Abstract
Current FDA regulations have resulted in a ban of flavored e-cigarette pods, with only menthol and tobacco flavored pods being exempted. Previous work using menthol and tobacco-flavored e-cigarettes have been shown to induce mitochondrial reactive oxygen species. We hypothesized that exposure to pod-based JUUL Menthol and Virginia Tobacco aerosols will alter mitochondrial respiration and electron transport chain protein levels. We determined mitochondrial respiration by using a Seahorse technique and electron transport chain complexes by total OXPHOS antibodies after exposing lung epithelial cells, Beas-2b, to pod-based Menthol and Virginia Tobacco favored aerosols. Menthol pod exposure resulted in an immediate increase in proton leak and decrease coupling efficiency, as well as a decrease in complex I, II, and IV. Menthol pod exposure twenty-four hour post exposure resulted in a decrease in basal respiration, maximal respiration, and spare capacity, as well as a decrease in complex I. Tobacco pod exposure resulted in no significant alterations to mitochondrial respiration, but immediately post final exposure resulted in a significant increase in complex I, IV, and V. Our results indicate that exposure to Menthol flavored e-cigarette pods causes mitochondrial respiration dysfunction in lung epithelial cells.
Keywords: Mitochondrial Bioenergetics, E-cigarettes, Pod-based, Menthol, Tobacco
Graphical Abstract
1. Introduction
Electronic cigarettes (e-cigarettes) are devices that generate aerosols from a liquid typically composed of propylene glycol, vegetable glycerin, nicotine, and flavoring chemicals (Bals et al., 2019). As of 2018, there were more than 250 e-cigarette brands and over 8,000 different flavorings in the United States (Kaur et al., 2018). These flavorings composed of flavoring chemicals are classified as ‘Generally Recognized As Safe’ for ingestion, but the effects of inhalation of these chemicals are still relatively unknown (Kaur et al., 2018).
Current e-cigarette use has remained stable from 2014 to 2018 at roughly 3%. Despite this, use in young adults (18–24 years old) has increased from 5.1% to 7.6%, along with a significant increase in use among never smokers (Dai and Leventhal, 2019). A recent epidemiological study also found that current e-cigarette use in high school students was 27% and 10% in middle school students (Cullen et al., 2019). Within these current users, 59 % of high school students and 54% of middle school students reported that JUUL was the usual e-cigarette device used (Cullen et al., 2019). In 2018, JUUL had a 70% market share of US convenient store vapor product sales (Ramamurthi et al., 2018). JUUL e-liquids are packaged in pods, composed of a mixture of propylene glycol, vegetable glycerin, nicotine, benzoic acid, and flavoring chemicals (Ramamurthi et al., 2018). These pods were initially sold in one of eight flavors with 5% nicotine (Omaiye et al., 2019).
In the recent Population Assessment of Tobacco and Health Study, the most common flavor used by either adults or youth participants in the past 30 days was fruit flavor (Schneller et al., 2019). Meanwhile, menthol/mint flavors had a 17% preference and tobacco flavors had a 24% preference in adult participants (Schneller et al., 2019). While menthol/mint flavors had a 10.8% preference and tobacco flavors have a 5% preference in youth participants (Schneller et al., 2019). Another epidemiology study observing the flavor preference in a cohort over time showed menthol/mint flavor preference remained stable with the preference of 11% at baseline to 9% at follow up (Du et al., 2020).
Studies have begun to observe the effects of both e-cigarettes and flavoring chemicals on mitochondrial function. Cinnamaldehyde, a flavoring chemical used in e-cigarette liquids, effects on mitochondrial respiration was measured using seahorse technique (Clapp et al., 2019). Beas-2b lung epithelial treated with cinnamaldehyde resulted in a dose-dependent decrease in mitochondrial respiration and glycolysis (Clapp et al., 2019). Other studies observed a dose-dependent increase in mitochondrial superoxide (mitosox) production in cells that were treated with tobacco and menthol e-liquid (Zahedi et al., 2019). Similarly, another study showed an increase in mitosox in 16-HBE epithelial cells due to aerosol exposure of e-cigarette pod-based devices (Muthumalage et al., 2019).
Due to recent FDA regulation, flavored e-cigarette pods except for menthol and tobacco flavors have been banned from sale in the United States. From recent reports, it has been shown that flavoring chemicals and exposure to e-liquids have resulted in mitochondrial release of reactive oxygen species (Lerner et al., 2016). Therefore, we hypothesize that exposure to pod-based Menthol and JUUL Virginia Tobacco flavored e-cigarette exposures will result in alteration of mitochondrial respiration and electron transport chain complex protein levels in lung epithelial cells.
2. Material and Methods
2.1. Scientific Rigor and Reproducibility
We used a rigorous and unbiased approach in experimental planning and analyzing the data to produce reproducible data along with a full and detailed report of methods and analyzed data. All biological and chemical resources used in this study were validated and authenticated.
2.2. Ethical approval: Institutional biosafety approvals
All experiments performed in this study were approved and in accordance with the University of Rochester Institutional Biosafety Committee.
2.3. Procurement of JUUL Pods
JUUL pod flavor, “Menthol” and “Virginia Tobacco” with 5% nicotine were purchased from online store as well as local retail store.
2.4. Determining liquid and vapor phase constituents
A previous study from this lab has been conducted on the chemical composition of JUUL pods e-liquids (Muthumalage et al., 2019). To quantify vapor phase constituents, JUUL Menthol and Virginia Tobacco pods were aerosolized and collected in 1L vacuum bottles (3 puffs per minute over 10 minutes). These samples were sent to ALS Environmental, CA, for analysis. Vapor phase constituents quantified by EPA method TO-15 and mass spectral library search for tentatively identified compounds. Determined chemical constituents were then categorized by functional group.
2.5. Cell Culture
Lung epithelial cells, Beas2b cells, (ATTC, Virginia) were cultured in complete media in DMEM: F12 complete media (Corning, ref #16–405-CL, Arizona) with 5% FBS, 1% pen/strep, and 15 mM HEPES. Prior to exposure, cells were cultured in a 5% CO2 incubator to 80% confluency in T-75 flasks
2.6. In Vitro Exposure
JUUL device was connected to one end of the Scireq inExpose e-cig exposure system pump (Scireq, Canada). The other end of the pump was then connected to the Enzyscreen chamber (Enzyscreen, Netherlands). Cell culture plates were then exposed to either air or Menthol or Virginia Tobacco pods with 5% nicotine for 22 minutes using a puffing profile with 3 puffs per minute for a total of 66 puffs with 55ml/min puff volume. Cells then kept in vapors for 8 minutes in order to be exposed for a total of 30 minutes before being returned to the incubator. Two more exposure sessions occurred afterward at 12-hour intervals.
2.7. Mitochondrial Bioenergetics using Seahorse XFp Analyzer
To determine mitochondrial respiration, approximately 20,000 cells per well were plated in seahorse mini-plate (Agilent Technologies, Cat #103022–100, California), with well A and well H left blank for background control, and cells were allowed to grow to roughly 90% confluency. Immediately prior to exposure, cells were serum-deprived to DMEM: F12 complete media with 0% FBS. At either immediate or 24-hours post final exposure, media in the plate was removed and 180μl seahorse media, composed of 10 mM glucose, 1mM pyruvate, and 2 mM glutamine in Agilent Seahorse XF DMEM Media, was added per well and incubated for 1 hour in a non-CO2 37°C incubator. The night prior to running the assay, the Seahorse XFp analyzer was turned on and the XF cartridge was hydrated by adding seahorse XF calibrant. On the day of the assay, three of the four ports were loaded with mitochondrial inhibitor drugs to obtain a concentration of 2μM oligomycin, 1.5μM FCCP, and 0.5 μM Rotenone/Antimycin A after injection. MitoStress test kit (Agilent Technologies, cat#103010–100, California) was run on Seahorse xFP analyzer. Afterward, cell counts were performed using acridine orange/propidium iodide stain to normalize data. Data were analyzed using Wave software.
