ABSTRACT
For centuries, the eye has fascinated scientists and philosophers alike, and as a result the visual system has always been at the forefront of integrating cutting-edge technology in research. We are again at a turning point at which technical advances have expanded the range of organisms we can study developmentally and deepened what we can learn. In this new era, we are finally able to understand eye development in animals across the phylogenetic tree. In this Review, we highlight six areas in comparative visual system development that address questions that are important for understanding the developmental basis of evolutionary change. We focus on the opportunities now available to biologists to study the developmental genetics, cell biology and morphogenesis that underlie the incredible variation of visual organs found across the Metazoa. Although decades of important work focused on gene expression has suggested homologies and potential evolutionary relationships between the eyes of diverse animals, it is time for developmental biologists to move away from this reductive approach. We now have the opportunity to celebrate the differences and diversity in visual organs found across animal development, and to learn what it can teach us about the fundamental principles of biological systems and how they are built.
KEY WORDS: Evolution, Eye, Morphogenesis, Retina, Visual system
Summary: This Review discusses the lens-containing eyes of a variety of Metazoa with a focus on ontogeny and highlights what evolutionary developmental biology continues to teach us about fundamental principles of biological systems.
Introduction
‘The eye is like a mirror and the visible object is like the thing reflected in the mirror'
Avicenna, Danish Nama-I ‘ala'I (11th century)
The eye has fascinated scientists and philosophers since the emergence of the discipline of biology. In each new technological era that has intersected biology (e.g. the advent of electron microscopy, advances in neurophysiology, developmental genetics and molecular phylogenetics), the visual system has been one of the first and most in-depth subjects studied. In each case, discoveries made about the visual system have been fundamental in furthering our understanding of the living world. There is no example clearer than that of the discovery of the transcription factor Pax6 (Cvekl and Callaerts, 2017).
Before the molecular revolution, biologists hypothesized that the eye evolved independently at least 40 times (Salvini-Plawen and Mayr, 1977). However, in 1994, it was discovered that the gene eyeless in Drosophila was a homolog of the gene small eye (Pax6) in mice and aniridia (PAX6) in humans (Quiring et al., 1994). This finding was expanded to a group of genes, the retinal determination network (RDN), that are required for eye development in Drosophila [eyeless (ey; Pax6 in mouse), twin of eyeless (toy; Pax6 in mouse), sine oculis (so; Six1/2 in mouse), Optix (Six3/6 in mouse), dachshund (dac) and eyes absent (eya)] and are directly associated with eye formation in other species (Gehring, 2014). This discovery fundamentally shifted our understanding of the evolutionary relationship between vertebrates and other distantly related organisms. It suggested the existence of ancient genetic networks that have a common relationship to patterning and organ formation across animals (Carroll, 2008; Shubin et al., 2009). This premise has ruled the study of evolutionary developmental biology for over 25 years.
Again, we find ourselves in a new technological era in biology. Barriers to study organisms beyond traditional genetic models have fallen, which enables us to better understand the diversity of life. To consider the evolution of form and function, it is crucial that we go beyond the study of homology. Organogenesis is undeniably a significant contributor to the diversity that we see across taxa, and photoreception is one of the most ubiquitous sensory modalities found on Earth. The eye provides an excellent comparative context to address issues regarding how morphogenesis and developmental genetics contribute to the evolution of both novelty and complexity.
In this Review, we discuss six areas in which a greater knowledge of visual organ development is advancing our understanding of evolutionary change (Fig. 1). Although the scope of this Review is limited by space constraints, fortunately, the field enjoys a wealth of excellent reviews (Arendt et al., 2009, 2016; Erclik et al., 2009; Jonasova and Kozmik, 2008; Kozmik, 2005; Lamb et al., 2007; Land, 2018; Land and Nilsson, 2012; Nilsson, 2013, 2009, 2004; Oakley and Speiser, 2015; Sanes and Zipursky, 2010; Suzuki and Grillner, 2018).
