Abstract
Objective: The aim of this systematic review with meta-analysis was to describe the status on the effects of physical scar treatments on pain, pigmentation, pliability, pruritus, scar thickening, and surface area.
Design: Systematic review and meta-analysis.
Subjects: Adults with any kind of scar tissue.
Interventions: Physical scar management versus control or no scar management.
Outcome measures: Pain, pigmentation, pliability, pruritus, surface area, scar thickness.
Results: The overall results revealed that physical scar management is beneficial compared with the control treatment regarding the management of pain (p = 0.012), pruritus (p < 0.001), pigmentation (p = 0.010), pliability (p < 0.001), surface area (p < 0.001), and thickness (p = 0.022) of scar tissue in adults. The observed risk of bias was high for blinding of participants and personnel (47%) and low for other bias (100%).
Conclusions: Physical scar management demonstrates moderate-to-strong effects on improvement of scar issues as related to signs and symptoms. These results show the importance of specific physical management of scar tissue.
Keywords: cicatrix, conservative treatment, meta-analysis, physical therapy modalities, skin
Introduction
Physical scar management represents an important field in science, as scars can negatively impact the quality of life of patients.1,2 Disturbing perceptions such as pain, tenderness or itchiness on the one hand, and functional limitations in the form of contractures on the other, are consequences of problematic scars. In addition, scar esthetics can also have a negative influence on psychosocial factors.3–6 The restoration of injured skin requires a complex sequence of physiological interactions to form appropriate scar tissue and repair the dermal lesion.7 Any dysfunction in the wound healing process may result in excessive scar tissue formation.8 Hypertrophic scars or keloids are the results of such deviant wound healing.9 Different therapy options, described in the literature include chemical, physical, and surgical methods.10 The physiotherapist focuses on conservative modalities in the treatment of scar tissue. These physical scar management options can be grouped into mechanotherapy, occlusive and hydrogenatic therapies, and light therapy, whereby often combinations are used.11 The purpose of physical scar management concentrates primarily on the prevention of an aberrant healing process of the skin.12 To date, the effects of physical scar management are still controversially discussed in literature and previous reviews focus on the treatment of hypertrophic scars and keloids after burn injuries.11,13,14 Consequently, the aim of this systematic review and meta-analysis was to evaluate the effectiveness of physical scar management on different symptoms in adults with any kind of scar tissue.
Methods
Research question
The research question was defined following the PICO model.15 Population: Adults with scar tissue; Intervention: Physical scar management; Comparator: Control intervention or no treatment; Outcome: Pain ratings, pigmentation, pliability, pruritus, scar surface area, and scar thickness. The choice for the outcome variables was based upon the fact that physical interventions will have a direct influence on gaining functional, physical, and psychological improvements12 and because these parameters show valuable signs that account for therapy progression.16
Search strategy
An electronic search was conducted up to January 2020 on the databases PubMed (Medline), Cochrane Central Register of Controlled Trials (CENTRAL), and Physiotherapy Evidence Database (PEDro). Tissue-related keywords were combined with treatment-related keywords by using the Boolean operator “AND.” Tissue or treatment-related keywords themselves were combined with the function “OR.” The keywords used were proven for MeSH-terms (Table 1).
Table 1.
Overview of Keywords and Combinations
| Tissue related keywords | Treatment related keywords | Exclusion criteria | ||
|---|---|---|---|---|
| Scar (tissue) (MeSH) | AND | Casting physical activity | NOT | Surgery grafting |
| Burns (MeSH) | Compression (bandages) | |||
| Cicatrix (MeSH) | Cream | |||
| Keloid | Exercise | |||
| Hypertrophic scar | Gel sheet(ing) | |||
| Hydration | ||||
| Inserts | ||||
| Laser therapy | ||||
| Massage | ||||
| Mechanical treatment | ||||
| Mobilization | ||||
| Moisturizer | ||||
| Ointment | ||||
| Physical treatment | ||||
| Physiotherapy | ||||
| Pressure therapy/garment | ||||
| Rehabilitation stretching | ||||
| Silicone gel | ||||
| Skin cream | ||||
| Splint(ing) | ||||
| Tissue treatment | ||||
| Topical treatment | ||||
| Transdermal patch |
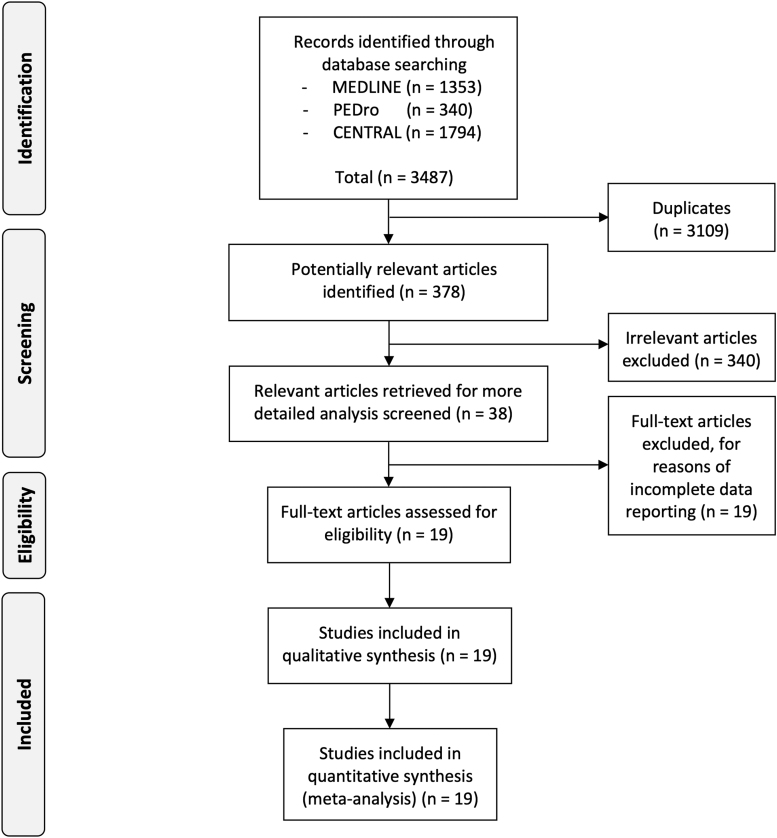
The a priori set inclusion criteria were (1) randomized controlled trial, controlled clinical trial, or controlled trial, (2) German, French, Dutch, or English full-text availability, (3) human participants, (4) any kind of physical burn or scar tissue management, (5) control intervention or no treatment, (6) outcome parameters comprising pain and/or pruritus rating scales, subjective and/or objective scar/burn tissue evaluations. Title and abstracts of the (k = 3487) articles found were independently screened by three researchers (C.D., E.H., R.S.). A total of 19 articles were eligible for this meta-analysis (Fig. 1).
FIG. 1.
PRISMA flow chart describing the selection process.
