SUMMARY
Objective:
HDL contains functional proteins that define single subspecies, each comprising 1 to 12% of the total HDL. We studied the differential association with coronary heart disease (CHD) of 15 such subspecies.
Approach and Results:
We measured plasma apolipoprotein A1 (apoA1) concentrations of 15 protein-defined HDL subspecies in four US-based prospective studies. Among participants without CVD at baseline, 932 developed CHD during 10 to 25 years. They were matched 1:1 to controls who did not experience CHD. In each cohort, hazard ratios (HRs) for each subspecies were computed by conditional logistic regression, and combined by meta-analysis. Higher levels of HDL subspecies containing alpha-2 macroglobulin, complement C3, haptoglobin, or plasminogen were associated with higher relative risk compared to the HDL counterpart lacking the defining protein (HR range 0.96 to 1.11 per 1 SD increase vs. 0.73 to 0.81, respectively; p heterogeneity <0.05). In contrast, HDL containing apoC1 or apoE were associated with lower relative risk compared to the counterpart (HR 0.74, p=0.002 and 0.77, p=0.001, respectively).
Conclusions:
Several subspecies of HDL defined by single proteins that are involved in thrombosis, inflammation, immunity and lipid metabolism are found in small fractions of total HDL and are associated with higher relative risk of CHD compared to HDL that lacks the defining protein. In contrast, HDL containing apoC1 or apoE are robustly associated with lower risk. The balance between beneficial and harmful subspecies in a person’s HDL sample may determine the risk of CHD pertaining to HDL and paths to treatment.
Keywords: High density lipoproteins, apolipoproteins, coronary heart disease
Subject terms: Lipids and cholesterol, coronary artery disease, epidemiology, cardiovascular disease, risk factors
INTRODUCTION
High-density lipoprotein (HDL) is a circulating protein-lipid complex that ranges in diameter from about 7 to 12 nm, much smaller than LDL and VLDL.1 The concentration of HDL, measured either by its principal protein component apolipoprotein A1 (apoA1) or cholesterol, strongly and consistently predicts cardiovascular disease (CVD),2 and is used for risk assessment.3
A typical HDL has 3–5 molecules of apoA1.4 ApoA1 is vital to the classical function of HDL because it activates steps in cholesterol transfers from peripheral cells to HDL and from HDL to the liver for excretion.5–7 ApoA2, the next most prevalent protein in HDL, is in about 60–80% of plasma HDL particles8, 9 and is inversely associated with CHD risk.10 HDL contains proteins in addition to apoA1 and apoA2,11, 12 many present on only a small percentage of the plasma total HDL.13 There is little known about most of these minor HDL proteins as regards risk of CHD, the exception being apoC3.14, 15 ApoC3 is present on 5–15% of HDL particles, more in people who are overweight, diabetic, or lacking regular exercise.16, 17 HDL that contains apoC3 predicts higher relative risk of a first coronary heart disease (CHD) event in four independent population-based cohorts.14
Proteins affect the functioning of HDL, not only in cholesterol metabolism, but also several other properties ascribed to HDL such as thrombosis, inflammation, oxidation, and innate immunity.12, 13, 18, 19 The correlation patterns of the HDL proteins appear to show functional networks that are localized to specific parts of the HDL size range.13, 19–21 It is possible that therapeutic attempts to prevent CHD by drugs that increase HDL failed22, 23 because they adversely affected the balance of beneficial and harmful HDL subspecies. Knowing the relationship to disease of protein-based HDL subspecies may improve risk prediction and targeting of treatments.24
We recently quantified and characterized the proteomes of 15 stable apoA1-containing HDL subspecies defined by content of a particular protein other than apoA1 or apoA213, comprising 1–12% of the total plasma apoA1 and involved in diverse metabolic processes. We call them “minor” HDL subspecies because they comprise a small percentage of the total apoA1. They are HDL that contain apoA4, apoC1, apoC2, apoC3, apoE, apoJ, alpha-1-antitrypsin (A1AT), alpha-2-macroglobulin (A2M), plasminogen (PLMG), fibrinogen (FBG), ceruloplasmin (CP), haptoglobin (HP), paraoxonase-1 (PON1), apoL1, or complement C3 (CoC3). We hypothesized that the HDL subspecies defined by content of a specific functional protein differ in their association with CHD compared to HDL that lacks the protein.
METHODS
Four cohorts were included in this study. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T. H. Chan School of Public Health, and participants gave informed consent. The data that support the findings of this study are available from the corresponding author on reasonable request.
Populations
We created nested case-control studies of CHD within four US-based cohorts, allowing each study to maintain a prospective design.25
MESA:
Between 2000 and 2002, the Multi-Ethnic Study of Atherosclerosis (MESA) enrolled 6,814 men and women aged 45–84 years from six regions in the U.S. who were free of clinical CVD.26 Information on demographics, anthropometrics, and medications was obtained at baseline through questionnaires and physical examinations. A random set of 1000 participants were used for piloting several assays and thus not available for this study. Among the remaining 5771 participants who were free of CHD, 275 incident CHD events were identified to have occurred by December 2013. In MESA, whites, blacks and Hispanics were included.
NHS:
The Nurses’ Health Study (NHS) enrolled 121,700 female nurses aged 30 to 55 years in 1976. Participants filled out questionnaires on lifestyle and medical history and have since been followed with biennial questionnaires to record newly diagnosed illnesses and to update lifestyle information.27 Between 1989 and 1990, a blood sample was requested from all active participants in NHS and collected from 32,826 women. Among participants who were free of CVD or cancer at blood draw, we identified incident cases of CHD and chose 235 women who were the youngest age at blood draw among those with 10 years or less of follow-up.
NHS-II:
The Nurses’ Health Study II (NHS-II) is a longitudinal cohort of 116,429 female nurses aged 25–42, followed with methods similar to NHS. The present study is based on the subset of 29,611 NHS-II participants who provided a blood sample between 1996–1997. Blood samples were collected when almost all the nurses were premenopausal. The blood samples were collected in the late luteal phase and only a small percentage were current users of oral contraceptives. Among nurses free of CVD or cancer at blood draw, we identified 144 women with incident CHD.
HPFS:
The Health Professionals Follow-Up Study (HPFS) enrolled 51,529 males aged 40 to 75 years in 1986. Blood samples were requested between 1993 and 1995 and obtained from 18,225 HPFS participants. This study comprises a subset of 18,018 HPFS members who provided a blood sample between April 1993 and August 1995 and who were not appreciably different from the total cohort.28 Among men free of CVD or cancer at blood draw, we identified 278 men with incident CHD.
Diagnosis of CHD
The diagnosis of CHD included first nonfatal myocardial infarction and fatal CHD without prior nonfatal myocardial infarction. In MESA, incident CHD events (myocardial infarction, resuscitated cardiac arrest, and CHD death) were ascertained and adjudicated as previously reported.29 In the NHS, NHS-II, and HPFS, the diagnosis of myocardial infarction was confirmed on the basis of the criteria of the World Health Organization (symptoms plus either diagnostic electrocardiographic changes or elevated levels of cardiac enzymes).30 Deaths were identified from state vital records and the National Death Index or reported by the participant’s next of kin or the postal system. Fatal CHD was confirmed by an examination of hospital or autopsy records, by the listing of CHD as the cause of death on the death certificate, if CHD was the underlying and most plausible cause, and if evidence of previous CHD was available.25, 31
Identification of controls
In each cohort, using risk-set sampling, controls were selected randomly and matched in a 1:1 ratio on age (1 year), smoking (never, past, current), and month of blood sample return, among participants who were free of cardiovascular disease at the time CHD was diagnosed in the case. In MESA, samples were additionally matched on racial group.
Blood collection and storage
In MESA, fasting blood was sampled at the baseline study visit and plasma stored at −70°C. In NHS, NHS-II, and HPFS cohorts, each interested participant was sent a blood collection kit containing instructions and needed supplies (blood tubes, tourniquet, gauze, bandage, needles). In the HPFS, blood was collected in tubes containing liquid EDTA; and in the NHS and NHS-II, heparin. The participants arranged for the blood to be drawn and then sent the sample back by overnight courier. Over 95% of the samples arrived within 24 hours of being drawn. A frozen water bottle was used as the coolant during transport of the blood to the laboratory for processing. Upon arrival in the laboratory, the whole blood samples were centrifuged and aliquoted into cryotubes as plasma, buffy coat, and red blood cells. These cryotubes are stored in the vapor phase of liquid nitrogen freezers; the highest temperature is - 130°C. All nitrogen freezers are alarmed and monitored continuously. Lifestyle and dietary characteristics are similar between participants who did and did not return blood samples in both cohorts except that those in the blood cohort were on average 1 year younger and were slightly less likely to smoke. In NHS, NHS-II and HPFS, approximately 75% bloods were drawn after ≥8-hour fast. A detailed study of measurements of lipids and apolipoproteins in freshly drawn blood and under conditions that simulated the blood sampling in the cohort showed no difference in the concentrations.28
The lipid laboratory at Harvard T. H. Chan School of Public Health received plasma samples on dry ice from all four studies.
Selection and measurement of HDL subspecies
As described in detail, we established a group of 15 HDL subspecies that spanned a range of HDL functions pertaining to atherosclerosis and CHD.13 The inclusion of a subspecies required that apoA1 concentrations were at least 1% of the plasma total apoA1 as determined by immunoaffinity chromatography containing antibody to a candidate protein, and ELISA to measure apoA1 in the bound and unbound fractions. Availability of antibodies that functioned properly in immunoaffinity column chromatography and ELISA was required. All told, 45 proteins were screened to obtain the panel of 15 HDL subspecies.
