Abstract
Embryo surface disinfection is utilized in aquaculture to decrease the risk of pathogen introduction into established colonies. Zebrafish embryos are commonly disinfected with unbuffered sodium hypochlorite at 25–50 ppm for 10 min with or without concurrent treatment with chemicals, including pronase (Pron), sodium thiosulfate, and/or methylene blue; however, the impact of these chemicals on embryo survival and development has not been evaluated. In this study, AB and casper embryos were exposed to disinfection protocols that used Pron, sodium thiosulfate, and/or methylene blue (given alone, in various combinations, or all three combined) with 50 and 100 ppm sodium hypochlorite performed 6 and 24 h postfertilization (HPF). All groups were evaluated for survival, hatching, and malformations at 5 days postfertilization. Maximal survival (69%–97%) and hatching rates (66%–94%) were generally observed with sodium hypochlorite disinfection followed by exposure to both Pron and sodium thiosulfate and maintenance in standard embryo medium without methylene blue. Methylene blue had variable effects on survival and hatching. Higher survival and hatching rates were seen in AB embryos disinfected at 6 HPF and casper embryos disinfected at 24 HPF. Susceptibility to sodium hypochlorite toxicity differed by strain, emphasizing the need to test disinfection protocols on small embryo cohorts.
Keywords: zebrafish, sodium hypochlorite, methylene blue, pronase, sodium thiosulfate, embryo disinfection
Introduction
The use of zebrafish (Danio rerio) as a biomedical research model has led to widespread sharing of unique fish lines among scientists. The importation of fish into an existing colony requires vigilant biosecurity practices, most notably quarantining newly imported fish and only introducing surface-disinfected embryos into quarantine and/or established colonies to minimize pathogen introduction.1–4
Zebrafish embryo disinfection protocols commonly use ∼10 min of exposure to unbuffered sodium hypochlorite solutions (NaOCl) at concentrations of 25–50 ppm.5,6 However, studies have demonstrated that exposure to these concentrations for 10 min is not effective at eliminating all known zebrafish pathogens or preventing vertical transmission to offspring.7,8 Additionally, exposure of embryos to high chlorine concentrations has been associated with embryo malformations and mortality.
Ferguson et al. discovered that exposure to 100 ppm NaOCl buffered to a pH of 7.0 for 10 min was required to destroy >99% of Pseudoloma neurophilia spores in vitro7; however, the use of this disinfection protocol was associated with developmental delay; notochord malformation; pericardial edema; yolk sac, eye, and snout abnormalities; and, increased embryo mortality.9 Kent et al. recommends treating embryos for 5 min with unbuffered (pH of 8.3) NaOCl solutions at 100 ppm to avoid the associated malformations and mortalities that occur with increased exposure time and buffered solutions.9 The ideal surface disinfection protocol would eliminate the greatest number of pathogens with minimal malformations and a high survival rate.
The age of the embryo at the time of surface disinfection also influences morbidity and mortality. Kent et al. evaluated toxicity in the 5D line of wild-type zebrafish embryos at 6 and 24 h postfertilization (HPF), and found the younger embryos were more resistant to the toxic effects of chlorine.9 These two time points were selected because embryos are usually treated within a few hours of fertilization (6 HPF), but sometimes treatment occurs following shipment (24 HPF). It is generally recommended that embryos not be treated later than 28 HPF since the chorion has been partially degraded by the hatching enzymes at this time,10 increasing the likelihood that the developing embryos are exposed to the disinfectant.
Pronase (Pron) and sodium thiosulfate (NaThio) are commonly used with NaOCl in embryo surface disinfection protocols.11 Exposure of embryos to NaOCl is thought to harden the chorion making larval hatching more difficult, potentially decreasing hatching rates.6 To address this concern, Pron, an enzyme used to degrade proteins, is applied to the embryos after NaOCl treatment to assist with hatching.6,10 Residual NaOCl may result in unnecessarily prolonged chlorine exposure. Sodium thiosulfate, a sulfuric acid derivative, can be used to neutralize residual NaOCl that remains on the chorion after surface disinfection.12 Although both additives are frequently included in surface disinfection protocols, their benefits have not been scientifically evaluated.
Following surface disinfection, embryos are incubated in a small container for several days. Methylene blue (MethB) is commonly added to the embryo water media (EM) to suppress bacterial and fungal growth. Methylene blue was shown to be neuroprotective in the zebrafish ALS model due to suppression of mTDP-43 and mFUS—two DNA/RNA-binding proteins that can be found in protein aggregates associated with neurodegenerative diseases. Exposure to MethB corrected motor deficits and reduced the level of oxidative stress associated with the expression of those mutant proteins.13
Methylene blue has also been shown to be a photosensitizer promoting reactive oxygen species generation, which leads to single-strand DNA breaks and base oxidation.14 These properties have led to its use as a biocide in aquaculture. However, an in vitro study by Costa et al. demonstrated sensitivity of zebrafish hepatocytes to the photodynamic action of MethB through DNA damage and induction of apoptosis.14 Methylene blue also has documented teratogenic effects. For example, exposure resulted in swim bladder inflation failure in 4-day posthatching angelfish larvae and axial skeleton and neural tube defects in mouse fetuses.15,16 Zebrafish embryos may have an increased risk of developing morphological defects when exposed to this agent during critical developmental stages, although this potential has not been evaluated.
Most evaluations of toxicity and embryo viability following embryo surface disinfection have been conducted with embryos from wild-type lines, such as AB, TU, or 5D.9,17 The influence of fish strain on the incidence of embryo malformations or survival following surface disinfection is unknown.
This study evaluated whether the use of Pron, NaThio, and MethB, during surface disinfection and embryo incubation, affected embryo development (based on presence of malformations) or survival. Embryos were evaluated at two different ages using two concentrations of NaOCl. We hypothesized that hatching and survivability would be increased in groups exposed to Pron, while morphological defects would be decreased in groups exposed to NaThio compared with the groups exposed to NaOCl alone. Moreover, MethB would have no or minimal impact on the variables evaluated. These evaluations were conducted in the commonly used AB wild-type line and the casper (nacre–/–, roy–/–) mutant. Abbreviations are detailed in Table 1.
Table 1.
List of Abbreviations
| Abbreviations | |
|---|---|
| DPF | Days postfertilization |
| EM | Embryo medium |
| High NaOCl | 100 parts per million sodium hypochlorite |
| HPF | Hours postfertilization |
| Low NaOCl | 50 parts per million sodium hypochlorite |
| MethB | Methylene blue |
| MethB+EM | Methylene blue with embryo medium |
| MSK | Memorial Sloan Kettering Cancer Center |
| NaOCl | Sodium hypochlorite |
| NaThio | Sodium thiosulfate |
| Pron | Pronase |
| WCM | Weill Cornell Medicine |
Materials and Methods
Experimental design
AB or casper embryos (n = 100 embryos per group, 2 replicates of 50 embryos) were assigned to control (n = 2) or treatment (n = 16) groups. Treatment groups were surface disinfected at either 6 or 24 HPF with either a 50 ppm (low NaOCl) or 100 ppm (high NaOCl) sodium hypochlorite solution. Subsequently, embryos were disinfected with either NaThio, Pron, or NaThio+Pron solutions. Embryos were exposed to either EM or MethB+EM during the disinfection protocol's loading rinse and after disinfection. The loading rinse is an initial rinse to remove organic debris before NaOCl exposure. Control groups were rinsed with EM or MethB+EM after collection and incubated in the same medium; they were not subject to surface disinfection. A summary of the groups is provided in Table 2.
