometry and has been previously used in 2- to 6-week-old infants.9 Therefore, we aimed to (a) determine the feasibility of assessing bfPWV using an oscillometric technique in neonates and (b) measure the association between bfPWV and normative cfPWV data from the meta-regression. We hypothesized that (a) measuring bfPWV would be feasible using an oscillometric technique in neonates and (b) mean bfPWV values from neonates would overlap with the intercept (year 0) from the normative data generated from the meta-regression.
We measured bfPWV in 5 neonates (1–2 days old; mean weight 3.65 kg [95% CI: 3.10, 4.36 kg]) from healthy women. We used 2.5 cm wide oscillometric cuffs placed on the upper right arm and right thigh (Figure 1). Cuffs were inflated to subdiastolic blood pressures (~60 mm Hg), and pressure waveforms were recorded with the Vicorder (Skidmore Medical, Bristol, UK). The pulse wave path distance was measured from the substernal notch to the umbilicus. The cuffs were inflated for 3–5 heart cycles. If the neonate appeared distressed, the cuffs were deflated. Three measures were taken, and the closest 2 were averaged.
Figure 1.
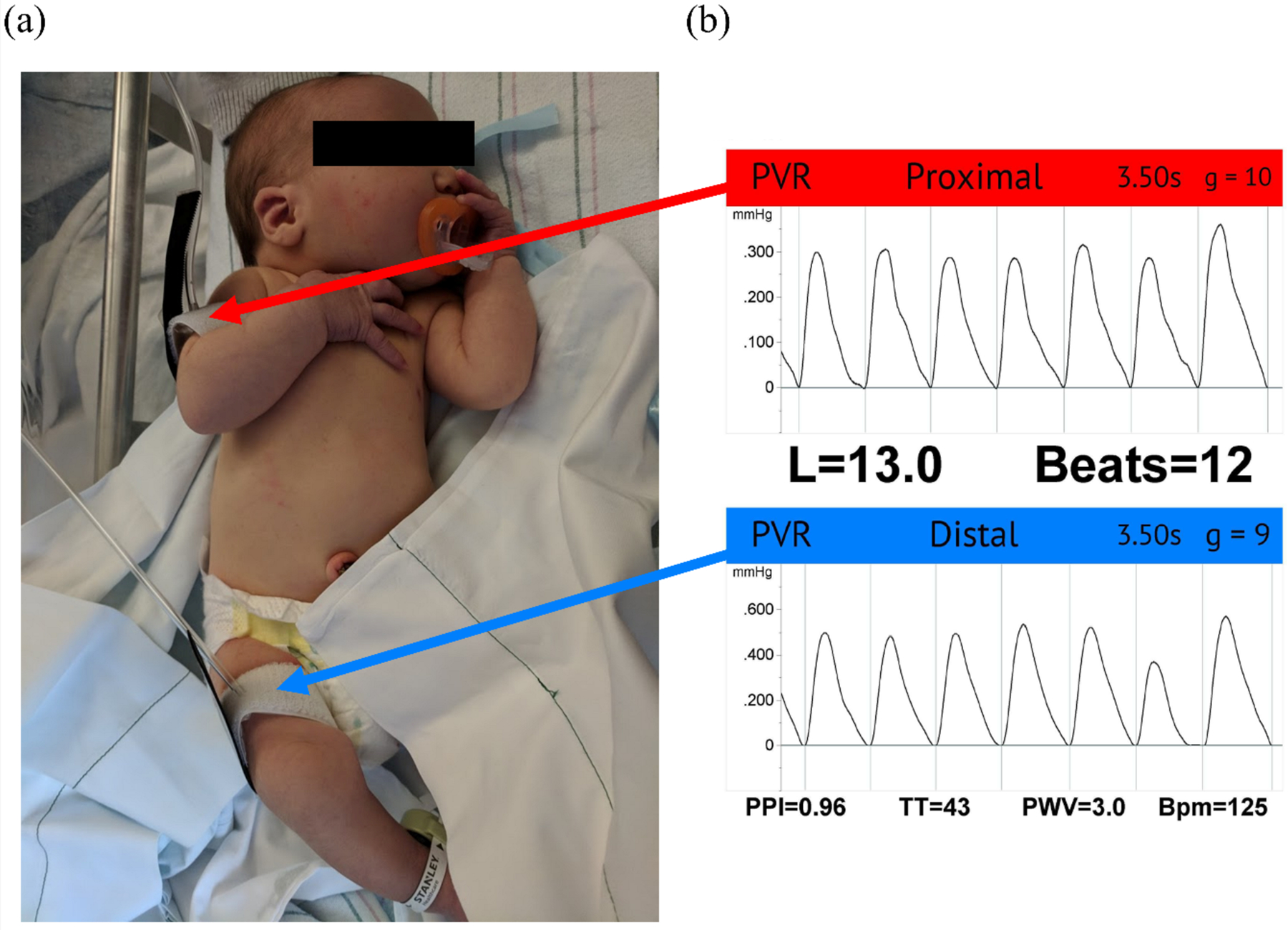
Neonate set-up and example waveform. (a) Neonate set-up: bfPWV was assessed using oscillometric cuffs, attached to the upper right arm and thigh. (b) Example waveform: (top) proximal cuff measurement located on the upper right arm, (bottom) distal cuff measurement located on the thigh.
