Abstract
Epithelial-to-mesenchymal transition (EMT) is a dynamic process that produces migratory cells from epithelial precursors. However, EMT is not binary; rather it results in migratory cells which adopt diverse strategies including collective and individual cell migration to arrive at target destinations. Of the many embryonic cells that undergo EMT, the vertebrate neural crest is a particularly good example which has provided valuable insight into these processes. Neural crest cells from different species often adopt different migratory strategies with collective migration predominating in anamniotes whereas individual cell migration is more prevalent in amniotes. Here we will provide a perspective on recent work towards understanding the process of neural crest EMT focusing on how these cells undergo collective and individual cell migration.
Keywords: Developmental biology, Neural Crest, Epithelial-to-mesenchymal transition, Cell migration, Collective migration
Introduction
During development, dynamic cellular events are critical for appropriate organization of the final animal body plan and reproducible differentiation of each organ system. Critical cell types are often specified in locations distant from the final site of differentiation. In order to overcome this challenge, many cells undergo an epithelial-to-mesenchymal transition (EMT) to adopt a migratory program, allowing these cells to leave their site of origin and move to their final destinations [1–5]. Migratory cells utilize diverse mechanisms to traverse their environment by following chemical gradients (chemotaxis), adhesive gradients (haptotaxis), and extracellular matrix stiffness (durotaxis)[6]. While EMT and migration are normal events during embryogenesis, precocious EMT is often a hallmark of disease states, as exemplified by cancer metastasis [2,7]. While the general principles of EMT have long been appreciated, recent technological advances have helped to uncover the heterogenous mechanisms of migration and the spectrum of cell states ranging from epithelial to mesenchymal cells [4,5,7,8].
The neural crest represents an archetypical cell type that undergoes EMT. This multipotent population is unique to vertebrate embryos and contributes to diverse organ systems including the peripheral and enteric nervous systems, the craniofacial skeleton, skin pigment, and the cardiovascular system [3,9–12]. Neural crest cells are specified during gastrulation at the border between neural and nonneural ectoderm (termed neural plate border), and undergo EMT during neurulation to leave the neuroepithelium and migrate extensively through the embryo (Figure 1a) [3,9]. Neural crest cells emerge from the neural tube at nearly all levels along the anterior-posterior body axis, though their behavior differs depending on their axial level of origin (Figure 1b)(reviewed in [9]). While many of the molecular mechanisms controlling neural crest EMT are conserved between vertebrates, interesting heterogeneities are apparent during their migratory phase. For example, neural crest cells undergo individual cell migration in amniotes, while neural crest cells derived from the same axial level in anamniotes migrate collectively, with more extensive interactions with their neighbors (Figure 2) [13–16]. These events are of critical interest in the neural crest field and are the subject of many recent comprehensive reviews (see [1–3,9,17–21]). Here we will provide a brief perspective on some recent concepts and outstanding questions in the field of neural crest EMT and migration.
Figure 1. Neural crest development and axial levels of origin.
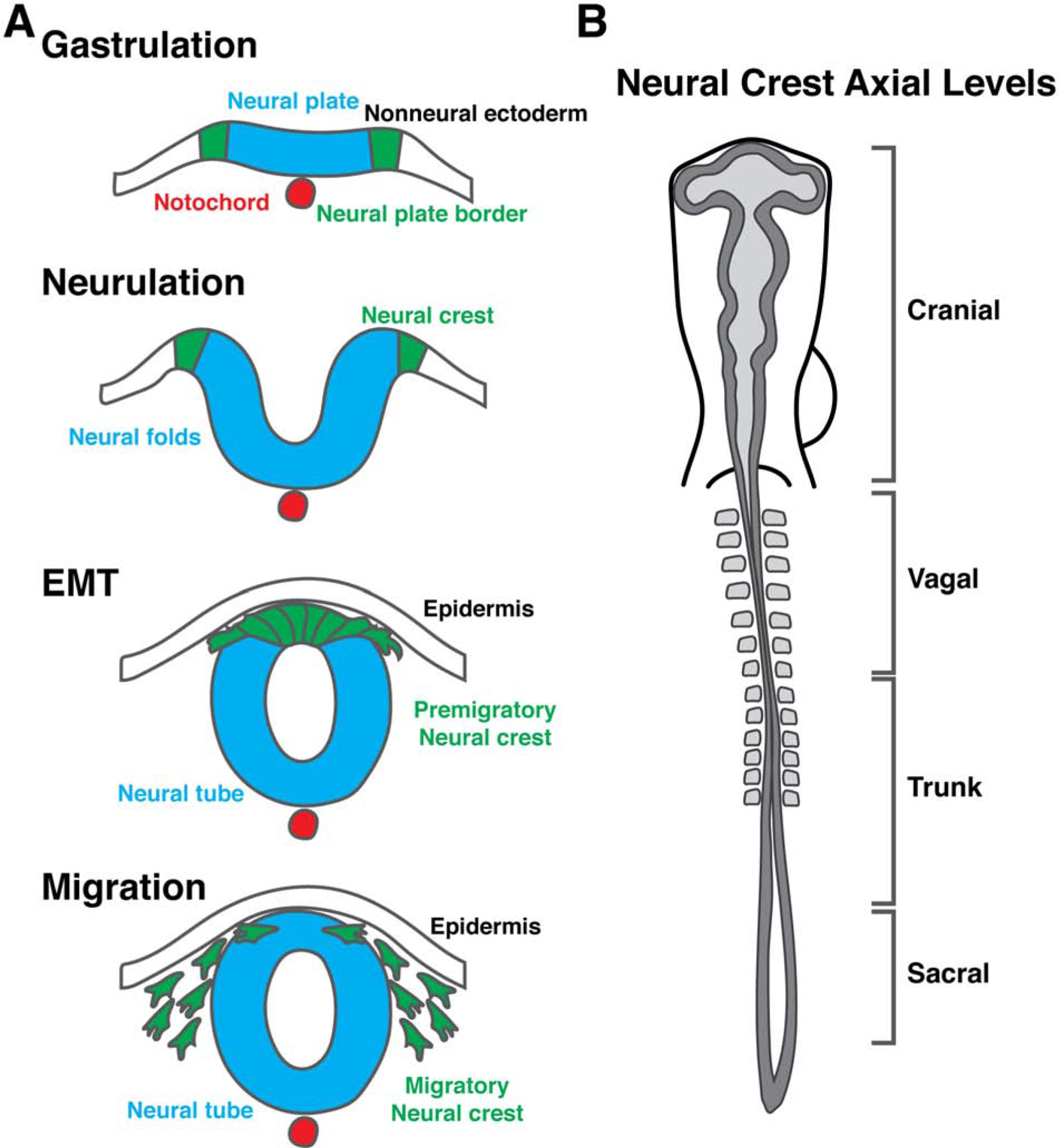
(a) Neural crest cells are induced during gastrulation in the neural plate border between the neural plate and the nonneural ectoderm. During neurulation, the neural crest becomes specified and the neural folds reposition the neural crest to the dorsal midline. Following neural tube closure, premigratory neural crest cells undergo an epithelial-to-mesenchymal transition and delaminate from the neural tube to migrate throughout the embryo. (b) Neural crest cells arise from nearly all axial levels in the vertebrate embryo, and are categorized into four broad groups (cranial, vagal, trunk, and sacral) based on their level of origin along the anterior-posterior axis.
