Abstract
Background and Aim
Fecal microbiota transplantation (FMT) is a highly effective therapy for recurrent or refractory Clostridioides difficile infection (rCDI). Despite inclusion in society guidelines, the uptake of FMT therapy has been variable. Physician and patient attitudes may be a barrier to evidence‐based uptake of therapies; however, data assessing attitudes regarding FMT for rCDI are limited.
Methods
The South Australian FMT for CDI database prospectively recorded patient outcomes of FMT for CDI from August 2013 to January 2019. A total of 93 consecutive patients who underwent FMT for rCDI in South Australia were invited to participate in a 20‐question survey regarding the patient experience of FMT. All gastroenterologists and infectious disease physicians practicing in South Australia were invited to participate in an online survey comprised of 22 questions that addressed referral experience, indications for referral, perceived risks, and regulation and funding.
Results
Fifty‐four patients (54/93, 58%) returned the survey, of whom 52 (96%) would recommend FMT to others, and 51 (94%) were satisfied with treatment outcome. Fifty physicians returned the online survey (50/100, 50%), of whom 23 (46%) were concerned about disease transmission risk, and 15 (30%) believed that the risk of FMT would outweigh the benefit. Infectious diseases physicians and advanced trainees had significantly greater concern regarding the potential alteration of the microbiome than gastroenterology physicians and advanced trainees (8/17 (47%) vs 6/33 (18%); P = 0.047).
Conclusion
Despite high levels of patient‐reported satisfaction following FMT, physician‐reported reservations exist and may present a barrier to uptake of this therapy.
Keywords: Clostridioides difficile, fecal microbiota transplantation, perception, physician, stool bank

Fecal microbiota transplantation (FMT) is a highly effective therapy for recurrent or refractory Clostridioides difficile infection; however, physician and patient attitudes may be a barrier to uptake. Of 54 surveyed FMT recipients, 52 (96%) would recommend FMT treatment to others. In contrast, 15 of 50 (30%) physicians believed the risk of FMT outweighed the benefit.
Introduction
Clostridioides difficile infection (CDI) is the most common cause of health‐care‐associated diarrhea and is associated with significant morbidity, mortality, and costs worldwide. 1 Recurrence of CDI following standard first‐line antibiotics is common and occurs in approximately 35%. 2 In those patients who do relapse, further antibiotic treatments give diminishing rates of cure; after a second recurrence, the chance of further recurrence increases to 60% and is even greater for subsequent recurrences. 3 Fecal microbiota transplantation (FMT), the transfer of stool from a healthy individual to a person with disease, has emerged over the last decade as the most effective therapy for recurrent or refractory CDI (rCDI), with rates of primary cure between 81 and 96%. 4 , 5 FMT is well tolerated and safe, with very few serious adverse effects, 4 even in the elderly and immunocompromised, and is more cost‐effective than traditional antibiotic therapy for rCDI. 6
Despite a strong body of evidence supporting the efficacy and safety of FMT for CDI and inclusion in treatment guidelines, 7 , 8 , 9 , 10 , 11 the uptake of this therapy has been variable, with many centers worldwide still unable to provide the service. 10 Local logistical and regulatory issues exist, which serve as barriers to widespread and equitable access to FMT for patients with rCDI. 12 In addition to this, physician and patient attitudes may also be barriers to the uptake of FMT, with limited awareness, provider resistance, and lack of availability cited as potential contributing factors. 13
With the establishment of a state‐based stool bank in South Australia in 2013, 14 , 15 there has been universal access to this therapy within the public health system in the state. However, the degree of patient and physician awareness of, and experience with, FMT is not known. Furthermore, it is unknown whether inconsistencies between patient and physician attitudes toward FMT still exist and if attitudinal factors may affect the uptake of this therapy. The aim of this study was therefore to gain an understanding of patient and physician perception and experience with FMT for CDI in South Australia and identify attitudinal discrepancies to provide insight into potential barriers of uptake of this therapy in the future.
Methods
Study participants
The South Australian FMT for CDI database was interrogated, and the first 93 consecutive patients to undergo FMT for rCDI in South Australia from August 2013 to January 2019 were included and invited to participate in the survey. Patient surveys were posted and returned via mail.
