Abstract
As the brain develops, proliferating cells organize into structures, differentiate, migrate, extrude long processes, and connect with other cells. These biological processes produce mechanical forces that further shape cellular dynamics and organ patterning. A major unanswered question in developmental biology is how the mechanical forces produced during development are detected and transduced by cells to impact biochemical and genetic programs of development. This gap in knowledge stems from a lack of understanding of the molecular players of cellular mechanics and an absence of techniques for measuring and manipulating mechanical forces in tissue. In this review article we examine recent advances that are beginning to clear these bottlenecks, and highlight results from new approaches that reveal the role of mechanical forces in neurodevelopmental processes.
Keywords: Neural development, brain morphogenesis, mechanical forces, developmental biology, biomechanics, mechanotransduction
Introduction
A developing embryo grows, folds, and contorts in a series of complex but reproducible events to generate all organs of the human body. Each organ is shaped to the unique architecture necessary to support its function. Failure in this process results in spontaneous abortion or lifelong developmental defects. Thus, understanding organ patterning is critical to preventing and treating developmental disorders. Traditionally the field of developmental biology has focused on genetics and chemical signaling; however, emerging evidence shows that mechanical cues are as important as genetic and chemical cues.
D’Arcy Thompson proposed in a seminal treatise over a hundred years ago that organismal growth is governed by physical and mechanical principles [1]. Empirical observations in embryology provided evidence that mechanical cues were important for development [2]. However, the daunting technical challenges associated with studying physical forces in living tissue, and the lack of a molecular understanding of how mechanical forces are generated and transduced by cells, prohibited an in depth examination. Furthermore, with the advent of molecular biology the focus in the field of developmental biology through the 20th century shifted to understanding molecular and genetic mechanisms. In the last two decades, the area of developmental mechanics - the study of how forces arise during embryo development and how they shape organismal growth - has gathered momentum.
Vertebrate brain development begins when ectodermal cells are induced into the neural lineage, forming the neural plate, which folds over to generate the neural tube. The tube elongates and bends, then dilates and constricts at specific points. The neural stem/progenitor cells (NSPCs) lining hollow cavities called ventricles proliferate and then differentiate. The differentiated cells migrate, connect with each other, and generate specialized neural signaling centers. The biological processes that give rise to the brain and spinal cord are all dynamic processes that produce mechanical stresses and strains, which in turn affect cell behavior and organ patterning (Fig. 1).
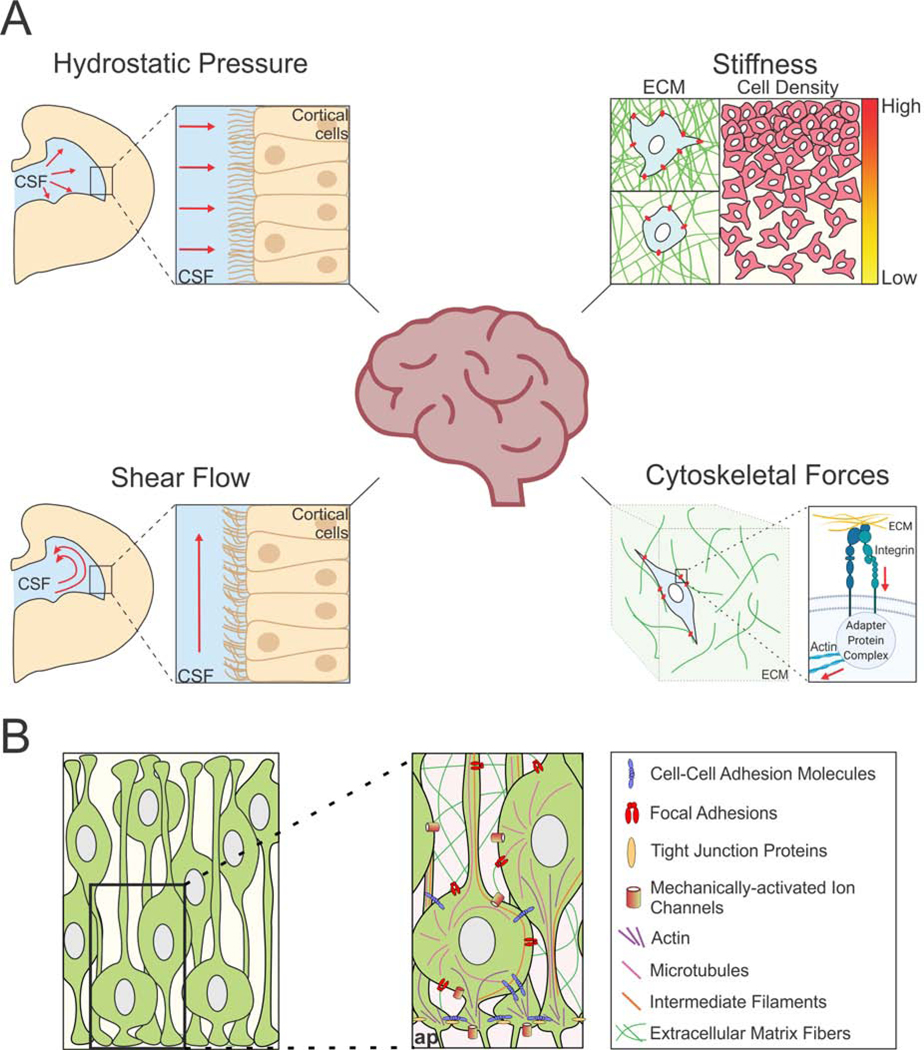
Figure 1. Mechanotransduction in the developing brain.
