Abstract
The main causes of acute pancreatitis (AP) are biliary disease, alcohol consumption, hypertriglyceridaemia (HTG) and endoscopic retrograde cholangiopancreatography (ERCP). The aim of this meta-analysis was to evaluate the effects of these aetiological factors on the severity and outcome of AP. Pubmed and Embase were searched between 01/01/2012 and 31/05/2020. Included articles involved adult alcoholic, biliary, HTG- or post-ERCP AP (PAP) patients. Primary outcome was severity, secondary outcomes were organ failures, intensive care unit admission, recurrence rate, pancreatic necrosis, mortality, length of hospital stay, pseudocyst, fluid collection and systematic inflammatory response syndrome. Data were analysed from 127 eligible studies. The risk for non-mild (moderately severe and severe) condition was the highest in HTG-induced AP (HTG-AP) followed by alcoholic AP (AAP), biliary AP (BAP) and PAP. Recurrence rate was significantly lower among BAP vs. HTG-AP or AAP patients (OR = 2.69 and 2.98, 95% CI 1.55–4.65 and 2.22–4.01, respectively). Mortality rate was significantly greater in HTG-AP vs. AAP or BAP (OR = 1.72 and 1.50, 95% CI 1.04–2.84 and 0.96–2.35, respectively), pancreatic necrosis occurred more frequently in AAP than BAP patients (OR = 1.58, 95% CI 1.08–2.30). Overall, there is a potential association between aetiology and the development and course of AP. HTG-AP is associated with the highest number of complications. Furthermore, AAP is likely to be more severe than BAP or PAP. Greater emphasis should be placed on determining aetiology on admission.
Subject terms: Gastrointestinal diseases, Pancreas, Gastrointestinal diseases
Introduction
Acute pancreatitis (AP) is a sudden inflammatory disease of the pancreas. In the last 20 years, the incidence of the disease has increased by more than 20%1,2. Nowadays, AP is one of the most common reasons for hospitalization in case of gastrointestinal diseases3.
Gallstones represent the main aetiological background of AP globally (42%), which are diagnosed by imaging techniques and liver function tests4. Gallstone-related or biliary AP (BAP) occurs twice as often as alcohol-induced AP (AAP)4. AAP is caused by regular, excessive alcohol consumption usually with a clinical history of > 5 years and > 50–100 g/day5. Hypertriglyceridaemia (HTG) with serum triglyceride concentrations > 11.3 mM is the third most common (9%) known aetiological factor of the disease6–8. Less frequent causes of AP include endoscopic retrograde cholangiopancreatography (ERCP), hypercalcaemia, pancreas divisum, tumours, genetic polymorphisms and drugs9. To date, no standardized diagnostic criteria exist for post-ERCP AP (PAP). The guidelines recommended by Cotton et al.10 are most commonly applied, which suggest PAP to be diagnosed if pancreatitis develops within 24 h after the procedure.
Based on the Revised Atlanta Classification (RAC), AP severity can be categorized into three groups: mild, moderately severe and severe11. Although the majority of cases are mild with a self-limiting course11, the mortality rate of severe AP can reach 30% which underlies the desperate need of finding proper treatment12. Organ failure (OF) is the most important determinant of this classification system11. Patients with mild AP have no organ dysfunction and usually recover within a week. Moderately severe AP resolves slower and might require interventions because of the presence of transient organ failure (TOF, < 48 h). Severe AP results in persistent organ failure (POF) which lasts > 48 h. Multiple organ failure (MOF) is defined as failure of two or more organ systems, which can be transient or persistent13. The three extrapancreatic organs most commonly affected by AP are the lungs, the heart and the kidneys11. Approximately 25% of AP patients develop severe complications and have to be admitted to an intensive care unit (ICU)14. Local complications can also occur in cases of moderately severe and severe AP, which include acute peripancreatic fluid collections, pancreatic pseudocysts, acute necrotic collections and walled-off necrosis11. About 20% of patients experience recurrent AP (RAP), which refers to a clinical condition defined by repeated episodes of AP15. 10% of AP patients with a single episode and 36% with RAP progress to chronic pancreatitis (CP)15. The risk of progression to CP increases with excessive alcohol consumption, smoking and male gender. 5% of CP patients develop pancreatic cancer16.
Although there are several risk factors, it is difficult to predict which patient will develop mild, moderately severe or severe AP. To date, numerous clinical studies have investigated the effect of aetiology on AP progression. However, to the best of our knowledge, there have been no efforts to summarize clinical data on how various aetiological backgrounds affect the severity and course of AP. Consequently, this study was undertaken to reveal the impact of the above-mentioned aetiologies (HTG-AP, AAP, BAP, PAP) by performing thorough literature search and meta-analysis on available clinical data.
Methods
This systematic review and meta-analysis followed the recommendations of Stroup et al.17 and was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines18 (Supplementary Table S1). The analysis was based on the Problem, Intervention, Comparison intervention and Outcome (PICO) model18 as follows: AP patients with alcoholic, biliary, hypertriglyceridaemic and post-ERCP aetiologies were compared in order to examine the effect of aetiology on disease outcomes. Primary outcome was severity, secondary outcomes were POF, MOF, TOF, ICU admission, recurrence rate, mortality, pancreatic necrosis, pulmonary failure (PUF), renal failure, length of hospital stay (LOS), pseudocyst, fluid collection, and systematic inflammatory response syndrome (SIRS).
The protocol for the meta-analysis was registered in the PROSPERO database on 15/05/2018 (https://www.crd.york.ac.uk/PROSPERO/, ID: CRD42018093574).
Search strategy
Literature search was conducted in the electronic databases Embase and Pubmed from publication date 01/01/2012 to 31/05/2020. The reason for the start date is that the RAC was introduced in 2012, which provides the most accepted and widespread criteria for determining AP severity. The following search query was used for Embase: (alcohol* OR ethanol* OR biliary OR gallstone OR cholelithiasis OR 'post-ercp' OR 'post ercp' OR idiopathic OR triglyceride OR hypertriglyceridemia OR hyperlipidemia OR severity OR severe OR mild OR moderate) AND acute AND pancreatitis NOT ('conference abstract'/it OR 'review'/it)) AND (2012:py OR 2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py OR 2020:py). In Pubmed, the following search terms were applied: (alcohol* OR ethanol* OR biliary OR gallstone OR cholelithiasis OR "post-ercp" OR "post ercp" OR idiopathic OR triglyceride OR hypertriglyceridemia OR hyperlipidemia OR severity OR severe OR mild OR moderate) AND acute AND pancreatitis NOT Review[ptyp] NOT Case Reports[ptyp]. The search was restricted to studies written in English or in Hungarian.
Eligibility criteria
All randomised trials, retrospective and prospective cohort studies were included that involved adult patients with AP and relevant data (primary and secondary outcomes) are categorized according to the aetiology of the disease. Four major disease backgrounds were included: alcohol abuse, HTG, biliary disease and post-ERCP. Articles that studied only one aetiological group or compared one aetiological group with another group called others or non-… (e.g. alcohol vs. non-alcohol) were excluded. Non-human studies or articles with data from patients younger than 18 years of age were not included. In case of cohort overlap between studies, only the most recent study was included unless a prior study had higher quality.
When assessing AP severity, only studies were included where severity was defined according to the RAC, because in this case it was crucial to present a consistent and clear definition for the analysis. Articles were also excluded if only one or two of the three severity groups were analysed. Both local complications and OFs could lead to serious conditions and death which are characteristic features of moderately severe and severe AP. Therefore, these two groups were combined in our study, and are referred to as “non-mild” disease forms and compared to the mild group. In cases of outcomes other than severity, using only the RAC was not in the criteria.
Study selection and data extraction
Titles and abstracts of publications were screened independently by two review authors (E.R.B. and G.F.) to identify studies that potentially meet inclusion criteria. The full texts of these potentially eligible studies were independently assessed for eligibility by the same two review authors. Disagreement between reviewers was resolved by discussion with other two colleagues (L.K. and Z.R.). E.R.B. and G.F. independently extracted study characteristics (author, title, journal, study location, inclusion period, number of centres involved, type of study, number of participants) and outcome data (severity, POF, MOF, TOF, ICU admission, recurrence rate, mortality, pancreatic necrosis, PUF, renal failure, LOS, pseudocyst, fluid collection, SIRS), which were recorded on a standardized Microsoft Excel spreadsheet. Discrepancies were resolved by discussion.
Quality assessment
Methodological quality of the articles was assessed by applying the Quality In Prognosis Studies (QUIPS) tool19 (Supplementary Table S2). This considers the following domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. All domains were scored by three individual researchers (E.R.B., G.F., L.K., each article was assessed by at least two of them). The overall risk of bias was considered:
low if < 3 domains were rated a moderate risk of bias and all others were rated a low risk of bias,
moderate if ≥ 3 domains were rated a moderate risk of bias and all others were rated a low risk of bias,
high if ≥ 1 domain was rated a high risk of bias, irrespective of all other domains.
Consensus was reached after classification by the individual researchers.
Data analyses
Statistical analysis was performed with Stata 11 SE (StataCorp LLC, College Station, TX, USA). The investigated aetiologies were analysed pairwise. Odds ratios (ORs) calculated from patient numbers were used to compare outcomes in different aetiologic groups. ORs were pooled using the random effects model with the DerSimonien–Laird estimation and displayed on forest plots. Summary OR estimation, p value and 95% confidence interval (CI) were calculated. P < 0.05 was considered as significant difference from summary OR = 1. BAP was defined as primary reference group, the other aetiologies were ranked in the following order: AAP, HTG-AP, PAP.
Statistical heterogeneity was analysed using the I2 statistic and the chi-square test to acquire probability values; p < 0.1 was defined to indicate significant heterogeneity. The small-study effect (in case of comparisons with at least 10 articles) was visually investigated on funnel plots and was also confirmed by Egger’s test. Sensitivity analysis was performed to examine the robustness of our results.