We successfully obtained acceptable-quality bfPWV measurements for all neonates (n=5), with a mean bfPWV of 3.64 (95% CI: 3.31, 3.97) m/s (Table 1). These measurements were completed within several minutes for all but 1 neonate, who began to cry following cuff inflation. Acceptable measurements were obtained following reinflation.
TABLE 1.
Neonate Characteristics
| Mean (n=5) | SD | |
|---|---|---|
| Age (days) | 1.40 | 0.54 |
| Body weight (kg) | 3.65 | 0.52 |
| HR (bpm) | 107.40 | 18.79 |
| Beats used for calculation (bpm) | 6.50 | 2.92 |
| TT (s) | 35.30 | 5.12 |
| Length of segment under study (cm) | 13.20 | 0.84 |
| bfPWV (m/s) | 3.64 | 0.37 |
Abbreviations: kg, kilograms; HR, heart rate; BPM, beats per minute; TT, transit time; cm, centimeter; PWV, bfPWV, brachial-femoral pulse wave velocity; m/s, meters per second.
Measuring bfPWV using an oscillometric technique is feasible in healthy-term neonates and yields results that are comparable to published cfPWV data in children. As hypothesized, the mean bfPWV of 3.64 (95% CI: 3.31, 3.97) m/s from the neonates overlapped with the intercept [3.61 (95% CI: 3.07, 4.16) m/s] from the meta-regression analysis.
We showed that measuring bfPWV is feasible in neonates, supporting its use in future studies. The ability to measure bfPWV would allow the design of studies to evaluate the effect of modifying lifestyle behaviors as early as infancy, taking a primordial prevention approach to CVD. Primordial prevention focuses on preventing the development of CVD risk factors rather than the treatment of existing risk factors once they occur. Assessing bfPWV since birth can provide a unique opportunity to track individuals across the lifespan. OBGYN and pediatric clinics would be the ideal clinical settings to implement these methodologies, considering prenatal care is the initial access to health care for most neonates and pediatricians evaluate neonates through their development into adulthood. Compared to the standard cfPWV measurement, bfPWV measurement requires minimal training, takes a shorter time to ascertain measurements, and resembles typical blood pressure measurements, allowing for easier implementation in clinical settings. Future directions would be to implement bfPWV measurements in OBGYN and pediatric clinics to track CVD risk, detect early abnormalities, and enable the implementation of a primordial approach.
Source of funding:
NIH Building Interdisciplinary Research Careers in Women’s Health (5K12HD001441).
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA & Dolinsky VW Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci 55, 71–101 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Nabel EG & Braunwald E A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med 366, 54–63 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Stoner L, Kucharska-Newton A & Meyer ML Cardiometabolic Health and Carotid-Femoral Pulse Wave Velocity in Children: A Systematic Review and Meta-Regression. J. Pediatr 218, 98–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J 27, 2588–605 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol 63, 636–646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattace-Raso FUS, Hofman A, Verwoert GC, Wittemana JCM, Wilkinson I, Cockcroft J, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J 31, 2338–2350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucharska-Newton AM, Stoner L & Meyer ML Determinants of vascular age: An epidemiological perspective. Clin. Chem 65, 108–118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Rourke MF & Jiang APXJ Pulse wave analysis. Br. J. Clin. Pharmacol 51, 507–522 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alwan NA, Cade JE, McArdle HJ, Greenwood DC, Hayes HE, Ciantar E, et al. Infant arterial stiffness and maternal iron status in pregnancy: A UK birth cohort (Baby VIP Study). Neonatology 107, 297–303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