(A) The developing brain experiences a variety of mechanical cues. Left panels show a schematic of a coronal cross section of half of the developing brain, and the fluid-based forces, hydrostatic pressure (upper left) and shear flow (lower left) impinging against cells that line the ventricles. Tissue stiffness (upper right) is modulated by extracellular matrix components or by cellular density. The actomyosin cytoskeleton (lower right) connects to the extracellular matrix through focal adhesions and is integral to cellular mechanotransduction during development. CSF = cerebrospinal fluid, ECM = extracellular matrix. (B) The molecules and cellular structures involved in the mechanotransduction in the developing neuroepithelium. ap = apical border.
Recent experiments employing novel bioengineering principles and methodologies have demonstrated the importance of mechanical cues in cell fate and differentiation. These findings generated new principles of neural development, and motivated the development of novel approaches for measurement and manipulation of mechanical cues in developing neural tissue. In this review, we examine developmental mechanics of the brain in mammalian and non-mammalian organisms, highlight the latest developments in this area and identify key areas of investigation spawned by these recent findings. We regret not being able to cite several related studies due to space constraints, and refer the interested reader to other review articles wherever possible.
Neural Tube Closure
The first major mechanical event in the development of the nervous system is neural tube closure (NTC). This is mediated by actomyosin force generation at the neural plate’s apical surface, which becomes the apical border once the neural tube closes. The neural plate bends at three specific locations called hinge points. The two ends of the folding neural plate then meet, and “zipper” the tube closed (Fig. 2). Failure in this process causes neural tube defects such as anencephaly and spina bifida.
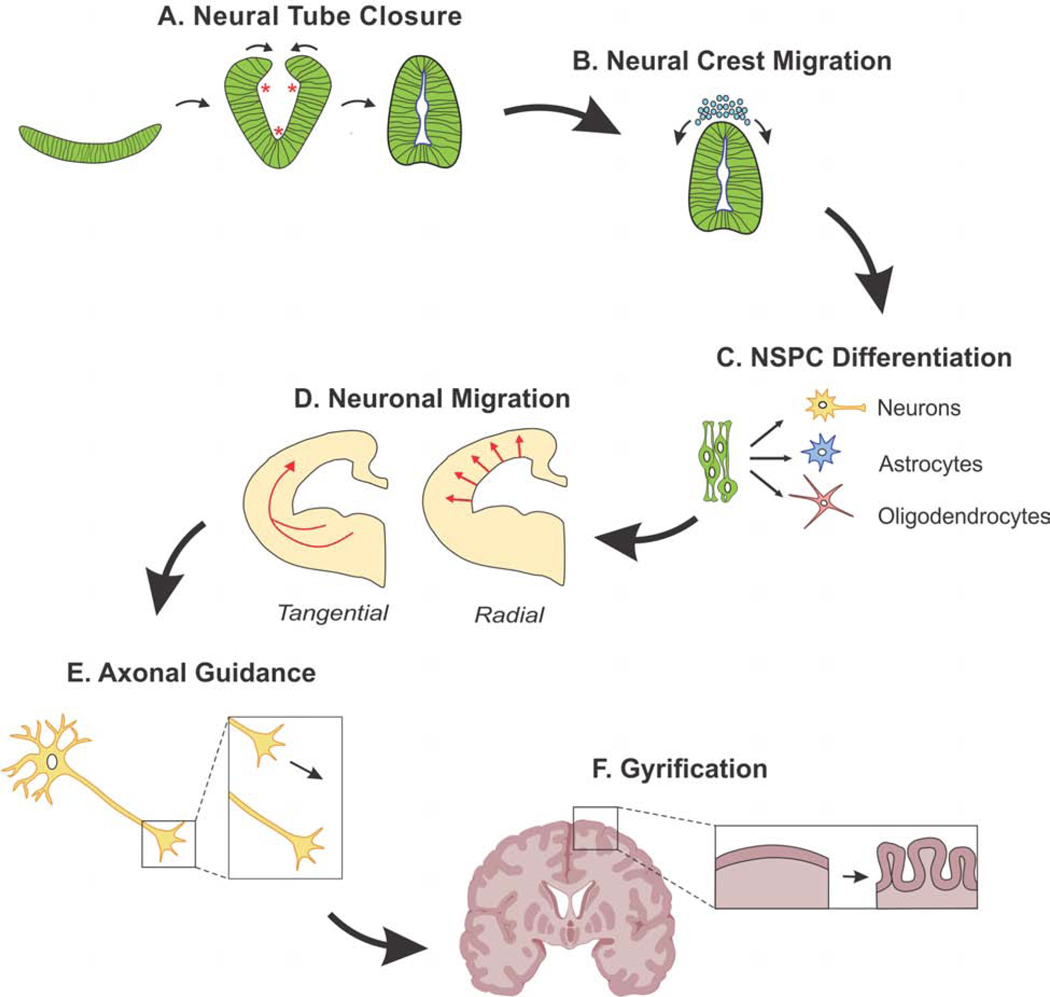
Figure 2. Timeline of mechanical events during neural development.
(A) The neural plate bends at three points, called hinge points (asterisks), and closes to form the neural tube.(B) Neural crest cells migrate away from the closed neural tube to form a variety of structures. (C) Neural stem/progenitor cells (NSPCs) differentiate into neurons, astrocytes, and oligodendrocytes. (D) Newly-formed neurons migrate radially or tangentially to their final destination. (E) Axons extend from the newborn neurons to form connections throughout the developing brain. (F) In some mammals including humans, the cortex folds to increase cortical surface area.
