Abstract
COVID-2019 pandemic is affecting people worldwide in the absence of an effective treatment strategy. Several suggestive therapeutic options through drug repurposing are recommended, but a complete consensus is not reached. A combination of Hydroxychloroquine (HCQ) and Azithromycin (AZM) has been widely tried and discussed but its administration has also led to potential adversities in patients. Studies are suggesting that most prominent adverse event with HCQ and AZM combination is QT interval prolongation. We studied interaction of HCQ with AZM and subsequent effect of this drug combination on QT interval prolongation. We performed system biological investigation of HCQ and AZM targets and screened important targets and pathways possibly involved in QT interval prolongation. The best core hub protein drug targets involved in QT interval prolongation were identified as HSP90AA1 exclusively associated with HCQ, while AKT1 exclusively associated with AZM on the basis of node degree value. It was found that PI3K/Akt, VEGF, ERBB2 pathways must be given consideration for understanding the role of HCQ and AZM in QT interval prolongation. Conclusion: Computational methods have certain limitations based on source database coverage and prediction algorithms and therefore this data needs experimental correlation to draw final conclusion, but current findings screen targets for QT interval prolongation associated with HCQ and AZM. These proteins and pathways may provide ways to reduce this major risk associated with this combination.
Keywords: Drug targets, COVID-19, Adverse reactions, Network pharmacology, Cardiac arrhythmia
Highlights
-
•
Hydroxychloroquine and azithromycin is widely tried for recent pandemic.
-
•
It is discussed to prolong QT interval causing severe adverse events.
-
•
Potential drug targets involved in this process were screened using system biology.
-
•
Pathways were also screened for drug combination mediated adverse events.
1. Introduction
Recent COVID-19 pandemic raised several challenges in front of scientific community in the absence of widely accepted therapeutic intervention. The use of Hydroxychloroquine (HCQ) was also debated globally during this pandemic with wide range of observations, making it difficult to reach complete consensus about potential of this drug as therapeutic intervention for COVID-19 [1]. It was suggested that use of HCQ has potential to manage COVID-19 [2,3] and azithromycin (AZM) enforce this ability [4]. In contrast, the use of this combination may cause several adverse events, including QT interval prolongation, hypoglycemia, and neuropsychiatric effects etc. [5]. Several statements were issued to draw the attention towards safety evaluation of this combination in COVID-19 management [6]. Among the adverse event associated with HCQ and AZM, the QT interval prolongation received major attention with the propensity of fatal cardiac arrhythmia leading to sudden cardiac death among susceptible individuals. Although, several suggestive articles are published for anti COVID-19 activities of HCQ and AZM combinations [7,8], but no data is available explaining mechanisms of these adverse events as per our literature search. The network pharmacology is an emerging bioinformatics approach to identify potential drug targets and its underlying mechanisms associated with potential actions of drugs [9].
In this article, we performed network pharmacology analyses of HCQ and AZM targets to understand the mechanisms of adverse events associated with this drug combination. We analyzed targets of these two drugs and their involvement in QT interval prolongation. We also identified targets linked with HCQ and/or AZM and involved in QT interval prolongation in addition to metabolic pathways modulated by these target proteins. Consequently, we present here a bioinformatics analyses of HCQ and AZM targets for understanding effect of these drugs alone and in combination for QT interval prolongation.
2. Materials and methods
2.1. Screening of HCQ and AZM targets
The well-known databases of pharmacological drug targets were used to obtain targets of HCQ and AZM. During the analysis, we used DrugBank [10], SuperPred [11], and Swiss Target Prediction [12] databases for listing of HCQ and AZM targets in order to understand their role in QT interval prolongation. In addition, the DisGeNET [13], and Genecards [14] were used to identify targets involved in QT interval prolongation. All the databases were last screened on or before Sep 2020.
2.2. Filtering of HCQ and AZM targets
The drug targets redundant with different databases were filtered to find unique targets associated with these two drugs. The unique targets of both drugs were further screened for identification of common and exclusive targets of AZM and/or HCQ.
2.3. Screening of targets involved in QT interval prolongation
The human pathological targets involved in QT interval prolongation were screened through DisGeNET and Genecard. The targets obtained from both databases were filtered to avoid redundant targets obtained from both databases.
