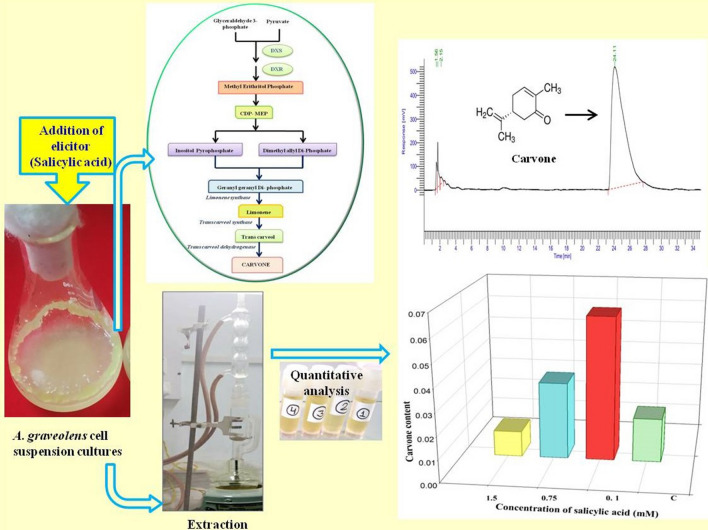
Abstract
The study illustrates the system for enhanced production of a medicinally important unexplored compound, carvone occurring naturally in Anethum graveolens. The effect of salicylic acid (SA) on biomass yield, carvone biosynthesis, growth and major enzymatic antioxidant parameters in A. graveolens was evaluated. The effects of different combinations of benzyl adenine (BA) and 1-Naphthalene acetic acid (NAA) were tested. Murashige and Skoog (MS) medium comprising 1.76 µM BA + 3.24 µM NAA was the best for friable callus induction. The friable callus was used for the initiation of cell suspension culture. MS salts in combination with 4.4 µM BA and 2.6 µM NAA, 3% sucrose was appropriate for cell growth and bioactive compound accumulation. The cell suspension cultures were then treated with SA (0.1, 0.75 and 1.5 mM) as an elicitor for four weeks. An up regulation of enzymatic antioxidants, ascorbate peroxidase (APX); superoxide dismutase (SOD) and catalase (CAT) activity with increasing concentrations of SA whereas a reduction in guaiacol peroxidase (GPX) activity was recorded at the end of the growth phase. The results also showed that higher concentrations of SA significantly increased malondialdehyde (MDA) and Proline content. Cell suspension culture was then subjected to extraction and isolation. The quantification of carvone through HPLC analysis revealed highest amount of carvone (0.063%) in cell suspension culture treated with 0.1 mM concentration of SA whereas higher concentration 0.75 mM SA showed reduction in amount (0.035%) of carvone. SA elicited cell suspension culture offered an effective and favorable in vitro method to improve the production of carvone for its potential use in pharmaceuticals.
Keywords: Anethum graveolens, Cell suspension cultures, Salicylic acid, Carvone, HPLC
Introduction
Anethum graveolens is an annual aromatic herb belonging to the family Apiaceae. Being one of the most important culinary herbs it’s been extensively used as a flavoring agent and spice from approximately 2000 years. There is a history of its use as an essential home remedy to treat infections related to the digestive system (Kaur and Arora, 2010; Jana and Shekhawat 2010a). A. graveolens possesses a broad range of naturally occurring secondary metabolites endowed with a wide variety of structural diversity and biological activities (Raghawan 2006; Dhir et al. 2014). The herb has gained renewed interest as it contains higher levels of carvone, fundamentally a monoterpene it is the most important constituent, synthesized in maximum proportion and responsible for imparting the plant it’s most important medicinal properties including its potential antiviral activity (De Carvalho et al. 2006; Coppens and Cesa 2014). It has now been widely used as a commercial product in food, cosmetic and pharmaceutical industry. Carvone is also used as flavor additive, fragrance enhancer, potato sprouting inhibitor, building block (Score et al. 1997), antimicrobial agent (Aggwart et al. 2002), anti tumor substance (Carter et al. 2000), insecticidal agent against fruit flies (Franzois 1997) and biochemical environmental indicator (Supuka and Berta 1998). Considering various applications of carvone and the mounting demand for this active component, the production of carvone needs to be improved using biotechnological approaches in an efficient way. In nature, the bioactive compound occurs in very small quantities therefore the production needs to be enhanced by in vitro culture techniques.
Plant cell culture is an effective feasible system to improve the production of secondary compounds of biological significance. In vitro cultivation of plant cells provides an additional advantage of manipulation of culture conditions (Jana and Shekhawat 2010b; Shekhawat et al. 2009, 2010). Biosynthetic pathways responsible for the synthesis of secondary metabolites are often inducible by exogenous addition of elicitor and precursor feeding leading to stimulation of secondary products accumulation (Barber et al. 2000; Gupta and Sharma 2014; Vats 2018). Hence elicitation has been considered as an effective strategy for enhanced accumulation of secondary metabolites.