Both gastroenterologists and gastroenterology trainees, as well as infectious disease (ID) physicians and ID trainees, practicing in public and private hospital systems were identified using existing practitioner registers and contacted via email. These registers contain all known practicing gastroenterology and ID doctors in the state. A total of 69 gastroenterologists and 31 ID physicians from South Australia were invited to participate in an online survey via email.
Survey development
A 20‐item paper survey was devised for the patient group (patient survey questions listed in (Table 1). An electronic survey (Table 2) was developed for the physician group using the online program SurveyMonkey. It was distributed via email in April and May 2018. It comprised 22 items and addressed referral experience, indications for referral, perceived risks, and regulation and funding. The Central Adelaide Local Health Network ethics committee approved the distribution of the surveys and collection of study data.
Table 1.
Patient survey questions
| Question | Options |
|---|---|
| What is your age? | |
| What is your gender? |
|
| What date was your FMT? | |
| How was your FMT delivered? |
|
| Had you heard of FMT prior to developing Clostridium difficile infection? |
|
| How did you first hear of FMT as a treatment? |
|
| What was your perception of FMT when first discussed with your doctor as a treatment for your C. difficile? |
|
| Did this perception change after FMT? |
If yes, how? |
| Would you recommend FMT to other patients with CDI? |
|
| How many relapses did you have prior to FMT? | |
| Have you had a relapse since FMT? |
If yes, how was this treated? |
| How long did it take for symptoms to resolve after FMT? |
|
| Have you developed any new diseases or symptoms following FMT? |
If yes, describe |
| Have you noticed improvement in any other medical conditions after FMT? |
If yes, describe |
| Are you concerned about infection risk from FMT? |
|
| Are you satisfied with your treatment outcome? |
|
| Who do you believe would be an ideal donor? |
|
| Do you think a third party (i.e. Medicare, private insurance or state government) should subsidise the costs to patients for recurrent or refractory FMT? |
|
| In your opinion, should FMT be classified as: |
|
CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation.
Table 2.
Physician survey questions
| Question | Options |
|---|---|
| What is your gender |
|
| What is your age? |
|
| What is your speciality? |
|
| What is the nature of the majority of your practice? |
|
| Are you aware of the existence of an FMT service in South Australia? |
|
| Have you ever referred a patient with CDI for FMT? |
|
If the above answer was yes:
|
|
| Have you seen any new diseases develop in your patients following FMT? |
|
| In you patients who have received FMT for CDI and who have other medical comorbidities, have you noticed any improvement or deterioration in these conditions following FMT? |
|
| For which of the following patients with C. difficile in an outpatient setting would you consider FMT? (may select more than one answer) |
|
| For which of the following patients hospitalised with C. difficile would you consider FMT? (may select more than one answer) |
|
| For which of the following patients with C difficile would you consider FMT? (may select more than one answer) |
|
| Do you believe most of your patients with recurrent or refractor CDI would consider FMT? |
|
| If above answer was no—what do you think would be their main reason for not considering FMT? |
|
| Are you concerned regarding potential alteration in the recipient's microbiome? |
|
| Are you concerned about disease transmission risk? |
|
| If above answer was yes, what are your main concerns? (may select more than one answer) |
|
| Do you believe these risks outweigh the potential benefits? |
|
| How do you believe FMT should be delivered? |
|
| Who do you believe would be an ideal donor? |
|
| Do you think a third party (i.e. Medicare, private insurance or state government) should subsidise the costs to patients for recurrent or refractory FMT? |
|
| There is a current debate about the regulation of FMT. In your opinion, should processed donor faeces for FMT be classified as: |
|
CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation; HDU, high dependency unit; ICU, intensive care unit.
Clinical data
The South Australian FMT for CDI database was established in 2013 and prospectively recorded patient demographic details, clinical details of CDI, and outcomes of FMT for CDI. Attempts were made to contact all patients within 3 months following FMT to assess clinical cure. In case a patient could not be contacted, medical records were reviewed. Primary cure was defined as resolution of symptoms or a negative C. Difficile toxin test following a single FMT; secondary cure was defined as resolution of symptoms or a negative C. Difficile toxin test following multiple FMTs.