Novel modified sandwich ELISAs were developed to quantify the concentrations of the HDL subspecies defined by selected proteins. Each capture antibody was carefully chosen based on its ability to capture the subspecies-defining protein when it is part of an intact lipoprotein complex, thus in the absence of detergent. Selection of an antibody required recognition and strong binding of regions of the protein that are exposed on the surface of a lipoprotein complex. Conditions for all ELISAs were established such that they would bind 100% of the subspecies-defining protein as determined by there being no detectable amounts of that protein present in the unbound fraction.
The ELISA measurements used an absolute standard of the apoA1 concentration of the subspecies, established by the reference method, immunoaffinity chromatography. These protocols were validated against and calibrated by the standard reference protocol and have been described in detail previously.13 Plasma samples were diluted in phosphate-buffered saline and loaded in duplicate into 15 prepared 96-well microplates, each one coated with a different antibody corresponding to one of the 15 subspecies-defining proteins. Following overnight incubation at 4°C, the unbound fraction depleted of the HDL subspecies that contains the defining protein was removed, and plates were washed gently three times with PBS then loaded with Tween-containing diluent to dissociate the bound lipoprotein complexes. The dissociated sample was transferred to a prepared 96-well microplate coated with anti-apoA1 antibody, one per HDL subspecies. Bound apoA1 was quantified by detection through sequential incubations with biotinylated anti-apoA1, streptavidin, and o-phenylenediamine substrate. The subspecies-defining proteins remained bound to the initial 96-well microplates following dissociation and were quantified by detection with biotin-avidin or HRP-conjugated antibodies and o-phenylenediamine substrate. To minimize batch effects, case-controls pairs were loaded side-by side in the same ELISA plate in random order. Each plate contained three samples in duplicate of large-volume control pools with concentrations established by the reference method that were used to monitor and standardize batch effects. The lab was blinded to case status. ELISAs were judged on the quality of the calibration curve, the correlation of obtained and expected values of the control samples (required r>0.7), and the coefficients of variation for the unknown samples. Extreme outliers could be removed from calibration curves, but curves had to be produced from at least 4 of the 7 calibration curve points and show a fit of r2 >0.95. The average coefficient of variation (%CV) for all replicate samples could not exceed 15% for a plate to be accepted. Individual replicates whose %CV exceeded 20% were repeated.
Stability during short- and long-term storage.
We compared concentrations of apoA1 in HDL that contains apoC3 of lab control pools created in 2006, 2010, and 2013. There were four control pools each year comprised of entirely different individuals. There was no suggestion of systemic degradation in the 2006 pools (ranging from 9.7 to 12.7 mg/dL of apoA1 that contains apoC3) as compared to the 2013 pools (ranging from 5.4 to 11.6 mg/dL). Additionally, samples that had been collected between 2000 and 2002, analyzed in 2018, produced mean values for apoA1 that contains and that lacks apoC3 of 8.75 mg/dL and 121 mg/dL. These concentrations are similar to those of the 2006 and 2010 lab control pools, ranging from 6.1 to 12.1 mg/dL for apoA1 that contains apoC3 and 104 to 159 mg/dL for apoA1 that lacks apoC3. Thus, there is no suggestion of degradation during storage. Finally, as part of an extensive validation study, we compared the concentrations of apoA1 that contains or lacks apoC3 on fresh versus frozen samples and found no significant difference (n=20, mean 4.4mg/dL vs 4.5mg/dL, respectively, NS), and no effect of up to 5 freeze-thaw cycles (first versus fifth cycle 4.5 and 4.3 mg/dL, respectively, NS).
Statistics
Analyses were performed by Dr. Liang and Ms. He under the direction of Dr. Cai. All the analyses were done in the HPFS (men), NHS (women) and NHS-II (women) separately. In MESA, because of small sample sizes, Chinese-Americans were excluded and Hispanics were combined with the white group. The final number of matched case-controls pairs in MESA was 275. Because of strong heterogeneity between white and black participants, the two ethnicities were analyzed separately. Thus, MESA was analyzed in four separate subcohorts (i.e., black men and women, and white men and women).
We first assessed participant characteristics of each cohort using means and proportions of covariates of interest (defined in Supplemental Table I), estimated via inverse probability weighting to account for the nested case-control sampling design. Specifically, each case or control sample is weighted inversely proportional to its estimated sampling probability to recover the population characteristics. We further ran the meta-analysis on the four white cohorts to combine the results. Heterogeneity in hazard ratios was tested across the 4 cohorts. This same analysis was done for the two black cohorts.
We examined the association between apoA1 concentration (mg/dL) of each HDL subspecies and relative risk of CHD using loge-linear transformation adjusting for covariates, to determine the best functional form to model each HDL subtype. We modeled concentrations of each HDL subtype as continuous variables, in units of 1 standard deviation. The relative risk of incident CHD was analyzed using conditional logistic regression, where participants contributed person time from baseline until the date of an event, death, or end of follow-up, whichever occurred first. Women and men were analyzed separately in white and black ethnicities. White women and men were combined in view of non-heterogeneity. We did not combine the black women and men because of apparent heterogeneity.
Our nested case-control designs are matched on age, smoking, and fasting status. In all models, unless specified otherwise, we adjusted for other potential baseline confounders, namely hypertension, diabetes, body mass index, physical activity, and use of medications for hyperlipidemia. Because HDL that contains and HDL that lacks a protein, e.g. apoC1, sum to total HDL, all models simultaneously included the two subspecies. Finally, meta-analysis was done over the 4 cohorts of whites. The final model for determining relative risk associated with HDL subspecies included the covariates, the concentration of apoA1 in HDL that contains the protein; the concentration of apoA1 in HDL that lacks the protein; and the plasma total concentration of the protein. Additional models included plasma triglyceride to test independence of risk for the subspecies and plasma triglyceride. This analysis was done in MESA, the only cohort with complete triglyceride data. The primary outcome was heterogeneity in CHD relative risk between HDL that contains vs. lacks a defining protein. This expresses the central hypothesis of the study that protein-based minor HDL subspecies have CHD risks distinct from the majority of HDL that lacks the protein.
To develop CHD risk prediction models based on the HDL subspecies, we refrained from including information on covariates during follow-up (time-varying covariates) as that would allow changes in covariates over time to predict a greater proportion of risk whereas information on HDL subspecies and traditional CHD risk factors would only be assessed at a single time-point in the clinical scenario.
Multiple comparison adjustment with control for false discovery rate (FDR) was made when simultaneously assessing the contribution of subspecies information for all the subspecies. We used the FDR because we expected the log hazard ratio estimates from these separate models of the subspecies to be highly correlated, as in the genomic literature.32, 33 The hazard ratios for CHD risk pertaining to the apoA1 concentrations of the individual HDL subspecies were adjusted, as were separately the heterogeneity tests of differences in hazard ratios between HDL that contains and lacks a defining protein.
Statistical analyses were performed using SAS 9.4 (SAS Institute; Cary, NC) and R (R Core Team).
RESULTS
Baseline characteristics
A total of 1864 participants, 932 controls and 932 cases were studied (Table 1). Women comprised 958 (51%), men 906 (49%), whites 1694 (91%), and blacks 170 (9%). At the start of follow-up, mean age in MESA and HPFS was 61–62 y; NHS 56y; and NHS-II 44y. Diabetes was present in 4–26% among cohorts, highest in the MESA black cohorts (26%) and lowest in NHS-II (4%) and HPFS (5%). Body mass index ranged from 22 kg/M2 in NHS1, 26 kg/M2 in NHS-II and HPFS and 27–30 kg/M2 in MESA. Use of lipid lowering drugs was 16–23% in MESA and 5–7% in the other cohorts. Mean HDL-cholesterol concentration was similar across cohorts, higher in the women (55–61 mg/dL) compared to the men (44–45 mg/dL). Mean triglycerides ranged from 85 to 132 mg/dL. Mean LDL-cholesterol ranged from 117 to 142 mg/dL.
Table 1.
Baseline characteristics by cohort. Data are weighted mean (SD) or n (%).
| MESA | NHS | NHS-II | HPFS | ||||
|---|---|---|---|---|---|---|---|
| White | Black | White | White | White | |||
| Women | Men | Women | Men | Women | Women | Men | |
| Total, n | 136 | 244 | 64 | 106 | 470 | 288 | 556 |
| Age (y), mean (SD) | 62 (10) | 62 (11) | 62 (10) | 62 (10) | 56 (7) | 44 (4) | 61 (7) |
| BMI (kg/m2), mean (SD) | 27 (5) | 28 (4) | 30 (7) | 29 (4) | 23 (3) | 26 (5) | 26 (4) |
| Postmenopausal, n (%) | 126 (93) | NA | 55 (86) | NA | 379 (81) | 72 (25) | NA |
| Diabetes, n (%) | 11 (8) | 37 (15) | 17 (27) | 28 (26) | 56 (12) | 11 (4) | 30 (5) |
| Hypertension, n (%) | 87 (64) | 124 (51) | 50 (78) | 71 (67) | 185 (39) | 62 (22) | 177 (32) |
| Use of hyperlipidemia drugs, n (%) | 31 (23) | 55 (22) | 15 (23) | 17 (16) | 22 (5) | 21 (7) | 39 (7) |
| current smoker, n (%) | 12 (9) | 34 (14) | 12 (19) | 21 (20) | 142 (30) | 20 (7) | 57 (10) |
| no alcohol intake, n (%) | 70 (52) | 158 (65) | 15 (23) | 58 (55) | 185 (39) | 120 (43) | 123 (22) |
| HDL- cholesterol (mg/dL), mean (SD) | 62 (16) | 44 (11) | 55 (20) | 45 (11) | 57 (16) | ND | 44 (12) |
| LDL- cholesterol (mg/dL), mean (SD) | 133 (137) | 128 (89) | 117 (28) | 142 (167) | 140 (39) | ND | 127 (33) |
| triglyceride† (mg/dL), mean (SD) | 122 (101) | 131 (110) | 96 (56) | 85 (41) | 118 (79) | 97 (71) | 132 (91) |
| apoA1 (mg/dL), mean (SD) | 186 (59) | 143 (46) | 161 (50) | 148 (57) | 166 (33) | 157 (40) | 145 (30) |
Each case or control sample is weighted inversely proportional to its estimated sampling probability to recover the population characteristics. ND: not determined.
triglyceride concentrations presented as weighted median (interquartile range). Triglycerides were available for 100% of MESA participants, 88% of NHS, 42% of NHS-II, and 81% of HPFS.
