Abstract
Mutations in the calcium channel gene Transient Receptor Potential cation channel subfamily V member 4 (TRPV4) cause autosomal dominant skeletal dysplasia, with phenotypes ranging from mild to perinatal lethality. A recent report detailed enhanced proplatelet formation and increased murine platelet count in the context of TRPV4 activation. No prior reports have described platelet count abnormalities in human TRPV4 disease. Here, we report a case of prolonged thrombocytosis in the context of TRPV4-associated metatropic dysplasia that was lethal in the infantile period.
Keywords: thrombocytosis, skeletal dysplasia, TRPV4, calcium channel
Introduction
Mutations in TRPV4 (Transient Receptor Potential cation channel subfamily V member 4) cause a clinically heterogenous metatropic skeletal dysplasia with autosomal dominant inheritance.1,2 Metatropic dysplasia is characterized by short extremities, short trunk, progressive kyphoscoliosis, and distinct craniofacial abnormalities. Phenotypic severity ranges from mild to perinatal lethal, with death typically due to thoracic insufficiency.1,2 Skeletal manifestations arise from altered TRPV4 calcium sensing and perturbed chondrocyte differentiation.2
In a cellular model, pharmacologic TRPV4 activation enhanced proplatelet formation.3 In vivo, decreasing extracellular matrix stiffness led to increased TRPV4 activation and increased platelet count.3 To our knowledge, no prior reports have described altered platelet counts in humans with metatropic dysplasia.
Here, we report a case of TRPV4 mutation with lethality around 4 months of age. The infant had lifelong thrombocytosis without known associated clinical complications directly related to the platelet count. This is the first report of thrombocytosis in human TRPV4-associated metatropic dysplasia. Thrombocytosis might be also observed in similar clinical cases.
Case
Prenatal testing for the female patient was consistent with arthrogryposis, including decreased thoracic circumference, spinal segmentation anomalies, thoracolumbar kyphoscoliosis, lumbosacral lordosis, and a tethered spinal cord. Upper and lower extremities were noted to have shortened long bones with concerns for contracture, with lower extremities more severely affected. MRI showed no significant structural anomalies of the brain or spine.
The patient was born at 32 weeks 1 day gestation by cesarean section, in the setting of prolonged premature rupture of membranes and recurrent fetal decelerations. She was admitted to our Level 4 neonatal and infant intensive care unit for respiratory support and multidisciplinary evaluation. Her clinical exam was consistent with the described prenatal imaging findings. Clinical exome sequencing identified a heterozygous de novo variant in TRPV4 (c.1303 G>A, p.E435K). This mutation was not previously reported in the ClinVar or Human Mutation Gene Database registries, but was deemed a ‘likely pathogenic variant’ per ACMG guidelines.4 Scores from in silico prediction tools indicated potential pathogenicity for this mutation (Supplemental Table 1).
The patient required non-invasive support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BIPAP) or high flow nasal cannula for her entire life. She was intubated in the setting of procedures, including a laparoscopic gastrostomy tube placement and fundoplication at 3 months of age. At almost 4 months of age, the infant acutely decompensated in the setting of abdominal compartment syndrome. She was unable to be resuscitated despite emergent exploratory laparotomy. The cause of this abdominal catastrophe was unknown, despite full autopsy.
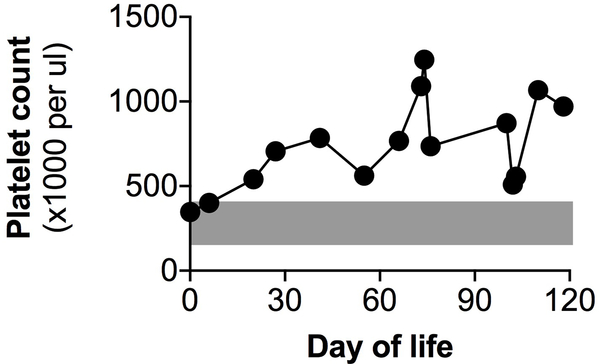
Thrombocytosis was noted throughout the infant’s life, with a maximum recorded platelet count more than 3 times the upper limit of normal (1,247,000 per μl, Figure 1). There were no other consistently abnormal hematologic parameters on complete blood counts (Table 1). Thrombocytosis was unexpected, as platelet count derangement had not been previously described in clinical descriptions of TRPV4-related disease.