Results
Patient survey
Patient demographics, disease, and treatment characteristics are shown in Table 3. Regarding patient perceptions prior to FMT, 37 of 54 (69%) patients reported having no concerns regarding FMT prior to the procedure. The remaining 17 (31%) patients had reservations when first offered FMT; in all 17 patients, the concerns were aesthetic (the “yuck factor”); 6 of these 17 patients were also concerned about infection, and 2 were concerned about the colonoscopy procedure. Awareness of FMT prior to developing CDI was relatively low, with only 20 (37%) of patients reporting prior knowledge of the procedure. Of the 20 patients under 60 years of age, 10 (50%) had prior awareness, compared with 10 of 34 (29%) of the patients over 60 years of age. Overall, 29 of 54 (54%) first heard of FMT from a medical specialist, 17 (31%) heard about FMT through the media, and only 2 (3.7%) heard through their general practitioner (GP).
Table 3.
Demographics of patient respondents and disease and treatment characteristics
| Total respondents | n = 54 |
|---|---|
| Female gender, n (%) | 36 (67) |
| Median age, n (IQR) | 65.5 (51–79) |
| Route of FMT administration | |
| Colonoscopy, n (%) | 51 (94) |
| Push enteroscopy, n (%) | 1 (2) |
| Colonoscopy and enteroscopy, n (%) | 1 (2) |
| Enema, n (%) | 1 (2) |
| Median number of relapses prior to FMT, n (range, IQR) | 3 (0–12, 2–4) |
| Primary cure rate in respondents, n (%) | 51 (94) |
| Timing of symptom response to FMT | |
| Within days, n (%) | 32 (59) |
| Within weeks, n (%) | 13 (24) |
FMT, fecal microbiota transplantation; IQR, interquartile range.
Almost all patients (52/54 [96%]) would recommend FMT to others, and 51 of 54 (94%) were satisfied with treatment outcome. Primary cure was achieved in 51 (94%) (compared with 26/31 [84%] of patients who did not return the survey).
The majority of respondents, 40 of 46 (87%), believed the ideal donor was anonymous to the recipient, with 2 (4%) preferring a sibling, 1 (2%) preferring a friend, and 3 (5%) happy with any donor, known or anonymous. When asked how FMT should be regulated, 33 of 54 (61%) thought FMT should be classified as a bodily tissue donation; only 7 (13%) thought FMT should be classified as a drug, and 14 (26%) were unsure. All 54 patients (100%) believed the cost should be covered by government or medical insurance providers.
Physician survey
Respondents
One hundred physicians were contacted, and 50 completed the online survey (50% response, male: female 32:18). Gastroenterology (GE) consultants or advanced trainees made up 66% (33/50); 17 (34%) were ID consultants or advanced trainees (Fig. 1).
Figure 1.
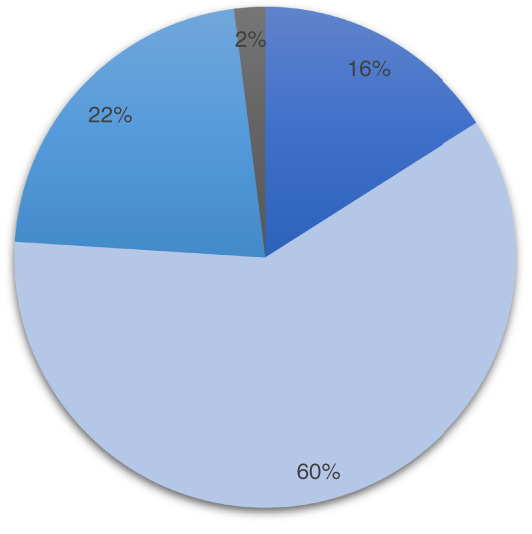
Job descriptions of physician respondents. ( ), Advanced trainee; (
), Advanced trainee; ( ), staff specialist; (
), staff specialist; ( ), private practice; (
), private practice; ( ), predominantly research.