MESA, Multiethnic Study of Atherosclerosis; NHS, Nurses’ Health Study; NHS-II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study.
ApoA1 concentrations of HDL subspecies
Because of differences in HDL subspecies concentrations and relative risks for the black cohorts compared to whites, the results for the two groups are shown separately. In whites, apoA1 concentrations of the minor HDL subspecies that contain a defining protein ranged from 1.2 mg/dL (apoC2) to 19 mg/dL (apoC1), 1% to 12% of plasma total apoA1 (Figure 1). White women compared to white men had higher apoA1 concentrations in total plasma and in most subspecies (Figure 1). If expressed as a percentage of total apoA1, the sex differences in the subspecies were smaller and mostly not significant. Concentrations for total plasma apoA1 and for apoA1 in each of the HDL subspecies are shown in Supplemental Table II.
Figure 1. ApoA1 concentrations of minor HDL subspecies defined by the indicated protein in whites.
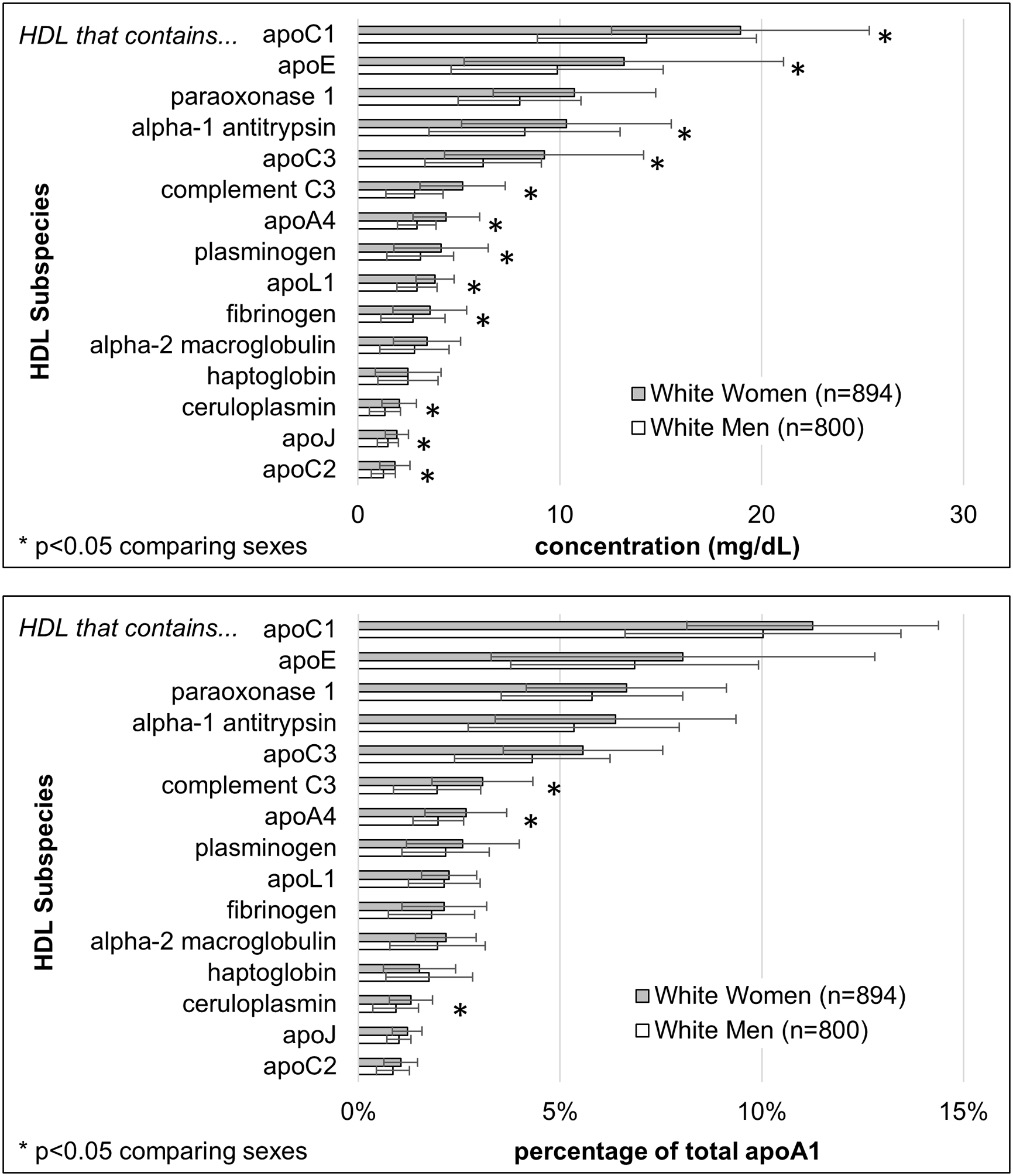
Upper panel: apoA1 concentrations (mean, SD); Lower panel: percentage of plasma total apoA1 (mean, SD). *p<0.05 comparing sexes. Data tables for the Black cohorts and the whites are in Supplemental Table II.
Associations with CHD
The primary analysis includes women and men combined (Figure 2). There were no significant differences in hazard ratios between white women and white men for the minor HDL subspecies (Supplemental Figure I). Tests of heterogeneity in hazard ratios across the 4 white cohorts for the 15 subspecies were not significant, p>0.5 for all subspecies. The primary outcome was heterogeneity in CHD relative risk between HDL that contains vs. lacks a defining protein.
Figure 2. Relative Risk of CHD for the apoA1 concentrations of HDL subspecies and of plasma total apoA1: white women and white men combined.
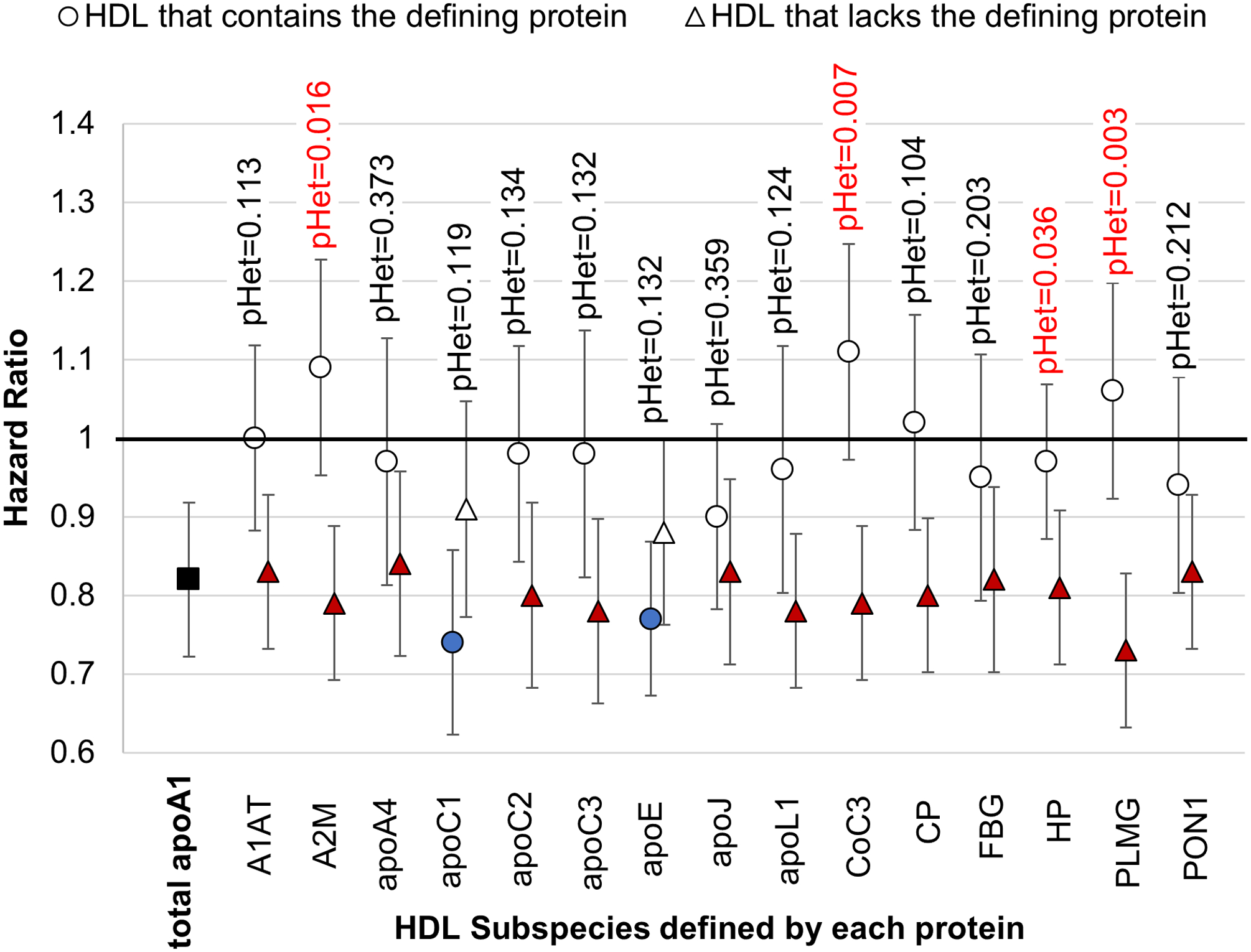
Hazard Ratios with 95% confidence intervals for HDL subspecies that contain or lack the indicated defining proteins with FDR-adjusted p-values for heterogeneity (pHet). Each subspecies was studied in a separate model (not mutually adjusted). Each model includes covariates, plasma total concentration of the protein that defines the subspecies, apoA1 concentration of the minor subspecies that contains the defining protein, and the apoA1 concentration of HDL that lacks the defining protein. Plasma total apoA1 is studied in a separate model that does not contain the subspecies.