Figure 2. Neural crest epithelial-to-mesenchymal transition produces migratory cells of varying degrees of mesenchymalization.
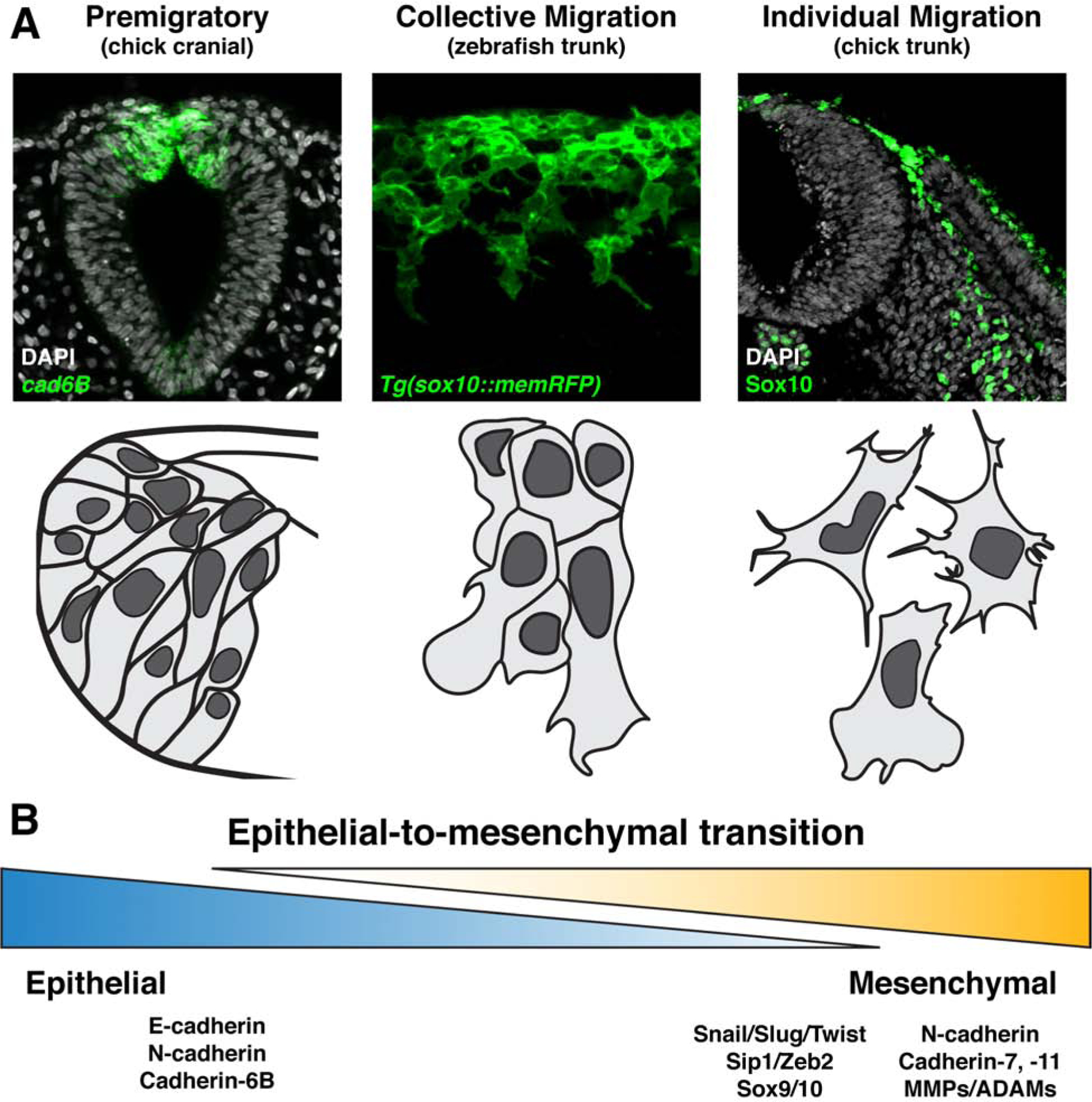
(a) Representative images (top panels) and schematics (bottom panels) displaying neural crest cells at varying degrees of epithelial-to-mesenchymal transition. Premigratory neural crest cells reside in the neuroepithelium exhibit highly epithelial characteristics including tight cell adhesions, as demonstrated by cadherin6B expression in chick cranial neural crest (left). Collective migration of neural crest cells occurs when there is a partial downregulation of epithelial, and upregulation of mesenchymal, characteristics. The resulting migratory cells traverse their environment as tight clusters or in chains with extensive cell-cell contacts, as illustrated by migrating zebrafish trunk neural crest (center). In contrast, individually migrating neural crest cells are more completely mesenchymalized and move through their migratory paths as free cells with more infrequent and transient interactions with one another, as is evident in the trunk of chick embryos (right). (b) Epithelial-to-mesenchymal transition is best considered as a spectrum of cell states ranging from epithelial to mesenchymal, rather than a binary switch between the two. Listed are a selection of common gene signatures expressed in more epithelial (left) and more mesenchymal (right) neural crest cells.
