To the editor
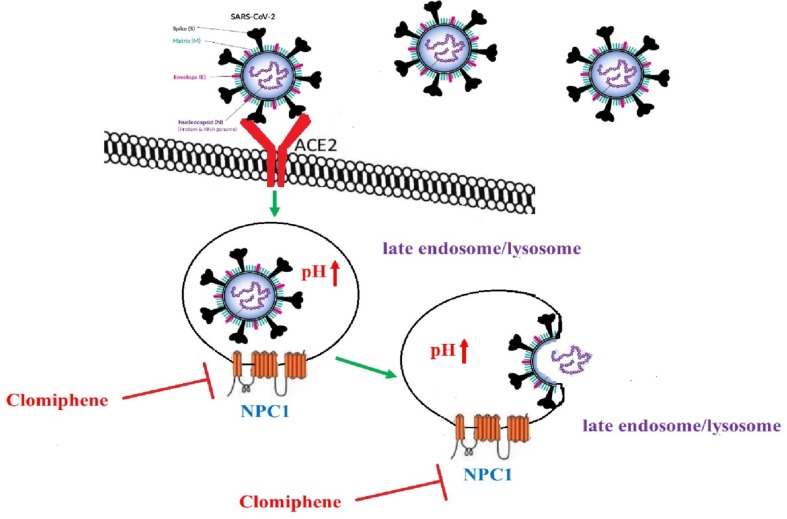
A novel coronavirus disease 2019 (COVID-19) outbreak is a global concern that has immeasurably impacted mankind's life. At present, there is no approved therapeutics available to treat this infection and only the symptomatic management is the base of clinical treatment [1]. Since, the drug development is a time-consuming process, repurposing the use of an old drug with low adverse effects to treat new infection could be a reasonable strategy to reduce the massive health and economic burden of the COVID-19 pandemic. The classical method to develop an antiviral agent is based on drugs affecting the functions of viral proteins that play essential role in the viral life cycles [2]. Clomiphene is a non‐steroidal triphenylethylene derivative belongs to a group of drugs known as selective estrogen receptor modulators (SERMs) which exerts both estrogenic agonist and estrogenic antagonist effects. This drug is used to treat female infertility due to anovulation. Furthermore, clomiphene is an efficient drug for reversing the impotence in men with hypogonadism due to low testosterone secretion [3]. Previous studies have shown that clomiphene has antiviral effects against Ebola virus. Screening studies for drug repurposing as antivirals indicated that the antiviral effect of clomiphene is related to cell-based mechanisms independent to the classical estrogen signaling pathway. In fact, this drug would interfere with a late stage of Ebola virus entry into target cells, likely affecting the triggering of fusion of the viral envelope with the endosomal limiting membrane. This drug showed EC50 values of 11 and 3.8 μM against the two strains EBOV-95 and EBOV-76, respectively, and a 90% of survival benefit for infected mice [4]. SARS-COV-2 is a lipid-enveloped virus encounter the endosomal/lysosomal host compartment in a critical step of infection [5]. Niemann-Pick type C (NP-C) disease is a rare neurodegenerative disease caused by deficient efflux of lipids from the late endosome/lysosome. The NP-C disease-causing gene (NPC1) has been intensively related with viral infection, as a filovirus receptor (e.g., Ebola) and through endosome/lysosome lipid trafficking [6]. It has been indicated that the intracellular trafficking of the SARS-CoV particle to NPC1-positive compartments of the endo/lysosomal system is crucial for establishing successful infection. Therefore, SARS-CoV-2, which has a same infectious life cycle as SARS-CoV would also be expected to require entry into NPC1-positive subcellular compartments, that is, late endosomes and lysosomes, in order for it to successfully establish infection. This suggests inhibitors of NPC1 could use as anti-SARS-CoV-2 drugs [7]. Previous studies have suggested that the clomiphene which inhibits EBOV entry exert its effects through an NPC1-dependent pathway [8], [9]. It’s possible that inhibition of NPC1 by clomiphene in SARS-CoV-2 disturbs viral infectivity via several lipid-dependent mechanisms such as cholesterol accumulation that impairs late endosome/lysosome function (e.g., increasing pH), which impair the microenvironment optimum for viral entry (Fig. 1 ) [10]. In summary, given the biological conservation of SARS-CoV-1 and SARS-CoV-2, and the role of the NPC1-dependent late endosome/lysosome lipid pathway in coronavirus infections, we suggest clomiphene as NPC1 inhibitor for treatment of patients with COVID-19. Since clomiphene is FDA-approved and clinically available, it could be a valuable adjuvant treatment in patients with COVID 19. However, clinical trials either as a single therapy or in combination with other anti-viral drugs are much needed. We hope that our hypothesis can provide a basis for future studies on the safe and effective use of clomiphene in patients with such infections.
Fig. 1.
Proposed inhibition of SARS-CoV-2 entry by clomiphene as inhibitor of NPC1. Inhibiting NPC1 by clomiphene leads to cholesterol accumulation that impairs late endosome/lysosome function (e.g., increasing pH), which impair the microenvironment for viral entry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The following authors have affiliations with organizations with direct or indirect financial interest in the subject matter discussed in the manuscript: Morteza Ghasemnejad-Berenji – Department of Pharmacology and Toxicology, Faculty of Pharmacy, Urmia University of Medical Sciences, Urmia, Iran. Sarvin Pashapour – Department of Pediatrics, Faculty of Medicine, Motahari hospital, Urmia , University of Medical Sciences, Urmia, Iran. Hojat Ghasemnejad-Berenji – Department of Anatomy and Reproductive Biology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Dhama K., Sharun K., Tiwari R. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Human Vacc Immunotherap. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Yang L., Liu F.-F. Overview of therapeutic drug research for COVID-19 in China. Acta Pharmacol Sin. 2020:1–8. doi: 10.1038/s41401-020-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scovell J.M., Khera M. Testosterone replacement therapy versus clomiphene citrate in the young hypogonadal male. Eur Urol Focus. 2018;4:321–323. doi: 10.1016/j.euf.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Nelson E.A., Barnes A.B., Wiehle R.D., Fontenot G.K., Hoenen T., White J.M. Clomiphene and its isomers block Ebola virus particle entry and infection with similar potency: potential therapeutic implications. Viruses. 2016;8:206. doi: 10.3390/v8080206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casalino L., Gaieb Z., Dommer A.C. Shielding and beyond: The roles of glycans in SARS-CoV-2 spike protein. bioRxiv. 2020 doi: 10.1021/acscentsci.0c01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons J.A., D'Souza R.S., Ruas M., Galione A., Casanova J.E., White J.M. Ebolavirus glycoprotein directs fusion through NPC1+ endolysosomes. J Virol. 2016;90:605–610. doi: 10.1128/JVI.01828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballout R.A., Sviridov D., Bukrinsky M.I., Remaley A.T. The lysosome: A potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications. FASEB J. 2020 doi: 10.1096/fj.202000654R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen L.M., Brannan J.M., Delos S.E. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005471. pp. 190ra179-190ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoemaker C.J., Schornberg K.L., Delos S.E. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturley S., Rajakumar T., Hammond N. Potential COVID-19 therapeutics from a rare disease: Weaponizing lipid dysregulation to combat viral infectivity. J Lipid Res. 2020 doi: 10.1194/jlr.R120000851. p. jlr. R120000851. [DOI] [PMC free article] [PubMed] [Google Scholar]