Abstract
Abnormally increased neuronal activity in the lateral habenula (LHb) is closely associated with depressive-like behavior. Despite the emphasis on the pathological importance of NMDA receptor (NMDAR)-dependent long-term depression (LTD) and the involvement of calcium permeable AMPA receptor (CP-AMPAR) as major Ca2+ source, the functions of NMDAR and CP-AMPAR on LTD modulation in the LHb still have not been fully investigated. Here, we found that NMDAR-dependent LTD by low frequency stimulation was induced in both synaptic and extrasynaptic regions in the LHb. In addition, CP-AMPAR was necessary for the activation of NMDAR in the induction phase of NMDAR-dependent LTD. The acute stress, which induced depressive behavior, had a blocked effect on synaptic NMDAR-dependent LTD but left extrasynaptic NMDAR-dependent LTD intact. These findings show that NMDAR-dependent LTD in LHb plays an important role in regulating neuronal activity, which is probable to be excessively increased by repeated stress, via maintaining homeostasis in both synaptic and extrasynaptic regions of the LHb. Moreover, NMDAR and CP-AMPAR may serve as a depression-related modulator and be regarded as a promising therapeutic target for treatment of psychopathology such as depression.
Subject terms: Neuroscience, Physiology
Introduction
Long-lasting suppression of glutamatergic transmission is linked with the homeostasis of basal synaptic transmission by the regulation of neuronal output transmitted into the monoaminergic system in the lateral habenula (LHb)1. Several studies have reported that alteration of long-term depression (LTD) in the LHb is an important molecular mechanism to understand psychopathological changes such as depression and addiction2,3. Low-frequency stimulation (LFS)-induced LTD in the LHb is dependently modulated by the activation of group I metabotropic glutamate receptor (mGluR)1,3. The mGluR-dependent LTD modulated the excitatory and inhibitory output signaling, and consequently, it engaged in modulating the neuronal activity in the LHb. Although calcium-permeable AMPA receptor (CP-AMPAR), considered to be a Ca2+ source, is involved in changing LTD to long-term potentiation (LTP) in the LHb neuron1,4, a molecular mechanism concerning the fundamental modulation of LTD has not been fully reported. It is generally believed that mGluR-dependent LTD causes CP-AMPAR to change into calcium-impermeable (CI)-AMPAR5–7. However, mGluR-dependent LTD is independently modulated without having an effect on CP-AMPAR expression1. An electrophysiological investigation concerning the generation of LTD in the LHb should be undertaken to clarify the functional roles of CP-AMPAR in modulating LTD8.
A small component of NMDA receptor-mediated excitatory postsynaptic current is shown in the excitatory synapse of the rat LHb9. Until now, a functional investigation concerning the modulation of NMDAR-mediated LTD in the LHb was excluded because the NMDAR-mediated responses are minimal during the confirmation process of the current–voltage (I–V) curve through voltage clamp recordings1,3,10. However, the functional roles of the NMDAR-mediated LTD should be considered as well based on the fact that a significant component of NMDAR-mediated excitatory postsynaptic potential is shown in the mouse LHb synapse1 and levels of NR1 and NR2B gene expression are significant in the rat LHb11,12. In general, the GluN2A and GluN2B subunits of NMDAR are key factors in determining the kinetics of NMDAR13. In addition, GluN2A is preferentially expressed at the synapse14, whereas GluN2B is expressed at both the synapse and extrasynapse13–15. Therefore, further investigation is required not only to confirm whether LTD induction is NMDAR-dependent but also to identify where NMDAR is located among the synaptic or extrasynaptic sites.
The LHb is a core brain region associated with depression3,11,16,17. In learned helplessness models, LHb neurons are found to be excessively activated9,16. In addition, mGluR-dependent LTD in the LHb is impaired by stress, and these synaptic alterations are found to be associated with depressive-like behavior as well3. A recent study reported that depressive-like behavior caused by repetitive stress is associated with an increase of bursting firing in the LHb, and inhibition of NMDAR by ketamine leads to an antidepressant effect17. However, in this report, ketamine has shown antidepressant effects by inhibiting NMDAR-dependent bursting, and there is still no research on the association between NMDA-dependent LTD in LHb and depressive behavior. In other words, we need to predict the functional role of NMDAR by examining the effect of NMDAR-dependent LTD on the LHb in the depression model.
In this study, we investigated overall properties of LFS-induced LTD and confirmed that the LFS-induced LTD was modulated by the activation of GluN2A- and GluN2B-containing NMDARs. In addition, we predicted the physiological and pathological function of NMDAR by determining NMDAR-dependent glutamatergic transmission in the synaptic and extrasynaptic regions of the LHb in normal and stressed rats.
Materials and methods
Animals
All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Ewha Womans University (EWU) and carried out in accordance with local guidelines and regulations. This study was approved by the IACUC committee at Ewha Womans University (IACUC 18-010). Male Sprague Dawley (SD) rats (aged 17–24 days, weighing 20–35 g) were purchased from Orient Bio Inc. (Seongnam, South Korea). The animal center maintained constant humidity of 60%, temperature of 20–22 °C, and air ventilation, with a 12 h light–dark cycle (lights on at 7:00 a.m.).
