Abstract
The prognostic role of marital status on colorectal signet ring cell carcinoma (SRCC) has not been studied. In this study, the correlation of marital status with prognosis of colorectal SRCC was analyzed. Eligible subjects were extracted from the Surveillance, Epidemiology, and End Results (SEER) dataset from 2004 to 2015, followed by comparison of cancer-specific survival (CSS) and overall survival (OS) between married and unmarried group. 3152 patients were identified including 1777 married patients (56.38%). Married populations tended to be more patients aged < 65, male, receiving chemotherapy, and less black race and large tumor size compared to unmarried group (all P < 0.05).Moreover, 5-year CSS (30.04% vs. 28.19%, P = 0.0013) and OS rates (26.68% vs. 22.94%, P < 0.0001) were superior in married population. Multivariate analysis revealed that marital status was an independent favorable prognostic indicator, and married population had better CSS (HR: 0.898; 95% CI: 0.822–0.980; P = 0.016) and OS (HR: 0.898; 95%CI: 0.827–0.975; P = 0.011).In addition, CSS as well as OS were superior in married populations than unmarried ones in most subgroups. Marital status was an independent prognostic factor for survival in patients with colorectal SRCC. Additionally, married patients obtained better survival advantages.
Subject terms: Cancer, Oncology, Risk factors
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-associated mortality in the USA, also greatly threatening the global health1. Despite the diverse subtypes of CRCs, accumulative attention has been recently paid to colorectal signet ring cell carcinoma (SRCC), which was initially proposed by Saphir as well as Laufman in 19512. Stomach is considered to be the most common organ for primary SRCC, while colorectal SRCC is less frequent3. Colorectal SRCC is a very rare and special type of CRCs, accounting for only 0.3 to 4.6% of all types of CRCs4–8.
The prognostic factors of colorectal SRCC have intensively explored, mostly including clinicopathological features as well as therapeutic strategies9–12. However, the present attention has also been paid to social factors, which might be involved in disease progression13,14. Among them, marital status as an important social factor has attracted more and more attention. To be specific, marital status has been identified as an independent prognostic indicator in several types of malignancies, including colorectal cancer, pancreatic, breast, lung and prostate cancer, with superior survival in married population15–19. However, there is no study concerning the role of marital status on colorectal SRCC survival specially.
The National Cancer Institute (NCI)’s Surveillance, Epidemiology and End Results (SEER) database reports data from 18 population-based cancer registries by covering nearly 30% of the US population20. Therefore, we could investigate the correlation of marital status with survival in rare tumors by extracting data from SEER14,18,21. This research was designed to examine the association of marital status with survival in colorectal SRCC patients by utilizing SEER database.
Materials and methods
Ethics statement
For acquisition of relevant data from the database, we signed the SEER Research Data Agreement (No. 19817-Nov2018) and further searched for data according to the approved guidelines. All extracted data were publicly accessible and de-identified, and data analysis was considered to be non-human subjects by Office for Human Research Protection, therefore, no approval was requested by institutional review board.
Study population
SEER*State v8.3.6 (released on August 8, 2019) was employed to select and identify qualified subjects, which includes 18 SEER regions during the period of 1998–2015 (2018 submission). The inclusion criteria were as follows: (1) it should be primary colorectal SRCC patients; (2) the diagnosis of SRCC was based on ICD-O-3; coded as 8490/3. Patients were excluded if they had: (1) more than one primary malignancies; (2) reported diagnosis source from autopsy or death certificate or without pathological diagnosis; (3) without certain necessary clinicopathological data, including: AJCC stage, surgical style and marital status; (4) without prognostic information. The remaining qualified populations were included.