Data extraction and quality assessment
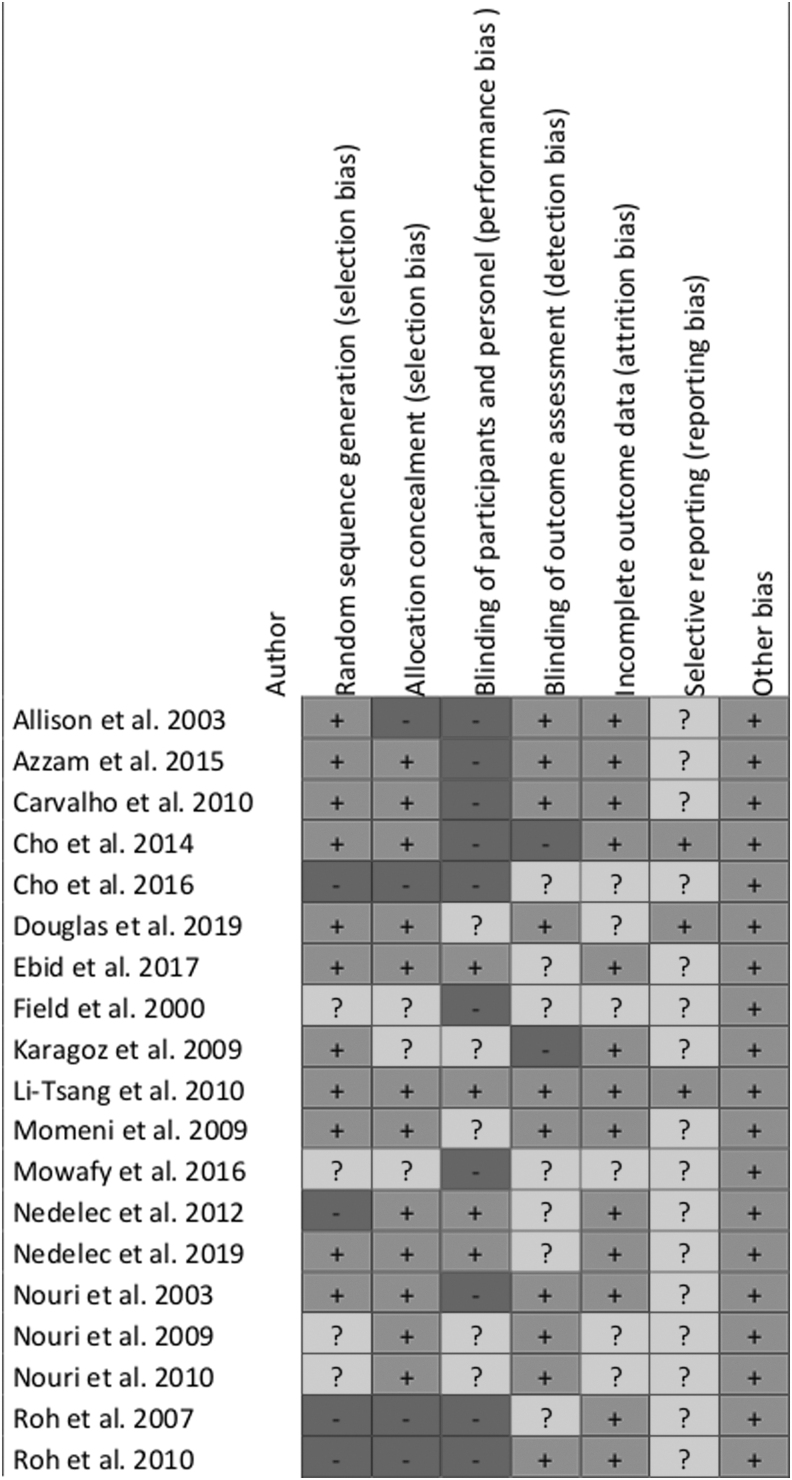
Data were manually extracted from the included studies by three researchers (C.D., E.H., R.S.), independently from each other. In case of disagreement, a fourth researcher (R.C.) checked the variable and agreement was sought by consensus. The methodological quality of the studies was assessed with the Cochrane Risk of Bias Tool (Version 1).17 Two researchers independently evaluated the 19 studies (E.H., R.S.). A third researcher (R.C.) rated in case of disagreement.
Data analysis
Comprehensive Meta-Analysis II software (CMA II; Biostat, Inc., Englewood, NJ) was used to conduct the meta-analysis calculations. A random-effects model was used to account for the fact that the included studies were not exact replicates of each other. Weighting factors were calculated based on the DerSimonian and Laird inversed-variance method.18 As most of the eligible studies used small samples, the individual studies' effect sizes were standardized and expressed as Hedges' g. The corresponding 95% confidence intervals (95% CI) around the individual studies' effect sizes as well as around the overall weighted estimate were calculated. Cohen's benchmarking for effect size interpretation was followed: g < 0.20 (negligible effect), g between 0.20 and 0.49 (small effect), g between 0.50 and 0.79 (moderate effect), and g > 0.80 (large effect).19 The null hypothesis of no heterogeneity (i.e., that all studies have a common effect size) was tested using the Cochran's Q-test. The Q-value, the corresponding degrees of freedom (df(Q)) as well as the corresponding exact p-value were reported. Because it has been described that the Q-test has a low statistical power, we set the significance level of the Q-test at 10%, as suggested.20 Higgins' I2 value was calculated to evaluate the amount of the total observed variance that can be explained by the true effect between studies' variance (rather than random sampling error). Higgins suggested that benchmarking values for the interpretation of heterogeneity be followed: I2 around 25% (low), I2 around 50% (moderate), and I2 around 75% or more (high).20
If a study showed an extreme effect size, a sensitivity analysis was conducted by excluding this study from the meta-analysis. An extreme effect size was defined, when the study's CI does not overlap with the CI of the pooled effect. The likelihood for publication bias was not tested because of less than 10 studies included in the different meta-analyses.21
Results
Risk of bias analysis
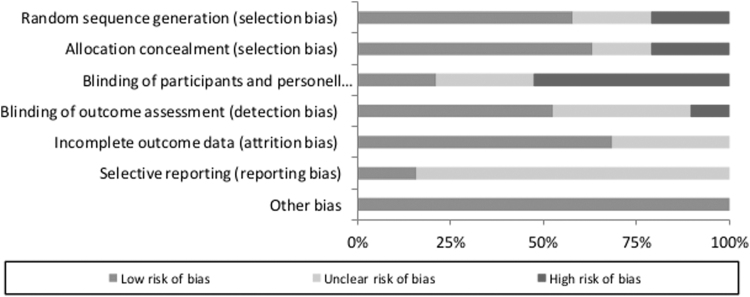
The risk of bias analysis demonstrated a high risk for performance bias with 47% (blinding of participants and personnel). The reporting bias stayed unclear in 84% of the observed studies due to unclear or insufficient information. A low risk of selection (63%), attrition (68%), and other bias (100%) could be observed throughout the studies (Figs. 2 and 3).
FIG. 2.
Risk of bias graph for each included study.
FIG. 3.
Risk of bias summary for all included studies.
