Abstract
Traumatic brain injury is among the leading causes of death in individuals under 45 years of age. However, since trauma mechanisms and survival times differ enormously, the exact mechanisms leading to the primary and secondary injury and eventually to death after traumatic brain injury (TBI) remain unclear. Several studies showed the versatile functions of microglia, the innate macrophages of the brain, following a TBI. Earlier being characterized as rather neurotoxic, neuroprotective capacities were recently demonstrated, therefore, making microglia one of the key players following TBI. Especially in cases with only short survival times, immediate microglial reactions are of great forensic interest in questions of wound age estimation. Using standardized immunohistochemical methods, we examined 8 cases which died causatively of TBI with survival times between minutes and 7 days and 5 control cases with cardiovascular failure as the cause of death to determine acute changes in microglial morphology and antigen expression after TBI. In this pilot study, we detected highly localized changes in microglial morphology already early after traumatic damage, e.g., activated microglia and phagocyted erythrocytes in the contusion areas in cases with minute survival. Furthermore, an altered antigen expression was observed with increasing trauma wound age, showing similar effects like earlier transcriptomic studies. There is minute data on the direct impact of shear forces on microglial morphology. We were able to show localization-depending effects on microglial morphology causing localized dystrophy and adjacent activation. While rodent studies are widespread, they fail to mimic the exact mechanisms in human TBI response. Therefore, more studies focusing on cadaveric samples need to follow to thoroughly define the mechanisms leading to cell destruction and eventually evaluate their forensic value.
Electronic supplementary material
The online version of this article (10.1007/s00414-020-02308-x) contains supplementary material, which is available to authorized users.
Keywords: Chronic traumatic encephalopathy, Immunohistochemistry, Inflammation, Microglia, Traumatic brain injury
Introduction
Traumatic brain injuries (TBI) have a biphasic course [1]. While tissue destruction and necrosis occur immediately and directly at the impact zone together with intracranial or intracerebral hemorrhages as well as cortical contusions (primary phase), numerous changes and pathways in inflammation, edema, and blood flow disturbances start subsequently within minutes, hours, or days due to pathophysiological regulatory processes of the brain tissue. These mechanisms are usually summarized as the secondary injury [2, 3]. Due to the heterogeneity of the initial trauma, a reconstruction of the precise events leading from primary to secondary injury is very difficult. Therefore, the exact molecular and cellular mechanisms at acute, subacute, or chronic survival times after TBI remain to be defined in more detail [1, 3]. Given the high numbers of traumatic fatalities including head injuries worldwide, diagnosing the fatal injuries and estimating the TBI survival time are the main aspects of legal medicine and, therefore, were the aim of many forensic studies before [4–8]. Microglial cells, the resident macrophages of the brain, are activated in the course of traumatic brain injuries, producing cytokines and causing an influx of peripheral immune cells [1, 9]. While being a silent observer surveying the brain parenchyma in their ramified phenotype, microglia can target focal brain injury within minutes by projecting their processes towards the sites of damage, subsequently followed by alterations in morphology and protein expression [10, 11]. Histological studies in humans suggest that microglia activation can be sustained for many years after TBI [12], and their changes are therefore attributed to chronic traumatic encephalopathy stages; however, minute data are available for acute microglial changes in traumatized human brain tissue.
The verification of a TBI during the autopsy is relatively easy when severe intracerebral injuries are found. However, especially with only short survival times or in cases with severe destructions due to polytrauma, these findings are not necessarily established macroscopically. Since reliable and unique biomarkers of traumatic damage to the central nervous system (CNS) are still lacking despite years of active research [5, 13, 14], a closer look and detailed evaluation of immediate microglial reactions are of great forensic interest. Additional knowledge about time-dependent microglial changes after TBI may be used for a determination of trauma severity, survival time estimation, and a deeper understanding of mechanisms of traumatic death cases.
Materials and methods
Brain tissue samples from the pericontusional zones, macroscopically unaffected areas of the contralateral cortex, and the cerebellum were taken from eight individuals, who died causatively after suffering a TBI, and five control cases during forensic autopsies, which are displayed in detail in Table 1. If the cerebellum was traumatized by contusions, also intracerebellar controls were taken. None of these cases suffered from any clinically relevant neurodegenerative disease, either known from the medical history of the deceased or autopsy results. No brain tissue sample showed signs of putrefaction.