Galea et al. described the complex dynamics of mechanical forces during mammalian NTC [3]. They showed that laser ablation of the closing neural tube at a single point was sufficient to re-open a portion of the neural tube longitudinally. Furthermore, after this re-opening, tissues distant from the neural tube underwent expansions or compressions, suggesting a mechanical coupling of these tissues with the closing neural tube through the actin cytoskeleton [3].
Additionally, recent work suggests there are variations in tissue stiffness during NTC: the zippering points are softer than the immediately surrounding tissue of the neural tube [4]. This work utilizes a new non-invasive technique called Brillouin microscopy, which provides stiffness measurements in 3D, as opposed to the traditional 2D surface stiffness measurements via Atomic Force Microscopy (AFM). If this observation holds across different species, it would be interesting to examine whether the observed stiffness differences during NTC are an epiphenomenon of the process, or whether they are key for its normal progression.
Neural Crest Cell Migration
The first major migratory event during development of the nervous system involves neural crest (NC) cells. This specialized neural stem cell population migrates away from the neural tube over relatively long distances and generates diverse structures such as cartilage, peripheral nerves, and smooth muscles.
NC migration is guided by chemotaxis [5]. However, substrate mechanical cues also play an important role in cellular migration [6,7]. A recent study by Barriga et al. examined the contribution of tissue stiffness to the migratory patterns of the NC in vivo [8 ••]. They showed that the initiation of NC migration is regulated by stiffening of the surrounding tissue. When the stiffness of the tissue was mechanically or pharmacologically perturbed, NC migration did not commence. Moreover, the stiffness cues were largely found to arise from changes in cellular density [8 ••]. Additionally, Scarpa et al. found that when NC migration begins, adhesion forces between cells reduce and traction forces applied to the substrate at the leading edge of the NC increases [9]. The few NC cells that initially migrate (termed “leader cells”) appear transcriptionally different from those that follow [10], suggesting a functional difference between leader and follower cells. It remains to be determined whether leader and follower cells differ in their response to the mechanical environment.