), predominantly research.
Experience with FMT
The majority of physicians, 48 of 50 (96%), were aware of the existence of the FMT service, and 29 (58%) had referred at least one patient with CDI for FMT (median 2, interquartile range [IQR] 1–2), with 28 of the 29 (97%) witnessing primary cure in their patient(s). All physicians could envisage using the service again in the future. Regarding previous patient referrals, 21 of 33 (64%) of gastroenterologists had prior experience, compared with 8 of 17 (47%) of ID physicians. None noted new diseases developing in their patients following FMT.
Indications for FMT
In the outpatient setting, 57% of clinicians would consider FMT after the second recurrence, and 55% would consider FMT after three or more recurrences (Table 4). In the inpatient setting, this was 61 and 47%, respectively. After the first recurrence in the outpatient setting, 18% of clinicians would consider FMT, compared with 41% in the inpatient setting. Only one clinician (2%) would consider referring first line, that is, prior to antibiotics, in either setting. Following a severe episode of CDI requiring supportive care, 66% would consider FMT upfront, and 96% would consider FMT in a patient not responding to antibiotics.
Table 4.
Number of physicians, n (%), who would refer for fecal microbiota transplantation (FMT) for each indication
| Indication | Outpatient | Inpatient |
|---|---|---|
| Prior to antibiotics | 1 (2%) | 1 (2%) |
| Immediately after first course of antibiotics | 1 (2%) | 6 (12%) |
| Following first recurrence | 9 (18%) | 20 (41%) |
| Following second recurrence | 28 (57%) | 30 (61%) |
| Following ≥3 recurrences | 27 (55%) | 23 (47%) |
Concerns regarding patient perception of FMT
The majority of physicians, 45 of 50 (90%), believed their patients would consider FMT a treatment. Among the small group of physicians who thought that their patients would not consider FMT, all predicted that this would be for aesthetic reasons rather than for risk of infection or disease transmission.
Concerns regarding safety of FMT
A minority of physicians, 14 of 50 (28%), expressed concern regarding potential alteration of the microbiome, as shown in Figure 2. ID physicians had significantly greater concern regarding potential deleterious alteration of the microbiome than gastroenterologists (8/17 [47%] vs 6/33 [18%]; P = 0.047). Nearly half of physicians, 23 of 50 (46%), were concerned about disease transmission risk; there was no difference between the specialties for this question. Of these physicians, 17 of 23 (76%) were concerned about infection, 12 (57%) were concerned about metabolic risk (e.g. obesity, insulin resistance), and 9 (43%) were concerned about autoimmune disease risk. When asked whether the risk of FMT would outweigh the benefit, 15 of 50 (30%) responded “yes”; however, 8 of these physicians had referred patients for FMT, with primary cure in all but one.
Figure 2.
Clinicians (% per specialty) concerned about potential alteration of microbiome in fecal microbiota transplantation recipients. ( ), Infectious diseases; (
), Infectious diseases; ( ), gastroenterology.
), gastroenterology.
Classification and regulation of FMT
The majority of physicians, 36 of 50 (72%), thought that FMT should be classified as a bodily tissue donation; 12 (24%) thought it should be classified as a drug, and all 50 physicians (100%) believed FMT should be funded by a third party.
