Abstract
By definition total synthesis is the art and science of making the molecules of living Nature in the laboratory, and by extension, their analogues. Although obvious, its application to the synthesis of molecules for biology and medicine was not always the purpose of total synthesis. In recent years, however, the field has acquired momentum as its power to reach higher molecular complexity and diversity is increasing, and as the demand for rare bioactive natural products and their analogues is expanding due to their recognised potential to facilitate biology and drug discovery and development. Today this component of total synthesis endeavors is considered highly desirable, and could be part of interdisciplinary academic and/or industrial partnerships, providing further inspiration and momentum to the field. In this review we provide a brief historical background of the emergence of the field of total synthesis as it relates to making molecules for biology and medicine. We then discuss specific examples of this practice from our laboratories as they developed over the years. The review ends with a conclusion and future perspectives for natural products chemistry and its applications to biology and medicine and other added-value contributions to science and society.
Graphical Abstract

1. Introduction
The serendipitous preparation of urea, from silver cyanate (more accurately, following today’s nomenclature, silver isocyanate)1 and ammonium chloride, or lead cyanate and ammonia, respectively, by Friedrich Wöhler in 18282 had a profound impact on chemistry. It gave birth to organic synthesis and its flagship, total synthesis of natural products, of which urea was the first. It also helped shape the concept of isomerism,3 a fundamental principle and important feature of the molecular sciences. As the power of total synthesis improved, more complex natural products were replicated in the laboratory while the main purpose of the endeavour evolved to become the means for confirming the hypothetical structures of natural products.4 This purpose remained as the main objective of the continuously growing in power discipline of total synthesis until well in the twentieth century when R. B. Woodward emerged as the leader of the field in the 1940s and 1950s. With his important contributions he turned synthesis into an art, with one complex molecule after another falling as he was marching over them in glory, mesmerising his admirers with his aesthetically pleasing synthetic strategies. Then came E. J. Corey, who turned total synthesis into a systematic art and science through his logic-based approach by introducing the retrosynthetic analysis concept and method development as integral parts of total synthesis endeavours. Corey also demonstrated the crucial value of total synthesis to biology and medicine by synthesising scarce naturally occurring molecules, and other molecular structures hypothesised to be naturally occurring, thereby rendering them readily available for structural confirmation, elucidation, and biological investigation. His landmark accomplishments included a number of prostaglandins5-7 and leukotrienes,8-13 among others. By the 1970s, the seeds were in the ground for the field of making molecules for biology and medicine through total synthesis to emerge and gather momentum in the following decades. In the meantime, isolation chemists, both from academia and the pharmaceutical industry, were busy harvesting the molecules of Nature as potential drug candidates, a revolution that gathered strong momentum with the discovery of penicillin, and accelerated during the second World War as incentivised by the need to treat and save the lives of wounded soldiers in the field. The second half of the twentieth century was indeed a golden era for natural products isolation and total synthesis, not to mention the myriad drugs derived from or inspired by them that were approved for clinical use, with a good number of them, or their descendants, still in use today.
Then random combinatorial chemistry kicked in, to some perhaps an unfortunate occurrence for both natural products isolation and total synthesis. Based on assumptions, some believed that random combinatorial chemistry would provide all the drugs needed to treat every disease, and there was perhaps no need for rational drug design or natural products anymore. Although combinatorial chemistry had its positive impact in academia and the pharmaceutical industry, depending on how it was practiced, it left a wound and a void in terms of exploring Nature for more of its hidden treasures, molecules that perhaps, can teach and inspire us more about chemistry, biology and medicine than any other source of knowledge. A sign of hope, however, is on the horizon that can be translated into action, with total synthesis incorporating a biology and medicine component in its endeavours and, hopefully, re-establishment of the isolation of natural products as a priority that will surely provide inspiration, reigniting and fuelling new momentum to the field. The community of total synthesis practitioners with this philosophy is on the move, both in terms of numbers and new initiatives as evidenced from the collection of the articles in this issue of Natural Product Reports.
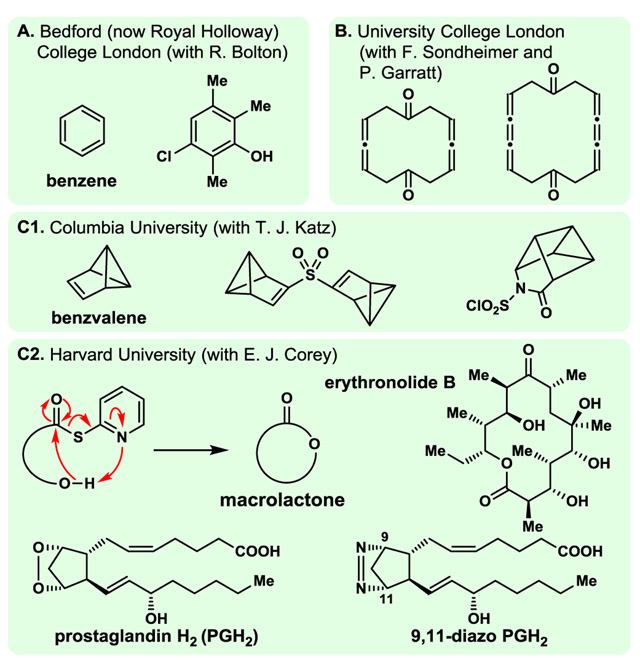
The design, synthesis and biological evaluation of natural product analogues and other molecules for biology and medicine in academia was rather sporadic and rare in the 1970s. One of us (KCN), as a postdoctoral fellow in the Corey group at Harvard University, had the opportunity to be given one of the first projects in the lab to synthesise an envisioned stable analogue (9,11-diazo-PGH2, Fig. 1C2) of the labile prostaglandin endoperoxide (PGH2, Fig. 1C2), the biosynthetic precursor of prostaglandins from arachidonic acid.14 With teammate Dr Yoshi Machida, we completed its synthesis from PGF2α in short order, and Professor Corey promptly dispatched a sample to Professor Bengt Samuelsson at the Karolinska Institute in Stockholm for biological evaluation. The results came back shortly thereafter, and for the first time I (KCN) felt the magical joy of biology intertwined with that of chemical synthesis. Our molecule turned out to be not only more stable but also more potent than its “parent” compound (PGH2) with regards to its ability to induce platelet aggregation and release of serotonin when added to human platelet-rich plasma.14 It also demonstrated higher potency than the naturally occurring PGG2 (15-hydroperoxy-PGH2)14 in inducing muscle contraction in an isolated rabbit aorta strip assay. As a result of this exciting and inspirational experience, the idea of organic synthesis merged with biology and medicine was embodied within our research programs in total synthesis from the very beginning of my (KCN) independent career at the University of Pennsylvania (1976). Before I (KCN) was granted the privilege to join the Corey group (1973), my research as an undergraduate student with Roger Bolton at Bedford (now Royal Holloway) College London (1966–1969), as a graduate student with Franz Sondheimer and Peter Garratt (1969–1972) at University College London,15 and as a postdoctoral fellow with Thomas J. Katz at Columbia University (1972–1973)8 involved the synthesis of theoretically interesting molecules such as those shown in Fig. 1A,B and C1. My work (KCN) in the Corey group in the 1970s included method development (e.g., double activation macrolactonisation, Fig. 1C2) and total synthesis of complex natural products and their analogues (e.g., erythronolide B, and 9,11-diazo prostaglandin H2, respectively, Fig. 1C2), an experience that anchored my interests within the field of total synthesis, and expansion of its periphery to include contributions to chemistry, biology and medicine.
Fig. 1.
Highlights of KCN’s studies in the UK and USA. Predoctoral (A), doctoral (B), and postdoctoral (C1 and C2). From benzene to 3-chloro-2,5,6-trimethylphenol at Bedford (now Royal Holloway) College London to cyclic bis-diallenes and bis-cumulenes at University College London, and from benzvalene and its derivatives at Columbia University, to erythronolide B, method development (double activation macrolactonisation), and analogues for biology and medicine (9,11-diazo-PGH2) at Harvard University.
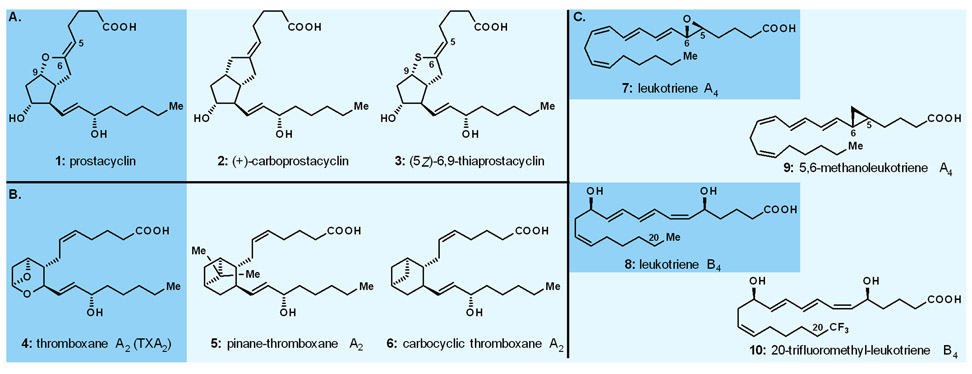
The opportunity to practice this paradigm in our laboratories at the University of Pennsylvania, where KCN started his independent career in 1976, came quickly with the arrival on the scene of prostacyclin (PGI2, 1, Fig. 2A), a new prostaglandin reported by Vane and his group in 1977.16 Processing potent antithrombotic and vasodilatory properties, prostacyclin ignited excitement in the biology, chemistry and medicine communities and despite its short lifetime at ambient temperature, became in great demand from biologists and clinicians due to its potential in treating cardiovascular disorders. In order to address the demand from the biologists and pharmacologists we devised a semisynthesis of prostacyclin from PGF2α and supplied it to the many of them who requested it for biological investigations. In parallel, we designed and synthesised a number of stable analogues of prostacyclin, including its methylene (6,9-CH2, carboprostacyclin, 2, Fig. 2A)17 and sulphur [(5Z)-6,9-thiaprostacyclin, 3, Fig. 2A]18 analogues, with the former proving stable, albeit somewhat less potent,17 and the latter demonstrating higher stability and equipotency to the natural product, both as antithrombotics and vasodilators.19
Fig. 2.
Highlights of our work on prostacyclin, thromboxanes, and leukotrienes. A. Prostacyclin and analogues; B. Thromboxane A2 and analogues; C. Leukotrienes A4 and B4 and analogues.
Inspired by the structure and important biological properties (i.e., vasoconstricting and thrombotic) of the highly labile thromboxane A2 (TXA2, 4, Fig. 2B),20 we also initiated a program directed towards the design, synthesis and biological evaluation of its carbocyclic analogues, pinane-thromboxane A221 (pinane TXA2, 5, Fig. 2B) and carbocyclic thromboxane A222 (CTA2, 6, Fig. 2B), the former exhibiting inhibitory activity against coronary artery contraction induced by stable PGH2 analogues, and the latter demonstrating significant increase in coronary perfusion pressure in cat arteries.23 Within the same program we also synthesised a number of designed analogues of the naturally occurring leukotrienes A4 (inflammation inhibitor, 7, Fig. 2C) and B4 (chemotactic agent, 8, Fig. 2C), namely, 5,6-cyclopropyl leukotriene A4 (9, Fig. 2C)24,25 and 20-trifluoromethyl leukotriene B4 (10, Fig. 2C),26 respectively, the former acting as an inhibitor of the enzymatic production of leukotriene B4.24,25
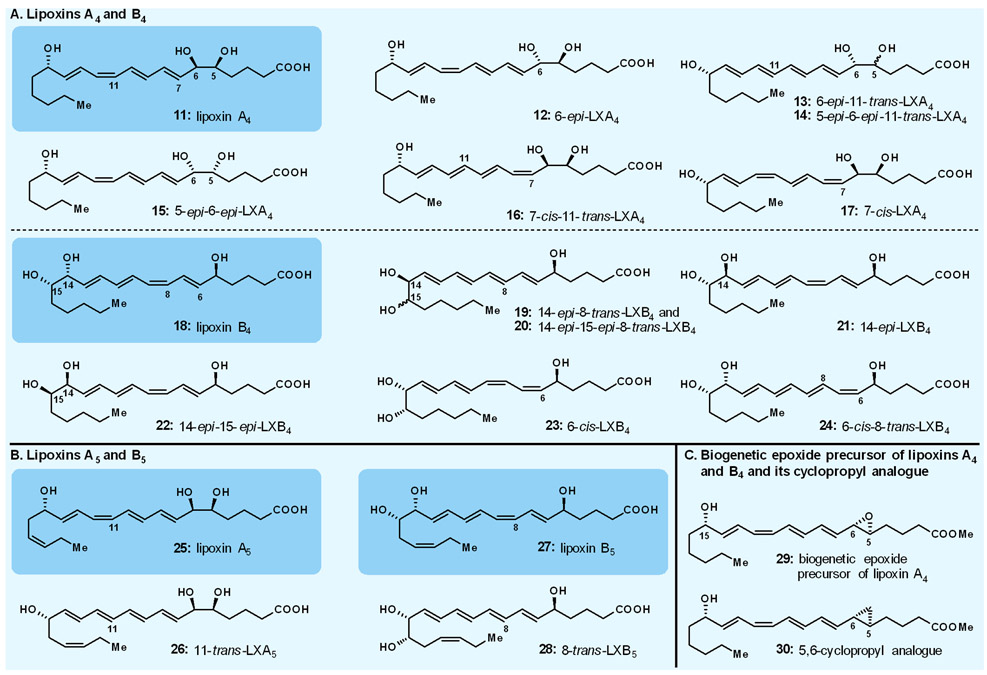
In the 1980s, a new family of eicosanoids was discovered in the Karolinska Institute by the Samuelsson group.27,28 Derived from metabolism of arachidonic acid as facilitated by lipoxygenase enzymes, and isolated in minute amounts, these new naturally occurring compounds were named lipoxins. Although suspected, their structures could not be fully determined or confirmed, and their biological properties and physiological role remained speculative until larger quantities of them became available from our laboratories. Our collaborative work on the lipoxins with Charles Serhan (one of Samuelsson’s co-workers on the lipoxin project at the Karolinska, and later an independent investigator at Harvard) was intended to make these compounds readily available for structural determination and biological investigation purposes. Thus, we synthesised all possible variations of the suspected lipoxins A4 and B4 structures (i.e., 11–17 and 18–24, respectively, Fig. 3A),29,30 comparison of which with the enzymatically produced compounds revealed their molecular structures (i.e., 11 for lipoxin A4, and 18 for lipoxin B4, Fig. 3A), including their absolute configurations. We also synthesised, through similar strategies, the corresponding lipoxins A5 (25, 26) and B5 (27, 28),31 suspected to be derived biosynthetically from eicosapentaenoic acid, and confirmed their structures (i.e, 25 and 27, respectively, Fig. 3B) by comparison with the enzymatically derived materials. In addition to confirming their molecular structures, rendering these scarce molecules readily available through chemical synthesis allowed their thorough biological evaluation and elucidated their action as modulators of human physiology and pathophysiology, thereby providing understanding of their role and opening opportunities for further research and drug discovery and development.32-43
Fig. 3.
Highlights of our work on lipoxins. A. Lipoxins A4 and B4; B. Lipoxins A5 and B5; C. Biogenetic epoxide precursor of lipoxins A4 and B4 and its cyclopropyl analogue.
Our expanded collaborative studies in the eicosanoid field resulted in several additional contributions that facilitated biological investigations. Thus, besides synthesising leukotriene B4 (8, Fig. 2C) and several of its analogues, we also synthesised various mono-hydroxyeicosatetraenoic acids (HETEs)44-48 and a number of their analogues as well as the 15-hydroxy 5,6-epoxytetraene hypothesised biosynthetic precursor (i.e., 29, Fig. 3C) of lipoxins A4 (11) and B4 (18, Fig. 3A).49 The latter precursor was shown to be enzymatically convertible to lipoxins A4 and B4 (11 and 18, Fig. 3A), thereby confirming its biochemical role.50 In addition, we synthesised the 5,6-methano counterpart (i.e., 30, Fig. 3C);49 this stable analogue was shown to inhibit the enzymatic conversion of the corresponding epoxide biogenetic precursor (i.e., 29, Fig. 3C) of lipoxins A4 and B4.25 These contributions of total synthesis to biology, and potentially medicine, are exemplary for the field in general. Many more are out there in the literature and, no doubt, myriad others may emerge in the future as rare natural products are unearthed and taken on by synthetic chemists as challenges and opportunities.