Covariates and endpoint
We analyzed the patients’ characteristics according to the following factors: year of diagnosis (2004–2007, 2008–2011, 2012–2015); insured status (uninsured/unknown, any medicaid/insured); age (< 65, ≥ 65); marital status (unmarried, married); gender (female, male); race (black, white or others); primary site (cecum–transverse colon, descending colon–sigmoid, multiple, rectum and unknown); grade (grade I/II, grade III/IV, unknown); tumor size (≤ 5 cm, ˃5 cm, unknown); AJCC stage (stage I, II, III, IV); surgery (no surgery, local tumor excision/partial colectomy, total colectomy), lymph node dissections (none or biopsy, 1–3 regional lymph nodes removed, ≥ 4 regional lymph nodes removed, unknown), chemotherapy (no/unknown, yes), radiotherapy (no/unknown, yes).The widowed or single (never married or having a domestic partner) or divorced or separated patients were classified as unmarried. The primary tumor site was classified as cecum–transverse colon (including the cecum, appendix, ascending colon, hepatic flexure and the transverse colon), descending colon–sigmoid (including the splenic flexure and descending and sigmoid colons), multiple, rectum and unknown. Year of diagnosis was equally divided into 2004–2007, 2008–2011, 2012–2015, which was referred to the previous papers22,23. The grouping of the age and tumor size also refers to the published studies24,25. In addition, the staging of cancer is based on the 6th edition of AJCC stage system, which adapted to patients in the SEER database with a diagnosis time of 2004–2015.
The endpoint included cancer-specific survival (CSS) and overall survival (OS). The former was defined as the duration from diagnosis to colorectal SRCC-caused death, and the latter was referred to the duration from diagnosis to all-cause death.
Statistical analyses
Kaplan–Meier (K–M) method was employed to estimate the univariate analysis, followed by log-rank test for assessing the differences of CSS and OS. Notably, if variables had P values ≤ 0.1 in univariate analysis, they were incorporated into multivariate Cox proportional hazard analysis. Similarly, Cox regression analysis was also used for stratified analysis. SPSS software (SPSS Inc., Chicago, USA, version 19.0) was used for statistical analysis, and GraphPad Prism 5 was utilized for plotting survival curves as well as generating forest plots. A two-sided P < 0.05 indicated statistical significance.
Results
Patient features
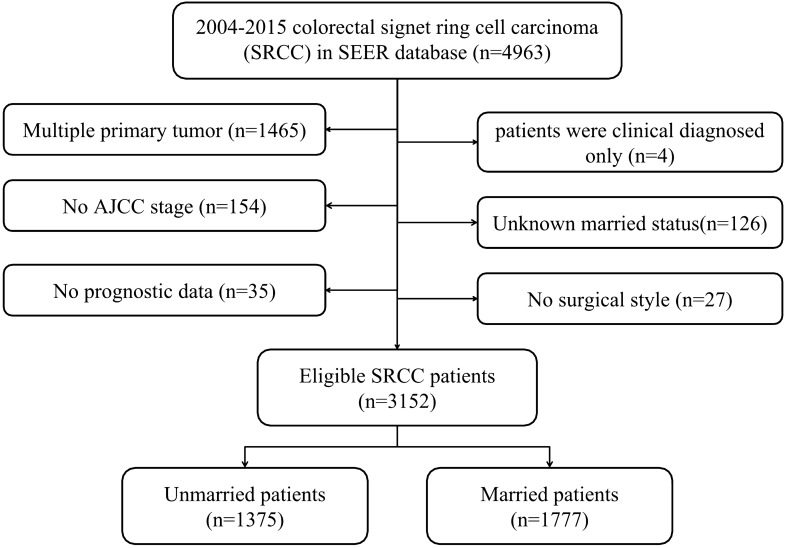
In total, 3152 eligible patients were identified from the SEER database between 2004 and 2015, with a median follow-up duration of 16 months (range: 0–155 months). Afterwards, subjected were categorized into unmarried group (n = 1375, 43.62%) and married group (n = 1777, 56.38%), with specific screening process shown in Fig. 1.Moreover, the baseline characteristics of patients stratified by marital status were summarized in Table 1. To be specific, age (P = 0.002), gender (P < 0.001), race (P < 0.001), tumor size (P = 0.002) and chemotherapy (P < 0.001) were significantly different between the two groups. Additionally, married populations tended to be more patients aged < 65 (56.95% vs. 48.36%), male (58.98% vs. 41.45%), receiving chemotherapy (63.53% vs. 51.71%), and were less to be black race (7.09% vs. 12.58%) and tumor size ˃5 cm (36.18% vs. 42.04%) in comparison with unmarried ones.