2.4. Identification of HCQ and AZM targets involved in QT interval prolongation
The Venn diagram was constructed to find HCQ and AZM targets involved in QT interval prolongation. This was used to identify QT interval targets exclusively or commonly affected by HCQ and AZM.
2.5. Screening of important HCQ and AZM targets involved in QT interval prolongation and interrelated network construction
The protein-protein interaction maps of HCQ and AZM targets involved in QT interval prolongation was prepared with STRING database. The STRING database screen interactions and gives combined score to each interaction on the basis of different evidences including experimental after adding probability to each channel. Cytoscape v.3.8.0 was used to generate combined interaction map in order to find interrelation between HCQ and AZM targets. The cytoscape network analyzer was used to analyze topological parameters like degree of nodes and betweenness centrality in network. The best targets influenced by HCQ and AZM and possibly involved in QT interval prolongation were filtered as hub proteins using degree value of nodes in the network. The filtering was performed to separate nodes with degree values above than twice fold of the median degree value [15].
2.6. Functional processes and molecular pathways analysis using gene ontology
Gene Ontology (Biological Process) of HCQ and AZM target involved in QT interval prolongation were separately analyzed using functional enrichment analysis tool GOlorize [16]. We used cytoscape plugin GOlorize for gene ontology (biological process) based functional gene set enrichment analysis. The GOlorize worked on the BINGO [17] and the analysis was done with default Hypergeometric statistical test with Benjamini and Hochberg FDR correction and significance value 0.05. We also performed pathway analysis of QT interval targets associated with HCQ and/or AZM using REACTOME [18] in order to understand their role in different pathway modulation during single or co-administration of both drugs.
3. Results
3.1. Identification of drugs targets
Our analyses found total 202 protein targets for HCQ, including 11 protein targets identified from DrugBank, and 91 and 100 targets identified from SuperPred and Swiss Target Prediction respectively. In addition, DrugBank found one target as nucleotide. These 202 protein targets correspond to 207 protein IDs in database. There were several redundant targets among the proteins identified from all 3 databases. Total 176 unique protein entries were identified from all 3 databases after removing 31 redundant entries.
Total 206 targets were found during analysis of drug targets of AZM in above mentioned 3 databases (5 targets from DrugBank, 101 from SuperPred, and 100 from Swiss Target Prediction), including 1 nucleotide identified from DrugBank and therefore proteins targets were 205 corresponding to 207 protein IDs. These targets found 190 unique protein targets after removing 17 redundant protein entries. Tables S1 and S2 represents targets of HCQ and AZM collected through different databases and their analysis.
3.2. Screening of common targets of HCQ and AZM
Total 366 targets of HCQ and AZM were analyzed for their common interactions with both drugs or exclusive interaction with either HCQ or AZM. These targets included total 257 unique values, indicating exclusive 67 HCQ, 81 AZM targets and 109 targets common among both HCQ and AZM. Table S3 represent human targets common and exclusive with HCQ and AZM.
3.3. Screening of pathological targets of QT interval prolongation
Screening of DisGeNET for targets involved in prolonged QT interval found 38 targets while Genecard found 1071 targets. Filtering redundant targets from both databases gave 1073 unique targets out of 1109 targets obtained from DisGeNET and Genecard (36 redundant). Table S4 gives detail about targets obtained from both databases.