Salicylic acid (SA), a potent signaling molecule in plants, plays different regulatory roles in growth, metabolism, development, interaction and biotic and abiotic stresses (Senaratna et al. 2000). These responses vary depending on the concentration and time duration of SA treatment. Although moderate doses of SA are known to improve the antioxidant activities and stimulate stress tolerance; higher concentrations lead to activation of the cell death pathway and develop sensitivity to stress (Yang et al. 2004). Furthermore, it is well recognized that stress tolerance is strictly associated with the antioxidant enzymatic activities, such as superoxide dismutase, ascorbate peroxidase and guaiacol peroxidase. The antioxidant activities in plants get activated in response to damage caused by the generation of ROS during stress conditions (Gupta and Huang 2014; Rao and Shekhawat 2016; Dwivedi et al. 2016). These biochemical estimations suggest a view about the developmental changes occurring during the growth phase of plant cell suspension cultures (Jana and Shekhawat 2010c; Jana and Shekhawat 2012a, b). Several studies report SA induced elicitation in plants; taxol synthesis in Taxus chinensis cell cultures (Wang et al. 2004); hypericin and pseudohypericin in Hypericum perforatum cell suspension cultures (Gadzovska et al. 2013); ginsenoside accumulation in Panax ginseng (Ali et al. 2006); Elutherosides in Eleutherococcus koreanum (Lee et al. 2015); Gymnemic acid in Gymnema sylvestre (Bhuvaneswari et al. 2014); Withanolides in Withania somnifera (Sivanandan et al. 2012); bioactive compounds in Orostachys cartilaginous (Wen et al. 2019) cell suspension cultures. However, insufficient information is available on the accumulation of carvone and its anti-oxidative mechanisms upon SA treatment. Meanwhile, no report could be traced regarding elicitation of carvone. The article gives detailed insights to the effect of SA on carvone production in cell suspension cultures of A. graveolens at shake flask scale.
Materials and methods
Collection of plant material and callus induction
Murashige and Skoog medium (Murashige and Skoog 1962) was used as the basal medium including 3% (w/v) sucrose and 0.7% (w/v) agar (chemicals for MS medium preparations were procured from HiMedia Laboratories, Merck, Germany). Phytohormones (HiMedia) from previously prepared stock solutions were added to the MS medium and pH (ELICO LI 120 pH meter) of the medium was maintained to 5.8 ± 2 by using 1 M KOH (Loba Chemie, Mumbai, India) / 1 M HCl (Fishers Scientific Co.). Leaf tissues from plantlets developed in vitro (Bulchandani and Shekhawat 2018) were utilized as explants for callus induction. The leaves were sliced into small pieces and inoculated on culture medium supplemented with different concentrations of BA and NAA. The inoculated flasks were incubated in thermostatically controlled culture rooms with a temperature of 26 ± 2 °C, a 12 h photoperiod provided by white fluorescent tubes (Philips, India Ltd.) with 40–50 µmole m−2 s−2 of light intensity; 55–60% RH.
Suspension culture development
Mature callus (0.5–1 g) obtained from the multiplication medium that comprised of MS augmented with a combination of 4.4 µM BA and 2.6 µM NAA was used as initial inoculums for the development of cell suspension cultures of A. graveolens. Three different concentrations of SA (molecular weight = 138.12; HiMedia, 250 g): 0.1 mM, 0.75 mM, 1.5 mM were incorporated into the MS liquid multiplication medium. The suspension cultures were incubated in an orbital shaker (Infors HT CH-4103 Bottmingen) with continuous agitation at a rate of 110 rpm. The samples from suspension cultures were collected after every seven days to study the biomass yield and packed cell volume.
Determination of lipid peroxidation
Lipid peroxidation was determined according to the procedure given by De Vos et al. (1989). The LP level was estimated by determining MDA using the extinction coefficient of 155 mM−1 cm−1. 0.5 g of freshly isolated suspension cells from 7, 14, 21 and 28 days old elicited suspension cultures of SA were subjected to homogenization in 10 ml of thiobarbituric acid and trichloroacetic acid. The absorbance of the supernatant was recorded at 532 and 600 nm.
Determination of proline accumulation
Estimation of proline was carried out by the method given by Bates et al. (1973). 0.5 g of sample was homogenized in 5 ml of sulfosalicylic acid. After centrifugation (Sigma Centrifuge, Sigma Aldrich) at 3000 × g for the duration of 20 min, the supernatant was reacted with glacial acetic acid (2 ml). After incubation at 100 °C for 60 min in a water bath, the reaction was terminated on ice bath. The mixture was spin for 1 min after subsequent addition of 4 ml toluene. The absorbance of chromatophores was recorded at 520 nm.
Enzymatic antioxidant assay
For the extraction of anti-oxidative enzymes, 0.5 g of freshly isolated suspension cultures were homogenized in 5 ml of pre chilled 50 mM phosphate buffer. The homogenate was subjected to centrifugation at 12,000 rpm for 10 min at 4℃. The supernatant so obtained was utilized for enzymatic estimation.
The SOD (superoxide dismutase) (EC 1.15.1.1) activity was estimated by the method given by Beauchamp and Fridovich (1971). The reduction of NBT by superoxide radicals to blue colored formazan was measured at 560 nm. The activity of Catalase enzyme CAT (EC 1.11.1.6) was determined by adopting the protocol given by Aebi (1974). The estimation was based on the disappearance of H2O2 in the presence of the enzyme source which was followed at 505 nm.
Ascorbate peroxidase activity, APX (EC 1.11.1.11) was calculated by the procedure suggested by Chen and Asada (1989). The rate of oxidation of ascorbic acid was assayed by observing the absorbance at 290 nm. The Guaiacol peroxidase enzyme activity, GPX (EC 1.11.1.7) was estimated by recording the absorbance at 436 nm according to the method given by Putter (1974).
Extraction procedure
The extraction of Carvone was achieved by the method given by Ahmed et al. (1980) with some minor modifications. Air-dried samples of suspension cultures were defatted with petroleum ether. Followed by treatment with KOH, the extracts were refluxed on steam bath for 90 min. Thereafter, they were extracted thrice with ethanol. After filtration, the samples were dried over anhydrous sodium sulfite; the extracts were dissolved in ethanol (2 ml) and stored at – 4 °C for HPLC analysis.