2.8. Immunoblot Analysis of Electron Transport Chain (ETC) Protein Levels
To determine ETC protein levels, 300,000 cells per well were plated in a 6-well plate and allowed to grow to roughly 80% confluency. Immediately prior to first exposure, cells were serum-deprived to DMEM: F12 media with 0% FBS. After final exposure, cell pellets were collected at an immediate and 24 hour time point and stored at −80°C. Whole-cell lysate from air, Menthol, and Virginia Tobacco exposed cells protein levels were determined using a BCA protein assay. Equal concentrations of sample protein were added to a 12% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were probed with total OXPHOS human antibody cocktail (Abcam, ab110411, 1:1000, United Kingdom) and anti-GAPDH (Santa Cruz, Sc365062, 1:2000, Texas) overnight at 4˚C. Following probing, anti-mouse (1:5000) secondary antibody was incubated for 1 hour at room temperature. Membranes were then detected using enhanced chemiluminescence imaging reagent (ThermoFisher, ref #32106, Massachusetts) and detected by Bio-Rad ChemiDoc MP imaging system (Bio-Rad, Hercules, CA). Band intensities were analyzed using ImageJ software.
2.9. Statistical Analysis
Statistical analysis of the data was conducted by performing unpaired two-tailed t-test to analyze comparisons between the two groups using GraphPad 8.1.1. Data are represented as mean ± SD. Statistical significance was reported as *p<0.05, **p<0.01, and ***p<0.001.
3. Results
3.1. Menthol and Virginia Tobacco flavor pod-based vapor phase constituents
Quantitative chemical composition of JUUL Menthol and Virginia Tobacco flavor aerosols showed slight differences with unique chemical constituents to these Menthol and Virginia Tobacco pods. There were sixteen overlapping chemical constituents between both Menthol and Virginia Tobacco pods. The unique chemical constituents of Menthol pod were found to be 1,4-Dioxane, 1-Propanol, 2-Ethyl-1-hexanol, and 2-(2-Ethoxyethoxy) Ethanol (Table 1). The unique Virginia Tobacco pod chemical constituents were found to be Propene, 2-Propanol, 1-Hydroxy-2propanone, 2,2,4-Trimethyl-1,3-dioxolane, 2-Hydroxy-, Enthylpropanoic Acid, and 2,2-Dimethly-1,3-dioxolane-4-methanol (Table 2).
Table 1:
Chemical Composition of Menthol flavor pod-based Aerosol
| Chemical Compounds | Functional Group | Average Concentration (μg/m3) |
|---|---|---|
| Dichlorodifluoromethane | Alkyl Halide | 2.45 |
| Ethanol | Alcohol | 3300 |
| 1,4-Dioxane | Ether | 8.5 |
| Toluene | Benzene Ring | 23 |
| Ethylbenzene | Benzene Ring | 25.5 |
| Styrene | Alkene/Benzene Ring | 56.5 |
| Sulfur Dioxide | Sulfide | >34.5 |
| Acetaldehyde | Aldehyde | 18 |
| 2-Methylbutane | Alkane | 420 |
| n-Pentane | Alkane | 1250 |
| tert-Butanol | Alcohol | 7.8 |
| 1-Propanol | Alcohol | 6 |
| Cyclopentane | Alkane | 140 |
| Trimethylsilanol | Alcohol | 70.5 |
| Dimethyl ester carbonic acid | Ester | 16 |
| Propylene Glycol | Alcohol | 2400 |
| 2-Ethyl-1-hexanol | Alcohol | 3.45 |
| 2-(2-Ethoxyethoxy)Ethanol | Ether/Alcohol | 17 |
| Unknown Siloxane | Siloxane | 12.5 |
| Levomenthol | Alcohol | 3 60 |
Aerosol collection occurred from 30 puffs of Menthol flavor pods in a 1L vacuum bottle and analyzed by ALS environmental. Average concentration of chemical compounds was obtained from two separate aerosol collections.
Table 2:
Chemical Composition of Virginia Tobacco flavor pod-based Aerosol
| Chemical Compounds | Functional Group | Average Concentration (μg/m3) |
|---|---|---|
| Propene | Alkene | 3.35 |
| Dichlorodifluoromethane | Alkyl Halide | 2.25 |
| Ethanol | Alcohol | 9850 |
| 2-Propanol | Alcohol | 6.5 |
| Toluene | Benzene Ring | 23 |
| Ethylbenzene | Benzene Ring | 33.5 |
| Styrene | Alkene/Benzene Ring | 99 |
| Sulfur Dioxide | Sulfide | >38.5 |
| Acetaldehyde | Aldehyde | 23.5 |
| 2-Methylbutane | Alkane | 220 |
| n-Pentane | Alkane | 675 |
| tert-Butanol | Alcohol | 6 |
| Cyclopentane | Alkane | 81 |
| Trimethylsilanol | Alcohol | 16 |
| Dimethyl ester carbonic acid | Ester | 16 |
| 1-Hydroxy-2-propanone | Ketone/Alcohol | 7 |
| 2,2,4-Trimethyl-1,3-dioxolane | Ether | 10.35 |
| Propylene Glycol | Alcohol | 3350 |
| 2-Hydroxy-, Ethylpropanoic Acid | Ester/Alcohol | 30.5 |
| 2,2-Dimethyl-1,3-dioxolane-4-methanol | Alcohol/Ether | 10.3 |
| unknown Siloxane | Siloxane | 56 |
| Levomenthol | Alcohol | 285 |
Aerosol collection occurred from 30 puffs of Virginia Tobacco flavor pods in a 1L vacuum bottle and analyzed by ALS environmental. Average concentration of chemical compounds was obtained from two separate aerosol collections.
3.2. Menthol pod exposure alters mitochondrial bioenergetics
To determine JUUL Menthol pod exposure effects on mitochondrial oxidative phosphorylation, by performing cell mitochondria stress test using Seahorse XFp analyzer. At immediate and 24-hour time points, Menthol flavor pod exposure resulted in an increase in the extracellular acidification rate, potentially indicating a shift to glycolysis (Figure 1A & 1C). Immediately post-final exposure, non-mitochondrial oxygen consumption and proton leak was significantly increased and coupling efficiency was significantly decreased compared to air-exposed cells (Figure 1B). Twenty-four hours post-final exposure, non-mitochondrial oxygen consumption was significantly increased, and basal respiration, maximal respiration, and spare capacity was significantly decrease compared to air exposed cells (Figure 1D).
Figure 1: Menthol flavor pod exposure alter mitochondrial respiration in lung epithelial cells immediately and 24-hours post-exposure.