3.4. Screening of pathological targets involved in QT interval prolongation affected by HCQ and/or AZM
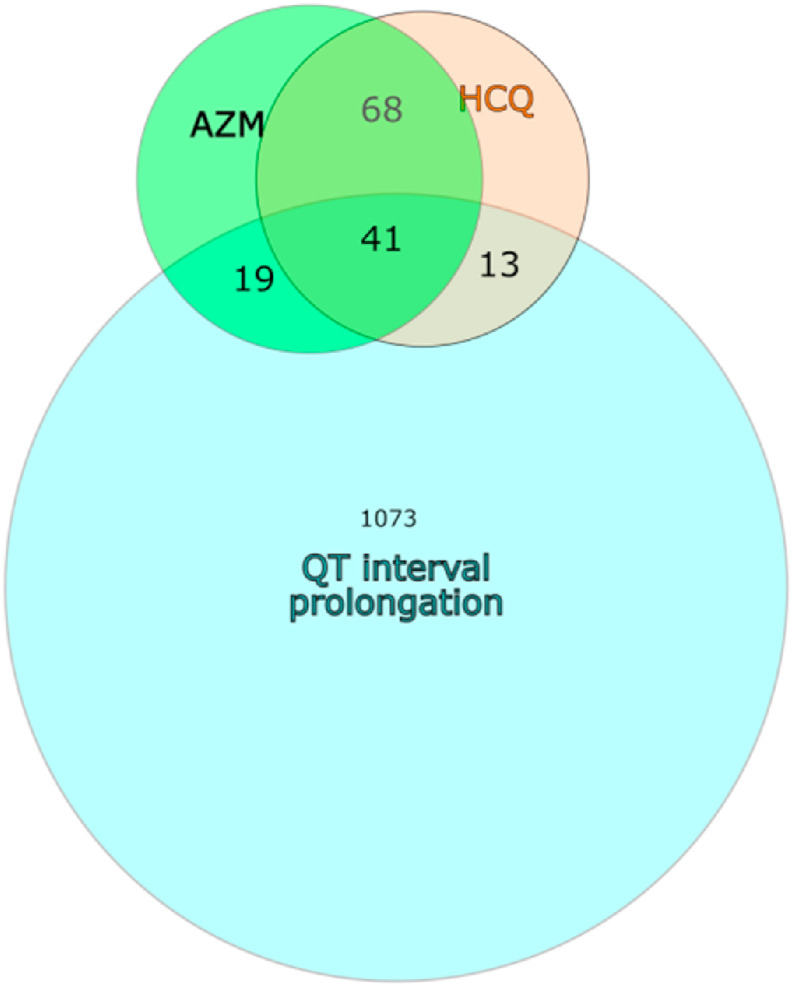
During analysis of QT interval prolongation targets affected by HCQ and AZM, we found common targets between HCQ, AZM and QT interval prolongation. Table 1 gives detail about target proteins involved in QT interval prolongation that could be affected by HCQ and/or AZM. Total 73/1073 QT interval prolongation targets were found to get affected by HCQ and/or AZM. Fig. 1 indicates about Venn diagram of targets involved in QT interval prolongation and their common interactions with HCQ and/or AZM.
Table 1.
QT interval prolongation targets with the chances to get affected by HCQ and/or AZM.
| Sr. No. | Targets affected by both HCQ and AZM | Targets affected by only HCQ | Targets affected by only AZM |
|---|---|---|---|
| 1 | LMNA | CACNA2D1 | F10 |
| 2 | KCNH2 | NOS1 | TSHR |
| 3 | ADRB1 | HSP90AA1 | PIK3CG |
| 4 | ADRB2 | CCND1 | HSP90B1 |
| 5 | ALB | NOS2 | DPP4 |
| 6 | SRC | CHKA | LGALS3 |
| 7 | ADRA2C | CDK2 | AKT1 |
| 8 | ELANE | HTR1A | ROCK1 |
| 9 | MC4R | HTR3A | LGALS4 |
| 10 | CYP1A2 | KIF11 | PDE4B |
| 11 | PRKCA | CYP2C8 | NPPA |
| 12 | EGFR | SPHK1 | DHFR |
| 13 | ESR1 | ROCK2 | NQO1 |
| 14 | PRKDC | SLC5A2 | |
| 15 | MMP9 | ITGA4 | |
| 16 | CYP2D6 | MLNR | |
| 17 | DRD2 | KDM1A | |
| 18 | CYP3A4 | TRAP1 | |
| 19 | MAPK14 | HTR4 | |
| 20 | ACHE | ||
| 21 | ABCB1 | ||
| 22 | SLC6A4 | ||
| 23 | CCR5 | ||
| 24 | HTR2A | ||
| 25 | CYP2C19 | ||
| 26 | CYP2C9 | ||
| 27 | HMGCR | ||
| 28 | INSR | ||
| 29 | CHRM3 | ||
| 30 | SYK | ||
| 31 | CYP2E1 | ||
| 32 | AKT3 | ||
| 33 | PDE5A | ||
| 34 | CDK4 | ||
| 35 | HRH1 | ||
| 36 | IGF1R | ||
| 37 | SLC6A2 | ||
| 38 | TACR1 | ||
| 39 | FYN | ||
| 40 | ADORA1 | ||
| 41 | AVPR1A |
Fig. 1.