Quantitative estimation
Samples prepared by above-described procedure were further analyzed by HPLC by HPLC instrument (Perkin Elmer Series 200). The samples were passed through C18 column (150 × 4.6 mm), 0.5 micron. The samples were detected by PDA detector. The solvent system comprised of methanol and 1 ml TFA in 1000 ml filtered degas HPLC water. The quantitative estimation of Carvone production in cell suspension cultures of A. graveolens treated with or without SA was calculated using the formula:
For calibration, the analytical grade standard of carvone (with 99% purity) were procured from Sigma (22,060–1 ml), Sigma Aldrich, USA.
Statistic analysis
All the data is presented as mean ± S.E. The effects of different concentration of SA on biochemical parameters and carvone content were analyzed statistically by Sigma plot (Version: 12). The level of significance was determined by ANNOVA by SPSS software (Version: 16) at p ≤ 0.05.
Results and discussion
Explant and callus induction
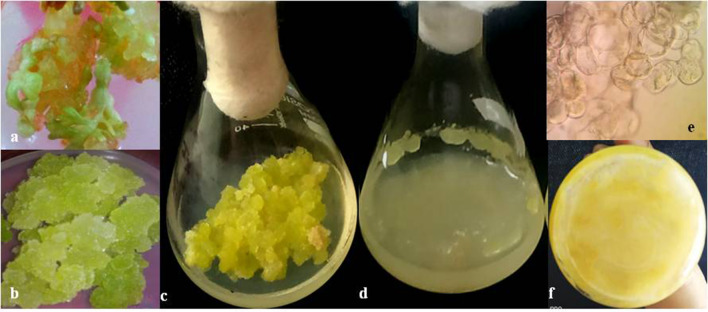
Callus was induced from leaf explants of A. graveolens. MS medium devoid of plant growth hormones did not show callus initiation from explants. Callus growth initiation was observed on MS basal medium augmented with BA and NAA in combination. The growth of calli was observed from the incised edges of explants (Fig. 1.a). Lower concentration of BA gave rise to compact nodular callus whereas higher concentrations of BA in combination with NAA induced green friable callus (Fig. 1.b, c). The highest frequency of leaf disc showing callus formation (77%) was with BA (1.76 μM) and NAA (3.24 μM), respectively (Table 1). Similar results of callus proliferation on MS medium fortified with a combination of auxin and cytokinin have been reported in Ceropegia bulbosa (Dhir and Shekhawat 2013; 2014), Salvadora persica (Mathur et al. 2002a, b; Mathur et al. 2008).
Fig. 1.
Establishment of callus and cell suspension cultures of A. graveolens. a Initiation of callus from leaf explants b mature callus in MS + BA at 1.76 µM + NAA at 3.24 µM c sub cultured callus for seeding cell suspension d suspension culture of A. graveolens in flask e photomicrograph of cell suspension growth during exponential phase (10X) f cell aggregates at the bottom of flask after third subculture
Table 1.
Effects of different combinations of BA and NAA on callus induction from leaf explants
| S. no | PGR’s (μM) | % Callogenesis | Nature of callus | |
|---|---|---|---|---|
| BA | NAA | |||
| 1 | 1.32 | – | – | – |
| 2 | 1.76 | – | 58 ± 0.0462c | Green compact |
| 3 | 1.76 | 3.24 | 77.70 ± 0.0267a | Green friable |
| 4 | 2.2 | – | 69. ± 0.0300b | Green compact |
| 5 | 2.2 | 5.4 | 44 ± 0.0300d | Green friable |
| 6 | 2.2 | 8.1 | 72 ± 0.0300ab | Green friable |
| 7 | 3.08 | 2.7 | 72 ± 0.0737ab | Green friable |
| 8 | 3.96 | 2.7 | 30 ± 0.0533e | Green compact |
| 9 | 4.4 | 2.7 | 69.3 ± 0.0567b | Multiple shoots |
| 10 | 5.28 | 2.7 | 30 ± 0.0267e | Green compact |
Results recorded after 3 weeks of culture. Data represents mean ± SE. Means having the same letter in each column do not differ significantly at P ≤ 0.05 (DMRT)
Determination of fresh weight, dry weight and PCV in cell suspension cultures
Cell suspension cultures of A. graveolens were initiated from healthy friable callus. The suspension cultures were maintained in MS liquid augmented with a combination of BA (4.4 μM); NAA (2.6 μM) and 3% sucrose (Fig. 1.d). Approximately 1 g of inoculum was sub-cultured from mother cultures for further multiplication of suspension cultures. The advantage of suspension cultures over other techniques is to minimize associated complex factors implicated in the organogenesis processes (Kim et al. 1995; Choi et al. 2000).
The suspension cultures analyzed for a period of four weeks determined that the cell growth exhibited a lag phase up to second week and subsequently the cells entered their exponential phase with a maximum dry weight of 3.49 mg/L on 21st day (Fig. 2 left axis). The production of terpenoids (carvone in the present study) is directly related to cell growth. A stationary phase was observed between 14 and 21 days during which maximum accumulation of carvone content was obtained. The cell cultures chiefly comprised of uniform cells of smaller size dispersed in medium and dense friable cell aggregates at the bottom of the flask (Fig. 1e, f). The cell suspension comprised of distinct types of cells ranging from spherical to elliptical. During the exponential phase of growth, the number of large spherical cells increases in cell cultures (Mathur and Shekhawat 2013). The growth of cell suspension cultures gets affected by the availability of macronutrients in the MS basal medium. Both, PCV and fresh weight of the cell cultures remained unaffected till 7th day of seeding the suspension. Later, PCV and fresh weight increased gradually with a significant increase in cell suspension cultures upto 21st day which was maximum with 4.95 ml/ 10 ml; insignificant reduction in packed cell volume (4.3 ml/10 ml) was observed on 28th day (Fig. 2 right axis). The initial appearance of culture cells was green which subsequently turned brown while approaching 28th day of the culture period. Similar results were also reported in suspension cultures of Stevia rebaudiana which showed higher packed cell volume until 21st day of the growth cycle (Gupta 2014). Browning of the cell suspensions was also observed by Gadzovska et al. (2013) at the end of the elicitor treatment period for each SA concentration tested.