Figure 1.
Recorded platelet counts in this patient. Gray zone indicates normal reference range (150–400 ×1000 platelets per μl).
Table 1.
Hematologic trait values for this patient with associated normal reference ranges. Shown are the full range of laboratory values obtained, as well as the mean ± standard deviation for each parameter (n = 15 complete blood counts). PLT, platelet count. MPV, mean platelet volume. RBC, red blood cell count. Hb, hemoglobin. Hct, hematocrit. MCV, mean corpuscular volume. MCH, mean corpuscular hemoglobin. MCHC, mean corpuscular hemoglobin concentration. RDW, red cell distribution width. WBC, white blood cell count.
| Trait | Normal Range | Range | Mean ± SD |
|---|---|---|---|
| PLT | 150 – 400 ×1000/ul | 347 – 1247 | 743 ± 266 |
| MPV | 9.0 – 10.9 fL | 8.4 – 11.1 | 9.3 ± 0.9 |
| RBC | 3.1 – 4.5 ×10^6/ul | 3.3 – 5.2 | 4.1 ± 0.6 |
| Hb | 9.5 – 13.5 g/dL | 10.2 – 14.8 | 12.1 ± 1.8 |
| Hct | 29 – 41 % | 30.3 – 44.8 | 35.9 ± 5.1 |
| MCV | 74 – 108 fL | 81.5 – 100.5 | 87.8 ± 5.2 |
| MCH | 25 – 35 pg | 26.6 – 36.0 | 29.7 ± 2.7 |
| MCHC | 30. – 36 g/dL | 31.1 – 36.5 | 33.8 ± 1.3 |
| RDW | 35.2 – 45.1 fL | 35.9 – 61.0 | 44.1 ± 8.1 |
| WBC | 6.0 – 13.3 ×1000/ul | 7.7 – 29.6 | 14.7 ± 5.3 |
Though the etiology remained unclear despite subspecialist discussions, there was never an indication for intervention, treatment or other alteration in clinical management as a result of the high platelet count alone. The patient underwent one sepsis evaluation, which was triggered by acute respiratory decompensation where thrombocytosis was noted as evidence supporting the use of empiric antibiotics to rule out possible bacterial infection. When her blood culture remained negative after 48 hours, antibiotics were discontinued.
Discussion
TRPV4 mutations can cause autosomal dominant, life-limiting metatropic dysplasia. Clinical descriptions of the related skeletal manifestations are well documented,1,2 as are potential associated clinical neuromuscular5,6 and neuropathic7,8 manifestations. To our knowledge, aberrancies in hematologic parameters have not previously been reported in association with human TRPV4 mutation. However, TRPV4 activation was recently linked to increased platelet production in mice.3
Clinically relevant TRPV4 mutations have been described throughout the gene. Based on the recently solved TRPV4 protein structure, the mutation in this case falls in a region where other mutations have been associated with skeletal dysplasia.9 We suspect that the E435K mutation represents a gain-of-function variant, based on autosomal dominant inheritance, its location, and related phenotypes. However, future molecular studies are needed to clarify the functional impact of this mutation.
Benign thrombocytosis may be a clinical component of TRPV4-associated metatropic dysplasia. Thrombocytosis observed in this case may have resulted from altered extracellular matrix stiffness and megakaryocyte reactivity, in agreement with cellular and murine phenotypes.3 However, we cannot exclude chronic inflammation or respiratory support as potential confounders. Reactive thrombocytosis can occur in the setting of inflammation10 or lung injury.11 However, the magnitude and duration of this patient’s thrombocytosis is outside the range of what we typically encounter in infants with lung disease. Notably, the platelet count was within the normal range for the first few days of this patient’s life (Figure 1). This may represent relatively depressed platelet count values shortly after birth in this preterm infant, or alternatively reflect a postnatal reactive thrombocytosis. Indeed, one might expect a TRPV4 gain-of-function mutation to have made our patient more susceptible to reactive thrombocytosis. A small focus of extramedullary hematopoiesis was identified on autopsy of the lung in this patient, but we suspect that this was most likely an incidental finding of limited clinical consequence.