Venn diagram indicating QT interval prolongation targets affected by HCQ and AZM.
3.5. Screening of important HCQ and AZM targets involved in QT interval prolongation and interrelated network construction
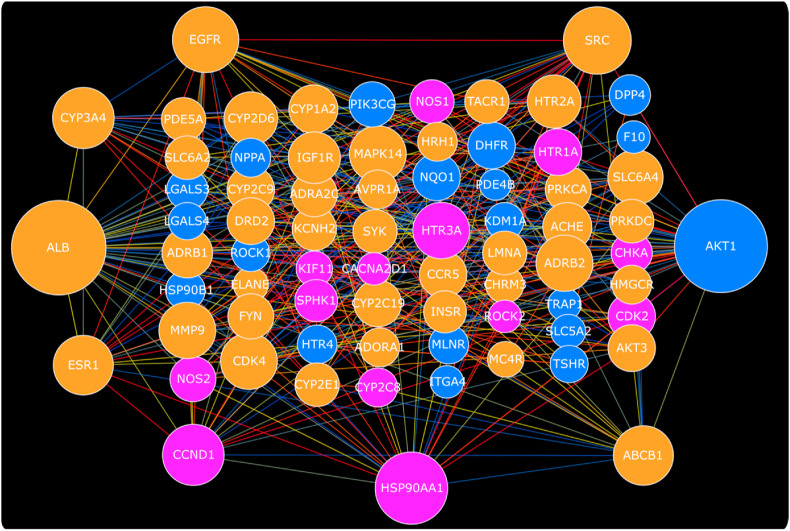
The construction of protein-protein interaction map of all 73 QT interval targets mentioned in Table 1 through STRING database and common interactions of both drugs with important targets are shown in Fig. 2 . The median degree of nodes for all HCQ and AZM QT targets was found to be 9. Therefore best targets were selected with the degree value ≥ 18 for combined HCQ and AZM QT target PPI network. ALB (41), AKT1 (40), HSP90AA1 (27), SRC (24), EGFR (23), CCND1 (20), CYP3A4 (20), ESR1 (19), ABCB1 (19) were found to satisfy degree value criteria of best target in combined HCQ and AZM prolonged QT interval target network. AKT1, ALB, HSP90AA1, KCNH2, SCR were screened as top nodes on the basis of betweenness centrality. The AZM target PDE4B was not interacting with other AZM targets but found to interact with HCQ targets. Table S5 represent PPI screened through STRING database with the computed score of each interaction for all HCQ and AZM QT interval target. The CYP3A4, SRC, HSP90AA1, ALB were found to be important hub targets for HCQ, while ADRB2, HTR2A, SLC6A4, ABCB1, CYP3A4, EGFR, AKT1, ALB, SRC, ESR1, MAPK14 were found as important hub targets for AZM during analysis of separate PPI network of HCQ and AZM on the basis of node degree (Fig. 3, Fig. 4 ).
Fig. 2.
Combined protein-protein interaction map of HCQ and AZM targets involved in QT interval prolongation. The QT interval prolongation targets of HCQ and AZM are shown with pink and blue color respectively, while protein targets interacting with both drugs are shown with orange color. The node size is arranged as per their comparative degree value while the colors of edges represent combined interaction score obtained from STRING (blue » yellow » red representing low » medium » high). Refer data for more details. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
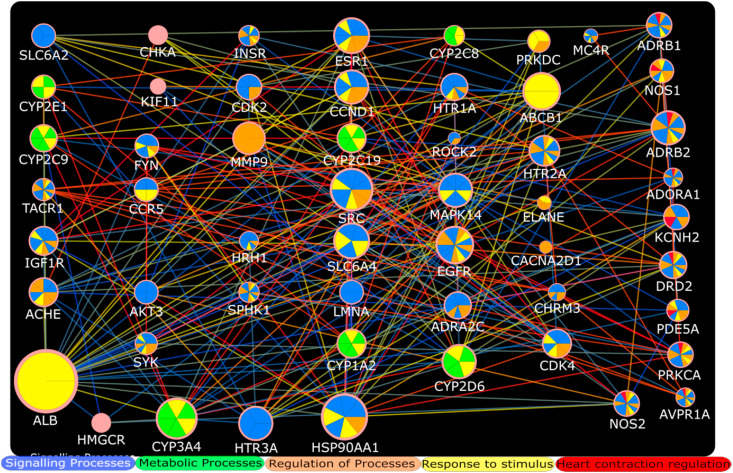
Fig. 3.