Neural Progenitor Proliferation
Once the neural tube closes, fluid mechanical cues become a powerful determinant of the continual development of the nervous system. In a classic experiment almost half a century ago, Desmond and Jacobson showed that relieving pressure in the brain ventricle of 3-day old chick embryos for a few hours results in a collapse of the entire central nervous system [11]. The intervention also reduced proliferation of the neural stem/progenitor cells (NSPCs). This experiment elegantly demonstrated that ventricular pressure is a defining aspect of early brain development. Although the molecular mechanism associated with this process in embryonic development is unclear, work is beginning to identify the molecular signaling involved. Desmond et al. identified focal adhesion kinase (FAK) as part of the mechanoresponsive signaling pathway that reacts to increased ventricular pressure [12]. They found that artificially increasing neural tube pressure by increasing intraluminal osmolarity caused activated FAK to localize to the apical border [12].
Recent work has now shown shear flow to be another important regulator of NPSC proliferation. The epithelial sodium channel (ENaC) was found to be a direct mechanotransducer of CSF shear flow in adult mouse NSPCs [13]. Through time-lapse imaging, the Gotz group found that ENaC allowed the influx of sodium ions into the cell in response to CSF flow. When this channel was functionally inhibited, NSPC proliferation decreased [13]. These studies set the stage for further investigations to understand the magnitude, temporal dynamics, and origin of the fluid mechanics in neural development.
Neural Progenitor Differentiation
The neural tube is composed of radial glial cells (RGCs), a class of NSPCs. These bipolar cells span the neuroepithelium, extending processes from the apical to the basal surface, with the cell bodies staggered to form a pseudostratified layer. These cells initially divide rapidly to expand the NSPC pool, and then differentiate first into neurons, then into glial cells. Studies examining mechanical regulation of NSPC fate have largely focused on substrate stiffness in vitro, in part due to the ease of tuning this mechanical cue using polymer chemistry [14–19].
Seminal work from the Discher group demonstrated that mesenchymal stem cell lineage choice is powerfully affected by substrate stiffness: soft substrates promoted neurogenic differentiation, moderately stiff substrates induced myogenic differentiation, and hard substrates triggered osteogenic differentiation [20]. This mechanosensitive lineage specification was found to require cellular contractility through Myosin II motors, suggesting the model that stem cells use Myosin II-mediated traction forces to probe substrate stiffness [20]. This paradigm-shifting study highlighted the importance of mechanical cues for stem cell fate and launched the new field of stem cell mechanobiology.
NSPC lineage choice into neurons or glia is also modulated by substrate stiffness [21–23]. Keung et al showed that the Rho GTPases RhoA and Cdc42 were important for NSPC specification, and also identified RhoA as important for neurogenesis in the adult rat brain [24]. Recently, dynamic hydrogels have been used to resolve temporal dynamics of this fate decision [25]. By reversing the stiffness of substrates at different time points, Rammensee et al. identified a time window of 12–36 hours in which NSPCs were receptive to stiffness cues that informed fate decisions. Reversal of substrate stiffness within this time window resulted in the reversal of fate commitment; however, after this time window passed, NSPCs were generally committed to their fate [25].
Our group discovered that Piezo1, a mechanically-activated ion channel, transduces substrate stiffness to direct the mechanosensitive lineage specification of NSPCs [23]. Specifically, cell-generated traction forces elicit Piezo1 Ca2+ flickers in spatially localized hotspots, suggesting a model wherein cells generate traction forces to probe substrate stiffness, and Piezo1 tranduces these traction forces into spatially-regulated Ca2+ flickers to determine cell fate [26]. When Piezo1 was pharmacologically inhibited or genetically knocked down, human brain-derived fetal cortical NSPCs (hNSPCs) showed a decrease in neurogenesis and an increase in astrogenesis [23]. Interestingly, this result differed from a previous report from adult rat hippocampal neural stem cells, which showed increased neuron formation on soft substrates versus stiff substrates [21,23], suggesting that the biological origin of the stem cells may influence how they respond to mechanics.
Piezo1 also regulated the nucleo-cytoplasmic localization of the transcriptional co-activator Yap in hNSPCs: Yap was excluded from the nucleus on soft substrates (which elicited reduced Piezo1 activity) and also when Piezo1 was knocked down [23]. This suggests that Piezo1-mediated Yap signaling may be involved in shaping the mechanical response of NSPCs. In rat, adult hippocampal neural stem cells, Rammensee et al showed that Yap was involved in stiffness-mediated neurogenesis, but not through the Yap nucleo-cytoplasmic localization mechanism. Rather, they found that Yap interacts with B-catenin on stiff substrates to negatively regulate neurogenesis [25]. While the two studies demonstrate a role for Yap in neural stem cells, they suggest that the underlying mechanisms may differ with neural stem cells from different brain regions, developmental stages, or species.