Results
Study selection
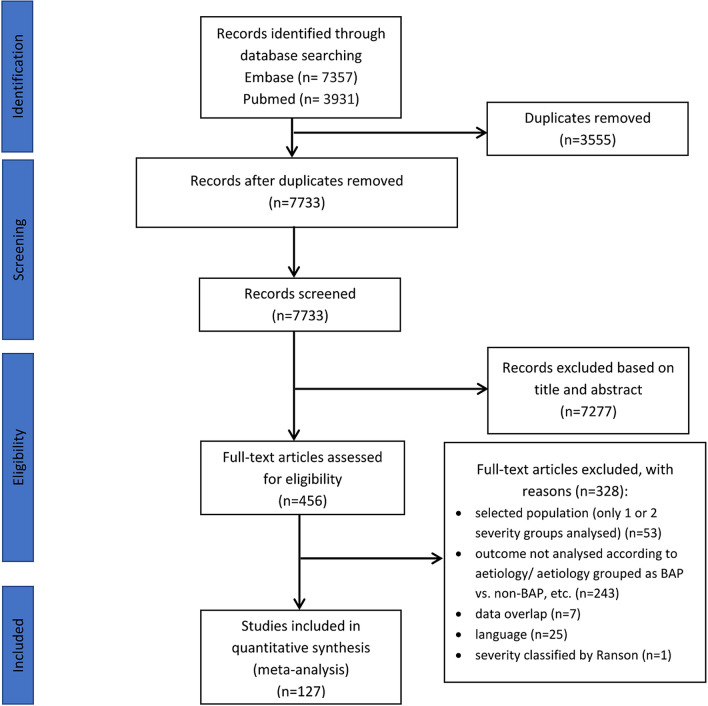
The search strategy identified 11,288 records. After removing duplicates 7733 articles were retrieved. Out of these, 456 records seemed to be relevant to the study question based on screening by title or abstract. After assessing the articles in full text, 328 records had to be excluded with different reasons (see details in Fig. 1). Finally, 127 publications fulfilled the eligibility criteria.
Figure 1.
PRISMA 2009 flow diagram for identification of relevant articles.
Characteristics of studies included
The majority of the included cohort studies (108 out of 128) collected data from the 2010′s. Our meta-analysis contains 102 single20–121 and 23 multicentre studies122–144. In two cases, there were no relevant data regarding the number of centres involved145,146. Sample sizes ranged from 11 to 1,165,777. Only the data of the four types of AP (AAP, BAP, HTG-AP, PAP) were analysed. Detailed characteristics of the included studies are provided in Supplementary Table S3. During quality assessment, we evaluated patient selection, comparability of the groups, and outcome data, which are presented in Supplementary Table S4.
Risk of bias assessment
According to the QUIPS checklist, most of the included studies had an overall moderate risk of bias (80, 63%; Supplementary Figure S1). 30 studies (23.4%) had low and 17 (13.3%) had high risk of bias. High risk was mainly due to the confounding factors which showed significant difference between the analysed aetiological groups. Moderate risk of bias resulted mainly from „Study confounding” and „Statistical Analysis and Reporting”, furthermore „Prognostic factor measurement” was also missing in a relatively high number (66.9%) of included studies. A detailed analysis can be found in Supplementary Table S4.
Clinical outcomes
Severity
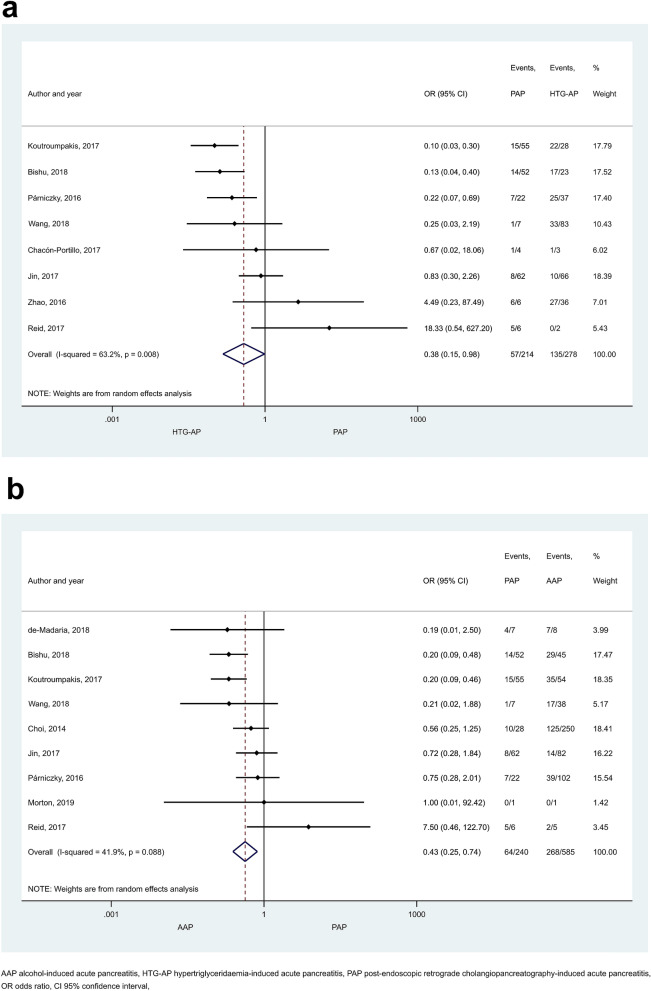
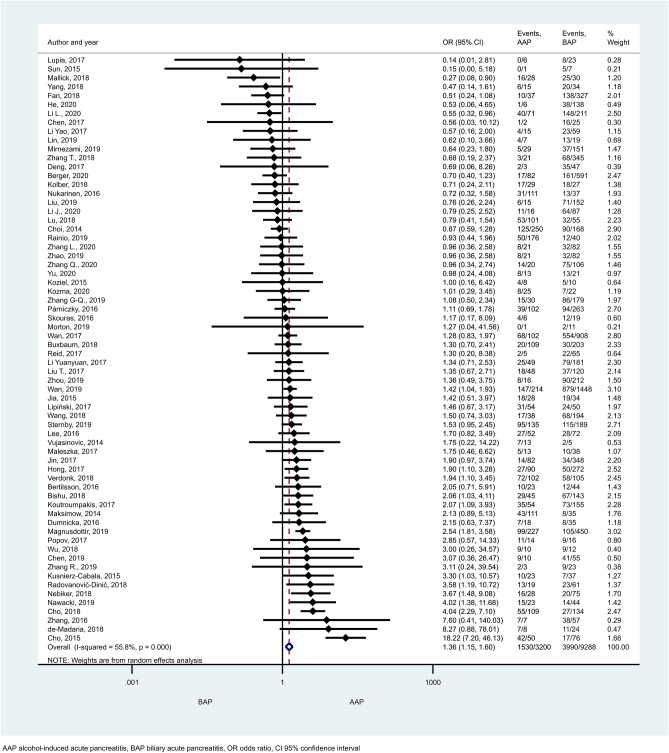
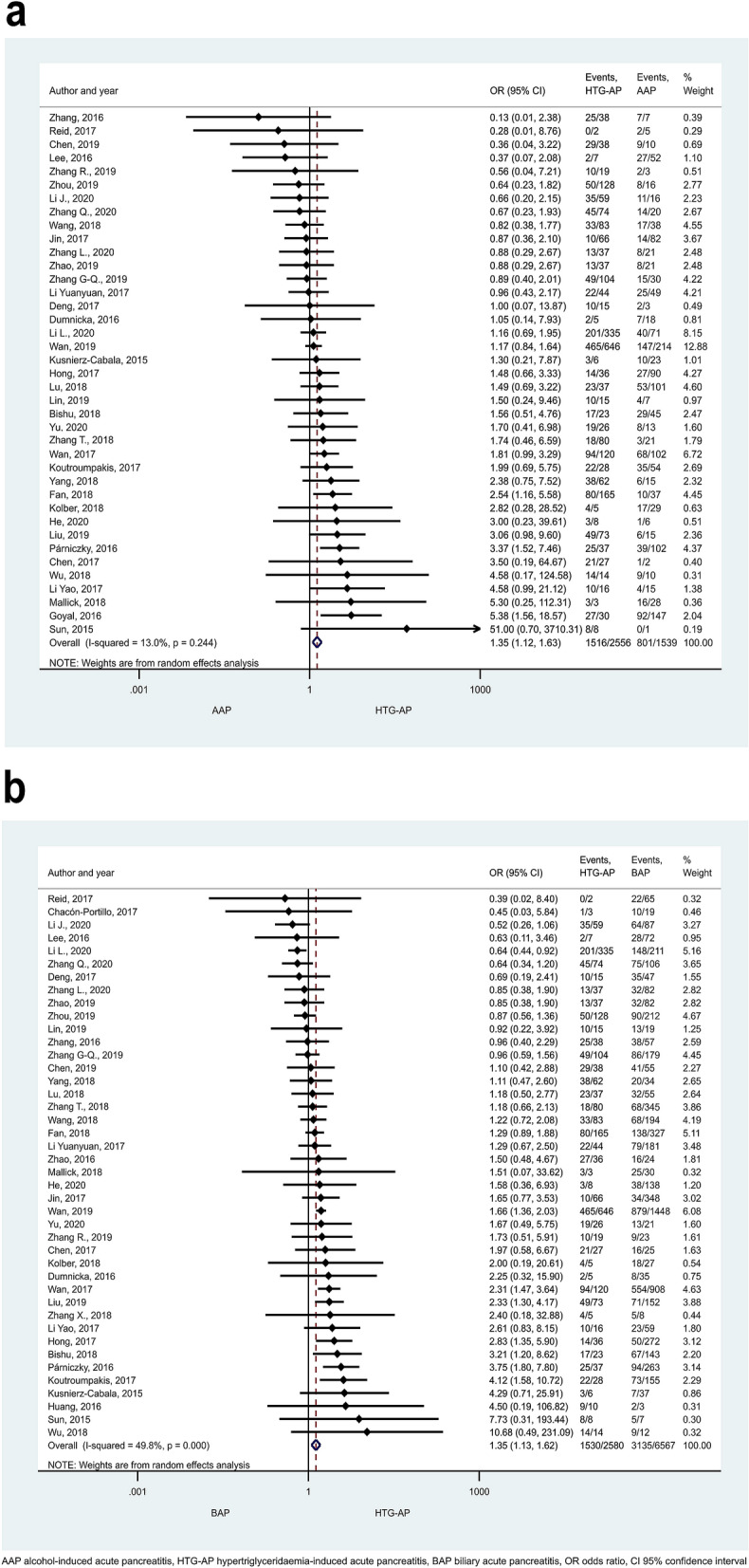
HTG proved to induce non-mild AP in a significantly higher number of cases than the other aetiological factors (Figs. 2, 3). ORs of non-mild cases in HTG-AP were 1.35 [CI 1.12–1.63] and 1.35 [CI 1.13–1.62] vs. AAP and BAP, respectively (Fig. 2a, b). PAP also appeared to be significantly less severe compared to HTG-AP (Fig. 3a; OR: 0.38 [CI 0.15–0.98]) or AAP (Fig. 3b; OR: 0.43 [CI 0.25–0.74]), while no significant difference could be detected between the severities of BAP and PAP (Supplementary Figure S2]). Alcoholic aetiology significantly increased AP severity compared to biliary-related events (Fig. 4; OR: 1.36 [CI 1.15–1.60]). We found heterogeneity in the comparison of HTG-AP vs. BAP, HTG-AP vs. PAP, AAP vs. BAP, PAP vs. AAP, BAP vs. PAP (Figs. 2b, 3, 4 and Supplementary Figure S2). No signs of small-study effect were detected in any comparison (Supplementary Figure S3).