Table 2.
Both AB and casper Embryos Were Randomly Assigned to 16 Experimental and 2 Control Surface Disinfection Protocol Groups (n = 100 Embryos, 2 Replicates of 50 Embryos)
Groups 17 and 18 were controls. Embryos from each group were treated at 6 or 24 h postfertilization.
All surface disinfection experiments with the AB fish line in MethB+EM were conducted at the Memorial Sloan Kettering Cancer Center (MSK) and all surface disinfection experiments with the AB fish line in EM without MethB were conducted at Weill Cornell Medicine (WCM). All experiments with casper fish were conducted at MSK.
At 5 days postfertilization (DPF), embryos and free-swimming larvae were assessed for survival, hatching success, and morphological defects using a dissecting microscope (Leica KL 200 LED, Wetzlar, Germany). Survival was expressed as a percentage determined by the number of live embryos/larvae (as determined by a heartbeat) divided by the total number of embryos present at the start of the study. Hatching was defined as a percentage determined by the number of larvae that emerged from their chorion (either partially or whole) divided by the total number of embryos present at the start of the study. Embryos/larvae were deemed to have morphological defects when edema (pericardial, yolk sac, ocular, and/or generalized), spinal deformities (bent spine and/or truncated tail), and/or eye malformations were seen. Following assessment, embryos were euthanized using a dilute NaOCl solution (1 part 8.25% NaOCl [Clorox Concentrated Germicidal Bleach, Oakland, CA, USA] to 5 parts housing system water [pH 7.2–7.8]). Embryos remained in this solution for at least 5 min to ensure death.
Solution preparation
Unbuffered NaOCl soak solutions were prepared at either a 50 or 100 ppm concentration by adding 1–1.15 mL or 2–2.25 mL Reagent grade 5% NaOCl (Acros Organics™; Fisher Scientific, Hampton, NH, USA), respectively, to 1.0 L reverse osmosis (RO) water (experiments conducted at WCM) or RO/deionized (RO/DI) water (experiments conducted at MSK).9,11 The chlorine concentration was verified using a portable chlorine meter (Extech CL200, Camarillo, CA, USA) and chlorine test strips (LaMotte, Chestertown, MD, USA).
The 500 ppm NaThio soak solution was prepared by dissolving 500 mg of NaThio pentahydrate (Fisher Scientific, Hampton, NH, USA) in 1.0 L of RO or RO/DI water. The 40.8 ppm Pron soak solution was prepared by adding 0.68 mL of the Pron stock (concentrated—30 mg/mL) solution to 500 mL of RO or RO/DI water. The Pron stock solution was created by dissolving 30 mg of Pron E (Protease from Streptomyces griseus, P6911-1G; Sigma-Aldrich®, Saint Louis, MO, USA) in 1.0 mL of RO or RO/DI water. All solutions were made fresh daily before the experiment. The various solutions were placed in 6-oz. glass bowls (Anchor Hocking®, Libertyville, IL, USA), except the loading and rinse solutions were placed in 16-oz. glass bowls (Crisa Moderno; Libbey®, Toledo, OH, USA).
Surface sanitation procedures
Embryo disinfection was carried out by placing 50 embryos in a 3″ stainless steel strainer (OXO, New York, NY, USA). According to the assigned group, the strainer was transferred through the various solutions as follows: (1) Loading solution (containing either EM [pH 6.30–7.02] or MethB+EM [pH 6.38–6.64]) for 2 min. Mechanical rinsing of the embryos before surface disinfection was performed by continuously swirling the strainer in the solution. (2) Sodium hypochlorite solution (low NaOCl [pH 8.09–8.40] or high NaOCl [pH 8.45–8.84]) for 10 min. The strainer was swirled for 5 s every minute to ensure the chorion had adequate exposure to the disinfectant solution. (3) The embryos were swirled continuously in the rinse solution (RO [pH 6.5–6.8] or RO/DI [pH 6.64–6.79]) water for 5 min to rinse off any NaOCl residue. (4) Sodium thiosulfate solution (for applicable groups). The strainer was swirled continuously for 5 min. (5) Pron solution (for applicable groups). The strainer was swirled continuously for 1 min. (6) Rinse solution (RO or RO/DI water). The strainer was swirled continuously for 5 min.
Upon completion of the assigned disinfection protocol, the embryos were visually inspected for viability under a dissecting microscope and live embryos were counted and each embryo was placed into one well in a sterile untreated 96-well culture plate (Denville®, Holliston, MA, USA) filled with 100 microliter EM or MethB+EM, and incubated at 28°C for 5 days.
Fish
Embryos were obtained from AB and casper zebrafish crosses (mixed sex; 6–11 months of age) maintained at the core zebrafish facilities at MSK and WCM. Colony health surveillance monitoring is conducted quarterly on sentinel fish (6–15 months of age) housed in tanks supplied with untreated system water and every 7–8 months on sentinel fish (7–16 months of age) receiving treated system water that are housed with the remainder of the colony. Additionally, sick and aged fish are periodically evaluated.
Skin scrape, gill and fin clip, aerobic and anaerobic renal bacterial culture, and histopathology are performed on untreated water sentinels collected following 6, 9, and 12 months of exposure and on treated water sentinels collected following 7–8 months of exposure (10 fish per time point, per holding system). Half of the untreated and treated water sentinels collected after 15–16 months of exposure are processed as described above, and half (5 fish per group) are pooled and evaluated by polymerase chain reaction for Edwardsiella ictaluri, Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium haemophilum, Mycobacterium marinum, Mycobacterium peregrinum, Mycobacterium spp., Myxidium streisingeri, Pleistophora hyphessobryconis, Pseudocapillaria tomentosa, and P. neurophilia.
Both colonies are endemically infected with P. neurophilia, with low levels of M. chelonae isolated from fish housed at MSK. A variety of aquatic opportunistic bacteria (e.g., Shewanella putrefacians, Plesiomonas shigelloides, and Aeromonas spp.) are occasionally isolated from fish from both facilities. All fish evaluated were free of E. ictaluri, M. abscessus, M. fortuitum, M. haemophilum, M. marinum, M. peregrinum, M. streisingeri, external parasites, P. tomentosa and other internal parasites, and P. hyphessobryconis.
Adult zebrafish were housed at a density of 5–10 fish/L in mixed-sex groups in 2.8 or 6.0 L tanks on a recirculating aquatic housing system (Aquaneering, Inc.) with 10%–15% system water exchange/day. Full-spectrum light (64.2–68.1 foot candles at 1 m above the floor) was provided with a 14-h light/10-h dark photoperiod.
The MSK system is supplied with calcite-filtered RO/DI water and the WCM system is supplied with RO water. Recirculated water was mechanically filtered by passage through either 25-μm bag filters (MSK) or a mechanical backwashing sand filter (WCM) for mechanical filtration, chemically filtered by carbon, biologically filtered by a fluidized bed biofilter, and exposed to UV sterilizers providing a minimum of 100,000 μW/cm2/s. Water on both systems was maintained at 27°C–29°C, conductivity 700–900 μS, pH 7.2–7.8, ammonia <0.2 ppm, nitrite <0.1 ppm, and nitrate <30 ppm.