2. Miscellaneous Strategies for Natural Product Analogue Construction, Including Compound Libraries
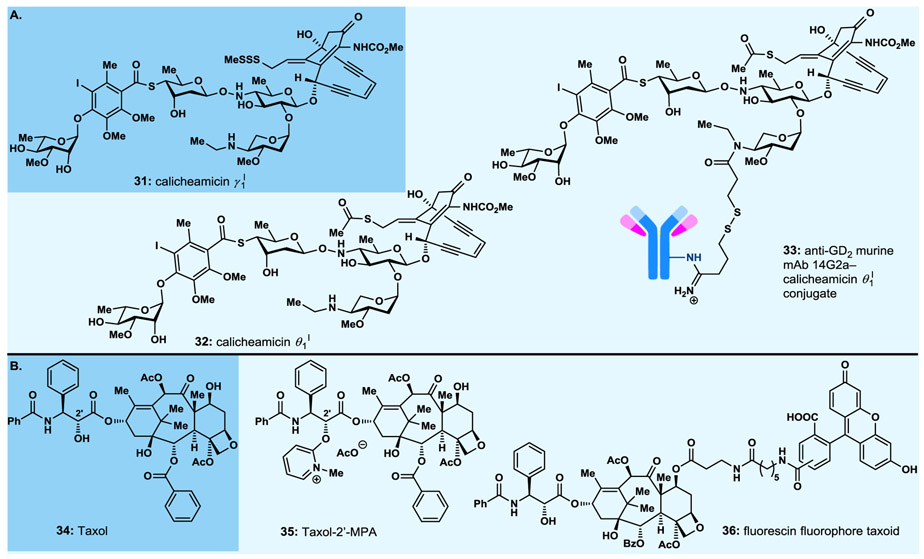
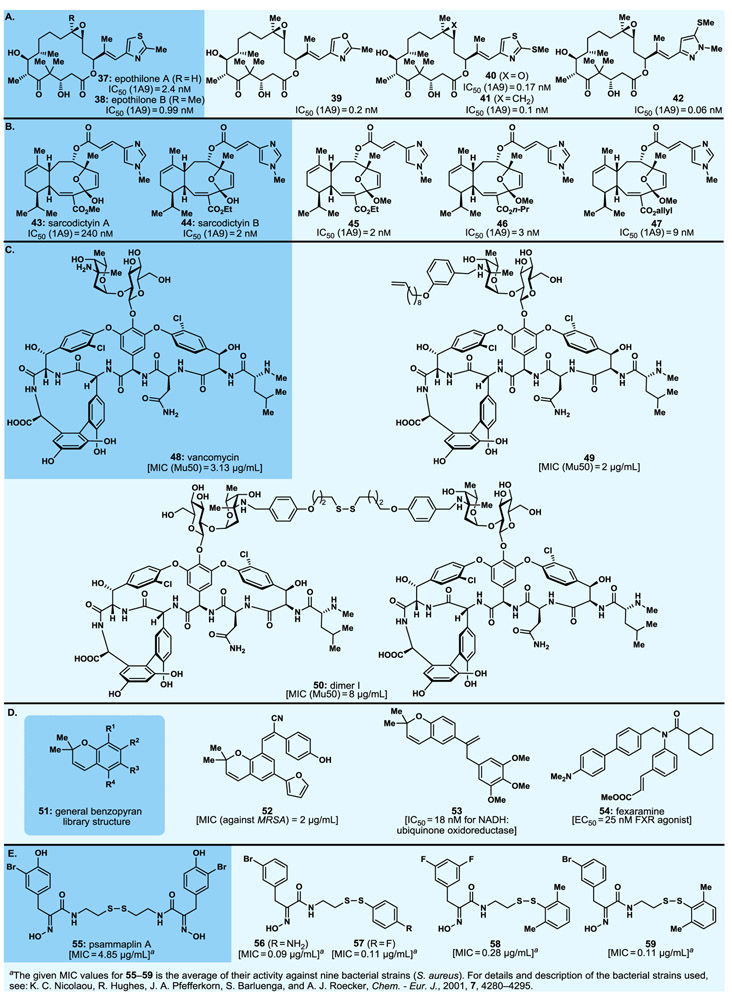
We would be remiss if we do not mention briefly a number of exemplary projects that led to a rich harvest of molecules for biology and medicine during the 1990s and early 2000s in our group at The Scripps Research Institute. These programs (e.g., calicheamicin γ1I,51 Taxol52-56) involved, in addition to specific analogue construction, the systematic design and synthesis, through either parallel solution or combinatorial solid phase methods, of analogue libraries of numerous natural products, including the antitumor agents epothilones A and B57-76 and sarcodictyins,77,78 the antibiotic of last resort vancomycin,79-85 the bioactive benzopyran-type natural products,86-91 and the antibiotic psammaplin A.92,93 Several lead compounds were discovered and optimised from these studies that employed specially designed and synthesised resins and MicroKans94 or NanoKans89 equipped with encoding systems readable by radiofrequency (chips) or light (laser). The Kans themselves consisted of a reactor vessel of differing size with an optically readable laser-etched grid or containing a radiofrequency tag for identification purposes and a suitable resin. This allowed for automated computer-guided pooling. Ranging from tens to thousands of compounds, these compound libraries were prepared on milligram scale per compound so as to allow full structural characterisation of each member as discrete and of high purity compounds. These efforts yielded a number of impressively bioactive compounds (more often than not, possessing higher potencies than their parent naturally occurring molecules) that emerged as powerful biological tools, with some becoming, or considered as, drug candidates. More details of these investigations can be found in the original publications given above and in our 2005 review.95,96
Among the most important molecules synthesised was the calicheamicin γ1I (31, Fig. 4A) analogue, calicheamicin θ1I (32, Fig. 4A),97 that proved more potent against a number of cancer cell lines than its parent compound, calicheamicin γ1I (31), and was attached, in collaboration with the Reisfeld group at Scripps, to an antibody targeting a specific cancer cell line to produce an antibody–drug conjugate (ADC).98 Antibody–drug conjugates, still a rather new paradigm for the treatment of cancer, consist of a cytotoxic payload attached through a molecular chain (the chemical linker) to an antibody. The entire assembly binds selectively to the surface receptor of the target cancer cell, is then internalized through endocytosis, and eventually releases its payload within the latter where is exerts its cytotoxic action, thus killing the cancer cell. The anti-GD2 murine mAb 14G2a–calicheamicin θ1I ADC (33, Fig. 4A) exhibited impressive growth suppression and dissemination of liver metastases in a syngeneic model of murine neuroblastoma.98 Published in 1998, this ADC served as a harbinger to Mylotarg, the first ADC to be approved by the FDA in 2000 as a targeted cancer therapy.99-102
Fig. 4.
Select highlights of designed natural product analogues synthesised (1990s–early 2000s). A. Calicheamicin γ1I, and calicheamicin θ1I and its antibody–drug conjugate; B. Taxol and analogues.
From the numerous Taxol (34, Fig. 4B) analogues we designed and synthesised, we highlight here the Taxol 2’-methyl pyridinium acetate (Taxol-2’-MPA, 35, Fig. 4B)53 and the fluorescein fluorophore taxoid (36, Fig. 4B),56 the former showing high antitumor potencies and higher than Taxol solubility for improved delivery purposes,103 the second serving as a biological tool employed for imaging purposes probing the destination of Taxol upon administration to cells, animal or human.
The epothilones [e.g., A (37) and B (38), Fig. 5A] provided a long-lasting platform for synthetic and biological investigations since the mid-1990s. Investigations within this family of naturally occurring compounds led to not only the syntheses of numerous designed analogues (e.g., 39−42, Fig. 5A) but also to the discovery of important structure–activity relationships (SARs) within this class of compounds57 (more about epothilones below, see section 3.11).
Fig. 5.
Select highlights of designed natural product analogues synthesised (1990s–early 2000s). A. Epothilones A and B and analogues; B. Sarcodictyins A and B and analogues; C. Vancomycin and analogues; D. Benzopyran natural products and analogues; E. Psammaplin A and analogues.
Similar studies within the sarcodictyin (e.g., 43, 44, Fig. 5B)77,78,104,105 and eleutherobin104-106 family of marine natural products employing solution and solid phase synthetic strategies led to a plethora of analogues, from which 45, 46 and 47 were identified among the most potent against a number of cancer cell lines as indicated in Fig. 5B.78
Our vancomycin (48, Fig. 5C) total synthesis campaign79-82 included combinatorial synthesis of monomeric and dimeric vancomycin analogues84,85 made possible by several synthetic strategies. These included the so-called “target accelerated” combinatorial synthesis83 in which a library of building blocks was allowed to react in the presence of the vancomycin binding target domain (l-Lys-d-Ala-d-Ala), with the most “potent” combinations of building blocks expected to first assemble onto the l-Lys-d-Ala-d-Ala fragment and then couple through covalent bond formation as the favoured products. These studies resulted in numerous potent vancomycin analogues such as the ones displayed, together with their impressive antibacterial MIC values, in Fig. 5C (e.g., 49 and 50).
Comprising a large number of natural products (general structure 51, Fig. 5D) and exhibiting an impressive range of biological activities, the benzopyran family of compounds attracted our attention as a worthy class of molecules for chemical synthesis and biological investigations. Thus, an analogue library of over 10000 compounds89 was designed and synthesised, in single digit milligram scale for structural elucidation and biological screening, through combinatorial chemistry using MacroKans94 and NanoKans89 with radiofrequency or light-read encoding and robotic manipulation. From this large library, and through several collaborations with biologists, we identified a number of lead compounds whose optimisation led to more potent agents than their parent compounds. Among them antibacterial agent 52 (Fig. 5D, MIC=2 μM against MRSA),91 ubiquinone oxidoreductase inhibitor 53 (Fig. 5D, IC50=18 nM),107 and fexaramine (54, Fig. 5D), a farnesyl X receptor (FXR) agonist (EC50=25 nM),108 the latter facilitating structural biology studies as a crystalline FXR-ligand complex revealing the structure of the receptor and the binding side of the ligand.109 These efforts provided important biological tools that served well biology, and possibly, medicine upon pending further optimisation and development.
A designed combinatorial analogue library of the naturally occurring antibiotic psammaplin A (55, Fig. 5E) was synthesised and screened for antibacterial activity, leading to the eventual establishment of SARs and the discovery of highly potent antibacterial agents, examples of which are depicted in Fig. 5E (i.e., 56: MIC=0.09 μg/mL; 57: MIC=0.11 μg/mL; 58: MIC=0.28 μg/mL, and 59: MIC=0.11 μg/mL).92,93
Requiring the development of sophisticated solution and/or solid phase technologies, and often coupled with robotic systems, these rationally designed combinatorial/optimisation studies collectively demonstrated the power of these automated strategies for making valuable molecules for biology and medicine as evidenced from numerous lead and optimised compounds discovered. Robotics in organic synthesis are currently on the move and are expected to become a popular means to replace human hands in the laboratory.110-114
3. Selected Total Synthesis Endeavours
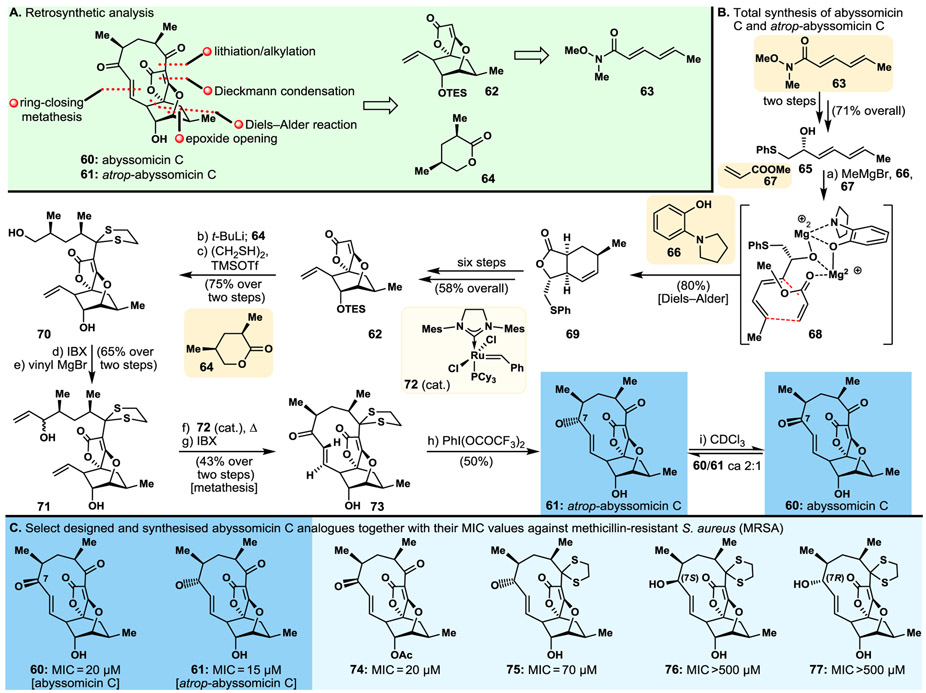
3.1. Abyssomycin and atrop-Abyssomycin
The intriguing molecular structure and potent antibacterial activities reported for abyssomicin C (60, Fig. 6) in 2004115,116 prompted our interest as a synthetic challenge. Our total synthesis117,118 of this fascinating molecule, and its congeners atrop-abyssomicin C (61, Fig. 6) and abyssomicin D (structure not shown),119 turned out to be even more intriguing than the initial discovery of abyssomicin C itself115,116 in that it revealed the existence of two atropisomers of the originally reported structure. Separated chromatographically, these distinct isomers exhibited different NMR spectral data and were distinguished and defined by X-ray crystallographic analysis.117,118 Furthermore, atrop-abyssomicin (61, Fig. 6B) exhibited even higher antibacterial potencies than those of the originally reported abyssomicin C (60, Fig. 6B). To their credit, the discoverers of abyssomicin C made, later on, the discovery of atrop-abyssomicin C from the same producing species, marine-derived actinomycete strain Verrucosispora.120 Our synthetic strategy towards the abyssomicins as derived from the retrosynthetic analysis depicted in Fig. 6A, defined building blocks 62, to be obtained from Weinreb amide 63,121 and the readily available lactone 64, in their enantiopure forms as the key starting materials for the projected total synthesis. The total synthesis of abyssomycin C (60) and atrop-abyssomycin C (61) proceeded as summarised in Fig. 6B. Thus, Weinreb amide 63 was converted to phenylthiol hydroxydiene 65 by standard chemistry (i.e., displacement with PhSCH2Li and asymmetric reduction, 71% yield over two steps). The latter was reacted with methyl acrylate (67) in a controlled Diels–Alder reaction in the presence of MeMgBr and 2-(pyrrolidine-1-yl) phenol (66, acting as an auxiliary ligand) to afford desired product 69, through transient assembly 68, in 80% yield. This intermediate was then converted to tricyclic system 62 through a six-step sequence and 58% overall yield as indicated in Fig. 6B. Exposure of lactone 64 to t-BuLi followed by reaction of the resulting anion with tricyclic lactone 62 furnished the corresponding ketone, whose protection and further elaboration afforded bis-terminal olefin 71, via intermediate 70, as shown in Fig. 6B. Ring closure metathesis within 71 under the influence of precatalyst 72 followed by oxidation of the resulting cyclic alcohol furnished protected abyssomicin precursor 73. Both abyssomicin C (60) and its atropoisomer, atrop-abyssomicin C (61), were generated upon removal of the dithiane protecting group [PhI(OCOCF3)2] and equilibration of the two products under acidic conditions, as shown in Fig. 6B.
Fig. 6.
Highlights of the abyssomicin C project. A. Retrosynthetic analysis; B. Total synthesis of abyssomicin C (60) and atrop-abyssomicin C (61); C. Select designed and synthesised abyssomicin C analogues and their potencies against methicillin-resistant S. aureus (MRSA).
Employing our developed synthetic strategies and tactics,119,122 we synthesised a series of designed analogues of abyssomicins118 60 and 61, including those exhibited in Fig. 6C {i.e., 74 (acetylated abyssomicin C); 75 (dithiane protected atrop-abyssomicin C); 76 and 77 [(7S)- and (7R)-epimers of hydroxy dithiane abyssomicin C, respectively]}. Biological evaluation of these compounds revealed slight potency superiority (against MSRA, see Fig. 6C) of atrop-abyssomicin C (61, MIC=15 μm) over abyssomicin (60, MIC=20 μm), loss of some activity upon acetylation of the hydroxyl group of atrop-abyssomicin (analogue 74), and significant loss of potency upon reduction of the enone carbonyl group of the molecule (analogues 76, >500 μm; and 77, MIC >500 μm) as depicted in Fig. 6C. Finally, it is worth noting that the type of isomerism detected in abyssomicins C (60) and atrop-abyssomicin C (61) is rare and speaks volumes of the power and value of total synthesis for structural elucidations, even in these days of supreme analytical techniques and instrumentation.