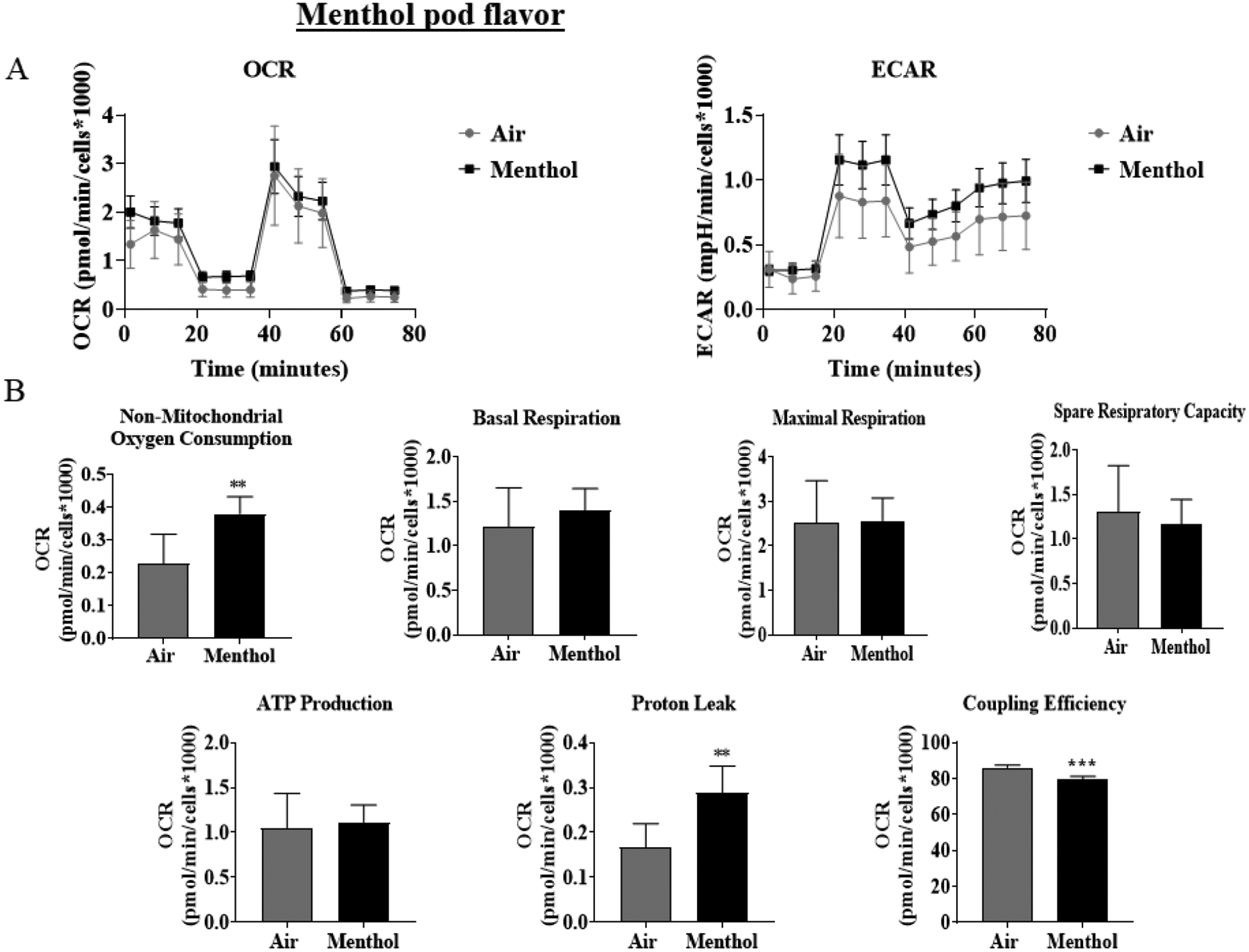
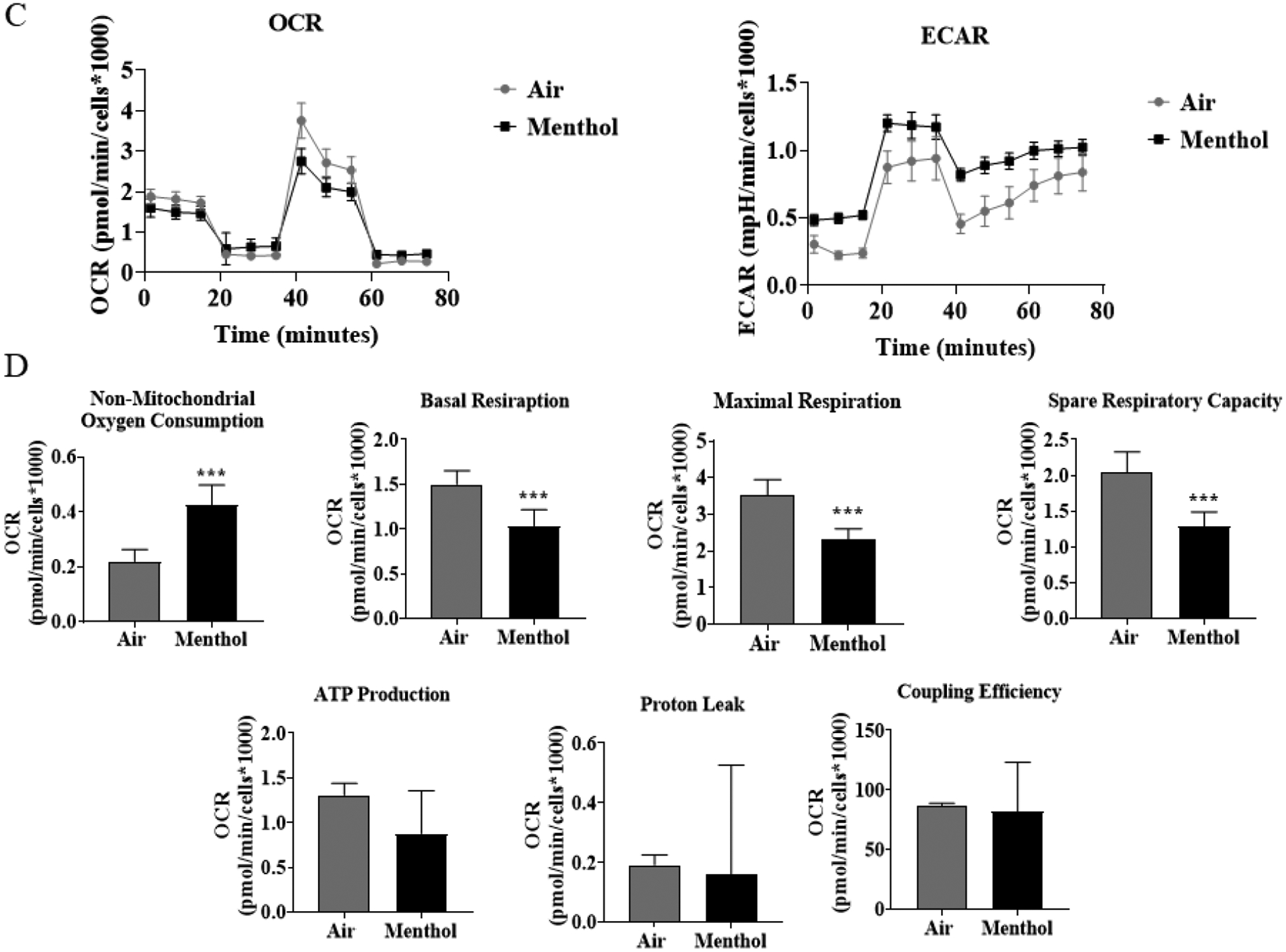
Beas2b cells with 20,000 cells per seahorse well were grown to 90% confluency. Immediately after serum deprivation, the wells were exposed to a three-session exposure to JUUL Menthol pods with 12-hour intervals between sessions. (A) Immediate post final exposure graphs of oxygen consumption rate and extracellular acidification rate. (B) Immediate post final exposure values for mitochondrial respiration. ** p < 0.01, or *** p < 0.001 vs air exposed, unpaired t-test N = 6 wells per group. (C) 24 hours post final exposure graphs of oxygen consumption rate and extracellular acidification rate. (D) 24 hours post final exposure values for mitochondrial respiration. *** p < 0.001 vs air exposed, unpaired t-test N = 6 wells per group.
3.3. Virginia Tobacco pod exposure does alter mitochondrial bioenergetics
To determine JUUL Virginia Tobacco flavor exposure effects on mitochondrial oxidative phosphorylation by performing cell mitochondria stress test using Seahorse XFp analyzer. At both the immediate and 24-hour time points, Virginia Tobacco pod resulted in a significant increase in non-mitochondrial oxygen consumption compared to air-exposed cells (Figure 2B & 2D). Immediately post-final exposure resulted in a significant decrease in coupling efficiency compared to air-exposed cells (Figure 2B). Twenty-four hours post-final exposure, did not result in any alteration in mitochondrial bioenergetics (Figure 2D).
Figure 2: Virginia Tobacco pod exposure do not alter mitochondrial respiration in lung epithelial cells immediately or 24-hours post-exposure.