Functional overrepresentation analysis of HCQ targets involved in QT interval prolongation. Their role in different biological processes is presented with different colors. Figure only represents top biological processes on the basis of p value. Different node sizes represent comparative degree value in the network while colors of edges indicate combined interaction score (same as Fig. 2) of each interaction. The HCQ target proteins involved in regulation of heart contraction are presented at one side of network indicating red color. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
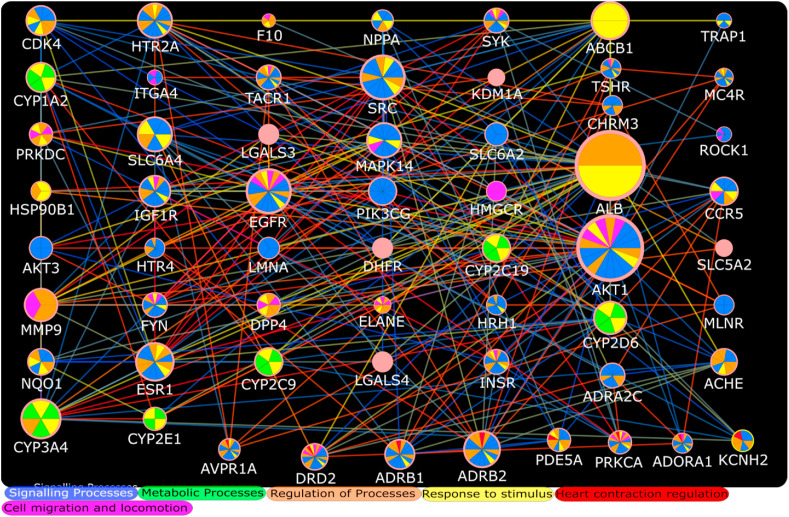
Fig. 4.
Functional overrepresentation analysis of AZM targets involved in QT interval prolongation. Their role in different biological processes is presented with different colors. Figure only represents top biological processes on the basis of p value. Different node sizes represent comparative degree value in the network while colors of edges indicate combined interaction score (same as Fig. 2) of each interaction. The AZM target proteins involved in regulation of heart contraction are presented at one side of network indicating red color. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Functional processes and molecular pathways analysis using gene ontology
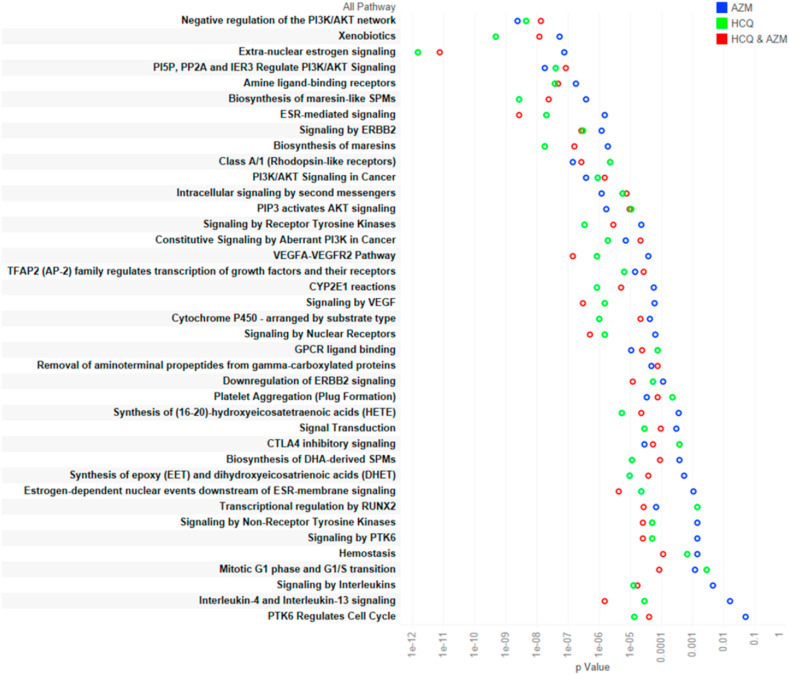
The gene set enrichment analyses of HCQ and AZM targets are presented in Tables S6 and S7 respectively. Fig. 3 represents top biological processes (on the basis of p value) in which HCQ QT interval prolongation targets are involved. Fig. 4 indicates top biological processes (on the basis of p value) involved with AZM QT interval prolongation targets as per gene set enrichment analysis. The Reactome pathways associated with HCQ, AZM, and combined HCQ and AZM are presented in Tables S8–S10 respectively. The top pathways on the basis of p-value are presented in Fig. 5 .