Restraint stress (RS) model and forced swimming test (FST)
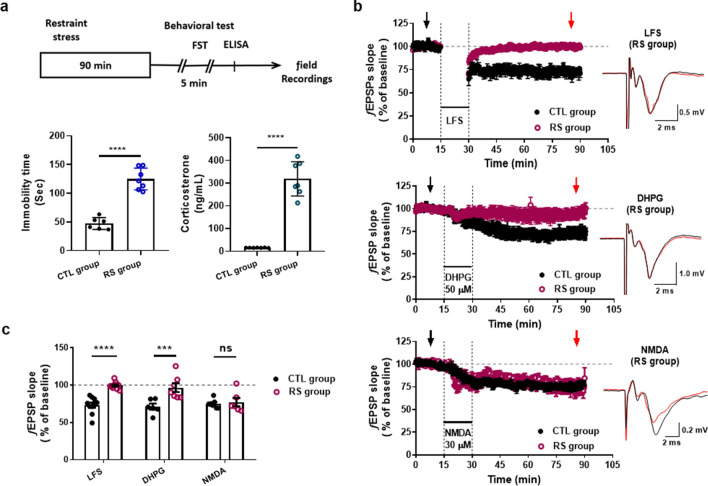
Based on the method reported in a previous study18, we had male SD rats in their postnatal days 17–24 implanted into a well-ventilated 50-ml polypropylene conical tube. The rat’s bodies were forcibly compressed in the tube to immobilize its legs and tails. This control process was conducted from 10 a.m. for 90 min a day (see Fig. 4a). The stressed rats were subsequently forced to swim for 6 min, and the immobility time was measured for 5 min (except for 1 min of adaptation time), based on Porsolt’s study19. The RS model was composed of the rats that displayed an immobility time of more than 100 s during FST.
Figure 4.
The effects of acute stress on NMDAR-dependent LTD. (a) Top, Restraint stress paradigm. Restraint stress was conducted for 90 min a day and then FST was carried out. Before extracellular recording, enzyme-linked immunosorbent assay (ELISA) was used to measure stress hormone corticosterone levels in the control (CTL) and acute restraint stress (RS) groups. Bottom Left, Immobility time (CTL group; 47.4 ± 4.2 s, n = 6, RS group; 124.8 ± 7.2 s, n = 7). Bottom Right, Level of corticosterone (CTL group, 15.6 ± 0.3 ng/mL, n = 7; RS group, 319.1 ± 28.5, n = 7). Static significance was determined using an unpaired t-test for comparisons between two groups (****P < 0.0001). (b) Top, LFS-LTD was completely blocked with rats exposed to restraint stress (99.6 ± 1.5%, n = 8). Middle, The acute stress-inhibited mGluR-dependent LTD which was induced by DHPG (50 μM) (CTL group, 73.6 ± 3.0%, n = 5; RS group, 96.8 ± 3.3%, n = 7). Bottom, Chemical LTD determined by NMDA (30 μM) was not affected and was well induced in the RS group (74.5 ± 7.2%, n = 6). (c) Summary histogram for differences in synaptic plasticity in the CTL group and RS group (two-way ANOVA; Holm-Sidak multiple comparisons, ****P < 0.0001, ***P = 0.0003, ns; P = 0.9722).
Corticosterone assay
To determine the concentration of corticosterone (CORT), Corticosterone HS EIA kit (Immunodiagnostic Systems Ltd, IDS Ltd; Boldon, United Kingdom) was used. Blood was collected from normal and experimental groups and blood was centrifuged at 3,000 rpm for 15 min in a centrifuge (Eppendorf centrifuge 5810R, Brinkmann Instruments Co., Westbury, NY) to obtain blood-serum only. The experimental procedure was based on the experimental guide of the corticosterone HS EIA kit.
Slice preparation
The rats were anesthetized with isoflurane aspiration. Sagittal sections of LHb slices (400-µm thick) were cut in ice-cold sucrose solution containing (in mM): sucrose, 201; NaHCO3, 26; glucose, 10; KCl, 3; NaH2PO4, 1.25; MgCl2, 3; and CaCl2, 1 (saturated with 95% O2/5% CO2). They were then transferred to a holding chamber filled with artificial cerebrospinal fluid (aCSF), containing (in mM): NaCl, 126; NaHCO3, 26; glucose, 10; MgSO4, 1.3; NaH2PO4, 1.25; KCl, 3; and CaCl2, 2.4. Freshly cut slices were placed in an incubating chamber containing aCFS and recovered at 30–31 °C for 1 h. Slices were then maintained in aCSF at room temperature (21–23 °C) prior to recording. For recording, each slice was transferred into a recording chamber and continuously superfused with oxygenated aCSF at a flow rate of 2–3 ml/min and temperature of 30 ± 2 °C using an in-line heater (Warner Instruments, Hamden, CT, USA). In the low-Mg2+ aCSF experiment, only 1.3 mM MgSO4 was changed to 0.5 mM concentration. To enhance the low osmolality level, glucose was added.
