Abstract
The application of neonicotinoid insecticides (neonics) has increased dramatically as a replacement for organophosphate pesticides (OPs) in recent years. Nevertheless, little is known about human exposure to these pesticides in various countries. In this study, concentrations of 14 neonics and six dialkylphosphate metabolites (DAPs) were determined simultaneously in 566 urine samples collected from nine countries during 2010–2014. The highest sum concentration of 14 neonics was found in urine from Vietnam (median: 12.2 ng/mL) whereas that of six DAPs was from China (18.4 ng/mL). The median concentrations of Σ6 DAPs were twice higher than those of Σ14 neonics across the nine countries, which suggested a greater exposure to OPs than neonics. The overall pattern of urinary pesticide concentrations was similar among the nine countries with dimethylphosphate (DMP) and dimethylthiophosphate (DMTP) accounting for 51–89% of the total pesticide concentrations. Differences in urinary pesticide concentrations between genders (female and male), age groups (≤20, 21–49, and ≥50 years), and regions (cities of Shanghai, Guangzhou and Qiqihar) were examined. Total daily exposure doses to OPs were highest in China (515 μg/day) with 15% of the samples exceeding the U.S. Environmental Protection Agency’s reference dose for chlorpyrifos (18 μg/day). This is the first study to establish baseline levels of OP and neonics exposure in general populations across nine countries.
Keywords: Neonicotinoid, Organophosphate, Metabolite, Chlorpyrifos, Urine, Biomonitoring
Graphical Abstract
1. Introduction
Neonicotinoid insecticides (hereafter “neonics”) have emerged as alternatives to organophosphate (OP) pesticides, and their usage in agriculture has increased due to their broad-spectrum insecticidal activity, unique mode of neurotoxic action and presumed low mammalian toxicity (Casida and Durkin, 2013; Rundlöf et al., 2015). The application of neonics has also expanded to non-agricultural fields, such as homes, lawns, gardens and animal welfare (Morrissey et al., 2015; Zhang et al., 2018). The market share of neonics grew rapidly from the launch in the 1990s to >25% of the pesticide use in 2014 (Bass et al., 2015; Jeschke et al., 2011). The annual global production of neonic active ingredients was estimated at 20 thousand tonnes in 2010 (Wang et al., 2018). Thiamethoxam (THX, 37.6% of the total market share of $3.2 billion in 2012), imidacloprid (IMI, 33.5%), clothianidin (CLO, 14.7%), acetamiprid (ACE, 7.2%), thiacloprid (THI, 3.8%), dinotefuran (DIN, 2.9%) and nitenpyram (NIT, 0.3%) are the most commonly used neonics in over 140 crops in 120 countries, especially in Asia, North and South America (75% of total neonics use) and Europe (11%) (Bass et al., 2015; Sadaria et al., 2016).
Given their widespread use and physicochemical properties (systemic action and persistence in soil and water), neonics are ubiquitous in soil (Morrissey et al., 2015; Stewart et al., 2014), water (Klarich et al., 2017; Sadaria et al., 2016), house dust (Bennett et al., 2019), air (Tapparo et al., 2012; Ikenaka et al., 2019) and food (Mitchell et al., 2017; Chen et al., 2020). In spite of the original notion that neonics have low mammalian toxicity, evidences suggest adverse effects on non-target organisms such as honey bees (Rundlöf et al., 2015; Mitchell et al., 2017), insectivorous birds (Hallmann et al., 2014) and aquatic invertebrates (Morrissey et al., 2015), and further raising concerns about human health. Epidemiologic studies have linked human exposure of neonics with adverse developmental and neurological outcomes (e.g., congenital heart defects, anencephaly, autism spectrum disorders and memory loss) (Cimino et al., 2017; Zhang et al., 2018).
Human exposure to pesticides arises primarily via the ingestion of contaminated food and water (Bennett et al., 2019; Jusko et al., 2019; Klarich et al., 2017; Mitchell et al., 2017). Following enteric absorption, both neonics and OPs are metabolized by phase I enzymes, and some of the phase I metabolites undergo phase II conjugation to facilitate excretion in urine (Li et al., 2020a; Li and Kannan, 2018). Some neonics are excreted in urine as unchanged compounds due to their high water solubility whereas 70–75% of OPs are metabolized to dialkylphosphates (DAPs) (Ueyama et al., 2015) (Table S1). Very few studies have reported urinary concentrations of neonics and DAPs in general populations (Table S2; Table S3). An understanding of exposure patterns of neonics and DAPs in populations from various countries is lacking.
The objectives of this study were to establish baseline levels of exposure of general populations to neonics and OPs in nine countries (Table S1), and to elucidate the determinants of exposure and potential risks. An analytical method that was capable of measuring 14 neonics and 6 DAPs was developed and applied in the analysis of 566 urine samples collected from populations in nine countries (Table S4). By investigating geographic patterns in pesticide exposure profiles and demographic variables (i.e., gender and age), we established baseline levels needed for investigating future trends in exposures. On the basis of the concentrations measured in urine, daily exposure doses to target pesticides were calculated, which were then compared against available reference values.
2. Materials and methods
2.1. Study population
Spot urine samples (n = 566) were collected from nine countries during 2010–2014: the USA (n = 44; number of samples of males/females/unknown gender: 29/15/0), Greece (n = 118; 54/64/0), China (n = 84; 31/24/29), India (n = 41; 3/10/28), Saudi Arabia (n = 61; 28/33/0), Japan (n = 35; 27/8/0), Korea (n = 100; 47/53/0), Vietnam (n = 17; 8/9/0) and Kuwait (n = 66; 27/39/0) (Table S4). The samples originated from 12 cities in the nine countries, i.e., Albany (New York State), Athens, Guangzhou/Shanghai/Qiqihar, Mettupalayam (Tamil Nadu State), Jeddah, Ehime/Kumamoto, Seoul, Hanoi, and Kuwait. The age of the donors ranged from 1 to 87 years (mean ± standard deviation, 41.5 ± 19.7 years), with age groups of ≤20 (n = 66; 13%), 21–49 (n = 244; 48%) and ≥50 years (n = 198; 39%); the ratios of males to females were 0.9, 1.1 and 0.9, respectively, for the three age groups. These samples were collected as a part of our previous studies and all samples were void of personal identifiers. Institutional Review Board approvals were obtained from the New York State Department of Health for the analysis of urine samples. All samples were kept at −20 °C in polypropylene tubes until analysis.
2.2. Determination of neonicotinoids and dialkylphosphates in urine
Details pertaining to analytical standards, internal standards, and reagents used in this study are provided in the Supplementary Information and Table S5. The target neonics and DAPs were extracted from urine by a solid phase extraction (SPE) method, similar to that described earlier (Li et al., 2020a; 2020b). Briefly, urine samples (250 μL) were spiked with 2.5 ng each of stable isotopically labeled internal standard mixture (seven neonics and six DAPs; Table S5) and mixed with 2% formic acid (1.5 mL). The samples were then passed through SPE cartridges (Bond Elut Plexa, 3 mL, 60 mg, Agilent; Lexington, MA, USA) that were conditioned with 5% ammonium hydroxide in methanol (3 mL) and water (3 mL). After loading samples, cartridges were washed with 2 mL of formic acid/methanol/water (2:10:88, v/v/v) and vacuum dried for 1 min. The eluates (3 mL of methanol) were dried under a gentle stream of nitrogen (Organomation Associates Inc.; West Berlin, MA, USA) and reconstituted with 250 μL of methanol/water (25:75, v/v).