Figure1.
Flow chart of patient selection.
Table 1.
The clinicopathological characteristics and treatment of the included 3152 colorectal signet ring cell carcinoma patients.
| Characteristic | Total | Unmarried | Married | P-value |
|---|---|---|---|---|
| Year at diagnosis | 0.068 | |||
| 2004–2007 | 1066 (33.82%) | 440 (32.00%) | 626 (35.23%) | |
| 2008–2011 | 1022 (32.42%) | 443 (32.22%) | 579 (32.58%) | |
| 2012–2015 | 1064 (33.76%) | 492 (35.78%) | 572 (32.19%) | |
| Insured status | 0.405 | |||
| Uninsured/unknown | 969 (30.74%) | 412 (29.96%) | 557 (31.34%) | |
| Any medicaid/insured | 2183 (69.26%) | 963 (70.04%) | 1220 (68.66%) | |
| Age | < 0.001 | |||
| < 65 | 1677 (53.20%) | 665 (48.36%) | 1012 (56.95%) | |
| ≥ 65 | 1475 (46.80%) | 710 (51.64%) | 765 (43.05%) | |
| Gender | < 0.001 | |||
| Female | 1534 (48.67%) | 805 (58.55%) | 729 (41.02%) | |
| Male | 1618 (51.33%) | 570 (41.45%) | 1048 (58.98%) | |
| Race | < 0.001 | |||
| Black | 299 (9.49%) | 173 (12.58%) | 126 (7.09%) | |
| White | 2584 (81.98%) | 1115 (81.09%) | 1469 (82.67%) | |
| Other | 269 (8.53%) | 87 (6.33%) | 182 (10.24%) | |
| Primary site | 0.213 | |||
| Cecum–transverse colon | 1901 (60.31%) | 841 (61.16%) | 1060 (59.65%) | |
| Descending colon–sigmoid | 493 (15.64%) | 206 (14.98%) | 287 (16.15%) | |
| Multiple | 48 (1.52%) | 28 (2.04%) | 20 (1.13%) | |
| Rectum | 623 (19.77%) | 262 (19.05%) | 361 (20.32%) | |
| Unknown | 87 (2.76%) | 38 (2.76%) | 49 (2.76%) | |
| Grade | 0.233 | |||
| Grade I/II | 171 (5.43%) | 64 (4.65%) | 107 (6.02%) | |
| Grade III/IV | 2419 (76.74%) | 1067 (77.60%) | 1352 (76.08%) | |
| Unknown | 562 (17.83%) | 244 (17.75%) | 318 (17.90%) | |
| Tumor size | 0.002 | |||
| ≤ 5 cm | 1173 (37.21%) | 494 (35.93%) | 679 (38.21%) | |
| > 5 cm | 1221 (38.74%) | 578 (42.04%) | 643 (36.18%) | |
| Unknown | 758 (24.05%) | 303 (22.04%) | 455 (25.60%) | |
| AJCC stage | 0.968 | |||
| I | 147 (4.66%) | 64 (4.65%) | 83 (4.67%) | |
| II | 444 (14.09%) | 192 (13.96%) | 252 (14.18%) | |
| III | 1156 (36.68%) | 511 (37.16%) | 645 (36.30%) | |
| IV | 1405 (44.57%) | 608 (44.22%) | 797 (44.85%) | |
| Surgery | 0.053 | |||
| No surgery | 650 (20.62%) | 287 (20.87%) | 363 (20.43%) | |
| Local tumor excision /Partial colectomy | 828 (26.27%) | 332 (24.15%) | 496 (27.91%) | |
| Total colectomy | 1674 (53.11%) | 756 (54.98%) | 918 (51.66%) | |
| Lymph node dissection | 0.991 | |||
| None or biopsy | 847 (26.87%) | 368 (26.76%) | 479 (26.96%) | |
| 1–3 | 83 (2.63%) | 36 (2.62%) | 47 (2.64%) | |