Table 1.
Characteristics of all cases used in this study
| Case no. | Gender | Age | Trauma mechanism | Trauma type | Cortical contusion | Survival time | Cause of death | Brain weight | Degree of brain edema | PMI |
|---|---|---|---|---|---|---|---|---|---|---|
| Traumatic brain injury cases (n = 8) | ||||||||||
| 1 | m | 18 | Car accident | Blunt force (impact) | Pons | Few min | Pons dehiscence | 1380 g | None | 44 h |
| 2 | m | 32 | High height fall | Blunt force (fall) | Cerebellum | 10 min | Subarachnoidal hemorrhage | 1100 g | None | 36 h |
| 3 | w | 73 | Falling branch | Blunt force (hit) | Parietal | 27 min | Opened TBI | 1230 g | None | 44 h |
| 4 | w | 53 | Car accident | Blunt force (impact) | Temporal | 38 min | Blood aspiration | 1280 g | Moderate | 72 h |
| 5 | m | 58 | Motorcycle accident | Blunt force (fall) | Temporal | 9.5 h | Brain edema | 1570 g | Severe | 35 h |
| 6 | w | 73 | Pedestrian accident | Blunt force (impact) | Parietal | 2 d | Brain edema | 1400 g | Severe | 48 h |
| 7 | m | 30 | Motorcycle accident | Blunt force (impact) | Temporal | 2.8 d | Brain edema | 1610 g | Severe | 46 h |
| 8 | w | 78 | Tram accident | Blunt force (fall) | Temporal | 7 d | Multi-organ failure | 1260 g | None | 64 h |
| Control cases (n = 5) | ||||||||||
| 9 | m | 42 | None | Acute myocardial infarction | 1310 g | Moderate | 89 h | |||
| 10 | m | 67 | None | Acute myocardial infarction | 1250 g | None | 94 h | |||
| 11 | m | 47 | None | Ruptured aortic aneurysm | 1580 g | Severe | 48 h | |||
| 12 | w | 27 | None | Sudden cardiac death | 1410 g | Moderate | 93 h | |||
| 13 | m | 50 | None | Acute myocardial infarction | 1390 g | Moderate | 40 h | |||
Before embedding in paraffin, the tissues were fixed in a 4% aqueous solution of formaldehyde. Using serial sections (18 μm), we performed Nissl and hematoxylin eosin (HE) staining for routine evaluation, as well as immunohistochemistry for a variety of microglia/macrophage markers (Supplementary Table 1). Furthermore, we examined the occurrence of tau and beta-amyloid using the corresponding antibodies (Supplementary Table 1).
Antigen retrieval was achieved by using microwave pretreatment in citrate buffer (95 °C, 5 min). Sections were then treated with 1.5% H2O2 in methanol for 20 min. Blocking the tissue with 1% bovine serum albumin (BSA; A7906, Sigma-Aldrich, St. Louis, USA) in PBS/Triton (0.03%, PBS-T) prevented further nonspecific binding. Subsequently, primary antibodies were incubated overnight at 4 °C in the adequate dilution in 0.5% BSA in PBS-T. After rinsing with PBS-T, application of the biotinylated secondary antibodies followed, and the slices were incubated for 90 min at room temperature (1:100, Supplementary Table 1).
Slices were then treated with peroxidase (ExtrAvidin-Peroxidase; E2886, Sigma-Aldrich). Its activity was developed using 3,3′ diaminobenzidine tetrahydrochloride (DAB; D5905, Sigma-Aldrich) as a chromogen. After counterstaining using hematoxylin, sections were washed, dehydrated, and mounted.
To verify the antibodies specificity and to eliminate unspecific staining, positive and negative controls were made constantly for the staining charges.
Results
This study detected clear changes in microglial morphology and expression profiles in TBI fatalities with increased survival times compared to control cases of fatal cardiovascular diseases, starting already after a short period between trauma and death.
The brain samples of all control cases showed comparable ramified morphology without signs of microglial activation (Supplementary Table 2). Furthermore, microglial cells appeared to be evenly distributed in the brain samples of cardiovascular fatalities.