Extracellular recording
Field EPSPs (ƒEPSPs) were recorded in the LHb with aCSF-filled 1–2 MΩ glass pipettes using an A-M Systems microelectrode AC amplifier and A/D converter (Digidata 1322A, Molecular Devices, Sunnyvale, CA, USA). Current stimuli were delivered to a constant stimulus isolation unit (150-µs pulse duration) (World Precision Instruments, Inc., Sarasota, FL, USA) by a Master-8 pulse generator (AMPI, Jerusalem, Israel). The stimulation rate was 0.067 Hz for all ƒEPSP recording experiments. After obtaining a stable baseline for 15 min, LTD was induced by applying 900 pulses at 1 Hz (LFS) through a concentric bipolar electrode (FHC, Bowdoin, Me, USA; 125 µm/Rnd/25 µm Pt-lr) with the same stimulating intensity used during baseline. The initial slope of the response was used to assess changes in synaptic strength.
Drugs
For drug application, the aCSF solution was switched to the drug solution for at least 15 min before the induction of LFS. D-2-amino-5-phosphonovalerate (D-AP5), (S)-3,5-Dihydroxyphenylglycine ((S)-3,5-DHPG)), DL-threo-β-benzyloxyaspartate (DL-TBOA), (S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), (+)-5-methyl-10,11-dihydroxy-5H-dibenzo(a,d)cyclohepten-5,10-imine[(+)-MK-801],N-[3-[[4-[(3-Aminopropyl)amino]butyl]amino]propyl]-1-naphthaleneacetamide (NASPM) trihydrochloride, N-Methyl-D-aspartic acid (NMDA), (αR,βS)-α-(4-Hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidinepropanol (Ro 25-6981) maleate, and (1R*,2S*)-erythro-2-(4-Benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol (Ifenprodil) hemitartrate were obtained from Tocris Cookson (Bristol, UK). Aminoacetic acid, Aminoethanoic acid (Glycine) and [[[(1S)-1-(4-Bromophenyl)ethyl]amino](1,2,3,4-tetrahydro-2,3-dioxo-5-quinoxalinyl)methyl] phosphonic acid (PEAQX, NVP-AAM 077) tetrasodium hydrate were purchased from Sigma Aldrich (St. Louis, MO).
Statistical analyses
Recordings were filtered at 1 Hz and 5 kHz, digitized at 10 kHz, and analyzed using pClamp9 (Molecular Devices). The ƒEPSP slope was calculated as the change at 10–90% of the baseline. The mean change in ƒEPSP slope was normalized to each slice’s baseline period. The sweep was presented by averaging 10 consecutive ƒEPSPs. Data were analyzed using GraphPad Prism (GraphPad Prism 8.0, USA). Data are presented as mean ± S.E.M. Two-way analysis of variance (ANOVA) was performed for comparison of all continuous variables between groups with Sidak’s multiple comparisons. Static significance was determined using an unpaired Student’s t-tests for comparisons between two groups. One-way ANOVA was performed for comparison of all continuous variables between groups; when indicated, a Dunnett’s correction was performed for post-hoc comparisons within and between groups (vs. baseline). Probability values of P < 0.05 were considered to represent significant differences. A dose–response relationship curve was analyzed using GraphPad Prism 8.0., and the curve was fit by a nonlinear regression.
Results
Long-term modulation of glutamatergic transmission in the LHb
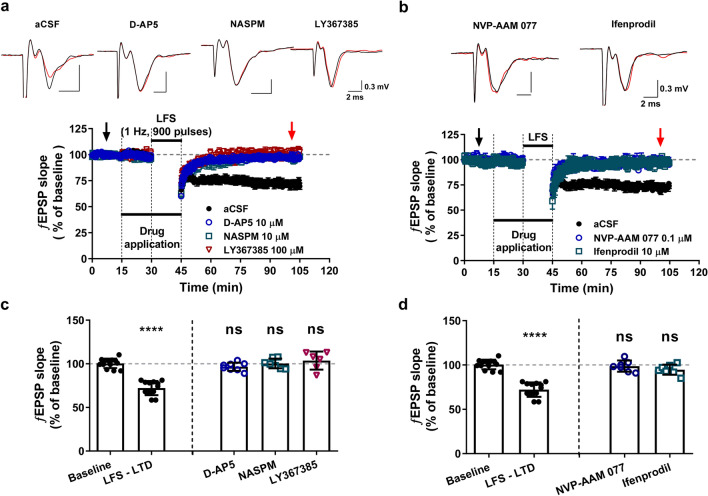
To determine which glutamate receptors are mainly involved in the long-term modulation of excitatory transmission in the LHb, we examined the effect of various glutamate receptor antagonists on LFS-induced LTD (Fig. 1). Basically, the changes in the slope of evoked ƒEPSPs was analyzed to avoid the interference of population spike in observing AMPA-mediated synaptic transmission (Fig. S1). The LFS (900 pulses each with a pulse duration of 150 μs at 1 Hz) did not induce the LTD at all under the presence of LY3677385 (100 μM) (Fig. 1a,c), a selective mGluR1 antagonist, confirming previous findings that the LFS-induced LTD in LHb could be mediated by the activation of mGluR11,3. In addition, either a CP-AMPAR antagonist (NASPM, 10 μM) or an NMDAR blocker (D-AP5, 10 μM) suppressed the LFS-induced LTD as much as LY3677385 did (Fig. 1a,c), suggesting that, in addition to the activation of mGluR1, the activation of NMDAR and CP-AMPAR lead to a long-lasting suppression of the excitatory transmission from the stria medullaris fibers into the LHb regions.