Neural crest epithelial-to-mesenchymal transition
Prior to migration, neural crest cells undergo EMT during which premigratory cells release epithelial adhesions and delaminate from the neuroepithelium as migratory cells. This process is regulated by a conserved gene regulatory network, and triggered by instructive cell signaling events [9]. Premigratory neural crest cells initiate expression of pro-EMT transcription factors Snai1/2 and Twist, along with neural crest specifier and promigratory transcription factors such as FoxD3, Sip1/Zeb2, and Sox9/10 [9]. Together, these transcription factors mediate a “cadherin switch” to downregulate epithelial cadherins and Cadherin6B, and initiate expression of more mesenchymal cadherins [19]. Recent studies have addressed how this switch is temporally regulated by cell signaling events and tissue-mediated biophysical changes.
Wnt/β-catenin signaling plays multiple roles in regulating neural crest EMT. Wnt/β-catenin directly feed into Snai2 expression [22], which directly suppresses E-cadherin and Cadherin-6B expression [23,24]. Furthermore, Wnt/β-catenin signaling activates expression of mesenchymal Cadherin-7 and Cadherin-11 [25]. Recent evidence in chick identifies the secreted Wnt antagonist, Draxin, as a temporal rheostat that mediates the timing of EMT in the chick cranial neural crest [26]. While Draxin is expressed by premigratory neural crest cells, Draxin transcripts are quickly downregulated at the onset of EMT to allow for Wnt/β-catenin signaling to initiate Snai2 expression, delamination, extracellular matrix (ECM) remodeling, and migration [26,27]. In the trunk, the scaffold protein Dact1/2 mediates β-catenin subcellular localization to similarly control the timing of Wnt activity during EMT [28].
Cues to trigger EMT in the neural crest are not limited to classical signaling events, but also include neural crest interactions with adjacent tissues and ECM. Neural crest cells secrete proteases in order to degrade cadherin junctions, penetrate the basement membrane, and leave the neuroepithelium [19]. Accordingly, matrix metalloproteinases MMP-2, MMP-9, and MMP-14, and a disintegrin and metalloproteinases ADAM-10 and ADAM-19 function is required for neural crest migration [29–32]. These proteases act to cleave cadherins (N-cadherin, Cadherin-6B) and the ECM proteins fibronectin and laminin [30,32,33]. Interestingly, the ectodomain shed by Cadherin-6B cleavage acts to increase MMP-2 activity and is sufficient to trigger precocious neural crest delamination [33].
While it is clear that neural crest cells modify their external environment, the biophysical effect of neighboring tissues during EMT has only recently been uncovered. In Xenopus embryos, convergent-extension movements of the head mesoderm during gastrulation are in close juxtaposition with the premigratory neural crest [34]. This results in a stiffening of the adjacent tissue as mesodermal cell density increases, and this stiffening is necessary and sufficient to trigger neural crest delamination [34]. Inhibition of the integrin β1/vinculin/talin complex in neural crest cells blocks their migration, similar to loss of tissue stiffening, suggesting a mechanism by which neural crest cells sense their biophysical environment [34]. In avian embryos, the presence of head mesoderm increases the directionality of neural crest cells in vitro, and mesoderm proliferation appears to precede neural crest invasion into the branchial arches in vivo [35]. While the molecular mechanism underlying these interactions remains to be conclusively demonstrated, it is interesting question whether mesoderm proliferation may similarly increase tissue stiffness as in Xenopus to facilitate avian neural crest migration [34,35].
While these mechanisms, along with others, promote neural crest EMT, not all EMTs result in individual migrating cells. Rather, EMT represents a spectrum between epithelial and mesenchymal cell states, with intermediate states, termed “partial EMT” between the two [4,5,7,8]. With differing degrees of mesenchymalization comes different modes of migration, including collective and individual cell migration (Figure 2) [18,36]. In the following sections we will discuss how neural crest cells in amniotes adopt individual cell modes of migration [15,16,37], while those in anamniotes prefer a more collective migration strategy [13–15,38,39].
Individual cell migration
After EMT, neural crest cells can migrate as individuals, as has been observed in the hindbrain and trunk region of amniotes [15,16,37]. To date, most of our mechanistic understanding of this phenomenon comes primarily from studies of cultured fibroblast cells. A prerequisite for individual cell migration is the acquisition of front-to-back cell polarity, a process by which a broad fan-shaped lamellipodium protrudes at the leading edge and a rounded swollen cell body retracts at the trailing edge [40]. This cell polarization is controlled by the distribution of small GTPases and cytoskeletal components [40]. Rac1 and Cdc42 are restricted to the anterior side to regulate actin assembly to pull the cell body; meanwhile, RhoA, located on the cell posterior side, promotes the contractility of myosin to push the cell body forward (Figure 3a) [40]. These two modules coordinate with each other to drive the net forward displacement of the cell. Remarkably, this operating principle is maintained by collectively migrating streams, but on a larger scale (Figure 3b), as will be discussed in the following section. Additional cytoskeleton elements, microtubules, function as tracks for polarized transportation of the small GTPases [41]. One immediate challenge for the neural crest field is to test these proposed mechanisms in developing embryos.