Figure 2.
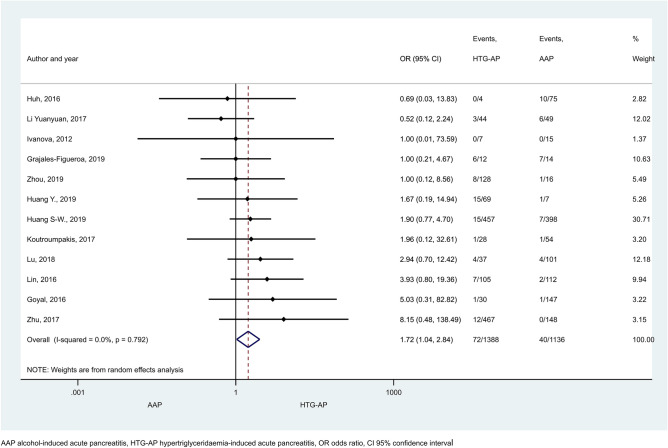
Forest plot showing the comparison of disease severity in (A) HTG-AP and AAP, p = 0.001; (B) HTG-AP and BAP, p = 0.001. Filled diamonds represent the ORs derived from the articles analysed. Horizontal bars represent CI. Empty diamond shows the overall OR (middle of the diamond and CIs are the edges) for non-mild (moderately severe and severe groups based on the Revised Atlanta Classification) disease. Heterogeneity of the results was presented by I-square and p value.
Figure 3.
Forest plot showing the comparison of disease severity in (A) HTG-AP and PAP, p = 0.045; (B) AAP and PAP, p = 0.002. Filled diamonds represent the ORs derived from the articles analysed. Horizontal bars represent CI. Empty diamond shows the overall OR (the middle of the diamond, CIs are the edges) for non-mild disease.
Figure 4.
Forest plot showing the comparison of disease severity in AAP and BAP, p < 0.001. Filled diamonds represent the ORs derived from the articles analysed. Horizontal bars represent CI. Empty diamond shows the overall OR (middle of the diamond, CIs are the edges) for non-mild disease.
Organ failures, intensive care unit admission, and systematic inflammatory response syndrome
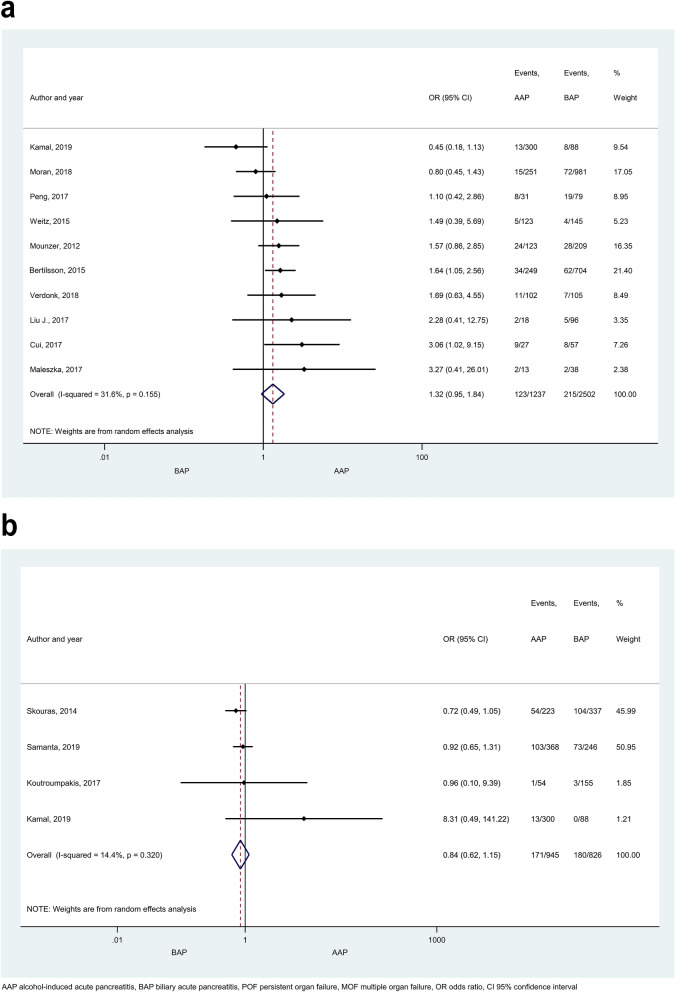
No significant difference was found in POF between any aetiological groups (AAP vs. BAP, HTG-AP vs. AAP, HTG-AP vs. BAP; Fig. 5a; Supplementary Figure S4). No signs of small-study effect were found in POF in the comparison of AAP vs. BAP (Supplementary Figure S5a). There were no significant differences in the occurrences of MOF, TOF or renal failure between AAP and BAP (Fig. 5b and Supplementary Figures S6a,b, respectively). PUF occurred more frequently in HTG-AP patients compared to BAP (Supplementary Figure S7a; OR: 2.39 [CI 1.06–5.39]), while AAP and BAP patients did not differ in this respect (Supplementary Figure S7b). The frequency of ICU admission was similar in AAP and BAP patients (Supplementary Figure S8). More AAP patients developed SIRS than PAP patients (Supplementary Figure S9a, OR: 0.40 [CI 0.21–0.77]. The rate of SIRS did not differ when comparing other patient groups (Supplementary Figure S9b,c). Heterogeneity was found in the comparison of renal failure, PUF and ICU admission between AAP and BAP (Supplementary Figures S6b, S7b, S8).
Figure 5.
Forest plot showing the effect of different disease aetiologies on POF and MOF. The effects of BAP vs. AAP on (A) POF, p = 0.102; and (B) MOF, p = 0.284. Filled diamonds represent the ORs derived from the articles analysed. Horizontal bars represent CI. Empty diamond shows the overall OR (the middle of the diamond, CIs are the edges).
Recurrence rate and length of hospital stay
Recurrence rate was significantly higher in AAP vs. BAP patients (Supplementary Figure S10a; OR: 2.98 [CI 2.22–4.01]) and in HTG-AP vs. BAP patients (Supplementary Figure S10b; OR: 2.69 [CI 1.55–4.65]). However, AP did not reoccur more frequently due to alcoholic aetiology than HTG or post-ERCP (Supplementary Figure S11a,b). Recurrence rate was also similar in BAP and PAP patients (Supplementary Figure S11c). Patients of the analysed aetiologies were hospitalized for a similar length of time (Supplementary Figure S12a,b). We found heterogeneity in the comparison of all cases of LOS and all cases of recurrence rate, except for the comparison between AAP and HTG-AP (Supplementary Figure S11a). No signs of small-study effect could be detected in case of recurrence rate or LOS (Supplementary Figure S5b,c).
Mortality and pancreatic necrosis
Mortality rate proved to be significantly higher in HTG-AP than in AAP (Fig. 6; OR: 1.72 [CI 1.04–2.84]), but no statistical difference was found between any other patient groups (Supplementary Figures S13 and S14). In the comparison of AAP and BAP a large proportion of patients came from one study contributing 1,165,777 subjects (accounting for 12.76% weight, Supplementary Figure S13a). However, sensitivity analysis showed that the results remained similar when this study was excluded (OR = 0.96 [CI 0.75–1.23]; Supplementary Figure S15). Pancreatic necrosis was reported more often in AAP than BAP patients (Fig. 7a, OR = 1.58 [CI 1.08–2.30]). No significant difference was detected in any other comparisons regarding necrosis (Fig. 7b,c). Heterogeneity was found in the comparison of mortality rate between BAP and HTG-AP, AAP and BAP (Supplementary Figure S13), and in case of necrosis when AAP and BAP groups were compared (Fig. 7a). We found signs of the small-study effect in case of mortality in the comparison of AAP and BAP (Supplementary Figure S16).
Figure 6.
Forest plot showing the effect of HTG-AP and AAP on mortality, p = 0.034. Filled diamonds represent the ORs derived from the articles analysed. Horizontal bars represent CI. Empty diamond shows the overall OR (the middle of the diamond, CIs are the edges).
Figure 7.