Early and highly localized activation of microglia
In cases with short survival times (less than 1 h), microglial activation was already visible, and red blood cells were found to be phagocytosed by microglia within the directly affected area (Fig. 1). This phenomenon was detectable in survival times of 10 min at the earliest. Already in short distance to the contusion area, the so-called pericontusional zone, microglial morphology stayed ramified and seemingly unaffected.
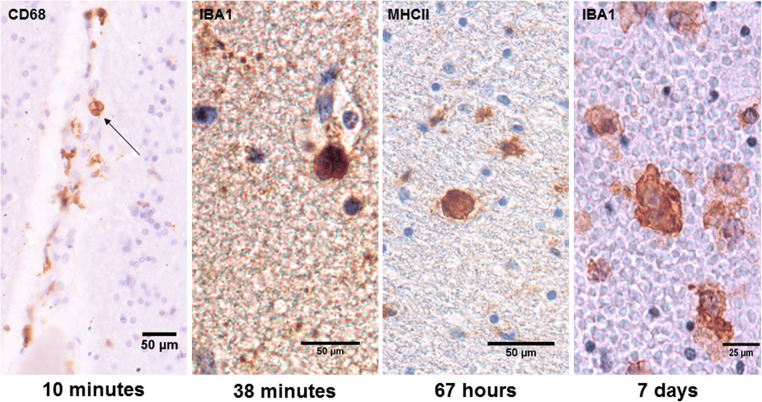
Fig. 1.
Hemophagocytosis by microglial cells in traumatic brain injury cases with different survival times. Already in cases with minute trauma survival, red blood cells are phagocytosed by microglia/macrophages in the contusion zones. This phenomenon seemingly appeared more frequently with increasing survival time and expansion of bleeding but stayed continuously inside the damaged brain area
By examination of sections deriving from contusion areas and their contralateral hemispheres, we detected that in cases with longer survival times (at a minimum 9 h), microglial morphology appeared to be similar within both brain hemispheres, mostly independent from their distance to the cortical contusion.
Change of microglial protein expression
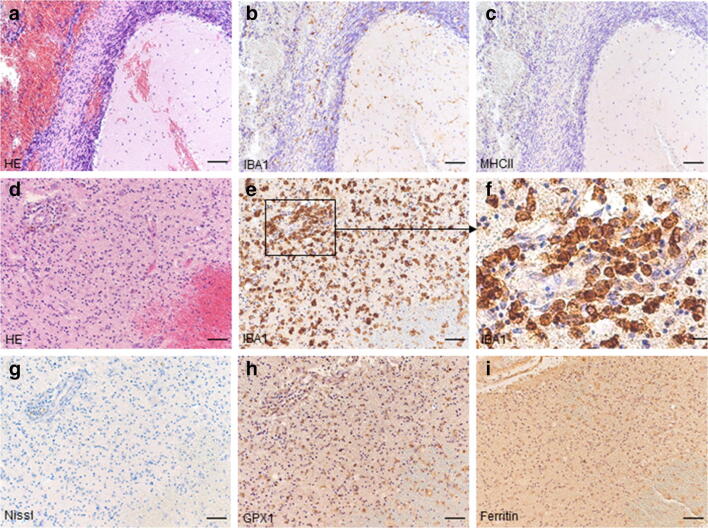
In cases with shorter survival times (at the earliest in a case with 10 min survival), we detected morphologically activated microglia expressing IBA1 in acute vicinity to the traumatic lesions, while MHCII stayed negative in these areas (Fig. 2a–c). The case with the longest survival time (7 days) was characterized by meaningful changes in microglial morphology and an altered antigen expression. In this case, a positive staining for GPX1 and ferritin mirrored alterations in the transcriptional profile of microglia (Fig. 2d–i, Supplementary Table 2).
Fig. 2.
