Abstract
Despite many hypothesized benefits of dietary isoflavone genistein (GEN) deriving from soy-based products, questions surrounding GEN’s developmental effects are increasing. To understand if in utero GEN exposure modulated postnatal respiratory allergies in the middle age, we conducted a time course study in the B6C3F1 offspring (PND 240–330) using a common household allergen (house dust mites: HDM; 10 μg/mouse for PND 240 and 290, and 50 μg/mouse for PND 330, a middle age in mice) following intranasal instillation, a physiological route of allergen exposure. GEN was administered to dams by gavage from gestational day 14 to parturition at a physiologically relevant dose (20 mg/kg body weight). Female and male offspring were sensitized with HDM allergens beginning about one month prior to sacrifice followed by challenges with three weekly dosings of HDM extracts, and they were euthanized at day 3 following the final HDM exposure. In utero exposure to GEN decreased HDM allergen-induced respiratory allergy in male B6C3F1 offspring at PND 330 as reflected by decreases in airway hyperresponsiveness (e.g., Penh value), HDM-specific IgG1 (a Th2 type Ab) and the activity of eosinophil peroxidase in the lung (an indication of eosinophil recruitment to the lungs). However, in utero exposure to GEN had minimal effects on HDM allergen-induced respiratory allergy in the middle-aged female offspring. Changes in serum total IgE, HDM-specific IgE, and lung histopathology scores in both male and female offspring were not biologically significant. Overall, in utero GEN exposure exerted a protective effect on respiratory allergy in the middle-aged male, but not female, B6C3F1 offspring following later-life HDM exposures.
Keywords: Genistein, house dust mites, respiratory allergy, in utero exposure, IgE
Introduction
Genistein (GEN), a major isoflavone in soy products, can interact with estrogen receptors (Guo, Auttachoat, & Chi, 2005; Martin, Horwitz, Ryan, & McGuire, 1978). Despite the hypothesized beneficial effects of GEN, e.g., higher GEN intake in adults is associated with better lung function (Bime, Wei, Holbrook, Smith, & Wise, 2011; Smith et al., 2004), there are concerns about the potential long-term effects of this compound on human health, especially that of infants and young children. Infants fed soy milk formulas have plasma isoflavone levels that are orders of magnitude higher than those of infants fed human or cow’s milk (Katchy et al., 2014; Patisaul & Jefferson, 2010; Setchell, ZimmerNechemias, Cai, & Heubi, 1997). The possible long-term effects of these relatively high levels of phytoestrogens during infancy are unknown. Phytoestrogens have been detected in amniotic fluid (Doerge, Churchwell, & Delclos, 2000; Jefferson, Patisaul, & Williams, 2012), suggesting that in utero exposure indeed occurs.
The prevalence of asthma has doubled in the past decades and continues to rise (Greenwood, 2011; Robinson, Larche, & Durham, 2004). High titers of IgE antibody to common house allergens such as house dust mite (HDM) significantly increased the risk for acute wheezing provoked by infection (e.g., rhinovirus) among asthmatic children (Soto-Quiros et al., 2012). In our previous studies, we have demonstrated that in utero exposure to GEN increased IgE production in young B6C3F1 offspring, e.g., postnatal day (PND) 56–120, following adult exposure to a respiratory sensitizer trimellitic anhydride (TMA) (Guo et al., 2005) or HDM (Guo & Meng, 2016). There is evidence that midlife systemic inflammation is associated with frailty in later life (Walker et al., 2019). To further understand how in utero GEN exposure modulated respiratory allergy, we hypothesized that exposure to GEN during a sensitive period (e.g., in utero exposure) would differentially modulate allergic sensitization in middle-aged male and female offspring to the respiratory allergen HDM. To this end, we have conducted a time course study in the middle-aged offspring at three time points (PND 240, 290, and 330) following in utero GEN and later intranasal HDM exposures.
In this study, we have evaluated the effects of in utero GEN exposure through dosing dams from gestation day 14 (GD14) to parturition on various allergic responses following HDM stimulation in middle-aged B6C3F1 offspring, including airway hyperresponsiveness (AHR), the total and antigen-specific IgE responses, and eosinophil peroxidase (EPO) activity. The period of GD14 until birth is the period of colonization and establishment of the bone marrow and thymus in mice (Landreth, 2002). The B6C3F1 mouse, a hybrid of male C3H/HeN and female C57BL/6J mice, was selected over randomly bred mice to decrease the variation between individual responses and reduce the number of animals for each experiment, and yet have the vigor associated with the heterozygosity. This model has been widely used for studies of estrogenic effects (Frawley et al., 2011; Ng, Steinetz, Lasano, & Zelikoff, 2006; Papaconstantinou, Goering, Umbreit, & Brown, 2003). Furthermore, our studies on several strains of mice including B6C3F1, C57BL/6, BDF1 and BALB/c have suggested that the B6C3F1 mice was the best responder (e.g., the highest production of IgE and IL-4) following respiratory allergen exposure, and has the potential to detect respiratory sensitization by various treatments (Guo et al., 2002).
Materials and Methods
Animals and animal exposure
Both female C57BL/6 and male C3H mice (8–12 weeks old) were obtained from Charles River Breeding Laboratories (Portage, MI). Timed pregnant primiparous C57BL/6 mice were generated through housing two female C57BL/6 mice and one male C3H mouse in one cage (plug date = gestational day 0). Pregnant mice were housed individually in polycarbonate cages with hardwood chip bedding, and the animal room was maintained at 21–24°C and the relative humidity between 40 and 70%. The mice consumed Harlan Teklad Laboratory Diets (NIH 07; Madison, WI) and tap water from water bottles ad libitum. Previous studies reported that a negligible amount of bisphenol A leached from new or used polycarbonate cages maintained at room temperature (Delclos et al., 2014; Johnson et al., 2016; Thigpen et al., 2013). It has been reported that the dietary concentration of isoflavones in the NIH-07 diet is approximately 33 ppm (Brown & Setchell, 2001). In our previous studies (Guo et al., 2005), two different diets, e.g., the phytoestrogen free 5K96 diet and NIH 07 rodent diet, were compared, and it was found that in utero GEN exposure by gavage increased serum total IgE in both cases with more enhancement observed in NIH 07 diet-fed female mice. Thus, the NIH 07 rodent diet was used in this study.
GEN solutions were prepared fresh daily in 25 mM Na2CO3 at a concentration of 2 mg/ml (Guo et al., 2005). Mice were administered the GEN solution or the vehicle (VH) by gavage (0.1 ml/10 g body) via an 18 G gavage needle from GD 14 to parturition, which produced a dose of 20 mg/kg body. The offspring were weaned at PND 22, and at this time the offspring were housed up to four same-sex littermates per cage. All animal procedures were conducted under an animal protocol approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Dosing of house dust mite allergen
At each time point, each mouse in a group was randomly collected from different litters to control for bias due to litter effect, and there were no significant differences in the initial body weights between the groups that were assigned to different time points. About one month prior to sacrifice, mice were sensitized with one dose of HDM extract (Dermatophagoides farinae, Greer Laboratories, Lenoir, NC) followed by challenges with three dosings of HDM allergens (e.g., four dosings of HDM extract were given once a week except for a two-week interval between dosings 1 and 2; Figure 1). Mice were anesthetized with an intraperitoneal (ip) injection of ketamine (100 mg/kg)/xylazine (10 mg/kg body), followed by intranasal instillation of HDM extract (10 μg/mouse for PND 240 and 290, and 50 μg/mouse for PND 330) in 50 μl (25 μl/nostril) of physiological Hank’s balanced salt solution (HBSS). The rationale for the exposure protocol was based on the immunology paradigm that the primary immune response (e.g., the allergy sensitization phase) was approximately 7–14 days following the initial exposure (doses 1 to 2). The second exposure was 14 days after the first, and thus, it was on the boundary between sensitization and challenge. The last two exposures were challenge exposures. This dosing regimen has been used extensively for various allergens including HDM (Guo & Meng, 2016; Ward, Chung, Copeland, & Doerfler, 2010).
Figure 1.
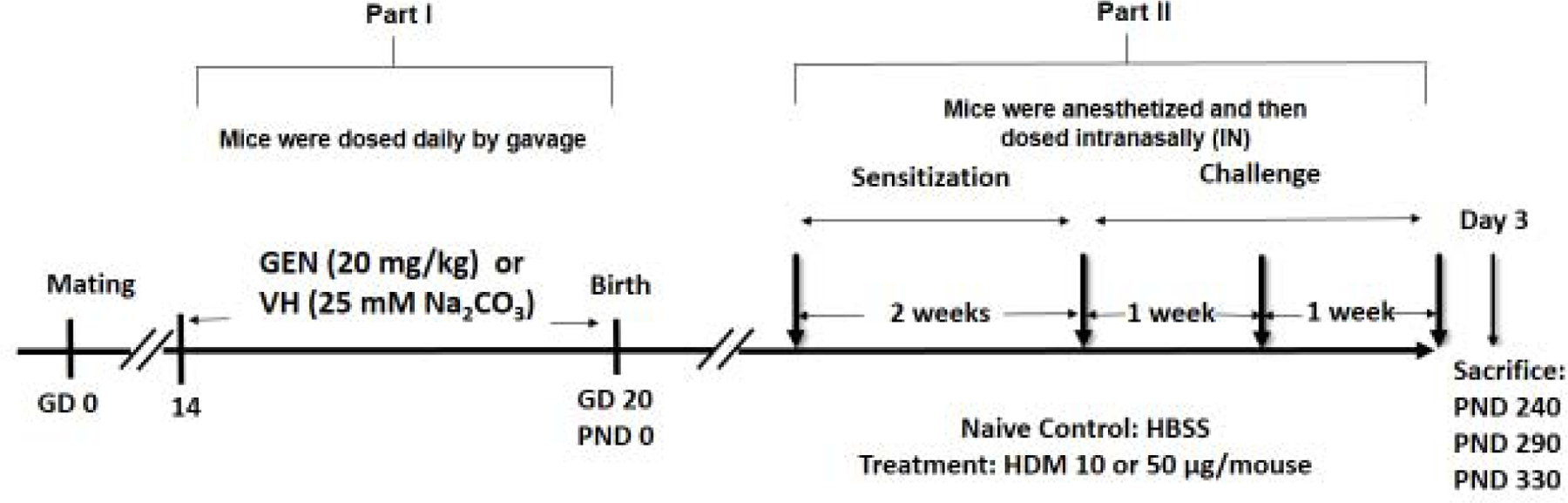
Experimental design for animal treatments. In our studies with young mice (Guo & Meng, 2016), the dose of 10 μg/mouse of HDM was considered the optimal dose to be used for studies because of less variation, and it also permitted further increases. However, as the mice became aged, the HDM seemed to be less effective in eliciting IgE production when compared to IgE levels in the naïve mice. Thus, for the PND 330 time point, the HDM dose was increased to 50 μg/mouse. PND = postnatal day; gd = gestation day.