Figure 1.
Determining NMDAR-dependent LTD in the LHb regions. (a, b) Top, The proposed ƒEPSP sweep shows the change in baseline (black trace, from black arrow in bottom) and after 55 min of LFS transmission (red trace, from red arrow in bottom). Bottom, The LFS (900 pulses at 1 Hz) elicited robust LTD. The regulation of LFS-induced LTD by selective glutamate receptor antagonists (a) and NMDAR subtype antagonists (b). (c) Summary histogram showing modulation of LTD by selective glutamate receptor antagonists (one-way ANOVA, F[4, 42] = 39.14; Dunnett’s post-hoc test as condition vs. baseline; LFS-LTD, 71.9 ± 4.2%, n = 10, ****P < 0.0001; D-AP5, 96.7 ± 1.7%, n = 8, ns, P = 0.5884; NASPM, 100.4 ± 2.1%, n = 7, ns, P > 0.9999; LY367385, 103.7 ± 4.2%, n = 6, ns, P = 0.7828). (d) Bar graph shows LTD is regulated by selective NMDAR subtype antagonist (one-way ANOVA, F[4, 42] = 37.97; Dunnett’s post-hoc test as condition vs. baseline; NVP-AAM 077, 99.1 ± 2.7%, n = 7, ns, P = 0.9533; ifenprodil, 97.4 ± 3.0%, n = 7, ns, P = 0.2148).
Despite synaptic NMDA signals not being noticed functionally in rat LHb9, the main components of NMDAR, GluN1, and GluN2A/B are known to exist meaningfully11. We thus investigated LFS-induced LTD under treatment with either an GluN2A- or an GluN2B-containing NMDAR antagonist to determine the specificity for receptor subtypes of NMDAR-dependent LTD in LHb (Fig. 1b,d). The LFS-induced LTDs were completely inhibited under treatment with NVP-AAM 077 (0.1 μM), an GluN2A-containing NMDAR antagonist and under ifenprodil (10 μM), GluN2B-containing NMDAR antagonist (Fig. 1b,d). All these results clearly show that the activation of GluN2A- or GluN2B- containing NMDARs leads to LTD induction triggered by LFS in LHb.
Roles of CP-AMPAR on NMDAR-dependent LTD in LHb
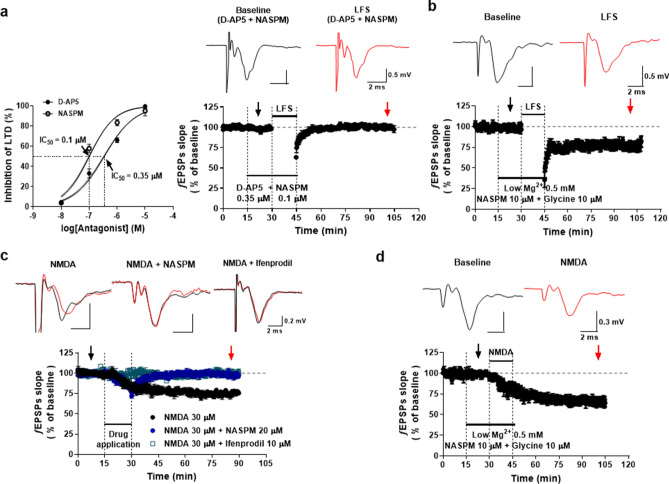
It has been generally reported that CP-AMPAR is concerned as a crucial regulator in NMDAR-dependent LTD8. We thus focused on the possible relation of CP-AMPAR with NMDAR-dependent LTD in LHb. First, as AMPAR and NMDAR-dependent LTD were not reported directly and systematically in the LHb, we investigated the actions of NASPM and D-AP5 on LFS-induced LTD. Both NASPM and D-AP5 suppressed the LFS-induced LTD in a concentration-dependent manner and their IC50 values were determined as 0.1 µM and 0.35 µM, respectively (Fig. 2a, Left). Such a dose–response relationship shows that the contribution of CP-AMPAR and NMDAR to LTD generation is authentic. We roughly predicted that if the actions of CP-AMPAR and NMDAR on LTD generation occur independently, the partial suppression effects of NASPM and D-AP5 would appear to be a simple arithmetic sum, while if there was interdependence, they would be larger or smaller than the arithmetic sum. We found that D-AP5 (0.35 µM), which partially suppressed the LFS-induced LTD (Fig. 2a, Left), inhibited the LTD completely when applied together with NASPM (0.1 µM) (Fig. 2a, Right). This strongly suggests an interdependence of CP-AMPAR and NMDAR activity in LTD generation.