Study characteristics
In the present work, 13 studies investigated burn scars. These included seven studies with burn scars,22–29 five with hypertrophic burn scars,28,30–33 and one with hypertrophic scars after burns, scalds, or other skin traumata.34 Five studies focused on postsurgical scars,35–39 of which one concentrated specifically on hypertrophic and keloid scars.39 Finally, one study included all kinds of hypertrophic and keloid scars.40 Seven studies divided the scars into two or three halves to perform the intervention and control treatment on the same subject,13,29,32,36–38,40 whereas the other 12 studies analyzed scars of independent groups of subjects.22,24–28,30,31,33–35,41 The intervention methods can be categorized into (1) mechanotherapy (massage,26–28,30,33 extracorporeal shockwave therapy (ESWT)24,25), (2) occlusion and hydration therapy (silicone application,31,32,34 moisturizing cream with protease enzymes41), and (3) light therapy (noninvasive laser22,29,35–40). Table 2 gives a summary of the included studies.
Table 2.
Characteristics of the Included Studies
| Author year | Sample size, n Population specification | Control condition | Intervention versus control treatment | Outcome variable and assessment tool |
|---|---|---|---|---|
| Alster and Williams (1995)39 |
n = 16 (10 m, 6 f) FA IG n = 16, CG n = 16 |
Hypertrophic and keloidal postsurgical scars Scar divided into two halves |
IG: PDL CG: no treatment |
Pliability: Likert scale, Surface area: Magiscan digital image processing system, Thickness: caliper |
| Azzam et al. (2016)40 |
n = 30 (15 m, 15 f) FA IG n = 30, CG n = 30 |
Hypertrophic and keloidal scars Scar divided into two halves |
IG: CO2 laser CG: no treatment |
Surface area: biopsy |
| Carvalho et al. (2010)35 |
n = 30 FA IG n = 14, CG n = 15 |
Postsurgical scars Independent subject groups |
IG: LLLT GA1As CG: no treatment |
Pain: VAS |
| Cho et al. (2014)30 |
n = 146 FA IG n = 76 (61m, 15 f), CG n = 70 (50 m, 20 f) |
Hypertrophic scars after burn Independent subject groups |
IG: massage therapy and standard therapy CG: standard therapy alone |
Pain: VAS Pruritus: itching scale Thickness: ultrasonography |
| Cho et al. (2016)24 |
n = 43 FA IG n = 20, CG n = 20 (34 m, 6 f) |
Burn scars Independent subject groups |
IG: ESWT and standard therapy CG: sham and standard therapy |
Pain: NRS |
| Douglas et al. (2019)29 |
n = 19 (15 m, 4 f) FA IG n = 19, CG n = 19 |
Burn scars Scar divided into two halves |
IG: CO2 laser CG: standard therapy |
Pain: VSS Pruritus: VSS |
| Ebid et al. (2017)22 |
n = 49 (30 m, 19 f) FA IG n = 24, CG n = 25 |
Burn scars Independent subject groups |
IG: pulsed HILT and standard therapy CG: placebo HILT and standard therapy |
Pain: VAS |
| Field et al. (2000)27 |
n = 20 (14 m, 6 f) FA IG n = 10, CG n = 10 |
Burn scars Independent subject groups |
IG: massage therapy and standard therapy CG: standard medical care |
Pain: VAS |
| Karagoz et al. (2009)31 |
n = 32 (12 m, 20 f) FA IG 15 scars, IG 15 scars |
Hypertrophic scars after burn Independent subject groups |
IG: silicone gel CG: topical onion extract |
Pigmentation: VSS Pliability: VSS Thickness: VSS |
| Li-Tsang et al. (2010)34 |
n = 104 (63 m, 41 f) FA IG n = 22, CG n = 12 |
Hypertrophic scars after burns, scalds, or other skin trauma Independent subject groups | IG: silicone gel sheet and lanolin massage CG: lanolin massage |
Pain: VAS Pliability: VSS Pruritus: VAS Thickness: TUPS |
| Momeni et al. (2009)32 |
n = 38 (16 m, 18 f) FA IG n = 38, CG n = 38 |
Hypertrophic scars after burn Scar divided into two halves |
IG: silicone gel sheet CG: placebo sheet |
Pain: VSS Pliability: VSS Pruritus: VSS Pigmentation: VSS |
| Mowafy et al. (2016)25 |
n = 30 (m, f NM) FA IG n = 15, CG n = 15 |
Burn scars Independent subject groups |
IG: ESWT and medical treatment CG: medical treatment alone |
Pain: VAS |
| Nedelec et al. (2012)41 |
n = 23 FA IG n = 9, CG n = 9 |
Burn scars Independent subject groups |
IG: moisturizer with Provase® CG: standard moisturizer |
Pruritus: VAS |
| Nedelec et al. (2019)28 |
n = 70 (41 m, 29 f) FA IG n = 70, CG n = 70 |
Hypertrophic scars after burn Independent subject group |
IG: massage therapy CG: usual care |
Pigmentation: light absorption Thickness: ultrasonography |
| Nouri et al. (2003)37 |
n = 11 (4 m, 7 f) FA IG n = 11, CG n = 11 |
Postsurgical scars Scar divided into two halves |
IG: PDL and adhesive tape CG: no treatment and adhesive tape |
Pigmentation: VSS Pliability: VSS Thickness: VSS |
| Nouri et al. (2009)38 |
n = 15 (11 m, 4 f) with 21 scars in total FA n = 14 with 19 scars |
Postsurgical scars Scar divided into three parts |
IG: PDL CG: no treatment |
Pigmentation: VSS Thickness: VSS |
| Nouri et al. (2010)36 |
n = 20 (10 m, 4 f) FA IG n = NM, CG n = NM |
Postsurgical scars Scar divided into three parts |
IG: PDL CG: no treatment |
Pliability: VSS Thickness: VSS |
| Roh et al. (2007)33 |
n = 35 (26 m, 9 f) FA IG n = 18, CG n = 17 |
Hypertrophic scars after burn Independent subject groups |
IG: SRMT CG: no treatment |
Pliability: VSS Thickness: VSS |
| Roh et al. (2010)26 |
n = 26 (24 m, 2 f) FA IG n = 13 (12 m, 1 f), CG n = 13 (12 m, 1 f) |
Burn scars Independent subject groups |
IG: SRMT CG: standard treatment |
Thickness: ultrasonography |
CG, control group, CO2, carbon dioxide, ESWT, extracorporeal shockwave therapy, f, females, FA, final analysis, HILT: high-intensity laser therapy, IG, intervention group, LLLT GA1As, low-level laser therapy, m, males, n, number of subjects, NRS, numeric rating scale, PDL: pulsed dye laser, SRMT, skin rehabilitation massage therapy, TUPS: tissue ultrasound palpation system, VAS, visual analog scale, VSS, Vancouver scar scale.