Figure 3. Individually and collectively migrating neural crest cells utilize many of the same cellular mechanisms for migration.
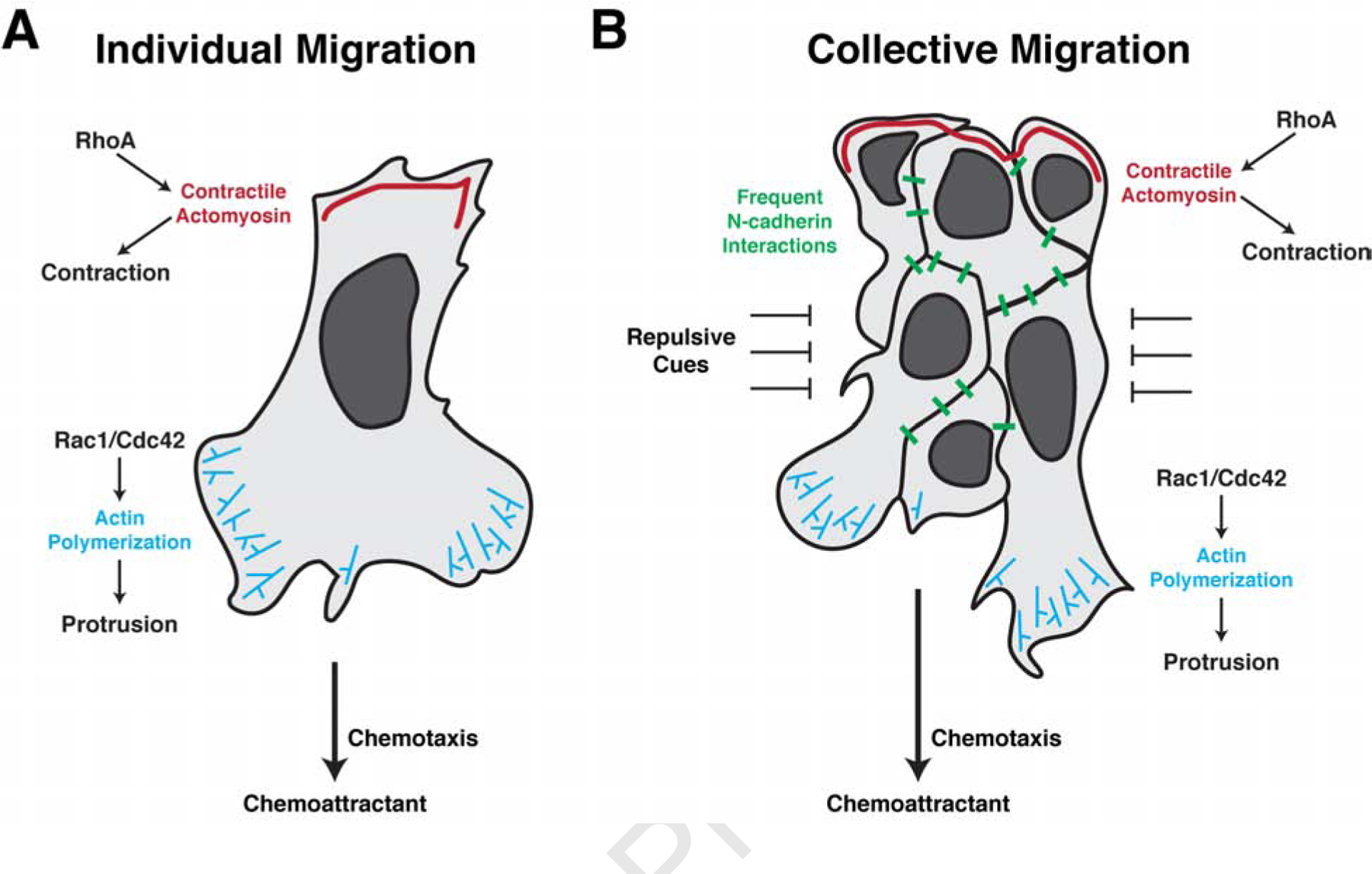
(a) Individually migrating neural crest cells undergo chemotaxis to maintain directional movement. Binding of the chemokine to its receptors on the cell surface polarize the activity of the small GTPases Rac1, Cdc42, and RhoA. At the leading edge, Rac1/Cdc42 activation promotes actin polymerization to extend lamellipodia and filopodia, allowing for new adhesions with the neighboring environment. RhoA activity is restricted to the trailing edge where it controls myosin phosphorylation and actomyosin contractions, propelling the cell forward. Infrequent N-cadherin interactions between individually migrating cells further shape their directionality. (b) In collectively migrating neural crest cells, chemotaxis similarly promotes leading edge Rac1/Cdc42 activation and actin polymerization in the leading cells. Trailing cells, however, activate RhoA to mediate supracellular contractions to move the migratory cluster forward. Simultaneously, frequent N-cadherin interactions maintain cluster spreading and fluidity through contact inhibition of locomotion. Collectively migrating cells are tightly confined by adjacent repulsive cues which aid to maintain cohesiveness of the migratory tissue.