Mice were sacrificed and sera were collected at day 3 following the final HDM exposure. The animals in an additional group were treated with 50 μl of HBSS. These HBSS-only mice are herein referred to as “naïve group” to differentiate them from the group of mice that received 25 mM Na2CO3 in utero, the vehicle for GEN.
Enzyme linked immunosorbent assay (ELISA)
ELISA for total IgE was performed according to the manufacturer’s instructions (BD Pharmigen, San Diego, CA). Briefly, 100 μl of diluted capture antibody in the bicarbonate/carbonate coating buffer (100 mM) were added to each well in a 96-well plate (NUNC MaxiSorp flat-bottom), and allowed to adhere overnight at 4°C. Plates were washed, then blocked with 10% fetal bovine serum–phosphate buffered saline (FBS-PBS) for 1 h at room temperature. After washing, serial dilutions of the standard and samples (from 1:16 to 1:128) were prepared in the plates, and then allowed to adhere for 2 h at room temperature. After washing, 100 μl of working solution including detector antibody and avidin-horseradish peroxidase (HRP) reagent were added to each well, then incubated for 1 h at room temperature. The detector antibody was biotinylated anti-mouse IgE monoclonal antibody. After washing, 100 μl of tetramethylbenzidine (TMB) substrate solution were added to each well. After the incubation in the dark for 30 min at room temperature the absorbance was read at 450 nm within 30 min of adding stop solution (2N H2SO4).
Eosinophil peroxidase (EPO) assay
The EPO assay is a colorimetric assay used for detecting eosinophils by measuring the eosinophil peroxidase activity (Davoine et al., 2013; Lintomen et al., 2008; Strath, Warren, & Sanderson, 1985). Mouse spleen and lung were homogenized and then sonicated (30–45 seconds per sample) until the cells were disrupted. The organ homogenates were centrifuged and the resulting supernatants were used for assay. Briefly, Tris-HCl buffer (50 μl) was added to 96-well plates initially except for the wells for undiluted samples (100 μl). The samples were serially diluted from 1:2 to 1:4096 in the plates. The diluted samples were incubated for 30 min with 150 μl of substrate solution containing 1.5 mM o-phenylenediamine (2 mg/ml, Sigma, St Louis, MO) and 6.6 mM H2O2 (1.3 μl/ml) in 0.05 M Tris-HCl. The reaction was stopped by adding 2N H2SO4, and the optical density measured at 490 nm on an ELISA plate reader (Molecular Devices Thermomax Plate Reader, Rockville, MD).
Determination of airway responsiveness
Measurements of the physiological response to a standard methacholine (MCh) challenge (0 and 5, 20 and 50 mg/ml MCh) were conducted using whole body plethysmographic techniques, similar to those described previously (Rasid, Chirita, Iancu, Stavaru, & Radu, 2012; Whitehead, Walker, Berman, Foster, & Schwartz, 2003). Briefly, the mice were individually placed in a whole-body plethysmograph (Buxco Electronics, Sharon, CT) that contained built-in pneumotachographs at day 2 following the final HDM exposure. The mice were ventilated by 0.8 L/min regulated bias airflow (PLY1040; Buxco Electronics) through the plethysmograph. A differential pressure transducer was used to measure the pressure differential across the pneumotachograph to determine flow. The flow through the pneumotachograph was linear to the differential pressure and integrated. Transducer signals were conditioned using an amplifier (Emka Technologies; France), digitized, and processed in real time. Real-time calculations of frequency and breath waveform [expiratory time (Te), relaxation time (Tr), peak expiratory flow (PEF), peak inspiratory flow (PIF)] were performed and recorded electronically by computer software (Emka Technologies). Estimates of airway responsiveness, expressed as enhanced pause (Penh), were derived from the ventilation and flow-derived parameters. Penh was calculated as [(Te - Tr/Tr) x (PEF/PIF)]. Penh values were averaged every 30 s and recorded for a minimum of 3-min intervals at baseline and after stimulation with each concentration of MCh. The different concentrations of aerosolized MCh were administered for 1 min using an ultrasonic nebulizer and nebulizer control unit (Emka Technologies). The dilution flow through the mixing chamber was 10 L/min. This noninvasive physiological approach offered the advantages of eliminating the effects of anesthesia and surgical trauma and permitting repeated assessment of the same mice while they breathed spontaneously. The noninvasive index of airway hyperresponsiveness, Penh, is an empirically derived, unit-less value based on the pressure waveform in the plethysmograph box (Albertine et al., 2002).
HDM-specific IgE, IgG1, IgG2a and IgG2b levels in sera
The removal of IgG has been shown to increase the sensitivity of detecting antigen-specific IgE (Fischer et al., 2005). On day 1, dilutions of serum samples (1:8 and 1:16) in blocking solution (PBS + 10% FBS) were first depleted of IgG by overnight incubation at 4°C in protein G-coated 96-well plates (Reacti-Bind plates; Pierce, Rockford, IL). Protein G is a surface protein found on the bacteria Staphylococcus aureus that binds Fc portion of IgG. Meanwhile, HDM extract diluted with coating buffer was plated on 96 well (NUNC) plates and incubated overnight at 4°C. On day 2, HDM extract (50 μg/ml) coated plate was washed, then blocked with 10% FBS-PBS for 1 h at room temperature. After additional washings, the IgG depleted sera were transferred to the HDM coated plates and incubated for 2 h at room temperature. After washing, 100 μl of working solution including detector antibody (biotinylated anti-mouse IgE monoclonal) and avidin-HRP reagent were added to each well, then incubated for 1 h at room temperature. After washing, 100 μl of TMB substrate solution were added to each well. After the incubation in the dark for 30 min at room temperature the absorbance was read at 450 nm within 30 min of adding stop solution (2N H2SO4). For the measurement of HDM-specific IgG1, IgG2a and IgG2b levels, sera without IgG depletion with higher dilutions were used.
Histopathology
Lung tissues were fixed in 10% phosphate-buffered formalin, sectioned and then stained with hematoxylin and eosin, and examined for pathological findings. The sections were also stained with mucicarmine for enumeration of mucin secreting cells in similar peribronchial regions (Cai, Zhou, & Webb, 2012). Mucicarmine is specific to the mucins of epithelial origin, and has been used for the assessment of goblet cells in human specimens (Osho, Wang, Horn, & Adeola, 2017). Grading was performed within the study and a score was assigned based the total lesion severity (4= Marked; 0 = no lesion).
Statistical analysis
Results are presented as mean ± SEM. To determine the type of analysis to be used, the Bartlett’s Test for homogeneity was conducted. The software used was JMP Pro 10. Homogenous data were analyzed using a one-way analysis of variance, and the Dunnett’s t test was used to determine differences between the control and experimental groups. Non-homogenous data were analyzed using a nonparametric analysis of variance and the Wilcoxon Rank Test to determine differences between the vehicle control group and exposure groups. A group was considered statistically significant from the control group if p ≤ 0.05.
Results
Effect of in utero GEN exposure on the body weight and spleen weight in middle-aged female and male offspring
To determine if in utero GEN exposure affected allergic responses in middle-aged mice, a time course study at three different time points (PND 240, 290, and 330) using HDM allergens was conducted in the B6C3F1 offspring (Figure 1). All dams receiving GEN by gavage from GD 14 to parturition at doses of 20 mg/kg body survived the experimental period and showed no overt signs of toxicity as manifested in changes in gait, fur status, drainage from orifices or pregnancy complications. All dams delivered their pups successfully, and total pups per litter, female pups per litter and litter sex (F:M) ratios were not significantly affected by GEN treatment. However, male pups per litter was significantly decreased by GEN (Table 1). In general, there was an age-related increase in body weight in both female and male offspring (Table 2), and no significant differences were observed among the groups in females at any timepoints. Treatment with HDM decreased terminal body weight in male offspring at PND 240, 290 and 330 when compared to naïve mice that received HBSS during intranasal dosing. However, in utero GEN treatment in the males did not have a significant effect on body weight when compared with vehicle group.
Table 1.
Effect of GEN exposure during pregnancy on the offspring parameters
| Treatment | Litter # | Female # | Male # | Total pups | Sex ratio (F:M) |
|---|---|---|---|---|---|
| VH | 1 | 4 | 4 | 8 | 1.00 |
| 2 | 6 | 4 | 10 | 1.50 | |
| 3 | 4 | 6 | 10 | 0.67 | |
| 4 | 5 | 5 | 10 | 1.00 | |
| 5 | 2 | 7 | 9 | 0.29 | |
| 6 | 3 | 8 | 11 | 0.38 | |
| 7 | 5 | 6 | 11 | 0.83 | |
| 8 | 1 | 7 | 8 | 0.14 | |
| 9 | 2 | 7 | 9 | 0.29 | |
| 10 | 6 | 3 | 9 | 2.00 | |
| 11 | 5 | 6 | 11 | 0.83 | |
| 12 | 7 | 6 | 13 | 1.17 | |
| Average | 4.17 ± 0.53 | 5.75 ± 0.43 | 9.92 ± 0.42 | 0.84 ± 0.16 | |
| GEN | 1 | 1 | 7 | 8 | 0.14 |
| 2 | 6 | 5 | 11 | 1.20 | |
| 3 | 6 | 4 | 10 | 1.50 | |
| 4 | 6 | 5 | 11 | 1.20 | |
| 5 | 6 | 4 | 10 | 1.50 | |
| 6 | 0 | 2 | 2 | 0.00 | |
| 7 | 5 | 5 | 10 | 1.00 | |
| 8 | 6 | 4 | 10 | 1.50 | |
| 9 | 5 | 5 | 10 | 1.00 | |
| 10 | 1 | 2 | 3 | 0.50 | |
| 11 | 1 | 5 | 6 | 0.20 | |
| 12 | 4 | 6 | 10 | 0.67 | |
| 13 | 7 | 4 | 11 | 1.75 | |
| 14 | 6 | 5 | 11 | 1.20 | |
| Average | 4.28 ± 0.65 | 4.50 ± 0.36* | 8.79 ± 0.80 | 0.95 ± 0.15 | |
Pregnant mice were treated with genistein (GEN) or vehicle (VH) from GD14 to birth by gavage. Female or male pups were counted, and the sex ratio determined.
p ≤ 0.05 when compared to VH.