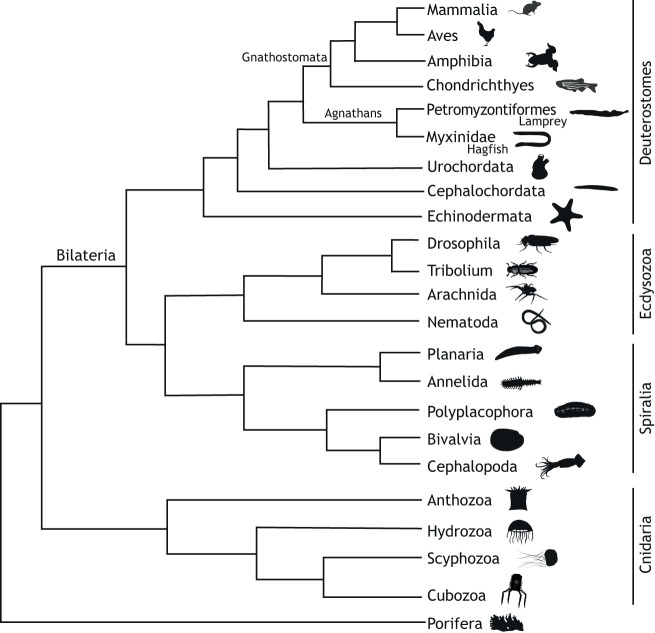
Fig. 1.
Phylogenetic tree of the Metazoa. Tree showing relative placements of metazoans, with an emphasis on animals discussed in this Review. Importantly, this tree is intended to help the reader with relationships between the organisms discussed. Branch lengths are only approximations. Images taken from http://phylopic.org/ under https://creativecommons.org/licenses/by/3.0/. Specific attributions: Lamprey, Gareth Monger; Urochordate, Mali'o Kodis; Cephalochordate, Bennet McComish; Planaria and Polyplacophora, Noah Schlottman; Scyphozoa, Michelle Site; Cubozoa image based on University of California, Berkeley (www.ucmp.berkeley.edu/cnidaria/cubozoa.html) Tribolium image based on Young et al. (2013). Tree based on Kocot et al. (2020); Marlétaz et al. (2019); Giribet and Edgecombe (2019); Kayal et al. (2018); Miyashita et al. (2019).
Comparative morphogenesis of single-chambered visual organs and the evolution of complex morphologies
Single-chambered visual organs, like the vertebrate eye, have evolved multiple times. These complex organs are composed of multiple cell and tissue types that, through the process of morphogenesis, become arranged in an exacting morphology to enable specific visual functions. The components include a single lens (see Glossary, Box 1) in the anterior and a retina (see Glossary, Box 1) in the posterior. In addition, a pigment layer behind or within the retina is necessary, and a cornea (see Glossary, Box 1) is often found anterior to the lens. Animals have evolved varied morphogenetic processes to arrange these tissues during development. Here, we discuss eye morphogenesis in Vertebrates, Arachnids and Cephalopods (Fig. 2). This comparative understanding of morphogenesis can tell us about convergence in the evolution of tissue fates, organization and movement in development.
Box 1. Glossary.
Anterior segment. The lens and surrounding tissue composing the anterior of the eye.
Cornea. Transparent tissue located at the front (anterior) of the eye that can function to refract light.
Crystallin. Water-soluble structural proteins in the cornea and lens that pack tightly in a precise fashion to increase the refractive index of the tissue and enable transparency; there has been incredible taxon-specific recruitment of diverse crystallin proteins across Metazoans.
Ganglion cell. The primary output neurons in many vertebrate retinae that convey light information received by photoreceptors to higher visual centers.
Interneuron. Neurons in many retinae that process visual information before it is conveyed to higher visual centers.
Lens. Transparent tissue that refracts and focuses light onto photoreceptors.
Ommatidium. Single optical unit of a compound eye; typically composed of one or more photoreceptors, pigment cell(s), support cell(s) and a lens.
Opsin. Light-sensitive proteins found in photoresponsive cells (see Porter et al., 2012).
Outer segment. The expanded membrane of the photoreceptor cell in which opsin proteins primarily function.
Photoreceptor. A light-sensitive/responsive cell with an expanded cell membrane. This expansion can be derived from the primary cilium (ciliary) or can be microvillar in nature.
Pigmented cell. A cell that is often associated with photoreceptors to block light from angles other than the primary one(s) that activate the photoreceptors.
Retina. A light-sensitive tissue in the eye that contains photoreceptors and, depending on the organism, can also contain pigmented cells, glia or support cells, interneurons and output neurons.