Discussion
These data give insight into the views of patients who have undergone FMT for CDI and physicians who have had access to this therapy for their patients via a stool bank. FMT for CDI has a strong evidence base from multiple randomized controlled trials 3 , 5 , 16 and has been accepted as the standard of care in major international treatment guidelines. 9 , 11 , 17 , 18 However, evidence of efficacy and safety alone have not been sufficient to facilitate the widespread uptake and availability of FMT therapy. In additional to current regulatory frameworks hampering the availability of FMT, patient and physician views on FMT may have a bearing on prescribing and could diverge from best practice guideline recommendations. 19 , 20 , 21
The results of this survey suggest that perceived risks of FMT by many physicians may be a potential barrier to the use of this therapy. These perceived risks do not appear to be due to physicians' experience with adverse events as none of the physicians surveyed reported noting new diseases developing in their patients following FMT, and most achieved primary cure. Despite very few reported adverse events in many thousands of treated patients in the literature, a concerning 30% of physicians still reported that the potential risks of FMT outweigh the benefit. The findings of this survey are in keeping with previous Australian data: Paramsothy et al., 22 in a survey of 52 gastroenterologists in 2015, found that over half of the surveyed physicians were concerned about lack of evidence for efficacy, and 15% did not believe FMT was an effective therapy, even in the setting of CDI. Two thirds believed their patients would be concerned by the aesthetic factor despite patients' willingness to consider FMT being well established. Severe or fulminant CDI carries a high mortality, reported to be between 36 and 58%, which is not mitigated by colectomy. 23 , 24 , 25 A recent study of FMT in this setting demonstrated that the number needed to treat with FMT to prevent one death was 3.2 relative to standard antibiotic therapy. 26 A lack of utilization of FMT, particularly in this context, is life threatening and of great concern.
Almost half (46%) of physicians were concerned about disease transmission risk, and the majority of these concerns centered on infection risk. Much lower proportions of patients shared these concerns, with only 11% concerned about infection risk. Interestingly, this survey was undertaken prior to reports of transmission of antibiotic‐resistant bacteria producing an extended‐spectrum beta‐lactamase (ESBL) via FMT, which may have further raised concern among physicians and patients alike. 27 A considerable number of physicians also harbored concerns regarding metabolic and autoimmune disease risk, but again, this was not a concern held by most patients in this cohort. ID physicians were particularly concerned about altering the gut microbiota with FMT and associated risks. This is surprising given that ID physicians routinely prescribe and monitor antibiotic prescriptions and therefore may be more aware of the disease associations, with loss of microbiota diversity as a result of antibiotic use. 28 , 29 , 30 , 31 , 32 There is, however, far less evidence that the gain in microbial diversity, or change in microbial composition following FMT, results in disease. Although short‐term data are reassuring, larger case control studies and registry data will be required to properly assess long‐term unknown risks of disease transmission via FMT.
In contrast to physicians, patients in this survey reported high rates of acceptance for FMT as a therapy for rCDI. Patients noted rapid improvement in their symptoms following FMT, and adverse events were minor and infrequent. Despite a third of patients having concerns regarding aesthetics prior to the procedure, almost all patients were satisfied with the treatment and would recommend it to others. This is also consistent with previous studies. While patient aversion to the aesthetics of FMT is cited by clinicians as a barrier to its use, 33 in a large survey of 192 patients, when provided with efficacy data for CDI treatments, 85% of patients chose FMT, with only 4% deterred by its fecal composition. 34 Furthermore, high symptom burden and morbidity has been shown to be a powerful motivator for acceptance of FMT as a treatment, 35 and high patient satisfaction has previously been reported. 13
Awareness of this therapy seems to be increasing over time, with a third of patients in this cohort first hearing of FMT through the media, as opposed to their general practitioner or medical specialist. Increased public and media interest in the procedure may have resulted in increased acceptability as the procedure is normalized. Very few patients had heard of FMT from their GP, and this may reflect a lack of awareness among primary care doctors or that these patients are primarily being treated in the hospital system. Physician experience is also increasing, with 58% of our cohort having referred patients for FMT, which is much higher than previous similar reports, particularly by Paramsothy et al. 22 4 years ago, where only 21% of the 52 Australian gastroenterologists surveyed had referred a patient for FMT, despite 90% reporting that they would refer patients if FMT was easily available.
The majority (72%) of physicians believed that FMT should be regulated as a tissue product and not a drug, and both patients and physicians wanted FMT to be funded by a third‐party payer. Regulatory uncertainty continues to pose as a barrier for service delivery, and the development of a regulatory framework is essential for the efficient and safe delivery of this therapy. 12 , 36
This study had a number of limitations. First, a third of patients did not respond to the survey, and primary cure was more common in those who responded, which may overestimate the reports of satisfaction with treatment. Only half of physicians responded, and there is potential for bias here as those who took the time to respond may be more interested in or more experienced with FMT. Secondly, all physicians were practicing in South Australia, and so, there are potential limitations on the generalizability of the responses. However, given that South Australia has had uninterrupted access to a stool bank since 2013, limiting the survey to this jurisdiction is informative as it removes the important variable of a lack of access to therapy. There was also no standardized method of reporting adverse events in this survey, although global satisfaction was more the focus.