| ≥ 4 | 2222 (70.49%) | 971 (70.62%) | 1251 (70.40%) | |
| Chemotherapy | < 0.001 | |||
| No/unknown | 1312 (41.62%) | 664 (48.29%) | 648 (36.47%) | |
| Yes | 1840 (58.38%) | 711 (51.71%) | 1129 (63.53%) | |
| Radiotherapy | 0.077 | |||
| No/unknown | 2726 (86.48%) | 1206 (87.71%) | 1520 (85.54%) | |
| Yes | 426 (13.52%) | 169 (12.29%) | 257 (14.46%) |
Marital status and survival
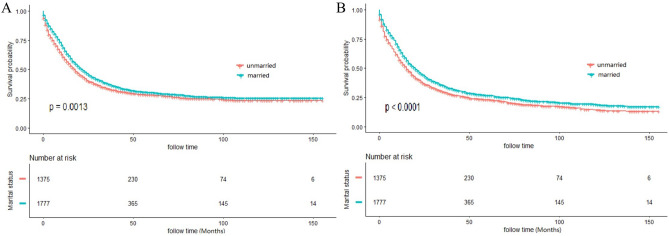
K–M curves revealed significant difference of CSS and OS stratified by marital status (Fig. 2), with superior OS and CSS in married populations than unmarried ones. The 3, 5, 10-year CSS rate was 37.54%, 30.04% and 25.49% in married patients, which was 33.20% ,28.19% and 23.47% in unmarried group (P = 0.0013). Meanwhile, the 3, 5, 10-year OS rate was 34.54%, 26.68% and 18.92% in married patients, which was 29.28% ,22.94% and 14.40% in unmarried group (P < 0.0001). Univariate log-rank test identified that marital status, primary site, grade, tumor size, AJCC stage, surgery, lymph node dissections and chemotherapy were significantly relate to CSS (P < 0.05). After the adjustment of the above variables in the Cox proportional hazards regression model, marital status remained as an independent prognostic indicator, with superior CSS in married populations than unmarried ones (HR: 0.898; 95% CI: 0.822–0.980; P = 0.016]. Meanwhile, all aforementioned variables including age and radiotherapy also had significant relationship with OS, and multivariate analysis also found that marital status was a favorable independent prognostic indicator of OS (HR: 0.898; 95%CI: 0.827–0.975; P = 0.011). Table 2 showed the detailed results of univariate and multivariate analysis.
Figure2.
Kaplan–Meier (K–M) curves for cancer-specific survival (CSS) (A) and overall survival (OS) (B) between unmarried and married patients.
Table 2.
Univariate and multivariate analyses of cancer special survival (CSS) and overall survival (OS) for patients with colorectal SRCC.
| Variables | CSS | OS | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||
| P value | HR (95%CI) | P value | P value | HR (95%CI) | P value | |
| Year at diagnosis | 0.826 | NI | 0.962 | NI | ||
| 2004–2007 | ||||||
| 2008–2011 | ||||||
| 2012–2015 | ||||||
| Insured status | 0.443 | NI | 0.620 | NI | ||
| Uninsured/unknown | ||||||
| Any medicaid/insured | ||||||
| Age | 0.731 | NI | < 0.001 | < 0.001 | ||
| < 65 | Reference | |||||
| ≥ 65 | 1.486 (1.359, 1.624) | |||||
| Gender | 0.176 | NI | 0.506 | NI | ||
| Female | ||||||
| Male | ||||||