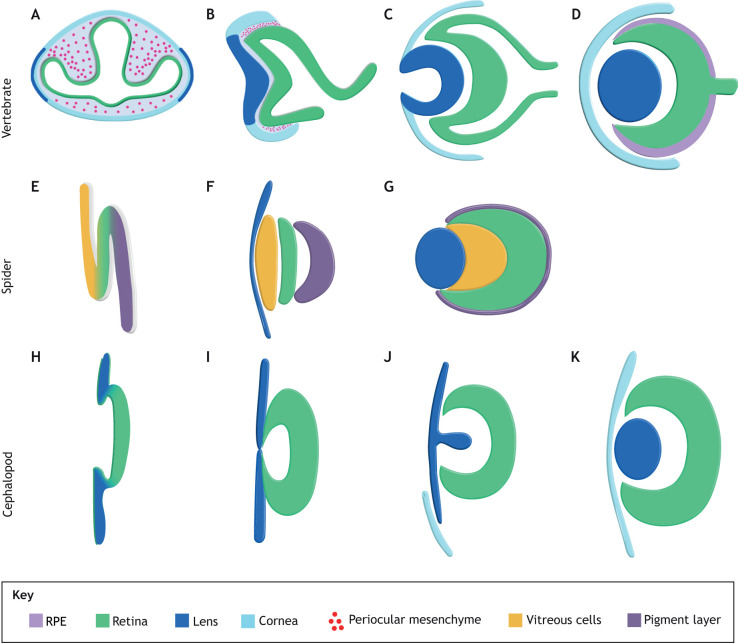
Fig. 2.
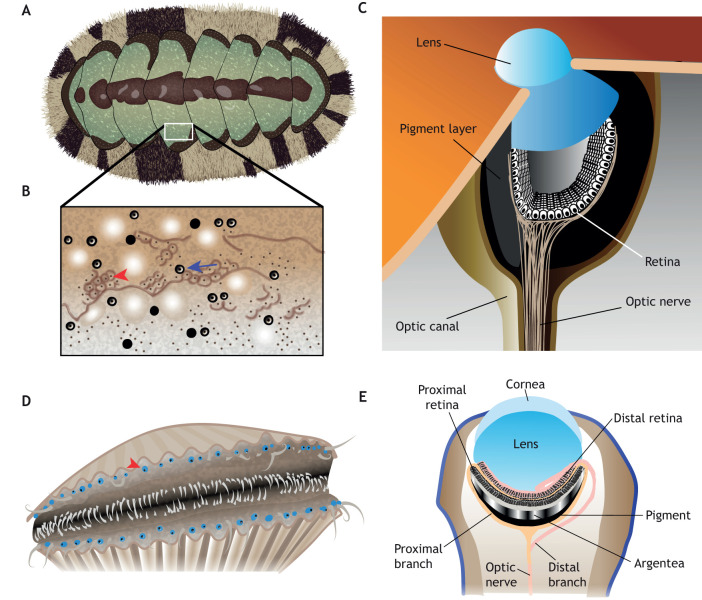
Development of vertebrate, spider and cephalopod eyes. (A-D) Generic vertebrate eye (modified from Dash et al., 2015). (A) In all vertebrates, eye development begins with the evagination of the optic primordia (OP) from the diencephalic region of the forebrain. Thereafter, the neuroectodermal OP reorient such that they begin to adopt a vesicle or vesicle-like structure. (B) Lens induction results from an interaction between the optic vesicle (OV) and the overlying surface ectoderm that results in the formation of the lens placode, from which the lens will eventually arise. POM cells invade the eye (red). (C) The OVs undergo a dramatic series of species-dependent morphogenetic movements that generally involve invagination to generate a bilayered optic cup (OC). (D) The OC, through a series of intrinsic regulatory programs and extrinsic cell-cell and cell-ECM interactions, segregates into the retinal pigment epithelium (RPE) and retina. (E-G) Invagination and morphogenesis of the principal eyes of the spider, Heteropoda venatoria based on Homann (1971). Cross-section view, anterior is left. (E) The principal eye is formed from an invagination of the anterior ectodermal tissue. Invagination starts from the bottom of the future eye, moving upward to make an ‘s’ shape. (F) Three layers of tissue result, the vitreous cells, the photoreceptors and the pigmented cells. (G) A cuticular lens grows over these layers. (H-K) Invagination and internalization of the retina in the squid D. pealeii, as described by Koenig et al. (2016). Cross-section view, anterior is right. (H) Stage 19: retinal placode has formed and lateral lip cells are internalizing the placode. (I) Stage 21: internalization is complete and the eye vesicle is formed. (J) Stage 27: cup-shaped retina and teardrop lens has formed, and corneal tissue begins to grow to internalize the eye. (K) Stage 29: eye is functional (stages as described by Arnold, 1967).