Fig. 2.
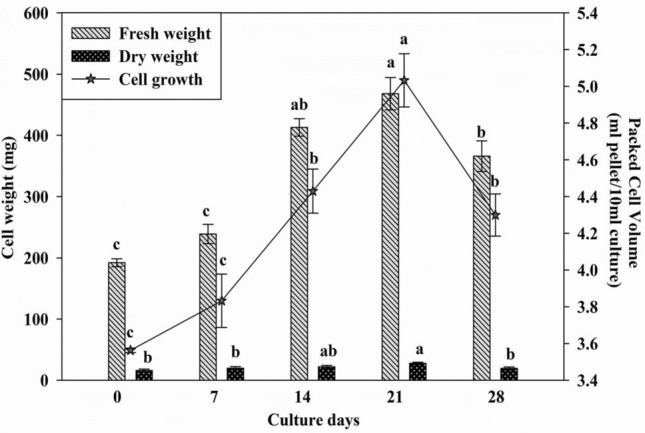
Growth kinetics of the optimized cell suspension cultures of A. graveolens. Data represent mean ± SEM, n = 3. Means with same letter do not differ significantly at P ≤ 0.05
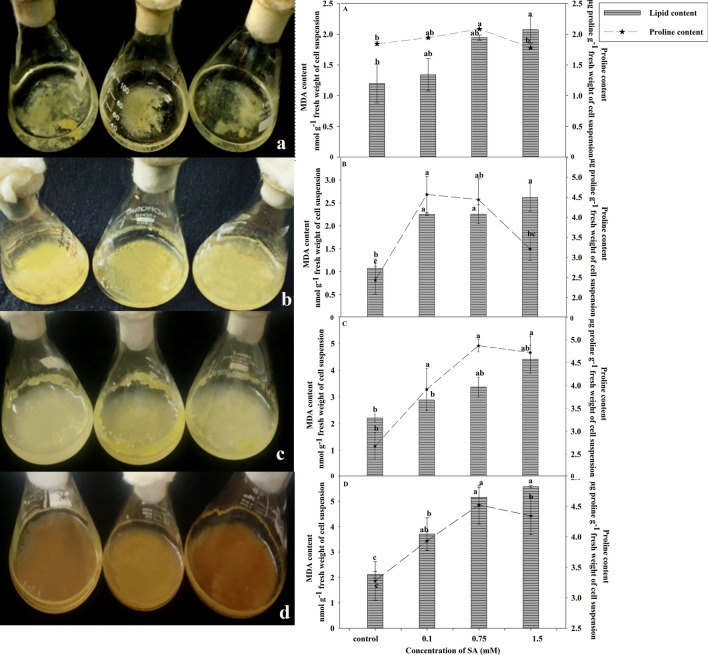
Non enzymatic parameters
The SA treated suspension cultures of A. graveolens showed a continuous rise in MDA content irrespective of the concentration of SA. Following 7th day, maximum increase (2.07 folds) was observed at 1.5 mM concentration of SA than in control (Fig. 3a). After two weeks, a significant rise (45%) was noticed in total MDA content at 1.5 mM concentration in SA treated cultures when compared to control. Similarly, after three weeks interval, 1.5 mM SA treated cultures increased twofold of non treated cultures (Fig. 3b, c). The observations of the present study are in corroboration with Ali et al. (2006), which showed that the formation of MDA content increased in the SA exposed roots of P. ginseng compared to the control. Through the growth phase, the highest level of lipid peroxidation was observed at 1.5 mM SA concentration during the fourth week which was 2.54 times higher than MDA content during first week (Fig. 3d). ROS generation due to induced stress brings about peroxidation of membrane lipids, leading to membrane damage (Scandalios 1993). In accordance with these early studies, exposure to SA significantly increased the MDA content, an index of lipid peroxidation. Elicitor induced ROS generation has been reported previously by Gomes et al. (2006) which showed enhancement in MDA content at 28th day of the growth cycle. However, lower MDA increases observed in SA-treated suspension cultures during initiation of cell growth suggest a better protection from oxidative damage.
Fig. 3.
MDA and proline content in cell suspension cultures of A. graveolens. The cell cultures were elicited with 0.1, 0.75 and 1.5 mM SA for a period of 4 weeks a Lag phase b; c exponential growth phase (second and third week) d declining phase (after fourth week) of SA elicitation. Control represents non treated cell line. Vertical bar and horizontal line represents mean ± standard error (n = 3) and were analyzed by one way ANOVA. The values are significant at 0.01 level
Proline has been reported to improve plant’s resistance to oxidative stress by scavenging ROS, by means of increasing anti-oxidative enzyme activity, thereby maintaining redox equilibrium (Zafar et al. 2020). The proline content showed a significant increase at 0.75 mM concentration after 21 days in cell suspension with respect to initial days of the culture period. 58% increase in proline content at 0.75 mM concentration of SA was observed on 21st day with respect to 7th day of the culture period. During the concluding phase of the culture period, the maximum accumulation of proline was observed at 0.75 mM SA concentration (Fig. 3). The elevation in proline accumulation could be directly correlated to the onset of stress conditions for plants. Progressive culture period leads to a reduction in essential nutrients for further development of the cell. According to Misra and Misra (2012), SA induces the activity of γ-glutamyl kinase and pyrroline-5-carboxylate reductase enzymes responsible for proline biosynthesis under stress conditions resulting in an enhanced level of proline. SA has already been reported to cause growth enhancement and stimulate proline accumulation in wheat plants (Shakirova et al. 2003).