Zebrafish larvae were fed an irradiated commercial pelleted diet (Larval AP100 < 50 μM; Zeigler Brothers, Gardners, PA) 1.75 g three times daily starting at ∼5 DPF. At 10 DPF, the frequency of the pelleted food diet decreased to twice daily and newly hatched Artemia nauplii 1.0 mL were given three times daily. At 14 DPF, larvae were fed a larger irradiated commercial pelleted diet (Larval AP100 100–150 μM; Zeigler Brothers, Gardens, PA, USA) 1.75 g twice daily along with the newly hatched A. nauplii 1.0 mL three times daily. At 25 DPF, fish were fed an irradiated adult commercial pelleted diet (Adult Zebrafish Complete Diet; Zeigler Brother, Gardens, PA, USA) 3.5 g twice daily at MSK, whereas fish at WCM were fed an irradiated adult commercial pelleted diet (Adult Zebrafish Complete Diet; Zeigler Brother) 3.5 g once daily and newly hatched A. nauplii 4.0 mL twice daily.
All animal care and experimental procedures were reviewed and approved by the MSK Institutional Animal Care and Use Committee (IACUC) and were performed in AAALAC-accredited facilities in compliance with the Guide for the Care and Use of Laboratory Animals.18
Embryo production and housing
The afternoon before spawning (14:00–16:00), a minimum of five 1.0-L breeding tanks per strain (Model #ZHCT100; Aquaneering, Inc., San Diego, CA, USA) were prepared using system water and dividers to separate fish by sex. Either AB or casper adult zebrafish from the same housing tank were randomly selected and placed in the various breeding tanks as trios (1–2 M:1–2 F), separated by sex and left overnight. In the morning, the dividers were removed and the tanks tilted at an ∼45° angle immediately after the initiation of the light phase (08:30). Fish were allowed to spawn for 2 h, after which they were returned to their home tank and viable embryos from each tank were collected with a 3″ nylon mesh strainer (HIC Harold Import Company, Lakewood, NJ, USA).
Embryos collected from each breeding tank containing one to two clutches, based on the number of females that released eggs in the tank, were divided among 60 mm × 15 mm Petri dishes as needed to achieve a density of 150 live embryos per dish with EM (15.8 ppm sea salt) (Instant Ocean® Aquarium Sea Salt Mixture; Instant Ocean Spectrum Brands, Blacksburg, VA) in RO (experiments conducted at WCM) or RO/DI water (experiments conducted at MSK) with or without 0.15 ppm MethB. At 6 or 24 HPF, embryo viability in each dish was assessed and surviving embryos were assigned to experimental groups of 50 live embryos. Replicates were conducted using embryos collected from different breeding tanks.
Statistics
Within each disinfection age and methylene blue (MethB) exposure group, groups of 100 live embryos (2 replicates of 50) that were exposed to different NaOCl concentrations, Pron, and/or NaThio were compared with their respective control to determine whether surface disinfection impacted hatching and survival, and/or caused morphological defects. Additionally, within each disinfection age and NaOCl concentration group, experimental groups were compared with their respective NaOCl-only group in standard EM (low NaOCl + EM or high NaOCl + EM) to evaluate the impact of these additives alone or in combination on survivability, the hatching rate, and the morphological defect rate on the disinfected embryos.
Survival, hatching, and morphological defect rates were compared between groups using a Fisher's exact test. p-Values were adjusted for multiple comparisons using the method of Benjamini and Hochberg.19 p-values could not be calculated when the group rates were both 0% or 100%. p-Values ≤0.05 were considered statistically significant. All calculations were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Effects of different surface disinfection protocols on AB embryos
All experimental groups of AB embryos disinfected with low NaOCl at 6 HPF in EM alone had significantly reduced survivability and hatching rates when compared with EM controls, irrespective of exposure to Pron and/or NaThio (p < 0.0001; Fig. 1A); however, the AB 6 HPF embryos exposed to MethB+EM and low NaOCl had significantly reduced hatching rates only when compared with the MethB+EM controls (p < 0.0001; Fig. 1B). The morphological defect rates were not significantly different between controls and the various experimental groups.
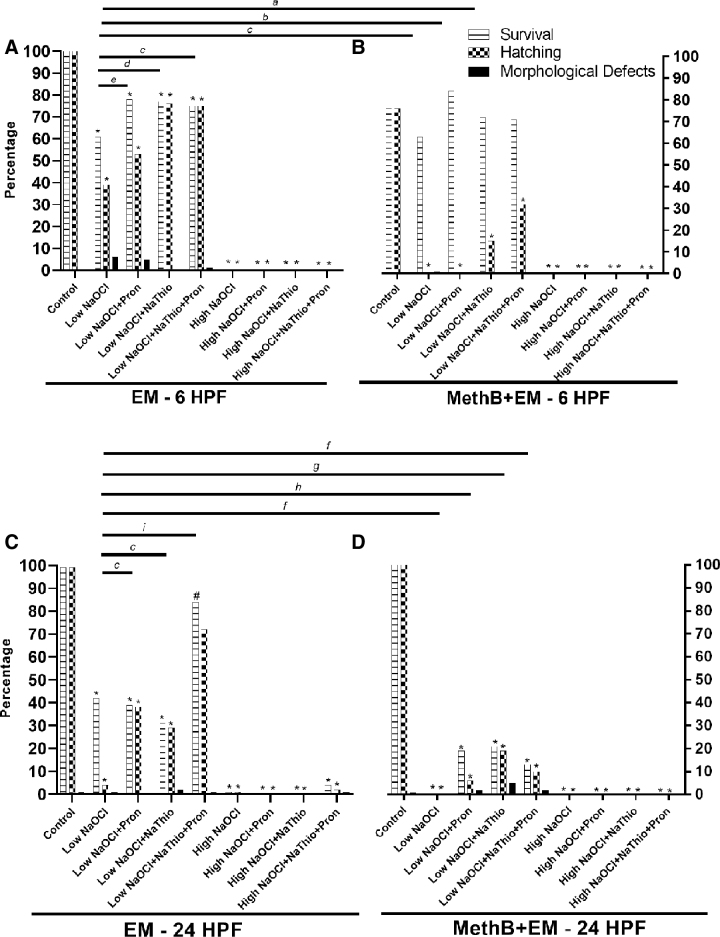
FIG. 1.
Survival, hatching, and morphological defect rates for AB embryos exposed to low NaOCl or high NaOCl surface disinfection protocols. (A) Data from 6 HPF AB embryos exposed to EM alone during surface disinfection. (B) Data from 6 HPF AB embryos exposed to MethB+EM during surface disinfection. (C) Data from 24 HPF AB embryos exposed to EM alone during surface disinfection. (D) Data from 24 HPF AB embryos exposed to MethB+EM surface disinfection. Statistical significance of survival or hatching when experimental groups are compared with control groups are indicated by * and #. Groups with and without MethB were evaluated separately (*p < 0.0001 and #p = 0.004, Fisher's exact test). a, b, c, d, e, f, g, h, and i with bars denotes statistical significance when low NaOCl group in EM is compared with experimental groups with and without MethB (a: p = 0.0005 for hatching, b: p = 0.001 for survival and p < 0.0001 for hatching, c: p < 0.0001 for hatching, d: p = 0.04 for survival and p < 0.0001 for hatching, e: p = 0.03 for survival, f: p < 0.0001 for survival, g: p = 0.003 for hatching and p = 0.0049 for survival, h: p = 0.0015 for survival, and i: p < 0.0001 for survival and hatching, Fisher's exact test). EM, embryo medium; high NaOCl, 100 ppm sodium hypochlorite; HPF, hours postfertilization; low NaOCl, 50 ppm sodium hypochlorite; MethB, methylene blue; MethB+EM, methylene blue with embryo medium; NaThio, sodium thiosulfate; Pron, pronase.