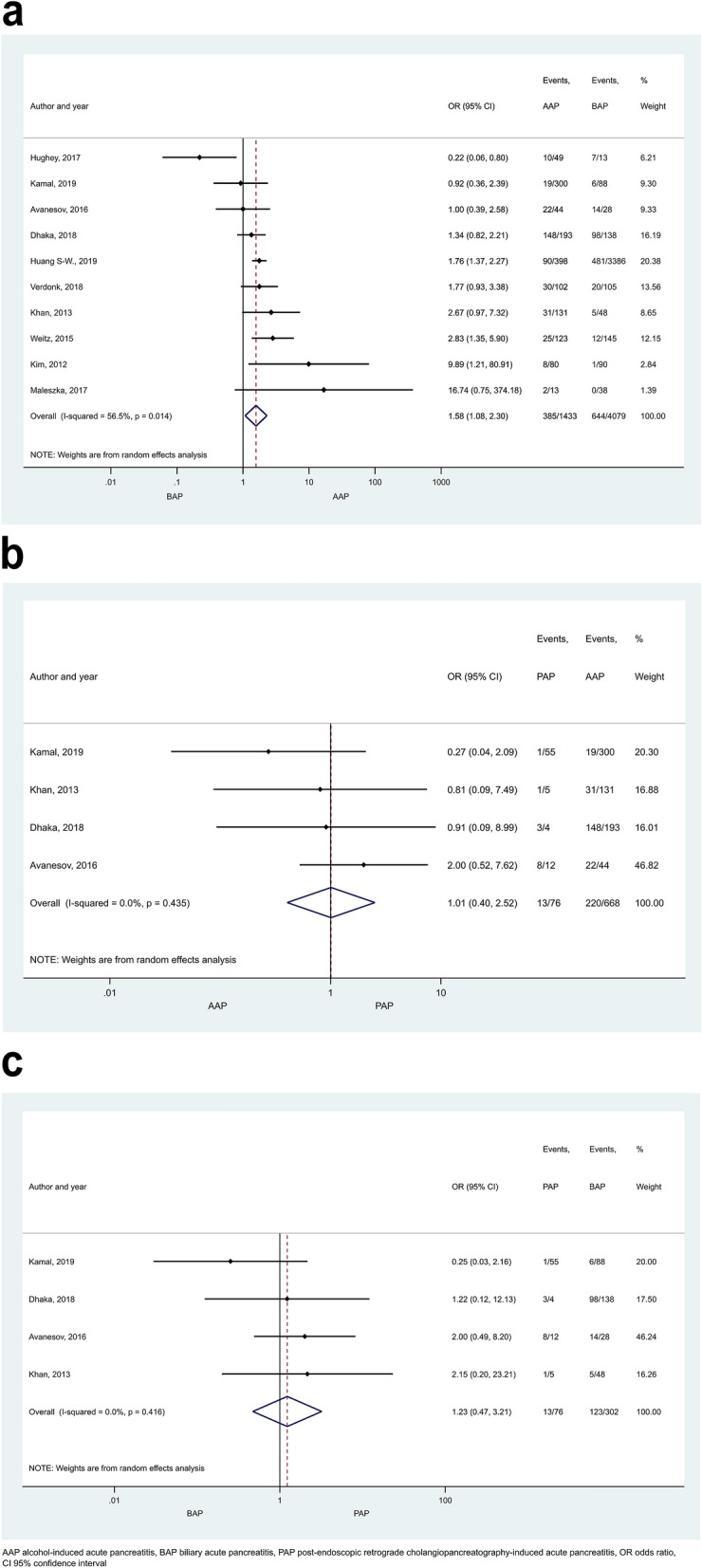
Forest plot showing the effects of different disease aetiologies on pancreatic necrosis. The effects of (A) BAP vs. AAP, p = 0.019; (B) AAP vs. PAP, p = 0.982; (C) BAP vs. PAP, p = 0.674. Filled diamonds represent the ORs derived from the articles analysed. Horizontal bars represent CI. Empty diamond shows the overall OR (the middle of the diamond, CIs are the edges).
Pseudocyst, fluid collection
There was no significant difference in the presence of fluid collection or pseudocysts in the available comparisons (Supplementary Figure S17). We found heterogeneity in case of fluid collection (AAP vs. BAP) and pseudocyst (HTG-AP vs BAP).
Discussion
This is the first detailed meta-analysis investigating the relationship between different aetiologies (alcohol abuse, biliary, HTG, post-ERCP) and the course of AP. Our study revealed that the prevalence of severe and moderately severe (non-mild) disease forms was highest in case of HTG-AP which was followed by AAP, BAP and PAP (Table 1). Due to the large number of included articles and patients, our results have strong evidence in case of the severity outcome. These are also in accordance with our earlier observations147 and the data of Wang et al.148. However, previously we compared the severe disease category to moderately severe and mild groups, which has less relevance than merging the severe and moderately severe groups as we did in the current study. Furthermore, our previous study and that of Wang et al.148 compared the characteristics and outcome of HTG-AP to non-HTG-AP patients, without classifying non-HTG-AP group any further according to aetiology. Importantly, non-HTG-AP group is rather heterogenous and it cannot be decided whether all aetiological subgroups included in non-HTG-AP are less severe than HTG-AP or some of the subgroups are basically more severe than HTG-AP but due to other much milder subtypes, HTG-AP turns out to be the most severe disease form. In addition, non-HTG-AP included all aetiologies (“idiopathic” and “other” as well) except HTG-AP, while we only analysed well-defined and clear aetiologies. Therefore, the current meta-analysis provides a more refined picture of the outcomes. Wang et al.148 evaluated AP severity using the APACHE-II scoring system, but this does not specifically define AP severity.
Table 1.
Summary of the results of our study.
| Severity | HTG-AP > AAP > BAP ≸ PAP |
| POF |
AAP ≸ BAP HTG-AP ≸ [AAP/BAP] |
| MOF | AAP ≸BAP |
| TOF | AAP ≸BAP |
| PUF | HTG-AP > BAP ≸ AAP |
| Renal failure | AAP ≸BAP |
| ICU admission | AAP ≸ BAP |
| SIRS |
BAP ≸ AAP > PAP BAP ≸ PAP |
| Recurrence rate |
[HTG-AP/AAP] > BAP HTG-AP ≸ AAP PAP ≸ [AAP/ BAP] |
| Mortality |
HTG-AP > AAP [HTG-AP/AAP/PAP] ≸ BAP AAP ≸ PAP |
| Necrosis |
AAP > BAP PAP ≸ [AAP/BAP] |
| Pseudocyst |
HTG-AP ≸ BAP AAP ≸ BAP |
| LOS | [HTG-AP/AAP] ≸ BAP |
AAP alcohol-induced acute pancreatitis, BAP biliary acute pancreatitis, HTG-AP hypertriglyceridaemia-induced acute pancreatitis, ICU intensive care unit, LOS length of hospital stay, MOF multiple organ failure, PAP post endoscopic retrograde cholangiopancreatography-induced acute pancreatitis, POF persistent organ failure, PUF pulmonary failure, SIRS systematic inflammatory response syndrome, TOF transient organ failure.
Statistically siginificant difference (p < 0.05) was presented with < ; ≸ shows no significant difference.
In our study, no difference could be observed between any aetiological groups in POF (Table 1). Although HTG-AP and AAP exhibited the most severe forms of AP from an aetiologic point of view, the data of POF does not support it. This can be explained by the fact that POF associated with AP was assessed in HTG-AP patients only in three of the articles included in our meta-analysis38,77,90. In the study of Wang et al.148 POF was most commonly observed in HTG-AP, which is in accordance with our results for severity. Although several articles evaluate characteristic features of severe AP such as POF, they focus exclusively on severe AP patients. For this reason, these could not be utilized in our analysis.
MOF is another distinctive feature of severe and moderately severe AP. In the current study, no significant difference could be detected in MOF between any of the analysed groups. Similarly, in a previous study, we did not find differences in MOF among HTG-AP vs. non-HTG-AP patients147. Tai et al.149 also found a higher risk for the severe form of AP in HTG-AP patients compared to BAP. They diagnosed MOF more frequently in BAP patients, however, there was no difference in single OFs (renal, heart, pulmonary).
AP patients with systemic complications eventually end up in ICU. In case of this outcome, only one comparison could be performed: no significant difference was found between AAP and BAP (Table 1), which is supported by our previous findings147.
The 27% recurrence rate of AP in the 1990s150 has nowadays decreased to about 20%151, which could be explained by better diagnosis and treatment after the first attack. In our study, alcoholic and hypertriglyceridaemic aetiologies caused more AP recurrence than biliary, while the repeated hospitalization for AAP and HTG-AP patients was similar. Tai et al. also found higher recurrence rate of HTG-AP than BAP149. Other studies drew the conclusion that alcohol is the most frequent aetiological factor for recurrent AP150,151. Suchsland et al.151 analysed the risk factors for readmittance in AP, most of which were related to alcohol abuse, so these patients have a higher risk for disease recurrence after discharge. In case of BAP, delayed cholecystectomy could be responsible for recurrence152,153.
Our study has shown that HTG-AP led to significantly higher mortality rate than AAP. However, no significant difference could be detected between the other aetiological groups. BAP used to have a higher mortality than AAP; however, this rate has decreased in the last decade due to improved supportive care154. Several studies have reported that mortality rate was not influenced by aetiological factors155,156. Other studies stated that HTG-AP did not cause significantly higher mortality rate, even though it led to higher severity and complication rates compared to other aetiological factors157,158. Wang et al.148 concluded that HTG-AP caused higher mortality rate than non-HTG-AP, while Kiss et al.147 did not find significant difference in this respect. Based on the current study, there is no strong relationship between aetiology of AP and mortality.
HTG carried the greatest risk for non-mild (moderately severe and severe) AP, which was followed by AAP; the least severe disease forms were observed in BAP and PAP. One of the possible pathomechanisms is that lipotoxicity mediated by unsaturated fatty acids contributes to necrosis, OF (eg. cardiovascular diseases) and mortality159. Experimental studies also demonstrated that HTG exacerbates the severity of AP159,160. Fatty acid administration resulted in elevated intracellular Ca2+ levels in pancreatic acinar cells and impaired mitochondrial function161,162. HTG-AP is often accompanied by one or more secondary factors (alcoholism, medications, uncontrolled diabetes mellitus, physical inactivity), which can further aggravate the severity of the disease163–166. Furthermore, elevated serum chylomicron concentration during HTG increases viscosity, causing reduced blood flow in microvessels and resulting in ischemic conditions. This could be an additional risk factor for a severe form of AP161,162.