Pain ratings
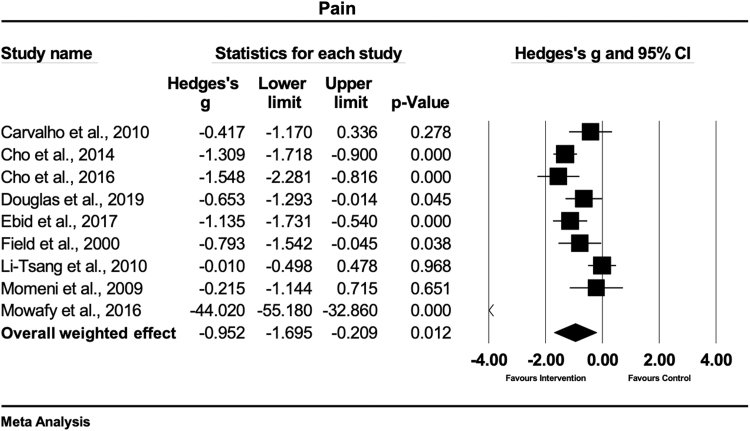
Pain ratings were evaluated in nine studies.22,24,25,27,29,30,32,34,35 Of these, six studies measured pain with the visual analog scale (VAS),22,25,27,30,34,35 two applied the Vancouver scar scale (VSS),29,32 and one the numeric rating scale.24 Two studies each treated the scars with massage therapy,27,30 ESWT,24,25 laser therapy,22,35 and gel sheets,32,34 and one study with a CO2 laser.29 To test the hypothesis that physical therapy interventions had an enhancing effect to decrease pain as compared with control, an overall meta-analysis was conducted. Figure 4 shows that, in this set of sampled studies, physical scar management had a large and statistically significant effect on pain reduction compared with the control interventions (Hedges' g = −0.95 [95% CI: −1.69 to −0.20]). The observed heterogeneity was high and statistically significant (Q = 81.5; df(Q) = 8; p < 0.001; I2 = 90.1%) suggesting that about 90.1% of the variance of observed effects reflect the variance of true effects.
FIG. 4.
Forest plot of the meta-analysis illustrating the overall weighted effect size of physical therapy versus control on pain in patients with scar tissue. The diamond on the bottom of the forest plot represents the overall weighted estimate. CI, confidence interval.
A sensitivity analysis was conducted since one study showed an extreme effect size.25 The results of the sensitivity analysis show a moderate and statistically significant effect on pain reduction compared with the control interventions (Hedges' g = −0.77 [95% CI: −1.18 to −0.36]). The sensitivity analysis decreased the observed heterogeneity, but remained high and statistically significant (Q = 23.95; df(Q) = 7; p = 0.001; I2 = 70.77%).
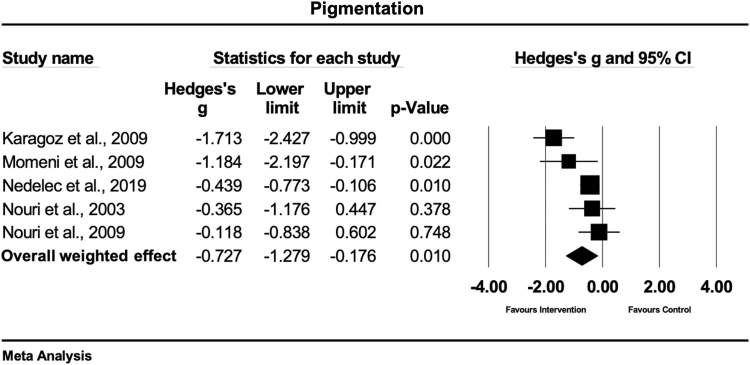
Pigmentation
Five studies investigated the effects of physiotherapy on pigmentation, using the VSS.28,31,32,37,38 One study treated scars with massage therapy,28 two with silicone applications,31,32 while two studies used laser therapy.37,38 Figure 5 shows that, based on this set of studies, there is evidence that physical scar management has a moderate and statistically significant effect compared with the control treatment on pigmentation (Hedges' g = −0.72 [95% CI: −1.27 to −0.17]). The heterogeneity was moderate and statistically significant (Q = 13.5; df(Q) = 4; p = 0.009; I2 = 70.4%).
FIG. 5.
Forest plot of the meta-analysis illustrating the overall weighted effect size of physical therapy versus control on pigmentation in patients with scar tissue. The diamond on the bottom of the forest plot represents the overall weighted estimate.
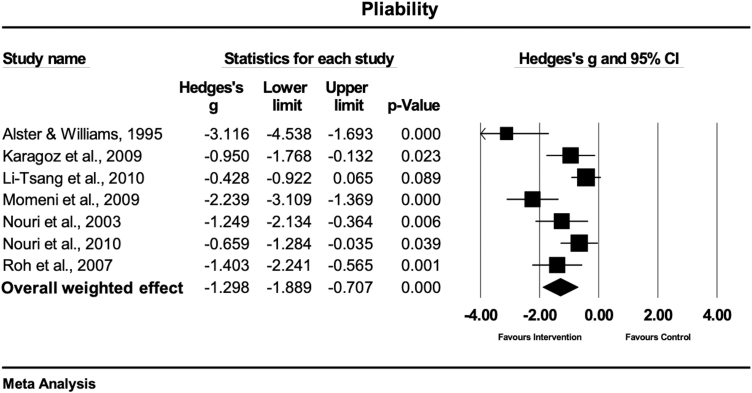
Pliability
Pliability was evaluated in seven studies.31–34,36,37,39 Six studies used the VSS,31–34,36,37 while one study used a 5-point Likert scale,39 for the quantification of pliability. Three studies treated the scars with laser therapy,36,37,39 three studies with gel sheets,31,32,34 and one study with massage therapy.33 The overall weighted effect size of physical (conservative) treatment as compared with control treatment in this set of scar studies, was large and statistically significant (Hedges' g = −1.29 [95% CI: −1.88 to −0.70]), favoring the physical scar management (Fig. 6). The observed heterogeneity was moderate but statistically significant (Q = 23.7; df(Q) = 6; p = 0.001; I2 = 74.7%).
FIG. 6.
Forest plot of the meta-analysis illustrating the overall weighted effect size of physical therapy versus control on pliability in patients with scar tissue. The diamond on the bottom of the forest plot represents the overall weighted estimate.