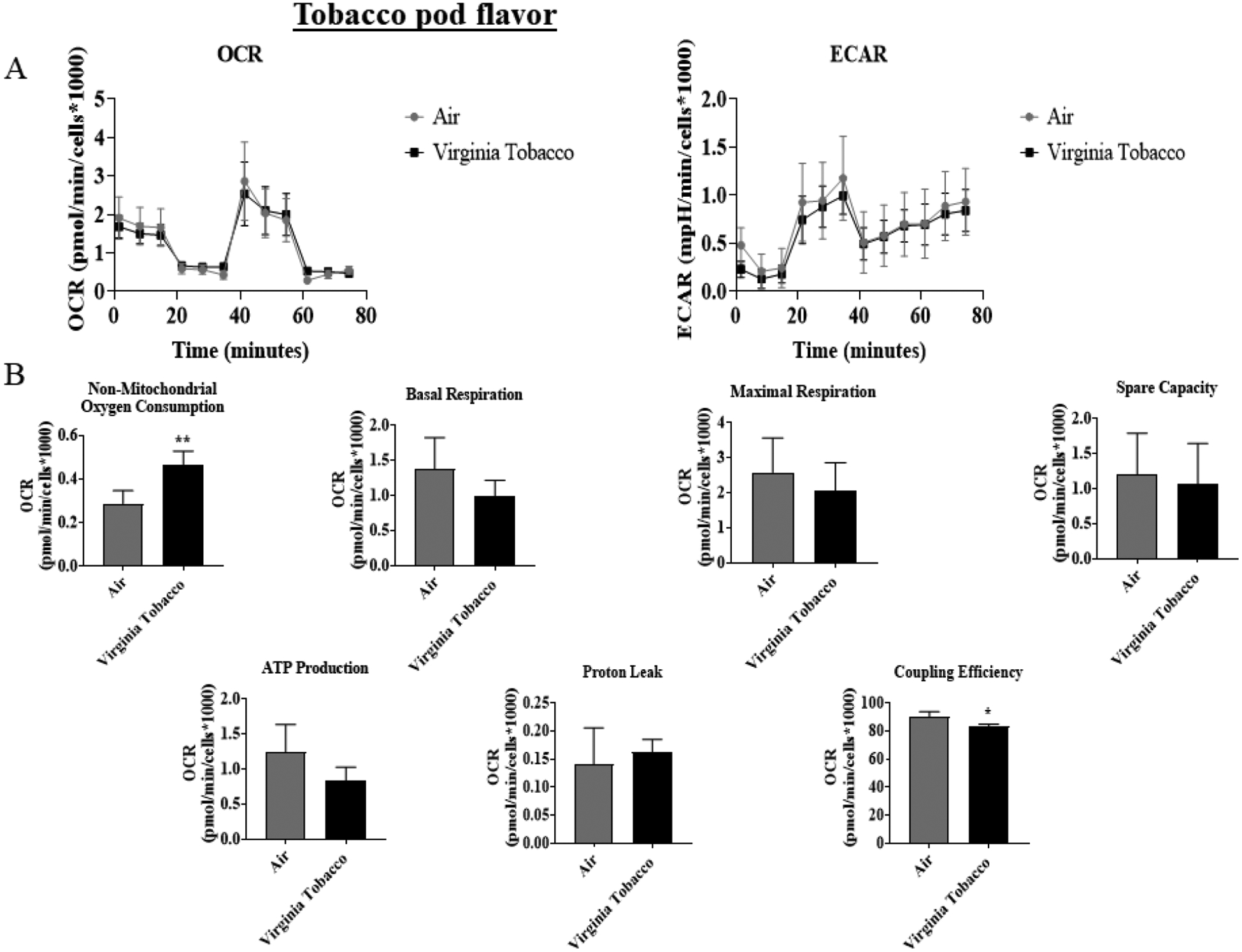
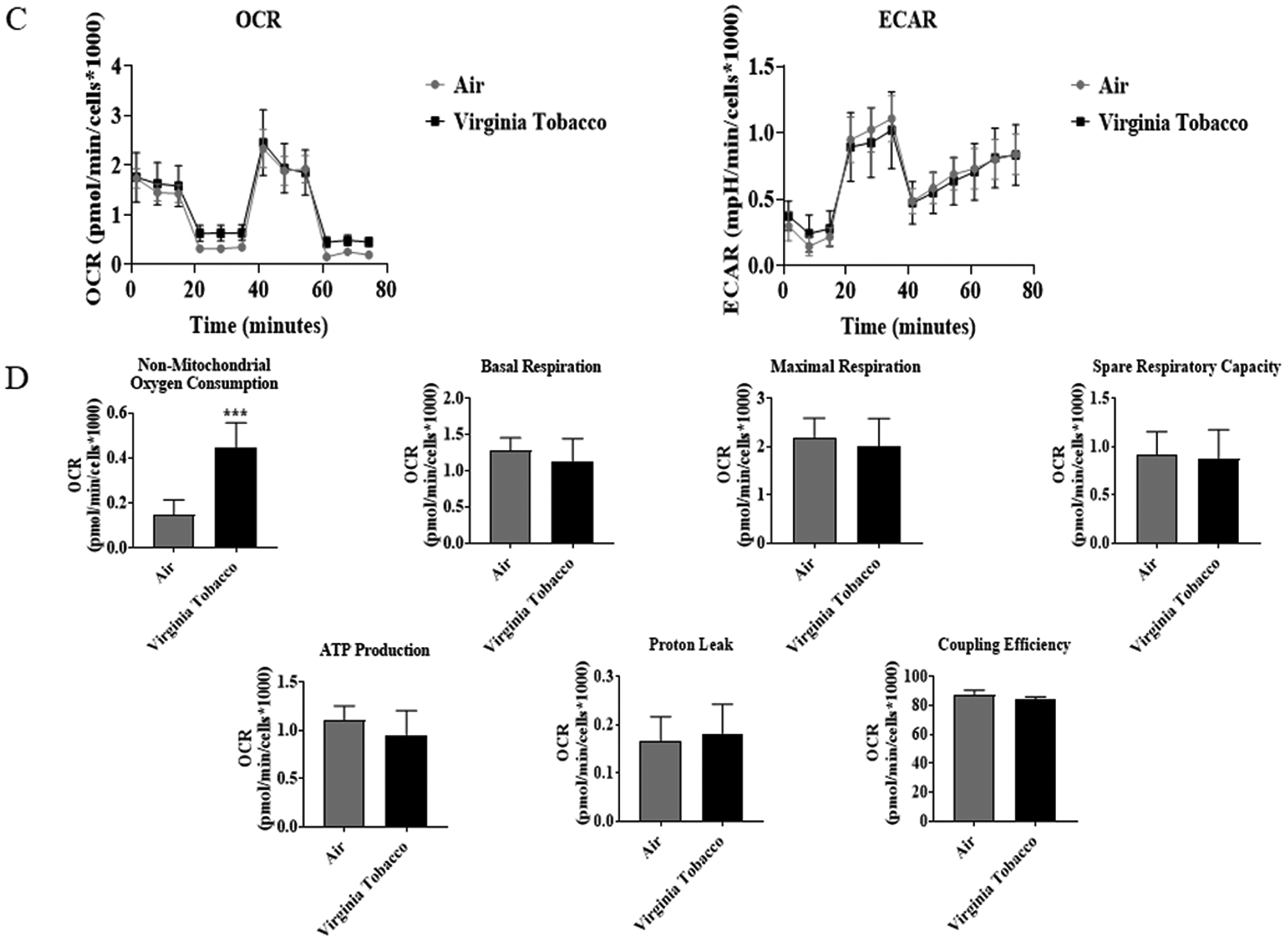
Beas2b cells with 20,000 cells per seahorse well were grown to 90% confluency. Immediately after serum deprivation, the wells were exposed to a three-session exposure to JUUL Virginia Tobacco pods with 12-hour intervals between sessions. (A) Immediate post final exposure graphs of oxygen consumption rate and extracellular acidification rate. (B) Immediate post final exposure values for mitochondrial respiration. * p < 0.05, or ** p < 0.01 vs air exposed, unpaired t-test N = 6 wells per group. (C) 24 hours post final exposure graphs of oxygen consumption rate and extracellular acidification rate. (D) 24 hours post final exposure values for mitochondrial respiration. *** p < 0.001 vs air exposed, unpaired t-test N = 4–6 wells per group.