Determining the exact aetiology of AP may be challenging in some cases. For example, alcohol is not only known as an independent risk factor for AP but can also increase serum TG concentrations, as mentioned before. In addition, mild-to-moderate elevation in TG concentrations can be observed in the early phase of AP, regardless of aetiology167. Since TG concentrations can rapidly decrease during fasting state after the diagnosis of AP, the measurement of TG concentrations on (or shortly after) admission is crucial.
The number of events, which refer to positive outcomes in certain aetiologies were relatively high in case of severity (1516 severe events occurred out of 2556 HTG-AP patients in Fig. 2a) and partly in mortality (10,161 events/620,027 BAP patients in Supplementary Figure S13a) and recurrence rate outcomes (1671 events/5254 AAP patients in Supplementary Figure S10a). Smaller number of events (9–97) could be included in the analysis of other outcomes (Table 1). Low event rates can have detrimental influence on the reliability of the results168,169. Based on the studies mentioned above, the results of all severity comparisons, mortality and recurrence rates in comparisons of AAP vs. BAP are strongly reliable. Most of the other calculations have lower reliability but there is no precedent to contradict the results of severity.
The current meta-analysis has strengths and limitations that should be noted. The major strengths are the following: we included a large number of articles. Four major aetiologies were analysed, leaving out miscellaneous or idiopathic backgrounds. For the analysis of severity, we only included articles where severity was defined according to the RAC, which provided a clear and consistent base for the comparisons. In addition, we compared mild to moderately severe and severe (“non-mild”) AP groups, which further refined our analysis. The quality of the involved articles determines the value of pooled data. There has been high variability in methodology of the studies which may have unintended effects on the final results and interpretation, study populations were diverse in age and gender, which might cause heterogeneity in aetiologic distribution. Aetiologies were not necessarily defined the same way. Certain outcomes (e.g. necrosis) were only evaluated by a limited number of studies, especially in case of HTG-AP, which may be the reason that no statistically significant difference could be detected between HTG-AP and other aetiologies or no statistical analysis could be performed. One article analysed data from 1975 to 2010, which was only applied for the assessment of recurrence rate. Another article contributed 1,165,777 patients to the analysis, which was only used for the evaluation of mortality. In addition, only articles published in English or Hungarian were included.
Conclusions
AP is a complex disorder mediated by metabolic, environmental and genetic factors, which can lead to death in the most severe forms. Therefore, clinicians should be more alert for a severe disease course in the at-risk patients. Our observations highlight the importance of disease aetiology. We found association between aetiology and the development and course of AP. HTG proved to carry the highest risk for non-mild (moderately severe and severe) AP, which was followed by AAP; the least severe disease forms were observed in BAP and PAP. It is essential to determine the cause of the disease in time to apply the most appropriate therapy. Based on the results, greater emphasis should be placed on determining aetiology on admission, especially in case of HTG-AP.
Supplementary information
Acknowledgements
This work was supported by Hungarian Ministry of Human Capacities (EFOP-3.6.2–16–2017–00006), National Research, Development and Innovation Office (GINOP-2.3.2-15-2016-00048, NKFIH K119938), and the Hungarian Academy of Sciences (LP2014-10/2014). The funders did not influence the interpretation of results in any way.
Abbreviations
- AP
Acute pancreatitis
- AAP
Alcohol-induced/alcoholic acute pancreatitis
- BAP
Biliary acute pancreatitis
- CI
95% Confidence interval
- ERCP
Endoscopic retrograde cholangiopancreatography
- HTG-AP
Hypertriglyceridaemia-induced acute pancreatitis
- HTG
Hypertriglyceridaemia
- ICU
Intensive care unit
- LOS
Length of hospital stay
- MOF
Multiple organ failure
- OF
Organ failure
- OR
Odds ratio
- PAP
Post-endoscopic retrograde cholangiopancreatography-induced acute pancreatitis
- POF
Persistent organ failure
- PUF
Pulmonary failure
- RAC
Revised Atlanta Classification
- SIRS
Systematic inflammatory response syndrome
- TOF
Transient organ failure
Author contributions
E.R.B.: study planning, data collection, quality assessment, data interpretation, drafting manuscript, critical revision of the manuscript, G.F.: data collection, quality assessment, L.K.: planning and conduct of the study, critical revision of the manuscript, D.I.N., A.S.: statistical analysis, P.H.: conduct of the study, critical revision of the manuscript, Zs.Sz.: coordination of quality assessment, critical revision of the manuscript, B.T., P.V., Á.V., B.E., J.C., Z.Sz., G.V.: data interpretation, critical revision of the manuscript, Á.V.: data interpretation, critical revision of the manuscript, Z.R.: study concept, planning and conduct of the study, data interpretation, critical revision of the manuscript. All authors approved the final draft submitted.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-74943-8.
References
- 1.Peery AF, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156:254–272.e11. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forsmark CE, Swaroop Vege S, Wilcox CM. Acute Pancreatitis. N. Engl. J. Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 3.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16:175–184. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Geenen EJM, van der Peet DL, Bhagirath P, Mulder CJJ, Bruno MJ. Etiology and diagnosis of acute biliary pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:495–502. doi: 10.1038/nrgastro.2010.114. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 6.Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology. 2016;16:469–476. doi: 10.1016/j.pan.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med. 2014;25:689–694. doi: 10.1016/j.ejim.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Mosztbacher D, et al. Hypertriglyceridemia-induced acute pancreatitis: a prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology. 2020;20:608–616. doi: 10.1016/j.pan.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Guda NM, Trikudanathan G, Freeman ML. Idiopathic recurrent acute pancreatitis. Lancet Gastroenterol. Hepatol. 2018;3:720–728. doi: 10.1016/S2468-1253(18)30211-5. [DOI] [PubMed] [Google Scholar]
- 10.Cotton PB, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest. Endosc. 1991;37:383–393. doi: 10.1016/S0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 11.Banks PA, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 12.Hammad AY, Ditillo M, Castanon L. Pancreatitis. Surg. Clin. North Am. 2018;98:895–913. doi: 10.1016/j.suc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Schepers NJ, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 14.Pavlidis P, et al. Improved outcome of severe acute pancreatitis in the intensive care unit. Crit. Care Res. Pract. 2013;2013:1–5. doi: 10.1155/2013/897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaran SJ, et al. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149:1490–1500.e1. doi: 10.1053/j.gastro.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 16.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am. J. Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 17.Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA283, 2008–12 (2000). [DOI] [PubMed]
- 18.Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647–g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 19.Hayden JA, van der Windt DA, Cartwright JL, Co P. Research and reporting methods annals of internal medicine assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 20.Avanesov M, et al. MDCT of acute pancreatitis: Intraindividual comparison of single-phase versus dual-phase MDCT for initial assessment of acute pancreatitis using different CT scoring systems. Eur. J. Radiol. 2016;85:2014–2022. doi: 10.1016/j.ejrad.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Avanesov M, et al. Clinico-radiological comparison and short-term prognosis of single acute pancreatitis and recurrent acute pancreatitis including pancreatic volumetry. PLoS ONE. 2018;13:1–19. doi: 10.1371/journal.pone.0206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badhal SS, Sharma S, Saraya A, Mukhopadhyay AK. Prognostic significance of D-dimer, natural anticoagulants and routine coagulation parameters in acute pancreatitis. Trop. Gastroenterol. 2012;33:193–199. doi: 10.7869/tg.2012.48. [DOI] [PubMed] [Google Scholar]
- 23.Bertilsson S, Swärd P, Kalaitzakis E. Factors that affect disease progression after first attack of acute pancreatitis. Clin. Gastroenterol. Hepatol. 2015;13:1662–1669.e3. doi: 10.1016/j.cgh.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Bertilsson S, et al. Microproteinuria predicts organ failure in patients presenting with acute pancreatitis. Dig. Dis. Sci. 2016;61:3592–3601. doi: 10.1007/s10620-016-4335-7. [DOI] [PubMed] [Google Scholar]
- 25.Bishu S, et al. The −251 A/T polymorphism in the IL8 promoter is a risk factor for acute pancreatitis. Pancreas. 2017;47:1. doi: 10.1097/MPA.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdan J, Elsaftawy A, Kaczmarzyk J, Jabłecki J. Epidemiological characteristic of acute pancreatitis in Trzebnica District. Polish J. Surg. 2012;84:70–75. doi: 10.2478/v10035-012-0011-6. [DOI] [PubMed] [Google Scholar]