Comparison of microglial antibody expression after different trauma survival times. While IBA1 is expressed in a lethal traumatic brain injury case with a survival time of 10 min, showing activated microglia in acute vicinity of lesion and ramified cells in adjacent areas, MHCII immunostaining stayed negative (a–c, cerebellar contusion). Another fatal head injury with a survival time of 7 days showed changes in microglial morphology, migrating macrophages, and also altered antigen expression (d–i, frontal contusion). Scale bars a–e; g–i, 100 μm; f, 25 μm
Microglial activation and dystrophy due to physical forces
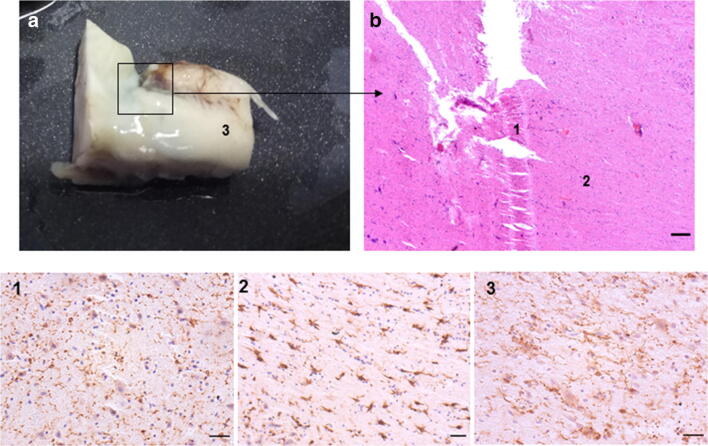
In one case, the pons was ruptured incompletely in an accident (Fig. 3a) with death occurring within minutes. The HE staining showed tissue rupture and injuries caused by shear forces (Fig. 3b). IBA1-stainings of microglia in immediate surroundings of the tissue damage showed pronounced dystrophy, while in close vicinity microglial activation, characterized by thickened somata and shortened processes, already appeared. However, these effects were also within a restricted area, since a shorter distance away, microglia presented a ramified morphology (Fig. 3(1–3)), comparable to the presentation in unaffected and non-traumatized tissue.
Fig. 3.
Shear forces have direct impact on microglial morphology. The pons was ruptured incompletely during an accident causing immediate death (a, macroscopic, and b, in hematoxylin eosin staining, scale bar 250 μm). (1) IBA1 immunostaining of microglia in immediate surrounding of the tissue dehiscence show pronounced dystrophy. (2) In close vicinity to the damaged area, microglial activation appears, characterized by thickened somata and shortened processes. (3) In short distance, microglia present a ramified morphology. Scale bars 1–3: 50 μm
No fast deposition of tau or beta-amyloid associated with contusion
Several studies have linked the appearance of TBI with the development of tau and amyloid depositions and therefore the development of neurodegenerative injuries [15]. Given that human studies were able to detect axonal tau depositions within 24 h post-injury [16], the here presented cases were examined for the occurrence of such deposits. There was not a single accumulation of tau or amyloid in the vicinity to contusion areas in the survival timeframe investigated between minutes and 7 days.
Discussion
This pilot study aimed to investigate the initial changes of microglial cells surrounding cortical contusions and in hypoxia-sensible areas of the brain to get a deeper understanding of the acute microglial activation and typical distribution patterns in the contusion area and the pericontusional zones of a TBI. The main results indicate a certain microglial morphology depending on severity, trauma mechanism, and survival time. These microglial changes could not only be easily examined with standard histological and immunohistochemical methods but also be used for daily forensic wound age estimation of TBI. A transformation in microglial protein expression towards a more reactive phenotype was also shown in transcriptional studies in rodents after a post-traumatic time course of 2 to 14 days [17].
Considering the biphasic course in the pathophysiology of TBI, microglia were shown to play a multifaceted role in the mechanisms following a trauma to the head. The depletion of microglia was shown to attenuate dendritic spine loss and neuronal apoptosis in the acute stage of TBI [18], therefore suggesting an active role of microglia in the early TBI pathophysiology. However, participation in repairing mechanisms [17] and maintaining the integrity of CNS barrier structures in dependence of purinergic receptors, e.g., the P2Y12R [19, 20], is one of the many neuroprotective capabilities. In future studies, especially the role of P2Y12R needs to be examined thoroughly, as it was characterized as a highly specific microglial marker, which is less expressed in the activated state [21]. Own research on purinergic signaling in TBI cases indicate a significant role of such receptors not only on microglia but also in many cell compartments in the TBI pathways [22]. The continuation of these receptor type characterizations showed potential for neuroprotective therapies (yet unpublished data).