HDL subspecies associated with higher CHD risk compared to the complementary subspecies:
Thirteen of the 15 minor HDL subspecies that contain a defining protein had higher hazard ratios (HRs) than the complementary HDL subspecies that lack the defining protein of which four showed statistical heterogeneity (Figure 2). The HR per each SD of HDL that contains complement C3 was 1.11 (95% CI 0.97–1.25), which was statistically significantly different from the HR of 0.79 (95% CI 0.69–0.89) for HDL that lacks complement C3 (p=0.007 for heterogeneity); similarly for alpha-2 macroglobulin, the HR of 1.09 (95% CI 0.95–1.23) was different from 0.79 (95% CI 0.69–0.89), (p-het=0.016); for plasminogen the HR of 1.06 (95% CI 0.92–1.20) was different from 0.73 (95% CI 0.63–0.83), (p-het=0.003); and finally for haptoglobin, the HR of 0.97 (95% CI 0.87–1.07) was significantly less inverse than the HR of 0.81 (95% CI 0.71–0.91) (p-het =0.036). The adjusted p-values for heterogeneity were 0.11 for HDL that contains alpha1 antitrypsin, 0.13 for apoC2, 0.13 for apoC3, 0.12 for apoL1, and 0.10 for ceruloplasmin. The remaining 4 subspecies, which contain apoA4, apoJ, fibrinogen, or PON1, had smaller differences in HRs between the subspecies that contain or lack the protein, and heterogeneity p-values of > 0.15 (Figure 2). These results also show that when one of the 4 minor HDL subspecies with significant p-values for heterogeneity was removed from the plasma sample by immunochemical methods, the HR for the remaining HDL that lacks the protein, comprising the majority of HDL, became more inverse, and lower than that of the total apoA1. This indicates that the relative risk for CHD of total apoA1 HDL concentration is affected by its concentration of minor subspecies.
HDL subspecies associated with lower relative risk:
HDL that contains apoC1 had the lowest HR (0.74 per SD increase; 95% CI 0.62–0.86; p=0.002) (Figure 2). In contrast, HDL that lacks apoC1, about 90% of plasma total HDL, was not associated with CHD risk (HR 0.91; 95% CI 0.78–1.06, p=0.32). The adjusted p for heterogeneity value was 0.12 (testing whether the HRs were the same). Similarly, the hazard ratio for HDL that contains apoE was 0.77 (95% CI 0.68–0.88, p=0.001) and for HDL that lacks apoE 0.88 (95% CI 0.76–0.99, p=0.10) (p for heterogeneity for the difference = 0.13).
In addition to the analysis of the 15 minor HDL subspecies, we found that HDL that contains apoA2, the most prevalent HDL subspecies comprising 80% of total apoA1, was associated inversely with CHD; HR = 0.81 (95% CI 0.67–0.95, p=0.032), and similar to that for plasma total apoA1, HR = 0.82, p=0.001. HDL that lacks apoA2 was not significantly associated with CHD, HR = 0.89 (95% CI 0.75–1.03, p= 0.18). However, their HRs, 0.81 for HDL that contains apoA2 and 0.89 for HDL that lacks apoA2, were not significantly different, p=0.68.
Independence of HDL subspecies from plasma total apoA1
The correlation between the concentration of plasma total apoA1 and the apoA1 concentrations of minor subspecies that contain a defining protein ranged from 0.2 to 0.5 (Table 2), indicating that the apoA1 concentrations of the minor subspecies are largely independent of that of the total apoA1. The hazard ratios for the minor HDL subspecies were not affected by adjustment for plasma total apoA1 rather than the complementary major subspecies (Supplemental Figure II).
Table 2.
Correlation coefficients between plasma apoA1 concentrations of HDL subspecies. White women and men combined.
| HDL that contains… | |||||||||||||||||
| A1AT | apoA4 | A2M | apoC1 | apoC2 | apoC3 | CoC3 | CP | apoE | FBG | HP | apoJ | apoL1 | PLMG | PON1 | WPA1 | ||
| HDL that contains… | A1AT | 1 | 0.39 | 0.45 | 0.35 | 0.43 | 0.27 | 0.52 | 0.52 | 0.17 | 0.37 | 0.31 | 0.41 | 0.19 | 0.37 | 0.22 | 0.22 |
| apoA4 | 1 | 0.39 | 0.52 | 0.53 | 0.32 | 0.40 | 0.46 | 0.45 | 0.51 | 0.20 | 0.52 | 0.22 | 0.40 | 0.34 | 0.50 | ||
| A2M | 1 | 0.32 | 0.50 | 0.28 | 0.56 | 0.61 | 0.31 | 0.40 | 0.22 | 0.48 | 0.23 | 0.49 | 0.40 | 0.27 | |||
| apoC1 | 1 | 0.40 | 0.48 | 0.38 | 0.42 | 0.51 | 0.38 | 0.31 | 0.49 | 0.13 | 0.32 | 0.40 | 0.49 | ||||
| apoC2 | 1 | 0.26 | 0.47 | 0.52 | 0.20 | 0.50 | 0.28 | 0.40 | 0.27 | 0.54 | 0.38 | 0.32 | |||||
| apoC3 | 1 | 0.31 | 0.31 | 0.31 | 0.31 | 0.24 | 0.33 | 0.22 | 0.32 | 0.30 | 0.33 | ||||||
| CoC3 | 1 | 0.52 | 0.26 | 0.52 | 0.25 | 0.47 | 0.11 | 0.46 | 0.39 | 0.25 | |||||||
| CP | 1 | 0.25 | 0.48 | 0.27 | 0.38 | 0.23 | 0.53 | 0.48 | 0.32 | ||||||||
| apoE | 1 | 0.21 | 0.06 | 0.50 | 0.04 | 0.15 | 0.22 | 0.33 | |||||||||
| FBG | 1 | 0.25 | 0.44 | 0.25 | 0.49 | 0.43 | 0.25 | ||||||||||
| HP | 1 | 0.18 | 0.23 | 0.17 | 0.17 | 0.24 | |||||||||||
| apoJ | 1 | 0.19 | 0.36 | 0.49 | 0.34 | ||||||||||||
| apoL1 | 1 | 0.14 | 0.24 | 0.23 | |||||||||||||
| PLMG | 1 | 0.40 | 0.28 | ||||||||||||||
| PON1 | 1 | 0.26 | |||||||||||||||
Combining all 15 minor subspecies and plasma total apoA1
A secondary analysis included the plasma concentrations of the 15 minor HDL subspecies and plasma total apoA1 altogether in a single multivariable model. The HR for the minor HDL subspecies (Figure 3) are qualitatively similar to those in models in which only one of the subspecies was included (Figure 2). ApoL1 was an exception, having a significant HR of 1.25 in the combined model compared to 0.96 in the model that included only the apoL1 subspecies. A log-likelihood ratio test showed that this model explains significantly more of the variance in risk of CHD compared to the model that has just plasma total apoA1, true for both women (p=0.021) and men (p=0.01). The beta coefficients and hazard ratios for all variables from this all-inclusive model are provided in Supplemental Table III.
Figure 3. All minor HDL subspecies and total apoA1 included in one model.