3.4. Menthol exposure alters protein levels of the ETC
To determine JUUL Menthol flavor pod exposure effects on ETC protein level, western blot analysis was performed on whole cell lysate. Immediately post final exposure resulted in a significant decrease in complex I, complex II, and complex IV, and a non-significant decrease in complex V (Figure 3B). Twenty-four hours post final exposure resulted in a significant decrease in complex I, and a non-significant decrease in complex II, complex IV, and complex V (Figure 3C). Complex III was not able to be quantified due to running over of bands from other ETC subunits.
Figure 3: Menthol pod exposure alter ETC protein levels.
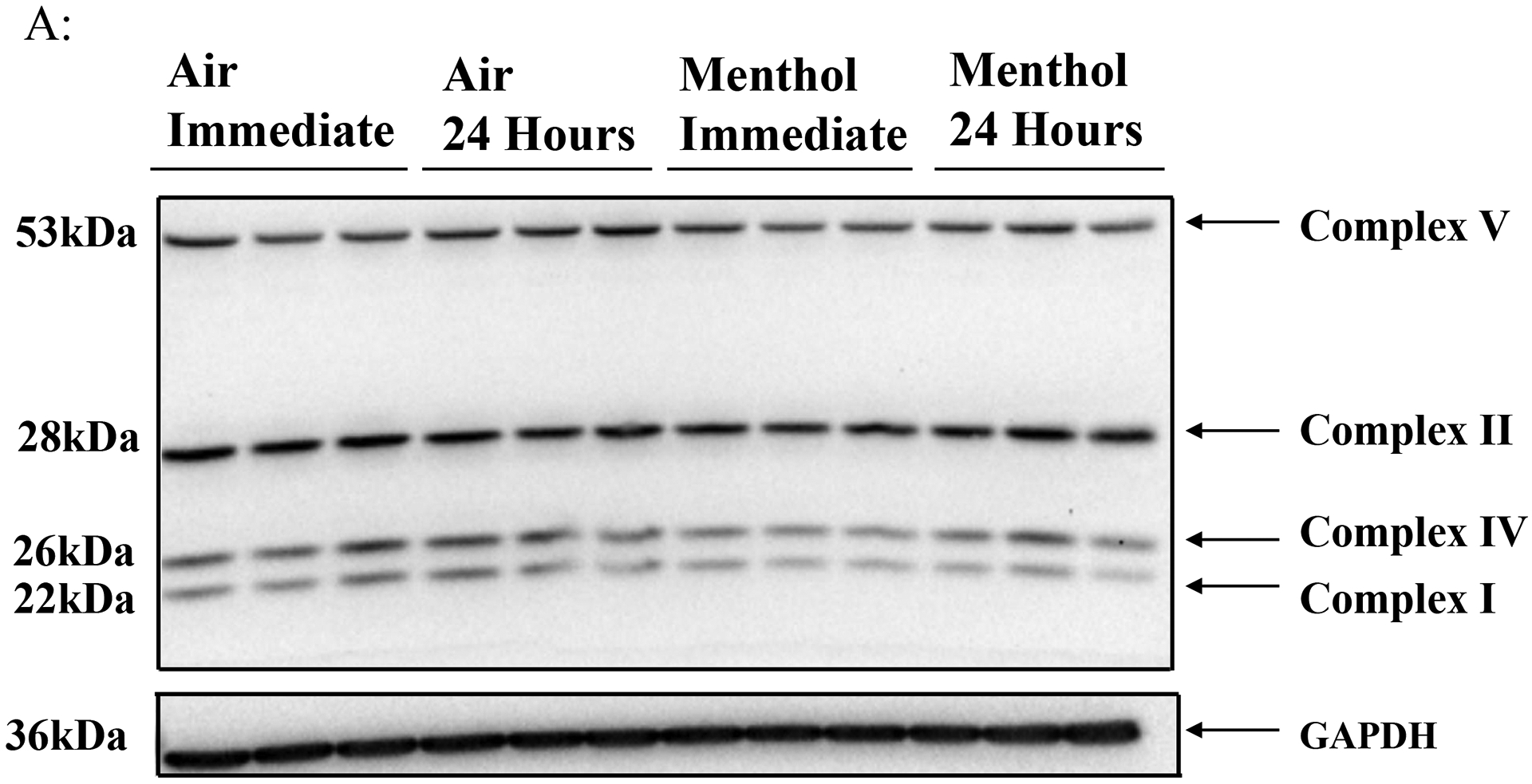
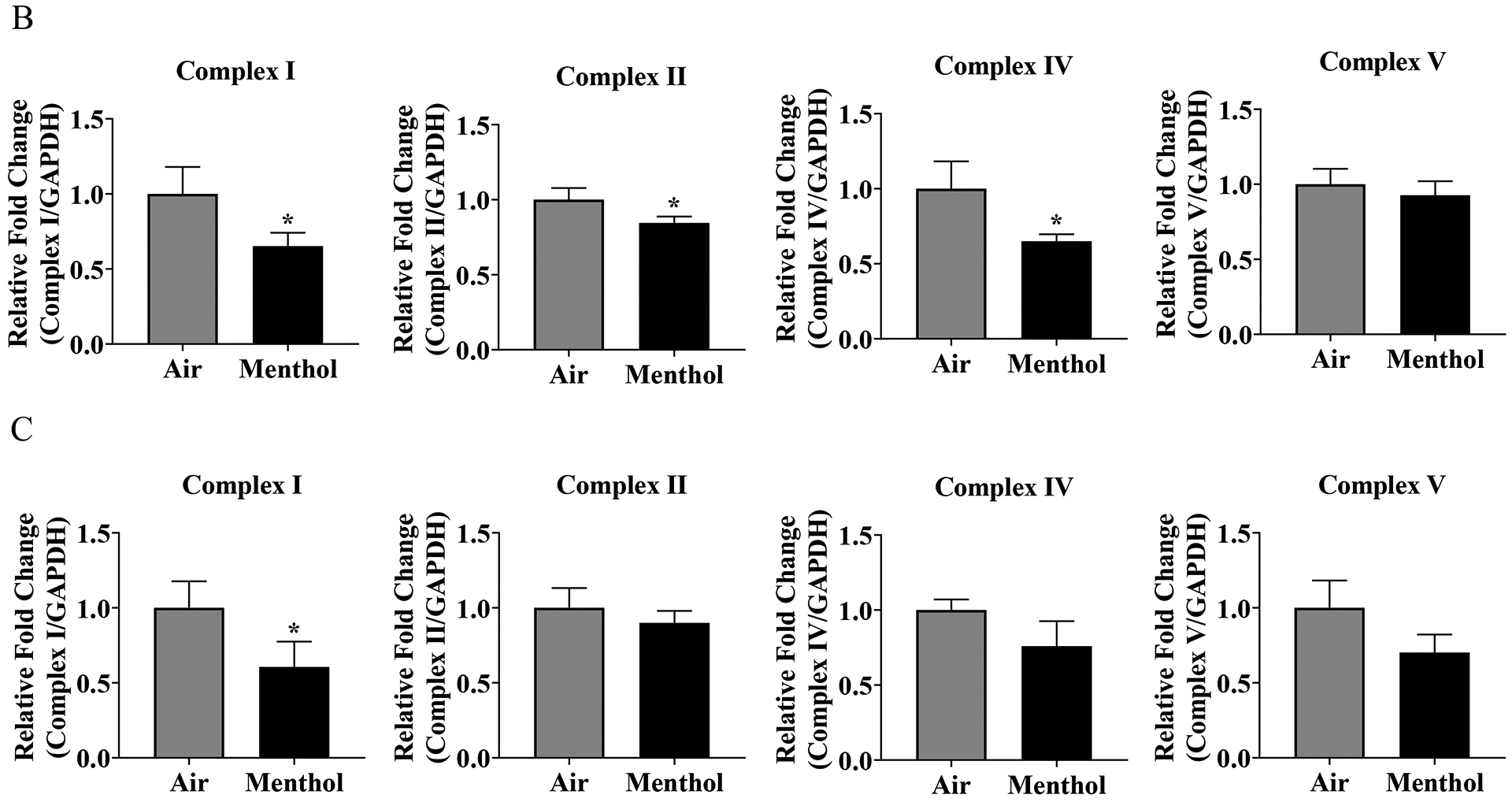
Beas2b cells with 300,000 cells per well were grown to 80% confluency. Immediately after serum deprivation the wells were exposed to a three-session exposure to JUUL Menthol pods with 12-hour intervals between sessions (A) Image of the western blot for human total OXPHOS protein and GAPDH. (B) Immediate post final exposure fold change of ETC protein levels. * p < 0.05 vs air exposed, unpaired t-test N = 3 wells per group. (C) 24 hours post final exposure fold change of ETC protein levels. * p < 0.05 vs air exposed, N = 3 unpaired t-test wells per group.