The active interactions between these intracellular components provide cells with the ability to undergo random locomotion; however, to achieve directional migration, extrinsic cues are required. In general, there are two classes of environmental guidance cues: chemotaxis and cell-cell contact. During chemotaxis, cells migrate along a chemical gradient [42]. Exposure to this ligand gradient creates minor differences of both receptor(s) and GTPases along the major axis of cells. Through a positive-feedback mechanism, this small difference is further amplified and maintained such that the asymmetrical localization of the two downstream mechanical components, polymerized actin in the anterior and contractile myosin in the posterior, is established (Figure 3a). By this means, cells advance by recycling protrusions in the leading edge and retracting the trailing edge. Presently, chemical cues that may instruct individual neural crest migration at the trunk level remain unknown. Novel sequencing approaches and imaging techniques will pave the way to address this long-standing and interesting question.
In addition to chemotaxis, individually migrating cells are heavily influenced by their neighboring cells. While cell-cell contact plays a fundamental role in controlling the streaming behavior of cell migration, it is equally important for individual cell migration. Compared with chemotaxis, the role of cell-cell contact has been relatively well studied in neural crest cells. Locomoting neural crest cells frequently collide with each other and subsequently display different morphological changes and motility based on the region of cell-cell contact. If the contact occurs between the lamellipodia, cells immediately repel each other and disperse, a phenomenon called contact inhibition of cell locomotion [43]. On the other hand, if it occurs between cell bodies, the cells tend to associate with each other, changing their shapes from a stretched and polarized form into a rounder form (contact attraction), and then either separate or move as a doublet [37]. In both scenarios, contacting cells establish adhesion junctions and then disassemble them; however, junctions formed during contact inhibition are less stable than those formed during contact attraction. This is likely to result from the differential distribution of myosin and cadherins on the cell membrane, which as a consequence, modulate stiffness and viscosity of the cells. If the attraction force is larger than the repulsion force in a contacting events involving many cells, the cells will move as a cohesive group. As a result, individual cell migration and collective cell migration are interchangeable under some circumstances [44,45].
Collective migration
Collective cell migration is a migratory mode in which neural crest cells move together with frequent physical contacts between neighboring cells. Studies in Xenopus and zebrafish embryos suggest this is the primary mechanism for neural crest migration in anamniotes (see [17,21] for recent detailed reviews). Briefly, collectively migrating neural crest cells integrate attractive and repulsive signaling events to maintain directionality and confinement to discrete migratory streams [38,39,46,47], while N-cadherin-mediated cell junctions mediate contact inhibition of locomotion to maintain cell spreading and tissue fluidity (Figure 3b) [14,43,48]. Collectively migrating clusters in Xenopus and zebrafish display “supracellular” behaviors. While leading cells show extensive activation of Rac1 to promote filopodial and lamellipodial protrusions, the trailing cells do not activate Rac1 and extend minimal filopodia [13]. The rear-most cells use RhoA to drive myosin light chain phosphorylation and actomyosin contractile forces, propelling the migratory cluster forward (Figure 3b) [13].
Directionality and confinement of collective neural crest cell migration is mediated by attractive and repulsive chemokines and environmental signals (Figure 3b). While the necessity of these individual cues has been demonstrated previously (reviewed in [17,21]), recent studies have begun to address how migrating cells simultaneously integrate multiple cues and further translate them into stereotypical cellular behaviors at both individual and population levels. Comprehensive analysis of combinations of attractive Sdf1 cues and repulsive Sema3A cues indicate that these two signals provide reciprocal inputs into the same cell, and together coordinate directional movements [47]. At the leading edge of a migratory cluster, Sdf1 binding to its receptor CXCR4 activates Rac1 and subsequent directional actin polymerization and cell-matrix adhesions [47]. Simultaneously, cells at the trailing edge of the migrating cluster experience Semaphorin-mediated repulsive cues to inhibit Rac1 activity, leading to de-adhesion from the matrix [47].
While chemotaxis in response to secreted ligands has been a primary focus in the neural crest field, it is likely that migrating neural crest cells also respond to extracellular matrix (ECM)-mediated migratory mechanisms including durotaxis and haptotaxis. ECM stiffness clearly influences neural crest EMT [34], suggesting that durotaxis may also play an important role in later neural crest migration. In parallel, neural crest cells are likely to use haptotaxis to migrate in response to ECM adhesiveness. Further studies that combine complex signaling events with three-dimensional ECM components are needed to address how neural crest cells navigate their complex environments, and how these mechanisms vary between species is of great interest.
While migrating clusters are directed through external signals, cell-cell interactions amongst neural crest cells maintain tissue cohesiveness, fluidity, and polarity. Many of these mechanisms are mediated by N-cadherin interactions (Figure 3b). N-cadherin junctions between two migrating cells trigger contact inhibition of locomotion, which stimulates RhoA activity at the junction site, and polarizes Rac1 activity away from the junction, thus promoting neural crest cells to spread [14,43,49]. PDGF signaling, both as a chemoattractant and activator of N-cadherin transcription, is necessary for contact inhibition of locomotion during migration [50]. In addition, the gap junction protein Connexin 43 is expressed in premigratory and migratory neural crest cells; however, migratory cells translate a truncated isoform of Connexin 43 from an internal ribosomal entry site [51]. Surprisingly, this Connexin 43 isoform complexed with basic transcription factor 3 enters the nucleus to directly activate N-cadherin transcription [51]. While these mechanisms maintain N-cadherin expression, the strong adhesion property of N-cadherin interactions may decrease tissue spreading; thus, contact inhibition of locomotion interactions must be transient. Lysophosphatidic acid (LPA) signaling through the receptor LPAR2 acts to promote internalization of N-cadherin from junction sites to facilitate deadhesion [48]. LPAR2 signaling is necessary to maintain tissue fluidity, and loss of LPAR2 increases tension across the migrating cell cluster leading to failure to invade into confined streams [48]. Together, these mechanisms help to regulate N-cadherin function to allow for successful collective migration.