Figure 2.
Roles of CP-AMPAR in NMDAR-dependent LTD. (a) Left; Dose response relationship of D-AP5 and NASPM on LFS-induced LTD (IC50 and Hill’s coefficient; D-AP5, 0.35 µM and 0.78 ± 0.1, n = 3; NASPM, 0.1 µM and 0.92 ± 0.1, n = 3). Right; Treatment with both DAP5 (0.35 μM) and NASPM (0.1 μM) completely inhibited LTD induction in the LHb (97.4 ± 3.2%, n = 6). (b) NASPM failed to inhibit LFS-induced LTD in aCSF containing low Mg2+ (0.5 mM) and glycine (10 μM) (LFS-LTD, 77.9 ± 3.9%, n = 5). (c) Chemically NMDA-induced LTD was induced in the LHb after 15 min of treatment with NMDA (30 μM)(76.9 ± 2.2%, n = 6) and was completely inhibited by NASPM (20 μM)(96.3 ± 2.5%, n = 6) and by ifenprodil (10 μM)(98.6 ± 2.6%, n = 4). (d) NASPM did not block chemically NMDA-induced LTD (65.5 ± 5.6%, n = 5).
Because CP-AMPAR is first introduced at an early stage of synaptic plasticity8, we hypothesized that CP-AMPAR might be involved in the initial process at NMDAR-dependent LTD as well. NMDAR activation requires unblocking of Mg2+ from the pore of NMDAR. The preemptive activation of CP-AMPAR by periodic electrical stimulation could unplug Mg2+ in NMDAR by inducing the membrane depolarization. To determine this issue, we investigated the LTD induction under a low Mg2+ concentration (0.5 mM) with high glycine concentration (10 μM). No block with NASPM (10 μM ) on the LTD generation was observed in the conditions under which the activity of NMDAR was maximized, implying that the NMDAR-induced LTD can be produced without CP-AMPAR activation as long as the NMDAR is off the Mg2+ pore block. In addition, NASPM failed to control LFS-induced LTD under the low Mg2+ and high glycine conditions (Fig. 2b).
To test this issue more, we examined the effect of NASPM on a chemically NMDA-induced LTD that was generated by applying NMDA (30 μM) alone for 15 min. The chemically NMDA-induced LTD was almost completely inhibited by the co-application of NASPM (20 μM) (Fig. 2c), indicating that the CP-AMPAR activity is required for the initiation of the NMDAR-induced LTD. It also completely blocked by ifenprodil, suggesting that chemically NMDA-induced LTD is NMDAR-dependent LTD (Fig. 2c). Moreover, NASPM (10 μM) also failed to chemically NMDA-induced LTD under the low Mg2+ and high glycine conditions (Fig. 2d). Taken together, we concluded that the CP-AMPAR activation may contribute to the initial process of NMDAR-dependent LTD by removing Mg2+ from NMDAR pore.
Characteristics of NMDAR-dependent LTD in LHb
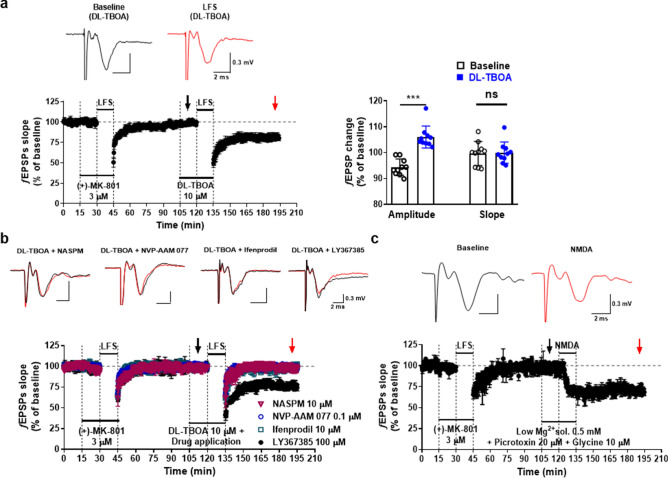
Recent microarray data12 indicate that the presence of GluN1 and GluN2B component of NMDAR in LHb is obvious, and our results confirmed that the expression of GluN2A as well as the expression of GluN2B was shown in LHb (Fig. S1b). Because GluN2A is found primarily within the synapses, wherease GluN2B is not, which is extrasynapses20, the following experiments were conducted to understand whether GluN2B NMDAR exists extrasynapse and, if so, whether its function induces synaptic plasticity. To block synaptic NMDAR in the LHb synapses, we applied LFS under the presence of MK-801, a well-known noncompetitive and irreversible blocker of NMDAR by exploiting the open channel blocking and “trapping” properties. No LTD was induced by LFS under MK-801 (3 μM) (Fig. 3a, left), confirming that the LFS-induced LTD in the LHb was synaptic NMDAR-dependent. No LTD was induced by another LFS given after more than 1 h of MK801 washout (data not shown), showing that the NMDARs activated by the previous LFS were irreversibly and completely blocked by MK-801.