These in vitro studies conducted over the course of a decade uncovered the importance of mechanics in stem cells of the neural lineage and identified key underlying mechanisms. However, an open question remained as to whether tissue stiffness played a role in vivo. While studies have yet to examine the role of stiffness on NSPC differentiation in the embryonic brain, recent work by Segel et al. showed how stiffness affects proliferation and differentiation of adult rat oligodendrocyte progenitor cells (OPCs). They showed that the rat brain stiffens with age, and found that OPCs in stiffer adult brains have significantly lower rates of proliferation and differentiation than OPCs in softer newborn brains, an observation recapitulated in vitro with hard and soft substrates [27 ••]. Furthermore, this “ageing” effect was reversible if the stiffer environments were softened pharmacologically in vivo. Consistent with our group’s findings, they also observed that matrix stiffness is transduced by Piezo1.
Many in vitro studies have used stiffness ranges on the scale of thousands of pascals, whereas the in vivo stiffness ranges in adult and embryonic brains are much smaller, on the scale of a few hundred pascals [28–31]. Interestingly, the study by Segel et al. suggests that even small stiffness ranges seem to powerfully affect cell behavior [27 ••]. A study by Kjell et al found that one of the neurogenic niches in the adult mouse brain, the subventricular zone, is 100 Pa stiffer than surrounding tissues [32 •]. To test whether this small difference was sufficient to induce higher rates of neurogenesis, they placed NSPCs onto gels that only differed by 100 Pa in stiffness and found that the stiffer gel resulted in double the rate of neuroblast formation.
Another mechanical cue, substrate stretch, has also been found to play a role in NSPC lineage choice. NSPCs on substrates that were stretched preferentially increased oligodendrocyte specification [33]. Further studies will be required to evaluate the interplay of different mechanical cues in modulating NSPC differentiation.
Neuronal Migration
Newly-formed neurons must position themselves appropriately in the developing brain. They typically do so through two modalities of migration. The first modality, radial migration, which is the major form of neuronal migration in the cerebrum, occurs when neurons migrate perpendicular to the ventricular surface along the projections of RGCs to their appropriate layer. A subset of neurons, many of which are inhibitory interneurons from the ganglionic eminences, display another form of migration, tangential migration, whereby the cells move parallel to the ventricular surface (Fig. 2).
During migration, traction forces are generated by the cell to allow translocation [34]. Using traction force microscopy, Jiang et al. demonstrated that migrating neurons in vitro exhibit three centers of traction force generation, one in the trailing process, and two in the leading process - one near the growth cone and the other near the soma [35]. Neurons must translocate their soma during migration, which was thought to involve both “pulling forces” at the leading process, and “pushing forces” from the trailing process of the neuron. However, Jiang et al. only found pulling forces, and no evidence of pushing forces in migrating neurons [35]. It will be interesting to examine to what extent in vivo mechanisms reflect the in vitro observations.
Neuronal Wiring
Once neurons migrate to their final destination, axons extend and connect to their target. This process, termed axon guidance or pathfinding, is tightly regulated since precise targeting of axonal processes is critical to the generation of normal neural circuits. While work initially focused on the biochemical aspects of this process, studies have demonstrated mechanical cues to also be vital in vitro [36]. However, until recently not much was known regarding the extent to which these findings are relevant in vivo.
Koser et al. showed the presence of differential stiffnesses in the developing frog brain. Their work suggests that stiff tissues dictate a straight axonal trajectory, stiffness gradients guide axon turning toward softer tissues, and softer tissues inform axons to slow down and splay out [37]. They also showed that Piezo1 transduces these matrix stiffness cues to inform the axonal pathfinding. Either disruption of normal stiffnesses in the brain or inhibition of Piezo1 resulted in abnormal axonal outgrowth [37]. A follow-up study by the same group showed that cell density is responsible for producing differences in tissue stiffness [38 ••].
A new mechanism of wiring termed “retrograde axon growth” was recently proposed, wherein the synaptic end of the axon is fixed in place and the cell body migrates away to its destination [39]. Breau et al. show in zebrafish olfactory placode, that cells are compressed by neighboring cells, forcing the neuronal soma away from the axon tip and initiating the growth of the axon in a retrograde manner [39]. This is a fascinating new mechanism that requires the coordinated mechanical action of neighboring cells. It will be important to explore whether this is conserved in other animal model systems.