Table 2.
Effect of in utero GEN exposure on body weight, absolute and relative spleen weight in middle-aged B6C3F1 offspring
| PND | Parameters | Naïve | Vehicle | Genistein | |
|---|---|---|---|---|---|
| Female | 240 | Body wt (g) | 33.43 ± 1.88 | 35.12 ± 2.59 | 33.93 ± 1.74 |
| (7) | (6) | (7) | |||
| Spleen (mg) | 81.25 ± 2.46 | 105.50 ± 8.79 a | 87.86 ± 2.76 b | ||
| %Body Wt | 0.244 ± 0.014 | 0.307 ± 0.030 | 0.263 ± 0.018 | ||
| 290 | Body wt (g) | 43.49 ± 2.38 | 41.02 ± 1.59 | 39.91 ± 1.96 | |
| (8) | (6) | (7) | |||
| Spleen (mg) | 106.88 ± 8.82 | 113.83 ± 5.64 | 98.14 ± 5.32 | ||
| %Body Wt | 0.247 ± 0.016 | 0.279 ± 0.017 | 0.253 ± 0.025 | ||
| 330 | Body wt (g) | 42.16 ± 2.06 | 40.97 ± 2.74 | 36.43 ± 2.42 | |
| (8) | (6) | (7) | |||
| Spleen (mg) | 105.50 ± 5.27 | 103.17 ± 2.65 | 126.29 ± 8.74 b | ||
| %Body Wt | 0.253 ± 0.016 | 0.258 ± 0.018 | 0.356 ± 0.032 a,b | ||
| Male | 240 | Body wt (g) | 44.48 ± 0.97 | 41.79 ± 0.30 a | 42.17 ± 0.72 |
| (8) | (8) | (8) | |||
| Spleen (mg) | 89.63 ± 5.15 | 80.63 ± 1.59 | 80.25 ± 2.85 | ||
| %Body Wt | 0.201 ± 0.009 | 0.193 ± 0.005 | 0.190 ± 0.006 | ||
| 290 | Body wt (g) | 46.25 ± 1.29 | 43.98 ± 0.91 | 43.34 ± 0.46 a | |
| (6) | (8) | (8) | |||
| Spleen (mg) | 99.17 ± 5.84 | 90.25 ± 6.26 | 95.25 ± 3.00 | ||
| %Body Wt | 0.214 ± 0.008 | 0.204 ± 0.011 | 0.220 ± 0.006 | ||
| 330 | Body wt (g) | 47.66 ± 1.18 | 44.25 ± 0.67 a | 43.29 ± 0.87 a | |
| (8) | (8) | (8) | |||
| Spleen (mg) | 113.13 ± 9.86 | 110.13 ± 10.25 | 105.88 ± 5.73 | ||
| %Body Wt | 0.236 ± 0.016 | 0.249 ± 0.023 | 0.244 ± 0.010 | ||
Pregnant mice were treated with genistein or vehicle from GD14 to birth by gavage. Female or male offspring were sacrificed at three different time points (PND 240, 290, and 330) following HDM dosing, and their body weight and spleens were weighed. Naive = untreated, non-sensitized naïve animals.
significantly different from naive;
significantly different from vehicle. The number in parenthesis indicates the number in each group.
For absolute spleen weight, there was an increasing trend with time in the female offspring except that the spleen weight leveled off at PND 330. This leveling off effect was seen in both naïve and vehicle groups, but was not observed in GEN-exposed female offspring (Table 2). In addition, at PND 240, there was a significant increase of spleen weight in HDM-treated vehicle female offspring when compared to naïve group, and this increase was abrogated by in utero GEN exposure. When the spleen weights were expressed as relative values (%body), GEN treatment significantly increased relative weights of spleen at PND 330 in female offspring when compared to either the NAF group or VHF group. The spleen weight in male offspring also increased with age in all treatment groups (Table 2); however, no significant changes were observed at any time points among the three groups in either absolute or relative spleen weights.
Total IgE levels in middle-aged female and male offspring following in utero GEN exposure
Total serum IgE levels were measured using ELISA. When compared to the vehicle group, in utero GEN exposure induced significant increases in IgE levels in female offspring at PND 240, but not at PND 290 and 330 (Figure 2A, top panels); however, there was no difference between NAF and VHF groups at PND 240. When compared to naïve group, significant increases were observed in the vehicle group at PND 330 (right panel) and in the GEN treatment group at all three time points (PND 240, 290 and 330). Taken together, in utero GEN exposure had no biologically significant increase of total IgE levels in middle-aged female offspring (Figure 2A).
Figure 2.
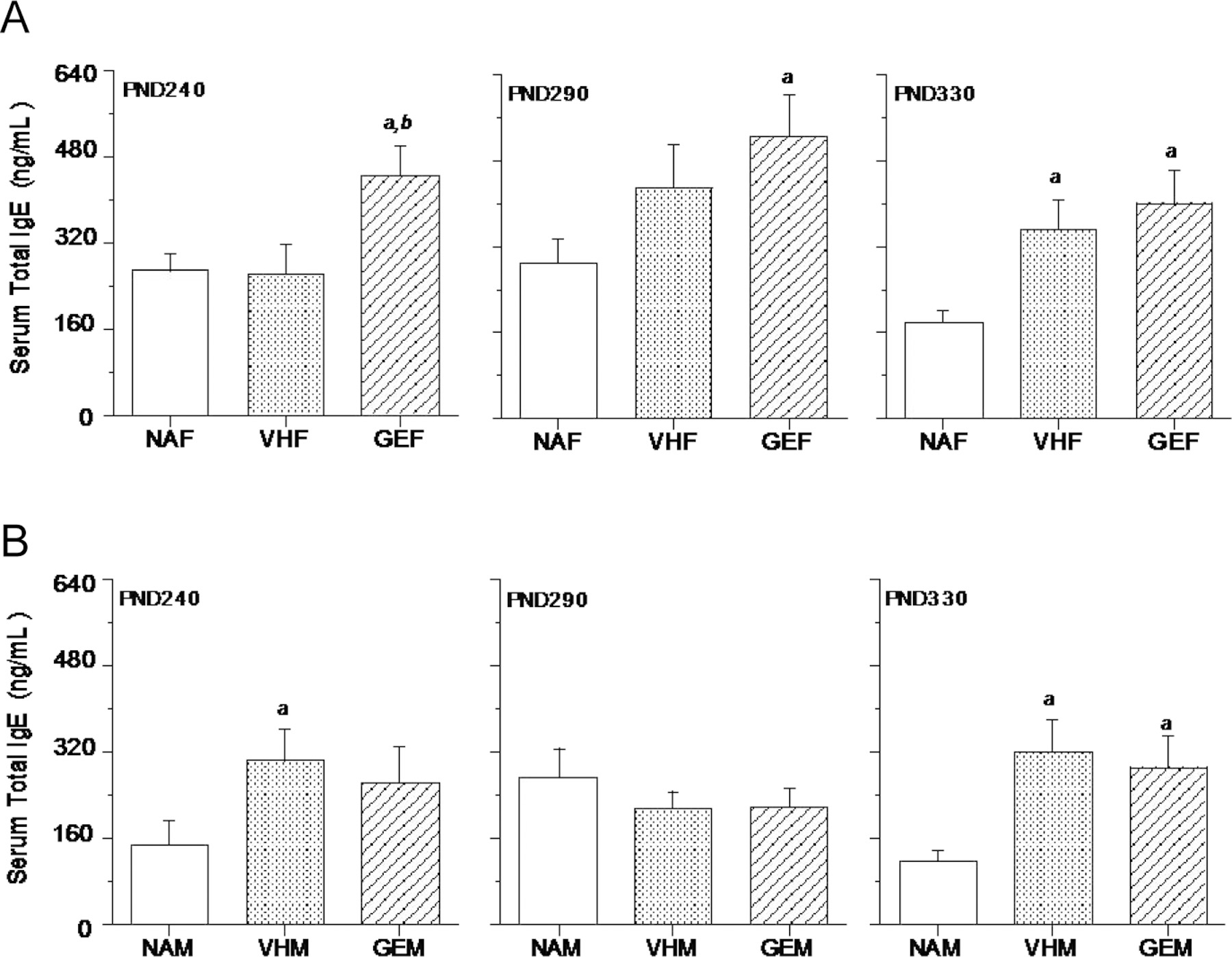
Effect of in utero GEN exposure on serum total IgE levels in female (A) and male (B) B6C3F1 offspring. Pregnant mice were treated with genistein (GEN) or vehicle (VH) from GD14 to birth by gavage. Female or male offspring were dosed with HDM at intervals as described (Figure 1) and sacrificed 3 days after the last intranasal HDM dosing at three different time points (PND 240, 290, and 330). The serum total IgE levels were measured using ELISA. NAF = untreated, non-sensitized naïve females, NAM = untreated, non-sensitized naïve males, VHF = VH females dosed with HDM, VHM = VH males dosed with HDM, GEF = GEN females dosed with HDM, GEM = GEN males dosed with HDM. a, significantly different from naïve group; b, significantly different from VH group. The number of mice in each group at each time point is shown in Table 2.
In male offspring, the HDM treatment (10 μg/mouse) in VHM group stimulated a significant increase in IgE levels when compared to the naïve mice at PND 240 (Figure 2B, left panel), while there was no significant difference between VHM and GEM groups. At PND 290 (Figure 2B, middle panel), there were no significant differences among the three groups. At PND 330, the HDM treatment (50 μg/mouse) stimulated a significant increase in IgE levels when compared to the naïve group (Figure 2B, right panel); however, the vehicle and GEN-treated offspring did not differ significantly in their IgE levels. These data suggest that in utero GEN exposure had minimal effects in total IgE production in middle-aged male B6C3F1 offspring.