Figure 3.
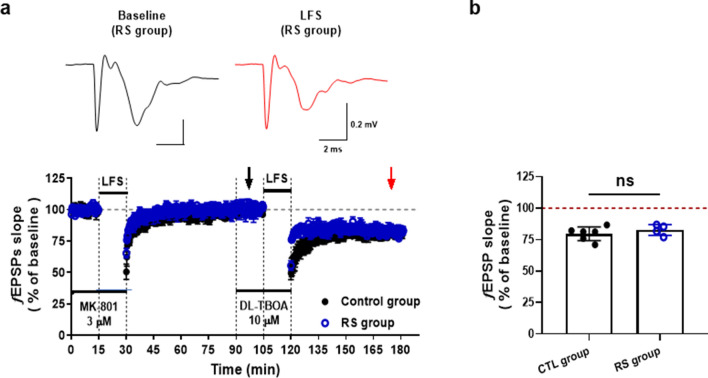
Function of CP-AMPAR and NMDAR for the induction of extrasynaptic LTD in the LHb. (a) Left; While the application of ( +)MK-801 (3 μM) completely suppressed LFS-induced LTD (95.1 ± 1.4%, n = 6), LFS steadily induced extrasynaptic LTD after the application of DL-TBOA (10 μM) (81.0 ± 4.0%, n = 6). Right; After the treatment of DL-TBOA without LFS, the change of amplitude showed a significant potentiation five minutes after the drug application of DL-TBOA but did not lead to the change of fEPSP slope. (b) LFS-induced LTD completely inhibited by the co-application of DL-TBOA with NASPM (20 μM; 97.5 ± 3.3%, n = 3), NVPAAM 077 (0.1 μM; 100.6 ± 1.7%, n = 5), ifenprodil (0.1 μM; 100.6 ± 1.7%, n = 5). However, the coapplication of DL-TBOA with LY367385 (100 μM) did not show any inhibitory effects on LFS-induced LTD (74.1 ± 3.7%, n = 3). (c) After MK-801 exposure, chemically NMDA-induced LTD was generated in aCSF containing low Mg2+ (0.5 mM) and glycine (10 μM) (69.9 ± 2.0%, n = 2).
Moreover, to determine the role of extrasynaptic NMDAR on NMDA-dependent LTD, in the presence of the glutamate-uptake inhibitor DL-TBOA, we examined the effect of spillover and temporal summation of glutamate by blocking glutamate uptake. Under treatment with DL-TBOA (10 μM), LFS dramatically induced an extrasynaptic LTD (Fig. 3a, left). The ƒEPSP amplitude showed weak potentiation five minutes after the drug application of DL-TBOA without LFS but did not lead to the change of fEPSP slope (Fig. 3a, right). NASPM (20 μM) fully inhibited the LFS-induced LTD under DL-TBOA (Fig. 3b), showing that the activation of CP-AMPAR was required for the induction of extrasynaptic LTD. Those extrasynaptic LTDs was fully blocked by ifenprodil (10 μM) and NVP-AAM 077 (0.1 μM) (Fig. 3b), showing that the LTD induction could be mediated by the activation of GluN2A- and GluN2B-containing NMDAR expressed outside the synapses. It also indicates that both GluN2A- and GluN2B-containing NMDAR exist at the extrasynapse, as in the synapse, of an LHb neuron. However, unlike synaptic LTD, LTD under DL-TBOA did not inhibited in the treatment with LY367385 (100 μM) (Fig. 3b), showing that the activation of mGluR1 was not required for the induction of the extrasynaptic LTD. Furthermore, after MK-801 exposure, under low Mg2+ condition, we found chemically NMDA-induced LTD, suggesting that chemically NMDA-induced LTD also includes extrasynptic NMDAR-dependent LTD (Fig. 3c).
Effect of acute stress on LTD generation in LHb
To determine how the NMDAR-dependent LFS-LTD is changed by stress, we examined the effect of LTD in the LHb of restraint stressed rats. We found that the exposure to restraint stress for 90 min induced depressive-like behaviors and caused an excessive increase in corticosterone (Fig. 4a). The exposure of acute stress completely inhibited the LFS-LTD (Fig. 4b, Top), which is comparable to results of a previous study3. However, because our experimental techniques limit effective distinction between mGluR-dependent LTD and NMDAR-dependent LTD, it was difficult to determine which of them resulted in a reduction in the LTD due to stress. To circumvent this problem, we examined the effect of acute stress on both chemically DHPG-induced and NMDA-induced LTD (Fig. 4b). We found that no LTD was induced by DHPG (50 μM) under acute stress (Fig. 4b Middle, 4c), showing that mGluR-dependent LTD is highly sensitive to stress3. By contrast, the chemically NMDA (30 μM NMDA)-induced LTD was unaffected by stress (Fig. 4b Bottom, 4c), strongly suggesting that NMDAR-dependent LTDs are not affected by stress. We then hypothesized that the extrasynaptic LTD could not be affected by stress because our data showed that synaptic LTD was caused by either NMDAR or mGluR activity, whereas extrasynaptic LTD was only caused by NMDAR activity. In fact, the stress selectively prevented the generation of synaptic LTD (Fig. 5a). In the stress group, LFS did not induce synaptic LTD, whereas adding DL-TBOA led the LFS to induce LTD comparable to those in the control group (Fig. 5b). Taken together, our results suggest that the acute stress had a blocked effect on synaptic NMDAR-dependent LTD but left extrasynaptic NMDAR-dependent LTD intact.