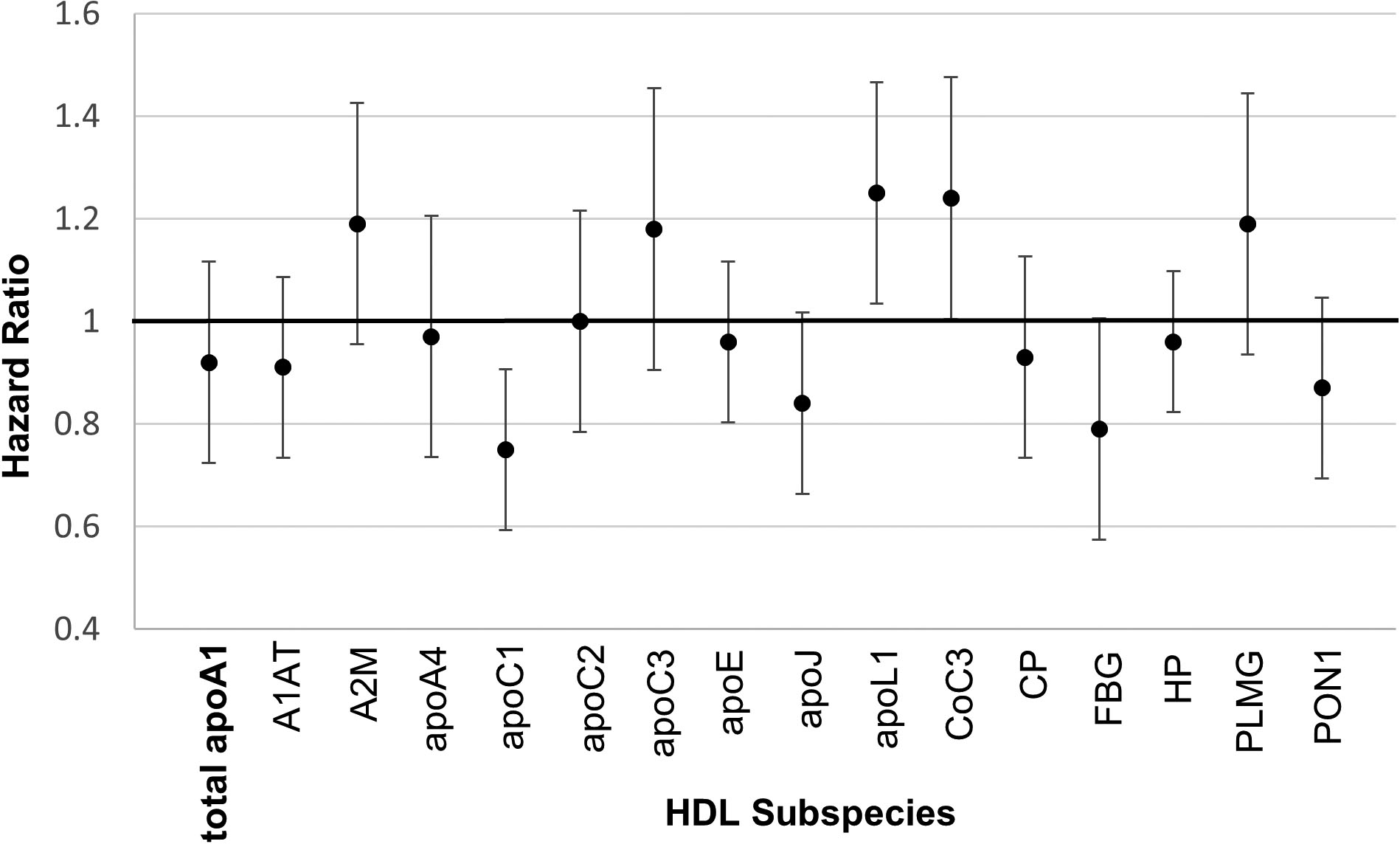
The nested case-control designs were matched on age, smoking, and fasting status.
HDL and apoE
Previous studies reported higher CHD risk or prevalence associated with the concentration of apoE in HDL34, 35 (as opposed to the concentration of the subspecies, the apoA1 concentration of HDL that contains apoE). Confirming the previous reports, the apoE concentration in HDL was associated with higher (HR=1.13, p=0.033), whereas the apoA-1 concentration of HDL that contains apoE was associated with lower relative risk (HR=0.77, p<0.001) (Figure 4). These findings suggest that a high concentration of apoE in the HDL subspecies that contains apoE impairs its protection against CHD. We explored this hypothesis by creating a variable, apoE density on HDL, defined as the concentration of apoE in HDL divided by the concentration of apoA-1 in HDL that contains apoE. A higher apoE density was associated with higher hazard ratio, 1.23 (p<0.001); and 1.17 (p=0.002) further adjusted for plasma total apoA1 (Figure 4).
Figure 4. ApoE and Relative Risk for CHD.
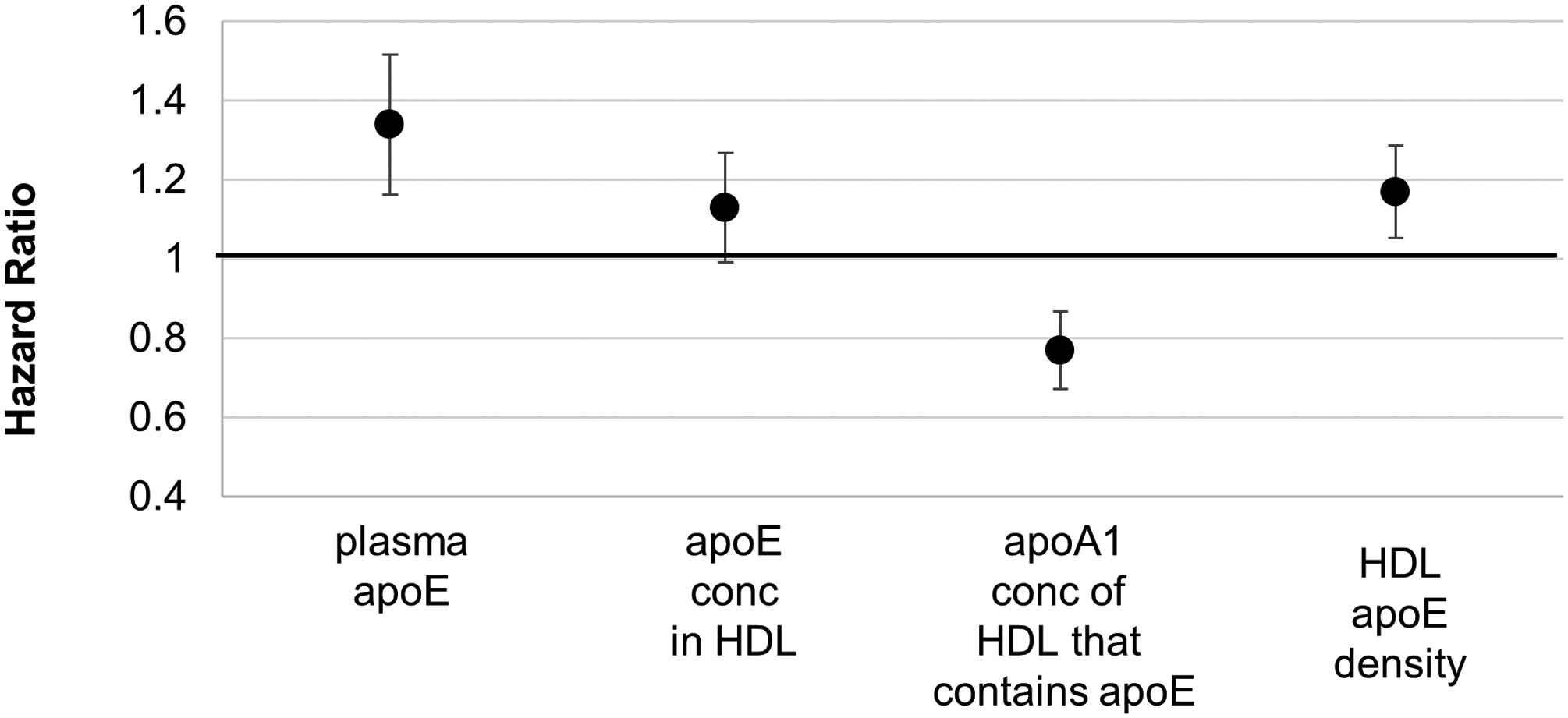
ApoE concentration in total plasma; in HDL; apoA1 concentration of HDL that contains apoE; and HDL apoE density (HR with 95% CI). ApoE density was defined as the ratio of apoE concentration divided by apoA1 concentration in HDL that contains apoE. Plasma total apoE and apoA1 concentration of HDL that contains apoE were studied in the same model that included the covariates. ApoE concentration in HDL and HDL apoE density were studied in separate models that included the covariates. The covariates were hypertension, diabetes, body mass index, physical activity, and use of medications for hyperlipidemia. The nested case-control designs were matched on age, smoking, and fasting status.
Triglycerides
Triglyceride concentration was added to the standard model. Only MESA was studied because the other cohorts did not have triglyceride data for all participants. Triglycerides as a covariate had no impact on the relative risks associated with the minor HDL subspecies (Figure 5).
Figure 5.