3.5. Virginia Tobacco exposure alters protein levels of the ETC
To determine JUUL Virginia Tobacco pod exposure effects on ETC protein level, western blot analysis was performed on whole cell lysate. Immediately post-final exposure resulted in a significant increase in complex V, complex IV, and Complex I (Figure 4B). 24 hours post final exposure did not result in a significant alteration of ETC protein levels (Figure 4C). Complex III was not able to be quantified for either time point.
Figure 4: Virginia Tobacco pod exposure alter ETC protein levels.
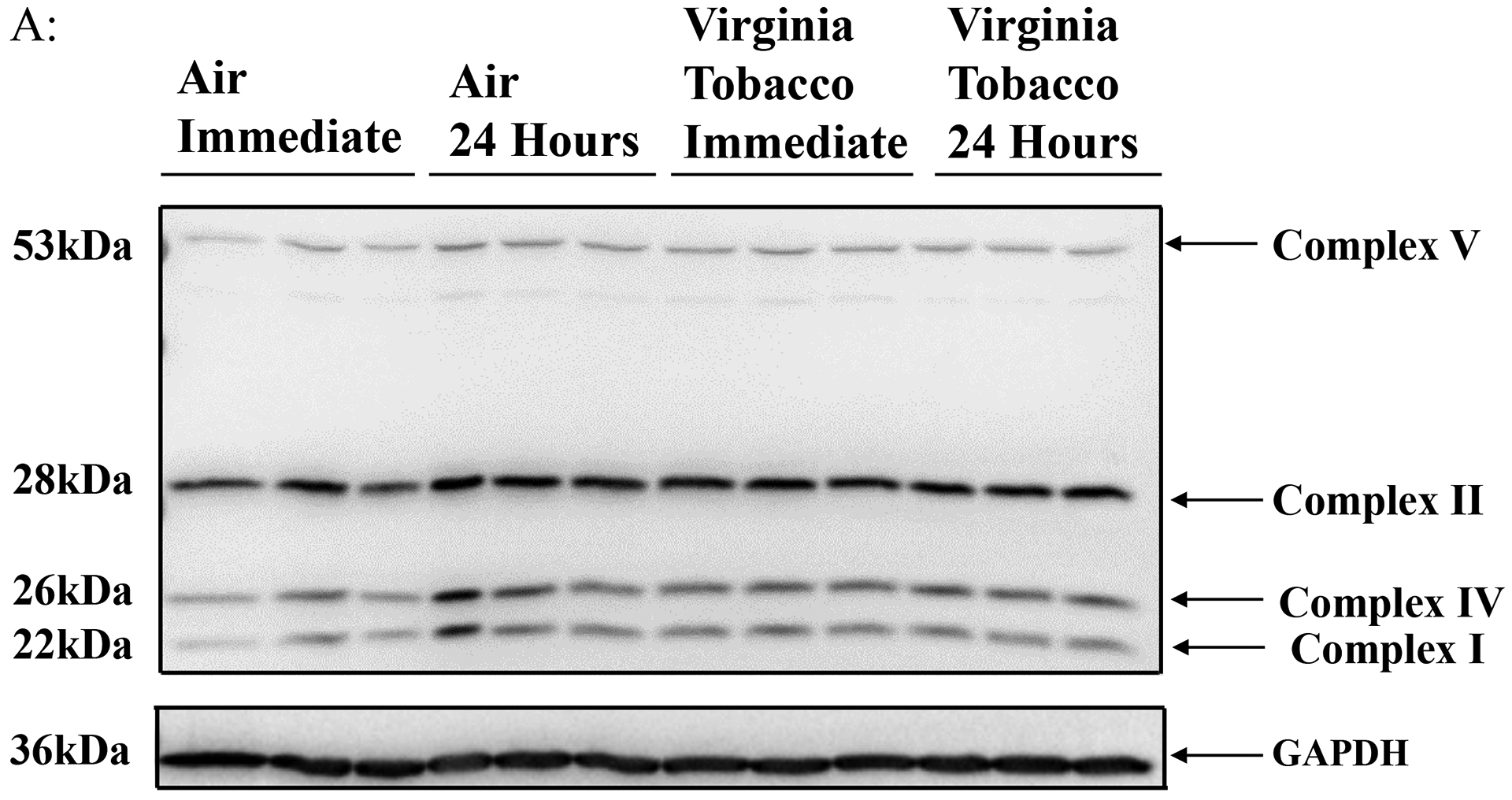
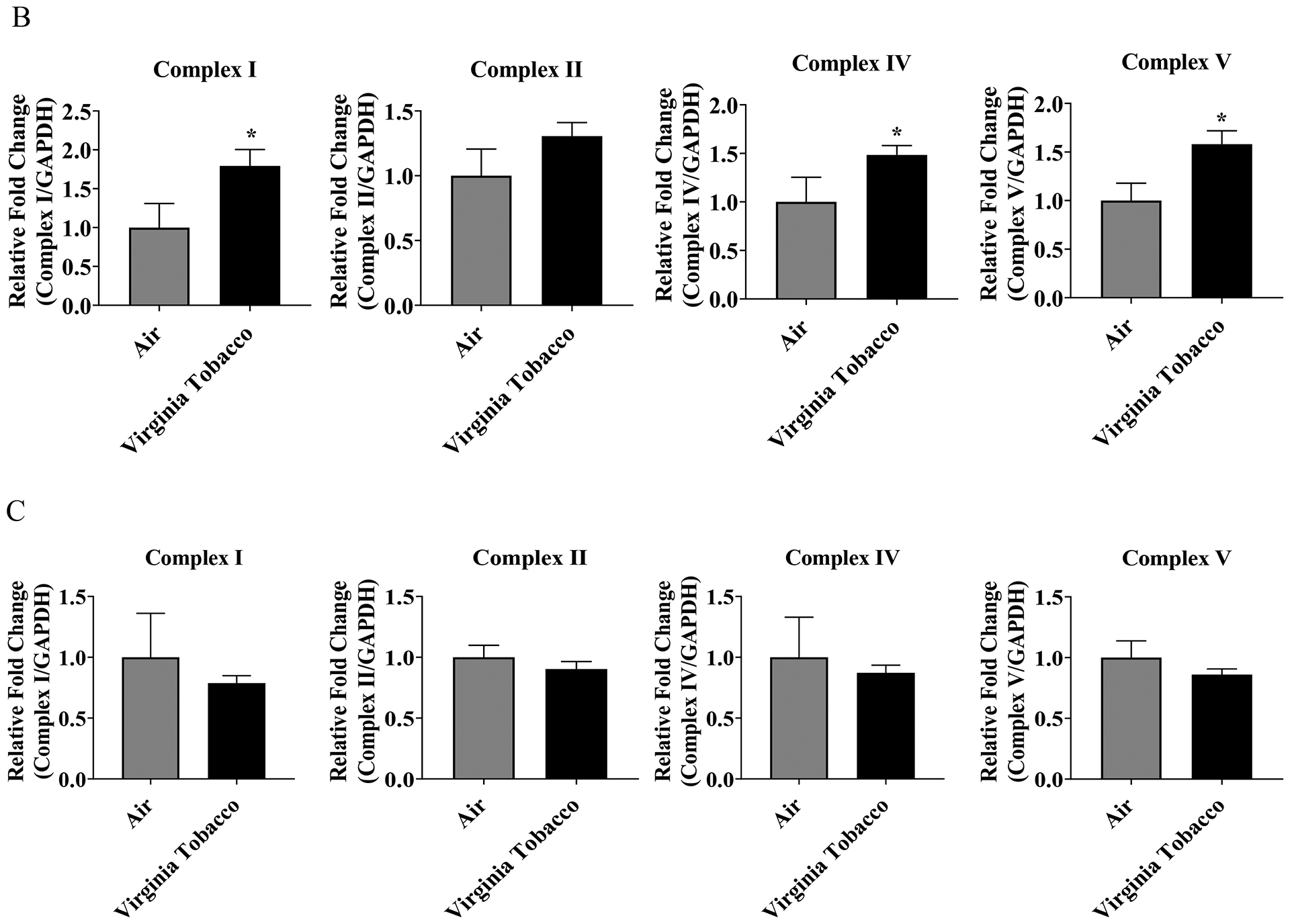
Beas2b cells with 300,000 cells per well were grown to 80% confluency. Immediately after serum deprivation the wells were exposed to a three-session exposure to JUUL Virginia Tobacco pods with 12-hour intervals between sessions (A) Image of the western blot for human total OXPHOS protein and GAPDH. (B) Immediate post final exposure fold change of ETC protein levels. * p < 0.05 vs air exposed, unpaired t-test N = 3 wells per group. (C) 24 hours post final exposure fold change of ETC protein levels. Unpaired t-test N = 3 wells per group.