The experimental groups that showed the highest survival and hatching rates with the lowest morphological defect rates were the low NaOCl+NaThio+Pron and low NaOCl+NaThio groups in EM alone. Embryos exposed to low NaOCl+NaThio+Pron had a significantly higher hatching rate (75%) compared with that of low NaOCl alone (39%); and embryos exposed to low NaOCl+NaThio had a significantly higher hatching rate (76%) and survival (77%) compared with that of low NaOCl alone (39% and 61%, respectively; Fig. 1A).
All experimental groups of AB embryos disinfected at 6 HPF with high NaOCl and housed in EM or MethB+EM had significantly reduced survivability and hatching rates compared with their respective controls (p < 0.0001; Fig. 1A, B). None of the embryos survived to 5 DPF when exposed to high NaOCl, irrespective of exposure to MethB, Pron, and/or NaThio.
All experimental groups of AB embryos disinfected at 24 HPF with either low NaOCl or high NaOCl and housed in EM or MethB+EM had significantly reduced survivability and hatching rates as compared with their respective controls (survivability rate p = 0.004 for low NaoCl+NaThio+Pron in EM group and p < 0.0001 for all other groups; Fig. 1C, D). The morphological defect rates were not significantly different between controls and the respective experimental groups.
The experimental group that showed the highest survival and hatching rates with the lowest morphological defect rates was the low NaOCl+NaThio+Pron group in EM. Embryos exposed to low NaOCl+NaThio+Pron had a significantly higher hatching rate (72%) and survival (84%) compared with that of low NaOCl alone (4% and 42%, respectively; Fig. 1C). A few embryos exposed to high NaOCl alone and high NaOCl+NaThio+Pron in EM survived to 5 DPF, but differences in survival, hatching rate, and morphological defect rate between the two groups were not statistically significant.
Effects of different surface disinfection protocols on casper embryos
All experimental groups of casper embryos disinfected at 6 HPF with low NaOCl or high NaOCl and housed in EM or MethB+EM had significantly reduced survivability and hatching rates compared with their respective controls, irrespective of exposure to Pron and/or NaThio (p < 0.0001; Fig. 2A, B). The morphological defect rates were not significantly different between controls and experimental groups. The experimental group that showed the highest survival and hatching rates with the lowest morphological defect rate was the low NaOCl+NaThio+Pron group in EM. Embryos exposed to low NaOCl+NaThio+Pron had a significantly higher hatching rate (66%) compared with that of low NaOCl alone (12%; Fig. 2A). None of the embryos survived to 5 DPF when exposed to high NaOCl, irrespective of exposure to MethB, Pron, and/or NaThio.
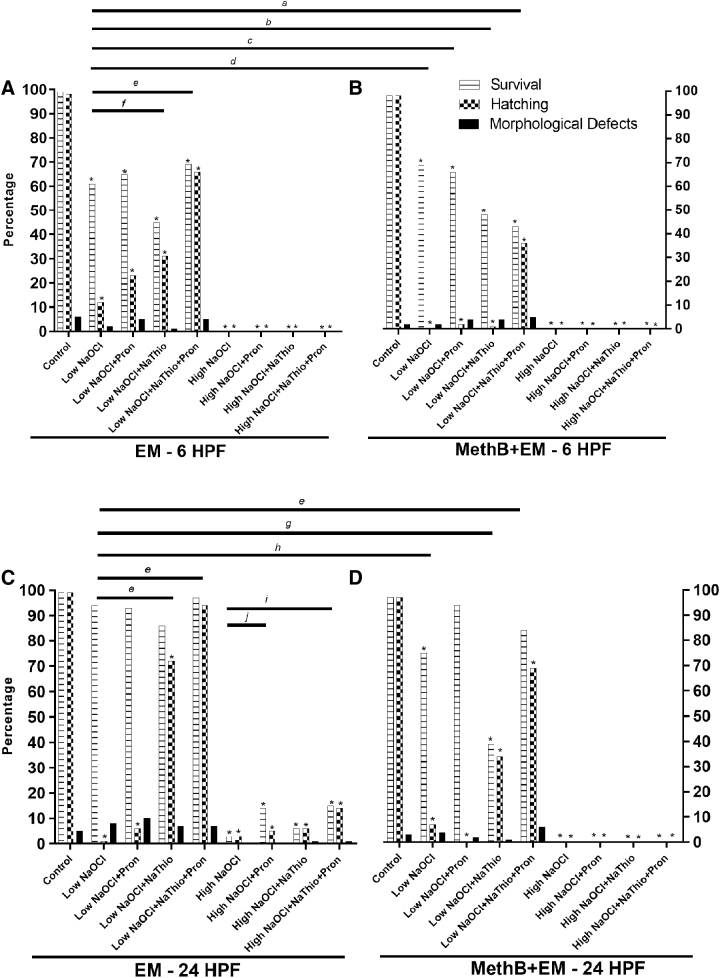
FIG. 2.
Survival, hatching, and morphological defect rates for casper embryos exposed to low NaOCl or high NaOCl surface disinfection protocols. (A) Data from 6 HPF casper embryos exposed to EM alone during surface disinfection. (B) Data from 6 HPF casper embryos exposed to MethB+EM during surface disinfection. (C) Data from 24 HPF casper embryos exposed to EM alone during surface disinfection. (D) Data from 24 HPF casper embryos exposed to MethB+EM during surface disinfection. Statistical significance of survival or hatching when experimental groups are compared with control groups are indicated by *. Groups with and without MethB were evaluated separately (*p < 0.0001, Fisher's exact test). a, b, c, d, e, f, g, and h with bars denotes statistical significance when low NaOCl in EM alone is compared with experimental groups with and without MethB (a: p = 0.03 for survival and p = 0.0003 hatching, b: p = 0.0056 for hatching, c: p = 0.02 for hatching, d: p = 0.0008 for hatching, e: p < 0.0001 for hatching, f: p = 0.004 for hatching, g: p = 0.0007 for survival and p < 0.0001 for hatching, and h: p < 0.0001 for survival, Fisher's exact test). i and j with bars denotes statistical significance when high NaOCl in EM alone is compared with experimental groups with and without MethB (i: p = 0.01 for survival and p = 0.02 for hatching, and j: p = 0.02 for survival, Fisher's exact test).
Casper embryos exposed to low NaOCl, low NaOCl+Pron, and low NaOCl+NaThio in EM at 24 HPF had significantly reduced hatching rates (1%, 6%, and 72%, respectively) compared with EM controls (99%; p < 0.0001; Fig. 2C). Survivability of embryos exposed to low NaOCl in EM, irrespective of exposure to Pron and/or NaThio, was not significantly different when compared with controls. Embryos exposed to low NaOCl and low NaOCl+NaThio in MethB+EM had significantly reduced survivals (75% and 39%, respectively) and hatching rates (7% and 34%, respectively) compared with MethB+EM controls (97% for both survival and hatching rate; p < 0.0001; Fig. 2D). Additionally, embryos exposed to low NaOCl+Pron and low NaOCl+NaThio+Pron in MethB+EM had significantly lower hatching rates (0% and 69%, respectively) compared with MethB+EM controls (34%; p < 0.0001; Fig. 2D). The morphological defect rates were not significantly different between controls and their respective experimental groups.
The experimental group that showed the highest survival and hatching rates with the lowest morphological defect rates was the low NaOCl+NaThio+Pron group in EM. Embryos exposed to low NaOCl+NaThio+Pron had a significantly higher hatching rate (94%) compared with that of low NaOCl alone (1%; Fig. 2C).