N-cadherin interactions between collectively migrating cells are also critical to control collective chemotaxis, in which a migratory cluster remains polarized toward a chemoattractant. While Sdf1/CXCR4 signaling potently attracts collectively migrating neural crest cells, N-cadherin loss strongly diminishes the directionality of neural crest migration [52]. These N-cadherin interactions act to suppress Rac1 activation and protrusion formation within the migrating cluster, effectively polarizing directionality by promoting a chemotactic response only at the free edge of the cell cluster [52].
Some recent studies have proposed a model in which migrating cell collectives adopt leader/follower cell behavior. In this scenario, leader cells at the distal migratory front respond to environmental cues to invade adjacent tissues while simultaneously attracting the trailing follower cells [46,53,54]. Leader cells in the chick embryo respond to environmental cues and display a distinct transcriptional signature from follower cells [53,54]. Consistent with this, leaders express aquaporin 1, a transmembrane channel protein located on lamellipodium, which promotes matrix metalloprotease activity and accordingly degrades the ECM, thus permitting the whole stream to move forward [55]. Zebrafish trunk neural crest cells display strict leader/follower commitment; when the leader cell is ablated, the neural crest stream halts migration and awaits a new cell to delaminate from the premigratory pool, overtake the follower cells, and resume migration as a new leader [15]. However, data from other organisms suggest that this is not a pan-vertebrate phenomenon. In Xenopus, for example, rear contractions drive trailing cells forward within migratory clusters, resulting in leader cell displacement toward the back and continuous exchange of leaders and followers [13]. In chick and zebrafish, migrating cranial neural crest cells frequently exchange positions and thus do not maintain a leader/follower identity [15,16]. Further single-cell analysis will be required to examine the transcriptional profile of leader/follower cells in different contexts and to determine if these expression profiles are autonomously committed or are nonautonomously maintained in leader cells.
Conclusions and Perspectives
Since its discovery over 150 years ago, defining the molecular and cellular principles of neural crest EMT and migration is both fascinating and challenging to developmental biologists. Recent work on the neural crest has supported the notion that EMT is not a strict transition from epithelial to mesenchymal cell types, but rather that neural crest cells in different species adopt migratory approaches with differing degrees of mesenchymalization (Figure 2). Studies over the next few years are expected to provide additional insights that will further our understandings of EMT. The majority of functional studies have focused on neural crest cells that migrate through well-defined streams such as the branchial arches or segmented by somites in the trunk, yet many interesting questions remain. For example, how is directionality maintained in less-confined environments such as for neural crest migrating from the midbrain level?
Neural crest cell migration in the zebrafish trunk shows clear leader-follower cell behaviors, suggesting that these identities are committed at the time of delamination [15]. As a leader cell is ablated, the trailers pause and wait for a new leader cell to delaminate and overtake the migratory stream in order to direct continued migration [15]. How do neural crest cells in this migratory chain communicate? One possibility is that functional gap junctions mediate intercellular communication of chemical signals across the stream, such that a leader cell passes information down the stream to the premigratory neural crest pool [51,56,57]. If so, ablation of the leader cell may disrupt this communication, signaling the delamination of a new leader cell. Alternatively, this intercellular communication could be maintained by a balance between tension forces and adhesion forces. The combination of mechanical sensors and fluorescence lifetime imaging will provide a solution to test this possibility.
Since neural crest cells are conserved across vertebrates, they represent an excellent model to ask how different modes of migration may have evolved. Many of the cues and gene networks controlling EMT are highly conserved, but it will be fascinating to uncover if there are unique contributors to EMT that promote collective migration in anamniotes and individual cell migration in amniotes. Is it possible to drive amniote-like EMT and migration in anamniotes, and vice versa? Finally, what were the evolutionary pressures that dictated the adoption of individual or collective cell migration modules in these species? These questions and more will help guide the future of EMT research in the neural crest field.
Acknowledgements
We would like to acknowledge Dr. Erica Hutchins for valuable discussion. This work was supported by the National Institutes of Health (K99DE029240 to M.L.P., R01DE027538 and R01DE027568 to M.E.B.). We apologize to those authors we were unable to cite due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
· special interest
·· outstanding interest
- 1.Kalcheim C: Epithelial: interestaiTransitions during Neural Crest and Somite Development. J Clin Med 2015, 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Weinberg RA: Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev Cell 2008, 14:818–829. [DOI] [PubMed] [Google Scholar]
- 3.Hutchins EJ, Kunttas E, Piacentino ML, Howard AGA, Bronner ME, Uribe RA: Migration and diversification of the vagal neural crest. Dev Biol 2018, 444:S98–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto MA, Huang RYYJ, Jackson RAA, Thiery JPP: Emt: 2016. Cell 2016, 166:21–45. [DOI] [PubMed] [Google Scholar]
- 5.Sha Y, Haensel D, Gutierrez G, Du H, Dai X, Nie Q: Intermediate cell states in epithelial-to-mesenchymal transition. Phys Biol 2019, 16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capuana L, Bostr, tiones in epithelial-to-, Multicellular scale front-to-rear polarity in collective migration. Curr Opin Cell Biol 2020, 62:114–122. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Weinberg RA: Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med 2018, 12:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell K, Casanova J: A common framework for EMT and collective cell migration. Development 2016, 143:4291–4300. [DOI] [PubMed] [Google Scholar]
- 9.Martik ML, Bronner ME: Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet 2017, 33:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noden DM: The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 1983, 96:144–165. [DOI] [PubMed] [Google Scholar]
- 11.Mayor R, Theveneau E: The neural crest. Cambridge University Press; 2012. [Google Scholar]
- 12.Horstadius S: The Neural Crest. Its Properties and Derivatives in the Light of Experimental Research. Q Rev Biol 1952, 27:221–221. [Google Scholar]
- 13.Shellard A, Szab, Its Properties and Der Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science (80- ) 2018, 362:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· This study uses in vivo and ex vivo experiments, combined with mathematical modeling, to demonstrate that collectively migrating neural crest cells employ a “supracellular” mechanism for forward movement. Contractility is inhibited in the leading cells, while trailing cells drive migration by contraction of a tensile actomyosin ring that spans the full migrating cluster.