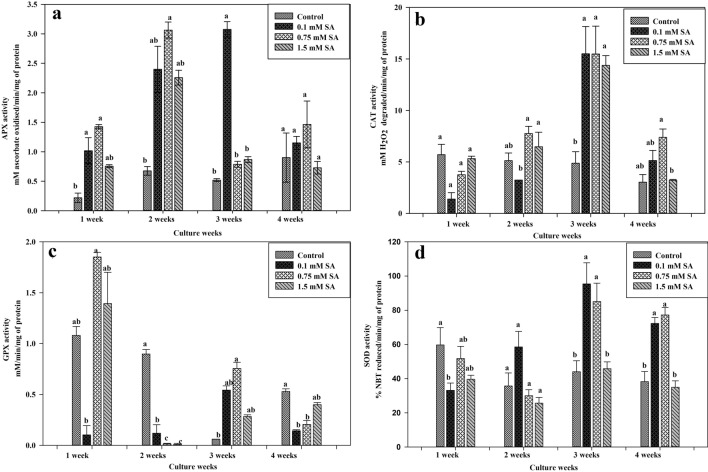
Enzymatic parameters
Visible changes in antioxidant enzymes were observed in SA treated cell suspension cultures when compared to control. Through the culture period, significant rise was observed in APX activity at 0.75 mM concentration after two weeks and 0.1 mM concentration after three weeks of the culture period (Fig. 4a). APX activity upregulated by 4.5 folds increase at 0.75 mM concentration of SA with respect to control whereas higher concentrations (1.5 mM) did not support APX activity. Therefore it appeared that APX played the most important role in offering resistance to H2O2 production. Similar increase in APX activity in suspension cultures of Jatropha curcas treated with Jasmonic acid was reported by Zaragoza et al. (2016). When compared to control, all the concentrations of SA improved the activity of Catalase. Among all the concentrations, maximum CAT activity with three folds increase compared to control was observed at 0.1 mM SA concentration (Fig. 4b). In the course of the culture growth period, the highest activity of CAT enzyme was recorded following the third week. SA-mediated stress responses are related to an increase in H2O2 generation by inhibition of CAT activity and promotion of peroxidase activity (Krantev et al. 2008). The amount of GPX was found to increase during initial days of culture period but showed a subsequent reduction with progressive culture growth. During the first week, highest GPX activity with 0.8% increase as compared to control was recorded at 0.75 mM SA concentration which continued till the third week of culture period (Fig. 4c). Studies have demonstrated that plants activate their defense systems by adjusting antioxidant molecule levels and inducing anti-oxidative enzymes to counteract oxidative stress (Noctor and Foyer 1998). Antioxidant defense enzymes such as APX, CAT and GPX are systems that are devised to minimize the ROS levels (Mittler 2002).
Fig. 4.
Antioxidant enzyme APX (a), CAT (b), GPX (c), and SOD (d) activity under SA pretreatments (0.1, 0.75 and 1.5 mM), exposure duration (7, 14, 21 and 28 day) in cell suspension cultures of A. graveolens. Data represents the mean ± standard error (n = 3) and were analyzed by one way ANOVA. The values are significant at 0.01 level
SOD activity is responsible for the degradation of the superoxide radical (O2•−), producing O2 and H2O2 (Ighodaro and Akinloye 2018). Here, enhancement in SOD concentration was recorded with progressing culture growth up to the third week which declined after the fourth week of the culture period (Fig. 4d). During the growth phase, the maximum SOD activity was noticed at 0.1 mM SA concentration which further got reduced at higher concentrations. In previous reports by Ali et al. (2006), SA induced SOD activity increased in roots of P. ginseng to maximum by 56% and retained the elevated levels in remaining SA-treated roots as compared to the control due to the increase of the scavenging capacity for O2− and prevents cellular damage.
Effect of SA on Carvone production
In the present investigation, the effect of different concentrations of SA on carvone content in cell suspension cultures of A. graveolens was examined on the 21st day of the growth phase. As reported in earlier studies by Bondarev (2001), the suspension cultures (control) collected after 3rd week and before 4th week produced the maximum amount of secondary metabolites (terpenoid) in comparison to initial culture weeks.
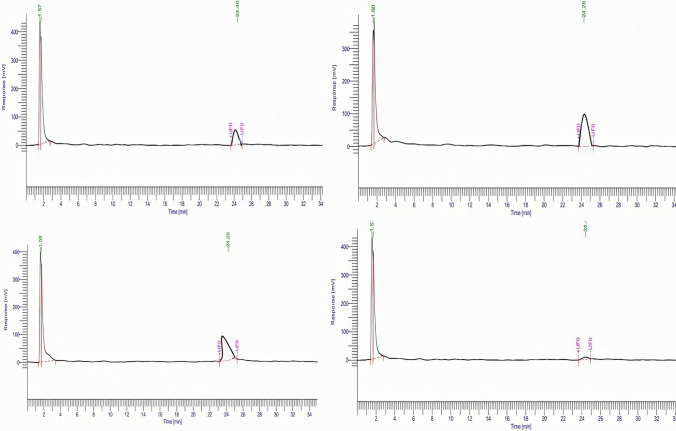
The content of carvone in the cell suspension cultures was significantly different among the treatments. The quantity of carvone production in suspension cultures treated with SA has been depicted in Fig. 5; 0.1 mM concentration of SA stimulated a threefold increase in carvone content with respect to control cultures (0.063%). Highest APX as well as CAT activity was also recorded at this particular concentration. The results in this regard are supported by Wen et al. (2019) that reported lower concentrations of SA (25–150 μM) result in enhanced production of secondary metabolites in cell cultures of Orostachys cartilaginous. According to the study, the total polysaccharide content increased to the maximum with 218.5 mg/g DW at 100 μM SA, phenolic compound 27.2 mg/g DW, and flavonoid content 35.4 mg/g DW. Further, it was observed that the content of carvone reduced to 0.035% concentration at 0.75 mM SA treatment which is 1.8% more than control cultures (Fig. 6a). At 1.5 mM concentration, the carvone content reduced to its minimum and reached below the control value (0.013%) as compared with all the samples collected on the 21st day which shows that only lower concentrations of SA support carvone accumulation in cell suspension cultures of A. graveolens. For instance, Kang et al. (2009) reported 3.1 and 6.1 times improved production of ginkgolides A and B, compared with control, in two weeks old SA (0.1 mM) treated cell cultures of Ginkgo biloba.