Vertebrates
In all vertebrates, eye development begins with the bilateral evagination of optic primordia from the diencephalic region of the forebrain (Chow and Lang, 2001). The distal optic primordia elongate and adopt vesicle-like structures, subsequently invaginating to form a bilayered optic cup, whereas the proximal region constricts and forms the optic stalk (Fig. 2A). The distal aspect of the optic cup generates the neural retina (Fig. 3A), whereas the proximal aspect generates the retinal pigment epithelium (RPE) (Fig. 2B). Cranial neural crest and head mesenchyme form the periocular mesenchyme (POM), a cell type that directly contributes to multiple ocular structures but also interacts with the developing optic cup and provides extrinsic cues to modulate morphogenesis (Fuhrmann, 2010; Williams and Bohnsack, 2015; Gage et al., 2005). The lens placode, from which the lens arises, is generated through an inductive event between the optic cup and surface ectoderm (Fig. 2B-D; Cvekl and Ashery-Padan, 2014).
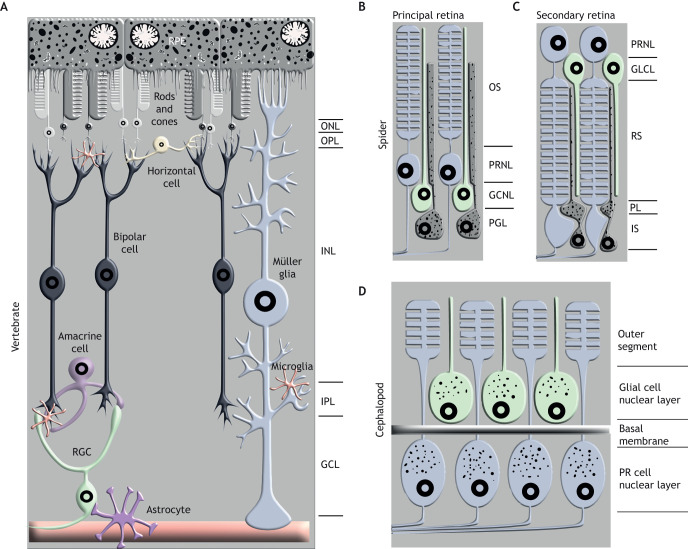
Fig. 3.
Retinal architecture in vertebrates, spiders and cephalopods. (A) The vertebrate retina is composed of seven cell types: RGCs, bipolar cells, horizontal cells, amacrine cells, rod and cone photoreceptors and Müller glia. These cell types are arranged into three cellular laminae: the ganglion cell layer (GCL); the inner nuclear layer (INL); and the outer nuclear layer (ONL). The laminae are separated by two synaptic layers: the inner plexiform layer (IPL) and outer plexiform layer (OPL). (B) The retina of the spider principal eye is found behind the vitreous cells and is composed of photoreceptor cells, unpigmented glia and pigmented glia. The outer segment (OS) of the photoreceptor cells is anterior to the photoreceptor nuclear layer (PRNL). The unpigmented glial cell nuclei (GCNL) are posterior to the photoreceptors and the pigmented glial cell nuclei (PGL) are at the retinal periphery. Anterior of the eye is up. (C) The secondary retina is also behind a layer of vitreous cells and has a PRNL, an unpigmented glial cell layer (GLCL) and pigmented cells. The nuclei of the photoreceptor cells and unpigmented glia are in the anterior of the retina. The photoreceptor segment (RS) is posterior to the GLCL. An accumulation of pigment is often found behind the RS (PL). This is followed by the photoreceptor intermediate segment (IS) and the pigmented glial cell nuclei. Figure is based on DeVoe et al. (1969), Blest et al. (1980), Blest (1983), Uehara et al. (1994), Schröer (2017) and Grusch et al. (1997). (D) Schematic of the Cephalopod retina (anterior is up). The PRNL and the glial cell/support cell nuclear layer are separated by the basal membrane. The photoreceptors penetrate the membrane and extend their outer segments anterior to the support cell bodies. Photoreceptors also synapse directly on the optic lobe. The support cells extend projections into the outer segment. Both the support cells and photoreceptor cells have pigment granules. Cartoon based on Koenig et al. (2016), Williams (1909) and Saibil (1990).