Both neonics and DAPs were chromatographically separated using a Waters Acquity Class I HPLC system (Waters; Milford, MA, USA) connected with a Kinetex Phenyl/Hexyl column (50 × 2.1 mm, 2.6 μm, Phenomenex; Torrance, CA, USA) for the analysis of 6-CN and SUF, with a Betasil C18 column (100 × 2.1 mm, 5 μm, Thermo Fisher Scientific; Waltham, MA, USA) for the analysis of remaining 12 neonics, and with a Luna HILIC column (100 × 3 mm, 3 μm, Phenomenex; Torrance, CA, USA) for the analysis of six DAPs. The target analytes were detected and quantified using an ABSCIEX 5500 electrospray triple quadrupole mass spectrometer (ESI-MS/MS; Applied Biosystems; Foster City, CA, USA) in either negative or positive ionization mode. Further details of the instrumental analysis and compound specific parameters are provided in the Supplementary Information and Table S6.
2.3. Determination of creatinine
Urine samples (10 μL) were diluted 160-times using HPLC-grade water, and 1200 ng of D3-creatinine was added. Creatinine concentrations were determined using a high performance liquid chromatography (Agilent 1100 series HPLC, Agilent Technologies; Santa Clara, CA, USA) coupled with an ABSCIEX 2000 ESI-MS/MS (Applied Biosystems; Foster City, CA, USA). The chromatographic separation was accomplished using a Betasil C18 column (100 × 2.1 mm, 5 μm, Thermo Fisher Scientific; Waltham, MA, USA). Further details of the instrumental analysis and compound specific mass spectrometric parameters are shown in the Supplementary Information and Table S6.
2.4. Method performance
Neonics and their metabolites were quantified using an isotope dilution method with a 10- to 11-point calibration curve prepared in synthetic urine at concentrations ranging from 0.02 to 50 ng/mL. The correlation coefficient (r) was >0.999 for all compounds. FLO (0.010 ng/mL) and N-DMA (0.005 ng/mL) were detected in procedural blanks and the concentrations found in blanks were subtracted from those measured for samples. The accuracy (% mean recovery) and precision (relative standard deviation; RSD) were measured by replicate analysis (n = 32) of a synthetic urine sample (Sigma-Aldrich; Round Rock, TX, USA) fortified at low (1 ng/mL), medium (10 ng/mL) and high concentrations (20 ng/mL) of target neionics. The recoveries of all target neonics were in the range of 85–110%, with RSDs of <14.6%. The limits of detection (LODs), defined as a signal-to-noise ratio of 3, ranged from 0.001 to 0.044 ng/mL (Table S7).
The six DAPs were quantified using an isotope dilution method with an 11 to 12-point calibration curve prepared at concentrations ranging from 0.02 to 100 ng/mL. The r was >0.999 for all compounds. Trace concentrations (ng/mL) of target compounds (0.043 for DMP, 0.006 for DMTP, 0.002 for DMDTP and 0.016 for DETP) were detected in procedural blanks, and these concentrations were subtracted from those measured for samples. The accuracy and precision were determined by replicate analysis (n = 32) of a synthetic urine sample fortified at low (1 ng/mL), medium (10 ng/mL) and high concentrations (20 ng/mL) of DAPs. The recoveries of the six DAPs were in the range of 85–110%, with RSDs <12%. The LODs for DAPs ranged from 0.002 to 0.053 ng/mL (Table S7).
Creatinine was quantified using an isotope dilution method with a nine-point calibration curve prepared at concentrations ranging from 100 to 2000 ng/mL (r = 0.9997). The concentration of creatinine in procedural blanks was below the LOD (19.4 ng/mL). The recovery of creatinine (fortified at 750 ng/mL in synthetic urine) through the analytical method was 99.6% (n = 10; RSD = 0.6%). Additionally, Standard Reference Materials (SRMs) 3672 and 3673 (creatinine in smokers’ and non-smokers’ urine, respectively), supplied by the National Institute of Standards and Technology (Gaithersburg, MD, USA), were analyzed for creatinine. The recoveries of creatinine from SRMs were from 97% to 102% (n = 8; RSDs <1.5%).
2.5. Data analysis
Data were analyzed using SPSS 19.0 (SPSS Inc.; Chicago, IL, USA). The concentrations below the LOD were assigned a value of LOD divided by the square root of 2. Prior to statistical analyses, data were log-transformed (χ + 1) to normalize their distributions. Creatinine-adjusted values were provided, as appropriate. Differences in urinary levels of neonics and DAPs among the nine countries and three age groups were examined by one-way ANOVA, if the data followed a normal distribution; otherwise, a non-parametric Kruskal-Wallis H test was applied. Differences in urinary levels of chemicals between genders were examined by a Student’s t-test, if the data followed a normal distribution; otherwise, a Mann-Whitney U test was applied. Pearson’s correlation analyses were conducted to explore associations between urinary concentrations of target pesticides. Values of p < 0.05 denote statistical significance.
3. Results and discussion
3.1. Urinary neonics and DAP concentrations in nine countries
The concentrations of Σ14 neonics and Σ6 DAPs were significantly different among the nine countries studied (Kruskal-Wallis H test, p < 0.001). The median urinary concentrations (ng/mL) of Σ14 neonics varied by an order of magnitude among the nine countries, with the highest concentration found in Vietnam (12.2), followed by Saudi Arabia (6.1), Greece (6.0), China (4.6), Japan (4.6), the USA (3.1), India (2.9), Kuwait (2.5), and Korea (1.9) (Fig. S4). The median concentrations (ng/mL) of Σ6 DAPs varied by a factor of five among the countries studied, and were the highest in China (18.4) followed in decreasing order by Korea (14.1), Kuwait (7.6), Saudi Arabia (7.3), Vietnam (6.9), Greece (6.2), Japan (4.0), the USA (3.8) and India (3.6) (Fig. S5). The differences in exposure patterns suggest varied usage patterns of neonics and OP pesticides among countries. We reported in our previous study that geographical differences existed in the distribution of urinary concentrations of 11 organophosphate and pyrethroid pesticides and phenoxy acid herbicides in eight countries, with Vietnam containing the highest concentrations (Li and Kannan, 2018).
The sum concentrations of Σ20 pesticides (sum of 14 neonics and 6 DAPs) differed significantly among the nine countries (Kruskal-Wallis H test, p < 0.001) in the decreasing order of China (median at ng/mL, 25.2), Vietnam (19.8), Korea (16.8), Greece (13.7), Saudi Arabia (13.6), Kuwait (11.3), Japan (9.6), India (8.5) and the USA (8.1) (Fig. 1). The median concentrations of Σ20 pesticides found in urine samples from China, Vietnam and Korea were 2–3 times higher than those found in Kuwait, Japan, India and the USA. The rate of annual pesticide use (kg/ha) during 2010–2014 was the greatest in Japan (18.9), followed by China (10.5), the USA (3.9) and India (0.3) (Zhang, 2018). The agricultural use of neonics was 23%, 22% and 11% of the total market share in 2012 in Asia, North America and Europe, respectively (Bass et al., 2015). Among all insecticides (94000 tonnes), OPs (30000 tonnes) were the most widely used in 2014 globally (Zhang, 2018). The rate of pesticide use among Chinese farmers reached three times the global average, leading to increased pollution (Zhang, 2018). In China, over 270000 tonnes of pesticides are used annually in agriculture, with OPs comprising approximately 70% of all pesticides used (Wang et al., 2017).