- 14.Scarpa E, Szabg that spans the full migrating cluster. trailing Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces. Dev Cell 2015, 34:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson J, Gauert A, Briones Montecinos L, Fanlo L, Alhashem ZM, Assar R, Marti E, Kabla A, Hichard S, Linker C: Leader Cells Define Directionality of Trunk, but Not Cranial, Neural Crest Cell Migration. Cell Rep 2016, 15:2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genuth MA, Allen CDC, Mikawa T, Weiner OD: Chick cranial neural crest cells use progressive polarity refinement, not contact inhibition of locomotion, to guide their migration. Dev Biol 2018, 444:S252–S261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shellard A, Mayor R: Integrating chemical and mechanical signals in neural crest cell migration. Curr Opin Genet Dev 2019, 57:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shellard A, Mayor R: Supracellular migration - Beyond collective cell migration. J Cell Sci 2019, 132. [DOI] [PubMed] [Google Scholar]
- 19.Taneyhill LA, Schiffmacher AT: Should I stay or should I go? Cadherin function and regulation in the neural crest. Genesis 2017, 55:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard CE, Taneyhill LA: The road best traveled: Neural crest migration upon the extracellular matrix. Semin Cell Dev Biol 2019, doi: 10.1016/j.semcdb.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shellard A, Mayor R: Chemotaxis during neural crest migration. Semin Cell Dev Biol 2016, 55:111–118. [DOI] [PubMed] [Google Scholar]
- 22.Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F: Cloning and Characterization of Three Xenopus Slug Promoters Reveal Direct Regulation by Lef/β-Catenin Signaling. J Biol Chem 2001, 276:30350–30358. [DOI] [PubMed] [Google Scholar]
- 23.Taneyhill LA, Coles EG, Bronner-Fraser M: Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 2007, 134:1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bol81–1490.nt 2007, itions of the neural crestithelial-y Lef/β-CateThe transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J Cell Sci 2003, 116:499Cell. [DOI] [PubMed] [Google Scholar]
- 25.Chalpe AJ, Prasad M, Henke AJ, Paulson AF: Regulation of cadherin expression in the chicken neural crest by the Wnt/β-catenin signaling pathway. Cell Adhes Migr 2010, 4:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchins EJ, Bronner ME: Draxin acts as a molecular rheostat of canonical Wnt signaling to control cranial neural crest EMT. J Cell Biol 2018, 217:3683–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchins EJ, Bronner ME: Draxin alters laminin organization during basement membrane remodeling to control cranial neural crest EMT. Dev Biol 2019, 446:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabadán MA, Herrera A, Fanlo L, Usieto S, Carmona-Fontaine C, Barriga EH, Mayor R, Pons S, Martí E: Delamination of neural crest cells requires transient and reversible Wnt inhibition mediated by Dact1/2. Development 2016, 143:2194–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalev-Altman R, Hanael E, Zelinger E, Blum M, Monsonego-Ornan E, Sela-Donenfeld D: Conserved role of matrix metalloproteases 2 and 9 in promoting the migration of neural crest cells in avian and mammalian embryos. FASEB J 2020, doi: 10.1096/fj.201901217RR. [DOI] [PubMed] [Google Scholar]
- 30.Monsonego-Ornan E, Kosonovsky J, Bar A, Roth L, Fraggi-Rankis V, Simsa S, Kohl A, Sela-Donenfeld D: Matrix metalloproteinase 9/gelatinase B is required for neural crest cell migration. Dev Biol 2012, 364:162–177. [DOI] [PubMed] [Google Scholar]
- 31.Garmon T, Wittling M, Nie S: MMP14 Regulates Cranial Neural Crest Epithelial-to-Mesenchymal Transition and Migration. Dev Dyn 2018, 247:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffmacher AT, Padmanabhan R, Jhingory S, Taneyhill LA: Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol Biol Cell 2014, 25:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffmacher AT, Adomako-Ankomah A, Xie V, Taneyhill LA: Cadherin-6B proteolytic N-terminal fragments promote chick cranial neural crest cell delamination by regulating extracellular matrix degradation. Dev Biol 2018, 444:S237–S251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barriga EH, Franze K, Charras G, Mayor R: Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 2018, 554:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· Combining classical embryology with in vivo atomic force microscopy and mechanical perturbations, this study shows that the cranial mesoderm in Xenopus increases in density during development, resulting in increased stiffness of the tissue surrounding the cranial neural crest. Increasing tissue stiffness activates the integrin/vinculin/talin complex in neural crest cells and is necessary and sufficient to induce delamination. These results demonstrate the critical importance of biophysical forces in regulating epithelial-to-mesenchymal transitions.
- 35.McKinney MC, McLennan R, Giniunaite R, Baker RE, Maini PK, Othmer HG, Kulesa PM: Visualizing mesoderm and neural crest cell dynamics during chick head morphogenesis. Dev Biol 2020, doi: 10.1016/j.ydbio.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· Live time-lapse microscopy of avian cranial neural crest and cranial mesoderm identifies dynamic interactions between these two populations. Importantly, the authors identify that interactions with the neural crest increase mesoderm migration speed and provide a model in which migrating neural crest and mesoderm interact to coordinate the rate of tissue growth.