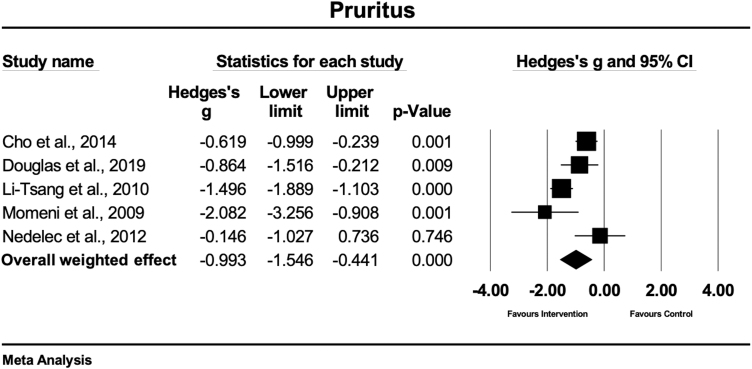
Pruritus
Five studies compared the effects between physical scar management and a control condition on pruritus.29,30,32,34,41 Two studies evaluated pruritus with the VSS,29,32 while the other studies used a VAS34,41 or an itching scale.30 Two studies treated the scars with gel sheets,32,34 one study each with massage therapy,30 CO2 laser therapy,29 and moisturizing cream with protease.41 In this sampled set of studies, the overall weighted effect revealed that conservative therapy had a large and statistically significant effect on pruritus compared with the control condition (Hedges' g = −0.99 [95% CI: −1.54 to −0.44]) (Fig. 7). The heterogeneity of the included studies was high and significant (Q = 17.0; df(Q) = 4; p = 0.002; I2 = 76.4%).
FIG. 7.
Forest plot of the meta-analysis illustrating the overall weighted effect size of physical therapy versus control on pruritus in patients with scar tissue. The diamond on the bottom of the forest plot represents the overall weighted estimate.
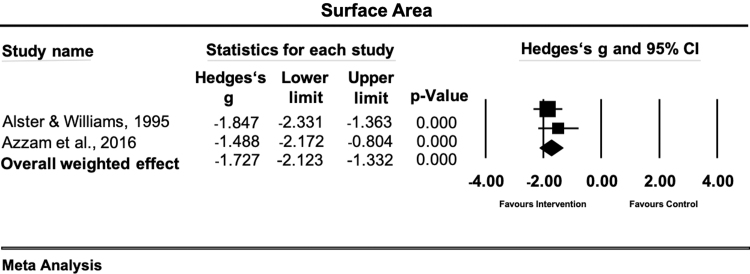
Surface area
Two studies met the inclusion criteria of evaluating the effects of conservative therapy versus a control condition on the surface area of the scar.39,40 All of them used different assessment methods comprising Magiscan digital image processing system39 and scar tissue biopsy.40 Each study applied laser therapy as physical scar management. The overall weighted effect size in this sample of studies was large and statistically significant (Hedges' g = −1.72 [95% CI: −2.12 to −1.33]) (Fig. 8). The observed heterogeneity was low and statistically not significant (Q = 0.70; df(Q) = 1; p < 0.401; I2 = 0.0%).
FIG. 8.
Forest plot of the meta-analysis illustrating the overall weighted effect size of physical therapy versus control on surface area in patients with scar tissue. The diamond on the bottom of the forest plot represents the overall weighted estimate.
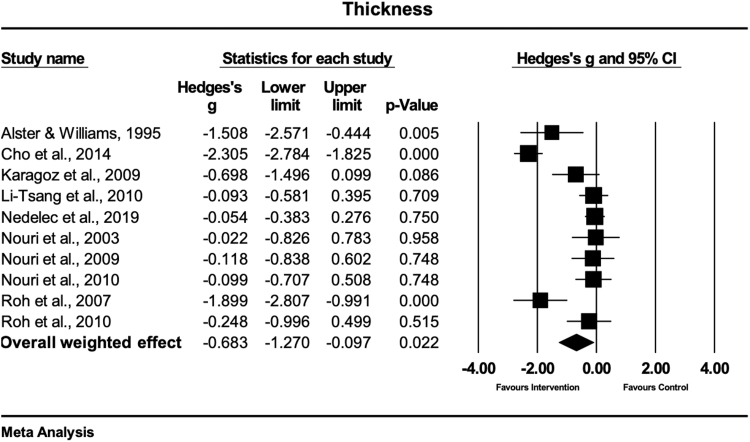
Scar thickness
A total of 10 studies evaluated the effects of physical scar management versus the control condition of scar thickness.26,28,30,32,33,34,36–39 The evaluation techniques were ultrasonography,26,28,30 tissue ultrasound palpation system,34 VSS subscale height,31,33,36–38 and caliper measurements.13,39 Four studies treated the scars with laser therapy,36–39 four studies with massage therapy,26,28,30,33 and two studies with gel sheets.31,34 The meta-analysis of the effect sizes extracted from this set of studies showed that physical scar management had a moderate and statistically significant effect on scar thickness reduction compared with the control group (Hedges' g = −0.68 [95% CI: −1.27 to −0.09)] (Fig. 9). The observed heterogeneity was high and statistically significant [Q = 81.0; df(Q) = 9; p < 0.001; I2 = 88.8%].
FIG. 9.
Forest plot of the meta-analysis illustrating the overall weighted effect size of physical therapy versus control on scar thickness in patients with scar tissue. The diamond on the bottom of the forest plot represents the overall weighted estimate.
Discussion
The aim of this review and meta-analysis was to evaluate the effectiveness of physical scar management on scar tissue. The main results of this analysis show significant overall effects in favor of the physical scar management compared with a control treatment for pain, pigmentation, pliability, pruritus, scar area, and thickness in adults suffering from any type of scar tissue.
However, this study has potential limitations. Although the performed literature search was conducted in three scientific databases, the authors are aware that publication bias might have occurred because no gray literature was screened. Insufficient data reporting might have led to potential over- or underestimation of the true effect as some authors did not show exact values (e.g., mean ± SD) but present the results as graphs only. Most of the included studies used small sample sizes, which limits the translation of these results into practice.
Pain
The main results of this meta-analysis reveal that physical scar management as compared with a control treatment has a significant positive influence on pain ratings (Hedges' g = −0.95 [95% CI: −1.69 to −0.20]). The strongest effects were seen in two studies,24,25 both using a combination of ESWT and medical or standard treatment. Both ESWT treatments were conducted according to the guidelines of the international society of medical shockwave therapy, with ESWT of 100 impulses per cm2 on the affected location. These strong effects (Hedges' g range = −1.54 to −44.02) were seen in patients suffering from burn scars. The combination of ESWT and medical treatment25 demonstrated stronger effects compared with the combination of ESWT and standard therapy.24 The study from Mowafy et al.25 consisted of medication, physical therapy, and burn rehabilitation massage therapy while the content of the medical treatment of the other study from Cho et al.24 was not further described. A study showed an extreme effect size for ESWT.25 Therefore, we performed a sensitivity analysis where the study was excluded. The statistical significance of the overall effect of physical therapy management on pain reduction was maintained but changed from large to moderate (Hedges' g = −0.78 [95% CI: −1.19 to −0.37]).
A moderate-to-strong effect on pain reduction was observed in two studies, where scar tissue was treated with massage therapy for the duration of 30 min.27,30 Both studies used massage therapy in combination with standard therapy in hypertrophic burn scars30 or burn scars,27 respectively. A possible explanation for the stronger effect of massage therapy in the study of Cho et al.30 (Hedges' g = −1.30) could be the higher treatment frequency of three times versus two times a week, as used in the study of Field et al.27 (Hedges' g = −0.79). Another possibility is that a mechanotherapy, such as massage, might lead to better results in hypertrophic compared with nonhypertrophic burn scars.