All experimental groups of casper embryos disinfected at 24 HPF with high NaOCl and housed in EM or MethB+EM had significantly reduced survivability and hatching rates compared with controls irrespective of exposure to Pron and/or NaThio (p < 0.0001; Fig. 2C, D). A few embryos exposed to high NaOCl alone or in combination with Pron and NaThio in EM survived to 5 DPF. Embryos exposed to high NaOCl+NaThio+Pron had a significantly higher hatching rate (14%) and survival (15%) compared with that of high NaOCl alone (3% for both hatching rate and survival; Fig. 2C). None of the embryos survived to 5 DPF when exposed to high NaOCl in MethB, irrespective of exposure Pron and/or NaThio.
Strain comparison
To evaluate for potential strain variability, results for AB and casper strains were compared within each age, NaOCl concentration, and MethB+EM exposure group (Fig. 3). AB embryos disinfected at 6 HPF and housed in EM had significantly higher hatching rates (39%, 53%, and 76%, respectively) than the casper embryos (12%, 23%, and 31%, respectively) when exposed to low NaOCl, low NaOCl+Pron, and low NaOCl+NaThio (p < 0.001 for low NaOCl and low NaOCl+NaThio, p = 0.0001 for LB+P; Fig. 3A). AB embryos also had significantly higher survival (77%) than the casper embryos (45%) when exposed to low NaOCl+NaThio in EM (p < 0.0001; Fig. 3A).
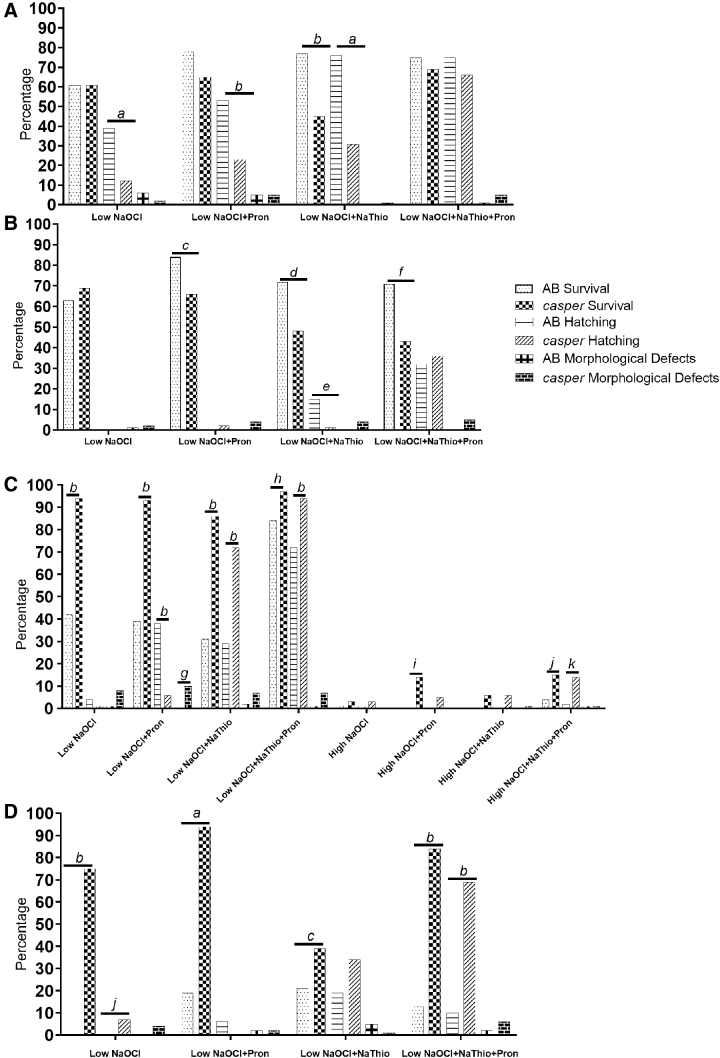
FIG. 3.
Percentage of embryo survival, hatching, and morphological defects for AB and casper embryos exposed to low NaOCl or high NaOCl surface disinfection protocols. (A) Data from 6 HPF embryos exposed to EM alone during surface disinfection. (B) Data from 6 HPF embryos exposed to MethB+EM during surface disinfection. (C) Data from 24 HPF embryos exposed to EM alone during surface disinfection. (D) Data from 24 HPF embryos exposed to MethB+EM during surface disinfection. a, b, c, d, e, f, g, h, i, j, and k denote statistical significance between the respective experimental groups and the two strains (a: p < 0.001, b: p ≤ 0.0001, c: p = 0.01, d: p = 0.002, e: p = 0.0008, f: p = 0.0003, g: p = 0.003, h: p = 0.006, i: p = 0.0002, j: p = 0.03 and k: p = 0.0067, Fisher's exact test).
AB embryos disinfected at 6 HPF and housed in MethB+EM had significantly higher survival (84%, 72%, and 71%, respectively) than the casper embryos (66%, 48%, and 43%, respectively) when exposed to low NaOCl+Pron, low NaOCl+NaThio, and low NaOCl+NaThio+Pron, respectively (p = 0.01, p = 0.002, and p = 0.0003, respectively; Fig. 3B). Lastly, AB embryos exposed to low NaOCl+NaThio in MethB+EM had a significantly higher hatching rate (15%) than the respective casper embryos (1%; p = 0.0008; Fig. 3B). The rest of the survival, hatching rate, and morphological defect rate comparisons did not differ significantly between the strains.
When evaluating disinfection performed at 24 HPF, higher survivals and hatching rates were seen in the casper groups. Casper embryos housed in EM alone had significantly higher survival (94%, 93%, 86%, and 97%, respectively) than AB embryos (42%, 39%, 31%, and 84%, respectively) when exposed to low NaOCl, low NaOCl+Pron, low NaOCl+NaThio, and low NaOCl+NaThio+Pron (p < 0.0001 for low NaOCl, low NaOCl+Pron, and low NaOCl+NaThio and p = 0.006 for low NaOCl+NaThio+Pron; Fig. 3C). The hatching rate for casper embryos (6%), however, was significantly less than the AB embryo hatching rate (38%) when exposed to low NaOCl+Pron in EM (p < 0.0001; Fig. 3C), but was significantly higher (72% and 94%, respectively) than the AB hatching rates (29% and 72%, respectively) when exposed to low NaOCl+NaThio and low NaOCl+NaThio+Pron in EM (p < 0.0001 and p = 0.0001 respectively; Fig. 3C). The casper embryos also had a higher morphological defect rate (10%) than the AB embryos (0%) when exposed to low NaOCl+Pron in EM (p = 0.003; Fig. 3C).
The casper embryos had significantly higher survival rates when compared with the AB embryos for all MethB+EM groups. Casper embryos exposed to low NaOCl in MethB+EM had significantly higher survival and hatching rate (75% and 7%, respectively) than the AB embryos (0% for both survival and hatching rate; p < 0.0001 and p = 0.03, respectively; Fig. 3D). Additionally, casper embryos housed in MethB+EM had significantly higher survival (94%, 39%, and 84%, respectively) than the AB embryos (19%, 21%, and 13%, respectively) when exposed to low NaOCl+Pron, low NaOCl+NaThio, and low NaOCl+NaThio+Pron, respectively (p < 0.001, p = 0.01, and p < 0.0001, respectively; Fig. 3D). The hatching rate for casper embryos in MethB+EM (69%) was significantly higher than the AB embryo hatching rate (10%) when exposed to low NaOCl+NaThio+Pron (p < 0.0001; Fig. 3D).