Gyrification
An aspect of neural development unique to a subset of mammals is cortical folding, thought to occur to increase the total surface area of the cerebral cortex and has been appreciated as a highly mechanical process [40]. While many studies have looked at gyrification using animal models such as ferrets and nonhuman primates and computational modeling, new studies have experimentally modeled this process in vitro to examine the mechanics of the process in greater detail.
Karzbrun et al, using human brain organoids, highlighted the importance of cytoskeletal forces in forming gyri, the “ridges” of the cerebral cortex [41 ••]. Upon pharmacologically inhibiting force-generating myosin motors, they found a reduction in the curvature of the brain organoid folds, suggesting these cell-generated forces maintain the appropriate shape of gyri [41 ••]. Additionally, AFM-measurements of gyri in human organotypic cultures show that the nascent gyri are stiffer, while the sulci, the furrows of the cerebral cortex, are softer by a few hundred pascals [31]. More studies will be required to determine whether this effect is an epiphenomenon of the process, or helps guide the process forward.
Future Directions
With the recent work described above, mechanical forces are now appreciated as crucial for neural development. Yet, the biomechanics of several aspects of brain development are still unclear and ripe for investigation. These include neural induction, neural tube morphogenesis, interkinetic nuclear migration of RGCs [42 •], glial cell migration, and synapse formation and pruning.
Matrix stiffness has emerged as a major regulatory process, likely because its tractability allowed extensive experimentation. Emerging techniques have recently been introduced to probe tissue stiffness in vivo in 3D, including the use of lipid droplets [43], and various optical techniques such as Brillouin Microscopy, Optical Coherence Tomography and Magnetic Resonance Elastometry [44]. These non-invasive approaches will provide a more accurate picture of the processes and take stiffness studies to the next level.
Many studies in the past decade have examined mechanical cues in the context of single cells, but it is now crucial to understand these forces in tissues. Furthermore, while much of the work has focused on how tissue mechanics influence developmental processes, it is also important to understand how these forces are generated in the first place and how different forces might influence each other. In some cases cells actively generate force as seen through contractility of the actomyosin cytoskeleton [41 ••]. In other cases, the forces arise as a byproduct of developmental morphogenesis, as seen in the case of cellular density [8,38]. Understanding passive or active forces generated and the interactions between them will be key to provide a comprehensive understanding of development.
Studying mechanical dynamics of neural development in vivo is still a challenging feat, especially in mammalian systems. Emerging brain organoid technologies can help bridge the gap. As a system that can recapitulate 3D developmental dynamics in vitro, it allows for mechanical measurements and manipulations. Thus, brain organoids can be used to test hypotheses that current technologies render untestable in vivo or that are simply not observed in model organisms. Brain organoids have limitations, including the lack of a vascular system, variability of outcomes, and differences in mechanics and metabolism compared to in vivo systems; however, several of these will likely be resolved as the technology improves. Questions that might be impossible to answer in organoids or in vivo could be modeled computationally, which can suggest future experiments to test new ideas on how mechanical forces shape neural development. Because development cannot be fully understood through the lens of any single discipline, work that integrates diverse fields and experimental systems will be paramount to further our understanding of these processes.
Acknowledgements
We would like to thank Drs. Edwin Monuki, Lisa Flanagan, Susana Cohen-Cory, Beth Schachter, and members of the Pathak lab for helpful feedback. Work in the PI’s laboratory is supported by grants DP2 AT010376 and R01 NS109810 from the National Institutes of Health. Figures created in part with BioRender.