4. Discussion
This study was attempted to determine whether e-cigarette pod-based JUUL Menthol and Virginia Tobacco flavor exposure alters the mitochondrial respiration in lung epithelial cells. Our study measured chemical constituents found in the vapor phase of Menthol and Virginia Tobacco pods, and found that a majority of the chemicals were shared between both flavors. This is similar to previous GC-MS data collected on JUUL Classic Menthol and JUUL Virginia Tobacco flavored e-liquids, with the majority of the chemicals found in Classic Menthol were shared by Virginia Tobacco pods (Muthumalage et al., 2019).
Our study also showed that Menthol flavor pod exposure resulted in a decrease in coupling efficiency and increase in proton leak at the immediate time point, and this decrease in coupling efficiency was also seen in Virginia Tobacco flavor pod at the immediate time point. Menthol pod exposure also resulted in mitochondrial dysfunction with a significant decrease in basal and maximal respiration and spare capacity at the 24-hour time points. Meanwhile, Virginia Tobacco pod exposure did not alter mitochondrial bioenergetics at the 24-hour time point. Both Menthol and Virginia Tobacco flavored pod exposures resulted in an increase in non-mitochondrial oxygen consumption. Menthol pod exposure also resulted in a potential increase in extracellular acidification rate, a measure of glycolysis, at both time points which would indicate a shift towards glycolysis. This alteration in extracellular acidification rate has also been seen in a previous study conducted on heat not burn cigarettes, i.e. IQOS exposure on Beas2b which resulted in a significant increase in extracellular acidification rate (Sohal et al., 2019). Along with this, a significant increase in proton leak was seen in Beas2b exposed to IQOS (Sohal et al., 2019), similar to the proton leak seen at the immediate time point of cells exposed to JUUL Menthol pod. We also showed that ETC subunit levels were significantly reduced, with complex I, II, and IV reduced at immediate time point and complex I reduced at 24-hour time point. Unlike Menthol flavor exposure, Virginia Tobacco flavor exposure resulted in a significant increase in complex I, II, and V at the immediate time point, but at the 24-hour time point no effect on ETC complexes were seen. A study conducted on the effect of Blu Classic Tobacco exposure on primary lung fibroblast showed that exposure to e-cigarettes exhibited complex IV sensitivity (Lerner et al., 2016). In another study, human lung fibroblasts were treated with e-cigarette condensate showed a reduction in complex I, II, and IV (Lei et al., 2017), similar to the results seen from our Menthol pod results.
One component of e-cigarettes that may result in mitochondrial dysfunction may be due to flavoring chemicals found in pod-based e-cigarettes since a previous study has shown that a flavoring chemical, such as cinnamaldehyde, can alter mitochondrial respiration/bioenergetics (Clapp et al., 2019). In this study, Beas2b cells were treated with various concentrations of cinnamaldehyde, which resulted in a dose-dependent decrease in basal respiration, ATP production, reserve capacity, proton leak, and maximal respiration (Clapp et al., 2019). Some of the unique chemical constituents of JUUL Menthol aerosols have been shown in previous studies to have some adverse health effects, e.g., inhalation of 1,4-dioxane has been found to induce nuclear enlargement in nasal respiratory epithelial cells from a 13-week exposure (Kasai et al., 2008). While in a two-year 1,4-dioxane inhalation study, preneoplastic lesions were found in the nasal cavity (Kasai et al., 2009). Nonneoplastic lesions in the nasal cavity showed a significant increase in nuclear enlargement, atrophy, and respiratory metaplasia, indicating 1,4-dioxane as a potential carcinogen (Kasai et al., 2009). A six-hour inhalation exposure of 2-ethyl-1-hexanol in Swiss mice, Wistar rats, and English Short Hair guinea pigs resulted in local irritation of the respiratory tract. Although the irritation was only temporary and all animals recovered with an hour (Wakayama et al., 2019). In this study, we were able to quantify many vapor phase compounds. However, there may be other responsible chemicals for mitochondrial dysfunction that we were unable to capture due to reasons, such as the volatility of compounds (temperature changes during sampling) and the auto-shutoff mechanism of JUUL devices.
The mitochondrial dysfunction seen in this study may potentially be due to an increase in mitophagy. A previous study conducted on the effects of menthol and tobacco e-liquids on neuronal stem cells have shown alterations in mitophagy, e.g. neuronal stem cells treated with 1% menthol for 4 hours resulted in an increase in mitophagy while treatment with 1% tobacco did not result in a significant increase in mitophagy (Zahedi et al., 2019). These results are similar to the observed results in this study with Menthol pod exposure and not Virginia Tobacco resulting in dysfunction of mitochondrial bioenergetics. Previous research in our lab has shown that CSE treatment leads to impaired mitophagy resulting in mitochondrial dysfunction by cigarette smoke extract (CSE) treatment to lung epithelial cells (Sundar et al., 2019). CSE treatment of lung epithelial cells resulted in an increase in DRP1 and a decrease in Mfn2 levels, along with a decrease in Miro1 and Pink1 (Sundar et al., 2019). CSE treatments also resulted in decreases in complex 1, II, III, and IV, along with alteration in mitochondrial respiration (Sundar et al., 2019). Since the mitophagy seen in cells treated with menthol e-liquids are similar to CSE treatments, which are associated with a decrease in mitochondrial respiration and ETC complex protein levels, there is a potential for an increase in mitophagy to be a cause of alteration in mitochondrial respiration. This highlights the need for future studies looking at the potential of mitophagy induced by e-cigarette exposure, particularly using the pod-based flavors, leading to alteration in mitochondrial respiration.
Despite e-cigarettes being marketed as a safer alternative to traditional cigarettes, similar results seen in our study have also been observed in cells treated with CSE (Sundar et al, 2019). CSE treatment of isolated mitochondria resulted in a decrease in mitochondrial oxygen consumption along with a reduction in complex I and II activity (van der Toorn et al., 2007). Other studies have indicated a reduction in complex IV activity in smokers compared to non-smokers (Miro et al., 1999). One study comparing 3R4F reference cigarette and tobacco heating system showed that both products resulted in a reduction in basal respiration and ATP production in a one-week exposure (Malinska et al., 2018). This result indicates that effects on mitochondria due to tobacco heating system are lower than traditional cigarette smoke (Malinska et al., 2018).