Fig. 5.
HPLC chromatogram for cell suspension cultures of A. graveolens after 21 days of SA pretreatment: control (a) 0.1 mM SA (b) 0.75 mM SA (c) 1.5 mM SA (d). Detection wavelength = 254 nm
Fig. 6.
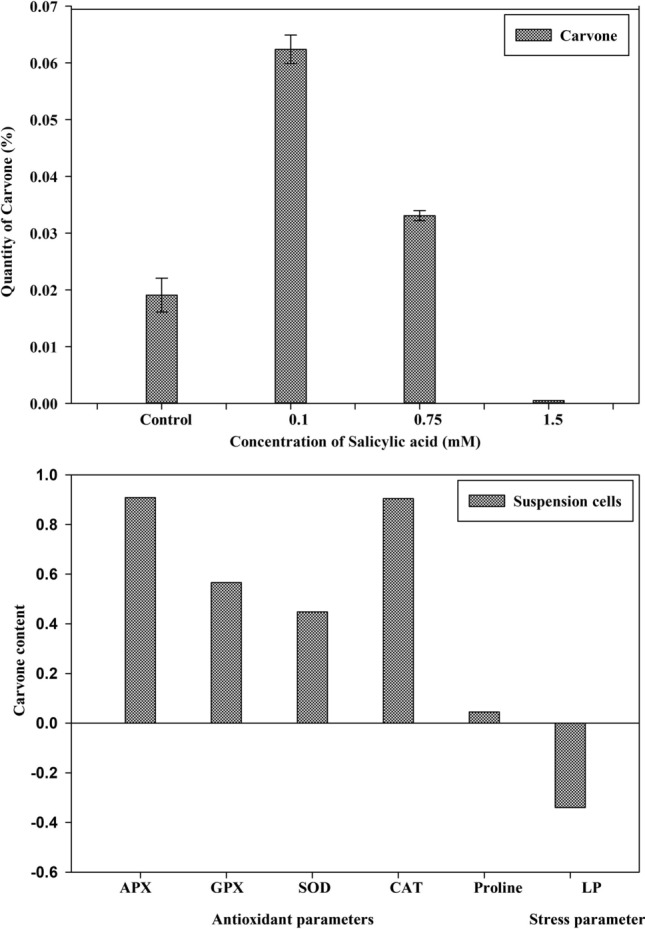
a Total carvone content in cell suspension cultures of A. graveolens after 21 days of SA (0.1, 0.75 and 1.5 mM) pretreatment b Linear correlation of carvone content with antioxidant parameters in cell suspension cultures of A. graveolens. The correlation values are significant at 0.01 level
The activated antioxidant defense mechanism at lower SA concentrations establishes the balance in antioxidant activities and nuetralization of free redicals but elevated concentrations and prolonged treatment duration affects the morphology and physiology of the cells. The results suggested a positive correlation between carvone accumulation and antioxidant activities at lower concentration of SA (Fig. 6b). However MDA content showed a negative correlation with metabolite accumulation which suggests that rise in SA concentration above the optimum levels for carvone accumulation leads to a negative impact on antioxidant activities as manifested by increase in lipid peroxidation levels. These results clearly confer that 0.1 mM concentration of SA could be used for the production of carvone on a large scale using cell suspension cultures of A. graveolens.
Conclusion
This is the first report on the subject of cell suspension culture studies in A. graveolens and analysis of the effect of SA during the growth period. The study demonstrated a feasible approach for SA induced enhanced accumulation of an overlooked therapeutically important compound; carvone in cell suspension cultures of A. graveolens. A synergistic correlation was manifested between the enzymatic parameters and carvone production at different levels of SA treatments. Through the growth phase, all the enzymatic antioxidant components maintained higher values except GPX in SA elicited cultures. However, the treatment of suspension cultures with SA as an elicitor showed a decrease in MDA content with increasing concentration of SA. The outcomes suggested that an optimum concentration of SA positively influences the production of carvone (Fig. 7). Present investigation confers SA as a promising elicitor for stimulation of the antioxidant defense system and carvone accumulation. The medicinal properties of carvone have refocused attention towards the improved production of this compound. Use of different elicitors for modification of plant secondary metabolism offers an alternative approach to induce some beneficial changes in the production of phytochemicals. The approach discussed in the present study could be efficiently utilized for exploring the potential for the commercial production of carvone from A. graveolens at pilot scale and subsequently commercial scale.
Fig. 7.
A descriptive model of targeted approach for elicitor mediated enhanced accumulation of carvone used in the study
Acknowledgements
Authors gratefully acknowledge University Grant Commission, New Delhi for providing financial assistance in the form of Centre for Advanced Study scheme.
Compliance with ethical standards
Conflict of interest
The authors declare none.