The results of our study indicate that mechanical therapies, such as ESWT and massage therapy, seem to have a positive effect on pain reductions in burn scar. The non-nociceptive mechanical stimuli can reduce pain through the stimulation of nociceptive fibers, which are known to transmit sharp, acute, diffuse, and burning pain sensations.42–44 However, these interpretations should be handled with care as both studies using ESWT demonstrated a high risk of bias.24,25
Different types of light therapy also demonstrated to be effective interventions for pain reductions in the treatment of burn scars.22,29 Using a CO2 laser led to a significant and moderate pain reducing effect (Hedges' g = −0.65),29 whereas pulsed high-intensity laser therapy (HILT) showed a significant and large pain reducing effect (Hedges' g = −1.13).22 Published studies already reported that the higher intensity and the greater depth reached by HILT might be one reason for its effectiveness to relieve pain compared with low-level laser treatments.45–47 The reduction of pain levels by HILT is probably based on the inhibition of Aδ- and C-fiber transmission48 and by enhancing the production and release of endorphins.22 The results of this analysis might reveal that the general effects of HILT might be strong in the management of burn scars for pain reduction. However, future studies should evaluate this issue for making a valid statement.
Small effects and nonsignificant treatment differences for pain reduction (p > 0.05) were seen for the treatments with silicone gel sheets combined with massage34 or placebo sheets.32
Pigmentation
The main results of this analysis reveal that the physical scar management compared with control has a significant positive effect on pigmentation (Hedges' g = −0.72 [95% CI: −1.27 to −0.17]). With respect to the other study results, the strongest effects were seen when applying a silicone gel twice a day on hypertrophic burn scars (Hedges'g = −1.71).31 A possible explanation might be that silicone applications are believed to positively influence the scar tissue through wound hydration,49 which is probably one reason that explains the great acceptance of this therapy since the early 1980s.9 Similarly to silicone gel applications, silicone gel sheets demonstrated to have a large and positive effect (Hedges'g = −1.18) on pigmentation of hypertrophic burn scars.32 Hydration and occlusion are known positive effects of silicone applications, while occlusion regulates epidermal cytokine and growth factor production in scar tissue.11,50 A 5-min massage treatment in burn patients showed a small and significant positive effect (Hedges'g = −0.43; p = 0.01) on pigmentation and melanin.28 This positive effect might result from an immediate increase in skin blood flow microcirculation.51 Longer lasting positive effects could be explained by the increased concentration of local transforming growth factor TGF-beta 1 and endothelin 1 that stimulates myofibroblast survival through protein kinase B activation.52
Pliability
The main results of this analysis show a significant positive effect on scar pliability with physical scar management compared with the control treatment (Hedges' g = −1.29 [95% CI: −1.88 to −0.70]). The largest effects were observed in studies, where pulsed dye laser (PDL) therapy was used to treat postsurgical scars.36,37,39 All studies used a laser wavelength of 585 nm with a pulse duration of 450 μs. However, the study of Alster and Williams39 used higher fluences per pulse (mean 7.0 J/cm2) compared with 4.0 and 3.5 J/cm2, respectively.36,37 Interestingly, PDL led to stronger effects on hypertrophic and keloid postsurgical scars,39 compared with early postsurgical scar treatment.36,37 The use of an adhesive tape in combination with the PDL treatment demonstrated an additional positive effect of the tissue pliability in postsurgical scar treatment.37
Silicone gel and silicone gel sheets demonstrated to be effective in the treatment of pliability in hypertrophic burn scars (p = 0.02 and p < 0.001).31,32 Nevertheless, the study of Li-Tsang et al.,34 using silicone gel sheets did not show significant positive pliability effects (p = 0.08).34 These nonsignificant results might be explained by the inclusion of other traumatic skin lesions beside hypertrophic burn scars, which could have negatively impacted the treatment's effectiveness.
Only one study investigated the effects of skin rehabilitation massage therapy on the pliability of burn scars, showing a large and significant effect (Hedges'g = −1.40, p = 0.001).33 A described reason for the massage to increase the pliability of scars could be the mechanical disruption of the fibrotic tissue. The application of mechanical stimuli can lead to changes in the expression of extracellular matrix proteins and proteases, and therefore may change the structural and signaling milieu.53,54
Pruritus
In general, the present analysis shows a significant positive effect of physical scar management compared with control treatments in the management of pruritus (Hedges' g = −0.99 [95% CI: −1.54 to −0.44]). The strongest effect in the reduction of pruritus or itching was seen for hypertrophic burn scars, treated with silicone gel sheets (Hedges'g = −1.49 and −2.08, respectively).32,34 Furthermore, the moisturizing effect of the silicone gel sheets on the stratum corneum layer also demonstrated to be effective to reduce pruritus in other traumatic skin lesions.34 A key factor seems to be the regulation of epidermal cytokines and growth factor production, which are evoked from occlusive therapy.50 Another effective method showing large and significant effects (Hedges'g = 0.86) in the treatment of pruritus was achieved with CO2 laser.29 Manstein et al.55 reported that the relief of itching can be explained by the undamaged columns of the skin between the microthermal treatment zones in CO2 laser treatment, resulting in rapid epithelialization.55
Further treatment that positively influenced pruritus in hypertrophic scar tissue was rehabilitation massage therapy, containing effleurage, friction, and petrissage techniques (p = 0.001).30 The observed moderate effect of massage therapy on pruritus (Hedges'g = −0.61) might be explained by the gate theory by Melzack and Wall56 or due to the release of beta endorphin levels.57 No positive effects were observed for the use of moisturizing creams (including proteases) in the reduction of pruritus in burn scar tissue (p > 0.05).41
Surface area
Physical scar management showed a large and significant positive effect on the scar surface area (Hedges' g = −1.72 [95% CI: −2.12 to −1.33]). Only two studies investigated the effects of laser therapy on scar surface area in patients suffering from hypertrophic and keloid scars.39,40 A stronger effect was found for the PDL treatment (Hedges'g = 1.84),39 in comparison to fractional CO2 laser (Hedges'g = 1.48)40 in the management of hypertrophic and keloid scars. The study using PDL investigated postsurgical hypertrophic and keloid scars, while the fractional CO2 laser study included hypertrophic and keloid scars from different origins. The different laser therapy specifications and treated scar types make a general recommendation for a specific treatment setting difficult. However, these results demonstrate that laser light therapy is a promising treatment option for reducing scar surface area, probably due to increased tissue repair processes58 and enhanced anti-inflammatory actions.59
Scar thickness
Physical scar management has a significantly positive effect on scar thickness management compared with control interventions (Hedges' g = −0.68 [95% CI: −1.27 to −0.09]).