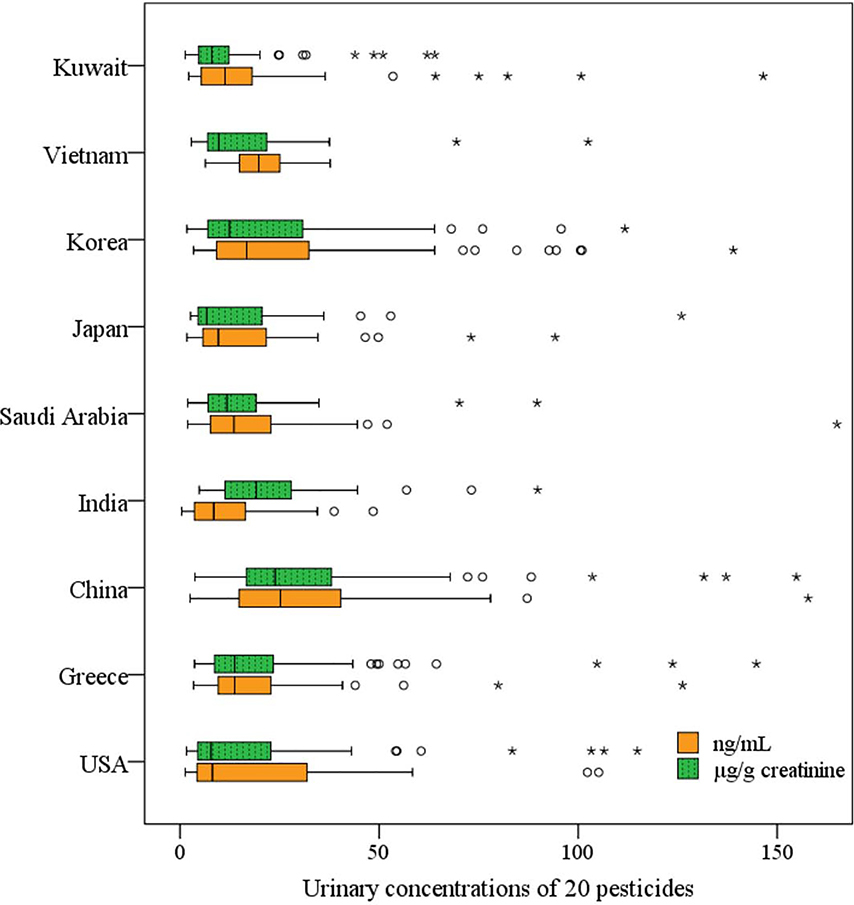
Fig. 1.
Urinary concentrations (ng/mL and μg/g creatinine) of pesticides (14 neonicotinoid insecticides and six dialkylphosphate metabolites) from nine countries. The vertical lines represent the minimum, 50th percentile, and maximum, and the boxes represent the 25th and 75th percentiles. Standard and extreme outliers are denoted by circles and stars, respectively, indicating values >1.5 and 3 times the interquartile range away from the 25th and 75th percentiles. Outliers’ concentrations of sum of 20 pesticides over 160 ng/mL and μg/g creatinine are not shown in the figure.
Urinary concentrations of neonics and DAPs were normalized for creatinine content. Pearson correlation analysis showed significant positive correlations between volume-based (ng/mL) and creatinine-adjusted (Σg/g creatinine) concentrations of pesticides for 191 out of 198 pairs (9 countries × (14 neonics + 6 DAPs + Σ14 neonics + Σ 6DAPs) (i.e., 9 × 22), regardless of individual or sum concentrations measured for the nine countries (r = 0.240–0.998, p < 0.05; Table S8). The volume-based and creatinine-adjusted concentrations of NIT, FLO, TA, SUF and DEDTP were not significantly correlated, which can be explained by their low detection frequencies in samples (2–37%) of these analytes. Significant positive correlations were found between volume-based and creatinine-adjusted concentrations of individual or total concentrations across the nine countries (r = 0.682–0.948, p < 0.01). These findings suggest that urine excretion volume at sampling (i.e., dilution factor) did not influence the measured concentrations of these pesticides (Fig. 1) (Li and Kannan, 2018). Further discussions on urinary pesticide concentrations were based on volumetric values (i.e., ng/mL), unless specified otherwise.
3.2. Profiles of urinary neonics and DAPs
The detection frequency (DF) and distribution of volume-based or creatinine-adjusted concentrations of neonics and DAPs in urine are presented (Tables 1 and S9). Among neonics, IMI, N-DMA (a metabolite of ACE) and 6-CN (a non-specific metabolite of IMI, ACE, THI, NIT and cycloxaprid) were found at a DF of >80% whereas other neonics were found at DFs in the range of 13–72%. Among six DAPs, DMP, DMTP, DMDTP and DETP were found in ≥92% of the urine samples while DEP and DEDTP were found in <70% of the samples analyzed. For further analysis, we selected those pesticides that were found at DFs of >80% in urine samples. Among the 20 pesticides analyzed, DMP was found at the highest concentration (median: 3.39 ng/mL), followed by DMTP (1.55), DETP (0.447), 6-CN (0.354), N-DMA (0.262), IMI (0.128) and DMDTP (0.112) (Table 1). Dimethyl phosphates were detected more frequently and at higher concentrations than those of diethyl phosphates (Berman et al., 2013; Jusko et al., 2019; Oya et al., 2020; Sokoloff et al., 2016). This may be ascribed to greater exposure to dimethyl OP pesticides such as malathion and longer half-lives of dimethyl phosphates (Sokoloff et al., 2016). The distribution pattern of 6-CN, N-DMA and IMI found in our study was similar to that reported previously from the USA, China and Japan (Ikenaka et al., 2019; Li et al., 2020a; Wang et al., 2015). The highest urinary concentrations of IMI (median: 1.13–2.56 ng/mL) and 6-CN (median: 0.45–1.38 ng/mL) were reported among Chinese residents living near orchards (Tao et al., 2019). A significant correlation between the concentrations of neonics and DAPs in urine (Table S10) suggested a common exposure pathway for these two classes of pesticides (Osaka et al., 2016). Diet is thought to be the major source of exposure to both of these classes of pesticides (Chen et al., 2020; Lu et al., 2006).
Table 1.