References
- Aebi H. Catalases. In: Bergmeyer HU, editor. Methods of enzymatic analysis, Verlags Chemie. New York: Academic Press Inc; 1974. p. 680. [Google Scholar]
- Aggarwal KK, Khanuja SPS, Ahmad A, Kumar TRS, Gupta VK, Kumar S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr J. 2002;17:59–63. [Google Scholar]
- Ahmed MS, Dobberstein RH, Farnsworth NR. Stevia rebaudiana: I. Use of p-bromophenacyl bromide to enhance ultraviolet detection of water-soluble organic acids (steviolbioside and rebaudioside B) in high-performance liquid chromatographic analysis. J Chromatogr A. 1980;192(2):387–393. [Google Scholar]
- Ali MB, Yu KW, Hahn EJ, Paek KY. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006;25(6):613–620. doi: 10.1007/s00299-005-0065-6. [DOI] [PubMed] [Google Scholar]
- Barber MS, McConnell VS, DeCaux BS. Antimicrobial intermediates of the general phenylpropanoid and lignin specific pathways. Phytochem. 2000;54:53–56. doi: 10.1016/s0031-9422(00)00038-8. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bondarev N, Reshetnyak O, Nosov A. Peculiarities of diterpenoid steviol glycoside production in in vitro cultures of Stevia rebaudiana Bertoni. Plant Sci. 2001;161:155–163. [Google Scholar]
- Bulchandani N, Shekhawat GS. Role of phytohormones and PGR's in micropropagation of A. graveolens L. through axillary shoots and evaluation of NaCl effect on growth parameters. Vegetos Int J Plant Res. 2018;31:76–81. [Google Scholar]
- Carter R, Hodgetts K, McKenna J, Magnus P, Wren S. Studies on the stereoselective synthesis of the marine antitumor agent eleutherobin. Tetrahedron. 2000;56:4367–4382. [Google Scholar]
- Carvalho D, Carla CCR, Manuela M, Da Fonseca R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006;95(3):413–422. [Google Scholar]
- Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Chodisetti B, Rao K, Gandi S, Giri A. Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules—methyl jasmonate and salicylic acid. Vitro Cell Dev Biol Plant. 2015;51(1):88–92. [Google Scholar]
- Choi SM, Son SH, Yun SR, Kwon OW, Seon JH, Paek KY. Pilotscale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tiss Organ Cult. 2000;62:187–193. [Google Scholar]
- Coppens C and Cesa Alliance SA (2014) Viral inhibitor compositions for in vivo therapeutic use comprising a combination of (-)-carvone, geraniol and a further essential oil component. US Patent 8,883,859
- De Vos CHR, Schat H, Vooijs R, Ernst WHO. Copper-induced damage to the permeability barrier in roots of silene cuiubalus. J Plant Physiol. 1989;135:164–179. [Google Scholar]
- Dhir R, Shekhawat GS. Production, storability and morphogenic response of alginate encapsulated axillary meristems and genetic fidelity evaluation of in vitro regenerated Ceropegia bulbosa: A pharmaceutically important threatened plant species. Indus Crops Prod. 2013;47:139–144. [Google Scholar]
- Dhir R, Shekhawat GS. In vitro propagation using transverse thin cell layer culture and homogeneity assessment in Ceropegia bulbosa Roxb. J Plant Growth Regul. 2014;33(4):820–830. [Google Scholar]
- Dhir R, Shekhawat GS, Alam A. Improved protocol for somatic embryogenesis and calcium alginate encapsulation in A. graveolens L.: a medicinal herb. Appl Biochem Biotechnol. 2014;173(8):2267–2278. doi: 10.1007/s12010-014-1032-x. [DOI] [PubMed] [Google Scholar]
- Dwivedi S, Alam A, Shekhawat GS. Antioxidant response of Stevia rebaudiana (Bertoni) Bertoni (Angiosperms; Asteraceae) during developing phase of suspension cell culture. Plant Sci Today. 2016;3(2):115–212. [Google Scholar]
- Franzios G, Mirotsou M, Hatziapostolou E, Kral J, Scouras ZG, Mavragani TP. Insecticidal and genotoxic activities of mint essential oils. J Agric Food Chem. 1997;45:2690–2694. [Google Scholar]
- Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagege D, Courtois D, Joseph C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tiss Organ Cult. 2013;113(1):25–39. [Google Scholar]
- Gomes Junior RA, Moldes CA, Delite FS, Pompeu GB, Gratao PL, Mazzafera P, Lea PJ, Azevedo RA. Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere. 2006;65(8):1330–1337. doi: 10.1016/j.chemosphere.2006.04.056. [DOI] [PubMed] [Google Scholar]
- Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom. 2014 doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Sharma S, Saxena S. Effect of salts (NaCl and Na2CO3) on callus and suspension culture of Stevia rebaudiana for steviol glycoside production. Appl Biochem Biotechnol. 2014;172(6):2894–2906. doi: 10.1007/s12010-014-0736-2. [DOI] [PubMed] [Google Scholar]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018;54(4):287–293. [Google Scholar]
- Jana S, Shekhawat GS. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacol Rev. 2010;4(7):190–195. doi: 10.4103/0973-7847.70915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S, Shekhawat GS. Plant growth regulators, adenine sulfate and carbohydrates regulate organogenesis and in vitro flowering of Anethum graveolens. Acta Physiol Plant. 2010;33:305–311. [Google Scholar]
- Jana S, Shekhawat GS. Phytochemical analysis and antibacterial screening of in vivo and in vitro extracts of Indian medicinal herb: Anethum graveolens. Res J Med Plant. 2010;4(4):206–212. [Google Scholar]
- Jana S, Shekhawat GS. RAPD analysis, antioxidant study during cell differentiation and standardization of in vitro regeneration protocol of Anethum graveolens: A medicinal herb and spice. Biol Plant. 2012;56(1):9–14. [Google Scholar]