Mitochondrial dysfunction due to exposure from cigarette smoke has been known to cause disease in users (Ahmad et al, 2015). Cigarette smoke has been known to induce cardiomyopathy in part due to mitochondrial dysfunction (Yang et al., 2007). Studies have shown that cigarette smoke can impair myocardial OXPHOS function in reperfusion injury and also increase sensitivity to heart ischemia/reperfusion injury (Yang et al., 2007). Other mitochondrial dysfunction such as alteration of mitochondrial membrane potential, mitochondrial respiration, and ATP content caused by traditional cigarette smoke has been implicated in pulmonary diseases like COPD (Fetterman et al., 2017). CSE treatment has also been shown to induce impairment in mitophagy, which leads to cellular senescence (Ahmad, et al 2015). Similar to the impact of CSE treatment, COPD patients small airway epithelial cells show impaired mitophagy and increased cellular senescence, potentially indicating the potential of CSE induced mitophagy impairment to induce chronic airway diseases (Ahmad et al., 2015). Since our JUUL pod exposure caused mitochondrial dysfunction comparable to traditional cigarette smoke, in the reduction of ETC complex protein level and decrease in mitochondrial respiration, there is a potential risk of e-cigarette chronic use to induce pulmonary and cardiovascular diseases. A recent longitudinal study showed that either current or former e-cigarette use was significantly associated with having a higher risk of developing respiratory diseases (Bhatta and Glantz, 2020).
Although mitochondrial dysfunction was seen in Beas-2b cell line in this study, it has been shown that different cell types react differently to oxidative stress (Thangboonijt, 2015). A previous study has observed that treatment with hydrogen peroxide resulted in Beas-2b cells having a lower production of intracellular reactive oxygen species than normal human bronchial epithelial cells, as well as, concluding that baseline mitochondrial function was different in different cell types (Thangboonijt, 2015). Based on this study, future studies will need to confirm the results seen in this study are also observed in primary lung epithelial cells.
In conclusion, our study has shown that exposure to currently available JUUL Menthol flavor pod exposure results in an alteration in mitochondrial respiration with a reduction in basal respiration and maximal respiration based upon the time post-exposure. Along with this, reduction in ETC subunits is seen based upon exposure to JUUL Menthol. Meanwhile, exposure to currently available JUUL Virginia Tobacco pods did not result in an alteration in mitochondrial bioenergetics. This study implicates the need for more stringent regulation on e-cigarette flavoring products with new emerging products like bar-based disposable e-cigarettes still not regulated by federal or state governments.
Highlights.
JUUL Menthol and tobacco flavored pod aerosols were tested on mitochondrial energetics
Menthol flavored e-cigarette pods cause mitochondrial dysfunction
Menthol pod exposure reduces basal and maximal respiration in mitochondria
Virginia Tobacco pods exposure did not cause alteration to mitochondrial bioenergetics
Menthol pod exposure reduces OXPHOS complexes
Acknowledgments
We would like to thank Dr. Krishna Maremanda for assisting in seahorse analysis and for adding input in the experimental process.
Funding
This study was supported by NIH 1R01HL135613 and WNY Center for Research on Flavored Tobacco Products (CRoFT) # U54CA228110.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I, 2015. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29, 2912–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Boyd J, Esposito S, Foronjy R, Hiemstra PS, Jimenez-Ruiz CA, Katsaounou P, Lindberg A, Metz C, Schober W, Spira A, Blasi F, 2019. Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J 53. [DOI] [PubMed] [Google Scholar]
- Bhatta DN, Glantz SA, 2020. Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am J Prev Med 58, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I, 2019. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol 316, L470–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, Creamer MR, Jamal A, Ambrose BK, King BA, 2019. e-Cigarette Use Among Youth in the United States, 2019. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Leventhal AM, 2019. Prevalence of e-Cigarette Use Among Adults in the United States, 2014–2018. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Bascom R, Fan T, Sinharoy A, Yingst J, Mondal P, Foulds J, 2020. Changes in Flavor Preference in a Cohort of Long-term Electronic Cigarette Users. Ann Am Thorac Soc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterman JL, Sammy MJ, Ballinger SW, 2017. Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology 391, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Kano H, Umeda Y, Sasaki T, Ikawa N, Nishizawa T, Nagano K, Arito H, Nagashima H, Fukushima S, 2009. Two-year inhalation study of carcinogenicity and chronic toxicity of 1,4-dioxane in male rats. Inhal Toxicol 21, 889–897. [DOI] [PubMed] [Google Scholar]
- Kasai T, Saito M, Senoh H, Umeda Y, Aiso S, Ohbayashi H, Nishizawa T, Nagano K, Fukushima S, 2008. Thirteen-week inhalation toxicity of 1,4-dioxane in rats. Inhal Toxicol 20, 961–971. [DOI] [PubMed] [Google Scholar]
- Kaur G, Muthumalage T, Rahman I, 2018. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol Lett 288, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Lerner C, Sundar IK, Rahman I, 2017. Myofibroblast differentiation and its functional properties are inhibited by nicotine and e-cigarette via mitochondrial OXPHOS complex III. Sci Rep 7, 43213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A, Rahman I, 2016. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun 477, 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinska D, Szymanski J, Patalas-Krawczyk P, Michalska B, Wojtala A, Prill M, Partyka M, Drabik K, Walczak J, Sewer A, Johne S, Luettich K, Peitsch MC, Hoeng J, Duszynski J, Szczepanowska J, van der Toorn M, Wieckowski MR, 2018. Assessment of mitochondrial function following short- and long-term exposure of human bronchial epithelial cells to total particulate matter from a candidate modified-risk tobacco product and reference cigarettes. Food Chem Toxicol 115, 1–12. [DOI] [PubMed] [Google Scholar]
- Miro O, Alonso JR, Jarreta D, Casademont J, Urbano-Marquez A, Cardellach F, 1999. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis 20, 1331–1336. [DOI] [PubMed] [Google Scholar]
- Muthumalage T, Lamb T, Friedman MR, Rahman I, 2019. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep 9, 19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P, 2019. High-Nicotine Electronic Cigarette Products: Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. Chem Res Toxicol 32, 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi D, Chau C, Jackler RK, 2018. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control. [DOI] [PubMed] [Google Scholar]
- Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, O’Connor RJ, 2019. Use of Flavored E-Cigarettes and the Type of E-Cigarette Devices Used among Adults and Youth in the US-Results from Wave 3 of the Population Assessment of Tobacco and Health Study (2015–2016). Int J Environ Res Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal SS, Eapen MS, Naidu VGM, Sharma P, 2019. IQOS exposure impairs human airway cell homeostasis: direct comparison with traditional cigarette and e-cigarette. ERJ Open Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar IK, Maremanda KP, Rahman I, 2019. Mitochondrial dysfunction is associated with Miro1 reduction in lung epithelial cells by cigarette smoke. Toxicol Lett 317, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangboonijt W, Barnes P, Durham A, Adcock I, 2015. Mitochondrial function in three different types of airway epithelial cells. European Respiratory Journal 46. [Google Scholar]
- van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, Koeter GH, van Oosterhout AJ, Kauffman HF, 2007. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol 292, L1211–1218. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Ito Y, Sakai K, Miyake M, Shibata E, Ohno H, Kamijima M, 2019. Comprehensive review of 2-ethyl-1-hexanol as an indoor air pollutant. J Occup Health 61, 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Harrison CM, Chuang GC, Ballinger SW, 2007. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutat Res 621, 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A, Phandthong R, Chaili A, Leung S, Omaiye E, Talbot P, 2019. Mitochondrial Stress Response in Neural Stem Cells Exposed to Electronic Cigarettes. iScience 16, 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]