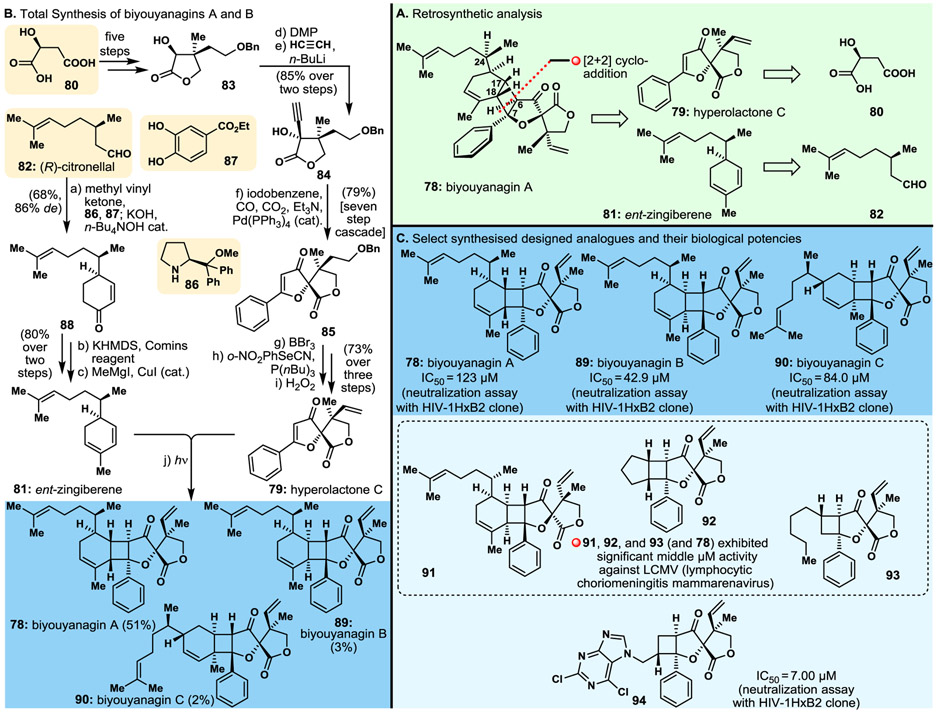
3.2. Biyouyanagins A and B
Isolated from a Hypericum genus (Clusiaceae) (i.e., H. Chinense), biyouyanagin A (78, Fig. 7A) exhibited interesting biological properties, including anti-HIV activity and inhibition of lipopolysaccharide-induced cytokine production, implying potential treatments of AIDS and inflammation, respectively.123 Attracted by its relevance to biology and medicine and its novel molecular structure, we initiated a program directed towards its total synthesis, despite the ambiguity of its C24-stereochemical configuration. Inspired by the proposed hypothesis for the biosynthesis of biyouyanagin A (78), we devised a synthetic strategy involving a photo-induced [2+2] cycloaddition to fuse the two advanced building blocks 81 (naturally occurring zingiberene or its antipode, ent-zingiberene) and 79 (naturally occurring hyperolactone C), generated by retrosynthetic rupture of the cyclobutane ring and traced back to citronellal [82, either (S) or (R)], and (S)-malic acid (80), respectively, as shown retrosynthetically in Fig. 7A. At this point we realised, using manual molecular modelling, that the [2+2] cycloaddition required to cast the reported structure was disfavoured on steric congestion grounds, leading to doubts as to the correctness of the configurations of the stereocentres around the cyclobutane moiety of the molecule (i.e., 78). Nevertheless, we proceeded with implementation of the devised strategy, leaving the last word for the final step to the experiment. Fig. 7B summarises the total synthesis of biyouyanagin A (78), and as it turned out, biyouyanagin B (89) and biyouyanagin C (90, see Fig. 7B for structures). Thus, (S)-malic acid (80) was elaborated to lactone 83 through a five-step sequence, and the latter was converted to acetylenic hydroxy lactone 84 by oxidation followed by addition of the acetylene unit, as shown in Fig. 7B. Hydroxy acetylene lactone 84 was then coached, via an aesthetically pleasing and remarkably efficient cascade of reactions initiated and sustained with Pd(PPh3)4 cat. in the presence of CO, CO2 and Et3N, to afford the desired bicyclic precursor 85, whose conversion to the targeted hyperolactone C (79) took only three steps, as summarised in Fig. 7B. The other required fragment, ent-zingiberene (81), was constructed from (R)-citronellal (82) as depicted in Fig. 7B. Thus, reaction of 82 with methyl vinyl ketone in the presence of proline-derived catalyst 86, co-catalyst 87, KOH, and n-Bu4NOH cat. furnished cyclohexenone 88 (via an enamine-mediated reaction of citronellal with methyl vinyl ketone). The latter was finally transformed to the desired ent-zingiberene (81) through a two-step process, as depicted in Fig. 7B.
Fig. 7.
Highlights of the biyouyanagins A and B project. A. Retrosynthetic analysis; B. Total synthesis of biyouyanagins A (78), B (89) and C (90); C. Molecular structures and cytotoxicity potencies of select designed and synthesised biyouyanagin analogues.
The much anticipated [2+2] cycloaddition of building blocks 79 and 81 induced by UV light proceeded as expected, and after careful isolation procedures yielded not only the initially targeted biyouyanagin A (78, 51% yield, Fig. 7B) but also, in lesser amounts, what turned out to be biyouyanagin B (89, 3% yield, Fig. 7B),124 isolated from the same Hypericum genus and reported as a natural product in 2009,125 four years after the report of its sibling biyouyanagin A.123 A third compound named by us as biyouyanagin C (90, Fig. 7B) was isolated from the reaction mixture (in 2% yield) and fully characterised.124 Formed by a [2+2] photo-cycloaddition of hyperolactone (79) to the endocyclic trisubstituted olefinic bond of ent-zingiberene (81), it will not be surprising if this synthesised biyouyanagin isomer (i.e., 90) is found in Nature one day, especially if the biosynthesis of these class of molecules involves a photo-induced reaction without the aid of enzymes.
It should be noted that the final synthetic strategies summarised in Fig. 7B do not tell the entire story of the biyouyanagins A and B chase, which was much more adventurous and intriguing in its entirety. It started with biyouyanagin A (78), the first shrouded in a structural mystery member of the class to be reported,123 and then included biyouyanagin B (89)125 that turned out to be a second structural puzzle, even though we must have made it in the laboratory before it was discovered in Nature, but never isolated it due to the minimal amounts formed. It was only after we exhausted all possible combinations of zingiberene/hyperolactone structural isomers in our final photo-induced fusion, and because of an astute observation regarding the 1H NMR signals of the various biyouyanagin stereoisomers around the cyclobutane ring, that we went back to our original synthesis of biyouyanagin A to search for, in a last and desperate attempt, and to possibly isolate the true structure of biyouyanagin B (89). And we did!
Thrilling as it was, the biyouyanagin campaign was not complete without the “dessert” of discovering molecules for biology and medicine. And so, a large number of biyouyanagin-type molecules were synthesised and, collaboratively, tested for their ability to neutralise the HI Virus, and for their activity against the LCMV (lymphocytic choriomeningitis mammarenavirus).126 A select number of biyouyanagins [A (78), B (89), and C (90)] and their analogues (e.g., 91, 92, 93, 94), together with their relevant biological activities, are shown in Fig. 7C. Most striking among them is analogue 94 that exhibited superior activity against HIV (HIV-1HxB1 clone) than any of the many compounds we synthesised for biological screening, including the two naturally occurring, biyouyanagins A (78) and B (89) as shown in Fig. 7C.126,127
3.3. Epidithio- and Bis(methylthio)diketopiperazines
The epidithio- and bis(methylthio)diketopiperazines are a diverse family of naturally occurring molecules endowed with a variety of disease relevant biological properties, including antiviral, antibacterial, antimalarial, and antitumor activities.128 Members of this family of molecules include epicoccin G (95, Fig. 8A), 8,8‘-epi-ent-rostratin B (96, the epimer of the enantiomer of the naturally occurring rostratin, Fig. 8A),128 gliotoxin (117, Fig. 8D),128 gliotoxin G (118, Fig. 8D),128 emethallicin E (119, Fig. 8D)128 and haematocin (120, Fig. 8D).128 These natural products, and others like them, presented, in addition to assembling their often complex molecular scaffolds, the special challenge of installing the epidisulphide and bis(methylthio) structural motifs within their diketopiperazine scaffolds. Their scarcity from natural sources, their important biological properties, and the methodology void for installing their sulphur structural motifs served as the motivation for us to initiate a research program that combined strategy design, method development, and design, synthesis and biological evaluation of analogues thereof.
Fig. 8.
Highlights of our epidithio- and bis(methylthio)diketopiperazines studies. A. Retrosynthetic analysis of epicoccin G and 8,8'-epi-ent-rostratin B; B. Discovery and development of a new sulphenylation method of diketopiperazines; C. Total synthesis of epicoccin G and 8,8'-epi-ent-rostratin B; D. Other naturally occurring totally synthesised epidithio, tetrathio and bis(methylthio)diketopiperazines; E. Select synthesised analogues of epidithio- and bis(methylthio)diketopiperazines.
Fig. 8 summarises our investigations in this field starting with the retrosynthetic analysis of epicoccin G (95) and 8,8'-epi-ent-rostratin B (96, Fig. 8A) defining N-Boc-l-tyrosine-derived building block 98 as our starting material, via advanced intermediate pentacyclic diketopiperazine 97, as indicated in Fig. 8A. As a prerequisite to our total synthesis, we first developed a new practical and efficient method for installing the epidithio- and bis(methylthio) structural motifs into their diketopiperazine frameworks (Fig. 8B). Thus, reaction of sulphur (S8, 99) with NaHMDS led mainly, via 100, to tetrasulphide 101, a reagent that reacted with diketopiperazine 102 in the presence of NaHMDS (enolate formation, 102→103), via 104, to afford tetrasulphide 105, which was diverted selectively to produce either bis(methylthio) diketopiperazine 107 (NaBH4; then MeI, 72% overall yield for the three steps from 101+102) or epidisulphide 108 (NaBH4; then NH4Cl; then KI3, 69% overall yield over the four steps from 102) as depicted in Fig. 8B.
Armed with this practical and versatile sulphenylation method, we completed the total syntheses of both epicoccin G (95) and 8,8'-epi-ent-rostratin B (96) starting with building block 98, the latter readily available from N-Boc-l-tyrosine, through intermediates 113 and 2,2’-epi-113, respectively, obtained as a separable mixture, as summarised in Fig. 8C. Intermediate 113 was diverted towards epicocin G (95), and 2,2’-epi-113 was funnelled through a different pathway towards 8,8’-epi-ent-rostratin B (95), as depicted in Fig. 8C. Thus, building block 98 was converted to hydroxy methyl ester amine TFA salt 109 (five steps), and separately, to hydroxy acid Boc-protected amine 110 (five steps). Stepwise coupling through amide bond formations gave first intermediate amide 111 (intermolecular), and finally diketopiperazine 112 (intramolecular). The latter was elaborated, in two steps, to tetraene diketopiperazine scaffold 97, whose sulphenylation and reduction/oxidation of the so-formed tetrasulphide furnished separable intermediates 113 (α C–S bonds) and 8,8’-epi-113 (β C–S bonds). Processing this mixture to the next step towards epicoccin G (reduction with NaBH4, followed by methylation of the resulting dithiol with MeI) furnished a mixture of the corresponding, chromatographically separable, bis(methylthio) products 114 and 2,2’-epi-114. Elaboration of the latter to the targeted natural product, epicoccin G (95), via intermediate bis-endoperoxide 115, formed by a Kornblum–DeLaMare singlet oxygen addition (O2, TPP; then DBU),129,130 proceeded as summarised in Fig. 8C. A similar photo-induced oxygenation of pure 2,2’-epi-113 resulted in the formation of the transient bis-endoperoxide 116 exposure of which to CuH(PPh3)6 followed by KI3 addition led to 8,8’-epi-ent-rostratin B (96), as shown in Fig. 8C.
The developed synthetic strategies and technologies were employed to synthesise the naturally occurring epidithio diketopiperazine natural products gliotoxin (117), gliotoxin G (118), bis(methylthio) diketopiperazine emethallicin E (119), and epitetrathio diketopiperazine hematocin (120, see Fig. 8D for structures). A number of designed analogues were also synthesised, including 121, 2,2’-epi-121, 122 and 123, whose structures and selected biological activities are shown in Fig. 8E. It is noteworthy that some of these analogues demonstrated superior bioactivities to those exhibited by epicoccin G, and pirodavir [EC50 (Poliovirus) = 1.58 μM (neutral red assay)],128 the standard anti-poliovirus drug, used in these studies as a control.
Blending total synthesis with method development and analogue design, synthesis and biological evaluation, this strategy proved delightfully fruitful in that it resulted in a) the total synthesis of a multi-member family of natural products; b) the development of a practical and useful sulphenylation procedure; and c) the discovery of potent lead compounds for further studies and optimisation directed towards potential treatments of the poliovirus and malaria diseases.
3.4. Platensimycin
The report of the discovery of platensimycin (124, Fig. 9A and B) by Merck Sharp & Dohme scientists in 2006,131 precipitated intense interest among biologists and chemists alike, not the least because of its potent antibacterial activities against drug resistant pathogens and its novel mechanism of action (i.e. selective blockage of bacterial fatty acid biosynthesis through inhibition of the elongation-condensing enzyme FabF). For those reasons, and because of the novelty of platensimycin’s molecular structure, we were prompted into a campaign to achieve its total synthesis and exploit the developed synthetic strategies and methods to construct novel analogues for biological evaluation studies. Our synthetic efforts towards this important target molecule yielded first a total synthesis of the racemic mixture, then an optimised asymmetric synthesis via two different approaches, and, finally, the synthesis of a number of its naturally occurring congeners (i.e., platencin,132,133 platensimycins B1, B2 and B3)134 and a series of designed analogues aiming to establish useful structure–activity relationships (SARs) within the resulting family of natural and designed platensimycins and related congeners.
Fig. 9.
Highlights of the platensimycin project. A. Retrosynthetic analysis; B. Total synthesis of platensimycin; C. Totally synthesised related natural products; D. Select designed and synthesised platensimycin analogues and their potencies against certain bacterial strains.
Fig. 9A summarises our two asymmetric synthetic approaches to platensimycin (124) in retrosynthetic format. Thus, disconnection of the molecule’s amide bond generated advanced aniline derivative 125 and fragment 127 (platensic acid,135 naturally occurring). The former was traced back to the readily available 2-nitroresorcinol (126), while the latter was unfolded to bis-spiroenone aldehyde 128. Serving as a common intermediate, enone aldehyde 128 was retro-diverted to the two planned asymmetric approaches, one (a) employing a catalytic asymmetric spirocyclisation/isomerisation to start with the easily obtained ethoxy enone 129, and the other (b) traced back to the readily available peptide fragment 130, and following a chiral auxiliary method to achieve selective alkylation followed by a downstream dearomatisation reaction, as summarised in Fig. 9A. Fig. 9B summarises the two approaches for the enantioselective synthesis of spiroaldehyde 128 and its conversion to platensimycin (124) via the naturally occurring platensic acid (127). Thus, enone 129 (top right) was alkylated with TBS-ether bromide 131 and the resulting product was converted to spirosystem 132, from which bis-enone 133 was generated in two simple steps. Upon considerable optimisation, it was found that substrate 133 could be converted, efficiently and enantioselectively, to the targeted spirocyclic aldehyde 128 through asymmetric cycloisomerisation as induced by [Rh(S)-BINAP]2SbF6 catalyst136-138 (86% yield, >99% ee). The same intermediate was obtained from building block 130 equipped with the (S,S)-pseudoephedrine moiety, through the sequence shown in Fig. 9B (left). Thus, alkylation of 130 with benzyl bromide 134 furnished derivative 135, in good yield and diastereomeric enrichment (87% yield, 85% de), from which the desired fragment 136 was obtained upon cleavage of the auxiliary and further elaboration (81% yield, four steps) as shown in Fig. 9B. The latter was converted to bis-enone 137 by PhI(OAc)2-induced dearomatisation (68% yield), and thence to the desired bis-enone aldehyde 128 under acidic conditions. The so-obtained aldehyde 128 was transformed to tricyclic compound 138 through the intermediacy of the corresponding ketyl radical (SmI2), and thence to the platencin scaffold 139 (46% overall yield) upon exposure to TfOH, as shown in Fig. 9B. Methylation of the latter followed by a second alkylation via enolate formation (KOt-Bu) and a 1,4-conjugate addition to tert-butyl acrylate (140) furnished, after acid-induced cycloetherification and tert-butyl ester cleavage, platensic acid (127), in 84% yield over the three steps as shown in Fig. 9B. The other required building block, aniline derivative 125, was prepared from 2-nitroresorcinol (126) through a five-step standard sequence and coupled with platensic acid (127) affording, upon methyl ester hydrolysis and MOM removal, platensimycin (124) in good overall yield, as depicted in Fig. 9B.
The synthetic strategies and methods developed in this program were applied to the total synthesis of several naturally occurring congeners of platensimycin, namely platensimycins B1 (141), B2 (142), and B3 (143), platencin (144, see structures in Fig. 9C), platensic acid (127, Fig. 9B), methyl platensinoate (methyl ester of 127), platensimide A, homoplatensimide A, and homoplatensimide methyl esters (structures not shown).139
The same synthetic strategies and methods also provided a platform from which numerous designed analogues of platensimycin were synthesised.140,141 Biological evaluation of these analogues revealed a number of potent compounds with comparable potencies to those of the natural product against methicillin- and vancomycin-resistant bacterial strains. Clear and valuable structure–activity relationships (SARs) consistent with the X-ray crystallographic analysis picture of the binding of the platensimycin molecule to its receptor131 emerged from these studies140,141 that could guide further investigations in the field if they were needed. Among the most potent of the many synthesised platensymicin analogues were carbaplatensimycin (145),141 adamantaplatensimycin (146)140 and analogue 147142 (for structures and potencies, see Fig. 9D). This collaborative program with biologists enriched our knowledge of the binding mode of the platensimycin molecule to its biological target, and provided strong guidance as to the design of analogues with high probability of possessing potent antibacterial properties as consistently reflected in the structures of not only the analogues synthesised but also those of the naturally occurring platensimycin congeners mentioned above (see Fig. 9).