- Jana S, Shekhawat GS. Critical review on medicinally potent plant species: Gloriosa superba. Fitoterap. 2012;82:293–301. doi: 10.1016/j.fitote.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Kang SM, Min JY, Kim YD, Karigar CS, Kim SW, Goo GH, Choi MS. Effect of biotic elicitors on the accumulation of bilobalide and ginkgolides in Ginkgo biloba cell cultures. J Biotechnol. 2009;139(1):84–88. doi: 10.1016/j.jbiotec.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Kaur GJ, Arora DS. Bioactive potential of A.graveolens, Foeniculum vulgare and Trachyspermum ammi belonging to the family Umbelliferae - Current status. J Med Plant Res. 2010;4(2):087–094. [Google Scholar]
- Kim SG, Soh WY, Cho DY. Saikosaponin content in adventitious root formed from callus of Bupleurum falcatum. Korean J Plant Tiss Cult. 1995;22:29–33. [Google Scholar]
- Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol. 2008;165(9):920–931. doi: 10.1016/j.jplph.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Park SY, Paek KY. Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant Cell Tiss Organ Cult. 2015;120(1):1–10. [Google Scholar]
- Mathur S, Shekhawat GS, Batra A. Micropropagation of Salvadora persica via cotyledonary nodes. J of Biotech. 2002;1:197–200. [Google Scholar]
- Mathur S, Shekhawat GS, Batra A. An efficient in in vitro method for mass propagation of Salvadora persica via apical meristem. J Biochem Biotech. 2002;11:125–127. [Google Scholar]
- Mathur S, Shekhawat GS, Batra A. Somatic embryogenesis and plantlet regeneration from cotyledon explants of Salvadora persica L. Phytomorp. 2008;58(1–2):57–63. [Google Scholar]
- Mathur S, Shekhawat GS. Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiol Plant. 2013;35(3):931–939. [Google Scholar]
- Misra N, Misra R. Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentina leaves grown under salinity stress. Am Eurasian JAgric Environ Sci. 2012;12:1601–1609. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;9:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Putter J. Methods of enzymatic analysis. New York: Academic Press Inc; 1974. Peroxidases; pp. 685–690. [Google Scholar]
- Raghavan S (2006) Handbook of spices, seasoning and flavourings. 2 nd edition. CRC Press Taylor and Franci group, Boca Raton, New York, p 63–64,107–109
- Rao S and Shekhawat GS (2016) Phytotoxicity and oxidative stress perspective of two selected nanoparticles in Brassica juncea. 3 Biotech 6(2): 244 [DOI] [PMC free article] [PubMed]
- Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101(1):7. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Score C, Lorenzi R, Ranall P. The effect of S- (+)-carvone treatments on seed potato tuber dormancy and sprouting. Potato Res. 1997;40:155–161. [Google Scholar]
- Senaratna T, Touvhell D, Bunn E, Dixon K. Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plant. J Plant Growth Regul. 2000;30:157–161. [Google Scholar]
- Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003;164:317–322. [Google Scholar]
- Shekhawat GS, Mathur S, Batra A. Role of phytohormones and various nitrogen inorganic and organic nutrients in induction of somatic embryogenesis in cell culture derived from leaflets of Azadirachta indica A. Juss Biol Plant. 2009;53(4):707–710. [Google Scholar]
- Shekhawat GS, Verma K, Jana S, Singh K, Teotia P, Prasad A. In vitro biochemical evaluation of cadmium tolerance mechanism in callus and seedlings of Brassica juncea. Protoplasma. 2010;239(1):31–38. doi: 10.1007/s00709-009-0079-y. [DOI] [PubMed] [Google Scholar]
- Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Dev GK, Ganapathi A. Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L) Dunal. Appl Biochem Biotechnol. 2012;168(3):681–696. doi: 10.1007/s12010-012-9809-2. [DOI] [PubMed] [Google Scholar]
- Supuka J, Berta F. The composition of terpenes in needles of white pine (Pinus strobus L.) growing in urban environment. Ekologia-Bratislava. 1998;17:419–433. [Google Scholar]
- Vats S. Larvicidal activity and in vitro regulation of rotenoids from Cassia tora L. 3 Biotech. 2018;8(1):13. doi: 10.1007/s13205-017-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YD, Yuan YJ, Wu JC. Induction studies of methyl jasmonate and salicylic acid on taxane production in suspension cultures of Taxus chinensis var. mairei. Biocheml Engg J. 2004;19:259–265. [Google Scholar]
- Wen T, Hao YJ, An XL, Sun HD, Li YR, Chen X, Lian ML. Improvement of bioactive compound accumulation in cell cultures of Orostachys cartilaginous A. Bor. through elicitation with salicylic acid and effect of cell extract on bioactive activity. Ind Crop Prod. 2019;139:111570. [Google Scholar]
- Yang Y, Qi M, Mei C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004;40(6):909–919. doi: 10.1111/j.1365-313X.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- Zafar N, Mujib A, Ali M, Tonk D, Gulzar B, Malik MK, Mamgain J, Sayeed R. Cadmium chloride (CdCl2) elicitation improves reserpine and ajmalicine yield in Rauvolfa serpentina as revealed by high-performance thin-layer chromatography (HPTLC) Biotech. 2020;10:344. doi: 10.1007/s13205-020-02339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza MF, Lucho CGG, Ponce NT, Esparza GF, Poggi VH, Cerda G, Rojas CM, Trejo TG, Ramos VAC. Jasmonic acid stimulates the oxidative responses and triterpene production in Jatropha curcas cell suspension cultures through mevalonate as biosynthetic precursor. Plant Cell Tiss Organ Cult. 2016;127(1):47–56. [Google Scholar]