Casper embryos exposed to high NaOCl in EM alone had significantly higher survival rates (14% and 15%, respectively) than the AB embryos (0% and 4% respectively) when exposed to high NaOCl+Pron and high NaOCl+NaThio+Pron, respectively (p = 0.0002 for high NaOCl+Pron and p = 0.03 for high NaOCl+NaThio+Pron; Fig. 3D). The rest of the survival, hatching rate, and morphological defect rate comparisons were not significantly different between the strains.
Effect of methylene blue on survival of control groups
Within each strain and age group, the survivability of control groups incubated with MethB+EM was compared with control groups incubated with EM alone. At 6 HPF, survival of AB embryos in MethB+EM was significantly lower than AB embryos in EM alone (76% vs. 100%; p < 0.0001, Fisher's test). The rest of the survival comparisons were not significantly different.
Discussion
This study demonstrated three key findings regarding the use of supplemental agents in NaOCl embryo surface disinfection protocols: (1) exposure to both Pron and NaThio during surface disinfection generally resulted in the lowest toxicity based on the variables measured; (2) MethB has the potential to impact the survival and/or hatching rates of zebrafish embryos; and, (3) there are strain differences in susceptibility to NaOCl toxicity.
Pron, a proteolytic enzyme, functions to soften or remove the chorion (depending on the concentration used) by fragmenting the chorion's extracellular matrix.20 Pron has been utilized primarily to dechorionate embryos before conducting toxicological studies as it facilitates embryo hatching before 3 DPF so that embryos can be directly exposed to the chemicals and drugs being studied.21,22 It has also been included in surface disinfection protocols to assist with hatching after exposure to NaOCl.6,10,12 When Pron is used for dechorionation, high concentrations, ranging from 0.1 to 1 mg/mL, are used for extended periods (7–20 min depending on the age of the embryo and concentrations of Pron used).6,21,22 When utilized to assist with hatching 24 h following surface disinfection, lower concentrations (0.04 mg/mL) are used for shorter durations (1 min).12
Sodium thiosulfate is used to neutralize residual NaOCl.12,23 Higher concentrations (3.175 g/L) are utilized following disinfection of equipment, whereas lower concentrations (0.5 g/L) are used to treat embryos after disinfection.12,23 Hu et al. reported that zebrafish embryo development was severely retarded and accompanied by multiorgan malformations when embryos were exposed to 0.1–1 mol/L NaThio for 48–72 h starting at 4 HPF; additionally, embryos exposed to 10 μmol/L–10 mmol/L NaThio had circulatory, nervous, and maxillofacial malformations.24 Sodium thiosulfate causes malformations by interfering with the cytoskeletal structure and inhibiting cell proliferation.24 Although the concentrations of NaThio typically used during embryo surface disinfection protocols (10 mmol/L) are within range that has been shown to lead to malformations, exposure times are significantly shorter (5 min compared with 48–72 h).
We utilized Pron and NaThio concentration exposure times specified in the Zebrafish International Resource Center Surface Disinfection Protocol.11 We hypothesized that hatching and survival would increase in groups exposed to Pron and morphological defects would be decreased in groups exposed to NaThio. When used with low NaOCl without NaThio, Pron facilitated embryo hatching by 5 DPF when EM was used, but were lower when embryos were exposed to MethB+EM. Embryo survival was similar when exposed to EM or MethB+EM, but was often statistically less when compared with their respective controls that were not disinfected with NaOCl. Morphological defects were infrequent in all experimental groups, irrespective of NaThio use, and were not different compared with their respective controls.
When AB and casper embryos were exposed to NaThio and Pron together in low NaOCl and EM, higher hatching and survival rates were seen as compared with the low NaOCl-only group; additionally, hatching rates trended higher when embryos were exposed to NaThio and Pron together as compared with the low NaOCl+Pron groups. Pron and NaThio may act synergistically to reduce embryo contact with NaOCl thus improving survival and hatching.
Although Pron is stable at a pH range of 3.0–9.0, it has optimal activity at pH 7.0–8.0.25 The pH of low NaOCl and high NaOCl is ∼8.25 and ∼8.65, respectively, thus the pH of the dilute NaOCl concentrations may not have been ideal for Pron's optimal activity. The addition of an NaThio soak before Pron exposure would neutralize residual NaOCl on the chorion, allowing the Pron to work more effectively. Future experiments should explore the relationship between Pron and NaThio to verify whether pH contributed to the lower hatching rates observed in the Pron-only groups.
The embryos were first exposed to NaThio and then Pron as described by the Zebrafish International Resource Center.11 This study did not randomize the order of the chemical solution exposure, nor analyze the impact of the order on embryo survival, hatching rate, or morphological defect rate. It is possible that results would differ from what was reported if embryos were first exposed to Pron and then NaThio. Future studies should analyze the order of exposure to determine whether survival and hatching is impacted by which chemical solution is used first in the surface disinfection process.
Across all experimental strain and age groups that underwent surface disinfection, embryos exposed to MethB+EM disinfected at 6 HPF trended lower in terms of hatching rates, whereas embryos exposed to MethB+EM and disinfected at 24 HPF trended lower in terms of survival and hatching rates when compared with the low NaOCl-only group. In addition, 6 HPF AB controls incubated with MethB+EM showed significantly lower survival than AB embryos incubated with EM alone. Although it could be postulated that the lower survival seen in the controls incubated with MethB+EM was due to clutch-specific sensitivity, similar results were seen in both replicates with embryos from different clutches.
Methylene blue, an inhibitor of nitric oxide synthase and guanylate cyclase, is commonly added to embryo medium due to its biocidal properties that can prevent fungal growth during incubation.26,27 While a concentration of 0.5 mg/L MethB in EM is recommended,11 0.15 mg/L was used in this study as this concentration has been historically used at our institution. Recent studies have shown that MethB fluoresces at ∼700 nm, which provides a mechanism to detect distribution of MethB within tissues.28 Methylene blue can potentially interfere with fluorescent imaging in larval zebrafish incubated in MethB-containing solutions.
Developmental defects have been described in embryos and/or fetuses of other species exposed to MethB.15,16 Developing angelfish larvae exposed to 0.5, 2.5, and 5 mg/L MethB upon hatching displayed unexpanded swim bladders, with significantly increased numbers reported in larvae exposed to 5 mg/L.15 The authors hypothesized that interference with the blood supply and oxygen-carrying capacity of the blood to the swim bladders, which resulted from methemoglobin formation and vasoconstriction caused by MethB, led to the swim bladder defect.29 Subcutaneous injections of MethB at doses ranging from 35 to 70 mg/kg to pregnant mice on gestation day 8 led to dose-dependent embryo lethality and axial skeleton and neural tube defects.16
It is unclear whether MethB has teratogenic effects on developing zebrafish embryos. Although increased DNA damage and cell death was noted in vitro in a zebrafish hepatocyte cell line 6–12 h after 5.6 or 38 μg/L MethB exposure,14 it remains unknown if MethB effects developing zebrafish embryos in vivo during incubation and/or surface disinfection. Exposure to both NaOCl and MethB may increase chorion hardness and/or developmental abnormalities leading to mortality and poor hatching rates. Additional studies are needed to better understand the combined interactions of these agents on the developing embryo.