Abbreviations:
- AFM
atomic force microscopy
- NC
neural crest
- NTC
neural tube closure
- NSPC
neural stem/progenitor cell
- RGC
radial glial cell
- CSF
cerebrospinal fluid
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson D: ‘Arcy: On growth and form. Cambridge: Univ Press, 1917, [Google Scholar]
- 2.Keller R: Developmental biology. Physical biology returns to morphogenesis. Science 2012, 338:201–203. [DOI] [PubMed] [Google Scholar]
- 3.Galea GL, Cho Y-J, Galea G, Molè MA, Rolo A, Savery D, Moulding D, Culshaw LH, Nikolopoulou E, Greene NDE, et al. : Biomechanical coupling facilitates spinal neural tube closure in mouse embryos. Proc Natl Acad Sci U S A 2017, 114:E5177–E5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Raghunathan R, Rippy J, Wu C, Finnell RH, Larin KV, Scarcelli G: Tissue biomechanics during cranial neural tube closure measured by Brillouin microscopy and optical coherence tomography. Birth Defects Res 2019, 111:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabó A, Mayor R: Mechanisms of Neural Crest Migration. Annu Rev Genet 2018, 52:43–63. [DOI] [PubMed] [Google Scholar]
- 6.Roca-Cusachs P, Sunyer R, Trepat X: Mechanical guidance of cell migration: lessons from chemotaxis. Curr Opin Cell Biol 2013, 25:543–549. [DOI] [PubMed] [Google Scholar]
- 7.Ladoux B, Mège R-M: Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol 2017, 18:743–757. [DOI] [PubMed] [Google Scholar]
- 8.Barriga EH, Franze K, Charras G, Mayor R: Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 2018, 554:523–527.•• This paper suggests that NC cells are poised to migrate, and require a change in the mechanical environment to begin this migration
- 9.Scarpa E, Szabó A, Bibonne A, Theveneau E, Parsons M, Mayor R: Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces. Dev Cell 2015, 34:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLennan R, Schumacher LJ, Morrison JA, Teddy JM, Ridenour DA, Box AC, Semerad CL, Li H, McDowell W, Kay D, et al. : Neural crest migration is driven by a few trailblazer cells with a unique molecular signature narrowly confined to the invasive front. Development 2015, 142:2014–2025. [DOI] [PubMed] [Google Scholar]
- 11.Desmond ME, Jacobson AG: Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev Biol 1977, 57:188–198. [DOI] [PubMed] [Google Scholar]
- 12.Desmond ME, Knepper JE, DiBenedetto AJ, Malaugh E, Callejo S, Carretero R, Alonso M-I, Gato A: Focal adhesion kinase as a mechanotransducer during rapid brain growth of the chick embryo. Int J Dev Biol 2014, 58:35–43. [DOI] [PubMed] [Google Scholar]
- 13.Petrik D, Myoga MH, Grade S, Gerkau NJ, Pusch M, Rose CR, Grothe B, Götz M: Epithelial Sodium Channel Regulates Adult Neural Stem Cell Proliferation in a Flow-Dependent Manner. Cell Stem Cell 2018, 22:865–878.e8. [DOI] [PubMed] [Google Scholar]
- 14.Pelham RJ Jr, Wang Y l.: Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 1997, 94:13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deroanne CF, Lapiere CM, Nusgens BV: In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res 2001, 49:647–658. [DOI] [PubMed] [Google Scholar]
- 16.Lo CM, Wang HB, Dembo M, Wang YL: Cell movement is guided by the rigidity of the substrate. Biophys J 2000, 79:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagan LA, Ju Y-E, Marg B, Osterfield M, Janmey PA: Neurite branching on deformable substrates. Neuroreport 2002, 13:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D: Substrate compliance versus ligand density in cell on gel responses. Biophys J 2004, 86:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Discher DE, Janmey P, Wang Y-L: Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310:1139–1143. [DOI] [PubMed] [Google Scholar]
- 20.Engler AJ, Sen S, Sweeney HL, Discher DE: Matrix elasticity directs stem cell lineage specification. Cell 2006, 126:677–689. [DOI] [PubMed] [Google Scholar]
- 21.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE: Substrate modulus directs neural stem cell behavior. Biophys J 2008, 95:4426–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leipzig ND, Shoichet MS: The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30:6867–6878. [DOI] [PubMed] [Google Scholar]
- 23.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT, Bernardis E, Flanagan LA, Tombola F: Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A 2014, 111:16148–16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S: Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 2011, 29:1886–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rammensee S, Kang MS, Georgiou K, Kumar S, Schaffer DV: Dynamics of Mechanosensitive Neural Stem Cell Differentiation. Stem Cells 2017, 35:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellefsen KL, Holt JR, Chang AC, Nourse JL, Arulmoli J, Mekhdjian AH, Abuwarda H, Tombola F, Flanagan LA, Dunn AR, et al. : Myosin-II mediated traction forces evoke localized Piezo1dependent Ca2+ flickers. Commun Biol 2019, 2:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, et al. : Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 2019, 573:130–134.•• The authors show that adult rat OPCs integrate stiffness cues from their environment in vivo and in vitro to influence rates of proliferation and differentiation. Genetically ablating Piezo1 in vivo caused adult OPCs to increase their rates of proliferation and differentiation to levels similar to those in newborn rat OPCs, suggesting Piezo1 underlies the “ageing” of OPCs