Despite the wealth of evidence supporting the safety and efficacy of FMT, and increasing patient awareness and acceptance as reflected in our survey, physician reservations may still present a barrier to uptake of this therapy, even in a region such as South Australia where access to FMT is readily available through a centralized stool bank. Publication of long‐term data on adverse effects, particularly infection risk and metabolic disease risk, will be important in assuaging these concerns and encouraging adherence to guideline recommendations. The establishment of national FMT registries will be important in this regard. Eliminating the regulatory ambiguity surrounding FMT and allowing for national standardization may also help to improve accessibility and acceptability of this life‐saving therapy.
Declaration of conflict of interest: Lisa M Dann is an employee of BiomeBank. Emily C Tucker is an employee of BiomeBank. Robert V Bryant has received grant/Research support/Speaker fees from AbbVie, Ferring, Janssen, Shire, Takeda, Emerge Health and holds shares in BiomeBank. Samuel P Costello has received educational grants, research support, speaker or consulting fees from Janssen, Ferring, Microbiotica, Pfizer, MSD and holds shares in BiomeBank.
References
- 1. Lessa FC, Mu Y, Bamberg WM et al Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015; 372: 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson L, Suda J, Evans T. Antibiotic treatment for Clostridium difficile‐associated diarrhoea in adults. Cochrane Database of Syst. Rev. 2017; 10.1002/14651858.cd004610.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cammarota G, Masucci L, Ianiro G et al Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015; 41: 835–43. [DOI] [PubMed] [Google Scholar]
- 4. Quraishi MN, Widlak M, Bhala N et al Systematic review with meta‐analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017; 46: 479–93. [DOI] [PubMed] [Google Scholar]
- 5. Ianiro G, Maida M, Burisch J et al Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta‐analysis. United European Gastroenterol. J. 2018; 6: 1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merlo G, Graves N, Brain D, Connelly LB. Economic evaluation of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection in Australia. J. Gastroenterol. Hepatol. 2016; 31: 1927–32. [DOI] [PubMed] [Google Scholar]
- 7. Cammarota G, Ianiro G, Tilg H et al European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017; 66: 569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Excellence NIfHaC . Faecal Microbiota Transplant for Recurrent Clostridium difficile Infection. London: NICE, 2014. [Google Scholar]
- 9. Trubiano JA, Cheng AC, Korman TM et al Australasian Society of Infectious Diseases updated guidelines for the management of Clostridium difficile infection in adults and children in Australia and New Zealand. Intern. Med. J. 2016; 46: 479–93. [DOI] [PubMed] [Google Scholar]
- 10. Quraishi MN, Segal J, Mullish B et al National survey of practice of faecal microbiota transplantation for Clostridium difficile infection in the UK. J. Hosp. Infect. 2017; 95: 444–5. [DOI] [PubMed] [Google Scholar]
- 11. Cammarota G, Ianiro G, Kelly CR et al International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019; 68: 2111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costello SP, Bryant RV. Faecal microbiota transplantation in Australia: bogged down in regulatory uncertainty. Intern. Med. J. 2019; 49: 148–51. [DOI] [PubMed] [Google Scholar]
- 13. Pakyz AL, Moczygemba LR, VanderWielen LM, Edmond MB. Fecal microbiota transplantation for recurrent Clostridium difficile infection: the patient experience. Am. J. Infect. Control. 2016; 44: 554–9. [DOI] [PubMed] [Google Scholar]
- 14. Costello SP, Tucker EC, La Brooy J, Schoeman MN, Andrews JM. Establishing a fecal microbiota transplant service for the treatment of Clostridium difficile infection. Clin. Infect. Dis. 2016; 62: 908–14. [DOI] [PubMed] [Google Scholar]