While macrophages can maintain their metabolism also in a hypoxic environment [23], microglia are highly dependent on the aerobic generation of ATP to sustain their functioning [24]. However, in our cases, changes in microglial morphology appeared already after very short survival times. Therefore, migration of microglia and their phagocytic activity seems to be possible without an adequate oxygen supply for a certain amount of time in the agony phase of the death process, where all cell types are confronted with a generalized hypoxic situation. Thus, one needs to consider the possibility of supravital reactions within the brain tissue and to further evaluate microglial capacities in a hypoxic penumbra. Due to putrefactive changes being an exclusion criterium, autolysis was not a confounding aspect of such staining results.
Given the different microglial morphology within the area of interest, the authors consider the reaction on physical powers such as shear forces as a direct impact on the microglial morphology and, therefore, as a “proof of live” sign rather than agonal changes alone. The alterations have a certain configuration, which might mirror the severity and mechanism of the injury allowing for a conclusion on the extent of physical injuries. Furthermore, even severe brain edema did not seem to affect microglial morphology in our control cases to a relevant extent, supporting our hypothesis of trauma-induced microglial changes.
However, the extent of microglial capacities after the physical death needs to be examined thoroughly in the future, to correctly interpret the traumatic findings after TBI and to differentiate them from cell changes accompanying the death progress.
Due to a large heterogeneity and the complex pathogenesis, microglial TBI studies in humans are rare. However, rodent studies fail to mimic the exact mechanisms since any model can fully recapitulate the entirety of mechanisms leading to primary and secondary injury, as well as the diversity of injury mechanisms in human TBI [25]. The potential link to neurodegenerative diseases via tau or amyloid deposits as a response to TBI could not be proven with the cases investigated here. Therefore, the authors propose that the changes leading to these deposits might rather mirror (i) long-term effects of TBI in survival times longer than 7 days, (ii) repeated traumatism, or (iii) unspecific deposits with no pathological/traumatological correlation.
In the future, more studies with higher case numbers and tighter intervals between the survival time categories are needed to further obtain profound knowledge of microglial morphology and mechanisms. Then, the characterization and interpretation of microglial changes after TBI are useful as informative indicators on TBI response, not only to achieve an applicability of microglial morphology in forensic TBI analysis but also to detect possible targets for clinical (“neuroprotective”) treatment.
Electronic supplementary material
(PDF 138 kb).
Acknowledgements
Open Access funding provided by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was granted by the medical faculty of the University of Leipzig, Germany (local number 117-12-230112012). Anonymization was performed according to the guidelines from the central ethic commission of the federal medical association, while all experiments were conducted in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Julia Lier and Benjamin Ondruschka contributed equally to this work as first authors
References
- 1.Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramlett HM, Dietrich WD. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32:1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreßler J, Hanisch U, Kuhlisch E, Geiger KD. Neuronal and glial apoptosis in human traumatic brain injury. Int J Legal Med. 2007;121:365–375. doi: 10.1007/s00414-006-0126-6. [DOI] [PubMed] [Google Scholar]
- 5.Krohn M, Dreßler J, Bauer M, Schober K, Franke H, Ondruschka B. Immunohistochemical investigation of S100 and NSE in cases of traumatic brain injury and its application for survival time determination. J Neurotrauma. 2015;32:430–440. doi: 10.1089/neu.2014.3524. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann R. Age determination of brain contusions. Forensic Sci Med Pathol. 2006;2:85–93. doi: 10.1385/FSMP:2:2:85. [DOI] [PubMed] [Google Scholar]