It is difficult to extrapolate generalized or mechanistic associations between TRPV4 mutations and thrombocytosis from this case study alone. To validate the clinical association of platelet count and TRPV4 mutation, it will be important to investigate hematologic phenotypes associated with other TRPV4 mutations. Specific functional TRPV4 perturbations may be reflected by altered platelet counts, similar to the spectrum of other TRPV4-associated clinical phenotypes.1
As an acute phase reactant, thrombocytosis can be a sign of infection, inflammation or other pathology.10 In some clinical scenarios, particularly in critically ill neonates, thrombocytosis can lead to sepsis workups and/or other invasive procedures. As such, recognition of conditions that can cause thrombocytosis is important. In the reported case, thrombocytosis did not cause known clinical complications and was not associated with acute pathology. We hope that presenting this case may ultimately inform diagnostic workup in similar cases. However, until a link between TRPV4 mutation and thrombocytosis can be confirmed in other cases or case series, our report should not preclude or deter a detailed workup related to clinically observed thrombocytosis.
There was prognostic uncertainty in this case of TRPV4-associated metatropic dysplasia, although the prognosis was guarded based on respiratory support needs early in life. It might also be difficult to predict disease severity for some individuals with TRPV4 variants of unknown significance. Platelet count might act as a non-invasive biomarker for TRPV4 function and/or disease severity in the right clinical context. This adds further importance for future studies to determine whether thrombocytosis correlates with TRPV4 function and/or TRPV4-associated disease severity.
Supplementary Material
Acknowledgements
We thank Alexandra Pomar, MSW, LSW for her assistance. We thank the family for their willingness to share this story.
Funding
This work was funded through T32HD043021 (CST) and an American Academy of Pediatrics Marshall Klaus Neonatal-Perinatal Research Award (CST).
Footnotes
Disclosures of interest
The authors declare no relevant conflicts of interest.
Consent
The patient’s family has given written consent to the inclusion of material pertaining to the patient’s case. They acknowledge that they cannot be identified via the paper, as the patient has been fully anonymized in this manuscript. The Children’s Hospital of Philadelphia Institutional Review Board deemed this study exempt from oversight.
References
- 1.Andreucci E et al. TRPV4 related skeletal dysplasias: A phenotypic spectrum highlighted byclinical, radiographic, and molecular studies in 21 new families. Orphanet J. Rare Dis 6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho N et al. Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am. J. Med. Genet. Part A 152, 1169–1177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbonante V et al. A new path to platelet production through matrix sensing. Haematologica 102, 1150–1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards S et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med 17, 405–424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auer-Grumbach M et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet 42, 160–164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biasini F et al. TRPV4 related scapuloperoneal spinal muscular atrophy: Report of an Italian family and review of the literature. Neuromuscul. Disord 26, 312–315 (2016). [DOI] [PubMed] [Google Scholar]
- 7.McEntagart M TRPV4 axonal neuropathy spectrum disorder. Journal of Clinical Neuroscience 19, 927–933 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Zimoń M et al. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 133, 1798–1809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Z et al. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat. Struct. Mol. Biol 25, 252–260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinger MHF & Jelkmann W Role of blood platelets in infection and inflammation. J. Interf. Cytokine Res 22, 913–922 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Weyrich AS & Zimmerman GA Platelets in Lung Biology. Annu. Rev. Physiol 75, 569–591 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng PC SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adzhubei IA et al. A method and server for predicting damaging missense mutations. Nature Methods 7, 248–249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircher M et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet 46, 310–315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rentzsch P, Witten D, Cooper GM, Shendure J & Kircher M CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 47, D886–D894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.