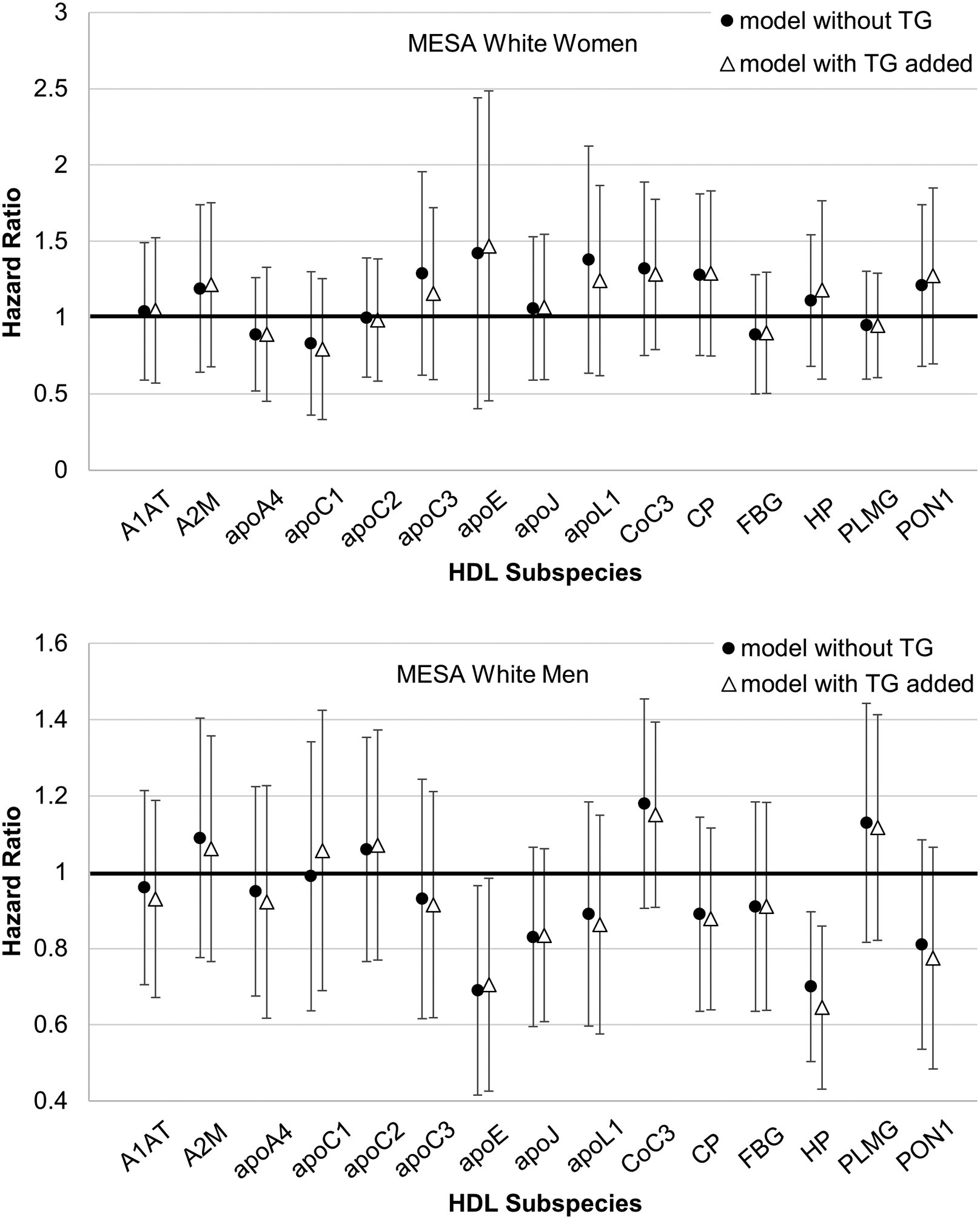
Plasma triglycerides added to the standard model described in Figure 2. Relative risk of CHD for minor HDL subspecies. Hazard ratio with 95% confidence interval.
Concentrations and associations with CHD in the black cohorts
Black women and men in MESA had similar HDL subspecies and plasma total apoA1 concentrations. These black cohorts had higher concentrations and percentages of the total of most of the minor HDL subspecies compared to whites, except for HDL that contains apoC1 (Supplemental Table II, Supplemental Figure III). In the black cohorts, plasma total apoA1 concentration was associated with higher relative risk (HR = 1.28, p=0.12), the opposite to the protective association in whites (HR 0.82, p<0.001; p-heterogeneity = 0.01) (Supplemental Figure IV). The relative risks of CHD associated with the minor HDL subspecies were not significant (Supplemental Figure V). The relatively small sample size of the black cohorts did not provide adequate power to detect moderate differences in relative risk between the subspecies containing and lacking the defining protein, to explore heterogeneity for blacks compared to whites, or to determine whether covariates other than race could explain the differences between the white and black cohorts.
DISCUSSION
HDL can be organized into many minor subspecies each defined by content of a specific protein that is active in cholesterol transport and other functions related to CHD and other conditions.13, 18–20 We call them “minor” because on average they comprise about 10% or less of total HDL apoA1 concentration. ApoC3 was the lead HDL protein that identified such a minor subspecies that predicts higher risk of CHD in several populations.14, 15, 36, 37 HDL that contains myeloperoxidase has a low plasma concentration but strong functional properties related to CHD.38 The current study extends the concept to 14 additional subspecies (other than HDL that contains apoC3) spanning a range of HDL functions. Two subspecies, HDL that contains apoC1 and HDL that contains apoE, are associated with lower risk of CHD; and several others are associated with higher risk. These associations of HDL subspecies are independent of plasma total HDL, as measured by the plasma total apoA1 level. The results are robust coming from a meta-analysis of four independent cohorts, and adjusted for multiple testing. Taken together, the HDL subspecies significantly improve our understanding of the meaning of protein-defined types of HDL circulating in plasma, linking them to higher or lower risk of CHD.
HDL subspecies associated with higher CHD risk compared to the complementary subspecies.
The 4 HDL subspecies that contain alpha-2 macroglobulin, complement C3, haptoglobin, or plasminogen were associated with higher risk than the complementary subspecies that lack the protein. Others, such as HDL that contains alpha1 antitrypsin, ceruloplasmin, apoA4, apoC2, or apoC3, have hazard ratios that approach 1.0, the null, in comparison with significant inverse associations of the complementary major HDL subspecies that lack the defining protein. The specific functions of these proteins have been extensively studied although much less so pertaining to their actions as a component of HDL. The adverse associations between these HDL subspecies and CHD could reflect specific functions of the defining proteins; a common structural interaction for example with apoA1 that could negate beneficial actions of HDL; or a reflection of an inflammatory state or other disease process.
Alpha-2 macroglobulin:
HDL that contains alpha-2 macroglobulin is associated with the highest CHD risk of the HDL subspecies studied. No other study has been published on this topic to our knowledge. Alpha-2 macroglobulin is a general protease inhibitor, and may interfere with the action of plasmin.39 It also binds to and inactivates LCAT.40 These actions to inhibit clot dissolving and cholesterol transport may be reasons why HDL that contains alpha-2 macroglobulin is associated directly with CHD.
Alpha-1 antitrypsin:
Like alpha-2 macroglobulin, alpha-1 antitrypsin is a serine protease inhibitor. It has been proposed that HDL inhibits vascular inflammation by delivering alpha-1 antitrypsin to inflammatory lesions.41 Alpha-1 antitrypsin bound to HDL reduces TNF-alpha production by macrophages whereas unbound alpha-1 antitrypsin does not.42 Thus, HDL may be necessary for the protective action of alpha-1 antitrypsin in atherosclerosis.41 Serum alpha-1 antitrypsin levels increase after myocardial infarction.43 Thus, high levels of HDL that contains alpha-1 antitrypsin could be a response to, and not a cause of vascular inflammation.
Plasminogen:
A high level of HDL that contains plasminogen, associated with higher risk compared to HDL that lacks plasminogen, might reflect a response to an ongoing thrombotic process. Plasmin also inhibits HDL-induced cholesterol efflux from macrophages.44 Little is known about plasminogen as a protein associated with HDL.
Complement C3:
HDL that contains complement C3 is associated with higher risk of CHD compared to HDL that lacks complement C3, as is serum total CoC3.45, 46 However, whether CoC3 acts to promote or reduce atherothrombosis is not established.47 As proposed by Gordon and Remaley41, HDL may affect pathophysiology of atherosclerosis by transporting biologically active molecules to active lesions and by facilitating their action by acting as a platform; or by sequestering CoC3 and other promoters of inflammation.
Haptoglobin:
ApoA1 on HDL binds haptoglobin in a complex with hemoglobin.48 Hemoglobin sequesters nitric oxide impairing its release from HDL and its vasodilating action on endothelial cells. High plasma levels of HDL-bound haptoglobin and hemoglobin are associated with CHD49 and impaired coronary endothelial vasodilation.50 HDL hemoglobin oxidatively modifies HDL lipids and proteins, impairing the function of apoA1 in reverse cholesterol transport. Haptoglobin binds to apoE and impairs its action to increase LCAT activity and deliver HDL cholesterol to hepatocytes.51 Thus, several mechanisms may account for the higher CHD risk associated with HDL that contains haptoglobin compared to HDL that lacks haptoglobin.
Little is known about apoA4, apoC2, and ceruloplasmin as components of HDL subspecies. Their concentrations are associated with nearly null hazard ratios for CHD risk. The presence of these proteins on HDL may nullify the normal protective association of HDL with CHD.
HDL subspecies associated with low risk of CHD: HDL that contains apoC1 or apoE.
HDL that contains apoC1 is the subspecies associated most strongly with lower risk of CHD. ApoC1 concentration in HDL was lower in a sample of 18 patients with CHD than in 20 controls35; and in another case control study of 10 CHD patients and controls52 --- the only two studies we found that reported an association between HDL apoC1 and CHD. ApoC1 has several properties that could improve the function of HDL in reverse cholesterol transport. ApoC1 increases ABCA1 mediated cholesterol efflux from macrophages53, 54; increases LCAT activity55, which esterifies cholesterol transferred from vascular cells to HDL; reduces the activity of CETP56, 57, which transfers cholesterol esters from HDL to VLDL and LDL; and protects LDL from oxidation.53 All told, these results support an interpretation that HDL that contains apoC1 is a strongly protective minor HDL subspecies.