Descriptive statistics of concentrations (ng/mL) of urinary neonicotinoids and organophosphates and their metabolites from nine countries (n = 566).
| Analyte | DF (%) | Mean (ng/mL) | Percentile (ng/mL) |
|||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 100th | |||
| NIT | 53.7 | 0.079 | <LOD | 0.006 | 0.094 | 3.89 |
| THX | 72.3 | 0.282 | <LOD | 0.114 | 0.344 | 6.57 |
| IMI | 89.4 | 0.193 | 0.051 | 0.128 | 0.230 | 2.78 |
| ACE | 21.2 | 0.014 | <LOD | <LOD | <LOD | 2.16 |
| THI | 13.1 | 0.003 | <LOD | <LOD | <LOD | 0.412 |
| CLO | 72.1 | 0.262 | <LOD | 0.105 | 0.237 | 24.3 |
| DIN | 66.8 | 3.05 | <LOD | 0.681 | 2.85 | 187 |
| FLO | 47.7 | 0.070 | <LOD | <LOD | 0.069 | 9.42 |
| N-DMT | 57.1 | 0.282 | <LOD | 0.035 | 0.222 | 16.6 |
| TA | 41.9 | 0.066 | <LOD | <LOD | 0.096 | 0.712 |
| IMZ | 69.1 | 0.425 | <LOD | 0.165 | 0.509 | 8.76 |
| N-DMA | 97.7 | 0.750 | 0.103 | 0.262 | 0.672 | 13.7 |
| 6-CN | 91.9 | 0.841 | 0.176 | 0.354 | 0.760 | 29.5 |
| SUF | 15.2 | 0.010 | <LOD | <LOD | <LOD | 1.38 |
| ∑14 neonics | 6.33 | 2.17 | 3.70 | 7.01 | 189 | |
| DMP | 98.6 | 9.21 | 1.41 | 3.39 | 8.77 | 216 |
| DMTP | 99.8 | 5.24 | 0.631 | 1.55 | 3.55 | 517 |
| DMDTP | 92.0 | 1.39 | 0.032 | 0.112 | 0.327 | 297 |
| DEP | 69.8 | 1.57 | <LOD | 0.244 | 1.48 | 47.3 |
| DETP | 97.7 | 1.52 | 0.186 | 0.447 | 1.34 | 132 |
| DEDTP | 8.5 | 0.068 | <LOD | <LOD | <LOD | 20.1 |
| ∑6 DAPs | 19.0 | 3.44 | 8.10 | 18.2 | 1035 | |
DF, detection frequency. LOD, limit of detection. ∑14 neonics, sum concentration of the 14 neonicotinoid insecticides. ∑6 DAPs, sum concentration of the six dialkylphosphate metabolites.
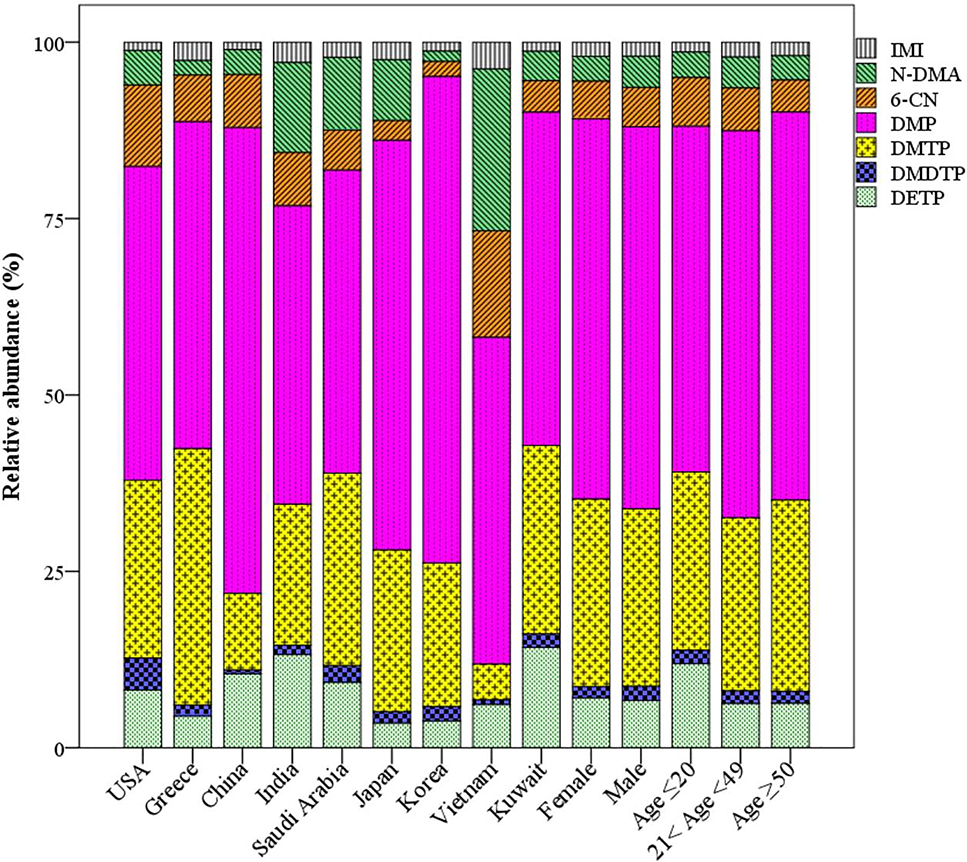
The overall distribution pattern of urinary pesticide concentrations was similar among the nine countries, three age groups and between genders (Fig. 2). The sum concentrations of DMP and DMTP collectively accounted for 51–89% of the seven major urinary pesticide concentrations whereas neonics, IMI, N-DMA and 6-CN accounted for 4.9–42% of the total concentrations. It has been reported that, following absorption, 75% of the registered OP pesticides get metabolized to DAPs in the body (Ueyama et al., 2015). OP pesticides were banned for most residential uses in the USA and western European countries in the early 2000s. Nevertheless, the general populations are exposed to OPs through the ingestion of agricultural products (Furlong et al., 2014). Our previous study showed that the general populations in the studied countries were exposed to (methyl) parathion and chlorpyrifos extensively as implied by the abundance of corresponding urinary metabolites, para-nitrophenol and 3,5,6-trichloro-2-pyridinol (Li and Kannan, 2018). IMI currently accounts for ~42% of neonics market and the demand for other neonics, ACE, THI, NIT and DIN is increasing (Jeschke et al., 2011). The use of OPs has been constantly declining, from 70 to 20 million pounds during the period of 2000–2012 in the USA (EPA, 2017) and from 946 to 44 thousand pounds during the period of 2001–2011 in Japan (Ueyama et al., 2015). This change in usage pattern was attributed to the emergence of resistance towards OPs and concomitant development of neonics (Hladik et al., 2018; Ueyama et al., 2015). However, the median concentrations of Σ6 DAPs were twice higher than those of Σ14 neonics across the nine countries, suggesting greater exposure to OPs than that of neonics (Ueyama et al., 2015). It should be noted that several neonics are metabolized to hydroxylated as well as olefin derivatives and analytical standards are not commercially available to quantify those metabolites (Zhang et al., 2019; Song et al., 2020). Further studies on the measurement of neonics metabolites are needed to assess cumulative exposure doses to these insecticides.
Fig. 2.
Composition profiles of urinary pesticides from nine countries (to sum of three neonicotinoid insecticides and four dialkylphosphate metabolites with detection frequencies >80%). IMI, imidacloprid; N-DMA, N-desmethyl acetamiprid; 6-CN, 6-chloronicotinic acid; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; DMDTP, dimethyldithiophosphate; DETP, diethylthiophosphate.