Scar thickening was significantly (p = 0.005) reduced when PDL (wavelength 585 nm, pulse duration 450 μs, and mean fluence per pulse of 7.0 J/cm2) was used in the treatment of hypertrophic and keloidal postsurgical scars compared with no treatment.39 However, these results do not corroborate the findings from other studies, using PDL to decrease scar thickening of the skin after postsurgical scars compared with no treatment.36–38 A reason for the different results might be due to the different laser settings. While one study used a fluence per pulse between 6.5 and 7.25 J/cm2,39 the other authors36–38 used lower pulses between 3.50 and 4.00 J/cm2. While in the study of Alster and Williams,39 the treated scar area was smaller compared with others (range: 7 to 10 mm),36–38 the totally applied laser energy applied was higher, which might have contributed to the significant positive effects.
Two studies demonstrated that massage therapy was effective in reducing scar thickening in hypertrophic and burn scars.30,33 However, also controversial results were found in our analysis. Two included studies, using skin rehabilitation massage in the treatment of hypertrophic and burn scars, showed no effects to reduce scar thickening.26,28 Besides the interindividual treatment intensities of massage therapy, the treatment frequency of the intervention might have also contributed to the different effects of massage therapy. While the two studies30,33 showing a positive effect of massage therapy on scar thickening used a treatment frequency of one to three times per week with a treatment duration of 30 min, the noneffective studies showed only treatment durations of 5 min28 or did not report26 the exact frequencies and durations. It seems that the combination of massage therapy with standard therapy (including joint mobilizations, silicone gel applications, etc.)30 has an additional beneficial effect compared with massage therapy alone33 (Hedges'g = −2.30 vs. −1.89).
Conclusion
In general, this meta-analysis shows that physical scar management has a significant positive effect to influence pain, pigmentation, pliability, pruritus, surface area, and scar thickness compared with control or no treatment.
Treatment modalities, such as ESWT, massage, as well as high-intensity light therapy, seem to be most effective agents in reducing pain in burn scars. Regarding the treatment of pliability and scar thickness, positive effects were seen for the use of PDL in postsurgical scars, while silicone gel and silicone gel sheets demonstrated to be effective in the management of pliability in hypertrophic scars. Our study further revealed strong effects in the reduction of pruritus, using silicone gel sheets in hypertrophic burn scars. Scar thickness was positively affected when hypertrophic and burn scars were treated with massage therapy, while scar surface area was positively influenced by laser therapy modalities (PDL and CO2 laser).
To investigate the most effective physical therapy strategy, further studies are needed, evaluating head-to-head comparisons of different physical scar therapy modalities.
Acknowledgment
The authors like to thank the “Thim van der Laan foundation” for the support.
Author Contributions
C.D., E.H., R.S., and R.C. were responsible for acquisition, interpretation, and drafting the article. J.T. and R.C. substantially contributed to the data analysis and critically revised the work for important intellectual content. U.v.D. was included in the article drafting and also critically revised the work. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Statement of Ethics
As this study was a review of published literature, no ethics approval by a Research Ethics Board was required to conduct our research.
Author Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Information
No sources of external funding were utilized for this project.
References
- 1. Bock O, Schmid-Ott G, Malewski P, et al. . Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 2006;297:433–438 [DOI] [PubMed] [Google Scholar]
- 2. Meirte J, van Loey NEE, Maertens K, et al. . Classification of quality of life subscales within the ICF framework in burn research: Identifying overlaps and gaps. Burns 2014;40:1353–1359 [DOI] [PubMed] [Google Scholar]
- 3. Bell L, McAdams T, Morgan R, et al. . Pruritus in burns: A descriptive study. J Burn Care Rehabil 1988;9:305–308 [PubMed] [Google Scholar]
- 4. Taal L, Faber AW. Posttraumatic stress and maladjustment among adult burn survivors 1 to 2 years postburn. Part II: The interview data. Burns 1998;24:399–405 [DOI] [PubMed] [Google Scholar]
- 5. Dorfmuller M. Psychological management and after-care of severely burned patients [In German]. Unfallchirurg 1995;98:213–217 [PubMed] [Google Scholar]
- 6. Robert R, Meyer W, Bishop S, et al. . Disfiguring burn scars and adolescent self-esteem. Burns 1999;25:581–585 [DOI] [PubMed] [Google Scholar]
- 7. Gurtner GC, Werner S, Barrandon Y, et al. . Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 8. Sephel GC, Woodward SC. Repair, regeneration, and fibrosis. In: Rubin E ed. Rubin's Pathology. Baltimore: Lippincott Williams & Wilkins, 2001:84–117. [Google Scholar]
- 9. Mustoe TA, Cooter RD, Gold MH, et al. . International clinical recommendations on scar management. Plast Reconstr Surg 2002;110:560–571 [DOI] [PubMed] [Google Scholar]
- 10. Alster TS, West TB. Treatment of scars: A review. Ann Plast Surg 1997;39:418–432 [DOI] [PubMed] [Google Scholar]
- 11. Anthonissen M, Daly D, Janssens T, et al. . The effects of conservative treatments on burn scars: A systematic review. Burns 2016;42:508–518 [DOI] [PubMed] [Google Scholar]
- 12. Paratz JD, Stockton K, Plaza A, et al. . Intensive exercise after thermal injury improves physical, functional, and psychological outcomes. J Trauma Acute Care Surg 2012;73:186–194 [DOI] [PubMed] [Google Scholar]
- 13. Alster TS, Tanzi EL. Hypertrophic scars and keloids: Etiology and management. Am J Clin Dermatol 2003;4:235–243 [DOI] [PubMed] [Google Scholar]
- 14. Zhang YT, Li-Tsang CWP, Au RKC. A systematic review on the effect of mechanical stretch on hypertrophic scars after burn injuries. Hong Kong J Occup Ther 2017;29:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 16. Balci DD, Inandi T, Dogramaci CA, et al. . DLQI scores in patients with keloids and hypertrophic scars: A prospective case control study. J Dtsch Dermatol Ges 2009;7:688–692 [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration 2011. Online document at: www.cochrane-handbook.org, accessed July26, 2019
- 18. Veroniki AA, Jackson D, Viechtbauer W, et al. . Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016;7:55–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen J. A power primer. Psychological bulletin 1992;112:155–159 [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis (Statistics and Practice). Chichester, UK: John Wiley & Sons, 2009 [Google Scholar]
- 22. Ebid AA, Ibrahim AR, Omar MT, et al. . Long-term effects of pulsed high-intensity laser therapy in the treatment of post-burn pruritus: A double-blind, placebo-controlled, randomized study. Lasers Med Sci 2017;32:693–701 [DOI] [PubMed] [Google Scholar]