Historically, zebrafish embryo disinfection protocols have utilized lower concentrations of NaOCl (25–50 ppm) to minimize pathogen introduction to colonies.5,6 Newer embryo disinfection protocols and recent studies use higher concentrations of NaOCl (∼100 ppm) since lower concentrations were not effective at eliminating zebrafish pathogens or preventing their vertical transmission.7,8 The effectiveness of NaOCl depends on two factors: (1) the concentration of available chlorine; and, (2) the pH of the solution.30
Hypochlorous acid (HOCI) is the active germicide in NaOCl solutions. Higher concentrations of NaOCl increase its germicidal effectiveness due to the increased presence of chlorine, which increases the concentration of HOCI. HOCI is able to penetrate the lipid bilayer of cellular plasma membranes, causing damage to the membrane, inhibiting enzymes essential for growth, and damaging DNA.30 While beneficial in eradicating unwanted pathogens, it can have detrimental effects on the host as was observed in embryos treated with high NaOCl in this study.9
This study also demonstrated that embryo susceptibility to NaOCl toxicity varied by both strain and age of disinfection. Two disinfection time points were chosen because embryos are usually treated within a few HPF (6 HPF), but sometimes treatment occurs following shipment of embryos (24 HPF). AB embryos had lower survival and hatching rates when surface disinfected at 24 HPF as compared with casper embryos. Kent et al. similarly reported that AB embryos were more sensitive to NaOCl as compared with 5D embryos.9 AB embryos, however, had improved survival and hatching rates when disinfected at 6 HPF. It is unclear why strains may differ in sensitivity. It may reflect differences in chorion permeability. Future studies should explore whether the chorion structure differs between strains or whether the concentration of hatching enzymes in the hatching gland on the embryo's pericardial membrane differs altering chorionic permeability.
All surface disinfection experiments with the AB fish line in MethB+EM were conducted at MSK with RO/DI water, while all surface disinfection experiments with the AB fish line in EM were conducted at WCM with RO water. Additionally, all experiments with casper fish were conducted at MSK with RO/DI water. Some experiments with the AB fish were conducted at WCM due to limited fish availability at MSK. Consequently, variables may have been inadvertently introduced to the AB studies since the two facilities had different parent fish, water purification systems, and husbandry practices. The AB embryos that were exposed to MethB+EM and EM originated from the same breeding colony, but were housed in two different facilities for more than one generation. Consequently, genetic drift may have resulted in the creation of two different strains of AB fish. The introduction of point mutations and/or varying environmental factors could also impact the overall fitness of the fish. Depending on the amount of inbreeding and degree of genetic monitoring performed, laboratories should be cognizant of the potential for intrastrain variability.
RO or RO/DI water was used to rinse the embryos and prepare chemical solutions in this study. Either RO or RO/DI was used based on its availability in the respective animal housing facility (RO was used at WCM and RO/DI was used at MSK). When surface disinfecting embryos with NaOCl, various aqueous solutions can be used for chemical baths, disinfection solutions, and rinsing, including embryo medium, Milli-Q® water, or RO water.11,17 RO water was used in this study since RO was utilized in a comparable surface disinfection experiment that examined chlorine toxicity in zebrafish embryos.9 One potential concern with using RO or RO/DI water is its low ion content and pH. RO water is produced by using a high pressure pump to force water across a semipermeable RO membrane, removing 95%–99% of dissolved salts.31 RO filtration effectively removes salts, heavy metals, and other particles. Deionization utilizes resin filters to exchange positive hydrogen and negative hydroxyl molecules for positive and negative contaminants present in the water.5 DI water is free from minerals but still contains uncharged organized particles and microbes. Consequently, the conductivity of RO and RO/DI can differ due to the amount of charged particles still remaining in the solutions.
At our facilities, the RO water had a conductivity of 3.2 μS/cm and RO/DI water had a conductivity of 0.75 μS/cm. Since the conductivity values were similar in the RO and RO/DI water, it is unlikely that the conductivity of the two different water sources had a significant impact on the embryos. Additionally, zebrafish are tolerant of a wide range of salinities, tolerating ranges from 0.3 up to 2 g/L. Although long-term maintenance of fish at low salinities (<0.2 g/L) can negatively impact egg production,5 there was no impact on the development or hatching of zebrafish embryos exposed to 0‰ (0 ppt) salinity for 1–2 h.32 Zebrafish embryos in this study were only exposed to the RO or RO/DI water for a maximum of 28 min during surface disinfection, thus any impact caused by exposure to the low salinity should be minimal to nonexistent.
When exposed to air, RO and RO/DI water interacts with carbon dioxide to create carbonic acid, causing the pH to drop from 7.0 to ∼5.5. This pH drop may be problematic because zebrafish are typically reared in water with a pH between 7.0 and 8.0, although most freshwater fish can tolerate pH between 6.0 and 9.5.5 However, pH has only been shown to impact the development of zebrafish embryos when pH was extreme with prolonged exposure times.
In a study evaluating the effect of water pH on early developmental responses, embryos incubated until hatching (at 2–4 DPF) at pH between 2.0 and 12.0 cleaved and developed normally, although the hatching rate was slightly lower when the pH was 4, 5, and 10, whereas none of the embryos developed normally at pH of 2 and 12.33 Another study exposed zebrafish embryos to pH ranges of 3–12 for 96 h to evaluate the effects of pH fluctuations on survival, hatching success, developmental delays, and morphological abnormalities. Embryos exposed to pH below 3.5 or above 10.5 showed 100% mortality after 24 h, and an alkaline pH (range between 7 and 12) resulted in hatching delays.34 Embryo survival started to decrease when pH was less than 4 or greater than 10.
Given that the AB and casper embryos in our study were only exposed to the RO and RO/DI water for a maximum of 28 min (time dependent on disinfection protocol) and the starting pH of the water was close to 7, it is unlikely that the pH of the water bath impacted the survival of the embryos. Future studies should evaluate the impact of pH and conductivity during embryo surface disinfection to determine whether RO or RO/DI water baths impact survival when compared with embryo medium.
With each spawning, zebrafish pairs can produce clutches that exhibit variations in survival, growth, hatching, numbers, and phenotype expression. Additionally, developmental differences can often be seen in embryos within the same clutch; however, variability is often higher among embryos from different clutches than within a single clutch.35 Consequently, the toxicity of NaOCl and the chemical additives can greatly vary across clutches, with some embryos exhibiting higher survival and fitness than others.
To minimize the impact of clutch variability on embryo survival and fitness, replicates were performed that originated from different clutches often on different days. Additionally, differences in survival and hatching rates were seen between the AB and casper embryos. It is plausible that these differences may not be due to strain, but a result of influence from other intrinsic or extrinsic factors such as reproductive fitness of contributing fish, clutch to clutch variability, or water supply. Additional studies should be performed to replicate the experiment using different populations of AB and casper fish to confirm if differences in susceptibility to NaOCl toxicity is consistent.
In conclusion, maximal survival and hatching rates were generally observed when zebrafish embryos were surface disinfected with low NaOCl and exposed to NaThio and Pron. For AB fish, higher survival and hatching rates were seen when embryos were surface disinfected at 6 HPF, whereas capser fish showed higher survival and hatching when embryos were surface disinfected at 24 HPF. Although the use of MethB in EM was associated with lower hatching and survival rates in this study, researchers should weigh its benefits as a biocide with its potential negative impact on embryo development. Toxicity from NaOCl varied between the AB and casper embryos used in this study; therefore, published surface disinfection protocols may not be ideal for all zebrafish strains. Researchers should ideally test disinfection protocols on a small cohort of embryos of a specific strain to assess survival and impact on development before use.