- 28.Elkin BS, Azeloglu EU, Costa KD, Morrison B 3rd: Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma 2007, 24:812–822. [DOI] [PubMed] [Google Scholar]
- 29.Iwashita M, Kataoka N, Toida K, Kosodo Y: Systematic profiling of spatiotemporal tissue and cellular stiffness in the developing brain. Development 2014, 141:3793–3798. [DOI] [PubMed] [Google Scholar]
- 30.Luque T, Kang MS, Schaffer DV, Kumar S: Microelastic mapping of the rat dentate gyrus. R Soc Open Sci 2016, 3:150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long KR, Newland B, Florio M, Kalebic N, Langen B, Kolterer A, Wimberger P, Huttner WB: Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron 2018, 99:702–719.e6. [DOI] [PubMed] [Google Scholar]
- 32.Kjell J, Fischer-Sternjak J, Thompson AJ, Friess C, Sticco MJ, Salinas F, Cox J, Martinelli DC, Ninkovic J, Franze K, et al. : Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell 2020, 26:277–293.e8.• This study demonstrates that the neurogenic niche in the adult brain is stiffer than the brain parenchyma by about ~100 Pa and this higher stiffness is sufficient to profoundly modulate NSPC behavior.
- 33.Arulmoli J, Pathak MM, McDonnell LP, Nourse JL, Tombola F, Earthman JC, Flanagan LA: Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci Rep 2015, 5:8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elson EL, Felder SF, Jay PY, Kolodney MS, Pasternak C: Forces in cell locomotion. Biochem Soc Symp 1999, 65:299–314. [PubMed] [Google Scholar]
- 35.Jiang J, Zhang Z-H, Yuan X-B, Poo M-M: Spatiotemporal dynamics of traction forces show three contraction centers in migratory neurons. J Cell Biol 2015, 209:759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangatharan G, Schneider-Maunoury S, Breau MA: Role of mechanical cues in shaping neuronal morphology and connectivity. Biol Cell 2018, 110:125–136. [DOI] [PubMed] [Google Scholar]
- 37.Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa L da F, Guck J, et al. : Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci 2016, doi: 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AJ, Pillai EK, Dimov IB, Foster SK, Holt CE, Franze K: Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. Elife 2019, 8.• This is one of the few articles thus far to investigate the origin of mechanical cues
- 39.Breau MA, Bonnet I, Stoufflet J, Xie J, De Castro S, Schneider-Maunoury S: Extrinsic mechanical forces mediate retrograde axon extension in a developing neuronal circuit. Nat Commun 2017, 8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Striedter GF, Srinivasan S, Monuki ES: Cortical folding: when, where, how, and why? Annu Rev Neurosci 2015, 38:291–307. [DOI] [PubMed] [Google Scholar]
- 41.Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, Reiner O: Human Brain Organoids on a Chip Reveal the Physics of Folding. Nat Phys 2018, 14:515–522.•• This report presents a unique model for studying cortical folding in vitro that allows for real-time imaging and biophysical measurements of the process
- 42.Yanakieva I, Erzberger A, Matejčić M, Modes CD, Norden C: Cell and tissue morphology determine actin-dependent nuclear migration mechanisms in neuroepithelia. J Cell Biol 2019, 218:3272–3289.• The authors show that interkinetic nuclear migration in the hindbrain of the developing zebrafish embryo involves the Rho-ROCK-myosin force-generating pathway.
- 43.Campàs O, Mammoto T, Hasso S, Sperling RA, O’Connell D, Bischof AG, Maas R, Weitz DA, Mahadevan L, Ingber DE: Quantifying cell-generated mechanical forces within living embryonic tissues. Nat Methods 2014, 11:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy BF, Wijesinghe P, Sampson DD: The emergence of optical elastography in biomedicine. Nat Photonics 2017, 11:215–221. [Google Scholar]
- 45.Nikolopoulou E, Galea GL, Rolo A, Greene NDE, Copp AJ: Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 2017, 144:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang HB, Dembo M, Wang YL: Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol 2000, 279:C1345–50. [DOI] [PubMed] [Google Scholar]
- 47.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. : Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell </References> [DOI] [PMC free article] [PubMed] [Google Scholar]