Figure 5.
The effects of acute stress on extrasynaptic LTD. (a) In RS groups, LFS-induced LTD was generated when treated with DL-TBOA (10 μM). (b) Comparison of the differences in extrasynaptic LTD between control and restraint stress groups (unpaired t-test; CTL group, 79.7 ± 2.2%, n = 6; RS group, 78.8 ± 1.5%, n = 4, ns, P = 0.3857). Data are presented as mean ± standard error of the mean (S.E.M.).
Discussion
In this study, we reported that various glutamate receptors are involved in the modulation of LFS-induced LTD in the LHb. First, NMDAR-dependent LTD in the LHb was specifically modulated by the NMDAR subtypes of GluN2A and GluN2B in the synaptic and extrasynaptic regions. The extrasynaptic NMDAR-dependent LTD had independent molecular mechanisms of group I mGluR. Second, we found that NMDAR and CP-AMPAR were dependently modulated in LTD for glutamatergic transmission. CP-AMPAR played a central role in activating NMDAR in the initial induction phase of NMDAR-dependent LTD. Third, acute stress exposure impaired synaptic NMDAR-dependent LTD but did not have an impact on extrasynaptic NMDAR-dependent LTD.
The projection of the excitatory synaptic response from the forebrain to the LHb containing CP-AMPAR displayed an inward rectification9. In Fig. 2, the activation of CP-AMPAR played an important role in NMDAR-dependent LTD and was involved in the long-lasting regulation of the excitatory synaptic response in the LHb. During the induction of NMDAR-dependent LTD in the hippocampal CA1 region, CP-AMPAR in the peri-synapse or the extrasynapse moves to the synapse and is involved in the activation of NMDAR, causing Ca2+ influx8. In our study, CP-AMPAR acted as an inducer to activate NMDAR and was necessary for NMDAR-dependent LTD.
The LTD generation was suppressed by MK-801, a selective synaptic NMDAR antagonist known as an open-channel blocker21, and LTD was not induced by LFS even after drug washout (data not shown). When synaptic NMDAR opened, MK-801 combined with the inside of synaptic NMDAR and blocked the flow of ions including Ca2+. During the suppression of NMDAR activation in the synaptic regions, DL-TBOA, a glutamate transporter (GluT) inhibitor, caused glutamate spillover in the synaptic cleft and was involved in the induction of extrasynaptic LTD. The extrasynaptic LTD was dependently modulated by the activation of GluN2A- and GluN2B-containing NMDAR as well (Fig. 3). Some studies reported that the GluN2A subtype is primarily expressed in the central portion of the synapse, whereas the GluN2B subtype is expressed in the peri-synaptic or extrasynaptic region22,23, while others reported that GluN2A- and GluN2B-containing NMDARs coexist in the extrasynaptic region24. Here, GluN2A- and GluN2B-containing NMDAR antagonists completely suppressed synaptic and extrasynaptic LFS-induced LTD (Figs. 1, 3). In immunohistochemistry assay, we also found that GluN2A and GluN2B subunits existed in LHb (Fig. S2a). Moreover, GluN2B did not affect the release probability of glutamate in the presynaptic sites (Fig. S2b). Therefore, NMDAR-dependent LTD in the LHb was specifically modulated by the NMDAR subtypes of GluN2A and GluN2B in the postsynaptic and extrasynaptic regions.
Generally, GluN2A-containing NMDAR has high open probability and leads to rapid inactivation in comparison with GluN2B-containing NMDAR. This property causes a relatively small amount of Ca2+ influx and facilitates AMPAR exocytosis. Meanwhile, GluN2B-containing NMDAR works opposite to GluN2A-containing NMDAR25. It has been reported that the GluN2A/GluN2B ratio determines the induction threshold of LTP and LTD26. When the synaptic GluN2A/GluN2B ratio is low following synaptic stimulation, LTP is more likely to occur rather than long-term depression (LTD). Conversely, when the GluN2A/GluN2B ratio is high, LTD is favored. The amount of Ca2+ influx from GluN2Bcontaining NMDAR, rather than from GluN2A-containing NMDAR, increases in low GluN2A/GluN2B synapses. However, LTD can be induced even in low GluN2A/GluN2B synapses. When the stimulation, such as LFS, is faintly transmitted in low GluN2A/GluN2B synapses, the amount of Ca2+ influx is insufficient for the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). The insufficient influx of Ca2+ triggers the activation of calcineurin rather than CaMKII and induces LTD26. This means that even in low GluN2A/GluN2B synapses, the induction threshold of LTP and LTD can be changed depending on the integrated postsynaptic response. LFS-induced LTD is shown to be regulated by GluN2A- and GluN2B-containing NMDARs in the extrasynaptic region in the LHb. As animals grow up, GluN2B is moved from the synaptic region to the extrasynaptic region13,14. As this study used rats at postnatal days 17–24, it seemed highly probable that the LHb contained immature synapses and that GluN2A and GluN2B were mixed across the LHb27.