Antigen-specific IgE in female and male offspring following in utero GEN exposure
Antigen-specific IgE assays were performed using IgG depleted sera from offspring at PND 240 and 290. At both time points tested, there were significant differences between naïve group and mice dosed with HDM (Figure 3 and data not shown), suggesting both male and female offspring developed sufficient respiratory sensitization. At PND 240, in utero GEN exposure had no effects on the levels of HDM-specific IgE in either female or male offspring (data not shown). At PND 290, a significant increase of HDM-specific IgE was observed in GEN exposed male offspring when compared to VH group (Figure 3B), but not in the female offspring (Figure 3A). However, the levels of HDM-specific IgE in male offspring were much lower than that in female offspring. Therefore, in utero GEN exposure did not induce a biologically meaningful increase of HDM-specific IgE levels in these male offspring.
Figure 3.
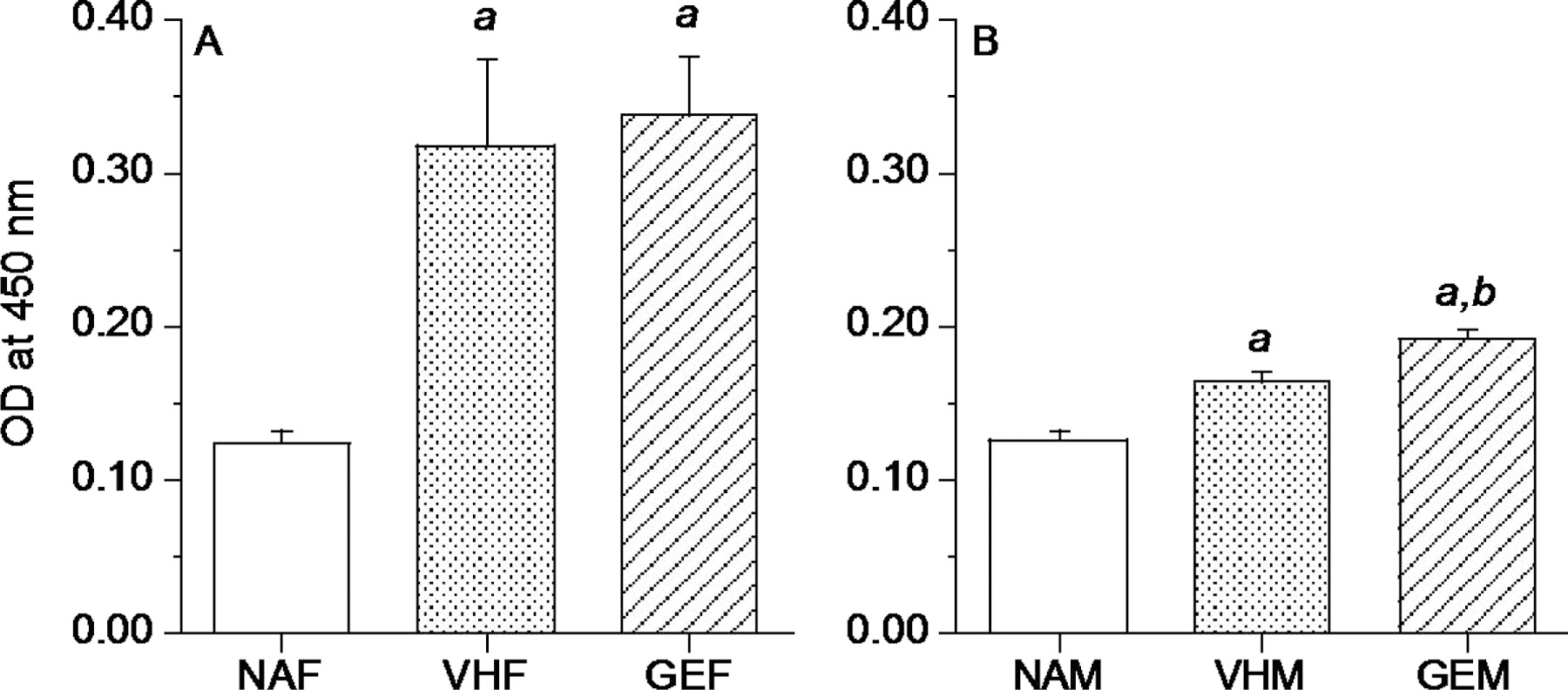
In utero GEN exposure had no effects on antigen-specific IgE in female B6C3F1 offspring (A) but increased antigen-specific IgE in male B6C3F1 offspring (B) at PND 290. Pregnant mice were treated with GEN or VH from GD14 to birth by gavage. Female or male offspring were dosed with HDM or HBSS (NA) at intervals as described in Figure 1 and sacrificed 3 days after the last intranasal dosing. HDM-specific IgE assays using IgG depleted sera were performed as described. NAF = untreated, non-sensitized naïve females, NAM = untreated, non-sensitized naïve males, VHF = VH females dosed with HDM, VHM = VH males dosed with HDM, GEF = GEN females dosed with HDM, GEM = GEN males dosed with HDM. a, significantly different from naïve group; b, significantly different from VH group. The number of mice in each group at each time point is shown in Table 2.
Antigen-specific IgG1, IgG2a and IgG2b levels in middle-aged female and male offspring
To determine if in utero GEN exposure affected the production of other antibodies in the offspring, the antigen-specific IgG1, IgG2a and IgG2b levels in the sera were measured. In females, in utero GEN exposure induced significant increases in the levels of antigen-specific IgG2b (a Th1 type Ab) at PND 330 when compared to vehicle group (Table 3). In males, in utero GEN exposure induced a significant increase in the levels of antigen-specific IgG1 (a Th2 type Ab) at PND 290 when compared to vehicle group (Table 3). In contrast, a significant decrease in the level of antigen-specific IgG1 at PND 330 was observed in male offspring following in utero GEN exposure (Table 3).
Table 3.
Effect of in utero GEN exposure on antigen-specific IgG1, IgG2a and IgG2b levels in middle-aged B6C3F1 offspring.
| PND | IgG1 |
IgG2a |
IgG2b |
||||
|---|---|---|---|---|---|---|---|
| VH GEN | VH GEN | VH GEN | |||||
| Female | 240 | 0.359 ± 0.106 | 0.236 ± 0.092 | 0.839 ± 0.3S5 | 0.457 ± 0.229 | 0.789 ± 0.247 | 0.365 ±0.123 |
| 290 | 0.193 ± 0.051 | 0.183 ±0.019 | 0.410 ± 0.229 | 0.196 ± 0.052 | 0.393 ± 0.023 | 0.358 ± 0.014 | |
| 330 | 0.534 ± 0.217 | 0.535 ± 0.147 | 0.569 ± 0.207 | 0.737 ± 0.128 | 0.409 ± 0.009 | 0.783 ± 0.176* | |
| Male | 240 | 0.162 ± 0.019 | 0.126 ±0.024 | 0.246 ± 0.062 | 0.320 ± 0.136 | 0.245 ± 0.026 | 0.415 ±0.182 |
| 290 | 0.120 ± 0.033 | 0.172 ±0.018* | 0.237 ± 0.071 | 0.263 ± 0.101 | 0.341 ± 0.014 | 0.609 ± 0.182 | |
| 330 | 0.413 ±0.039 | 0.245 ± 0.038* | 0.698 ± 0.129 | 0.519 ± 0.131 | 0.819 ± 0.187 | 0.634 ±0.118 | |
Pregnant mice were treated with genistein (GEN) or vehicle (VH) from GDI4 to birth by gavage. Female or male offspring were dosed with HDM at intervals as described in methods and sacrificed 3 days after the last intranasal dosing at three different time points (PND 240. 290. and 330). The serum levels of HDM-specific IgG subclasses were measured using ELISA as described at dilutions of 1:5,000 – 1:32.000 for IgG1, 1:100 – 1:800 for IgG2a, and at 1:100 – 1:200 for lgG2b.
p ≤ 0.05 when compared to VH.N = 6 – 8. Optical density (OD) values were shown as mean ± SEM.
Eosinophil peroxidase activity in middle-aged female and male offspring following in utero GEN exposure
Eosinophilia in the bronchial region is a sign of bronchial hyperreactivity (Afshar, Vucinic, & Sharm, 2007; Mathias et al., 2013). EPO assays (Figure 4A) were performed on homogenized lungs at PND 330, and in utero GEN exposure numerically (p > 0.05) increased lung EPO activities in female offspring (Figure 4B). However, in male offspring, in utero GEN exposure decreased lung EPO activity at PND 330 (Figure 4C). EPO assays on homogenized spleens were also tested at PND 330, and there were no significant differences between the GEN and vehicle groups in either male or female offspring (data not shown).
Figure 4.
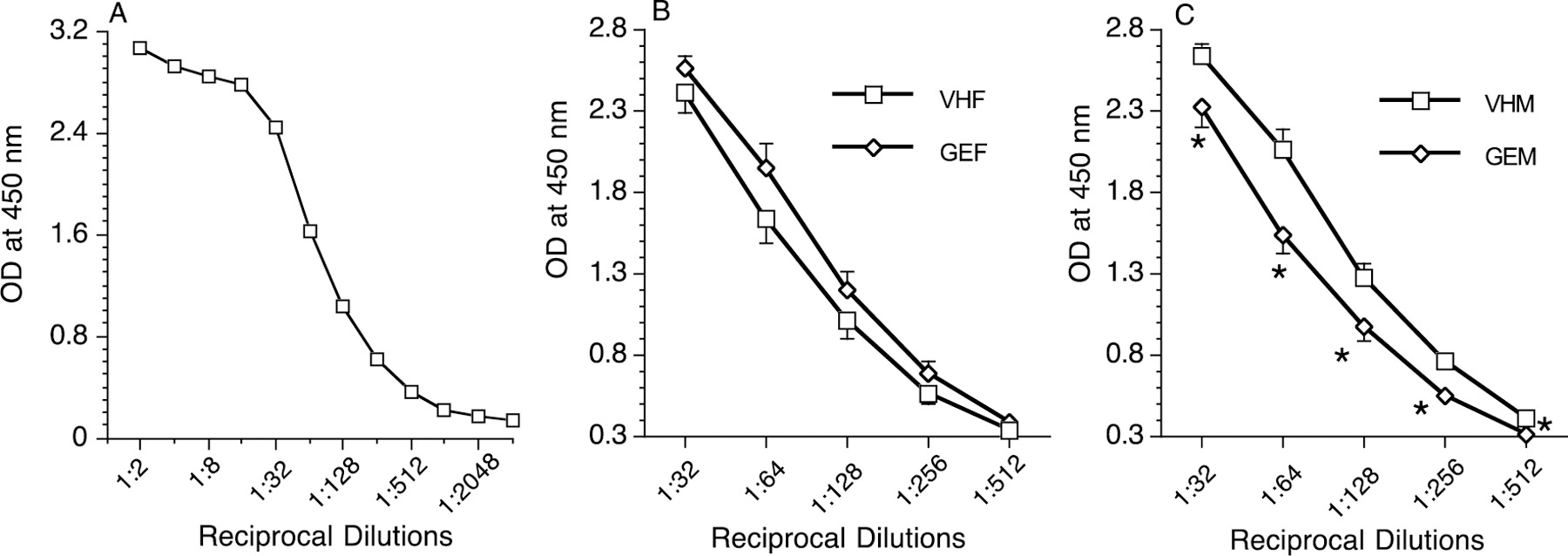
Eosinophil peroxidase activity in lungs of female and male B6C3F1 offspring at PND 330. (A) Optical density (OD) values decreased with dilution of samples from 1:2 to 1:4096; the linear region of the curve is where proper concentration of the analytes can be compared. In earlier regions where the concentration is in extreme excess (the prozone region), analytes can form large complexes and skew concentration. (B) Female offspring lung samples (both VHF and GEF) from dilutions of 1:32 to 1:512, the linear region of the curve. (C) Male lung samples (both VHM and GEM) from dilutions of 1:32 to 1:512. *, p ≤ 0.05 when compared to VH. VHF = VH females dosed with HDM, VHM = VH males dosed with HDM, GEF = GEN females dosed with HDM, GEM = GEN males dosed with HDM. The number of mice in each group at each time point is shown in Table 2.