3.5. Antibiotic CJ-16,264
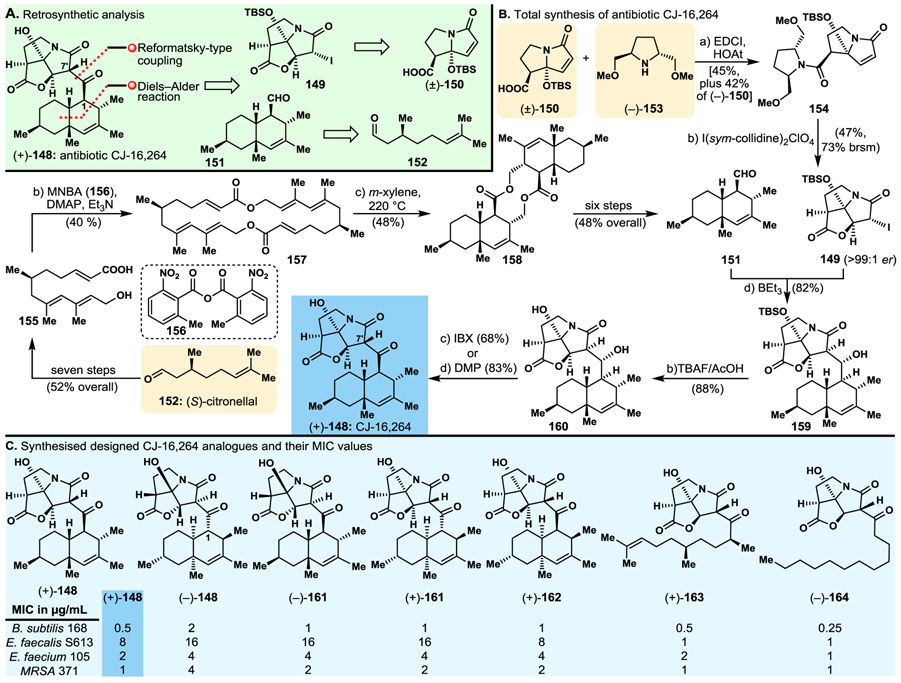
Decorated with two distinct molecular domains, its lipophilic terpenoid-like fused bicyclic moiety and its heterocyclic structural motif, and possessing potent antibacterial properties, antibiotic CJ-16,264 [(+)-148, Fig. 10] attracted our attention as a target of opportunity for total synthesis with a blend of biology, and perhaps medicine, through analogue design, synthesis and biological evaluation. Our retrosynthetic analysis (Fig. 10A) demanded bicyclic iodo lactam 149 and bicyclic aldehyde 151 as the prerequisite building blocks for the proposed synthetic strategy, making their construction the first priority for our drive towards the targeted molecule. Bicyclic aldehyde 151 and tricyclic iodide 149 were traced back to (S)-citronellal (152) and known racemic bicyclic lactam carboxylic acid (±)-150,143,144 respectively, as indicated retrosynthetically in Fig. 10A. Tricyclic iodide 149 required an especially sophisticated resolution, albeit short, synthetic approach for its enantioselective construction. Thus, and as indicated in Fig. 10B, carboxylic acid (+)-150 was preferentially trapped as an amide through kinetic resolution of its racemic form achieved by reaction with readily available amine (−)-153 to afford lactam 154 [originating from the (+)-enantiomer of 150] in good yield [leaving behind (−)-150]. Cyclisation of diastereoisomer 154 as facilitated by I(sym-collidine)2CIO4 then furnished the desired fragment (149, 47% yield, 73% brsm) as depicted in Fig. 10B. The construction of terpenoid aldehyde fragment 151 started from (S)-citronellal (152) and proceeded through intermediate hydroxy acid 155 and macrodiolide 157, as summarised in Fig. 10B. Thermally induced double intramolecular Diels–Alder reaction within 157 afforded pentacyclic dimeric product 158 (88% yield). Double cleavage of the latter diolide, and standard elaboration of the resulting monomeric hydroxy acid gave, in good overall yield, the desired terpenoid aldehyde fragment, 151, as depicted in Fig. 10B.
Fig. 10.
Highlights of the antibiotic CJ-16,264 project. A. Retrosynthetic analysis; B. Total synthesis of antibiotic CJ-16,264; C. Selected isomers and analogues of antibiotic CJ-16,264 and their potencies against certain bacterial strains.
Antibiotic CJ-16,264 [(+)-148] was then assembled from building blocks 149 and 151 through a short path involving enolate generation from iodo lactam 149 (BEt3) and addition of the resulting species to the aldehyde partner [i.e., 151] to furnish coupling product alcohol 159, whose desilylation, followed by oxidation of the so-formed tertiary hydroxyl secondary alcohol (IBX or DMP), led to the targeted natural product antibiotic CJ-16,264 [(+)-148] in good overall yield as depicted in Fig. 10B.
The practical and efficient synthetic strategies and technologies developed resulted in the total synthesis of six diastereoisomers [(+)-148, (−)-148, (+)-161, (−)-161, (+)-162 (Fig. 10C), and 1-epi-(−)-148 (structure not shown)] of the originally reported, apparently wrong, structure of the CJ-16,264 molecule, from which (+)-148 was proven to be the natural product’s true structure. In addition, two collections of CJ-16,264 analogues were synthesised, one of racemic mixtures and the other of enantiopure compounds. Biological evaluation of these substances led to important structure–activity relationships (SARs) and the identification of several potent antibacterial agents, some rivalling the activities of the natural product [(+)-148], despite their significantly simpler molecular structures [e.g., (+)-163, (−)-164, Fig. 10C]. It was also interesting to note that some of the synthesised racemic CJ-16,264 analogues (structures not shown) and enantiopure simplified analogues [e.g., 164], CJ-16,264 diastereoisomers (−)-161, (+)-161 and (+)-162, and the enantiomerically pure antipode [(−)-148] of the natural product exhibited comparable potencies (with the exception of some deviations with certain bacterial strains) to those of the natural product [(+)-148] (see Fig. 10C).
The CJ-16,264 total synthesis campaign demonstrated the value of total synthesis endeavours for discoveries and innovations, specifically in a) strategy design [i.e., the intramolecular Diels–Alder collapse of macrocyclic dimer 157 to two cleavable precursor units and their elaboration to the terpenoid aldehyde building block 151]; b) method development [i.e., the efficient kinetic resolution of racemic 150 through amide formation with the enantiopure amine (−)-153]; c) the revision of the originally assigned structure of CJ-16,264; d) discovery of significantly simpler, and yet equipotent to the natural product, antibacterial agents; and e) the derivation of important and useful SARs within the CJ-16,264 family of natural and designed molecules.
3.6. Brevetoxin B and Maitotoxin
The ladder-like marine derived neurotoxins elicited considerable interest from chemists and biologists alike since brevetoxin B (165, Fig. 11A) appeared on the scene in 1981.145 Since then, numerous new members were added to this class of marine natural products, with maitotoxin (168, Fig. 11B) being the most impressive with regards to a) molecular complexity and size (the largest non-polymeric natural product discovered as yet); b) potency as a neurotoxin (the most potent, non-proteinoic, neurotoxin known to date); and c) synthetic challenge. Synthetic efforts towards latter-like marine neurotoxins in our laboratories resulted in the total syntheses of hemibrevetoxin (1993, 175, Fig. 11C),146 brevetoxin B (1995, 165, Fig. 11A)147,148 and brevetoxin A (1998, 176, Fig. 11C).149-153 Our forays towards maitotoxin (168, Fig. 11B) led to confirmation of its originally assigned structure,154 after it was challenged,155 the total synthesis of several large domains of the molecule,156-161 and a number of new synthetic methods162,163 for the construction of its varying in size cyclic ether structural motifs, including the rings P and B’ that remain to be constructed (designated in red, see structure 168, Fig. 11B). Unfortunately, funding restrictions did not allow the completion of its total synthesis, although, in principle, it became feasible given the fact that all other 30 rings of the molecule were constructed as fused large fragments with their correct stereochemical configurations, and the methods to couple them and cast the remaining two rings were developed and tested successfully in our total syntheses of brevetoxins B (165)147,148 and A (176).149-153
Fig. 11.
Molecular structures and biological properties of select ladder-like marine neurotoxins and analogues thereof.
The brevetoxin B and maitotoxin campaigns gave us the opportunity to explore, collaboratively, their biological properties, propose binding modes of these fascinating neurotoxins to their receptors, and study structure–activity relationships of large segments of their molecular structures. Fig. 11 shows, in addition to the molecular structures of the naturally occurring ladder-like marine neurotoxins hemibrevetoxin (175), brevetoxins A (176) and B (165), and maitotoxin (168), the structures of a number of analogues and segments (166, 167, for brevetoxin B, Fig. 11A, and 169–174 for maitotoxin, Fig. 11B) of these molecules that we synthesised for biological investigations. Thus, synthesised truncated AFGHIJK brevetoxin B (167)164 (missing the BCDE domain of the parent molecule) and desoxycarbonyl brevetoxin B (166)165 (featuring a cyclic ether ring A, instead of the lactone ring A of the natural product) were investigated in collaborative efforts with biologists. Serving as a biological tool, the truncated version (i.e., 167) of brevetoxin B (165) revealed significant loss of binding affinity, as compared to brevetoxin B, to its receptor (IC50 = 15.02 nm) (i.e., site 5 of the voltage-gated sodium channel), as well as a modified action behaviour with regards to its biological role.165 The desoxybrevetoxin analogue 166 exhibited significantly weaker binding within the same receptor (IC50 = 36.5 nm) and lost its potency as a prolonging agent of the channel’s open time duration.165 These observations provided support for the previously proposed hypothesis of the “common pharmacophore” for brevetoxins B (165) and A (176), and their bioactive congeners, that envisioned it to be a “cigar shaped” structural motif of approximately 30 Å length, binding to its receptor site mainly through hydrophobic and non-polar solvation forces, with H-bonding of the ring A carbonyl group playing a possible role.165,166 Furthermore, these biological studies led to a better understanding of the role of the “cigar shape” of these molecules in their expression of their biological activities and provided further information on their precise mode and location of binding to their receptor.165,166
The maitotoxin project also yielded opportunities for biological investigations of numerous, more or less, complex fragments prepared during the total synthesis campaign, and which were subjected by our biology collaborators to assays testing their ability to inhibit maitotoxin-induced 45Ca2+ influx in rat C6 glioma cells.161 Fig. 11B displays a number of maitotoxin fragments with interesting capabilities to inhibit maitotoxin-elicited 45Ca2+ influx in rat C6 glioma cells. Thus, fragment 169 (QRSTUVWXYZA’) exhibited relatively strong inhibitory activity (IC50=3.2 μM), while its bis-benzyl ether (170, rings Q and W) exhibited only modest activity (IC50 ≈ 30 μM). Similarly, QRSTUVWXZA’ fragment 171 demonstrated significant potencies in this assay (IC50 = 5.4 μM) with its bis-benzyl derivative 172 (rings Q and W) showing lower potency (IC50 > 30 μM). Apparently the two benzyl moieties at the same locations within the structures of 169 and 171 had a detrimental effect on the binding of these molecules to their receptor as revealed consistently in these two examples (the terminal n-propyl and allyl groups on ring Q are irrelevant to the binding of the molecule’s polycyclic framework). On the other hand, compounds 173 and 174 (F’E’D’C’ fragments) revealed the opposite effect, in that the TBDPS protecting group on 174 enhanced its potency (IC50 = 2.3 μM) as an inhibitor in this assay, as compared to that (IC50 >30 μM) of its free hydroxyl counterpart (173). These observations may reflect the fact that the bulk and lipophilicity of the TBDPS moiety plays the role of an extension of the rather short lipophilic C’D’E’F’ domain (i.e., 173, 174) of the natural product, thereby fulfilling, at least partly, the requirements of proper binding to the receptor.161
A number of these synthesised maitotoxin fragments were also tested against the NCI-60 DTP Human Tumor Cell Line Panel. Interestingly, fragment QRSTUVWXYZA’ (170, Fig. 11B), that showed the second best inhibitory activity in the maitotoxin-elicited 45Ca2+ influx test (see above and Fig. 11B),161 demonstrated the highest growth inhibition (GI50) values against a number of cancer cell lines, including leukemia (cell line RPMI-8226: GI50 = 2.15 μM), non-small cell lung cancer (cell line HOP-92: GI50 = 2.44 μM), colon cancer (cell line HCT-116: GI50 = 2.34 μM), CNS cancer (cell line SF-539: GI50 = 4.50 μM), melanoma (cell line 5K-MEL-5: GI50 = 2.13/ μM), ovarian cancer (cell line OVCAR-3: GI50 = 384 μM), renal cancer (cell line SN12C: GI50 = 1.86 μM), prostate cancer (cell line PC-3: GI50 = 1.83 μM) and breast cancer (cell line MDA-MB-468: GI50 = 1.26 μM).161
The maitotoxin campaign may have not been completed in terms of reaching its destination as yet, but the valuable knowledge and wisdom gathered thus far should be an inspiration as to what can be done by total synthesis. Thus, in addition to confirming maitotoxin’s legendary molecular structure, the methods and strategies developed essentially assure the prospects of its total synthesis, given the opportunity. They also make credible the statement that “any molecule found in Nature can be replicated in the laboratory by chemical synthesis”. That does not mean by any means that improvements in the field cannot be made – on the contrary, we still have a long way to go before we can reach the elegance and performance of Nature in this regard. In terms of the biological investigations and their suggestive findings enabled by the synthesised molecules in this program, one can only speculate as to their value and importance to biology and wonder of potential applications. Could one develop antidotes to combat poisoning from certain marine neurotoxins such as the brevetoxins and maitotoxin? And as in many other instances, could a cure for cancer emerge one day from molecules such as the maitotoxin inspiring fragments as killers of cancer cells? And perhaps as neurotoxins can these gifts from Nature teach us something about how our brain works? And can such knowledge help in discovering a treatment for Alzheimer disease and other neurological disorders? In answering such questions, we must not forget that most inventions, if not all, stand on fundamental research.
3.7. Viridicatumtoxin B
As one of the most structurally complex members of the tetracycline class of antibiotics, and because of its reported high potencies against drug resistant bacterial strains and the questionable assignment of its molecular structure (i.e., hydroxy epoxide structural motif), viridicatumtoxin B167 (177, Fig. 12) tempted us into initiating a research program directed towards its total synthesis, one that also included full structural assignment and analogue design, synthesis and biological evaluation. Our first total synthesis168 delivered racemic viridicatumtoxin B [(±)-177] and resulted in the revision of its structure to what we believed it to be in the first place, namely the one with the hydroxy ketone structural motif (177, Fig. 12), rather than its isomeric hydroxy epoxide moiety reported.167 Our second, and enantioselective total synthesis of viridicatumtoxin B [(+)-177]169 dictated the development of a practical method for the required asymmetric anthrone alkylation (i.e., anthrone 180 with bromide 178, see Fig. 12A, retrosynthetic analysis). To this end we synthesised and screened a number of phase transfer catalysts and identified Corey’s quinine-derived catalyst 181 (Fig. 12B)169 as our lead for optimisation. Thus, from the numerous newly synthesised 181-related compounds, we discovered a number of optimised catalysts for a general asymmetric alkylation of anthrones (for both enantiomers) and eventually applied catalyst 182 (Fig. 12B) and its quinidine-derived pseudoenantiomer (192, Fig. 12C) to the synthesis of both enantiomeric forms of viridicatumtoxin B [182 for (+)-viridicatumtoxin B, that turned out to be the natural form of the molecule, and 192 for (−)-viridicatumtoxin B, the antipode of the natural product], respectively.169
Fig. 12.
Highlights of the viridicatumtoxin B project. A. Retrosynthetic analysis; B. Enantioselective total synthesis of viridicatumtoxin B; C. Molecular structures and antibacterial potencies of viridicatumtoxin B and designed and synthesised viridicatumtoxin B analogues.
Fig. 12B summarises the total synthesis of the naturally occurring viridicatumtoxin B [(+)-177]. Thus, alkylation of anthrone 180 with allylic bromide (S)-178 in the presence of catalyst 182 followed by crystallisation of the alkylated product furnished substituted anthrone (6R,17S)-183 (>99:1 dr). The latter underwent spirocyclisation upon exposure to BF3·Et2O leading to pentacyclic compound (6R,15S)-184 (>99:1 er), whose sequential treatment with PIDA and CSA afforded (+)-185 (via the corresponding keto dimethoxyketal). Coupling of (+)-185 with heterocyclic phenyl ester 179 in the presence of KOt-Bu and chiral catalyst 186 ensured the high stereoselectivity of the reaction that led to heptacyclic product 187 (via the corresponding dimethoxy enone), whose decarboxylative desilylation followed by hydroxylation with DMDO in the presence of Ni(acac)2 cat. furnished hydroxylated advanced intermediate 188, as shown in Fig. 12B. Reduction of the latter with NaCNBH3 followed by further elaboration of the resulting product (189) led to hydroxy diketone 190, whose further hydroxylation with Davis oxaziridine in the presence of KHMDS afforded dihydroxylated precursor 191 in good overall yield. Desilylation, oxidation and debenzylation of the latter then furnished (+)-viridicatumtoxin B [(+)-177] as summarised in Fig. 12B.169
Applying the developed synthetic strategies and methods we synthesised an array of (+)- and (−)-viridicatumtoxin B analogues. Biological evaluation of these compounds against E. faecalis, E. faecium, and MRSA revealed a number of potent antibacterial agents, including (+)-193 (E. faecalis: MIC=0.5 μg/mL; E. faecium: MIC=2 μg/mL; MRSA: MIC=2 μg/mL), (−)-193 (E. faecalis: MIC=1 μg/mL; E. faecium: MIC=1 μg/mL; MRSA: MIC=2 μg/mL), (+)-194 (E. faecalis: MIC=1 μg/mL; E. faecium: MIC=1 μg/mL; MRSA: MIC= 2 μg/mL), and (−)-194 (E. faecalis: MIC=1 μg/mL; E. faecium: 0.5 μg/mL; MRSA: MIC=2 μg/mL) (see Fig. 12C). It is worth pointing out that both enantiomers of viridicatumtoxin B (177) exhibited almost the same potencies in these assays [(+)-177: E. faecalis: MIC=2 μg/mL; E. faecium: MIC=2 μg/mL; MRSA: MIC= 4 μg/mL; (−)-177 (structure not shown): E. faecalis: MIC=4 μg/mL; E. faecium: MIC=4 μg/mL; MRSA: MIC= 8 μg/mL], an interesting phenomenon we also observed in several other projects on antibacterial agents in our group.
The viridicatumtoxin campaign was rich in delivery and impact in that it resulted in contributions in a) strategy design; b) structural revision of the originally erroneous structural assignment of the molecule; c) method discovery and development that filled a void at the time; d) development of valuable structure–activity relationships (SARs); and e) identification of several potential drug candidates. It was, indeed, a rewarding and exemplary total synthesis endeavour with multiple discoveries that made it, like so many others, worth pursuing in our laboratories.