| Race | 0.149 | NI | 0.183 | NI | ||
| Black | ||||||
| White | ||||||
| Other | ||||||
| Primary site | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| Cecum–transverse colon | Reference | Reference | ||||
| Descending colon–sigmoid | 1.196 (1.054, 1.358) | 0.005 | 1.211 (1.074, 1.365) | 0.002 | ||
| Multiple | 1.466 (1.050, 2.048) | 0.025 | 1.395 (1.008, 1.930) | 0.044 | ||
| Rectum | 1.334 (1.184, 1.503) | < 0.001 | 1.286 (1.119, 1.477) | < 0.001 | ||
| Unknown | 1.368 (1.075, 1.740) | 0.011 | 1.326 (1.049, 1.675) | 0.018 | ||
| Grade | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| Grade I/II | Reference | Reference | ||||
| Grade III/IV | 1.768 (1.417, 2.205) | < 0.001 | 1.742 (1.426, 2.128) | < 0.001 | ||
| Unknown | 1.763 (1.386, 2.242) | < 0.001 | 1.739 (1.397, 2.165) | < 0.001 | ||
| Tumor size | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| ≤ 5 cm | Reference | Reference | ||||
| > 5 cm | 1.253 (1.128, 1.392) | < 0.001 | 1.202 (1.091, 1.325) | < 0.001 | ||
| Unknown | 1.301 (1.143, 1.482) | < 0.001 | 1.294 (1.143, 1.465) | < 0.001 | ||
| AJCC stage | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| I | Reference | Reference | ||||
| II | 1.426 (0.976, 2.083) | 0.067 | 1.552 (1.149, 2.097) | 0.004 | ||
| III | 4.794 (3.390, 6.780) | < 0.001 | 3.921 (2.960, 5.193) | < 0.001 | ||
| IV | 12.086 (8.642, 17.099) | < 0.001 | 9.649 (7.271, 12.806) | < 0.001 | ||
| Surgery | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| No surgery | Reference | Reference | ||||
| Local tumor excision/partial colectomy | 0.595 (0.491, 0.722) | < 0.001 | 0.613 (0.510, 0.737) | < 0.001 | ||
| Total colectomy | 0.710 (0.579, 0.871) | 0.001 | 0.745 (0.614, 0.940) | 0.003 | ||
| Dissected lymph node | < 0.001 | 0.135 | < 0.001 | 0.042 | ||
| None or biopsy | Reference | Reference | ||||
| 1–3 | 0.914 (0.672, 1.244) | 0.568 | 0.913 (0.685, 1.216) | 0.325 | ||
| ≥ 4 | 0.832 (0.693, 1.000) | 0.050 | 0.807 (0.679, 0.959) | 0.015 | ||
| Chemotherapy | 0.084 | < 0.001 | < 0.001 | < 0.001 | ||
| No/unknown | Reference | Reference | ||||
| Yes | 0.514 (0.467, 0,565) | 0.513 (0.467, 0.563) | ||||
| Radiotherapy | 0.552 | NI | 0.096 | 0.132 | ||
| No/unknown | Reference | |||||
| Yes | 1.125 (0.965, 1.312) | |||||
| Marital status | 0.001 | 0.016 | < 0.001 | 0.011 | ||
| Unmarried | Reference | Reference | ||||
| Married | 0.898 (0.822, 0.980) | 0.898 (0.827, 0.975) | ||||
CSS cancer-specific survival, OS overall survival, NI not included in the multivariate survival analysis.