3.3. Demographic factors and urinary pesticide concentrations
Country-specific differences in urinary concentrations were examined for individual and sum concentrations of seven major pesticides (DFs >80%) for the nine countries. The individual and sum concentrations of pesticides differed significantly among the nine countries (Kruskal-Wallis H test, p < 0.01) (Table 2). The highest urinary concentrations of 6-CN (median: 1.11 ng/mL), DMP (9.73) and DETP (1.54) were found in China; IMI (0.202) and N-DMA (1.22) in Vietnam; and DMTP (2.44) and DMDTP (0.243) in Korea. Creatinine normalization of concentrations did not affect the geographic differences among countries except that India had the highest creatinine adjusted urinary concentration of IMI (median at μg/g creatinine, 0.232) and N-DMA (0.912) followed by Vietnam (IMI at 0.110; N-DMA at 0.667).
Table 2.
Country-wise concentrations (ng/mL and μg/g creatinine in bold italic) of urinary neonicotinoid insecticides and dialkylphosphate metabolites among the nine countries studied.
| IMI | N-DMA | 6-CN | DMP | DMTP | DMDTP | DETP | ∑7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Country | ** | ** | ** | ** | ** | ** | ** | ** | |
| USA (n = 44) | DF | 79.5 | 97.7 | 93.2 | 100 | 100 | 93.2 | 95.5 | |
| Median | 0.047 | 0.195 | 0.461 | 1.77 | 1.00 | 0.181 | 0.326 | 4.91 | |
| Median | 0.052 | 0.181 | 0.442 | 1.22 | 1.11 | 0.140 | 0.258 | 4.99 | |
| Greece (n = 118) | DF | 95.8 | 99.2 | 94.9 | 99.2 | 100 | 93.2 | 99.2 | |
| Median | 0.152 | 0.121 | 0.386 | 2.70 | 2.12 | 0.092 | 0.262 | 6.77 | |
| Median | 0.144 | 0.098 | 0.340 | 2.23 | 1.53 | 0.077 | 0.265 | 5.70 | |
| China (n = 84) | DF | 89.3 | 100 | 97.6 | 100 | 100 | 94.0 | 100 | |
| Median | 0.156 | 0.520 | 1.11 | 9.73 | 1.60 | 0.082 | 1.54 | 18.2 | |
| Median | 0.131 | 0.498 | 1.19 | 9.80 | 1.39 | 0.090 | 1.86 | 19.6 | |
| India (n = 41) | DF | 85.4 | 100 | 90.2 | 100 | 97.6 | 82.9 | 87.8 | |
| Median | 0.097 | 0.428 | 0.253 | 1.42 | 0.670 | 0.044 | 0.443 | 4.74 | |
| Median | 0.232 | 0.912 | 0.577 | 3.08 | 1.11 | 0.083 | 0.870 | 9.08 | |
| Saudi Arabia (n = 61) | DF | 85.2 | 95.1 | 98.4 | 91.8 | 100 | 96.7 | 96.7 | |
| Median | 0.138 | 0.659 | 0.363 | 2.75 | 1.74 | 0.153 | 0.591 | 8.78 | |
| Median | 0.113 | 0.458 | 0.324 | 1.67 | 1.81 | 0.137 | 0.504 | 7.01 | |
| Japan (n = 35) | DF | 88.6 | 100 | 60.0 | 100 | 100 | 82.9 | 100 | |
| Median | 0.080 | 0.279 | 0.090 | 1.87 | 0.740 | 0.052 | 0.112 | 3.59 | |
| Median | 0.059 | 0.210 | 0.085 | 1.45 | 0.477 | 0.053 | 0.086 | 2.91 | |
| Korea (n = 100) | DF | 99.0 | 99.0 | 96.0 | 100 | 100 | 96.0 | 98.0 | |
| Median | 0.152 | 0.178 | 0.253 | 8.27 | 2.44 | 0.243 | 0.454 | 14.3 | |
| Median | 0.108 | 0.152 | 0.195 | 6.46 | 1.57 | 0.189 | 0.391 | 10.1 | |
| Vietnam (n = 17) | DF | 100 | 100 | 100 | 100 | 100 | 76.5 | 94.1 | |
| Median | 0.202 | 1.22 | 0.803 | 2.46 | 0.270 | 0.035 | 0.326 | 6.19 | |
| Median | 0.110 | 0.667 | 0.538 | 1.67 | 0.195 | 0.024 | 0.266 | 4.41 | |
| Kuwait (n = 66) | DF | 74.2 | 89.4 | 81.8 | 97.0 | 100 | 90.9 | 100 | |
| Median | 0.080 | 0.258 | 0.276 | 2.92 | 1.65 | 0.118 | 0.879 | 7.94 | |
| Median | 0.064 | 0.209 | 0.181 | 3.04 | 1.34 | 0.089 | 0.645 | 6.60 | |
| Gender | |||||||||
| Female (n = 255) | DF | 89.8 | 98.0 | 94.1 | 98.4 | 100 | 92.2 | 98.4 | |
| Median | 0.133 | 0.234 | 0.355 | 3.58 | 1.77 | 0.107 | 0.470 | 9.00 | |
| Median | 0.125 | 0.250 | 0.324 | 3.22 | 1.59 | 0.129 | 0.504 | 8.63 | |
| Male (n = 254) | DF | 90.6 | 96.9 | 89.4 | 98.4 | 100 | 93.3 | 98.4 | |
| Median | 0.118 | 0.263 | 0.331 | 3.21 | 1.49 | 0.120 | 0.397 | 8.68 | |
| Median | 0.092 | 0.192 | 0.238 | 2.55 | 1.05 | 0.078 | 0.324 | 6.25 | |
| Age | |||||||||
| ≤20 (n = 66) | DF | 77.3 | 93.9 | 87.9 | 97.0 | 100 | 95.5 | 98.5 | |
| Median | 0.081 | 0.211 | 0.402 | 2.85 | 1.47 | 0.111 | 0.693 | 7.38 | |
| Median | 0.076 | 0.187 | 0.322 | 3.11 | 1.97 | 0.122 | 0.621 | 7.26 | |
| 21–49 (n = 244) | DF | 91.0 | 98.4 | 89.8 | 98.8 | 100 | 91.4 | 98.0 | |
| Median | 0.133 | 0.274 | 0.379 | 3.43 | 1.54 | 0.112 | 0.392 | 8.11 | |
| Median | 0.107 | 0.216 | 0.291 | 2.60 | 1.11 | 0.082 | 0.373 | 6.00 | |
| ≥50 (n = 198) | DF | 93.4 | 97.5 | 95.5 | 98.5 | 100 | 93.4 | 99.0 | |
| Median | 0.135 | 0.240 | 0.316 | 3.84 | 1.89 | 0.118 | 0.438 | 10.0 | |
| Median | 0.111 | 0.242 | 0.268 | 3.39 | 1.39 | 0.125 | 0.390 | 8.41 |
DF, detection frequency (%).
p < 0.01.