- 27.Bosques-Padilla FJ, et al. Hypertriglyceridemia-induced pancreatitis and risk of persistent systemic inflammatory response syndrome. Am. J. Med. Sci. 2015;349:206–211. doi: 10.1097/MAJ.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 28.Buxbaum J, et al. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am. J. Gastroenterol. 2018;113:755–764. doi: 10.1038/s41395-018-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavestro GM, et al. A single-centre prospective, cohort study of the natural history of acute pancreatitis. Dig. Liver Dis. 2015;47:205–210. doi: 10.1016/j.dld.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Ćeranić DB, Zorman M, Skok P. Interleukins and inflammatory markers are useful in predicting the severity of acute pancreatitis. Bosn. J. Basic Med. Sci. 2020;20:99–105. doi: 10.17305/bjbms.2019.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chacón-Portillo MA, et al. Abnormal cardiovascular findings in acute pancreatitis: are they associated with disease severity? Rev. Investig. Clínica. 2017;69:314–318. doi: 10.24875/RIC.17002361. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Wan J, Shu W, Yang X, Xia L. Association of serum levels of silent information regulator 1 with persistent organ failure in acute pancreatitis. Dig. Dis. Sci. 2019;64:3173–3181. doi: 10.1007/s10620-019-05647-x. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, et al. Endothelial markers are associated with pancreatic necrosis and overall prognosis in acute pancreatitis: a preliminary cohort study. Pancreatology. 2017;17:45–50. doi: 10.1016/j.pan.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Cho JH, Kim TN, Kim SB. Comparison of clinical course and outcome of acute pancreatitis according to the two main etiologies: alcohol and gallstone. BMC Gastroenterol. 2015;15:87. doi: 10.1186/s12876-015-0323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho SK, Jung S, Lee KJ, Kim JW. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterol. 2018;18:18. doi: 10.1186/s12876-018-0748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho JH, Jeong YH, Kim KH, Kim TN. Risk factors of recurrent pancreatitis after first acute pancreatitis attack: a retrospective cohort study. Scand. J. Gastroenterol. 2020;55:90–94. doi: 10.1080/00365521.2019.1699598. [DOI] [PubMed] [Google Scholar]
- 37.Choi J-H, et al. Clinical relevance of the revised Atlanta classification focusing on severity stratification system. Pancreatology. 2014;14:324–329. doi: 10.1016/j.pan.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Cui J, et al. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. J. Crit. Care. 2017;41:161–165. doi: 10.1016/j.jcrc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Deng YY, Wang R, Wu H, Tang CW, Chen XZ. Etiology, clinical features and management of acute recurrent pancreatitis. J. Dig. Dis. 2014;15:570–577. doi: 10.1111/1751-2980.12180. [DOI] [PubMed] [Google Scholar]
- 40.Deng, L.-H. et al. Plasma cytokines can help to identify the development of severe acute pancreatitis on admission. Medicine (Baltimore).96, e7312 (2017). [DOI] [PMC free article] [PubMed]
- 41.Dhaka N, et al. Impact of the site of necrosis on outcome of acute pancreatitis. JGH Open. 2018;2:295–299. doi: 10.1002/jgh3.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumnicka P, et al. Serum soluble Fms-like tyrosine kinase 1 (sFlt-1) predicts the severity of acute pancreatitis. Int. J. Mol. Sci. 2016;17:2038. doi: 10.3390/ijms17122038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esmer A, Ozsurmeli M, Kalelioglu I. Maternal and perinatal outcomes of acute pancreatitis during pregnancy. Gazi Med. J. 2012;23:133–137. [Google Scholar]
- 44.Fan, J. et al. Epidemiology and etiology of acute pancreatitis in urban and suburban areas in Shanghai: a retrospective study. Gastroenterol. Res. Pract.2018, (2018). [DOI] [PMC free article] [PubMed]
- 45.Goyal H, Smith B, Bayer C, Rutherford C, Shelnut D. Differences in severity and outcomes between hypertriglyceridemia and alcohol-induced pancreatitis. N. Am. J. Med. Sci. 2016;8:82. doi: 10.4103/1947-2714.177307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grajales-Figueroa G, et al. Increased mortality from extrapancreatic infections in hospitalized patients with acute pancreatitis. Gastroenterol. Res. Pract. 2019;2019:1–7. doi: 10.1155/2019/2789764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi TY, Gonoi W, Yoshikawa T, Hayashi N, Ohtomo K. Ansa pancreatica as a predisposing factor for recurrent acute pancreatitis. World J. Gastroenterol. 2016;22:8940–8948. doi: 10.3748/wjg.v22.i40.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He F, et al. Factors predicting the severity of acute pancreatitis in elderly patients. Aging Clin. Exp. Res. 2020;1:8–13. doi: 10.1007/s40520-020-01523-1. [DOI] [PubMed] [Google Scholar]
- 49.Hong W, et al. High-density lipoprotein cholesterol, blood urea nitrogen, and serum creatinine can predict severe acute pancreatitis. Biomed Res. Int. 2017;2017:1–7. doi: 10.1155/2017/1648385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang SW, et al. Clinical characteristics of 5375 cases of acute pancreatitis from a single Chinese center, 1996–2015. Chin. Med. J. (Engl) 2019;132:1233–1236. doi: 10.1097/CM9.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang C, et al. Clinical features and treatment of hypertriglyceridemia-induced acute pancreatitis during pregnancy: a retrospective study. J. Clin. Apher. 2016;31:571–578. doi: 10.1002/jca.21453. [DOI] [PubMed] [Google Scholar]
- 52.Hughey M, Taffel M, Zeman RK, Patel S, Hill MC. The diagnostic challenge of the sequelae of acute pancreatitis on CT imaging: a pictorial essay. Abdom. Radiol. 2017;42:1199–1209. doi: 10.1007/s00261-016-0986-2. [DOI] [PubMed] [Google Scholar]
- 53.Huh JH, et al. Diabetes mellitus is associated with mortality in acute pancreatitis. J. Clin. Gastroenterol. 2016;52:1. doi: 10.1097/MCG.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 54.Ikeura T, et al. A body mass index ≥25 kg/m 2 is associated with a poor prognosis in patients with acute pancreatitis: a study of Japanese patients. Hepatobiliary Pancreat. Dis. Int. 2017;16:645–651. doi: 10.1016/S1499-3872(17)60057-2. [DOI] [PubMed] [Google Scholar]
- 55.Ivanova R, et al. Triglyceride levels and apolipoprotein E polymorphism in patients with acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2012;11:96–101. doi: 10.1016/S1499-3872(11)60131-8. [DOI] [PubMed] [Google Scholar]
- 56.Jain, S., Mahapatra, S. J., Gupta, S., Shalimar & Garg, P. K. Infected Pancreatic Necrosis due to Multidrug-Resistant Organisms and Persistent Organ failure Predict Mortality in Acute Pancreatitis. Clin. Transl. Gastroenterol.9, (2018). [DOI] [PMC free article] [PubMed]
- 57.Jia R, et al. Increased interleukin-23/17 axis and C-reactive protein are associated with severity of acute pancreatitis in patients. Pancreas. 2015;44:321–325. doi: 10.1097/MPA.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 58.Jin Z, Xu L, Wang X, Yang D. Risk factors for worsening of acute pancreatitis in patients admitted with mild acute pancreatitis. Med. Sci. Monit. 2017;23:1026–1032. doi: 10.12659/MSM.900383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalaria R, Abraham P, Desai DC, Joshi A, Gupta T. Rate of recurrence in Indian patients presenting with acute pancreatitis and identification of chronicity on follow up: possible risk factors for progression. Indian J. Gastroenterol. 2018;37:92–97. doi: 10.1007/s12664-018-0818-0. [DOI] [PubMed] [Google Scholar]
- 60.Kamal A, et al. Does etiology of pancreatitis matter? Differences in outcomes among patients with post-endoscopic retrograde cholangiopancreatography, acute biliary, and alcoholic pancreatitis. Pancreas. 2019;48:574–578. doi: 10.1097/MPA.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 61.Khan J, Nordback I, Sand J. Serum lipid levels are associated with the severity of acute pancreatitis. Digestion. 2013;87:223–228. doi: 10.1159/000348438. [DOI] [PubMed] [Google Scholar]
- 62.Kim JE, et al. The clinical outcome of elderly patients with acute pancreatitis is not different in spite of the different etiologies and severity. Arch. Gerontol. Geriatr. 2012;54:256–260. doi: 10.1016/j.archger.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Kim YS, et al. Prolonged hyperamylasemia in patients with acute pancreatitis is associated with recurrence of acute pancreatitis. Med. (United States) 2020;99:1–6. doi: 10.1097/MD.0000000000018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolber W, et al. Serum urokinase-type plasminogen activator receptor does not outperform C-reactive protein and procalcitonin as an early marker of severity of acute pancreatitis. J. Clin. Med. 2018;7:305. doi: 10.3390/jcm7100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koutroumpakis E, et al. Management and outcomes of acute pancreatitis patients over the last decade: a US tertiary-center experience. Pancreatology. 2017;17:32–40. doi: 10.1016/j.pan.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 66.Koźma EM, et al. Significant remodeling affects the circulating glycosaminoglycan profile in adult patients with both severe and mild forms of acute pancreatitis. J. Clin. Med. 2020;9:1308. doi: 10.3390/jcm9051308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuśnierz-Cabala B, et al. Serum levels of unique miR-551-5p and endothelial-specific miR-126a-5p allow discrimination of patients in the early phase of acute pancreatitis. Pancreatology. 2015;15:344–351. doi: 10.1016/j.pan.2015.05.475. [DOI] [PubMed] [Google Scholar]
- 68.Lee KJ, et al. Comparison of predictive systems in severe acute pancreatitis according to the revised Atlanta classification. Pancreas. 2016;45:46–50. doi: 10.1097/MPA.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 69.Lew D, et al. Disease course differences in acute pancreatitis based on etiology using the pancreatitis activity scoring system. Pancreas. 2018;47:e40–e41. doi: 10.1097/MPA.0000000000001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J, et al. Predictive value of long non-coding RNA intersectin 1–2 for occurrence and in-hospital mortality of severe acute pancreatitis. J. Clin. Lab. Anal. 2020;34:1–8. doi: 10.1002/jcla.23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L, et al. Early rapid fluid therapy is associated with increased rate of noninvasive positive-pressure ventilation in hemoconcentrated patients with severe acute pancreatitis. Dig. Dis. Sci. 2020;65:2700–2711. doi: 10.1007/s10620-019-05985-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Lu Y, Shen J, Xu C. Elevated serum midkine in patients with acute pancreatitis. Am. J. Med. Sci. 2017;354:548–552. doi: 10.1016/j.amjms.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Zhao Y, Feng L, Guo R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open. 2017;7:e013206. doi: 10.1136/bmjopen-2016-013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin N, et al. Patient- and hospital-based factors at admission associated with outcomes of patients hospitalized for acute pancreatitis: a retrospective cohort study. Int. J. Clin. Exp. Med. 2016;9:23551–23559. [Google Scholar]