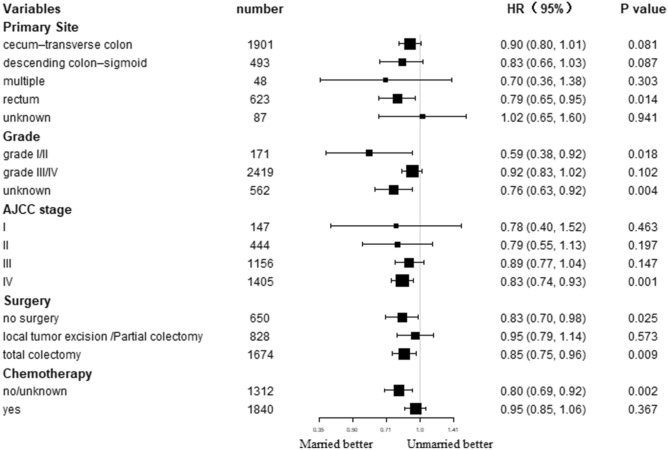
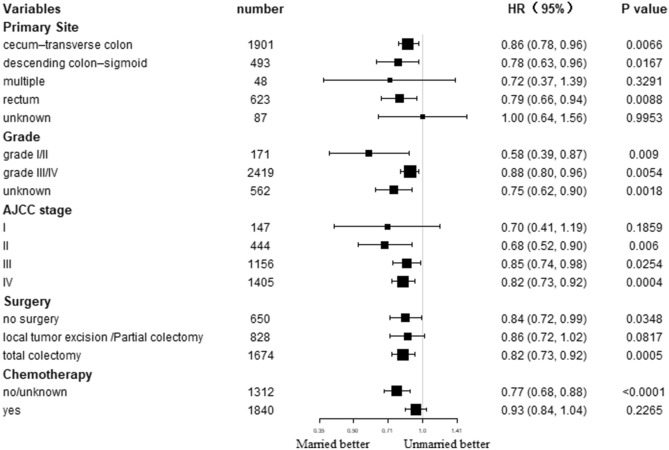
Subgroup analysis on marital status
The effects of marital status on survival were further determined in different subgroups. Subgroup analysis demonstrated superior OS as well CSS in married populations than unmarried ones in nearly all subgroups (Figs. 3 and 4). Specifically, primary site in rectum, grade I/II, AJCC stage IV, total colectomy, and no/unknown chemotherapy subgroups patients could significantly benefit from married status in terms of CSS (all P < 0.05). In addition, most subgroups could significantly benefit from married status in terms of OS (all P < 0.05).
Figure3.
Forest plot of subgroup analysis for cancer-specific survival (CSS).
Figure4.
Forest plot of subgroup analysis for overall survival (OS).
Discussion
To the best of our knowledge, this study is the first to specifically examine whether marital status has a significant impact on the survival of colorectal SRCC patients. By enrolling 3152 colorectal SRCC patients, we observed significantly lower risk of mortality in married populations compared to unmarried ones. After controlling for demographic and tumor characteristics, married populations had a 10.2% decreased death risk compared to unmarried patients with colorectal SRCC. In general, marital status was an independent favorable prognostic factor in colorectal SRCC populations.
Several studies have previously reported the correlation of marital status with prognosis in CRC26–28. Xiao et al. found marital status as an independent prognostic indicator in colorectal neuroendocrine neoplasm, with superior OS and CSS in married populations26. Furthermore, Ge et al. found worse OS in unmarried populations than married ones in CRC patients with metastasis27. By summarizing and analyzing 53 articles concerning the prognosis of CRC, Mozafar SH also confirmed marital status as a prognostic factor for CRC28. Taken together, these studies are basically consistent with the results of our study.
In addition to colorectal cancer, marital status has also been found to be significantly associated with prognosis in many other malignancies. For example, Zhou et al. proved that marital status was an independent prognostic risk factor for patients with pancreatic endocrine cancer18. Similar findings have also been discovered in breast cancer and nasopharyngeal carcinoma15,29. The above researches all exhibited that the prognosis of married patients is remarkably better than that of unmarried ones.
Two potential mechanisms may be used for explanation of the association of marital status with survival. For one thing, married populations have less distress and depression than unmarried patients following tumor diagnosis, because the emotional burden could be shared with their partners, who could also offer proper social support30,31. Loneliness and depression can down regulate the cellular immune response32, stimulate tumor angiogenesis and increase tumor burden and invasiveness33–35. For another thing, married patients with emotional and financial support from their spouses or children could have a better compliance from doctors36,37. Thus, they may be more likely to receive active treatments. Similarly, our study found that married patients had a higher rate of receiving chemotherapy. Therefore, social support as well as psychological interventions should be taken into consideration to attenuate the significant survival differences between married and unmarried tumor populations.