Acknowledgment
The authors thank Adedeji Afolalu for his valuable assistance with this study. We also thank the staff associated with the shared zebrafish facility care between Weill Cornell Medicine and Memorial Sloan Kettering.
Disclaimer
The content is solely the responsibility of the authors and does not represent the views of the NIH.
Disclosure Statement
No competing financial interests exist.
Funding Information
This research study was funded through the NIH/NCI Cancer Center Support Grant P30-CA008748 through Memorial Sloan Kettering.
References
- 1. Collymore C, Crim MJ, Lieggi C. Recommendations for health monitoring and reporting for zebrafish research facilities. Zebrafish 2016;13(Suppl 1):S138–S148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borges AC, Pereira N, Franco M, Vale L, Pereira M, Cunha MV, et al. Implementation of a Zebrafish Health Program in a Research Facility: a 4-year retrospective study. Zebrafish 2016;13(Suppl 1):S115–S126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mocho JP. Three-dimensional screen: a comprehensive approach to the health monitoring of zebrafish. Zebrafish 2016;13(Suppl 1):S132–S137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray KN, Varga ZM, Kent ML. Biosecurity and health monitoring at the Zebrafish International Resource Center. Zebrafish 2016;13(Suppl 1):S30–S38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harper C, Lawrence C: The Laboratory Zebrafish. CRC Press, Boca Raton, FL, 2011 [Google Scholar]
- 6. Westerfield M: The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th edition. University of Oregon Press, Eugene, OR, 2007 [Google Scholar]
- 7. Ferguson JA, Watral V, Schiwindt AR, Kent ML. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomala) are highly resistant to chlorine. Dis Aquat Org 2007;76:205–214 [DOI] [PubMed] [Google Scholar]
- 8. Chang CT, Colicino EG, DiPaola EJ, Al-Hasnawi HJ, Whipps CM. Evaluating the effectiveness of common disinfectants at preventing the propagation of Mycobacterium spp. isolated from zebrafish. Comp Biochem Phys C 2015;178:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kent ML, Buchner C, Barton C, Tanguay RL. Toxicity of chlorine to zebrafish embryos. Dis Aquat Org 2014;107:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nusslein-Volhard C: Zebrafish: A Practical Approach. Oxford University Press, Oxford, England, 2002 [Google Scholar]
- 11. Zebrafish International Resource Center (ZIRC): ZIRC protocols: egg bleaching. https://zebrafish.org/documents/protocols/pdf/Egg_Bleaching/Customer_Egg_Bleaching.pdf Accessed on October31, 2019
- 12. Varga ZM: Aquaculture and husbandry at the Zebrafish International Resource Center. In: The Zebrafish: Genetics, Genomics and Informatics. Detrich III HM, Westerfield M, and Zon LI (eds), pp. 471–473. Academic Press, Waltham, MA, 2011 [Google Scholar]
- 13. Vaccaro A, Patten SA, Ciura S, Maios C, Therrien M, Drapeau P, et al. Methylene blue protects against TDP-43 and FUS neuronal toxicity in C. elegans and D. rerio. PLoS ONE 2012;7:e42117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa SRd, Monteiro MdC, da Silva Júnior FMR, Sandrini JZ. Methylene blue toxicity in zebrafish cell line is dependent on light exposure. Cell Biol Int 2016;40:895–905 [DOI] [PubMed] [Google Scholar]
- 15. Perlberg ST, Diamant A, Ofir R, Zilberg D. Characterization of swim bladder non-inflation (SBN) in angelfish, Pterophyllum scalare (Schultz), and the effect of exposure to methylene blue. J Fish Dis 2008;31:215–228 [DOI] [PubMed] [Google Scholar]
- 16. Tiboni GM, Lamonaca D. Transplacental exposure to methylene blue initiates teratogenesis in the mouse: preliminart evidence for a mechanistic implication of cyclic GMP pathway disruption. Teratology 2001;64:213–220 [DOI] [PubMed] [Google Scholar]
- 17. Chang CT, Amack JD, Whipps CM. Zebrafish embryo disinfection with povidone–iodine: evaluating an alternative to chlorine bleach. Zebrafish 2016;13(Suppl 1):S96–S101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Research Council: Guide for the Care and Use of Laboratory Animals: Eighth Edition. The National Academies Press, Washington, DC, 2011 [Google Scholar]
- 19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300 [Google Scholar]
- 20. Yang IH, Lee D, Lee SH, Kang JY. Characterization of proteolytically digested zebrafish chorion as extracellular matrix. Conf Proc IEEE Eng Med Biol Soc 2008;2008:1837–1840 [DOI] [PubMed] [Google Scholar]
- 21. Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom 2012;17:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol 2011;691:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varga ZM: Aquaculture, husbandry, and shipping at the Zebrafish International Resource Center. In: The Zebrafish: Genetics, Genomics and Transcriptomics, 4th edition. Detrich III HM, Westerfield M, and Zon LI (eds), pp. 518–519, Academic Press, Waltham, MA, 2016 [DOI] [PubMed] [Google Scholar]
- 24. Hu W, Cheng L, Sun D, Li D, Li P, Song Y, et al. Teratogenic effects of sodium thiosulfate on developing zebrafish embryos. Front Biosci 2009;1:3680–3687 [DOI] [PubMed] [Google Scholar]
- 25. Sweeney PJ, Walker JM: Pronase (EC 3.4.24.4). In: Enzymes of Molecular Biology. Methods in Molecular Biology™, Vol. 16. Burrell MM (ed), Humana Press, New York, NY, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Noga EJ: Methods for treating fish diseases. In: Fish Disease Diagnosis and Treatment. Duncan LL (ed), pp. 289–290, Mosby, St. Louis, MO, 2000 [Google Scholar]
- 27. Ginimuge PR, Jyothi SD. Methylene blue: revisited. J Anaesthesiol Clin Pharamacol 2010;26:517–520 [PMC free article] [PubMed] [Google Scholar]
- 28. Mondal SB, Gao S, Zhu N, Liang R, Gruev V, Achilefu S. Real-time fluorescence image-guided oncologic surgery. Adv Cancer Res 2014;171–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cragan JD. Teratogen update: methylene blue. Teratology 1999;60:42–48 [DOI] [PubMed] [Google Scholar]
- 30. Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci 2006;11:147–157 [DOI] [PubMed] [Google Scholar]
- 31. Puretec Industrial Water. What is reverse osmosis? https://puretecwater.com/reverse-osmosis/what-is-reverse-osmosis Accessed on October31, 2019
- 32. Haque F, Farhana T, Amin FB, Islam MS. Effect of different salinity exposures on the embryonic development of zebrafish (Danio rerio. Proceedings of 5th International Conference on Environmental Aspect of Bangladesh. Paper ID E77. 2014, pp. 114–115 [Google Scholar]
- 33. Zahangir MM, Haque F, Mustakim GM, Khatun H, Islam MS. Effect of water pH on the early developmental responses in zebrafish (Danio rerio). Prog Ag 2015;26:85–89 [Google Scholar]
- 34. Andrade TS, Henriques JF, Almeida AR, Soared AMVM, Scholz S, Domingues I. Zebrafish embryo tolerance to environmental stress factors—concentration-dose response analysis of oxygen limitation, pH, and UV-light irradiation. Environ Toxicol 2016;36:682–690 [DOI] [PubMed] [Google Scholar]
- 35. Kimmel CB, Ballard WW, Kimmel SR, Ullman B, Chilling TF. Stages of embryonic development in zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]