- 23. Nedelec B, LaSalle L. Postburn itch: A review of the literature. Wounds 2018;30:E118–E124 [PubMed] [Google Scholar]
- 24. Cho YS, Joo SY, Cui H, et al. . Effect of extracorporeal shock wave therapy on scar pain in burn patients: A prospective, randomized, single-blind, placebo-controlled study. Medicine (Baltimore) 2016;95:e4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mowafy ZME, Monem MA, Khowailed KA-E, et al. . Efficacy of extracorporeal shock wave in the treatment of heterotopic ossification in burned patients. Int J PharmTech Res 2016;9:46–52 [Google Scholar]
- 26. Roh YS, Seo CH, Jang KU. Effects of a skin rehabilitation nursing program on skin status, depression, and burn-specific health in burn survivors. Rehabil Nurs 2010;35:65–69 [DOI] [PubMed] [Google Scholar]
- 27. Field T, Peck M, Scd, et al. Postburn itching, pain, and psychological symptoms are reduced with massage therapy. J Burn Care Rehabil 2000;21:189–193 [DOI] [PubMed] [Google Scholar]
- 28. Nedelec B, Couture MA, Calva V, et al. . Randomized controlled trial of the immediate and long-term effect of massage on adult postburn scar. Burns 2019;45:128–139 [DOI] [PubMed] [Google Scholar]
- 29. Douglas H, Lynch J, Harms KA, et al. . Carbon dioxide laser treatment in burn-related scarring: A prospective randomised controlled trial. J Plast Reconstr Aesthet Surg 2019;72:863–870 [DOI] [PubMed] [Google Scholar]
- 30. Cho YS, Jeon JH, Hong A, et al. . The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: A randomized controlled trial. Burns 2014;40:1513–1520 [DOI] [PubMed] [Google Scholar]
- 31. Karagoz H, Yuksel F, Ulkur E, et al. . Comparison of efficacy of silicone gel, silicone gel sheeting, and topical onion extract including heparin and allantoin for the treatment of postburn hypertrophic scars. Burns 2009;35:1097–1103 [DOI] [PubMed] [Google Scholar]
- 32. Momeni M, Hafezi F, Rahbar H, et al. . Effects of silicone gel on burn scars. Burns 2009;35:70–74 [DOI] [PubMed] [Google Scholar]
- 33. Roh YS, Cho H, Oh JO, et al. . Effects of skin rehabilitation massage therapy on pruritus, skin status, and depression in burn survivors. Taehan Kanho Hakhoe Chi 2007;37:221–226 [DOI] [PubMed] [Google Scholar]
- 34. Li-Tsang CW, Zheng YP, Lau JC. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res 2010;31:448–457 [DOI] [PubMed] [Google Scholar]
- 35. Carvalho RL, Alcantara PS, Kamamoto F, et al. . Effects of low-level laser therapy on pain and scar formation after inguinal herniation surgery: A randomized controlled single-blind study. Photomed Laser Surg 2010;28:417–422 [DOI] [PubMed] [Google Scholar]
- 36. Nouri K, Elsaie ML, Vejjabhinanta V, et al. . Comparison of the effects of short- and long-pulse durations when using a 585-nm pulsed dye laser in the treatment of new surgical scars. Lasers Med Sci 2010;25:121–126 [DOI] [PubMed] [Google Scholar]
- 37. Nouri K, Jimenez GP, Harrison-Balestra C, et al. . 585-nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatol Surg 2003;29:65–73; discussion 73. [DOI] [PubMed] [Google Scholar]
- 38. Nouri K, Rivas MP, Stevens M, et al. . Comparison of the effectiveness of the pulsed dye laser 585 nm versus 595 nm in the treatment of new surgical scars. Lasers Med Sci 2009;24:801–810 [DOI] [PubMed] [Google Scholar]
- 39. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet 1995;345:1198–1200 [DOI] [PubMed] [Google Scholar]
- 40. Azzam OA, Bassiouny DA, El-Hawary MS, et al. . Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: A clinical, histological, and immunohistochemical study. Lasers Med Sci 2016;31:9–18 [DOI] [PubMed] [Google Scholar]
- 41. Nedelec B, Rachelska G, Parnell LK, et al. . Double-blind, randomized, pilot study assessing the resolution of postburn pruritus. J Burn Care Res 2012;33:398–406 [DOI] [PubMed] [Google Scholar]
- 42. Djouhri L, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: A review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev 2004;46:131–145 [DOI] [PubMed] [Google Scholar]
- 43. Altun V, Hakvoort TE, van Zuijlen PP, et al. . Nerve outgrowth and neuropeptide expression during the remodeling of human burn wound scars. A 7-month follow-up study of 22 patients. Burns 2001;27:717–722 [DOI] [PubMed] [Google Scholar]
- 44. Wang C, Hah J, Mackey S, et al. . Quantitative sensory abnormalities in post-surgical cutaneous nerve injuries. J Pain 2010;11:S17 [Google Scholar]
- 45. Kheshie AR, Alayat MS, Ali MM. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: A randomized controlled trial. Lasers Med Sci 2014;29:1371–1376 [DOI] [PubMed] [Google Scholar]
- 46. Salli A, Akkurt E, Alparslan Ali İ, et al. . Comparison of high intensity laser and epicondylitis bandage in the treatment of lateral epicondylitis. Arch Rheumatol 2016;31:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ordahan B, Karahan AY, Kaydok E. The effect of high-intensity versus low-level laser therapy in the management of plantar fasciitis: A randomized clinical trial. Lasers Med Sci 2018;33:1363–1369 [DOI] [PubMed] [Google Scholar]
- 48. Chow R, Armati P, Laakso EL, et al. . Inhibitory effects of laser irradiation on peripheral mammalian nerves and relevance to analgesic effects: A systematic review. Photomed Laser Surg 2011;29:365–381 [DOI] [PubMed] [Google Scholar]
- 49. Shih R, Waltzman J, Evans GR, et al. . Review of over-the-counter topical scar treatment products. Plast Reconstr Surg 2007;119:1091–1095 [DOI] [PubMed] [Google Scholar]
- 50. Gallant-Behm CL, Mustoe TA. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen 2010;18:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goats GC. Massage—the scientific basis of an ancient art: Part 1. The techniques. Br J Sports Med 1994;28:149–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 2007;257:143–179 [DOI] [PubMed] [Google Scholar]
- 53. Bhadal N, Wall IB, Porter SR, et al. . The effect of mechanical strain on protease production by keratinocytes. Br J Dermatol 2008;158:396–398 [DOI] [PubMed] [Google Scholar]
- 54. Chan MW, Hinz B, McCulloch CA. Mechanical induction of gene expression in connective tissue cells. Methods Cell Biol 2010;98:178–205 [DOI] [PubMed] [Google Scholar]
- 55. Manstein D, Herron GS, Sink RK, et al. . Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004;34:426–438 [DOI] [PubMed] [Google Scholar]
- 56. Melzack R, Wall PD. Pain mechanisms: A new theory. Science 1965;150:971–979 [DOI] [PubMed] [Google Scholar]
- 57. Kaada B, Torsteinbo O. Increase of plasma beta-endorphins in connective tissue massage. Gen Pharmacol 1989;20:487–489 [DOI] [PubMed] [Google Scholar]
- 58. da Silva JP, da Silva MA, Almeida AP, et al. . Laser therapy in the tissue repair process: A literature review. Photomed Laser Surg 2010;28:17–21 [DOI] [PubMed] [Google Scholar]
- 59. Peplow PV, Chung T-Y, Baxter GD. Application of low level laser technologies for pain relief and wound healing: Overview of scientific bases. Phys Ther Rev 2010;15:253–285 [Google Scholar]