However, there are some limitations in this study, which mainly result from the restricted nature of SEER dataset. To begin with, the marital status extracted was recorded at diagnosis. Therefore, it remains unknown whether marital status changed throughout the follow-up, which might influence the outcomes as well. Secondly, the detailed quality of marriage was not available from the database, thereby affecting survival outcomes38. Thirdly, detailed therapeutic information is lacking, especially radiotherapy and chemotherapy. Finally, a causal correlation of marital status with survival cannot be proposed due to the research design, which requires further prospective cohort researches for validation. Nevertheless, our findings suggest that marital status has significantly impact on the survival of colorectal SRCC, highlighting the substantial as well as consistent effect of marriage, especially social support, on the detection, therapy and survival of cancer. Moreover, our outcomes also implicate that social support interventions targeting vulnerable populations, including unmarried populations, are likely to greatly enhance the cure probability. These types of interventions might be cost-effective to enhance clinical outcomes among unmarried tumor populations.
Conclusion
In conclusion, our study found that marital status was independent prognostic indicators of colorectal SRCC patients. Married patients have better CSS and OS than unmarried patients. The findings of the current study require further study.
Acknowledgements
We thank the staff members of the National Cancer Institute and their colleagues across the United States and at Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology, and End Results (SEER) Program.
Author contributions
L.F. and J.D. conceived the study. Y.Y. searched the database and literature. L.F., J.D., and Y.Y. discussed and analyzed the data. L.F. wrote the manuscript. All authors approved the final version.
Funding
No funding.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Shami K, et al. American cancer society colorectal cancer survivorship care guidelines. CA Cancer J. Clin. 2015;65:428–455. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch. Surg. 1951;62:79–91. doi: 10.1001/archsurg.1951.01250030082009. [DOI] [PubMed] [Google Scholar]
- 3.Almagro UA. Primary signet-ring carcinoma of the colon. Cancer. 1983;52:1453–1457. doi: 10.1002/1097-0142(19831015)52:8<1453::aid-cncr2820520819>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Nitsche U, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013;258:775–782. doi: 10.1097/SLA.0b013e3182a69f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima T, et al. Primary colorectal signet-ring cell carcinoma: clinicopathological features and postoperative survival. Surg. Today. 2010;40:234–238. doi: 10.1007/s00595-009-4057-y. [DOI] [PubMed] [Google Scholar]
- 6.Hugen N, et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. Int. J. Cancer. 2015;136:333–339. doi: 10.1002/ijc.28981. [DOI] [PubMed] [Google Scholar]
- 7.Thota R, Fang X, Subbiah S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database. J. Gastrointest. Oncol. 2014;5:18–24. doi: 10.3978/j.issn.2078-6891.2013.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalya PL, et al. Clinicopathological patterns and challenges of management of colorectal cancer in a resource-limited setting: a Tanzanian experience. World J. Surg. Oncol. 2013;11:88. doi: 10.1186/1477-7819-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moslehi R, Freedman E, Zeinomar N, Veneroso C, Levine PH. Importance of hereditary and selected environmental risk factors in the etiology of inflammatory breast cancer: a case-comparison study. BMC Cancer. 2016;16:334. doi: 10.1186/s12885-016-2369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lê MG, et al. Are risk factors for breast cancer similar in women with inflammatory breast cancer and in those with non-inflammatory breast cancer? Breast (Edinburgh, Scotland) 2006;15:355–362. doi: 10.1016/j.breast.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Schairer C, et al. Risk factors for inflammatory breast cancer and other invasive breast cancers. J. Natl Cancer Inst. 2013;105:1373–1384. doi: 10.1093/jnci/djt206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu SG, et al. Inflammatory breast cancer outcomes by breast cancer subtype: a population-based study. Fut. Oncol. (London, England) 2019;15:507–516. doi: 10.2217/fon-2018-0677. [DOI] [PubMed] [Google Scholar]
- 13.Pasek M, Dębska G, Wojtyna E. Perceived social support and the sense of coherence in patient-caregiver dyad versus acceptance of illness in cancer patients. J. Clin. Nurs. 2017;26(4985):4993. doi: 10.1111/jocn.13997. [DOI] [PubMed] [Google Scholar]