Fig. 5.
Metabolic pathways associated with use of HCQ and/or AZM (arranged as per p-value).
4. Discussion
The use of HCQ with AZM is globally tried for management of COVID-2019 in the absence of effective therapeutic regimen availability. However, the use of HCQ is speculated to be associated with high risk of QT interval prolongation and concurrent use of AZM can intensify these chances [[19], [20], [21]]. These studies emphasized to perform risk analysis of these two drugs for their application in the management of COVID-19, however the extensive mechanistic information about the involvement of HCQ and AZM in QT interval prolongation is not identified.
We used network pharmacology based approaches to identify interaction of these two drugs in modulation of QT interval prolongation. This approach has been used in several studies to identify potential targets and mechanisms associated with certain clinical condition [[22], [23], [24]]. The initial results of this study reveal that both drugs are interacting with QT interval prolongation targets (Fig. 1), and therefore it is plausible that use of this drug combination can intensify their effects on QT interval prolongation and correlate with clinical findings. During analysis of their common and exclusive interaction with QT interval prolongation hub targets, it is evident as per node degree distribution that HSP90AA1/CCND1 and AKT1 are major hub proteins involved in QT interval prolongation exclusively affected by HCQ and AZM respectively (Fig. 2). In contrast, SRC, EGFR, CYP3A4, ESR1, and ABCB1 and ALB are important hub proteins involved in QT interval prolongation that could be targeted by both HCQ and AZM according to combined PPI of HCQ and AZM targets. Both of these drugs may be involved in regulation of heart contraction, as indicated by separate gene set enrichment analysis of HCQ and AZM QT interval targets (Fig. 3, Fig. 4). The regulation of heart contraction as top biological process was enriched at 13th and 24th position in HCQ and AZM PPI network respectively (Tables S6 and S7). It will be interesting to verify these common targets in laboratory to understand their synergistic or inhibitory action under the simultaneous influence of HCQ and AZM. Among these targets, few are already suggested as potential targets for efficacy of HCQ and AZM combination, for example it has been suggested that ABCB1 provides synergistic effects of these two drug combination against COVID-19 [7]. Moreover, the functional enrichment analysis suggested that HCQ can directly interact with certain regulatory mediators of heart contraction including KCNH2 NOS2 ADORA1 PRKCA PDE5A ADRB1 NOS1 AVPR1A ADRB2 DRD2 (Fig. 3, Table S6). Whereas, AZM was found to interact with more hub proteins (Fig. 4) and support that HCQ may be directly involved in targeting mediators of cardiac arrhythmia, but AZM integrates more hub proteins in the process, and therefore it can intensify the chances of QT interval prolongation. These results are consistent with the finding obtained through clinical studies.