ApoE in HDL has a complex relation to CHD. On the one hand, in the present study, the apoA1 concentration of HDL that contains apoE, a measure of concentration of HDL particles that contain apoE, is associated with lower risk of CHD. This finding is concordant with the knowledge that apoE enhances classical cardioprotective functions of HDL like cholesterol uptake from macrophages, HDL particle enlargement, and rapid clearance of the cholesterol loaded HDL.15, 58–63 This HDL apoE system is stimulated by a diet high in unsaturated fat compared to carbohydrate.64 Together, these altered mechanisms may be reasons why dietary unsaturated fat lowers risk of CHD.65 On the other hand, the concentration of apoE itself in HDL is associated with higher risk in the present and two previous studies.34, 35 We showed that the ratio of apoE to apoA1 in HDL that contains apoE is associated with higher risk. A large quantity of apoE in an HDL particle, like some of the other proteins studied here, may interfere with actions of other proteins or lipids in the particle that protect against atherosclerosis. However, we consider this analysis to be exploratory, requiring confirmation and elaboration in additional populations.
HDL in the black cohorts
Plasma total apoA1 was associated with higher risk in the black cohorts of MESA and lower risk in whites (Supplemental Figure IV). There are no previous studies to our knowledge on apoA1 as a predictor of CHD in blacks. Also, the black cohorts had higher mean concentrations and percentages of HDL subspecies associated with higher risk than whites, similar to a previous finding on HDL that contains apoC3.66 Although it would appear that blacks have an adverse pattern of CHD risk associated with total apoA1 and its minor subspecies, the large confidence intervals rendered the risks of each subspecies nonsignificant. Further, this study is unable to determine whether covariates other than race that differ between the white and black cohorts, such as prevalence of diabetes or other attributes, could explain the differences. In this regard, some of the relative risks in the white cohorts in MESA differ from those in the other white cohorts. It is not clear why that should be so other than randomness. Heterogeneity testing across cohorts was not significant for any subspecies.
As regards HDL-C, several individual studies reported divergent findings that blacks had similar associations with CHD or higher risk of CHD than whites.67–70, consistent with the present study. A meta-analysis of 17 cohorts worldwide including MESA, having 7200 blacks and 662 CV events, myocardial infarction or stroke, found similar associations of HDL-C with CVD in whites (HR 0.67 [0.58–0.76]) and blacks (HR 0.64 [0.44–0.85]).71 This study is limited by not reporting separately stroke and myocardial infarction. Together, the present and previous studies underscore the need to learn more about HDL and its subspecies in highly powered studies in several cohorts of blacks.
Limitations
While we have shown different relative risks between HDL subspecies containing and lacking certain proteins, further research is needed to assess the ability of these HDL subspecies to improve risk prediction indices over total apoA1. This study did not measure concentrations in HDL of the proteins that define subspecies and other minor proteins of HDL which should be investigated in future work. Due to unstable estimates of the relative risks in extreme quantiles and quantile cut points varying across cohorts, further study of the individual HDL subspecies is needed to better understand the nature of the relationship, linear or otherwise, between the HDL subspecies and CHD risk. Although we did not find an effect of triglycerides concentrations on RR of HDL subspecies, we were only able to evaluate triglycerides in MESA, the only cohort that had complete triglycerides data. It would have been interesting to study the effect of renal function on the associations between HDL subspecies and CHD since renal function could affect HDL-associated marker levels or clearance, but these participants were healthy individuals and there was inadequate data on renal function to address this here. Finally, with over 90 proteins detected in the HDL proteome, it is likely that there are other protein-defined HDL subspecies, as well as specific lipids, that are associated with CHD risk.
Conclusion
Certain protein-based subspecies of HDL enhance, nullify or reverse the usual association of plasma total HDL with lower CHD risk. The beneficial association of high total HDL with CHD may be at least partly accounted for by strongly protective minor subspecies, like apoC1 and apoE, whose concentrations are mildly correlated with the total apoA1. Following similar logic, several HDL subspecies are associated with higher risk, and they are also mildly correlated with total apoA1. Thus, total HDL is not a good indicator of protective or harmful HDL subspecies. Drug development could target HDL to suppress the adverse or enhance the protective subspecies. Diet or drug treatments that raise HDL may have paradoxical effects on CHD if they increase harmful subspecies. Risk for CHD pertaining to HDL may reflect protective and harmful minor subspecies. Risk prediction and treatment, accounting for HDL subspecies, have the potential to be more accurate and personalized.
Supplementary Material
Highlights.
Protein-based HDL subspecies comprise 1–12% of total plasma apoA1 concentration
Higher levels of HDL subspecies containing alpha-2 macroglobulin, complement C3, haptoglobin, or plasminogen were associated with higher relative risk compared to the HDL counterpart lacking the defining protein.
In contrast, HDL containing apoC1 or apoE were associated with lower relative risk.
Risk for CHD may reflect the balance between protective and harmful minor subspecies of HDL.
Acknowledgments
We thank Sue Wong-Lee, Maria Gamez-Guerrero, and Nathaniel Smith for performing many of the laboratory analyses.
Sources of Funding
The research described in this article was supported by National Heart, Lung, and Blood Institute, National Institutes of Health Grant R01 HL123917 and the Harvard University Research Accelerator program (Dr. Sacks, PI). The NHS, NHS-II, and HPFS studies are supported by research grants UM1 CA186107, R01 CA49449, R01 HL034594, and R01 HL088521; UM1 CA176726 and R01 CA67262; UM1 CA167552 and HL35464, respectively, all from the National Institute of Health, Bethesda, MD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MESA is an NHLBI-funded study supported by grants R01 HL071739 and R21 HL091217 from the National Heart, Lung, and Blood Institute (NHLBI), T32 DK 007703 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources, and by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, HHSN268201500003I and N01-HC-95169 from NHLBI.
ABBREVIATIONS
- A1AT
alpha-1-antitrypsin
- A2M
alpha-2-macroglobulin
- apo
apolipoprotein
- CP
ceruloplasmin
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CoC3
complement C3
- FBG
fibrinogen
- HP
haptoglobin
- HPFS
Health Professionals Follow-up Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- NHS
Nurses Health Study
- PLMG
plasminogen
- PON1
paraoxonase-1
Footnotes
Disclosures: Drs. Sacks, Furtado, Rimm, and Jensen are Inventors of patents to Harvard University on use of HDL subspecies in cardiovascular disease. Dr. Sacks is a consultant to Abbvie, AstraZeneca, DalCor, CSL-Behring, and Pfizer; and expert witness for Abbvie and Pfizer
Supplemental Materials:
Supplemental Table I-III
Supplemental Figures I-V
Major Resources Table
References
- 1.Sacks FM, Jensen MK. From High-Density Lipoprotein Cholesterol to Measurements of Function: Prospects for the Development of Tests for High-Density Lipoprotein Functionality in Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2018;38:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1046–1081.30565953 [Google Scholar]
- 4.Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol. 2011;18:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. [DOI] [PubMed] [Google Scholar]
- 6.Rye KA, Barter PJ. Regulation of high-density lipoprotein metabolism. Circ Res. 2014;114:143–156. [DOI] [PubMed] [Google Scholar]
- 7.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melchior JT, Street SE, Andraski AB, Furtado JD, Sacks FM, Shute RL, Greve EI, Swertfeger DK, Li H, Shah AS, Lu LJ, Davidson WS. Apolipoprotein A-II alters the proteome of human lipoproteins and enhances cholesterol efflux from ABCA1. J Lipid Res. 2017;58:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung MC and Albers JJ. Distribution of high density lipoprotein particles with different apoprotein composition: particles with A-I and A-II and particles with A-I but no A-II. J Lipid Res.1982;23:747–753. [PubMed] [Google Scholar]
- 10.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. [DOI] [PubMed] [Google Scholar]
- 11.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9:5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtado JD, Yamamoto R, Melchior JT, Andraski AB, Gamez-Guerrero M, Mulcahy P, He Z, Cai T, Davidson WS, Sacks FM. Distinct Proteomic Signatures in 16 HDL (High-Density Lipoprotein) Subspecies. Arterioscler Thromb Vasc Biol. 2018;38:2827–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MK, Aroner SA, Mukamal KJ, Furtado JD, Post WS, Tsai MY, Tjonneland A, Polak JF, Rimm EB, Overvad K, McClelland RL, Sacks FM. High-Density Lipoprotein Subspecies Defined by Presence of Apolipoprotein C-III and Incident Coronary Heart Disease in Four Cohorts. Circulation. 2018;137:1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton AM, Koch M, Mendivil CO, Furtado JD, Tjonneland A, Overvad K, Wang L, Jensen MK, Sacks FM. Apolipoproteins E and CIII interact to regulate HDL metabolism and coronary heart disease risk. JCI Insight. 2018;3: e98045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talayero B, Wang L, Furtado J, Carey VJ, Bray GA, Sacks FM. Obesity favors apolipoprotein E- and C-III-containing high density lipoprotein subfractions associated with risk of heart disease. J Lipid Res. 2014;55:2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch M, Furtado JD, Jiang GZ, Gray BE, Cai T, Sacks F, Tjonneland A, Overvad K, Jensen MK. Associations of anthropometry and lifestyle factors with HDL subspecies according to apolipoprotein C-III. J Lipid Res. 2017;58:1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rye KA, Barter PJ. Predictive value of different HDL particles for the protection against or risk of coronary heart disease. Biochim Biophys Acta. 2012;1821:473–480. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SM, Deng J, Tomann AB, Shah AS, Lu LJ, Davidson WS. Multi-dimensional co-separation analysis reveals protein-protein interactions defining plasma lipoprotein subspecies. Mol Cell Proteomics. 2013;12:3123–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Gordon SM, Zhu X, Deng J, Swertfeger DK, Davidson WS, Lu LJ. Network-Based Analysis on Orthogonal Separation of Human Plasma Uncovers Distinct High Density Lipoprotein Complexes. J Proteome Res. 2015;14:3082–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SA, Andraski AB, Pieper B, Goh W, Mendivil CO, Sacks FM, Aikawa M. Multiple apolipoprotein kinetics measured in human HDL by high-resolution/accurate mass parallel reaction monitoring. J Lipid Res. 2016;57:714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 23.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med. 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 24.Altmann S, Davidson W. The continuing saga of HDL: Truth, fallacy, or something in between? LipidSpin. 2016;14:7–11. [Google Scholar]