- 14.Aizer AA, et al. Marital status and survival in patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:3869–3876. doi: 10.1200/jco.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YL, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res. 2019;178:379–388. doi: 10.1007/s10549-019-05385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8:103518–103534. doi: 10.18632/oncotarget.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan S, et al. The association of marital status and mortality among men with early-stage prostate cancer treated with radical prostatectomy: insight into post-prostatectomy survival strategies. Cancer Causes Control CCC. 2019;30:871–876. doi: 10.1007/s10552-019-01194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: an analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin. Res. Hepatol. Gastroenterol. 2017;41:476–486. doi: 10.1016/j.clinre.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, et al. The effect of marital status by age on patients with colorectal cancer over the past decades: a SEER-based analysis. Int. J. Colorectal Dis. 2018;33:1001–1010. doi: 10.1007/s00384-018-3017-7. [DOI] [PubMed] [Google Scholar]
- 20.Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am. J. Surg. Pathol. 2016;40:e94–e102. doi: 10.1097/pas.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, et al. Influence of marital status on small intestinal adenocarcinoma survival: an analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Cancer Manag. Res. 2018;10:5667–5676. doi: 10.2147/cmar.s177430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belli S, et al. Outcomes of surgical treatment of primary signet ring cell carcinoma of the colon and rectum: 22 cases reviewed with literature. Int. Surg. 2014;99:691–698. doi: 10.9738/intsurg-d-14-00067.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psathakis D, et al. Ordinary colorectal adenocarcinoma vs primary colorectal signet-ring cell carcinoma: study matched for age, gender, grade, and stage. Dis. Colon Rectum. 1999;42:1618–1625. doi: 10.1007/bf02236218. [DOI] [PubMed] [Google Scholar]
- 24.Stewart SL, Wike JM, Kato I, Lewis DR, Michaud F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer. 2006;107:1128–1141. doi: 10.1002/cncr.22010. [DOI] [PubMed] [Google Scholar]
- 25.Hyngstrom JR, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann. Surg. Oncol. 2012;19:2814–2821. doi: 10.1245/s10434-012-2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao K, et al. The effect of marital status on the survival of patients with colorectal neuroendocrine neoplasms: an analysis of the SEER database. Revista espanola de enfermedades digestivas organo oficial de la Sociedad Espanola de Patologia Digestiva. 2020;112:109–117. doi: 10.17235/reed.2019.6183/2019. [DOI] [PubMed] [Google Scholar]
- 27.Ge H, Yan Y, Xie M, Guo L, Tang D. Construction of a nomogram to predict overall survival for patients with M1 stage of colorectal cancer: a retrospective cohort study. Int. J. Surg. (London, England) 2019;72:96–101. doi: 10.1016/j.ijsu.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Mozafar Saadati H, Khodamoradi F, Salehiniya H. Associated factors of survival rate and screening for colorectal cancer in Iran: a systematic review. J. Gastrointest. Cancer. 2020;51:401–411. doi: 10.1007/s12029-019-00275-0. [DOI] [PubMed] [Google Scholar]
- 29.Xu C, et al. Impact of marital status at diagnosis on survival and its change over time between 1973 and 2012 in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Med. 2017;6:3040–3051. doi: 10.1002/cam4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissman MM, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. doi: 10.1001/jama.1996.03540040037030. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser NC, Hartoonian N, Owen JE. Toward a cancer-specific model of psychological distress: population data from the 2003–2005 National Health Interview Surveys. J. Cancer Surviv. Res. Pract. 2010;4:291–302. doi: 10.1007/s11764-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 32.Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav. Immun. 2006;20:169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Antoni MH, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaremka LM, et al. Cognitive problems among breast cancer survivors: loneliness enhances risk. Psycho Oncol. 2014;23:1356–1364. doi: 10.1002/pon.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kissane DW. Unrecognised and untreated depression in cancer care. Lancet Psychiatry. 2014;1:320–321. doi: 10.1016/s2215-0366(14)70345-1. [DOI] [PubMed] [Google Scholar]
- 36.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 37.Anderson JC, et al. Predictors of compliance with free endoscopic colorectal cancer screening in uninsured adults. J. Gen. Intern. Med. 2011;26:875–880. doi: 10.1007/s11606-011-1716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaremka LM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology. 2013;38:2713–2719. doi: 10.1016/j.psyneuen.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]