- 7.Goede A, Dreßler J, Sommer G, Schober K, Franke H, Ondruschka B. Wundalterdiagnostik nach letalem Schädel-Hirn-Trauma. Rechtsmedizin. 2015;25:261–267. doi: 10.1007/s00194-015-0040-7. [DOI] [Google Scholar]
- 8.Maxeiner H. Subduralblutungen. Rechtsmedizin. 2009;19:111–128. doi: 10.1007/s00194-008-0585-9. [DOI] [Google Scholar]
- 9.Das M, Mohapatra S, Mohapatra SS. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammation. 2012;9:236. doi: 10.1186/1742-2094-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 11.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 12.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ondruschka B, Pohlers D, Sommer G, Schober K, Teupser D, Franke H, Dreßler J. S100B and NSE as useful postmortem biochemical markers of traumatic brain injury in autopsy cases. J Neurotrauma. 2013;30:1862–1871. doi: 10.1089/neu.2013.2895. [DOI] [PubMed] [Google Scholar]
- 14.Trautz F, Franke H, Bohnert S, Hammer N, Müller W, Stassart R, Tse R, Zwirner J, Dreßler J, Ondruschka B (2019) Survival-time dependent increase in neuronal IL-6 and astroglial GFAP expression in fatally injured human brain tissue. Sci Rep 9:11771. 10.1038/s41598-019-48145-w [DOI] [PMC free article] [PubMed]
- 15.Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarze V, Huber BR, Alosco ML, Solomon TM, Nowinski CJ, McHale L, Cormier KA, Kubilus CA, Martin BM, Murphy L, Baugh CM, Montenigro PH, Chaisson CE, Tripodis Y, Kowall NW, Weuve J, McClean MD, Cantu RC, Goldstein LE, Katz DI, Stern RA, STein TD, McKee AC. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318:360–370. doi: 10.1001/jama.2017.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, Wojnarowicz MW, Casey N, Lu H, Kokiko-Chran ON, Saman S, Ericsson M, Onos KD, Veksler R, Senatorov VV, Jr, Kondo A, Zhou XZ, Miry O, Vose LR, Gopaul KR, Upreti C, Nowinski CJ, Cantu RC, Alvarez VE, Hildebrand AM, Franz ES, Konrad J, Hamilton JA, Hua N, Tripodis Y, Anderson AT, Howell GR, Kaufer D, Hall GF, Lu KP, Ransohoff RM, Cleveland RO, Kowall NW, Stein TD, Lamb BT, Huber BR, Moss WC, Friedman A, Stanton PK, McKee AC, Goldstein LE. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141:422–458. doi: 10.1093/brain/awx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzy S, Liu Q, Fang Z, Lule S, Wu L, Chung JY, Sarro-Schwartz A, Brown-Whalen A, Perner C, Hickman SE, Kaplan DL, Patsopoulos NA, Khoury JE, Whalen MJ. Time-dependent changes in microglia transcriptional networks following traumatic brain injury. Front Cell Neurosci. 2019;13:307. doi: 10.3389/fncel.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CF, Zhao CC, Liu WL, Huang XJ, Deng YF, Jiang JY, Li WP. Depletion of microglia attenuates dendritic spine loss and neuronal apoptosis in the acute stage of moderate traumatic brain injury in mice. J Neurotrauma. 2019;37:43–54. doi: 10.1089/neu.2019.6460. [DOI] [PubMed] [Google Scholar]
- 19.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 2016;113:1074–1079. doi: 10.1073/pnas.1520398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mildner A, Huang H, Radke J, Stenzel W, Priller J. P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia. 2017;65:375–387. doi: 10.1002/glia.23097. [DOI] [PubMed] [Google Scholar]
- 22.Bremicker K, Grohmann M, Becker K, Ondruschka B, Dreßler J, Weber M, Franke H. Humane Schädel-Hirn-Traumen – Beteiligung purinerger Rezeptoren an der Astrogliose. Rechtsmedizin. 2013;23:327–358. doi: 10.1007/s00194-013-0903-8. [DOI] [Google Scholar]
- 23.Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol. 2013;25:67–75. doi: 10.1093/intimm/dxs110. [DOI] [PubMed] [Google Scholar]
- 24.Gimeno-Bayón J, López-López A, Rodríguez MJ, Mahy N. Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res. 2014;92:723–731. doi: 10.1002/jnr.23356. [DOI] [PubMed] [Google Scholar]
- 25.Saatman KE, Duhaime AC, Bullock R, Maas AIR, Valadka A, Manley GT, Workshop Scientific Team and Advisory Panel Members Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 138 kb).