- 25.Jensen MK, Rimm EB, Furtado JD, Sacks FM. Apolipoprotein C-III as a Potential Modulator of the Association Between HDL-Cholesterol and Incident Coronary Heart Disease. J Am Heart Assoc. 2012;1: jah3-e000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, Sacks FM, Stampfer MJ. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35:2313–2316. [PubMed] [Google Scholar]
- 29.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 30.Rose GA. Cardiovascular survey methods. 2nd ed. Geneva and Albany, N.Y.: World Health Organization; WHO Publications Centre distributor; 1982. [Google Scholar]
- 31.Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, Rimm EB. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;128:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goeman JJ, Solari A. Multiple hypothesis testing in genomics. Stat Med. 2014;33:1946–1978. [DOI] [PubMed] [Google Scholar]
- 34.Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, Pfeffer MA, Braunwald E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–1892. [DOI] [PubMed] [Google Scholar]
- 35.Vaisar T, Mayer P, Nilsson E, Zhao XQ, Knopp R, Prazen BJ. HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin Chim Acta. 2010;411:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onat A, Hergenç G, Sansoy V, Fobker M, Ceyhan K, Toprak S, Assmann G. Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto R, Sacks FM, Hu FB, Rosner B, Furtado JD, Aroner SA, Ferrannini E, Baldi S, Kozakova M, Balkau B, Natali A, Jensen MK. High density lipoprotein with apolipoprotein C-III is associated with carotid intima-media thickness among generally healthy individuals. Atherosclerosis. 2018;269:92–99. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, DiDonato JA, Levison BS, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med. 2014;20:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaller J, Gerber SS. The plasmin-antiplasmin system: structural and functional aspects. Cell Mol Life Sci. 2011;68:785–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krimbou L, Marcil M, Davignon J, Genest J Jr. Interaction of lecithin:cholesterol acyltransferase (LCAT).alpha 2-macroglobulin complex with low density lipoprotein receptor-related protein (LRP). Evidence for an alpha 2-macroglobulin/LRP receptor-mediated system participating in LCAT clearance. J Biol Chem. 2001;276:33241–33248. [DOI] [PubMed] [Google Scholar]
- 41.Gordon SM, Remaley AT. High density lipoproteins are modulators of protease activity: Implications in inflammation, complement activation, and atherothrombosis. Atherosclerosis. 2017;259:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon SM, McKenzie B, Kemeh G, Sampson M, Perl S, Young NS, Fessler MB, Remaley AT. Rosuvastatin Alters the Proteome of High Density Lipoproteins: Generation of alpha-1-antitrypsin Enriched Particles with Anti-inflammatory Properties. Mol Cell Proteomics. 2015;14:3247–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilutz H, Siegel Y, Paran E, Cristal N, Quastel MR. Alpha 1-antitrypsin in acute myocardial infarction. Br Heart J. 1983;49:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindstedt L, Kovanen PT. Plasmin and kallikrein reduce HDL-induced cholesterol efflux from foam cells. Biochem Biophys Res Commun. 2000;277:552–557. [DOI] [PubMed] [Google Scholar]
- 45.Muscari A, Bozzoli C, Puddu GM, Sangiorgi Z, Dormi A, Rovinetti C, Descovich GC, Puddu P. Association of serum C3 levels with the risk of myocardial infarction. Am J Med. 1995;98:357–364. [DOI] [PubMed] [Google Scholar]
- 46.Szeplaki G, Prohaszka Z, Duba J, Rugonfalvi-Kiss S, Karadi I, Kokai M, Kramer J, Fust G, Kleiber M, Romics L, Varga L. Association of high serum concentration of the third component of complement (C3) with pre-existing severe coronary artery disease and new vascular events in women. Atherosclerosis. 2004;177:383–389. [DOI] [PubMed] [Google Scholar]
- 47.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9:428–440. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood. 2009;114:764–771. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, Fogelman AM, Reddy ST. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem. 2009;284:18292–18301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asleh R, Levy AP, Levy NS, Asleh A, Goldenstein H, Segol I, Gulati R, Lerman LO, Lerman A. Haptoglobin Phenotype Is Associated With High-Density Lipoprotein-Bound Hemoglobin Content and Coronary Endothelial Dysfunction in Patients With Mild Nonobstructive Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2019;39:774–786. [DOI] [PubMed] [Google Scholar]
- 51.Cigliano L, Pugliese CR, Spagnuolo MS, Palumbo R, Abrescia P. Haptoglobin binds the antiatherogenic protein apolipoprotein E - impairment of apolipoprotein E stimulation of both lecithin:cholesterol acyltransferase activity and cholesterol uptake by hepatocytes. FEBS J. 2009;276:6158–6171. [DOI] [PubMed] [Google Scholar]
- 52.Yan LR, Wang DX, Liu H, Zhang XX, Zhao H, Hua L, Xu P, Li YS. A pro-atherogenic HDL profile in coronary heart disease patients: an iTRAQ labelling-based proteomic approach. PLoS One. 2014;9:e98368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swertfeger DK, Li H, Rebholz S, Zhu X, Shah AS, Davidson WS, Lu LJ. Mapping Atheroprotective Functions and Related Proteins/Lipoproteins in Size Fractionated Human Plasma. Mol Cell Proteomics. 2017;16:680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith LE, Segrest JP, Davidson WS. Helical domains that mediate lipid solubilization and ABCA1-specific cholesterol efflux in apolipoproteins C-I and A-II. J Lipid Res. 2013;54:1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soutar AK, Garner CW, Baker HN, Sparrow JT, Jackson RL, Gotto AM, Smith LC. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: cholesterol acyltransferase. Biochemistry. 1975;14:3057–3064. [DOI] [PubMed] [Google Scholar]
- 56.de Barros JP, Boualam A, Gautier T, Dumont L, Verges B, Masson D, Lagrost L. Apolipoprotein CI is a physiological regulator of cholesteryl ester transfer protein activity in human plasma but not in rabbit plasma. J Lipid Res. 2009;50:1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautier T, Masson D, de Barros JP, Athias A, Gambert P, Aunis D, Metz-Boutigue MH, Lagrost L. Human apolipoprotein C-I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J Biol Chem. 2000;275:37504–37509. [DOI] [PubMed] [Google Scholar]
- 58.Koo C, Innerarity TL, Mahley RW. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J Biol Chem. 1985;260:11934–11943. [PubMed] [Google Scholar]
- 59.Blum CB, Deckelbaum RJ, Witte LD, Tall AR, Cornicelli J. Role of apolipoprotein E-containing lipoproteins in abetalipoproteinemia. J Clin Invest. 1982;70:1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikewaki K, Rader DJ, Zech LA, Brewer HB Jr., In vivo metabolism of apolipoproteins A-I and E in patients with abetalipoproteinemia: implications for the roles of apolipoproteins B and E in HDL metabolism. J Lipid Res. 1994;35:1809–1819. [PubMed] [Google Scholar]
- 61.Mahley RW, Huang Y, Weisgraber KH. Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest. 2006;116:1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Settasatian N, Barter PJ, Rye KA. Remodeling of apolipoprotein E-containing spherical reconstituted high density lipoproteins by phospholipid transfer protein. J Lipid Res. 2008;49:115–126. [DOI] [PubMed] [Google Scholar]
- 63.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morton AM, Furtado JD, Mendivil CO, Sacks FM. Dietary unsaturated fat increases HDL metabolic pathways involving apoE favorable to reverse cholesterol transport. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn LV. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 2017;136:e1–e23. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Sacks FM, Furtado JD, Ricks M, Courville AB, Sumner AE. Racial differences between African-American and white women in insulin resistance and visceral adiposity are associated with differences in apoCIII containing apoAI and apoB lipoproteins. Nutr Metab (Lond). 2014;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willey JZ, Rodriguez CJ, Carlino RF, Moon YP, Paik MC, Boden-Albala B, Sacco RL, DiTullio MR, Homma S, Elkind MS. Race-ethnic differences in the association between lipid profile components and risk of myocardial infarction: The Northern Manhattan Study. Am Heart J. 2011;161:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study). Am J Cardiol. 2015;115:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joshi PH, Toth PP, Lirette ST, Griswold ME, Massaro JM, Martin SS, Blaha MJ, Kulkarni KR, Khokhar AA, Correa A, D’Agustino RB Sr, Jones SR. Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur J Prev Cardiol 2016;23:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penson P, Long DL, Howard G, Howard VJ, Jones SR, Martin SS, Mikhailidis DP, Muntner P, Rizzo M, Rader DJ, Safford MM, Sahebkar A, Toth PP, Banach M. Associations between cardiovascular disease, cancer, and very low high-density lipoprotein cholesterol in the REasons for Geographical and Racial Differences in Stroke (REGARDS) study. Cardiovasc Res. 2019;115:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gijsberts CM, Groenewegen KA, Hoefer IE, et al. Race/Ethnic Differences in the Associations of the Framingham Risk Factors with Carotid IMT and Cardiovascular Events. PLoS One. 2015;10:e0132321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