To examine location-specific differences in urinary concentrations of neonics and DAPs within a country, samples collected from Shanghai, Guangzhou and Qiqihar in China, were examined. Significant differences were found in N-DMA (Kruskal-Wallis H test, p < 0.01; Shanghai > Guangzhou > Qiqihar) and DMDTP (Kruskal-Wallis H test, p < 0.01; Qiqihar > Guangzhou > Shanghai) concentrations among the three cities in China. The creatinine adjusted concentrations did not alter our conclusions for N-DMA and DMDTP (Kruskal-Wallis H test, p < 0.01). Additionally, DMP (one-way ANOVA, p < 0.05; Guangzhou > Qiqihar > Shanghai) and DMTP (Kruskal-Wallis H test, p < 0.05; Qiqihar > Guangzhou > Shanghai) also displayed significant differences among the three cities. Location-specific variations in urinary concentrations of 3,5,6-trichloro-2-pyridinol, trans/cis-3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid, 2,4-dichlorophenoxyacetic acid and DAPs have been reported in China and Japan previously (Li and Kannan, 2018; Ueyama et al., 2015). Our results reiterate region-specific differences in pesticide exposures.
No significant differences were found for individual or sum concentrations of urinary neonics and DAPs between females and males (Mann-Whitney U test, p > 0.05) (Table 2). However, females had significantly higher creatinine adjusted urinary concentrations than males for both individual and sum concentrations (Mann-Whitney U test, p < 0.01) except for N-DMA (Mann-Whitney U test, p > 0.05). A similar gender-related difference (females > males) in urinary DAPs (Berman et al., 2013) and pyrethroid pesticide metabolites (Barr et al., 2010; Li and Kannan, 2018) was found for the general populations previously. Higher exposure levels of pesticides in females may be related to dietary preference for vegetables/fruits, and also an artifact of lower creatinine content in urine of females than males (Barr et al., 2005; Berman et al., 2013; Sokoloff et al., 2016; Tao et al., 2019). Gender-related difference was not found in urinary concentrations of neonics (i.e., ACE, IMI, THI, THX, CLO, DIN and NIT) among Japanese children (Osaka et al., 2016).
We categorized the samples into three age groups, namely, ≤20, 21–49, and ≤50 years, for the examination of age-related differences in urinary concentrations of neonics and DAPs. For both individual and sum concentrations of seven pesticides, no significant difference was found among the three age groups (Kruskal-Wallis H test, p > 0.05) (Table 2). After creatinine adjustment, we found significant differences in DMTP, DMDTP and DETP concentrations (Kruskal-Wallis H test, p < 0.05). The highest concentrations of the three compounds were all detected in the age group of ≤20 years (median: 0.122–1.97 μg/g creatinine), followed by ≥50 years (0.125–1.39) and 21–49 years (0.082–1.11). It was reported in an earlier study that children and elderly were at a greater risk of exposure to OP pesticides and the pyrethroid metabolite 3-phenoxybenzoic acid (Barr et al., 2010; 2011). Age related variations in urinary pesticide concentrations may be attributed to the differences in dietary patterns and metabolism (Barr et al., 2010; Li and Kannan, 2018).
3.4. Human exposure to pesticides
Urinary biomonitoring data can be used in the assessment of daily exposure doses of environmental chemicals. We estimated daily exposure doses of IMI, N-DMA, 6-CN, DMP, DMTP, DMDTP and DETP, which were detected in >80% of the samples analyzed, as shown in Eq. (1) for the nine countries (Guo et al., 2011; Li and Kannan, 2018):
| (1) |
where DI is the daily intake of pesticide (μg/day), C is the median urinary pesticide concentration (ng/mL), V is the daily excretion volume of urine (L/day; 24 h average urine excretion volume at 1.7 L/day for adults) (Guo et al., 2011; Li and Kannan, 2018), M1 and M2 are the respective molecular weights of parent pesticide and its metabolite (g/mol), and f is the ratio of compound/metabolite excreted in urine relative to the total exposure dose of the parent compound. The f values were obtained from published studies: 0.127 for IMI, 0.307 for N-DMA (Harada et al., 2016), 0.8 for 6-CN (Uroz et al., 2001), 0.1 for DMP, DMTP and DMDTP (Chen et al., 2013), and 0.93 for DETP (Griffin et al., 1999). For this assessment, we presumed that 6-CN was the metabolite of IMI; DMP, DMTP and DMDTP were only from malathion; and DETP only from chlorpyrifos. Malathion is a registered dimethyl OP pesticide used in agriculture and public health programs (e.g., mosquito control) whereas chlorpyrifos is one of the most commonly used diethyl OP pesticide; residues of both of these pesticides were frequently found in fruits and vegetables (Hernández et al., 2019; Sokoloff et al., 2016).
The estimated daily exposure doses of neonics and OPs by the populations in the nine countries are shown in Table 3. The average body weight of 60 kg was used for adults, in the estimation of chronic reference doses (cRfDs) for daily intakes (μg/day), as suggested by the U.S. EPA (57, 71, 70 and 0.3 μg/kg body weight/day for IMI, ACE, malathion and chlorpyrifos, respectively) (EPA, 1994; 2000; 2006; 2009). The estimated median daily intakes to neonics, IMI and ACE among the nine countries were thousand-fold below the cRfD values of the U.S. EPA. The highest daily intake of neonics was found for populations in Vietnam (12.7 μg/day). None of samples collected from the nine countries were above the cRfDs values of IMI and ACE. The estimated exposure doses of OPs, malathion and chlorpyrifos were approximately 10-fold below the cRfD values of the U.S. EPA. The median daily exposure doses estimated for OP pesticides for populations in China were the highest (515 μg/day) among the nine countries studied, and 13 out of 84 samples from China exceeded the cRfD value for chlorpyrifos of 18 μg/day.
Table 3.
Median daily intake (DI; μg/day) of pesticides estimated from urinary neonicotinoid insecticides and dialkylphosphate metabolites for the nine countries studied.
| Imidacloprid | Acetamiprid | ∑ neonics a | Malathion | Chlorpyrifos | ∑ OPs b | Total c | |
|---|---|---|---|---|---|---|---|
| cRfD | 57 d | 71 | 70 | 0.3 | |||
| 3420 e | 4260 | 4200 | 18 | ||||
| Country | |||||||
| USA | 2.2 | 1.2 | 3.4 | 125 (1) f | 1.2 | 127 | 130 |
| Greece | 3.4 | 0.7 | 4.1 | 208 (1) | 1.0 (1) | 209 | 213 |
| China | 5.9 | 3.1 | 9.0 | 500 (2) | 5.8 (13) | 506 | 515 |
| India | 2.2 | 2.5 | 4.7 | 91.4 | 1.7 (1) | 93.1 | 97.8 |
| Saudi Arabia | 3.1 | 3.9 | 7.0 | 197 | 2.2 (5) | 199 | 206 |
| Japan | 1.4 | 1.6 | 3.0 | 115 | 0.4 | 115 | 118 |
| Korea | 2.9 | 1.1 | 4.0 | 474 (4) | 1.7 (6) | 476 | 480 |
| Vietnam | 5.5 | 7.2 | 12.7 | 122 | 1.2 (1) | 123 | 136 |
| Kuwait | 2.0 | 1.5 | 3.5 | 200 (1) | 3.3 (3) | 203 | 207 |
| Gender | |||||||
| Female | 3.4 | 1.4 | 4.8 | 262 (4) | 1.8 (13) | 263 | 268 |
| Male | 3.5 | 1.6 | 5.0 | 246 (4) | 1.5 (12) | 247 | 253 |
| Age | |||||||
| ≤20 | 3.0 | 1.2 | 4.3 | 212 (1) | 2.6 (2) | 214 | 219 |
| 21–49 | 3.6 | 1.6 | 5.2 | 250 (3) | 1.5 (13) | 252 | 257 |
| ≥50 | 3.4 | 1.4 | 4.8 | 280 (4) | 1.7 (10) | 282 | 287 |
| All g | 2.9 | 1.5 | 4.5 | 216 (9) | 1.7 (30) | 218 | 223 |
∑ neonics refers to sum DIs of imidacloprid and acetamiprid.