Airway hyperresponsiveness in middle-aged female and male offspring
Plethysmography was used to measure changes in lung volume and airway resistance in both female and male offspring at PND 330, and in utero GEN exposure did not significantly alter basal Penh values in either female (Figure 5A) or male offspring (Figure 5B). No significant changes in Penh values after MCh challenge were observed in the in-utero GEN exposed female offspring (Figure 5A). However, decreases in Penh value were observed in the male offspring with a significant change at MCh dose of 20 mg/kg following in utero GEN exposure (Figure 5B).
Figure 5.
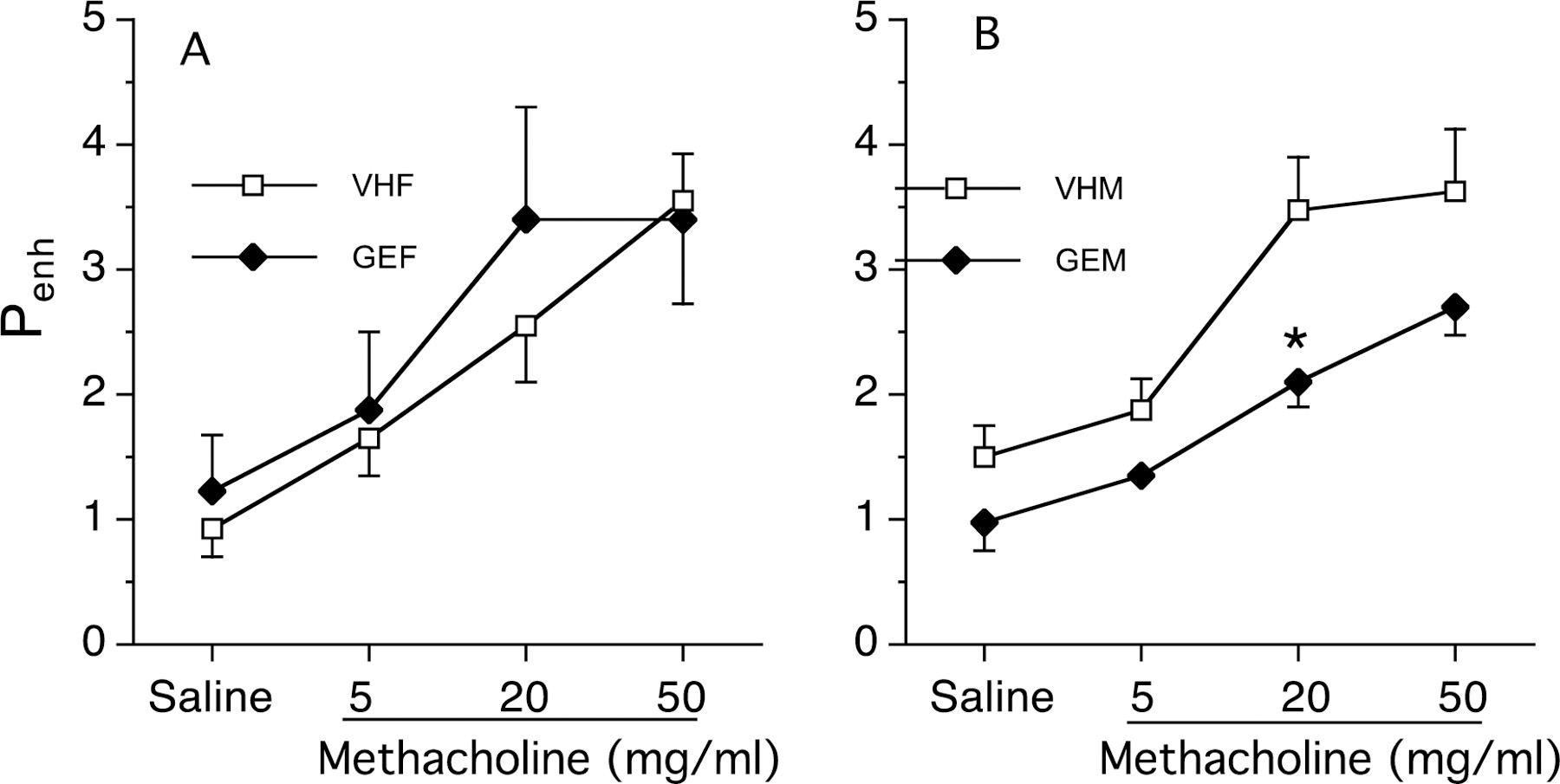
Effects of in utero GEN exposure on airway hyperresponsiveness in female (A) and male (B) B6C3F1 offspring at PND 330. Pregnant mice were treated with GEN or VH from GD14 to birth by gavage. Twenty-four hours after last HDM dosing, female or male offspring were individually placed in a whole-body plethysmograph. Estimates of airway responsiveness, expressed as enhanced pause (Penh), were derived from the ventilation and flow-derived parameters. Penh values were recorded for a minimum of 3-min intervals at baseline and after stimulation with each concentration of MCh (0 and 5, 20 and 50 mg/ml). NAF = untreated, non-sensitized naïve females, NAM = untreated, non-sensitized naïve males, VHF = VH females dosed with HDM, VHM = VH males dosed with HDM, GEF = GEN females dosed with HDM, GEM = GEN males dosed with HDM. a, significantly different from naïve group; b, significantly different from VH group. The number of mice in each group at each time point is shown in Table 2.
Lung histopathology in female and male offspring following in utero GEN exposure
Histopathological analysis of the lung samples (Figure 6A) included: peribronchial inflammatory infiltrate (Figure 6B), perivascular inflammatory infiltrate, prominent endothelial lining, subendothelial eosinophils, bronchi-associated lymphoid tissue and mucin producing cells (Figure 6C). At PND 290, the summed pathological score was higher in female offspring than male offspring (Figure 6D); however, there were no significant differences between the vehicle control and in utero GEN exposed female or male offspring (Table 4).
Figure 6.
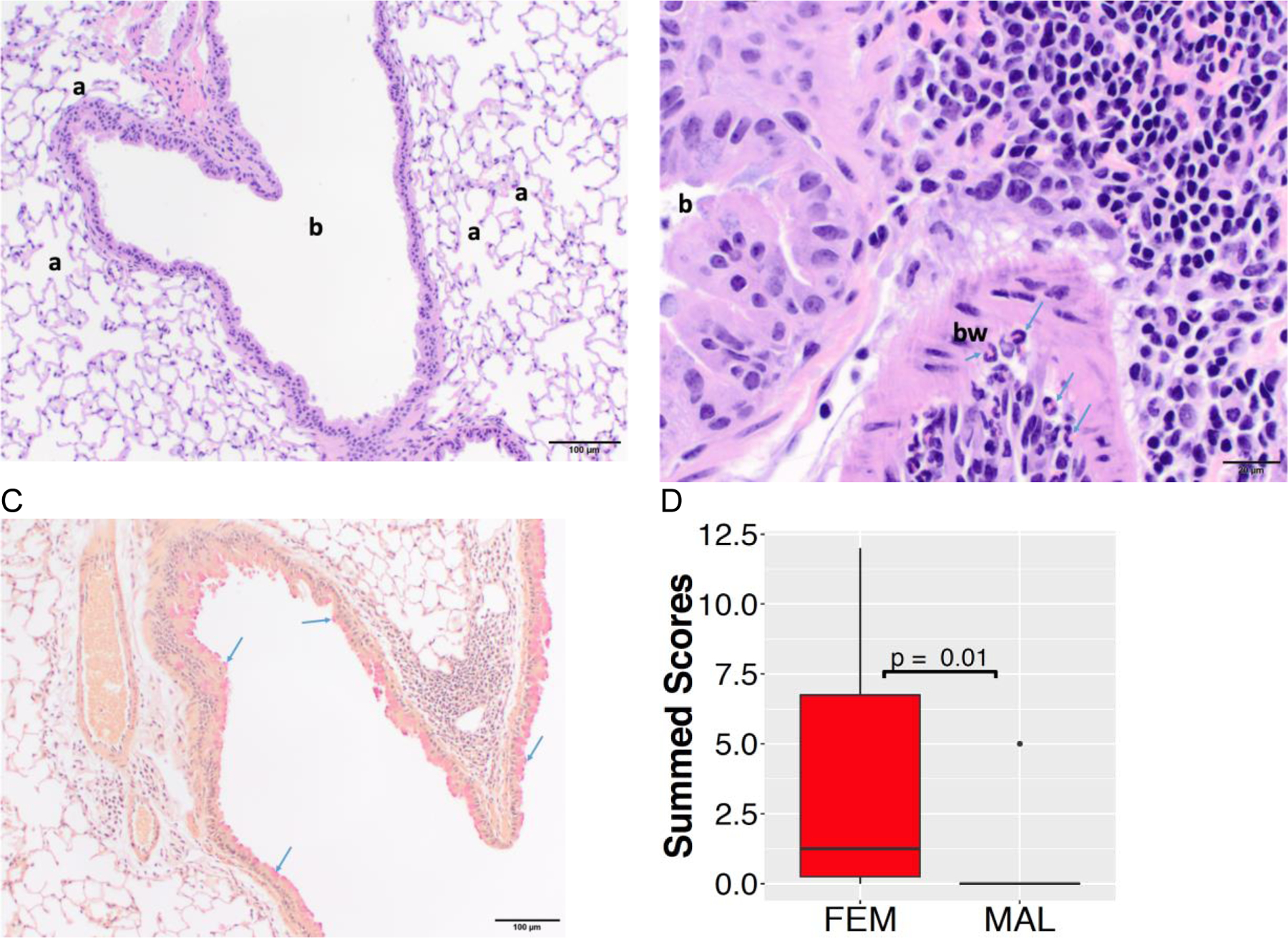
Lung histopathology in female and male offspring at PND 290. (A) Low magnification photomicrograph depicting a bronchiole (b) and surrounding alveoli (a) from a male mouse. The section is histologically normal. Hematoxylin and eosin staining, 100x original magnification, scale bar = 100µm. (B) High magnification photomicrograph of inflammatory infiltrate (right and top right sections of the image) around a bronchiole (b) and within the bronchiolar wall (bw) from a female mouse. Within the bronchiolar wall, there are numerous eosinophils (arrows). Hematoxylin and eosin staining, 400x original magnification, scale bar = 20µm. (C) Low magnification photomicrograph of mild goblet cell hyperplasia (bright red globules, arrows) in the bronchiolar epithelial lining from a female mouse. Mucicarmine staining, 100x original magnification, scale bar = 100µm. (D) The summed pathological score was higher in female offspring (FEM) than male offspring (MAL).