3.8. Prostaglandins J3 and J2
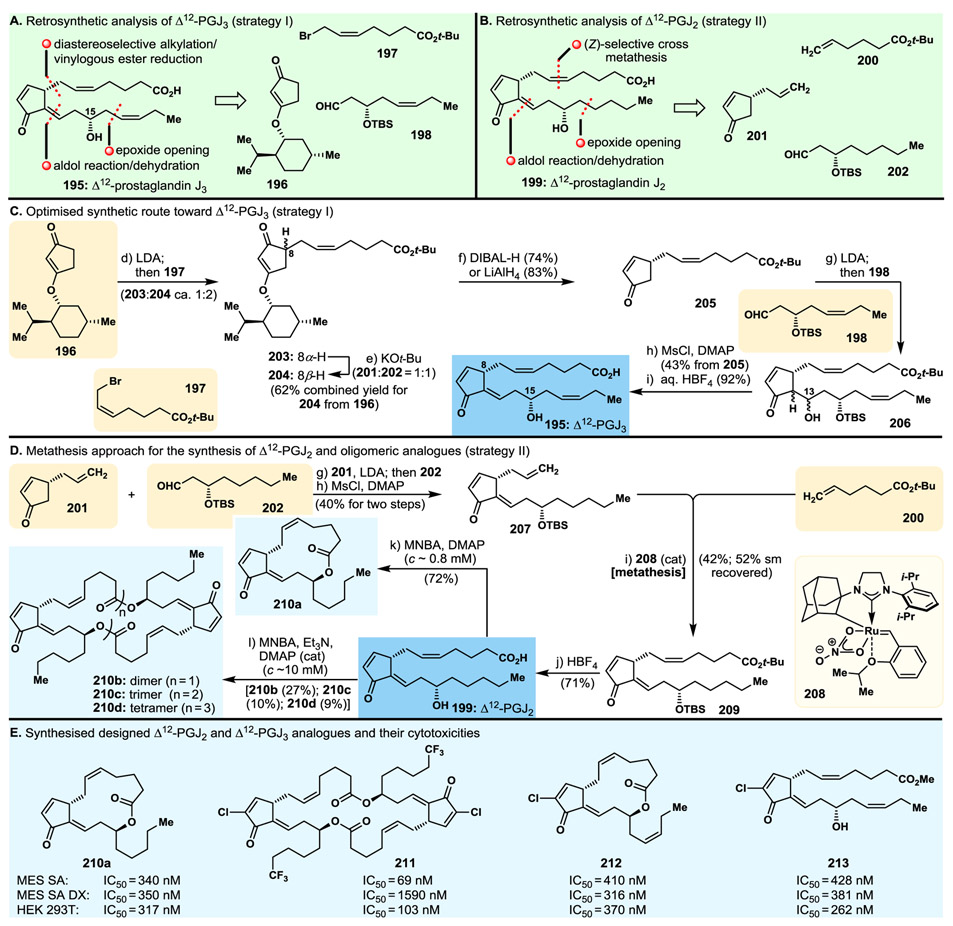
A rather latecomer and cousin of the previously known Δ12-prostaglandin J2 (Δ12-PGJ2, 199, Fig. 13B),170,171 Δ12-prostaglandin J3 (Δ12-PGJ3, 195, Fig. 13) was reported in the early 2010s as a naturally occurring secondary metabolite of eicosapentaenoic acid (EPA) and was claimed to possess impressive antileukemic properties by eradicating leukaemia stem cells.172,173 This exciting report prompted us to undertake a program directed towards the total synthesis of Δ12-PGJ3 (195) and its cousin molecule Δ12-PGJ2 (199), and apply the developed strategies and technologies to construct designed analogues of them for the purposes of biological investigations. Fig. 12A and C summarise our optimised total synthesis of Δ12-PGJ3 (195), which employed easily prepared key building blocks 196, 197 and 198, revealed by the retrosynthetic analysis as shown in Fig. 13A (strategy I). Their assembly proceeded as summarised in Fig. 13C. It involved first alkylation of 196 with 197 to afford 204 (and C8-epimer 203, which is convertible to 204) in good yield, then reductive cleavage of the menthol auxiliary group leading to enone 205. Aldol reaction of the latter with aldehyde 198 furnished hydroxy enone 206, from which Δ12-PGJ3 (195) was generated through elimination, via the corresponding mesylate, and ester hydrolysis, as summarised in Fig. 13C.
Fig. 13.
Highlights of the campaigns towards Δ12-prostaglandin J3 (PGJ3) and J2 (Δ12-PGJ2) and their analogues. A. and B. Retrosynthetic analyses I and II; C. Optimised synthetic route towards Δ12-PGJ3; D. Metathesis-based total synthesis of Δ12-PGJ2; E. Molecular structures and cytotoxicity potencies of designed and synthesised Δ12-PGJ2 and Δ12-PGJ3 analogues.
Our streamlined total synthesis approach to Δ12-prostaglandin J2 (Δ12-PGJ2, 199, Fig. 13B) relied on a different synthetic route. Involving a similar synthetic strategy for the construction of its “lower” chain as that employed in the synthesis of Δ12-PGJ3 (195), and an olefin metathesis tactic for the instalment of its “upper” chain, our approach to Δ12-PGJ2 (199) is shown retrosynthetically in Fig. 13B (strategy II, building blocks 200, 201, 202). Fig. 13D summarises the execution of this strategy starting with the readily available cyclopentenone 201, whose aldol coupling with aldehyde 202 followed by mesylation/elimination of the resulting mixture of alcohols led to terminal olefin 207. The latter was subjected to cross metathesis with terminal olefin 200, in the presence of catalyst 208, to afford protected Δ12-PGJ2 precursor 209, whose one-step global deprotection furnished Δ12-PGJ2 (199) as depicted in Fig. 13D.
Aiming to investigate the biological properties of these secondary metabolites (i.e., Δ12-PGJ3 and Δ12-PGJ2) and their variations, we exploited our synthetic strategies to construct a series of designed analogues,174-176 including those shown in Fig. 13D (210a and 210b–210d). Thus, exposing a number of the most potent PGJ3- and PGJ2-type synthesised compounds174-176 to standard Shiina macrolactonisation conditions, we prepared the corresponding 1,15-macrolactone analogues (199→210a, Fig. 13D), and, by using appropriate dilution conditions, their dimeric, trimeric and tetrameric counterparts (i.e., more concentrated, 199→210b–210d, Fig. 13D).175 Biological evaluation of these analogues led to interesting structure–activity relationships and the identification of a number of potent antitumor agents against a number of normal cancer cell lines (see Fig. 13E). Thus, depending on the cell line, the macrolactone and dimeric macrolactone analogues [e.g., 210a (Fig. 13D and E), 210b (Fig. 13D and E), 212174 (Fig. 13E)] were found to be more potent than their hydroxy acid precursors, with the dimeric analogues (e.g., 213,174 Fig. 13E) often, but not always, being somewhat more active than the monomers. Interestingly, and in contrast to their monomeric and dimeric macrolactones, the trimers (e.g., 210c, Fig. 13D) and tetramers (e.g., 210d, Fig. 13D) did not exhibit significant activities in those assays as compared to their precursor hydroxy acids or their monomeric cyclic counterparts. Tested at the NCI (National Cancer Institute, USA), analogue 212174 (Fig. 13E) demonstrated efficacy in treating cancer in mice. This program proved, once again, the importance of total synthesis in rendering extremely rare natural products readily available, and synthesising novel analogues of them for extensive biological investigations, not to mention confirming their molecular structures and preparing the ground for further investigations.
3.9. Tubulysin H
N14-Desacetoxytubulysin H (214) is a member of a large family of tubulin binding, naturally occurring molecules endowed with antitumor properties.177 Our research program in this area was directed towards the total synthesis of key members of the family and their designed analogues, aiming for high potencies as needed for antibody–drug conjugate (ADC) payloads.101,102 As an example, we describe here the total synthesis of N14-desacetoxytubulysin H (214, Fig. 14). Our retrosynthetic analysis (Fig. 14A) defined building blocks 218 (to be derived from fragments 219 and 220), 215, 216 and 217. The execution of the derived synthetic strategy is summarised in Fig. 14B. Thus, a C–H activation-based coupling of aldehyde 219 with thiazole derivative 220 under the influence of PhI(OCOCF3)2 and TMSN3178-181 furnished ketone 218 (top right, Fig. 14B), whose asymmetric reduction led to alcohol 221. A four-step standard sequence from the latter afforded acetoxy carboxylic acid 222, whose sequential peptide couplings with fragments 215, 216 and 217 gave advanced precursor 224, via 223, as summarised in Fig. 14B. The latter was then converted to N14-desacetoxytubulysin H (214) in two simple steps as shown in Fig. 14B.182,183
Fig. 14.
Highlights of the N14-desacetoxytubulysin H project. A. Retrosynthetic analysis; B. Total synthesis of N14-desacetoxytubulysin H; C. Molecular structures and cytotoxicity potencies of select designed and synthesised tubulysin analogues.
Employing the developed synthetic strategies and methods, a series of well over a hundred designed analogues were synthesised and biologically evaluated for their cytotoxicities against a number of cancer cell lines. Among the most potent were analogues 225,182 226,183 227183 and 228,183 whose structures, together with their potencies against certain cell lines, are shown in Fig. 14C. As two of the most active tubulysins, compounds 227 and 228 formed complexes with microtubules, whose X-ray crystallographic analysis elucidated their binding modes which were consistent with a previous study employing tubulysin M.184 These investigations led to a set of potential payloads to ADCs for targeted cancer therapies, provided a better understanding of the binding mode of tubulysins to their biological target, and expanded the established structure–activity relationships within the family.182,183
3.10. Thailanstatins
With its novel molecular structure and spliceosome inhibitory properties, thailanstatin A (229, Fig. 15) provided another challenge and opportunity for us to explore yet another promising natural product as a lead compound for ADC payloads, or “drug alone” (or in combination with other drugs). Our total synthesis proceeded according to the synthetic strategy derived from the retrosynthetic analysis shown in Fig. 15A) revealing building blocks 230, 231 and 233, with 231 traced back to pyrone 232, and 233 to hydroxy vinyl iodide 234. The total synthesis of thailanstatin A (229) proceeded as summarised in Fig. 15B. Thus, pyrone 232 was reacted with building block 235 (I2; then K2CO3) to afford ketone methyl ester 236, whose standard elaboration over four steps led to vinyl iodide methyl ester 237. The latter was converted to desired fragment, hydroxy epoxide 231, through the four-step sequence shown in Fig. 15B. In parallel, vinyl iodide building block 234, in the presence of allyl alcohol (239), was transformed to α,β,γ,δ-unsaturated hydroxy aldehyde 233, as summarised in Fig. 15B, and thence to dihydropyran 240 through a three-step standard sequence. The latter was selectively reduced to tetrahydropyran system 241 as summarised in Fig. 15B. Intermediate 241 was converted to the corresponding aldehyde, and the latter was reacted with the Tebbe reagent to afford, after further elaboration and coupling with carboxylic acid 230, amide 242, whose cross metathesis (Grubbs II catalyst) with vinyl boronate fragment 243 furnished vinyl boronate building block 244. The latter was joined through a Suzuki coupling with vinyl iodide 231 to furnish, after hydrolysis of the tert-butyl ester, the coveted thailanstatin A (229, Fig. 15B).
Fig. 15.
Highlights of the thailanstatin A project. A. Retrosynthetic analysis; B. Total synthesis of thailanstatin A; C. Molecular structures and cytotoxicity potencies of select designed and synthesised thailanstatin A analogues.
As part of this program an array of designed thailanstatin analogues were synthesised and tested for their antitumor properties, leading to optimisation beyond the potencies of previously known members of the family. Two of the most potent compounds synthesised thus far in this program, analogues 245 and 246, are depicted in Fig. 15C together with their potencies against certain cell lines, which are significantly higher than those of thailanstatin A (see Fig. 15B and 15C), making them suitable as antibody–drug conjugate payloads intended for targeted cancer therapies.101,102
3.11. Aziridinyl Epothilones
Beginning in 1996, our epothilone project continued through the ensuing period to the present time with numerous innovations and rewarding discoveries. Recently, we took advantage of the Ess–Kürti–Falck (EKF) aziridination reaction185 to produce a series of 12,13-azridinyl epothilone B analogues76 whose biological evaluation revealed a number of highly active compounds (i.e., 247, 248, 249 and 250, Fig. 16). Their short synthesis from epothilone B (38, see Fig. 5A) proceeded through a sequence involving ozonolysis (to cleave the side chain forming the corresponding methyl ketone), deoxygenation of the epoxide moiety (to reveal the trisubstituted olefinic bond), side chain attachment followed by EKF aziridination (to install selectively the aziridinyl structural motif), and optional placement of substituents on the N atom to generate the desired aziridinyl epothilone analogues. Fig. 16 depicts a number of highly potent analogues of this family of epothilones, together with their IC50 values against several cancer cell lines (see also section 2 and Fig. 5A).76 Such compounds or their decedents may find application as payloads for antibody–drug conjugates.
Fig. 16.
Select designed synthesised aziridinyl epothilone B analogues.
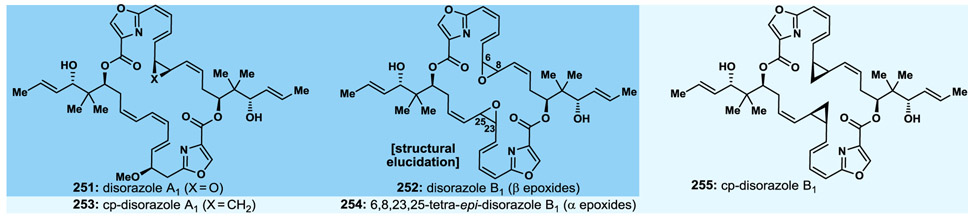
3.12. Disorazoles A1 and B1
The tubulin binding natural products disorazoles A1 and B1 (251 and 252, Fig. 17)186 are the most potent members of this family of compounds. Our recent total synthesis187 of these molecules provided opportunities to design, synthesise and biologically evaluate analogues of them, as well as to complete the structural assignment of the latter (disorazole B1, 252, Fig. 17) with regards to the epoxide moieties whose configurations were left undefined.186 Among the analogues synthesised are the ones depicted, together with their potencies against certain cell lines, in Fig. 17 (i.e., cyclopropyl analogues 253 and 255). It was of interest to observe the significant differences between the two diasteromeric bisepoxide structures 252 and 254 (Fig. 17) with regards to their biological activities, with the highly potent one (252) turning out to be the natural product and the essentially inactive one (254) relegated to an analogue, demonstrating the importance of the configuration of the two epoxide structural motifs to biological activity. Both cyclopropyl analogues, however, of disorazole A1 and B1 (i.e., 253 and 255, Fig. 17) turned out to be active,188 making them worth investigating further as potential leads to antibody–drug conjugates and/or as drugs alone or in combination with other anti-cancer drugs.
Fig. 17.
Disorazoles A1 and B1 and select synthesised designed analogues.
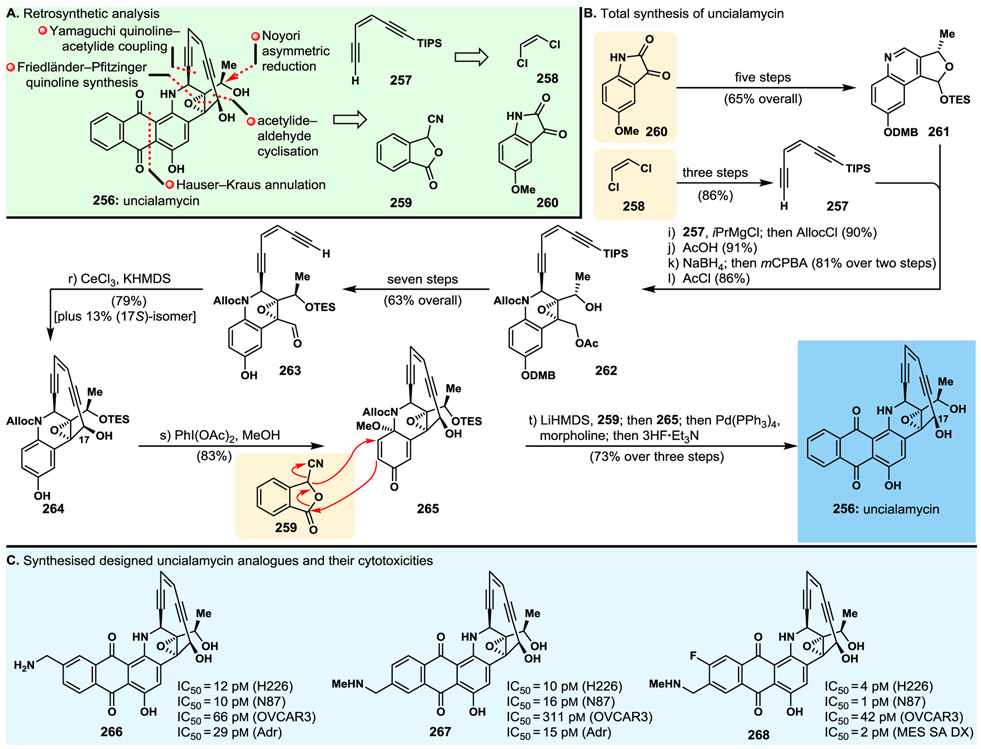
3.13. Uncialamycin
The discovery of the marine natural product uncialamycin (256, Fig. 18) in 2005189 elicited our interest as an ideal target molecule for total synthesis due to several reasons, including its challenging molecular structure, the fact that its structural assignment remained incomplete, leaving the relative stereochemistry of one of its stereocentres unassigned, and its phenomenal potencies as an antibiotic. Odd as it was, the disclosure of its isolation189 did not include any DNA cleavage studies, nor was any mention of cytotoxicity assays with cancer cells, an occurrence that could be explained by the meagre amounts (300 μg) isolated from its natural source. The natural scarcity of uncialamycin (256) provided yet another and compelling reason for undertaking its replication in the laboratory as a prelude to further biological investigations. Our first total synthesis (delivering racemic material) of uncialamycin was achieved in 2007.190 Our second, and enantioselective total synthesis,191 led to the molecule’s full structural assignment and made possible further biological investigations in our laboratories that revealed its expected DNA single and double strand cleavage of duplex DNA and potent cytotoxicities against several cancer cell lines. Later on, and as a consequence of a new method we discovered for the synthesis of substituted anthraquinones, we developed an optimised, streamlined total synthesis of uncialamycin192 and applied it to the synthesis of numerous designed analogues for the purposes of discovering suitable payloads for antibody–drug conjugates.101,102,192,193
Fig. 18.