- 36.De Pascalis C, Etienne-Manneville S: Single and collective cell migration: The mechanics of adhesions. Mol Biol Cell 2017, 28:1833–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Vieceli FM, Gonzalez WG, Li A, Tang W, Lois C, Bronner ME: In Vivo Quantitative Imaging Provides Insights into Trunk Neural Crest Migration. Cell Rep 2019, 26:1489–1500.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· The authors establish a method for in vivo time-lapse imaging of avian trunk neural crest cell migration over long time periods. This analysis indicates that avian trunk neural crest cells migrate individually in a biased random walk away from areas of neural crest density. As migrating neural crest interact, they reorient their directionality following a period of “contact attraction”.
- 38.Theveneau E, Steventon B, Scarpa E, Garcia S, Trepat X, Streit A, Mayor R: Chase-and-run between adjacent cell populations promotes directional collective migration. Nat Cell Biol 2013, 15:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szab- Cell Biol 2013, ons promotes directional collective migrationayor In vivo confinement promotes collective migration of neural crest cells. J Cell Biol 2016, 213:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etienne-Manneville S: Polarity proteins in migration and invasion. Oncogene 2008, 27:6970–6980. [DOI] [PubMed] [Google Scholar]
- 41.Etienne-Manneville S: Microtubules in Cell Migration. Annu Rev Cell Dev Biol 2013, 29:471–499. [DOI] [PubMed] [Google Scholar]
- 42.Roca-Cusachs P, Sunyer R, Trepat X: Mechanical guidance of cell migration: Lessons from chemotaxis. Curr Opin Cell Biol 2013, 25:543–549. [DOI] [PubMed] [Google Scholar]
- 43.Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R: Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 2008, 456:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo S, Housley MP, Weiner OD, Stainier DYR: Nodal signaling regulates endodermal cell motility and actin dynamics via Rac1 and Prex1. J Cell Biol 2012, 198:941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pézeron G, Mourrain P, Courty S, Ghislain J, Becker TS, Rosa FM, David NB: Live Analysis of Endodermal Layer Formation Identifies Random Walk as a Novel Gastrulation Movement. Curr Biol 2008, 18:276–281. [DOI] [PubMed] [Google Scholar]
- 46.Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, Parsons M, Lambris JD, Mayor R: Complement Fragment C3a Controls Mutual Cell Attraction during Collective Cell Migration. Dev Cell 2011, 21:1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajanca F, Gouignard N, Colle C, Parsons M, Mayor R, Theveneau E: In vivo topology converts competition for cell-matrix adhesion into directional migration. Nat Commun 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· This study addresses how individual cells integrate opposing migratory signals. Sdf1 and Sema3A have opposing effects on Rac1 activation, which modulate cell adhesion to extracellular fibronectin. While Sdf1 promotes adhesion formation, lateral Sema3A blocks adhesion to confine the migrating neural crest stream.
- 48.Kuriyama S, Theveneau E, Benedetto A, Parsons M, Tanaka M, Charras G, Kabla A, Mayor R: In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity. J Cell Biol 2014, 206:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roycroft A, Szab14,ration requires an LPAR2-dependent increase in ti Redistribution of Adhesive Forces through Src/FAK Drives Contact Inhibition of Locomotion in Neural Crest. Dev Cell 2018, 45:565–579.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahm I, Barriga EH, Frolov A, Theveneau E, Frankel P, Mayor R: PDGF controls contact inhibition of locomotion by regulating N-cadherin during neural crest migration. Development 2017, 144:2456elopme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotini M, Barriga EH, Leslie J, Gentzel M, Rauschenberger V, Schambon A, Mayor R: Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat Commun 2018, 9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]; · This work identifies a novel role for the gap junction protein Connexin 43, which is expressed as a truncated isoform in migrating neural crest cells. This truncated protein complexes with basic transcription factor 3 to enter the nucleus and directly activate N-cadherin expression, and this mechanism is conserved in Xenopus and mammalian cells.
- 52.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R: Collective Chemotaxis Requires Contact-Dependent Cell Polarity. Dev Cell 2010, 19:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLennan R, Schumacher LJ, Morrison JA, Teddy JM, Ridenour DA, Box AC, Semerad CL, Li H, McDowell W, Kay D, et al. : VEGF signals induce trailblazer cell identity that drives neural crest migration. Dev Biol 2015, 407:12–25. [DOI] [PubMed] [Google Scholar]
- 54.McLennan R, Schumacher LJ, Morrison JA, Teddy JM, Ridenour DA, Box AC, Semerad CL, Li H, McDowell W, Kay D, et al. : Neural crest migration is driven by a few trailblazer cells with a unique molecular signature narrowly confined to the invasive front. J Cell Sci 2015, 128:e1207–e1207. [DOI] [PubMed] [Google Scholar]
- 55.McLennan R, McKinney M, Teddy J, Morrison J, Kasemeier-Kulesa J, Ridenour D, Manthe C, Giniunaite R, Robinson M, Baker R, et al. : Neural crest cells bulldoze through the microenvironment using Aquaporin 1 to stabilize filopodia. Development 2020, 147:dev185231. [DOI] [PubMed] [Google Scholar]; · The authors identify the water channel protein Aquaporin 1 as enriched in leading migratory neural crest cells. In these cells, Aquaporin 1 functions to promote migratory stream speed by both enhancing focal adhesion formation and by increasing matrix metalloproteinase activity to “bulldoze” through the extracellular matrix.
- 56.Jourdeuil K, Taneyhill LA: The gap junction protein Connexin 43 controls multiple aspects of cranial neural crest cell development. J Cell Sci 2020, doi: 10.1242/jcs.235440. [DOI] [PMC free article] [PubMed] [Google Scholar]; · Using elegant dye injection experiments, this study demonstrates the presence of functional, Connexin 43-dependent, gap junctions between neural crest cells. Further, gap junction function controls the timing of neural crest specification and delamination.
- 57.Xu X, Francis R, Wei CJ, Linask KL, Lo CW: Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development 2006, 133:3629–3639. [DOI] [PubMed] [Google Scholar]