- 75.Lin, M., Huang, J., Huang, J., Liu, S. lan & Chen, W. C. Level of serum soluble Tim-3 expression in early-phase acute pancreatitis. Turkish J. Gastroenterol.30, 188–191 (2019). [DOI] [PMC free article] [PubMed]
- 76.Lipinski M, Rydzewska-Rosolowska A, Rydzewski A, Cicha M, Rydzewska G. Soluble urokinase-type plasminogen activator receptor (suPAR) in patients with acute pancreatitis (AP): progress in prediction of AP severity. Pancreatology. 2017;17:24–29. doi: 10.1016/j.pan.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Liu J, et al. Early prediction of organ failure under the revised Atlanta classification. Turkish J. Gastroenterol. 2017;28:46–52. doi: 10.5152/tjg.2016.0378. [DOI] [PubMed] [Google Scholar]
- 78.Liu T, et al. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br. J. Surg. 2017;104:1215–1225. doi: 10.1002/bjs.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu, G., Tao, J., zhu, Z. & Wang, W. The early prognostic value of inflammatory markers in patients with acute pancreatitis. Clin. Res. Hepatol. Gastroenterol.43, 330–337 (2019). [DOI] [PubMed]
- 80.Lu GW, Lu DW, Zhang YX. Predictive value of serum cyclophilin A concentrations after acute pancreatitis. Clin. Chim. Acta. 2018;484:237–245. doi: 10.1016/j.cca.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 81.Lupia E, et al. Thrombopoietin as early biomarker of disease severity in patients with acute pancreatitis. Pancreas. 2017;46:164–169. doi: 10.1097/MPA.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 82.Maksimow M, et al. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis*. Crit. Care Med. 2014;42:2556–2564. doi: 10.1097/CCM.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 83.Maleszka A, et al. The diagnostic usefulness of serum total bile acid concentrations in the early phase of acute pancreatitis of varied etiologies. Int. J. Mol. Sci. 2017;18:106. doi: 10.3390/ijms18010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mallick B, et al. Differences between the outcome of recurrent acute pancreatitis and acute pancreatitis. JGH Open. 2018;2:134–138. doi: 10.1002/jgh3.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Melitas C, et al. Factors predicting readmission within 30 days of acute pancreatitis attack: a prospective study. Pancreatology. 2019;19:805–806. doi: 10.1016/j.pan.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 86.Morton JC, et al. Altered bioenergetics of blood cell sub-populations in acute pancreatitis patients. J. Clin. Med. 2019;8:2201. doi: 10.3390/jcm8122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nawacki, Grabowska, U. & Głuszek, S. Can the matrix metalloproteinases 2 and 9 predict the course of acute pancreatitis in previously healthy patients? Acta Gastroenterol. Belg.82, 501–505 (2019). [PubMed]
- 88.Nebiker CA, et al. Cortisol outperforms novel cardiovascular, inflammatory, and neurohumoral biomarkers in the prediction of outcome in acute pancreatitis. Pancreas. 2017;47:1. doi: 10.1097/MPA.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 89.Nukarinen E, et al. Association of matrix metalloproteinases -7, -8 and -9 and TIMP -1 with disease severity in acute pancreatitis: a cohort study. PLoS ONE. 2016;11:e0161480. doi: 10.1371/journal.pone.0161480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng T, et al. Serum calcium as an indicator of persistent organ failure in acute pancreatitis. Am. J. Emerg. Med. 2017;35:978–982. doi: 10.1016/j.ajem.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Popov A, Ershova A, Podtaev S, Frick P, Zubareva N. Early differential diagnosis of the severity of acute pancreatitis. J. Clin. Monit. Comput. 2017;31:1289–1297. doi: 10.1007/s10877-016-9960-3. [DOI] [PubMed] [Google Scholar]
- 92.Pulkkinen J, et al. Statin use in patients with acute pancreatitis and symptomatic gallstone disease. Pancreas. 2014;43:638–641. doi: 10.1097/MPA.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 93.Quero G, et al. Acute pancreatitis in elderly patients: a single-center retrospective evaluation of clinical outcomes. Scand. J. Gastroenterol. 2019;54:492–498. doi: 10.1080/00365521.2019.1588369. [DOI] [PubMed] [Google Scholar]
- 94.Radovanović-Dinić B, Tešić-Rajković S, Ignjatovic A, Grgov S. Thrombin activatable fibrinolysis inhibitor as an indicator of the severity of acute pancreatitis. Turkish J. Gastroenterol. 2018;29:488–493. doi: 10.5152/tjg.2018.17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rainio M, et al. Serum serine peptidase inhibitor Kazal-Type 1, trypsinogens 1 to 3, and complex of trypsin 2 and α1-antitrypsin in the diagnosis of severe acute pancreatitis. Pancreas. 2019;48:374–380. doi: 10.1097/MPA.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 96.Reid GP, Williams EW, Francis DK, Lee MG. Acute pancreatitis: A 7 year retrospective cohort study of the epidemiology, aetiology and outcome from a tertiary hospital in Jamaica. Ann. Med. Surg. 2017;20:103–108. doi: 10.1016/j.amsu.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Samanta J, et al. Comparative study of the outcome between alcohol and gallstone pancreatitis in a high-volume tertiary care center. JGH Open. 2019;3:338–343. doi: 10.1002/jgh3.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seok AE, et al. Screening of sera from patients with pancreatitis by an apoptosis assay of skin-derived cells. Korean J. Gastroenterol. 2019;74:219–226. doi: 10.4166/kjg.2019.74.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Skouras C, et al. Early organ dysfunction affects long-term survival in acute pancreatitis patients. HPB. 2014;16:789–796. doi: 10.1111/hpb.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skouras C, et al. Lung ultrasonography as a direct measure of evolving respiratory dysfunction and disease severity in patients with acute pancreatitis. HPB. 2016;18:159–169. doi: 10.1016/j.hpb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun L, et al. Intra-abdominal pressure in third trimester pregnancy complicated by acute pancreatitis: an observational study. BMC Pregnancy Childbirth. 2015;15:223. doi: 10.1186/s12884-015-0651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takuma K, et al. Etiology of recurrent acute pancreatitis, with special emphasis on pancreaticobiliary malformation. Adv. Med. Sci. 2012;57:244–250. doi: 10.2478/v10039-012-0041-7. [DOI] [PubMed] [Google Scholar]
- 103.Wan J, et al. Serum D-dimer levels at admission for prediction of outcomes in acute pancreatitis. BMC Gastroenterol. 2019;19:1–7. doi: 10.1186/s12876-019-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wan J, et al. Stratified analysis and clinical significance of elevated serum triglyceride levels in early acute pancreatitis: a retrospective study. Lipids Health Dis. 2017;16:124. doi: 10.1186/s12944-017-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Qin L, Cao J. Value of the revised Atlanta classification (RAC) and determinant-based classification (DBC) systems in the evaluation of acute pancreatitis. Curr. Med. Res. Opin. 2018;34:1231–1238. doi: 10.1080/03007995.2017.1389705. [DOI] [PubMed] [Google Scholar]
- 106.Weitz G, et al. Does etiology of acute pancreatitis matter? A review of 391 consecutive episodes. JOP. 2015;16:171–175. doi: 10.6092/1590-8577/2959. [DOI] [PubMed] [Google Scholar]
- 107.Wu L, et al. Plasma mtDNA Analysis aids in predicting pancreatic necrosis in acute pancreatitis patients: a pilot study. Dig. Dis. Sci. 2018;63:2975–2982. doi: 10.1007/s10620-018-5227-9. [DOI] [PubMed] [Google Scholar]
- 108.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am. J. Gastroenterol. 2012;107:1096–1103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 109.Yang WQ, et al. Low FT3 is a valuable predictor of severe acute pancreatitis in the emergency department. J. Dig. Dis. 2018;19:431–438. doi: 10.1111/1751-2980.12609. [DOI] [PubMed] [Google Scholar]
- 110.Youn GJ, et al. The etiologic evaluation of acute pancreatitis in a general hospital of Seoul-Gyeonggi Province in Korea. Korean J. Gastroenterol. 2017;70:190. doi: 10.4166/kjg.2017.70.4.190. [DOI] [PubMed] [Google Scholar]
- 111.Yu S, et al. Identification of dysfunctional gut microbiota through rectal swab in patients with different severity of acute pancreatitis. Dig. Dis. Sci. 2020 doi: 10.1007/s10620-020-06061-4. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L, Qiao Z, Feng H, Shen J. The early predictive role of complement C3 and C4 in patients with acute pancreatitis. J. Clin. Lab. Anal. 2020;34:1–5. doi: 10.1002/jcla.23205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Q, et al. Soluble urokinase plasminogen activator receptor associates with higher risk, advanced disease severity as well as inflammation, and might serve as a prognostic biomarker of severe acute pancreatitis. J. Clin. Lab. Anal. 2020;34:1–7. doi: 10.1002/jcla.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y-P, et al. Early prediction of persistent organ failure by serum angiopoietin-2 in patients with acute pancreatitis. Dig. Dis. Sci. 2016;61:3584–3591. doi: 10.1007/s10620-016-4323-y. [DOI] [PubMed] [Google Scholar]
- 115.Zhang R, et al. Expanded CD14 hi CD16: immunosuppressive monocytes predict disease severity in patients with acute pancreatitis. J. Immunol. 2019;202:2578–2584. doi: 10.4049/jimmunol.1801194. [DOI] [PubMed] [Google Scholar]
- 116.Zhang GQ, et al. Plasma D-dimer level is an early predictor of severity of acute pancreatitis based on 2012 Atlanta classification. Med. Sci. Monit. 2019;25:9019–9027. doi: 10.12659/MSM.918311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang T, et al. Predicting the severity of acute pancreatitis with red cell distribution width at early admission stage. Shock. 2018;49:551–555. doi: 10.1097/SHK.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 118.Zhang X, et al. Underexpression of receptor for activated c kinase 1 (RACK1) in leukocytes from patients with severe acute pancreatitis. Tohoku J. Exp. Med. 2018;245:205–2015. doi: 10.1620/tjem.245.205. [DOI] [PubMed] [Google Scholar]
- 119.Zhao B, et al. Effect of S100A12 and soluble receptor for advanced glycation end products on the occurrence of severe acute pancreatitis. J. Dig. Dis. 2016;17:475–482. doi: 10.1111/1751-2980.12364. [DOI] [PubMed] [Google Scholar]
- 120.Zhou, H., Mei, X., He, X., Lan, T. & Guo, S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study. Medicine (Baltimore).98, e15275 (2019). [DOI] [PMC free article] [PubMed]