- 15. Costello SP, Conlon MA, Vuaran MS, Roberts‐Thomson IC, Andrews JM. Faecal microbiota transplant for recurrent Clostridium difficile infection using long‐term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment. Pharmacol. Ther. 2015; 42: 1011–8. [DOI] [PubMed] [Google Scholar]
- 16. van Nood E, Vrieze A, Nieuwdorp M et al Duodenal infusion of donor feces for recurrent Clostridium difficile . N. Engl. J. Med. 2013; 368: 407–15. [DOI] [PubMed] [Google Scholar]
- 17. Mullish BH, Quraishi MN, Segal JP et al The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018; 67: 1920–41. [DOI] [PubMed] [Google Scholar]
- 18. McDonald LC, Gerding DN, Johnson S et al Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018; 66: e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stenehjem E, Wallin A, Fleming‐Dutra KE et al Antibiotic Prescribing Variability in a Large Urgent Care Network: A New Target for Outpatient Stewardship. Clinical Infectious Diseases. 2020; 70: 1781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handy LK, Bryan M, Gerber JS, Zaoutis T, Feemster KA. Variability in antibiotic prescribing for community‐acquired pneumonia. Pediatrics. 2017; 139: e20162331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hicks LA, Blaser MJ. Variability in antibiotic prescribing: an inconvenient truth. J. Pediatr. Infect. Dis. Soc. 2015; 4: e136–8. [DOI] [PubMed] [Google Scholar]
- 22. Paramsothy S, Walsh AJ, Borody T et al Gastroenterologist perceptions of faecal microbiota transplantation. World J. Gastroenterol. 2015; 21: 10907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dudukgian H, Sie E, Gonzalez‐Ruiz C, Etzioni DA, Kaiser AM. C. difficile colitis–predictors of fatal outcome. J. Gastrointest. Surg. 2010; 14: 315–22. [DOI] [PubMed] [Google Scholar]
- 24. Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, West Midlands Research C . Systematic review and meta‐analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br. J. Surg. 2012; 99: 1501–13. [DOI] [PubMed] [Google Scholar]
- 25. Lamontagne F, Labbe AC, Haeck O et al Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann. Surg. 2007; 245: 267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tixier EN, Verheyen E, Ungaro RC, Grinspan AM. Faecal microbiota transplant decreases mortality in severe and fulminant Clostridioides difficile infection in critically ill patients. Aliment. Pharmacol. Ther. 2019; 50: 1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeFilipp Z, Bloom PP, Torres Soto M et al Drug‐resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019; 381: 2043–50. [DOI] [PubMed] [Google Scholar]
- 28. Blaser MJ. Our missing microbes: short‐term antibiotic courses have long‐term consequences. Cleve. Clin. J. Med. 2018; 85: 928–30. [DOI] [PubMed] [Google Scholar]
- 29. Kemppainen KM, Vehik K, Lynch KF et al Association between early‐life antibiotic use and the risk of islet or celiac disease autoimmunity. JAMA Pediatr. 2017; 171: 1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016; 352: 544–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blaser M, Bork P, Fraser C, Knight R, Wang J. The microbiome explored: recent insights and future challenges. Nat. Rev. Microbiol. 2013; 11: 213–7. [DOI] [PubMed] [Google Scholar]
- 32. Blaser M. Antibiotic overuse: stop the killing of beneficial bacteria. Nature. 2011; 476: 393–4. [DOI] [PubMed] [Google Scholar]
- 33. Brandt LJ. Editorial commentary: fecal microbiota transplantation: patient and physician attitudes. Clin. Infect. Dis. 2012; 55: 1659–60. [DOI] [PubMed] [Google Scholar]
- 34. Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin. Infect. Dis. 2012; 55: 1652–8. [DOI] [PubMed] [Google Scholar]
- 35. Zellmer C, De Wolfe TJ, Van Hoof S, Blakney R, Safdar N. Patient perspectives on fecal microbiota transplantation for Clostridium difficile infection. Infect. Dis. Ther. 2016; 5: 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mullish BH, Williams HR. Obstacles to establishing an NHS faecal transplant programme. BMJ. 2015; 351: h6043. [DOI] [PubMed] [Google Scholar]