∑ OPs refers to sum DIs of malathion and chlorpyrifos.
Total refers to sum DIs of imidacloprid, acetamiprid, malathion and chlorpyrifos.
cRfD, chronic reference doses of U.S. EPA (μg/kg body weight/day).
Estimated chronic reference doses from U.S. EPA (μg/day, the body weight assumed as 60 kg for adults).
Number of samples exceeded the estimated reference doses.
All refers to median DIs of pesticides estimated from urianry concentrations for the entire dataset from the nine countries.
4. Conclusions
This is the first study to describe exposure to 14 neonics and six DAPs in general populations from nine countries. An ultra-sensitive method was developed and applied in simultaneous analysis of 20 pesticides in urine. We found high exposure to OPs in China and Korea, and neonics in Vietnam. DMP and DMTP were the dominant urinary pesticide metabolites found in populations across the nine countries. Although this study is the first to report baseline levels of current use pesticides in populations in nine countries, our study has several limitations. We employed a convenience sampling approach by recruiting individuals within a small community with a small sample size. Considering location specific differences, large number of samples covering several locations in a country is needed for elucidating country-specific exposure doses. In addition, our exposure estimates were based on a single spot urine sample, which may not represent exposures over time (Li et al., 2020a). Pesticide exposures are often sporadic (Li et al., 2020a) and therefore multiple samples need to be collected from individuals to draw conclusions on individual’s exposure levels. Nevertheless, the data collected in this study can provide baseline information on exposures to neonics and OPs, which will lay the foundation for designing future studies to understand potential risks from exposure to pesticides.
Supplementary Material
Highlights.
Neonicotinoids and dialkylphosphates were measured in urine from nine countries.
The highest sum concentration of 14 neonicotinoids was found in Vietnam (12.2 ng/mL).
The highest sum concentration of six dialkylphosphates was found in China (18.4 ng/mL).
Dimethyl phosphates accounted for 51–89% of the total concentrations.
Daily exposure dose to organophosphates was highest in China (515 μg/day).
Acknowledgments
We thank all volunteers for kindly providing urine samples for this study. The method development portion of the research reported in this manuscript was supported in part by the National Institute of Environmental Health Sciences (NIEHS) of the NIH under Award Number U2CES026542-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found in a separate file.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD Jr., Magsumbol MS, Williams BL, Needham LL, 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect. 118, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL, 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 113, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wong L-Y, Bravo R, Weerasekera G, Odetokun M, Restrepo P, Kim D-G, Fernandez C, Whitehead RD Jr., Perez J, Gallegos M, Williams BL, Needham LL, 2011. Urinary concentrations of dialkylphosphate metabolites of organophosphorus pesticides: National Health and Nutrition Examination Survey 1999–2004. Int. J. Environ. Res. Public Health 8, 3063–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Denholm I, Williamson MS, Nauen R, 2015. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 121, 78–87. [DOI] [PubMed] [Google Scholar]
- Bennett B, Workman T, Smith MN, Griffith WC, Thompson B, Faustman EM, 2019. Longitudinal, seasonal, and occupational trends of multiple pesticides in house dust. Environ. Health Perspect. 127, 017003 10.1289/EHP3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman T, Goldsmith R, Göen T, Spungen J, Novack L, Levine H, Amitai Y, Shohat T, Grotto I, 2013. Urinary concentrations of organophosphate pesticide metabolites in aduts in Israel: demographic and dietary predictors. Environ. Int. 60, 183–189. [DOI] [PubMed] [Google Scholar]
- Casida JE, Durkin KA, 2013. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 58, 99–117. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhang Y, Lv B, Liu Z, Han J, Li J, Zhao Y, Wu Y, 2020. Dietary exposure to neonicotinoid insecticides and health risks in the Chinese general population through two consecutive total diet studies. Environ. Int. 135, 105399. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhao T, Pan C, Ross J, Ginevan M, Vega H, Krieger R, 2013. Absorption and excretion of organophosphorous insecticide biomarkers of malathion in the rat: implications for overestimation bias and exposure misclassification from environmental biomonitoring. Regul. Toxicol. Pharmacol. 65, 287–293. [DOI] [PubMed] [Google Scholar]
- Cimino AM, Boyles AL, Thayer KA, Perry MJ, 2017. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ. Health Perspect. 125, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, 1994. United States Environmental Protection Agency (EPA). Dietary exposure analysis for imidacloprid through the use on dried hops. https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-129099_17-Aug-94.pdf, Accessed date: 15 June 2020.
- EPA, 2000. United States Environmental Protection Agency (EPA). Human health risk assessment chlorpyrifos. https://archive.epa.gov/scipoly/sap/meetings/web/pdf/hed_ra.pdf, Accessed date: 16 June 2020.
- EPA, 2006. United States Environmental Protection Agency (EPA). Malathion: revised human health risk assessment for the reregistration eligibility decision document (RED). http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.174.8076&rep=rep1&type=pdf, Accessed date: 15 June 2020.
- EPA, 2009. United States Environmental Protection Agency (EPA). Petition for acetamiprid. https://nepis.epa.gov/Exe/ZyPDF.cgi/P1006ZZC.PDF?Dockey=P1006ZZC.PDF, Accessed date: 15 June 2020.
- EPA, 2017. United States Environmental Protection Agency (EPA). Pesticides industry sales and usage 2008–2012 market estimates. https://19january2017snapshot.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf, Accessed date: 16 June 2020.