Table 4.
Effect of in utero GEN exposure on selected lung findings in middle-aged B6C3F1 offspring at PND290
| Treatment | BALT Hyperplasia Score* | Muc Score | PBII Score | PVII Score | PEL Score | SeE Score | Comments: | |
|---|---|---|---|---|---|---|---|---|
| Female | VH | 1 | 0 | 0 | 0 | 0 | 0 | Normal, Scattered nuetrophil infiltration |
| VH | 1 | 0 | 0 | 1 | 0 | 0 | Normal, Perivascular inflammatory foci | |
| VH | 2 | 1 | 0 | 0 | 0 | 0 | Goblet cell hyperplasia | |
| VH | 0 | 3 | 2 | 2 | 0 | 0 | Lots of eosinophils | |
| VH | NA | 3 | 2 | 3 | 2 | 2 | Lots of eosinophils,Trickling inflammatory infiltrates shallowly into adjacent alveoli, acute vascular injury | |
| GEN | NA | 2 | 1 | 2 | 1 | 1 | Few eosinophils | |
| GEN | NA | 2 | 1 | 1 | 1 | 1 | Few eosinophils | |
| GEN | 1 | 0 | 0 | 0 | 0 | 0 | Normal, Scattered nuetrophil infiltration | |
| GEN | NA | 1 | 0 | 0.5 | 0 | 0 | Lots of eosinophils, normal vessels | |
| GEN | 1 | 0 | 0 | 0 | 0 | 0 | Single PV focus, no eosin | |
| Male | VH | 1 | 0 | 0 | 0 | 0 | 0 | Normal |
| VH | 1 | 0 | 0 | 0 | 0 | 0 | Normal | |
| VH | 1 | 0 | 0 | 0 | 0 | 0 | Normal | |
| VH | 1 | 0 | 0 | 0 | 0 | 0 | Normal | |
| VH | 0 | 0 | 0 | 0 | 0 | 0 | Normal, no score | |
| GEN | 0 | 0 | 0 | 0 | 0 | 0 | Normal, no score | |
| GEN | 0 | 0 | 0 | 0 | 0 | 0 | Normal, no score | |
| GEN | 0 | 0 | 0 | 0 | 0 | 0 | Normal, no score | |
| GEN | NA | 1 | 1 | 1 | 1 | 1 | Many Eosinophil | |
| GEN | 0 | 0 | 0 | 0 | 0 | 0 | Normal, no score | |
PBII Peribronchial Inflammatory Infiltrate
PVII PerivascularInflammatory Infiltrate
PEL Prominent Endothelial Lining
SeE Subendothelial eosinophils
BALT Bronchi-associated lymphoid tissue
Muc Mucin producing cells
Likely to be irrevelant
Discussion
Increases in asthma prevalence and severity over the last several decades are due at least in part to environmental factors (e.g., diet), and allergic reaction to airborne allergens is an important risk factor for severe asthma in adults (Zureik et al., 2002). In our evaluation of the relationship between soy isoflavone GEN and respiratory allergy, we have demonstrated that in utero exposure to GEN increased IgE production in young B6C3F1 offspring following adult exposure to a respiratory sensitizer TMA (Guo et al., 2005) or HDM (Guo & Meng, 2016). However, the effects in aging mice were unknown. Most aging studies focus on two or three specific life phases: mature adult, middle age, and old (Petry, 2002). Middle age refers to a phase of life during which senescent changes can be detected in some, but not all, biomarkers of aging. Using a survival curve that is based on a large cohort of C57BL/6J mice (150 males and 150 females), it has been suggested that mice should be approximately 10–15 months of age (corresponding to 38–47 years old in humans) to be considered the middle-aged group (Brinton, 2012). The mice in our studies were 8 −11 months old with the last time point (PND 330) being in the range of middle age. The other two time points PND 240 and 290 were between the mature adult (3–6 months of age in a mouse) and middle age.
In our studies with young adult mice (Guo & Meng, 2016), the dose of 10 μg/mouse of HDM was considered optimal because of less variation, and it also permitted further increases following exposures. However, as the mice became aged, the HDM seemed to be less effective in eliciting IgE production when compared to IgE levels in the naïve mice (e.g., PND 240 in male offspring and PND 290 in female offspring in Figure 2). Nonetheless, the PND 240 and 290 offspring did develop respiratory sensitization following HDM challenge because: (1) At both time points tested, there were significant differences in HDM-specific IgE between naïve group and mice dosed with HDM (Figure 3 and data not shown); (2) At both time points tested, there were significant increases in HDM-specific IgG antibodies between naïve group and mice dosed with HDM. For PND 330, the HDM dose was increased to 50 μg/mouse. We performed plethysmographic tests for the B6C3F1 offspring at PND 330, a middle age, and found that in utero GEN treatment decreased AHR (e.g., Penh value) at the 20 mg/ml MCh challenge in male offspring. At PND 330, in utero GEN treatment in male offspring had no effects on serum total IgE, while decreased the level of HDM-specific IgG1 (a Th2 type Ab). Furthermore, the EPO activity was decreased following in utero GEN exposure in male offspring at PND 330. Thus, in contrast to our findings in young adult offspring, in utero exposure to GEN decreased intranasal HDM allergen-induced respiratory allergic reactions in middle-aged male B6C3F1 offspring.
Pulmonary eosinophilia has been considered as one of the major mechanisms for allergen-induced bronchial hyperreactivity (Afshar et al., 2007; Mathias et al., 2013). Using extracts from whole lung homogenates, a significant decrease of EPO activity in the GEN treatment group over the vehicle was observed at PND 330 for male offspring. Consistent with our findings, there is evidence that GEN inhibits eosinophil leukotriene C4 synthesis (a key pathway that may contribute to asthma severity), reduces exhaled nitric oxide and ex vivo leukotriene C4 synthesis in a small group of patients with inadequately controlled asthma (Kalhan et al., 2008; Smith et al., 2015). In a male guinea pig model of asthma, GEN (15 mg/kg; i.p.) also reduced ovalbumin-induced eosinophil peroxidase activity and attenuated airway hyperresponsiveness to inhaled Mch (Duan et al., 2003). Similarly, dietary phytoestrogens also significantly attenuated ovalbumin-induced eosinophilia in the lung tissue of guinea pigs (Regal, Fraser, Weeks, & Greenberg, 2000). However, it should be noted that GEN exposure in our study was in early life, e.g., in utero, and there was not any dosed GEN left in the body when the HDM respiratory allergic reactions occurred.
As the immune system ages, the IgE response generally decreases (Busse & Mathur, 2010; Hanneuse, Delespesse, Hudson, Dehalleux, & Jacques, 1978; Yagi, Sato, Hayakawa, & Ide, 1997). Our total IgE data also showed a trend towards decline at a later time point. It is possible that GEN modulation of respiratory allergic responses is age-related. A retrospective multiple controlled cohort study has shown that there was an increase in the use of asthma or allergy drugs in young adults with significant changes observed in females who had been fed soy formula during infancy as compared to those who were fed cow milk formula from the age of less than 9 days old (Strom et al., 2001). This observation was somewhat confirmed in our animal studies that in utero exposure to GEN increased respiratory sensitization in both male and female young B6C3F1 offspring (Guo et al., 2005; Guo & Meng, 2016). In our study with middle-aged female offspring, however, in utero GEN treatment did not change AHR, which was consistent with the EPO activity that was not significantly altered following in utero GEN exposure in female offspring at PND330. In addition, in utero GEN treatment had no effects on serum total IgE in female offspring, while the level of antigen-specific IgG2b (a Th1 type Ab) was increased at PND 330. Thus, in utero exposure to GEN had minimal effect on intranasal HDM allergen-induced respiratory allergic responses in middle-aged female B6C3F1 offspring. Interestingly, an increase of spleen weight was observed in the in utero-GEN treated female offspring at PND 330. Although the phenotype of splenocytes and their functions in these mice were not examined in this study, developmental GEN exposure decreased CD5+ cells and IL-10, and did not affect CD4+CD25+ T cells, while increased CD24+ cells in our studies with the middle-aged female NOD mouse offspring (Huang et al., 2018). It was possible that IL-10 producing regulatory B cell subset might control the T cell-dependent inflammatory responses (Vighi, Marcucci, Sensi, Di Cara, & Frati, 2008).