During enrichment analysis of metabolic pathway modulation during single or co-administration of HCQ and/or AZM, we found that these two drugs can modulate several metabolic and other pathways associated with cardiac arrhythmia. It has been implicated that drug mediated long QT syndrome is associated with inhibition of PI3K/Akt signaling [25]. We found that HCQ and AZM both are able to inhibit this pathway (Fig. 5). It further indicates potential of HCQ and AZM in increasing QT interval prolongation through inhibiting PI3K/Akt pathway. In addition, these two drug targets are also involved in synthesis of maresins. These molecules are produced by leukocytes and platelets and found to be involved in post-inflammatory regenerative response after myocardial infarction [26] and therefore indicate towards therapeutic potential of HCQ and AZM. In addition to PI3K/Akt pathway, ErbB2 inhibitors are also known to induce QT prolongation [27]. Our study found that HCQ and AZM combination can modulate ERBB2 signaling and therefore, it can be another mechanism for induction of QT interval prolongation through concurrent use of these drugs and must be investigated experimentally. When used in combination, HCQ and AZM can also modulate VEGF signaling pathway, another mechanism that has been implicated in QT interval prolongation [28].
Nevertheless, this combination was also found to modulate interleukin-4 and interleukin-13 signaling. This signaling is very important in allergic inflammation ranging from atopic dermatitis, allergic rhinitis to life threatening allergic asthma. However, there is discrepancy about the role of IL-4/IL-13 in SARS-CoV-2 [29], but the effects of HCQ and AZM combination on this pathway must be studied in order to investigate the mechanistic role of such pathways in treatment and adverse reactions. Another metabolic pathway found to be modulated by HCQ and AZM was CTLA4 inhibitory signaling. Anti CTLA-4 therapy is already known to induce pneumonitis and therefore role in HCQ and AZM combination in treatment of COVID-19 and associated adverse events needs a separate study to understand the role of this combination through CTLA4 inhibitory signaling [30].
5. Conclusion
Although, all these studies present promising targets and pathways for their involvement in QT interval prolongation, but this work is based on existing information obtained from widely known databases. All the databases are continuously evolving and their coverage of certain information is not complete, in addition computational prediction algorithms are also having several limitations. Therefore we must consider limitations of computational predictions and these findings must be correlated with experimental evidences in order to draw final conclusion. In spite of that, this study shortlists several potential targets and mechanisms which can be useful to find management strategies for HCQ and AZM associated adverse events. Under current scenario, this study holds its value as this combination is widely discussed throughout the globe and attracted scientific community.
Funding
None.
Author contributions
AAK performed and analyzed the experiments, wrote the first draft of the manuscript. ZK participated in the analysis of the experiments, revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cbi.2020.109299.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wright C., Ross C., Mc Goldrick N. Are hydroxychloroquine and chloroquine effective in the treatment of SARS-COV-2 (COVID-19)? Evid. Base Dent. 2020;21:64–65. doi: 10.1038/s41432-020-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meo S.A., Klonoff D.C., Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee P., Anand T., Singh K., Rasaily R., Singh R., Das S., Singh H., Praharaj I., Gangakhedkar R., Bhargava B., Panda S. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J. Med. Res. 2020;151:459–467. doi: 10.4103/ijmr.IJMR_2234_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juurlink D.N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ (Can. Med. Assoc. J.) : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2020;192:E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor A., Pandurangi U., Arora V., Gupta A., Jaswal A., Nabar A., Naik A., Naik N., Namboodiri N., Vora A., Yadav R., Saxena A. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: a scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J. 2020;20:117–120. doi: 10.1016/j.ipej.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherrmann J.M. Intracellular ABCB1 as a possible mechanism to explain the synergistic effect of hydroxychloroquine-azithromycin combination in COVID-19 therapy. AAPS J. 2020;22:86. doi: 10.1208/s12248-020-00465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Ma X., Song Y., Zhang Y., Xiong W., Li L., Zhou L. Anti-colorectal cancer targets of resveratrol and biological molecular mechanism: analyses of network pharmacology, human and experimental data. J. Cell. Biochem. 2019;120(7):11265–11273. doi: 10.1002/jcb.28404. [DOI] [PubMed] [Google Scholar]