Highlights of the uncialamycin project. A. Retrosynthetic analysis; B. Total synthesis of uncialamycin; C. Molecular structures and cytotoxicity potencies of select designed and synthesised uncialamycin analogues.
Fig. 18 summarises our most recent and improved total synthesis of uncialamycin (256) and a number of analogues that exhibited higher potencies than their parent natural product.192 Retrosynthetic analysis of uncialamycin (256) as depicted in Fig. 18A defined building blocks 257 (to be derived from dichloride 258), 259 and 260. Thus, starting from 5-methoxy isatin (260), tricyclic intermediate 261 was constructed in five standard steps (65% yield), while dichloride 258 was converted to TIPS-enediyne 257 through a three-step sequence as indicated in Fig. 18B. Reaction of quinoline 261 with the acetylide generated from enediyne 257 and i-PrMgCl followed by quenching with Alloc chloride furnished, selectively, the corresponding Yamaguchi coupling product, whose elaboration as indicated in Fig. 18B led to hydroxy acetoxy epoxide 262. Epoxide 262 was sequentially processed, via intermediates 263 and 264, to stable methoxy quinone system 265, as indicated in Fig. 18B. The latter served well as a coupling partner with cyanophthalide 259 in a Hauser–Kraus-type fusion/annulation to afford, upon further elaboration, uncialamycin (256) in enantiopure form and good overall yield as depicted in Fig. 18B. These synthetic strategies and technologies were applied to synthesise several naturally occurring congeners of uncialamycin (i.e., tiancimycins A and B, yangpumicin A)194 and numerous analogues,194 from which those exhibited in Fig. 18C (266, 267 and 268) are among the most potent against certain cancer or cancer-like cell lines.192
3.14. Shishijimicin A
Although reported in 2003,195 shishijimicin A (269, Fig. 19) remained unexplored for more than a decade, despite its phenomenal cytotoxicity against certain cancer cell lines and, therefore, its potential as an antibody–drug conjugate payload. The reasons for this neglect most likely was its scarcity that made further, and well deserved, biological investigations virtually impossible. Prompted by the synthetic challenge posed by shishijimicin A and our desire to study its biological properties and those of analogues thereof, we initiated a synthetic program towards its total synthesis. Reported in 2015,196 our first total synthesis of shishijimicin A was later refined and improved, and employed to construct several analogues192 as shown in Fig. 19. Thus, retrosynthetic analysis (Fig. 19A) led to enediyne fragment 270, traced back to olefinic oxime 271, and carbohydrate carboline fragment 272, traced back to disaccharide ketoaldehyde 273 and serotonin hydrochloride (74). The streamlined total synthesis of shishijimicin A (269, Fig. 19)192 proceeded as summarised in Fig. 19B. Thus, oxidation of oxime 271 followed by spontaneous intramolecular [3+2] cycloaddition of the so formed olefin nitrile oxide (275) afforded bicyclic system 276. Elaboration of the latter to the corresponding ketone, and addition of the acetylide generated from enediyne 277 as summarised in Fig. 19B, furnished intermediate 278, whose further elaboration led to acetylenic aldehyde fragment 279. The latter was exposed to LiHMDS in the presence of LaCl3·2LiCl to induce double ring closure, first to form the 10-membered enediyne ring and thence the δ-lactone, leading to enediyne fragment 280 upon removal of the phthalide protecting group and elaboration of the resulting amino group to the desired carbamate, as shown in Fig. 19B. The latter was subsequently converted to the targeted thioacetate enediyne domain 270 through a standard four-step sequence, the latter ready to be coupled with carbohydrate-carboline domain 272. Trichloroacetimidate donor 272 was synthesised from disaccharide keto aldehyde intermediate 273197 by fusion with serotonin hydrochloride salt (274) through the sequential exposure to AcOH and O2 that led to intermediate 281, whose standard four-step elaboration furnished the targeted glycosyl donor 272, as summarised in Fig. 19B. Coupling of glycosyl donor 272 with carbohydrate acceptor 270 furnished thioacetate shishijimicin A precursor 282, whose sequential conversion to methyltrisulfide protected precursor (283), and thence to shishijimicin A (269), is summarised in Fig. 19B.197
Fig. 19.
Highlights of the shishijimicin A project. A. Retrosynthetic analysis; B. Total synthesis of shishijimicin A; C. Molecular structures and cytotoxicity potencies of select designed and synthesised shishijimicin A analogues.
Employing the developed synthetic strategies and methods a number of shishijimicin A analogues were synthesised, among which the most potent were the methyl disulfide 284 and the simpler shishijimicin A analogue 285, the latter lacking the MeS and the OH groups on carbohydrate ring A. Their potencies against the MES SA, MES SA DX and HEK 293T cell lines are shown in Fig. 19C, together with those of synthetic shishijimicin A (269).197 As can be seen, both these analogues turned out to be even more potent than the parent natural product, despite their less complex structures, especially true for analogue 285. Most importantly, rendering shishijimicin A (269) readily available through our total synthesis put us in a position to study its DNA binding and cleaving modes, in collaboration with the Tianhu Li group at the Nanyang University in Singapore, demonstrating its intercalating properties and powerful double strand cuts of duplex DNA.198
4. Conclusion and Future Perspectives
Introduced by Friedrich Wöhler in 1828 by accident, total synthesis and its more general version, organic synthesis, emerged from experimentation with inorganic substances [i.e., AgNCO (silver isocyanate, originally, and even today, called silver cyanate)1 and NH4Cl] that resulted in the formation of urea,2 an organic substance and a natural product (see ①, Fig. 20). Recognising its potential power to replicate the molecules of Nature and others like them in the laboratory, and inspired further by Hermann Kolbe’s intentional synthesis of acetic acid (1845),199 synthetic chemists adopted the field of total synthesis and expanded it to include all organic molecules, natural or designed, by introducing organic synthesis as the name of the art and science of making organic molecules. Thus, from an unintended purpose, total synthesis assumed the burden (others will say the privilege or the pleasure!) as the science of confirming the hypothetical structures of natural products4 (see ②, Fig. 20), a purpose that, ironically, remains valid to this day despite our sophisticated analytical and spectroscopic instrumentation and techniques. In the 1950s and 1960s total synthesis gained much appreciation as an art and was valued for its elegance and aesthetically pleasing bond making and breaking manoeuvres, as demonstrated so elegantly by R. B. Woodward (see ③, Fig. 20). This artistic aspect of total synthesis continuous to be admired today,200 and it is often accompanied by other advantages as is evident in cascade reactions in total synthesis201-203 where the benefits extend beyond the beauty, translating into practicality, saving economic cost and labour, and providing environmental protection through green chemistry (Fig. 20).
Fig. 20.
Evolution of the impact of total synthesis for science and society.
From the 1960s onwards, total synthesis entered into yet another era, when logic and generality took their proper place in planning strategies towards the ever increasing, in numbers and complexity, natural products unearthed from the forests, the soil, and the oceans. Introduced and championed by E. J. Corey with his contributions to the theory and methodology of total synthesis (see ④, Fig. 20), this last period moved forward steadily to systematically include retrosynthetic analysis, targeting rare biologically active natural products, and applications of the developed synthetic strategies and methods to the synthesis of designed analogues for biological investigations. These developments within the field of total synthesis, coupled with the trends of interdisciplinary research, collaborations and partnerships204 in more recent times, led to the present-day paradigm of total synthesis endeavours as already adopted by many practitioners around the world (see ⑤, Fig. 20).
The ideal modern paradigm of total synthesis endeavours includes, although not all obligatory or limited to, the following components interwoven together harmoniously, while maintaining the fundamentals of the science and its advancement to higher levels of sophistication and performance.
The endeavour should preferably include the discovery and invention of new chemistry, usually in the form of novel synthetic strategies and new methods. Such discoveries and inventions contribute to the advancement of the field of total synthesis, and organic synthesis in general, for their own sake, an important principle from the science point of view.
The overall project of total synthesis should preferably include a biology and, potentially, medicine, either within the same laboratory or, more often than not, as part of a collaboration/partnership with other academic/industrial groups. Such arrangements could result in added-value to the endeavour and provide opportunities for discoveries and inventions, all the way from the fundamentals (basic research) to the translational (drug discovery and development). This added-value of the endeavour could attract substantial funding and may result in lucrative benefits to science and society in terms of knowledge, understanding of biology, and faster translation of the fundamentals to the applied. These principles apply beyond biology and medicine and into other beneficiary fields such as agrochemicals, cosmetics, materials science and nanotechnology once the roles of the targeted natural products are recognised or suspected. One such example is that of the ladderanes as demonstrated recently by Noah Burns and his colleagues.205,206
Intended and/or unintended complete structural elucidations of natural products whose molecular structures were left incomplete for one reason or another are often added-value to total synthesis endeavours. From our experience involving almost 200 natural products total syntheses, at least 13% of them resulted in some sort of added value in terms of natural product structure elucidations, ranging from stereochemical configurations, relative or absolute, to synthesising naturally occurring molecules before their discovery from Nature, or prediction as naturally occurring molecules.207
We will be remiss if we do not mention the importance of total synthesis endeavours as platforms to educate and train young scientists in the art and science of making molecules for all intents and purposes. Being one of the most demanding endeavours in chemistry, total synthesis often presents what initially seem like intransigent problems beyond the limits of our knowledge and synthetic capabilities at the time. This is an opportunity “to challenge the challenge”, for it is only then that we can break the existing glass ceilings, for they are only temporary as it has been shown time and time again in the history of total synthesis and organic synthesis in general. It is during such challenging projects that students learn how to solve problems and develop immunity to disappointment and despair upon failing. Instead, resourcefulness, creativity and imagination, and hard work should come into play, with motivation and patience as supporting allies, to solve the problems often arising one after the other along the way.
It is gratifying to see that the new paradigm and philosophy of total synthesis (see ⑤, Fig. 20) is spreading around the world as the new model of practicing this fascinating and multifaceted discipline. Let us hope that funding agencies, and charities alike, recognise and appropriately honour the benefits to science and society derived from total synthesis endeavours, so as to provide the necessary support for the field to thrive in its newly and rapidly emerging form. For this to occur, isolation of natural products should also be rekindled as a means to provide the myriad treasure molecules of Nature waiting to be discovered, and inspire the new generation of practitioners of the art and science of total synthesis to exploit their potential as opportunities to increase our knowledge in science and apply it to discover new medicines for people.
Acknowledgements
KCN would like to thank his students and postdoctoral fellows who contributed decisively to the achievements described in this article and whose names appear in the references. We are grateful to the National Institutes of Health (USA), the Cancer Prevention & Research Institute of Texas (CPRIT), The Robert A. Welch Foundation (grant C-1819), Bristol-Myers Squibb, and AbbVie Stemcentrx for their generous support of our research. KCN is a CPRIT scholar in cancer research.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Britton D and Dunitz JD, Acta Crystallogr., 1965, 18, 424–428. [Google Scholar]
- 2.Wöhler F, Ann. Phys, 1828, 88, 253–256. [Google Scholar]
- 3.Esteban S, J. Chem. Educ, 2008, 85, 1201. [Google Scholar]
- 4.Hoffmann RW, Classical Methods in Structure Elucidation of Natural Products, Wiley-VHCA, Zürich, Switzerland, 2018. [Google Scholar]
- 5.Corey EJ, Weinshenker NM, Schaaf TK and Huber W, J. Am. Chem. Soc, 1969, 91, 5675–5677. [DOI] [PubMed] [Google Scholar]
- 6.Corey EJ, Schaaf TK, Huber W, Koelliker U and Weinshenker NM, J. Am. Chem. Soc, 1970, 92, 397–398. [DOI] [PubMed] [Google Scholar]
- 7.Corey EJ, Ann. N. Y. Acad. Sci, 1971, 180, 24–37. [DOI] [PubMed] [Google Scholar]
- 8.Hammarström S, Samuelsson B, Clark DA, Goto G, Marfat A, Mioskowski C and Corey EJ, Biochem. Biophys. Res. Commun, 1980, 92, 946–953. [DOI] [PubMed] [Google Scholar]
- 9.Lewis RA, Austen KF, Drazen JM, Clark DA, Marfat A and Corey EJ, Proc. Natl. Acad. Sci. U.S.A, 1980, 77, 3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rådmark O, Malmsten C, Samuelsson B, Clark DA, Goto G, Marfat A and Corey EJ, Biochem. Biophys. Res. Commun, 1980, 92, 954–961. [DOI] [PubMed] [Google Scholar]