Although the mechanisms underlying a fetal basis of adult effects are currently unclear, the immune-related sex difference, e.g., females tend to have an increased Th1 response in early life compared to males (Bao, Yang, Jun, & Yoon, 2002), and gut microbiota-driven hormone difference (Markle et al., 2013) might be responsible for our observations that male offspring exposed to GEN in utero showed a protection of respiratory sensitization. Similar sex-specific phenomena of developmental GEN exposure have been shown in our studies of middle-aged NOD mice in which a protective effect on type 1 diabetes in males while a detrimental effect in females was observed (Huang et al., 2018). It was concluded that the exacerbation of type 1 diabetes in NOD females was associated with gut microbiota-related immunomodulation. Interestingly, S-(−)equol, a biologically active gut microbiota-derived metabolite of the soy isoflavones daidzin/daidzein, is also developmentally regulated and related to early diet composition (Brown et al., 2014). In addition, the age-related epigenetic changes (e.g., methylation increases of estrogen receptor α at an average of 1% every 3 years) of the immune system (Issa, 2003; Liang et al., 2015) and differential sex maturation (e.g., earlier puberty in females) in female and male offspring might partially be responsible for our observations that female offspring following in utero GEN and postnatal HDM exposures showed a different pattern from similarly treated male offspring. There is evidence that prenatal exposure to phytoestrogen GEN triggers epigenetic changes in immune function genes (Vanhees et al., 2011). Rodents are sexually mature by 3–6 months of age and approach the endocrine equivalent of human perimenopause by 9 months of age (Brinton, 2012; Mobbs, Gee, & Finch, 1984). However, irregularity of cycle length, a reliable indicator of irregular fertility and impending reproductive senescence, starts occurring approximately 8 months of age (Finch, Felicio, Mobbs, & Nelson, 1984). It was possible that the female offspring (8 – 11 months old) were in perimenopause, and thus, the estrogen-dependent imprinting effects could not manifest.
Although it has been reported that higher GEN intake is associated with better lung function (Bime et al., 2011; Smith et al., 2004), this is the first study reporting a protective effect on respiratory allergic reactions in middle-aged male offspring following developmental GEN exposure. For a 4-month-old infant who consumes soy formula as directed by the manufacturers, approximately 6–11 mg/kg body weight of isoflavones can be obtained (Katchy et al., 2014; Setchell et al., 1997). The serum level of GEN (1.4–7.5 μM) in mice that have been fed 1000 ppm GEN-containing diet (~80 mg/kg body weight, which is higher than our 20 mg/kg dose) was equivalent to that in men who received 100 mg GEN/day (Bhandari, Crawford, Huang, & Reenstra, 2003; Djuric, Chen, Doerge, Heilbrun, & Kucuk, 2001; Yellayi et al., 2002), and in infants on soy formula (Cao et al., 2009). As the microbiome play a central role in respiratory allergy, and they are highly influenced by multiple environmental and dietary factors, further studies of the molecular mechanisms by determining how developmental GEN exposure creates a diet‑ linked, age‑ related microbiome modulation of the respiratory allergic phenotype are warranted.
Highlights:
In utero genistein exposure decreased lung EPO activity in middle-aged male offspring
In utero genistein exposure decreased airway hyperresponsiveness in middle-aged males
In utero genistein exposure decreased HDM-specific IgG1 in middle-aged males
Acknowledgments
This study was supported by the NIH R21ES012286, and in part by NIH R21ES24487, NIH R41AT009523, NIH R41DK121553 and USDA National Institute of Food and Agriculture [grant no. 2016-67021-24994/project accession no. 1009090]. The authors would like to thank R.D. Brown at Virginia Commonwealth University for her technical assistance.
Abbreviations used:
- AHR
airway hyperresponsiveness
- ELISA
enzyme linked immunosorbent assay
- EPO
eosinophil peroxidase
- FBS
fetal bovine serum
- GD
gestation day
- GEN
genistein
- HBSS
Hank’s balanced salt solution
- HDM
house dust mites
- HRP
horseradish peroxidase
- MCh
methacholine
- PBS
phosphate buffered saline
- Penh
enhanced pause
- PND
postnatal day
- TMA
trimellitic anhydride
- TMB
tetramethylbenzidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Afshar K, Vucinic V, & Sharm OP (2007). Eosinophil cell: pray tell us what you do! Current Opinion in Pulmonary Medicine, 13(5), 414–421. doi:DOI 10.1097/MCP.0b013e328224b90b [DOI] [PubMed] [Google Scholar]
- Albertine KH, Wang L, Watanabe S, Marathe GK, Zimmerman GA, & McIntyre TM (2002). Temporal correlation of measurements of airway hyperresponsiveness in ovalbumin-sensitized mice. American Journal of Physiology-Lung Cellular and Molecular Physiology, 283(1), L219–L233. doi: 10.1152/ajplung.00324.2001 [DOI] [PubMed] [Google Scholar]
- Bao M, Yang Y, Jun HS, & Yoon JW (2002). Molecular mechanisms for gender differences in susceptibility to T cell-mediated autoimmune diabetes in nonobese diabetic mice. Journal of Immunology, 168(10), 5369–5375. doi:DOI 10.4049/jimmunol.168.10.5369 [DOI] [PubMed] [Google Scholar]
- Bhandari A, Crawford SE, Huang LJ, & Reenstra WW (2003). Effects of oral genistein in mice. Pediatric Pathology & Molecular Medicine, 22(2), 131–141. doi: 10.1080/15227950307723 [DOI] [PubMed] [Google Scholar]
- Bime C, Wei CY, Holbrook J, Smith LJ, & Wise RA (2011). Dietary Intake Of Soy Genistein Is Associated With Asthma Control And Lung Function In Asthma Patients. American Journal of Respiratory and Critical Care Medicine, 183 Retrieved from <Go to ISI>://WOS:000208770304054 [Google Scholar]
- Brinton RD (2012). Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology, 153(8), 3571–3578. doi: 10.1210/en.2012-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NM, Galandi SL, Summer SS, Zhao X, Heubi JE, King EC, & Setchell KD (2014). S-(−)equol production is developmentally regulated and related to early diet composition. Nutr Res, 34(5), 401–409. doi: 10.1016/j.nutres.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NM, & Setchell KDR (2001). Animal models impacted by phytoestrogens in commercial chow: Implications for pathways influenced by hormones. Laboratory Investigation, 81(5), 735–747. doi:DOI 10.1038/labinvest.3780282 [DOI] [PubMed] [Google Scholar]
- Busse PJ, & Mathur SK (2010). Age-related changes in immune function: Effect on airway inflammation. Journal of Allergy and Clinical Immunology, 126(4), 690–699. doi: 10.1016/j.jaci.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YP, Zhou JS, & Webb DC (2012). Estrogen Stimulates Th2 Cytokine Production and Regulates the Compartmentalisation of Eosinophils during Allergen Challenge in a Mouse Model of Asthma. International Archives of Allergy and Immunology, 158(3), 252–260. doi: 10.1159/000331437 [DOI] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, … Rogan WJ (2009). Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol, 19(2), 223–234. doi: 10.1038/jes.2008.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoine F, Sim A, Tang C, Fisher S, Ethier C, Puttagunta L, … Moqbel R (2013). Eosinophils in human oral squamous carcinoma; role of prostaglandin D2. Journal of Inflammation-London, 10. doi:Artn 4 10.1186/1476-9255-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, … Thorn BT (2014). Toxicity Evaluation of Bisphenol A Administered by Gavage to Sprague Dawley Rats From Gestation Day 6 Through Postnatal Day 90. Toxicological Sciences, 139(1), 174–197. doi: 10.1093/toxsci/kfu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Chen G, Doerge DR, Heilbrun LK, & Kucuk O (2001). Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Letters, 172(1), 1–6. doi:Doi 10.1016/S0304-3835(01)00627-9 [DOI] [PubMed] [Google Scholar]
- Doerge DR, Churchwell MI, & Delclos KB (2000). On-line sample preparation using restricted-access media in the analysis of the soy isoflavones, genistein and daidzein, in rat serum using liquid chromatography electrospray mass spectrometry. Rapid Communications in Mass Spectrometry, 14(8), 673–678. doi:Doi [DOI] [PubMed] [Google Scholar]
- Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, & Wong WSF (2003). Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. American Journal of Respiratory and Critical Care Medicine, 167(2), 185–192. doi: 10.1164/rccm.200205-420OC [DOI] [PubMed] [Google Scholar]
- Finch CE, Felicio LS, Mobbs CV, & Nelson JF (1984). Ovarian and Steroidal Influences on Neuroendocrine Aging Processes in Female Rodents. Endocrine Reviews, 5(4), 467–497. doi:DOI 10.1210/edrv-5-4-467 [DOI] [PubMed] [Google Scholar]
- Fischer R, McGhee JR, Vu HL, Atkinson TP, Jackson RJ, Tome D, & Boyaka PN (2005). Oral and nasal sensitization promote distinct immune responses and lung reactivity in a mouse model of peanut allergy. American Journal of Pathology, 167(6), 1621–1630. doi:Doi 10.1016/S0002-9440(10)61246-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley R, White K, Brown R, Musgrove D, Walker N, & Germolec D (2011). Gene Expression Alterations in Immune System Pathways in the Thymus after Exposure to Immunosuppressive Chemicals. Environmental Health Perspectives, 119(3), 371–376. doi: 10.1289/ehp.1002358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood V (2011). Why Are Asthma Rates Soaring? Researchers once blamed a cleaner world. Now they are not so sure. Scientific American, 304(4), 32–33. doi: 10.1038/scientificamerican0411-32 [DOI] [PubMed] [Google Scholar]
- Guo TL, Auttachoat W, & Chi RP (2005). Genistein enhancement of respiratory allergen trimellitic anhydride-induced IgE production by adult B6C3F1 mice following in utero and postnatal exposure. Toxicol Sci, 87(2), 399–408. doi: 10.1093/toxsci/kfi268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, & Meng AH (2016). In Utero exposure to genistein enhanced intranasal house dust mite allergen-induced respiratory sensitization in young adult B6C3F1 mice. Toxicology Letters, 253, 17–26. doi: 10.1016/j.toxlet.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Zhang XL, Leffel EK, Peachee VL, Karrow NA, Germolec DR, & White KL (2002). Differential stimulation of IgE production, STAT activation and cytokine and CD86 expression by 2,4-dinitrochlorobenzene and trimellitic anhydride. Journal of Applied Toxicology, 22(6), 397–403. doi: 10.1002/jat.876 [DOI] [PubMed] [Google Scholar]