- 121.Zhu Y, et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8-year period. Pancreas. 2017;46:504–509. doi: 10.1097/MPA.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 122.Berger Z, et al. Acute pancreatitis in Chile: a multicenter study on epidemiology, etiology and clinical outcome. Retrospective analysis of clinical files. Pancreatology. 2020;20:637–643. doi: 10.1016/j.pan.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 123.Castoldi L, et al. Long term outcome of acute pancreatitis in Italy: results of a multicentre study. Dig. Liver Dis. 2013;45:827–832. doi: 10.1016/j.dld.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 124.Chen Y, Zak Y, Hernandez-Boussard T, Park W, Visser BC. The epidemiology of idiopathic acute pancreatitis, analysis of the nationwide inpatient sample from 1998 to 2007. Pancreas. 2013;42:1–5. doi: 10.1097/MPA.0b013e3182572d3a. [DOI] [PubMed] [Google Scholar]
- 125.Cui ML, et al. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig. Dis. Sci. 2014;59:1055–1062. doi: 10.1007/s10620-013-2967-4. [DOI] [PubMed] [Google Scholar]
- 126.de-Madaria, E. et al. Oleic acid chlorohydrin, a new early biomarker for the prediction of acute pancreatitis severity in humans. Ann. Intensive Care8, 1 (2018). [DOI] [PMC free article] [PubMed]
- 127.Huang Y, et al. Immature granulocytes: a novel biomarker of acute respiratory distress syndrome in patients with acute pancreatitis. J. Crit. Care. 2019;50:303–308. doi: 10.1016/j.jcrc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 128.Huang Y-X, et al. Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong China. Pancreas. 2014;43:548–552. doi: 10.1097/MPA.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 129.Jinno N, et al. Predictive factors for the mortality of acute pancreatitis on admission. PLoS ONE. 2019;14:1–12. doi: 10.1371/journal.pone.0221468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kikuta K. Impaired glucose tolerance in acute pancreatitis. World J. Gastroenterol. 2015;21:7367. doi: 10.3748/wjg.v21.i24.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koziel D, Gluszek S, Kowalik A, Chlopek M, Pieciak L. Genetic mutations in SPINK1, CFTR, CTRC genes in acute pancreatitis. BMC Gastroenterol. 2015;15:70. doi: 10.1186/s12876-015-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lankisch PG, Weber-Dany B, Maisonneuve P, Lowenfels AB. Pancreatic pseudocysts: prognostic factors for their development and their spontaneous resolution in the setting of acute pancreatitis. Pancreatology. 2012;12:85–90. doi: 10.1016/j.pan.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 133.Magnusdottir BA, Baldursdottir MB, Kalaitzakis E, Björnsson ES. Risk factors for chronic and recurrent pancreatitis after first attack of acute pancreatitis. Scand. J. Gastroenterol. 2019;54:87–94. doi: 10.1080/00365521.2018.1550670. [DOI] [PubMed] [Google Scholar]
- 134.Mirnezami A, et al. Population-based observational study of acute pancreatitis in southern England. Ann. R. Coll. Surg. Engl. 2019;101:487–494. doi: 10.1308/rcsann.2019.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moran RA, et al. Influence of age, body mass index and comorbidity on major outcomes in acute pancreatitis, a prospective nation-wide multicentre study. United Eur. Gastroenterol. J. 2018;6:1508–1518. doi: 10.1177/2050640618798155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mounzer R, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–1482. doi: 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 137.Párniczky A, et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS ONE. 2016;11:e0165309. doi: 10.1371/journal.pone.0165309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roberts SE, et al. Mortality following acute pancreatitis: social deprivation, hospital size and time of admission: record linkage study. BMC Gastroenterol. 2014;14:153. doi: 10.1186/1471-230X-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sternby H, et al. Mean muscle attenuation correlates with severe acute pancreatitis unlike visceral adipose tissue and subcutaneous adipose tissue. United Eur. Gastroenterol. J. 2019;7:1312–1320. doi: 10.1177/2050640619882520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sue LY, et al. Effect of serum triglycerides on clinical outcomes in acute pancreatitis. Pancreas. 2017;46:874–879. doi: 10.1097/MPA.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verdonk RC, et al. Short article: Presence, extent and location of pancreatic necrosis are independent of aetiology in acute pancreatitis. Eur. J. Gastroenterol. Hepatol. 2018;30:342–345. doi: 10.1097/MEG.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 142.Vujasinovic M. Pancreatic exocrine insufficiency, diabetes mellitus and serum nutritional markers after acute pancreatitis. World J. Gastroenterol. 2014;20:18432. doi: 10.3748/wjg.v20.i48.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yadav D, Lee E, Papachristou GI, O’Connell M. A population-based evaluation of readmissions after first hospitalization for acute pancreatitis. Pancreas. 2014;43:630–637. doi: 10.1097/MPA.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 144.Zádori N, Párniczky A, Szentesi A, Hegyi P. Insufficient implementation of the IAP/APA guidelines on aetiology in acute pancreatitis: Is there a need for implementation managers in pancreatology? United Eur. Gastroenterol. J. 2020;8:246–248. doi: 10.1177/2050640620918695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones MJ, Neal CP, Ngu WS, Dennison AR, Garcea G. Early warning score independently predicts adverse outcome and mortality in patients with acute pancreatitis. Langenbeck’s Arch. Surg. 2017;402:811–819. doi: 10.1007/s00423-017-1581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao Z, Shen J, Zhang D, Shen J. The prognostic role of peripheral lymphocyte subsets in patients with acute pancreatitis. Am. J. Med. Sci. 2019;357:242–246. doi: 10.1016/j.amjms.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 147.Kiss L, et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci. Rep. 2018;8:14096. doi: 10.1038/s41598-018-32337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Q, Wang G, Qiu Z, He X, Liu C. Elevated serum triglycerides in the prognostic assessment of acute pancreatitis. J. Clin. Gastroenterol. 2017;51:586–593. doi: 10.1097/MCG.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 149.Tai, W. et al. A Retrospective Research of the Characteristic of Hypertriglyceridemic Pancreatitis in Beijing , China. 2016, (2016). [DOI] [PMC free article] [PubMed]
- 150.Gullo L, et al. An update on recurrent acute pancreatitis: data from five European countries. Am. J. Gastroenterol. 2002;97:1959–1962. doi: 10.1111/j.1572-0241.2002.05907.x. [DOI] [PubMed] [Google Scholar]
- 151.Suchsland T, et al. Predictive factors for and incidence of hospital readmissions of patients with acute and chronic pancreatitis. Pancreatology. 2015;15:265–270. doi: 10.1016/j.pan.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 152.Kim SB, Kim TN, Chung HH, Kim KH. Small gallstone size and delayed cholecystectomy increase the risk of recurrent pancreatobiliary complications after resolved acute biliary pancreatitis. Dig. Dis. Sci. 2017;62:777–783. doi: 10.1007/s10620-016-4428-3. [DOI] [PubMed] [Google Scholar]
- 153.Stigliano S, et al. Recurrent biliary acute pancreatitis is frequent in a real-world setting. Dig. Liver Dis. 2018;50:277–282. doi: 10.1016/j.dld.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 154.Mandalia, A., Wamsteker, E. & DiMagno, M. Recent advances in understanding and managing acute pancreatitis. F1000Research7, 959 (2018). [DOI] [PMC free article] [PubMed]
- 155.Gislason H, et al. Acute pancreatitis in Bergen Norway. Scand. J. Surg. 2004;93:29–33. doi: 10.1177/145749690409300106. [DOI] [PubMed] [Google Scholar]
- 156.Uhl W, et al. Influence of etiology on the course and outcome of acute pancreatitis. Pancreas. 1996;13:335–343. doi: 10.1097/00006676-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 157.Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am. J. Gastroenterol. 2009;104:984–991. doi: 10.1038/ajg.2009.27. [DOI] [PubMed] [Google Scholar]
- 158.Nawaz H, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am. J. Gastroenterol. 2015;110:1497–1503. doi: 10.1038/ajg.2015.261. [DOI] [PubMed] [Google Scholar]
- 159.Navina S, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci. Transl. Med. 2011;3:107. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Acharya C, Navina S, Singh VP. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403–408. doi: 10.1016/j.pan.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Criddle DN, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 162.Maléth J, Rakonczay Z, Venglovecz V, Dolman NJ, Hegyi P. Central role of mitochondrial injury in the pathogenesis of acute pancreatitis. Acta Physiol. 2013;207:226–235. doi: 10.1111/apha.12037. [DOI] [PubMed] [Google Scholar]
- 163.Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis. J. Clin. Gastroenterol. 2014;48:195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adiamah A, Psaltis E, Crook M, Lobo DN. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin. Nutr. 2018;37:1810–1822. doi: 10.1016/j.clnu.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 165.Pascual I, et al. Association of elevated serum triglyceride levels with a more severe course of acute pancreatitis: cohort analysis of 1457 patients. Pancreatology. 2019;19:623–629. doi: 10.1016/j.pan.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 166.Hamada S, Masamune A, Kikuta K, Shimosegawa T. Clinical impact of elevated serum triglycerides in acute pancreatitis: validation from the nationwide epidemiological survey in Japan. Am. J. Gastroenterol. 2016;111:575–576. doi: 10.1038/ajg.2015.421. [DOI] [PubMed] [Google Scholar]
- 167.Ewald N. Hypertriglyceridemia-induced acute pancreatitis. Clin. Lipidol. 2013;8:587–594. doi: 10.2217/clp.13.44. [DOI] [Google Scholar]
- 168.Bennetts M, Whalen E, Ahadieh S, Cappelleri JC. An appraisal of meta-analysis guidelines: how do they relate to safety outcomes? Res. Synth. Methods. 2017;8:64–78. doi: 10.1002/jrsm.1219. [DOI] [PubMed] [Google Scholar]
- 169.Shuster JJ, Guo JD, Skyler JS. Meta-analysis of safety for low event-rate binomial trials. Res. Synth. Methods. 2012;3:30–50. doi: 10.1002/jrsm.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.