- 11.Corey EJ, Marfat A, Goto G and Brion F, J. Am. Chem. Soc, 1980, 102, 7984–7985. [Google Scholar]
- 12.Corey EJ, Pyne SG and Su W.-g., Tetrahedron Lett., 1983, 24, 4883–4886. [Google Scholar]
- 13.Marfat A and Corey EJ, Adv. Prostaglandin, Thromboxane, Leukotriene Res, 1985, 14, 155–228. [PubMed] [Google Scholar]
- 14.Corey EJ, Nicolaou KC, Machida Y, Malmsten CL and Samuelsson B, Proc. Natl. Acad. Sci. U.S.A, 1975, 72, 3355–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.See KCN’s CV at: https://nicolaou.rice.edu/classes.html#kcn [Google Scholar]
- 16.Moncada S, Gryglewski R, Bunting S and Vane JR, Nature, 1976, 263, 663–665. [DOI] [PubMed] [Google Scholar]
- 17.Nicolaou KC, Sipio WJ, Magolda RL, Seitz S and Barnette WE, J. Chem. Soc., Chem. Commun, 1978, 1067–1068. [Google Scholar]
- 18.Nicolaou KC, Barnette WE and Magolda RL, J. Am. Chem. Soc, 1981, 103, 3472–3480. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou KC, Barnette WE, Gasic GP and Magolda RL, J. Am. Chem. Soc, 1977, 99, 7736–7738. [DOI] [PubMed] [Google Scholar]
- 20.Hamberg M, Svensson J and Samuelsson B, Proc. Natl. Acad. Sci. U.S.A, 1975, 72, 2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolaou KC, Magolda RL, Smith JB, Aharony D, Smith EF and Lefer AM, Proc. Natl. Acad. Sci. U.S.A, 1979, 76, 2566–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefer AM, Smith EF, Araki H, Smith JB, Aharony D, Claremon DA, Magolda RL and Nicolaou KC, Proc. Natl. Acad. Sci. U.S.A, 1980, 77, 1706–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith III EF, Lefer AM and Nicolaou KC, Am. J. Physiol. Heart Circ. Physiol, 1981, 240, H493–H497. [DOI] [PubMed] [Google Scholar]
- 24.Koshishara Y, Murota S.-i., Petasis NA and Nicolaou KC, FEBS Lett., 1982, 143, 13–16. [DOI] [PubMed] [Google Scholar]
- 25.Koshihara Y, Murota S and Nicolaou KC, Adv. Prostaglandin, Thromboxane, Leukotriene Res, 1983, 11, 163–167. [PubMed] [Google Scholar]
- 26.Tsai BS, Keith RH, Villani-Price D, Haack RA, Bauer RF, Leonard R, Abe Y and Nicolaou KC, Prostaglandins, 1989, 37, 287–302. [DOI] [PubMed] [Google Scholar]
- 27.Serhan CN, Hamberg M and Samuelsson B, Biochem. Biophys. Res. Commun, 1984, 118, 943–949. [DOI] [PubMed] [Google Scholar]
- 28.Serhan CN, Hamberg M and Samuelsson B, Proc. Natl. Acad. Sci. U.S.A, 1984, 81, 5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolaou KC, Veale CA, Webber SE and Katerinopoulos H, J. Am. Chem. Soc, 1985, 107, 7515–7518. [Google Scholar]
- 30.Nicolaou KC and Webber SE, Synthesis, 1986, 1986, 453–461. [Google Scholar]
- 31.Nicolaou KC, Webber SE, Ramphal J and Abe Y, Angew. Chem. Int. Ed. Engl, 1987, 26, 1019–1021. [Google Scholar]
- 32.Greenberg R, Antonaccio MJ and Steinbacher T, Eur. J. Pharmacol, 1982, 80, 19–27. [DOI] [PubMed] [Google Scholar]
- 33.Ramstedt U, Serhan CN, Nicolaou KC, Webber SE, Wigzell H and Samuelsson B, J. Immunol, 1987, 138, 266–270. [PubMed] [Google Scholar]
- 34.Palmblad J, Gyllenhammar H, Ringertz B, Serhan CN, Samuelsson B and Nicolaou KC, Biochem. Biophys. Res. Commun, 1987, 145, 168–175. [DOI] [PubMed] [Google Scholar]
- 35.Badr KF, Serhan CN, Nicolaou KC and Samuelsson B, Biochem. Biophys. Res. Commun, 1987, 145, 408–414. [DOI] [PubMed] [Google Scholar]
- 36.Nicolaou KC and Petasis NA, in Handbook of Eicosanoids, Prostaglandins and Related Lipids, Part B (Chemical and Biochemical Aspects), ed. Willis AL, CRC Press, Boca Raton, Florida, USA, 1987, pp. 1–18. [Google Scholar]
- 37.Dahlén S-E, Franzén L, Raud J, Wikström E, Björck T, Matsuda H, Westlund P, Puustinen T, Haeggström J, Samuelsson B, Serhan CN, Webber SE, Veale CA and Nicolaou KC, in Lipoxins: Biosynthesis, Chemistry, and Biological Activities, eds. Wong PYK and Serhan CN, Springer US, Boston, MA, 1988, pp. 107–130. [Google Scholar]
- 38.Lefer AM, Stahl GL, Lefer DJ, Brezinski ME, Nicolaou KC, Veale CA, Abe Y and Smith JB, Proc. Natl. Acad. Sci. U.S.A, 1988, 85, 8340–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl GL, Tsao P, Lefer AM, Ramphal JY and Nicolaou KC, Eur. J. Pharmacol, 1989, 163, 55–60. [DOI] [PubMed] [Google Scholar]
- 40.Dahlén SE, Veale CA, Webber SE, Marron BE, Nicolaou KC and Serhan CN, Agents Actions, 1989, 26, 93–95. [DOI] [PubMed] [Google Scholar]
- 41.Brezinski ME, Gimbrone MA, Nicolaou KC and Serhan CN, FEBS Lett., 1989, 245, 167–172. [DOI] [PubMed] [Google Scholar]
- 42.Hedqvist P, Raud J, Palmertz U, Haeggström J, Nicolaou KC and Dahlén S-E, Acta Physiol. Scand, 1989, 137, 571–572. [DOI] [PubMed] [Google Scholar]
- 43.Nicolaou KC, Ramphal JY, Petasis NA and Serhan CN, Angew. Chem. Int. Ed. Engl, 1991, 30, 1100–1116. [Google Scholar]
- 44.Nicolaou KC and Webber SE, J. Am. Chem. Soc, 1984, 106, 5734–5736. [Google Scholar]
- 45.Nicolaou KC, Ladduwahetty T and Elisseou EM, J. Chem. Soc., Chem. Commun, 1985, 1580–1581. [Google Scholar]
- 46.Nicolaou KC, Ladduwahetty T, Taffer IM and Zipkin RE, Synthesis, 1986, 1986, 344–347. [Google Scholar]
- 47.Nicolaou KC, Ramphal JY, Palazon JM and Spanevello RA, Angew. Chem. Int. Ed. Engl, 1989, 28, 587–588. [Google Scholar]
- 48.Nicolaou KC, Ramphal JY and Abe Y, Synthesis, 1989, 1989, 898–901. [Google Scholar]
- 49.Nicolaou KC and Webber SE, J. Chem. Soc., Chem. Commun, 1986, 1816–1817. [Google Scholar]
- 50.Puustinen T, Webber SE, Nicolaou KC, Haeggström J, Serhan CN and Samuelsson B, FEBS Lett., 1986, 207, 127–132. [DOI] [PubMed] [Google Scholar]
- 51.Nicolaou KC, Hummel CW, Pitsinos EN, Nakada M, Smith AL, Shibayama K and Saimoto H, J. Am. Chem. Soc, 1992, 114, 10082–10084. [Google Scholar]
- 52.Nicolaou KC, Riemer C, Kerr MA, Rideout DR and Wrasidlo WW, Nature, 1993, 364, 464–466. [DOI] [PubMed] [Google Scholar]
- 53.Nicolaou KC, Guy RK, Pitsinos EN and Wrasidlo W, Angew. Chem. Int. Ed. Engl, 1994, 33, 1583–1587. [Google Scholar]
- 54.Nicolaou KC, Couladouros EA, Nantermet PG, Renaud J, Guy RK and Wrasidlo W, Angew. Chem. Int. Ed. Engl, 1994, 33, 1581–1583. [Google Scholar]
- 55.Nicolaou KC, Nantermet PG, Ueno H and Guy RK, J. Chem. Soc., Chem. Commun, 1994, 295–296. [Google Scholar]
- 56.Guy RK, Scott ZA, Sloboda RD and Nicolaou KC, Chem. Biol, 1996, 3, 1021–1031. [DOI] [PubMed] [Google Scholar]
- 57.Nicolaou KC, Vourloumis D, Li T, Pastor J, Winssinger N, He Y, Ninkovic S, Sarabia F, Vallberg H, Roschangar F, King NP, Finlay MRV, Giannakakou P, Verdier-Pinard P and Hamel E, Angew. Chem. Int. Ed. Engl, 1997, 36, 2097–2103. [Google Scholar]
- 58.Nicolaou KC, He Y, Vourloumis D, Vallberg H, Roschangar F, Sarabia F, Ninkovic S, Yang Z and Trujillo JI, J. Am. Chem. Soc, 1997, 119, 7960–7973. [Google Scholar]
- 59.Nicolaou KC, Ninkovic S, Ray VFinlay M, Sarabia F and Li T, Chem. Commun, 1997, 2343–2344. [Google Scholar]
- 60.Nicolaou KC, Vallberg H, King NP, Roschangar F, He Y, Vourloumis D and Nicolaou CG, Chem. - Eur. J, 1997, 3, 1957–1970. [Google Scholar]
- 61.Nicolaou KC, Sarabia F, Finlay MRV, Ninkovic S, King NP, Vourloumis D and He Y, Chem. - Eur. J, 1997, 3, 1971–1986. [Google Scholar]
- 62.Nicolaou KC, Sarabia F, Ninkovic S, Finlay MRV and Boddy CNC, Angew. Chem. Int. Ed, 1998, 37, 81–84. [Google Scholar]
- 63.Nicolaou KC, He Y, Roschangar F, King NP, Vourloumis D and Li T, Angew. Chem. Int. Ed, 1998, 37, 84–87. [DOI] [PubMed] [Google Scholar]
- 64.Nicolaou KC, Finlay MRV, Ninkovic S and Sarabia F, Tetrahedron, 1998, 54, 7127–7166. [Google Scholar]
- 65.Nicolaou KC, Finlay MRV, Ninkovic S, King NP, He Y, Li T, Sarabia F and Vourloumis D, Chem. Biol, 1998, 5, 365–372. [DOI] [PubMed] [Google Scholar]
- 66.Nicolaou KC, Hepworth D, Ray VFinlay M, Paul King N, Werschkun B and Bigot A, Chem. Commun, 1999, 519–520. [Google Scholar]
- 67.Nicolaou KC, King NP, Finlay MRV, He Y, Roschangar F, Vourloumis D, Vallberg H, Sarabia F, Ninkovic S and Hepworth D, Bioorg. Med. Chem, 1999, 7, 665–697. [DOI] [PubMed] [Google Scholar]
- 68.Nicolaou KC, Hepworth D, King NP, Finlay MRV, Scarpelli R, Pereira MMA, Bollbuck B, Bigot A, Werschkun B and Winssinger N, Chem. - Eur. J, 2000, 6, 2783–2800. [DOI] [PubMed] [Google Scholar]
- 69.Nicolaou KC, Scarpelli R, Bollbuck B, Werschkun B, Pereira MMA, Wartmann M, Altmann KH, Zaharevitz D, Gussio R and Giannakakou P, Chem. Biol, 2000, 7, 593–599. [DOI] [PubMed] [Google Scholar]
- 70.Nicolaou KC, Namoto K, Li J, Ritzén A, Ulven T, Shoji M, Zaharevitz D, Gussio R, Sackett DL, Ward RD, Hensler A, Fojo T and Giannakakou P, ChemBioChem, 2001, 2, 69–75. [DOI] [PubMed] [Google Scholar]
- 71.Nicolaou KC, Namoto K, Ritzén A, Ulven T, Shoji M, Li J, D’Amico G, Liotta D, French CT, Wartmann M, Altmann K-H and Giannakakou P, J. Am. Chem. Soc, 2001, 123, 9313–9323. [DOI] [PubMed] [Google Scholar]
- 72.Nicolaou KC, Ritzén A, Namoto K, Buey RM, Díaz JF, Andreu JM, Wartmann M, Altmann K-H, O’Brate A and Giannakakou P, Tetrahedron, 2002, 58, 6413–6432. [Google Scholar]
- 73.Nicolaou KC, Sasmal PK, Rassias G, Reddy MV, Altmann K-H, Wartmann M, O’Brate A and Giannakakou P, Angew. Chem. Int. Ed, 2003, 42, 3515–3520. [DOI] [PubMed] [Google Scholar]
- 74.Nicolaou KC, Pratt BA, Arseniyadis S, Wartmann M, O’Brate A and Giannakakou P, ChemMedChem, 2006, 1, 41–44. [DOI] [PubMed] [Google Scholar]
- 75.Nicolaou KC, Rhoades D, Wang Y, Totokotsopoulos S, Bai R and Hamel E, ChemMedChem, 2015, 10, 1974–1979. [DOI] [PubMed] [Google Scholar]
- 76.Nicolaou KC, Rhoades D, Wang Y, Bai R, Hamel E, Aujay M, Sandoval J and Gavrilyuk J, J. Am. Chem. Soc, 2017, 139, 7318–7334. [DOI] [PubMed] [Google Scholar]
- 77.Nicolaou KC, Kim S, Pfefferkorn J, Xu J, Ohshima T, Hosokawa S, Vourloumis D and Li T, Angew. Chem. Int. Ed, 1998, 37, 1418–1421. [DOI] [PubMed] [Google Scholar]
- 78.Nicolaou KC, Winssinger N, Vourloumis D, Ohshima T, Kim S, Pfefferkorn J, Xu JY and Li T, J. Am. Chem. Soc, 1998, 120, 10814–10826. [Google Scholar]
- 79.Nicolaou KC, Natarajan S, Li H, Jain NF, Hughes R, Solomon ME, Ramanjulu JM, Boddy CNC and Takayanagi M, Angew. Chem. Int. Ed, 1998, 37, 2708–2714. [DOI] [PubMed] [Google Scholar]
- 80.Nicolaou KC, Jain NF, Natarajan S, Hughes R, Solomon ME, Li H, Ramanjulu JM, Takayanagi M, Koumbis AE and Bando T, Angew. Chem. Int. Ed, 1998, 37, 2714–2716. [DOI] [PubMed] [Google Scholar]
- 81.Nicolaou KC, Takayanagi M, Jain NF, Natarajan S, Koumbis AE, Bando T and Ramanjulu JM, Angew. Chem. Int. Ed, 1998, 37, 2717–2719. [DOI] [PubMed] [Google Scholar]
- 82.Nicolaou KC, Mitchell HJ, Jain NF, Winssinger N, Hughes R and Bando T, Angew. Chem. Int. Ed, 1999, 38, 240–244. [Google Scholar]
- 83.Nicolaou KC, Hughes R, Cho SY, Winssinger N, Smethurst C, Labischinski H and Endermann R, Angew. Chem. Int. Ed, 2000, 39, 3823–3828. [DOI] [PubMed] [Google Scholar]
- 84.Nicolaou KC, Cho SY, Hughes R, Winssinger N, Smethurst C, Labischinski H and Endermann R, Chem. - Eur. J, 2001, 7, 3798–3823. [DOI] [PubMed] [Google Scholar]
- 85.Nicolaou KC, Hughes R, Cho SY, Winssinger N, Labischinski H and Endermann R, Chem. - Eur. J, 2001, 7, 3824–3843. [DOI] [PubMed] [Google Scholar]
- 86.Nicolaou KC, Pfefferkorn JA and Cao G-Q, Angew. Chem. Int. Ed, 2000, 39, 734–739. [PubMed] [Google Scholar]
- 87.Nicolaou KC, Cao G-Q and Pfefferkorn JA, Angew. Chem. Int. Ed, 2000, 39, 739–743. [PubMed] [Google Scholar]
- 88.Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao GQ, Barluenga S and Mitchell HJ, J. Am. Chem. Soc, 2000, 122, 9939–9953. [Google Scholar]
- 89.Nicolaou KC, Pfefferkorn JA, Mitchell HJ, Roecker AJ, Barluenga S, Cao GQ, Affleck RL and Lillig JE, J. Am. Chem. Soc, 2000, 122, 9954–9967. [Google Scholar]
- 90.Nicolaou KC, Pfefferkorn JA, Barluenga S, Mitchell HJ, Roecker AJ and Cao GQ, J. Am. Chem. Soc, 2000, 122, 9968–9976. [Google Scholar]
- 91.Nicolaou KC, Roecker AJ, Barluenga S, Pfefferkorn JA and Cao G-Q, ChemBioChem, 2001, 2, 460–465. [DOI] [PubMed] [Google Scholar]
- 92.Nicolaou KC, Hughes R, Pfefferkorn JA, Barluenga S and Roecker AJ, Chem. - Eur. J, 2001, 7, 4280–4295. [DOI] [PubMed] [Google Scholar]
- 93.Nicolaou KC, Hughes R, Pfefferkorn JA and Barluenga S, Chem. - Eur. J, 2001, 7, 4296–4310. [DOI] [PubMed] [Google Scholar]
- 94.Xiao X-Y, Li R, Zhuang H, Ewing B, Karunaratne K, Lillig J, Brown R and Nicolaou KC, Biotechnol. Bioeng, 2000, 71, 44–50. [DOI] [PubMed] [Google Scholar]
- 95.Nicolaou KC, J. Org. Chem, 2005, 70, 7007–7027. [DOI] [PubMed] [Google Scholar]
- 96.Nicolaou KC, J. Med. Chem, 2005, 48, 5613–5638. [DOI] [PubMed] [Google Scholar]
- 97.Nicolaou KC, Li T, Nakada M, Hummel CW, Hiatt A and Wrasidlo W, Angew. Chem. Int. Ed. Engl, 1994, 33, 183–186. [Google Scholar]
- 98.Lode HN, Reisfeld RA, Handgretinger R, Nicolaou KC, Gaedicke G and Wrasidlo W, Cancer Res., 1998, 58, 2925–2928. [PubMed] [Google Scholar]
- 99.Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE and Upeslacis J, Cancer Res., 1993, 53, 3336–3342. [PubMed] [Google Scholar]
- 100.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, Hallett W, Tsou H-R, Upeslacis J, Shochat D, Mountain A, Flowers DA and Bernstein I, Bioconjugate Chem., 2002, 13, 47–58. [DOI] [PubMed] [Google Scholar]
- 101.Nicolaou KC and Rigol S, Acc. Chem. Res, 2019, 52, 127–139. [DOI] [PubMed] [Google Scholar]
- 102.Nicolaou KC and Rigol S, Angew. Chem. Int. Ed, 2019, 58, 11206–11241. [DOI] [PubMed] [Google Scholar]
- 103.Wrasidlo W, Gaedicke G, Guy RK, Renaud J, Pitsinos E, Nicolaou KC, Reisfeld RA and Lode HN, Bioconjugate Chem., 2002, 13, 1093–1099. [DOI] [PubMed] [Google Scholar]
- 104.Nicolaou KC, Xu JY, Kim S, Ohshima T, Hosokawa S and Pfefferkorn J, J. Am. Chem. Soc, 1997, 119, 11353–11354. [Google Scholar]
- 105.Hamel E, Sackett DL, Vourloumis D and Nicolaou KC, Biochemistry, 1999, 38, 5490–5498. [DOI] [PubMed] [Google Scholar]
- 106.Nicolaou KC, Ohshima T, Hosokawa S, van Delft FL, Vourloumis D, Xu JY, Pfefferkorn J and Kim S, J. Am. Chem. Soc, 1998, 120, 8674–8680. [Google Scholar]
- 107.Nicolaou KC, Pfefferkorn JA, Schuler F, Roecker AJ, Cao GQ and Casida JE, Chem. Biol, 2000, 7, 979–992. [DOI] [PubMed] [Google Scholar]
- 108.Nicolaou KC, Evans RM, Roecker AJ, Hughes R, Downes M and Pfefferkorn JA, Org. Biomol. Chem, 2003, 1, 908–920. [PubMed] [Google Scholar]