- Hanneuse Y, Delespesse G, Hudson D, Dehalleux F, & Jacques JM (1978). Influence of Aging on Ige-Mediated Reactions in Allergic Patients. Clinical Allergy, 8(2), 165–174. doi:DOI 10.1111/j.1365-2222.1978.tb00461.x [DOI] [PubMed] [Google Scholar]
- Huang G, Xu J, Cai D, Chen SY, Nagy T, & Guo TL (2018). Exacerbation of Type 1 Diabetes in Perinatally Genistein Exposed Female Non-Obese Diabetic (NOD) Mouse Is Associated With Alterations of Gut Microbiota and Immune Homeostasis. Toxicol Sci, 165(2), 291–301. doi: 10.1093/toxsci/kfy162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa JP (2003). Age-related epigenetic changes and the immune system. Clinical Immunology, 109(1), 103–108. doi: 10.1016/S1521-6616(03)00203-1 [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Patisaul HB, & Williams CJ (2012). Reproductive consequences of developmental phytoestrogen exposure. Reproduction, 143(3), 247–260. doi: 10.1530/Rep-11-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Javurek AB, Painter MS, Ellersieck MR, Welsh TH, Camacho L, … Rosenfeld CS (2016). Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study. Hormones and Behavior, 80, 139–148. doi: 10.1016/j.yhbeh.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan R, Smith LJ, Nlend MC, Nair A, Hixon JL, & Sporn PHS (2008). A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clinical and Experimental Allergy, 38(1), 103–112. doi: 10.1111/j.1365-2222.2007.02862.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchy A, Pinto C, Jonsson P, Trang NV, Pandelova M, Riu A, … Williams C (2014). Coexposure to Phytoestrogens and Bisphenol A Mimics Estrogenic Effects in an Additive Manner. Toxicological Sciences, 138(1), 21–35. doi: 10.1093/toxsci/kft271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth KS (2002). Critical windows in development of the rodent immune system. Human & Experimental Toxicology, 21(9–10), 493–498. doi:DOI 10.1191/0960327102ht287oa [DOI] [PubMed] [Google Scholar]
- Liang LM, Willis-Owen SAG, Laprise C, Wong KCC, Davies GA, Hudson TJ, … Cookson WOCM (2015). An epigenome-wide association study of total serum immunoglobulin E concentration. Nature, 520(7549), 670–U188. doi: 10.1038/nature14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintomen L, Franchi G, Nowill A, Condino-Neto A, de Nucci G, Zanesco A, & Antunes E (2008). Human eosinophil adhesion and degranulation stimulated with eotaxin and RANTES in vitro: lack of interaction with nitric oxide. BMC Pulm Med, 8, 13. doi: 10.1186/1471-2466-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, … Danska JS (2013). Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science, 339(6123), 1084–1088. doi: 10.1126/science.1233521 [DOI] [PubMed] [Google Scholar]
- Martin PM, Horwitz KB, Ryan DS, & McGuire WL (1978). Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology, 103(5), 1860–1867. doi: 10.1210/endo-103-5-1860 [DOI] [PubMed] [Google Scholar]
- Mathias LJ, Khong SML, Spyroglou L, Payne NL, Siatskas C, Thorburn AN, … Heng TSP (2013). Alveolar Macrophages Are Critical for the Inhibition of Allergic Asthma by Mesenchymal Stromal Cells. Journal of Immunology, 191(12), 5914–5924. doi: 10.4049/jimmunol.1300667 [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Gee DM, & Finch CE (1984). Reproductive Senescence in Female C57bl/6j Mice - Ovarian Impairments and Neuroendocrine Impairments That Are Partially Reversible and Delayable by Ovariectomy. Endocrinology, 115(5), 1653–1662. doi:DOI 10.1210/endo-115-5-1653 [DOI] [PubMed] [Google Scholar]
- Ng SP, Steinetz BG, Lasano SG, & Zelikoff JT (2006). Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Experimental Biology and Medicine, 231(8), 1403–1409. doi:Doi 10.1177/153537020623100814 [DOI] [PubMed] [Google Scholar]
- Osho SO, Wang T, Horn NL, & Adeola O (2017). Comparison of goblet cell staining methods in jejunal mucosa of turkey poults. Poultry Science, 96(3), 556–559. doi: 10.3382/ps/pew324 [DOI] [PubMed] [Google Scholar]
- Papaconstantinou AD, Goering PL, Umbreit TH, & Brown KM (2003). Regulation of uterine hsp90alpha, hsp72 and HSF-1 transcription in B6C3F1 mice by beta-estradiol and bisphenol A: involvement of the estrogen receptor and protein kinase C. Toxicology Letters, 144(2), 257–270. doi: 10.1016/s0378-4274(03)00215-7 [DOI] [PubMed] [Google Scholar]
- Patisaul HB, & Jefferson W (2010). The pros and cons of phytoestrogens. Frontiers in Neuroendocrinology, 31(4), 400–419. doi: 10.1016/j.yfrne.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM (2002). A comparison of young, middle-aged, and older adult treatment-seeking pathological gamblers. Gerontologist, 42(1), 92–99. doi:DOI 10.1093/geront/42.1.92 [DOI] [PubMed] [Google Scholar]
- Rasid O, Chirita D, Iancu AD, Stavaru C, & Radu DL (2012). Assessment of Routine Procedure Effect on Breathing Parameters in Mice by Using Whole-Body Plethysmography. Journal of the American Association for Laboratory Animal Science, 51(4), 469–474. Retrieved from <Go to ISI>://WOS:000306772500014 [PMC free article] [PubMed] [Google Scholar]
- Regal JF, Fraser DG, Weeks CE, & Greenberg NA (2000). Dietary phytoestrogens have anti-inflammatory activity in a guinea pig model of asthma. Proceedings of the Society for Experimental Biology and Medicine, 223(4), 372-+. doi:DOI 10.1046/j.1525-1373.2000.22353.x [DOI] [PubMed] [Google Scholar]
- Robinson DS, Larche M, & Durham SR (2004). Tregs and allergic disease. Journal of Clinical Investigation, 114(10), 1389–1397. doi: 10.1172/Jci200423595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell KDR, ZimmerNechemias L, Cai JN, & Heubi JE (1997). Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet, 350(9070), 23–27. doi:Doi 10.1016/S0140-6736(96)09480-9 [DOI] [PubMed] [Google Scholar]
- Smith LJ, Holbrook JT, Wise R, Blumenthal M, Dozor AJ, Mastronarde J, … Clin, A. L. A. A. (2004). Dietary intake of soy genistein is associated with lung function in patients with asthma. Journal of Asthma, 41(8), 833–843. doi: 10.1081/Jas-200038447 [DOI] [PubMed] [Google Scholar]
- Smith LJ, Kalhan R, Wise RA, Sugar EA, Lima JJ, Irvin CG, … Clinical, A. L. A. A. (2015). Effect of a Soy Isoflavone Supplement on Lung Function and Clinical Outcomes in Patients With Poorly Controlled Asthma A Randomized Clinical Trial. Jama-Journal of the American Medical Association, 313(20), 2033–2043. doi: 10.1001/jama.2015.5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Quiros M, Avila L, Platts-Mills TAE, Hunt JF, Erdman DD, Carper H, … Heymann PW (2012). High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. Journal of Allergy and Clinical Immunology, 129(6), 1499-+. doi: 10.1016/j.jaci.2012.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strath M, Warren DJ, & Sanderson CJ (1985). Detection of Eosinophils Using an Eosinophil Peroxidase Assay - Its Use as an Assay for Eosinophil Differentiation Factors. Journal of Immunological Methods, 83(2), 209–215. doi:Doi 10.1016/0022-1759(85)90242-X [DOI] [PubMed] [Google Scholar]
- Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, … Hanson SA (2001). Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA, 286(7), 807–814. doi: 10.1001/jama.286.7.807 [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Kissling GE, Locklear J, Caviness GF, Whiteside T, … Grant M (2013). The Estrogenic Content of Rodent Diets, Bedding, Cages, and Water Bottles and Its Effect on Bisphenol A Studies. Journal of the American Association for Laboratory Animal Science, 52(2), 130–141. Retrieved from <Go to ISI>://WOS:000316159700002 [PMC free article] [PubMed] [Google Scholar]
- Vanhees K, Coort S, Ruijters EJB, Godschalk RWL, van Schooten FJ, & van Doorn-Khosrovani SBV (2011). Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. Faseb Journal, 25(2), 797–807. doi: 10.1096/fj.10-172155 [DOI] [PubMed] [Google Scholar]
- Vighi G, Marcucci F, Sensi L, Di Cara G, & Frati F (2008). Allergy and the gastrointestinal system. Clin Exp Immunol, 153 Suppl 1, 3–6. doi: 10.1111/j.1365-2249.2008.03713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Walston J, Gottesman RF, Kucharska-Newton A, Palta P, & Windham BG (2019). Midlife Systemic Inflammation Is Associated With Frailty in Later Life: The ARIC Study. Journals of Gerontology Series a-Biological Sciences and Medical Sciences, 74(3), 343–349. doi: 10.1093/gerona/gly045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MDW, Chung YJ, Copeland LB, & Doerfler DL (2010). A comparison of the allergic responses induced by Penicillium chrysogenum and house dust mite extracts in a mouse model. Indoor Air, 20(5), 380–391. doi: 10.1111/j.1600-0668.2010.00660.x [DOI] [PubMed] [Google Scholar]
- Whitehead GS, Walker JKL, Berman KG, Foster WM, & Schwartz DA (2003). Allergen-induced airway disease is mouse strain dependent. American Journal of Physiology-Lung Cellular and Molecular Physiology, 285(1), L32–L42. doi: 10.1152/ajplung.00390.2002 [DOI] [PubMed] [Google Scholar]
- Yagi T, Sato A, Hayakawa H, & Ide K (1997). Failure of aged rats to accumulate eosinophils in allergic inflammation of the airway. Journal of Allergy and Clinical Immunology, 99(1), 38–47. doi:Doi 10.1016/S0091-6749(97)81042-1 [DOI] [PubMed] [Google Scholar]
- Yellayi S, Naaz A, Szewczykowski MA, Sato T, Woods JA, Chang JS, … Cooke PS (2002). The phytoestrogen genistein induces thymic and immune changes: A human health concern? Proceedings of the National Academy of Sciences of the United States of America, 99(11), 7616–7621. doi: 10.1073/pnas.102650199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet L, Neukirch F, & Hlt ECR (2002). Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. Bmj-British Medical Journal, 325(7361), 411–414. doi:DOI 10.1136/bmj.325.7361.411 [DOI] [PMC free article] [PubMed] [Google Scholar]