- Furlong MA, Engel SM, Barr DB, Wolff MS, 2014. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ. Int. 70, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P, Mason H, Heywood K, Cocker J, 1999. Oral and dermal absorption of chlorpyrifos: a human volunteer study. Occup. Environ. Med. 56, 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Alomirah H, Cho H-S, Minh TB, Mohd MA, Nakata H, Kannan K, 2011. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 45, 3138–3144. [DOI] [PubMed] [Google Scholar]
- Hallmann CA, Foppen RPB, van Turnhout CAM, de Kroon H, Jongejans E, 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343. [DOI] [PubMed] [Google Scholar]
- Harada KH, Tanaka K, Sakamoto H, Imanaka M, Niisoe T, Hitomi T, Kobayashi H, Okuda H, Inoue S, Kusakawa K, Oshima M, Watanabe K, Yasojima M, Takasuga T, Koizumi A, 2016. Biological monitoring of human exposure to neonicotinoids using urine samples, and neonicotinoid excretion kinetics. PLoS One 11, e0146335. doi:10.1371/journal.pone.0146335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández AF, Lozano-Paniagua D, González-Alzaga B, Kavvalakis MP, Tzatzarakis MN, López-Flores I, Aguilar-Garduño C, Caparros-Gonzalez RA, Tsatsakis AM, Lacasaña M, 2019. Biomonitoring of common organophosphate metabolites in hair and urine of children from an agricultural community. Environ. Int. 131, 104997. [DOI] [PubMed] [Google Scholar]
- Hladik ML, Main AR, Goulson D, 2018. Environmental risks and challenges assocaited with neonicotinoid insecticides. Environ. Sci. Technol. 52, 3329–3335. [DOI] [PubMed] [Google Scholar]
- Ikenaka Y, Miyabara Y, Ichise T, Nakayama SMM, Nimako C, Ishizuka M, Tohyama C, 2019. Exposures of children to neonicotinoids in pine wilt disease control areas. Environ. Toxicol. Chem. 38, 71–79. [DOI] [PubMed] [Google Scholar]
- Jeschke P, Nauen R, Schindler M, Elbert A, 2011. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908. [DOI] [PubMed] [Google Scholar]
- Jusko TA, van den Dries MA, Pronk A, Shaw PA, Guxens M, Spaan S, Jaddoe VW, Tiemeier H, Longnecker MP, 2019. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring nonverbal IQ at age 6 years. Environ. Health Perspect. 127, 017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarich KL, Pflug NC, DeWald EM, Hladik ML, Kolpin DW, Cwiertny DM, LeFevre GH, 2017. Occurrence of neonicotinoid insecticides in finished drinking water and fate during drinking water treatment. Environ. Sci. Technol. Lett 4, 168–173. [Google Scholar]
- Li AJ, Banjabi AA, Takazawa M, Kumosani TA, Yousef JM, Kannan K, 2020b. Serum concentrations of pesticides including organophosphates, pyrethroids and neonicotinoids in a population with osteoarthritis in Saudi Arabia. Sci. Total Environ. 737, 139706. [DOI] [PubMed] [Google Scholar]
- Li AJ, Kannan K, 2018. Urinary concentrations and profiles of organophosphate and pyrethroid pesticide metabolites and phenoxyacid herbicides in populations in eight countries. Environ. Int. 121, 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Martinez-Moral MP, Kannan K, 2020a. Variability in urinary neonicotinoid concentrations in single-spot and first-morning void and its association with oxidative stress markers, Environ. Int. 135, 105415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R, 2006. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ. Health Perspect. 114, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EAD, Mulhauser B, Mulot M, Mutabazi A, Glauser G, Aebi A, 2017. A worldwide survey of neonicotinoids in honey. Science 358, 109–111. [DOI] [PubMed] [Google Scholar]
- Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K, 2015. Neonicotinoid contamination of global surface waters and assocaited risk to aquatic invertebrates: a review. Environ. Int. 74, 291–303. [DOI] [PubMed] [Google Scholar]
- Osaka A, Ueyama J, Kondo T, Nomura H, Sugiura Y, Saito I, Nakane K, Takaishi A, Ogi H, Wakusawa S, Ito Y, Kamijima M, 2016. Exposure characterization of three major insecticide lines in urine of young children in Japan - neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 147, 89–96. [DOI] [PubMed] [Google Scholar]
- Oya N, Ito Y, Ebara T, Kato S, Hioki K, Aoi A, Ueyama J, Oguri T, Shoji N, Sugiura-Ogasawara M, Saitoh S, Kamijima M, 2020. Exposure levels of organophosphate pesticides in Japanese diapered children: contributions of exposure-related behaviors and mothers' considerations of food selection and preparation. Environ. Int. 134, 105294. [DOI] [PubMed] [Google Scholar]
- Rundlöf M, Andersson GKS, Bommarco R, Fries I, Hederström V, Herbertsson L, Jonsson O, Klatt BK, Pedersen TR, Yourstone J, Smith HG, 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. [DOI] [PubMed] [Google Scholar]
- Sadaria AM, Supowit SD, Halden RU, 2016. Mass balance assessment for six neonicotinoid insecticides during conventional wastewater and wetland treatment: nationwide reconnaissance in United States wastewater. Environ. Sci. Technol. 50, 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff K, Fraser W, Arbuckle TE, Fisher M, Gaudreau E, LeBlanc A, Morisset A-S, Bouchard MF, 2016. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada - results from the MIREC study. Environ. Int. 94, 133–140. [DOI] [PubMed] [Google Scholar]
- Song S, Zhang T, Huang Y, Zhang B, Guo Y, He Y, Huang X, Bai X, Kannan K, 2020. Urinary metabolites of neonicotinoid insecticides: levels and recommendations for future biomonitoring studies in China. Environ. Sci. Technol. 54, 8210–8220. [DOI] [PubMed] [Google Scholar]
- Stewart SD, Lorenz GM, Catchot AL, Core J, Cook D, Skinner J, Mueller TC, Johnson DR, Zawislak J, Barber J, 2014. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci. Technol. 48, 9762–9769. [DOI] [PubMed] [Google Scholar]
- Tao Y, Dong F, Xu J, Phung D, Liu Q, Li R, Liu X, Wu X, He M, Zheng Y, 2019. Characteristics of neonicotinoid imidacloprid in urine following exposure of humans to orchards in China. Environ. Int. 132, 105079. [DOI] [PubMed] [Google Scholar]
- Tapparo A, Marton D, Giorio C, Zanella A, Soldà L, Marzaro M, Vivan L, Girolami V, 2012. Assessment of the environmental exposure of honeybees to particulate matter containing neonicotinoid insecticides coming from corn coated seeds. Environ. Sci. Technol. 46, 2592–2599. [DOI] [PubMed] [Google Scholar]
- Ueyama J, Harada KH, Koizumi A, Sugiura Y, Kondo T, Saito I, Kamijima M, 2015. Temporal levels of urinary neonicotinoid and dialkylphosphate concentrations in Japanese women between 1994 and 2011. Environ. Sci. Technol. 49, 14522–14528. [DOI] [PubMed] [Google Scholar]
- Uroz FJ, Arrebola FJ, Egea-González FJ, Martínez-Vidal JL, 2001. Monitoring of 6-chloronicotinic acid in human urine by gas chromatography-tandem mass spectrometry as indicator of exposure to the pesticide imidacloprid. Analyst, 126, 1355–1358. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu T, Liu F, Zhang J, Wu Y, Sun H, 2015. Occurrence and profile characteristics of the pesticide imidacloprid, preservative parabens, and their metabolites in human urine from rural and urban China. Environ. Sci. Technol. 49, 14633–14640. [DOI] [PubMed] [Google Scholar]
- Wang X, Anadón A, Wu Q, Qiao F, Ares I, Martínez-Larrañaga M-R, Yuan Z, Martínez M-A, 2018. Mechanism of neonicotinoid toxicity: impact on oxidative stress and metabolism. Annu. Rev. Pharmacol. Toxicol. 58, 471–507. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Ji L, Hu Y, Zhang J, Wang C, Ding G, Chen L, Kamijima M, Ueyama J, Gao Y, Tian Y, 2017. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ. Int 108, 119–126. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Z, Chang CH, Lou JL, Zhao MR, Lu C, 2018. Potential human exposures to neonicotinoid insecticides: a review. Environ. Pollut. 236, 71–81. [DOI] [PubMed] [Google Scholar]
- Zhang T, Song S, Bai X, He Y, Zhang B, Gui M, Kannan K, Lu S, Huang Y, Sun H, 2019. A nationwide survey of urinary concentrations of neonicotinoid insecticides in China. Environ. Int. 132, 105114. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, 2018. Global pesticide use: profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8, 1–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.