- 10.Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z., Assempour N., Iynkkaran I., Liu Y., Maciejewski A., Gale N., Wilson A., Chin L., Cummings R., Le D., Pon A., Knox C., Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickel J., Gohlke B.O., Erehman J., Banerjee P., Rong W.W., Goede A., Dunkel M., Preissner R. SuperPred: update on drug classification and target prediction. Nucleic Acids Res. 2014;42:W26–W31. doi: 10.1093/nar/gku477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daina A., Michielin O., Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinero J., Ramirez-Anguita J.M., Sauch-Pitarch J., Ronzano F., Centeno E., Sanz F., Furlong L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–D855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., Stein T.I., Nudel R., Lieder I., Mazor Y., Kaplan S., Dahary D., Warshawsky D., Guan-Golan Y., Kohn A., Rappaport N., Safran M., Lancet D. The GeneCards suite: from gene data mining to disease genome sequence analyses. Current protocols in bioinformatics. 2016;54:1 30 31–31 30 33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 15.Ge B., Guo C., Liang Y., Liu M., Wu K. Network analysis, and human and animal studies disclose the anticystitis glandularis effects of vitamin C. Biofactors. 2019;45:912–919. doi: 10.1002/biof.1558. [DOI] [PubMed] [Google Scholar]
- 16.Garcia O., Saveanu C., Cline M., Fromont-Racine M., Jacquier A., Schwikowski B., Aittokallio T. GOlorize: a Cytoscape plug-in for network visualization with Gene Ontology-based layout and coloring. Bioinformatics. 2007;23:394–396. doi: 10.1093/bioinformatics/btl605. [DOI] [PubMed] [Google Scholar]
- 17.Maere S., Heymans K., Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 18.Jassal B., Matthews L., Viteri G., Gong C., Lorente P., Fabregat A., Sidiropoulos K., Cook J., Gillespie M., Haw R., Loney F., May B., Milacic M., Rothfels K., Sevilla C., Shamovsky V., Shorser S., Varusai T., Weiser J., Wu G., Stein L., Hermjakob H., D'Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. doi: 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Vol. 2. JAMA cardiology; 2020. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) pp. 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramireddy A., Chugh H., Reinier K., Ebinger J., Park E., Thompson M., Cingolani E., Cheng S., Marban E., Albert C.M., Chugh S.S. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. Journal of the American Heart Association. 2020;9 doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C., Knotts R., Bar-Cohen R., Kogan E., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Stefano C., Chinitz L.A., Jankelson L. Vol. 17. Heart rhythm; 2020. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin; pp. 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F., Pan Q., Wang L., Yi S., Liu P., Huang W. Anticancer targets and mechanisms of calycosin to treat nasopharyngeal carcinoma. BioFactors. 2020;46:675–684. doi: 10.1002/biof.1639. [DOI] [PubMed] [Google Scholar]
- 23.Cheng X., Liu N., Liu H., Huang N., Sun X., Zhang G. Bioinformatic and biochemical findings disclosed anti-hepatic steatosis mechanism of calycosin. Bioorg. Chem. 2020;100:103914. doi: 10.1016/j.bioorg.2020.103914. [DOI] [PubMed] [Google Scholar]
- 24.Li R., Guo C., Li Y., Liang X., Yang L., Huang W. Therapeutic target and molecular mechanism of vitamin C-treated pneumonia: a systematic study of network pharmacology. Food & function. 2020;11:4765–4772. doi: 10.1039/d0fo00421a. [DOI] [PubMed] [Google Scholar]
- 25.Ballou L.M., Lin R.Z., Cohen I.S. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ. Res. 2015;116:127–137. doi: 10.1161/CIRCRESAHA.116.303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jadapalli J.K., Halade G.V. Unified nexus of macrophages and maresins in cardiac reparative mechanisms. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2018;32:5227–5237. doi: 10.1096/fj.201800254R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppola C., Rienzo A., Piscopo G., Barbieri A., Arra C., Maurea N. Management of QT prolongation induced by anti-cancer drugs: target therapy and old agents. Different algorithms for different drugs, Cancer treatment reviews. 2018;63:135–143. doi: 10.1016/j.ctrv.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Ghatalia P., Je Y., Kaymakcalan M.D., Sonpavde G., Choueiri T.K. QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br. J. Canc. 2015;112:296–305. doi: 10.1038/bjc.2014.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schett G., Sticherling M., Neurath M.F. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.