- 109.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer J-L, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JGA, Noel JP, Nicolaou KC and Evans RM, Mol. Cell, 2003, 11, 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Ballmer SG, Gillis EP, Fujii S, Schmidt MJ, Palazzolo AME, Lehmann JW, Morehouse GF and Burke MD, Science, 2015, 347, 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Granda JM, Donina L, Dragone V, Long D-L and Cronin L, Nature, 2018, 559, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Granda JM, Donina L, Dragone V, Long D-L and Cronin L, Nature, 2018, 562, E26–E26. [DOI] [PubMed] [Google Scholar]
- 113.Granda JM, Donina L, Dragone V, Long D-L and Cronin L, Nature, 2019, 570, E67–E69. [DOI] [PubMed] [Google Scholar]
- 114.Sanderson K, Nature, 2019, 568, 577–579. [DOI] [PubMed] [Google Scholar]
- 115.Riedlinger J, Reicke A, Zähner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Süssmuth RD and Fiedler HP, J. Antibiot, 2004, 57, 271–279. [DOI] [PubMed] [Google Scholar]
- 116.Bister B, Bischoff D, Ströbele M, Riedlinger J, Reicke A, Wolter F, Bull AT, Zähner H, Fiedler H-P and Süssmuth RD, Angew. Chem. Int. Ed, 2004, 43, 2574–2576. [DOI] [PubMed] [Google Scholar]
- 117.Nicolaou KC and Harrison ST, Angew. Chem. Int. Ed, 2006, 45, 3256–3260. [DOI] [PubMed] [Google Scholar]
- 118.Nicolaou KC and Harrison ST, J. Am. Chem. Soc, 2007, 129, 429–440. [DOI] [PubMed] [Google Scholar]
- 119.Nicolaou KC and Harrison ST, J. Am. Chem. Soc, 2007, 129, 429–440. [DOI] [PubMed] [Google Scholar]
- 120.Keller S, Nicholson G, Drahl C, Sorensen E, Fiedler H-P and Süssmuth RD, J. Antibiot, 2007, 60, 391–394. [DOI] [PubMed] [Google Scholar]
- 121.Tamejiro H, Bhaskar RG, Tatsuya M and Takeshi H, Bull. Chem. Soc. Jpn, 1995, 68, 350–363. [Google Scholar]
- 122.Nicolaou KC, Harrison ST and Chen JS, Synthesis, 2009, 2009, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka N, Okasaka M, Ishimaru Y, Takaishi Y, Sato M, Okamoto M, Oshikawa T, Ahmed SU, Consentino LM and Lee K-H, Org. Lett, 2005, 7, 2997–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nicolaou KC, Sanchini S, Wu TR and Sarlah D, Chem. - Eur. J, 2010, 16, 7678–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka N, Kashiwada Y, Kim SY, Hashida W, Sekiya M, Ikeshiro Y and Takaishi Y, J. Nat. Prod, 2009, 72, 1447–1452. [DOI] [PubMed] [Google Scholar]
- 126.Nicolaou KC, Sanchini S, Sarlah D, Lu G, Wu TR, Nomura DK, Cravatt BF, Cubitt B, de la Torre JC, Hessell AJ and Burton DR, Proc. Natl. Acad. Sci. U.S.A, 2011, 108, 6715–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nicolaou KC, Wu TR, Sarlah D, Shaw DM, Rowcliffe E and Burton DR, J. Am. Chem. Soc, 2008, 130, 11114–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nicolaou KC, Lu M, Totokotsopoulos S, Heretsch P, Giguère D, Sun Y-P, Sarlah D, Nguyen TH, Wolf IC, Smee DF, Day CW, Bopp S and Winzeler EA, J. Am. Chem. Soc, 2012, 134, 17320–17332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kornblum N and DeLaMare HE, J. Am. Chem. Soc, 1951, 73, 880–881. [Google Scholar]
- 130.Staben ST, Linghu X and Toste FD, J. Am. Chem. Soc, 2006, 128, 12658–12659. [DOI] [PubMed] [Google Scholar]
- 131.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio Á, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D and Singh SB, Nature, 2006, 441, 358–361. [DOI] [PubMed] [Google Scholar]
- 132.Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Genilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J and Singh SB, Angew. Chem. Int. Ed, 2007, 46, 4684–4688. [DOI] [PubMed] [Google Scholar]
- 133.Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, González I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF and Singh SB, Proc. Natl. Acad. Sci. U.S.A, 2007, 104, 7612–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang C, Ondeyka J, Zink DL, Burgess B, Wang J and Singh SB, Chem. Commun, 2008, 5034–5036. [DOI] [PubMed] [Google Scholar]
- 135.Herath KB, Zhang C, Jayasuriya H, Ondeyka JG, Zink DL, Burgess B, Wang J and Singh SB, Org. Lett, 2008, 10, 1699–1702. [DOI] [PubMed] [Google Scholar]
- 136.Lei A, Waldkirch JP, He M and Zhang X, Angew. Chem. Int. Ed, 2002, 41, 4526–4529. [DOI] [PubMed] [Google Scholar]
- 137.Lei A, He M, Wu S and Zhang X, Angew. Chem. Int. Ed, 2002, 41, 3457–3460. [DOI] [PubMed] [Google Scholar]
- 138.Lei A, He M and Zhang X, J. Am. Chem. Soc, 2002, 124, 8198–8199. [DOI] [PubMed] [Google Scholar]
- 139.Nicolaou KC, Li A, Edmonds DJ, Tria GS and Ellery SP, J. Am. Chem. Soc, 2009, 131, 16905–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nicolaou KC, Lister T, Denton RM, Montero A and Edmonds DJ, Angew. Chem. Int. Ed, 2007, 46, 4712–4714. [DOI] [PubMed] [Google Scholar]
- 141.Nicolaou KC, Tang Y, Wang J, Stepan AF, Li A and Montero A, J. Am. Chem. Soc, 2007, 129, 14850–14851. [DOI] [PubMed] [Google Scholar]
- 142.Nicolaou KC, Stepan AF, Lister T, Li A, Montero A, Tria GS, Turner CI, Tang Y, Wang J, Denton RM and Edmonds DJ, J. Am. Chem. Soc, 2008, 130, 13110–13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lambert TH and Danishefsky SJ, J. Am. Chem. Soc, 2006, 128, 426–427. [DOI] [PubMed] [Google Scholar]
- 144.de RM, Fröhlich Figueiredo R. and Christmann M, Angew. Chem. Int. Ed, 2007, 46, 2883–2886. [DOI] [PubMed] [Google Scholar]
- 145.Lin Y-Y, Risk M, Ray SM, Van Engen D, Clardy J, Golik J, James JC and Nakanishi K, J. Am. Chem. Soc, 1981, 103, 6773–6775. [Google Scholar]
- 146.Nicolaou KC, Reddy KR, Skokotas G, Sato F and Xiao X-Y, J. Am. Chem. Soc, 1992, 114, 7935–7936. [Google Scholar]
- 147.Nicolaou KC, Theodorakis EA, Rutjes FPJT, Tiebes J, Sato M, Untersteller E and Xiao X-Y, J. Am. Chem. Soc, 1995, 117, 1171–1172. [Google Scholar]
- 148.Nicolaou KC, Rutjes FPJT, Theodorakis EA, Tiebes J, Sato M and Untersteller E, J. Am. Chem. Soc, 1995, 117, 1173–1174. [Google Scholar]
- 149.Nicolaou KC, Yang Z, Shi G.-q., Gunzner JL, Agrios KA and Gartner P, Nature, 1998, 392, 264–269. [DOI] [PubMed] [Google Scholar]
- 150.Nicolaou KC, Bunnage ME, McGarry DG, Shi S, Somers PK, Wallace PA, Chu X-J, Agrios KA, Gunzner JL and Yang Z, Chem. - Eur. J, 1999, 5, 599–617. [Google Scholar]
- 151.Nicolaou KC, Wallace PA, Shi S, Ouellette MA, Bunnage ME, Gunzner JL, Agrios KA, Shi G.-q., Gärtner P and Yang Z, Chem. - Eur. J, 1999, 5, 618–627. [Google Scholar]
- 152.Nicolaou KC, Shi G.-q., Gunzner JL, Gärtner P, Wallace PA, Ouellette MA, Shi S, Bunnage ME, Agrios KA, Veale CA, Hwang C-K, Hutchinson J, Prasad CVC, Ogilvie WW and Yang Z, Chem. - Eur. J, 1999, 5, 628–645. [Google Scholar]
- 153.Nicolaou KC, Gunzner JL, Shi G.-q., Agrios KA, Gärtner P and Yang Z, Chem. - Eur. J, 1999, 5, 646–658. [Google Scholar]
- 154.Nicolaou KC and Frederick MO, Angew. Chem. Int. Ed, 2007, 46, 5278–5282. [DOI] [PubMed] [Google Scholar]
- 155.Gallimore AR and Spencer JB, Angew. Chem. Int. Ed, 2006, 45, 4406–4413. [DOI] [PubMed] [Google Scholar]
- 156.Nicolaou KC, Frederick MO, Burtoloso ACB, Denton RM, Rivas F, Cole KP, Aversa RJ, Gibe R, Umezawa T and Suzuki T, J. Am. Chem. Soc, 2008, 130, 7466–7476. [DOI] [PubMed] [Google Scholar]
- 157.Nicolaou KC, Aversa RJ, Jin J and Rivas F, J. Am. Chem. Soc, 2010, 132, 6855–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nicolaou KC, Gelin CF, Seo JH, Huang Z and Umezawa T, J. Am. Chem. Soc, 2010, 132, 9900–9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nicolaou KC, Baker TM and Nakamura T, J. Am. Chem. Soc, 2011, 133, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nicolaou KC, Seo JH, Nakamura T and Aversa RJ, J. Am. Chem. Soc, 2011, 133, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nicolaou KC, Heretsch P, Nakamura T, Rudo A, Murata M and Konoki K, J. Am. Chem. Soc, 2014, 136, 16444–16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nicolaou KC, Postema MHD and Claiborne CF, J. Am. Chem. Soc, 1996, 118, 1565–1566. [Google Scholar]
- 163.Nicolaou KC, Postema MHD, Yue EW and Nadin A, J. Am. Chem. Soc, 1996, 118, 10335–10336. [Google Scholar]
- 164.Nicolaou KC, Tiebes J, Theodorakis EA, Rutjes FPJT, Koide K, Sato M and Untersteller E, J. Am. Chem. Soc, 1994, 116, 9371–9372. [Google Scholar]
- 165.Gawley RE, Rein KS, Jeglitsch G, Adams DJ, Theodorakis EA, Tiebes J, Nicolaou KC and Baden DG, Chem. Biol, 1995, 2, 533–541. [DOI] [PubMed] [Google Scholar]
- 166.Rein KS, Baden DG and Gawley RE, J. Org. Chem, 1994, 59, 2101–2106. [Google Scholar]
- 167.Zheng C-J, Yu H-E, Kim E-H and Kim W-G, J. Antibiot, 2008, 61, 633–637. [DOI] [PubMed] [Google Scholar]
- 168.Nicolaou KC, Nilewski C, Hale CRH, Ioannidou HA, ElMarrouni A and Koch LG, Angew. Chem. Int. Ed, 2013, 52, 8736–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nicolaou KC, Liu G, Beabout K, McCurry MD and Shamoo Y, J. Am. Chem. Soc, 2017, 139, 3736–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fitzpatrick FA and Wynalda MA, J. Biol. Chem, 1983, 258, 11713–11718. [PubMed] [Google Scholar]
- 171.Mahmud I, Smith DL, Whyte MA, Nelson JT, Cho D, Tokes LG, Alvarez R and Willis AL, Prostaglandins, Leukotrienes Med., 1984, 16, 131–146. [DOI] [PubMed] [Google Scholar]
- 172.Hegde S, Kaushal N, Ravindra KC, Chiaro C, Hafer KT, Gandhi UH, Thompson JT, van den Heuvel JP, Kennett MJ, Hankey P, Paulson RF and Prabhu KS, Blood, 2011, 118, 6909–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nicolaou KC, Heretsch P, ElMarrouni A, Hale CRH, Pulukuri KK, Kudva AK, Narayan V and Prabhu KS, Angew. Chem. Int. Ed, 2014, 53, 10443–10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nicolaou KC, Pulukuri KK, Rigol S, Heretsch P, Yu R, Grove CI, Hale CRH, ElMarrouni A, Fetz V, Brönstrup M, Aujay M, Sandoval J and Gavrilyuk J, J. Am. Chem. Soc, 2016, 138, 6550–6560. [DOI] [PubMed] [Google Scholar]
- 175.Nicolaou KC, Pulukuri KK, Rigol S, Peitsinis Z, Yu R, Kishigami S, Cen N, Aujay M, Sandoval J, Zepeda N and Gavrilyuk J, J. Org. Chem, 2019, 84, 365–378. [DOI] [PubMed] [Google Scholar]
- 176.Nicolaou KC, Pulukuri KK, Rigol S, Peitsinis Z, Yu R, Kishigami S, Cen N, Aujay M, Sandoval J, Zepeda N and Gavrilyuk J, J. Org. Chem, 2019, 84, 2377–2378. [DOI] [PubMed] [Google Scholar]
- 177.Sasse F, Steinmetz H, Heil J, Höfle G and Reichenbach H, J. Antibiot, 2000, 53, 879–885. [DOI] [PubMed] [Google Scholar]
- 178.Matcha K and Antonchick AP, Angew. Chem. Int. Ed, 2013, 52, 2082–2086. [DOI] [PubMed] [Google Scholar]
- 179.Khemnar AB and Bhanage BM, Synlett, 2014, 25, 110–114. [Google Scholar]
- 180.Chatgilialoglu C, Crich D, Komatsu M and Ryu I, Chem. Rev, 1999, 99, 1991–2070. [DOI] [PubMed] [Google Scholar]
- 181.Yeung CS and Dong VM, Chem. Rev, 2011, 111, 1215–1292. [DOI] [PubMed] [Google Scholar]
- 182.Nicolaou KC, Yin J, Mandal D, Erande RD, Klahn P, Jin M, Aujay M, Sandoval J, Gavrilyuk J and Vourloumis D, J. Am. Chem. Soc, 2016, 138, 1698–1708. [DOI] [PubMed] [Google Scholar]
- 183.Nicolaou KC, Erande RD, Yin J, Vourloumis D, Aujay M, Sandoval J, Munneke S and Gavrilyuk J, J. Am. Chem. Soc, 2018, 140, 3690–3711. [DOI] [PubMed] [Google Scholar]
- 184.Wang Y, Benz FW, Wu Y, Wang Q, Chen Y, Chen X, Li H, Zhang Y, Zhang R and Yang J, Mol. Pharmacol, 2016, 89, 233–242. [DOI] [PubMed] [Google Scholar]
- 185.Jat JL, Paudyal MP, Gao H, Xu Q-L, Yousufuddin M, Devarajan D, Ess DH, Kürti L and Falck JR, Science, 2014, 343, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jansen R, Irschik H, Reichenbach H, Wray V and Höfle G, Liebigs Ann. Chem, 1994, 1994, 759–773. [Google Scholar]
- 187.Nicolaou KC, Bellavance G, Buchman M and Pulukuri KK, J. Am. Chem. Soc, 2017, 139, 15636–15639. [DOI] [PubMed] [Google Scholar]
- 188.Nicolaou KC, Buchman M, Bellavance G, Krieger J, Subramanian P and Pulukuri KK, J. Org. Chem, 2018, 83, 12374–12389. [DOI] [PubMed] [Google Scholar]
- 189.Davies J, Wang H, Taylor T, Warabi K, Huang X-H and Andersen RJ, Org. Lett, 2005, 7, 5233–5236. [DOI] [PubMed] [Google Scholar]
- 190.Nicolaou KC, Zhang H, Chen JS, Crawford JJ and Pasunoori L, Angew. Chem. Int. Ed, 2007, 46, 4704–4707. [DOI] [PubMed] [Google Scholar]
- 191.Nicolaou KC, Chen JS, Zhang H and Montero A, Angew. Chem. Int. Ed, 2008, 47, 185–189. [DOI] [PubMed] [Google Scholar]
- 192.Nicolaou KC, Wang Y, Lu M, Mandal D, Pattanayak MR, Yu R, Shah AA, Chen JS, Zhang H, Crawford JJ, Pasunoori L, Poudel YB, Chowdari NS, Pan C, Nazeer A, Gangwar S, Vite G and Pitsinos EN, J. Am. Chem. Soc, 2016, 138, 8235–8246. [DOI] [PubMed] [Google Scholar]
- 193.Poudel YB, Rao C, Kotapati S, Deshpande M, Thevanayagam L, Pan C, Cardarelli J, Chowdari N, Kaspady M, Samikannu R, Kuppusamy P, Saravanakumar P, Arunachalam PN, Deshpande S, Rangan V, Rampulla R, Mathur A, Vite GD and Gangwar S, Bioorg. Med. Chem. Lett, 2020, 30, 126782. [DOI] [PubMed] [Google Scholar]
- 194.Nicolaou KC, Das D, Lu Y, Rout S, Pitsinos EN, Lyssikatos J, Schammel A, Sandoval J, Hammond M, Aujay M and Gavrilyuk J, J. Am. Chem. Soc, 2020, 142, DOI: 10.1021/jacs.9b12522. [DOI] [PubMed] [Google Scholar]
- 195.Oku N, Matsunaga S and Fusetani N, J. Am. Chem. Soc, 2003, 125, 2044–2045. [DOI] [PubMed] [Google Scholar]
- 196.Nicolaou KC, Lu Z, Li R, Woods JR and Sohn T.-i., J. Am. Chem. Soc, 2015, 137, 8716–8719. [DOI] [PubMed] [Google Scholar]
- 197.Nicolaou KC, Li R, Lu Z, Pitsinos EN, Alemany LB, Aujay M, Lee C, Sandoval J and Gavrilyuk J, J. Am. Chem. Soc, 2018, 140, 12120–12136. [DOI] [PubMed] [Google Scholar]
- 198.Zhang H, Li R, Ba S, Lu Z, Pitsinos EN, Li T and Nicolaou KC, J. Am. Chem. Soc, 2019, 141, 7842–7852. [DOI] [PubMed] [Google Scholar]
- 199.Kolbe H, Ann. Chem. Pharm, 1845, 54, 145–188. [Google Scholar]
- 200.Nicolaou KC, Vourloumis D, Winssinger N and Baran PS, Angew. Chem. Int. Ed, 2000, 39, 44–122. [PubMed] [Google Scholar]
- 201.Nicolaou KC, Montagnon T and Snyder SA, Chem. Commun, 2003, 551–564. [DOI] [PubMed] [Google Scholar]
- 202.Nicolaou KC, Edmonds DJ and Bulger PG, Angew. Chem. Int. Ed, 2006, 45, 7134–7186. [DOI] [PubMed] [Google Scholar]
- 203.Nicolaou KC and Chen JS, Chem. Soc. Rev, 2009, 38, 2993–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nicolaou KC, Angew. Chem. Int. Ed, 2014, 53, 4730. [DOI] [PubMed] [Google Scholar]
- 205.Chen Z, Mercer JAM, Zhu X, Romaniuk JAH, Pfattner R, Cegelski L, Martinez TJ, Burns NZ and Xia Y, Science, 2017, 357, 475–479. [DOI] [PubMed] [Google Scholar]
- 206.Su JK, Feist JD, Yang J, Mercer JAM, Romaniuk JAH, Chen Z, Cegelski L, Burns NZ and Xia Y, J. Am. Chem. Soc, 2018, 140, 12388–12391. [DOI] [PubMed] [Google Scholar]
- 207.Nicolaou KC, Rigol S and Yu R, CCS Chemistry, 2019, 1, 3–37. [Google Scholar]