It has been suggested that the long-lasting inhibition of the excitatory synaptic response in the LHb may be closely related to stress and depression1,3,9. The molecular mechanism of LTD in the LHb is caused by the modulation of presynaptic neurons1, and the CBR1 signaling alteration mechanism caused by group I mGluR may be closely related to stress exposure3. Previous studies suggested that patients with major depression and animal models of depression showed decreased GluT in astrocytes28,29,30. The inhibition of GluT increases excitability in the LHb, which is likely to cause depressive-like behavior18. While the dysfunction of GluT in glial cells caused by stress may cause changes in depressive-like behavior, excessive glutamate spillover is likely to amplify LTD through the activation of extrasynaptic NMDAR (Fig. S3). The probability of glutamate release is increased in presynaptic neurons by stress3,9, and the amount of glutamate in the synaptic cleft may be excessively increased as GLT-1 dysfunction is caused in astrocytes18. Based on these findings, a glutamate increase in the synaptic cleft may inhibit synaptic LTD in the LHb but amplify extrasynaptic LTD (Fig. S3). As a result, extrasynaptic LTD can control neuronal activity in the LHb, which is excessively increased by stress, and play a key role in maintaining homeostasis.
Supplementary information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (NRF-2015R1D1A1A01057086 to JMC and NRF-2019R1A2C1002963 to JN).
Author contributions
M.K. and J.C. conceived the project idea and designed the study. M.K. conducted the experiment and drafted the manuscript. J.N. participated in the data analysis, and helped to draft the manuscript. M.K., J.N. and J.C. contributed to important intellectual content and revised the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jihyun Noh, Email: jihyun2@dankook.ac.kr.
Jun-mo Chung, Email: jmchung@ewha.ac.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-74496-w.
References
- 1.Valentinova K, Mameli M. mGluR-LTD at excitatory and inhibitory synapses in the lateral habenula tunes neuronal output. Cell Rep. 2016;16:2298–2307. doi: 10.1016/j.celrep.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J. Neurosci. 2012;32:12641–12646. doi: 10.1523/JNEUROSCI.2405-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Rhee J, Lee S, Chung C. Selectively impaired endocannabinoid-dependent long-term depression in the lateral habenula in an animal model of depression. Cell Rep. 2017;20:289–296. doi: 10.1016/j.celrep.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Meye FJ, Valentinova K, Lecca S, Marion-Poll L, Maroteaux MJ, Musardo S, Moutkine I, Gardoni F, Huganir RL, Georges F. Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nat. Neurosci. 2015;18:376–378. doi: 10.1038/nn.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 2006;9:636–341. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 6.Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat. Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- 7.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J. Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson JL, Gorski JA, Dell’Acqua ML. NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca2+-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron. 2016;89:1000–1015. doi: 10.1016/j.neuron.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J. Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner F, Weiss T, Veh RW. Electrophysiological properties of neurons and synapses in the lateral habenular complex (LHb) Pharmcol. Biochem. Behav. 2017;162:38–45. doi: 10.1016/j.pbb.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 14.Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- 15.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, Malinow R, Yates JR, III, Hu H. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 18.Magarin AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-I. [DOI] [PubMed] [Google Scholar]
- 19.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archi. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 20.Mony L, Kew JNC, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br. J. Pharm. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKay S, Bengtson CP, Bading H, Wyllie DJA, Hardingham GE. Recovery of NMDA receptor currents from MK-801 blockade is accelerated by Mg2+ and memantine under conditions of agonist exposure. Neuropharmacology. 2013;74:119–125. doi: 10.1016/j.neuropharm.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J. Neurosci. 2004;24:4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J. Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohrmann R, Hatt H, Gottmann K. Developmental regulation of subunit composition of extrasynaptic NMDA receptors in neocortical neurones. NeuroReport. 2000;11:1203–1208. doi: 10.1097/00001756-200004270-00012. [DOI] [PubMed] [Google Scholar]
- 25.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 26.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sans N, Petralia RS, Wang Y-X, Blahos J, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J. Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina A, Burke S, Thompson RC, Bunney W, Jr, Myers RM, Schatzberg A, Akil H, Watson SJ. Glutamate transporters: a key piece in the glutamate puzzle of major depressive disorder. J. Psychiatr. Res. 2013;47:1150–1156. doi: 10.1016/j.jpsychires.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–473. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Cui W, Mizukami H, Yanagisawa M, Aida T, Nomura M, Isomura Y, Takayanagi R, Ozawa K, Tanaka K, Aizawa H. Glial dysfunction in the mouse habenula causes depressive-like behaviors and sleep disturbance. J. Neurosci. 2014;34:16273–16285. doi: 10.1523/JNEUROSCI.1465-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.