Much has been learned about optic cup morphogenesis in many vertebrate species, with perhaps the most detailed information coming from studies in zebrafish (Kwan et al., 2012; Cavodeassi and Wilson, 2019). Although these studies have identified distinct morphogenetic movements contributing to lens, retina and RPE formation, some of which are conserved between avian, mammalian and teleost species (Bernstein et al., 2018), our understanding of morphogenesis in mammals is limited given their in utero development. Interestingly, aspects of optic cup morphogenesis seem to be intrinsically programmed into neural tissues as optic cups derived from induced pluripotent stem cells, which lack lenses and POM, nonetheless undergo invagination to generate a cup-shaped structure (Eiraku et al., 2011).
Arachnid
Spiders belong to the subphylum Chelicerata within arthropods. Spider visual systems vary in complexity. Some spiders are almost blind and primarily web-dwelling, using vibrational cues as their principal sensory modality (Foelix, 2011; Land, 1985). Others, like jumping spiders, are wandering hunters with high-acuity vision (Jackson and Pollard, 1996). Most spiders have four pairs of camera-type image-forming eyes, each with a cuticular cornea, lens, cellular vitreous and photoreceptor and pigmented cells (see Glossary, Box 1). Across species, a huge diversity of placement and specialization of each pair of eyes exists.
The two front eyes, the anterior median or principal eyes, are the largest and often most complex (Land, 1985). The remaining eyes, called secondary eyes, often have specific functions. There are notable differences between the principal and secondary eyes beyond their complexity. First, the principal eyes have muscles that can move the retina laterally to enable directional vision (Strausfeld and Barth, 1993). Principal eyes never have a tapetum, a reflective layer enhancing sensitivity, which is common in secondary eyes (Homann, 1971). The orientation of the cells also differs between the two eye types. Principal eyes have outer segments (see Glossary, Box 1) pointing toward the direction of the incoming light, whereas secondary eyes have outer segments that are oriented posterior to their cell bodies. This is likely a result of differences in the morphogenesis of the two eye types.
Development of the principal and secondary eyes has been described in multiple species (Homann, 1971; Locy, 1886; Yoshikura, 1955). The principal eye is formed from an invagination of the prosoma, an anterior ectodermal tissue. Invagination starts from the bottom of the future eye, moving upward. This makes an ‘s’ shape of three layers of tissue; the most anterior generates vitreous cells, the middle generates photoreceptors and the posterior generates pigmented cells (Homann, 1971; Paulus, 1979) (Fig. 2E-G). Secondary eyes develop from a simple invagination of lateral ectoderm (Homann, 1971). These invagination events lead to an everted principal eye and an inverted secondary eye (Fig. 3B,C). In all spider eyes, the main refractive surface is the cuticular cornea and nothing is known about its development or the specific cuticular proteins it contains.
Cephalopods
Coleoid cephalopods, including squid, octopus and cuttlefish, have a remarkable visual system with single-chambered eyes and large optic lobes for visual processing. The cephalopod retina has two identifiable cell types: photoreceptor cells and support cells (Fig. 3D). There are no interneurons (see Glossary, Box 1) found in the retina, as the photoreceptors synapse directly on the optic lobe.
The most in-depth description of cephalopod eye development comes from the squid, Doryteuthis pealeii (Koenig et al., 2016). The eye rudiments are among the first evidence of organ formation in the embryo. Two oval-shaped thickenings (placodes) form on each side of the embryo and, shortly thereafter, a lip of cells surrounding each placode emerges (Fig. 2H). Placodal cells contribute to the retina, whereas the lip cells contribute to the anterior segment (see Glossary, Box 1) and lens (Koenig et al., 2016). The lip begins to internalize placodal tissue and, once internalization is complete, the lip cells fuse and optic vesicles are generated (Fig. 2I). Shortly after vesicle fusion, lentigenic cells in the anterior of the vesicle begin to make the lens (Fig. 2J,K). The lens is made by long cellular processes wrapping around each other, forming fibrous layers (Meinertzhagen, 1990). These processes are made by three distinct populations of lentigenic cells arranged around the developing lens (Williams, 1909; Arnold, 1966, 1967; West et al., 1995). The lens itself is segmented, with distinct populations of lentigenic cells contributing to each segment (Arnold, 1967). The fibers from the lentigenic cells are eventually sheared from the cell bodies, making the lens acellular (Arnold, 1967).
What we see when comparing morphogenesis across species is that there is no universal way to execute this tissue organization. For example, cephalopod and spider lenses arise from neighboring tissue, are contiguous with the retina and are potentially neuroectodermally derived. The vertebrate lens is derived from the sensory pre-placodal ectoderm, is physically discontiguous from the retina and results from an inductive event. Beyond this tissue level of understanding, it will be interesting to identify cellular mechanisms of morphogenesis and their evolutionary conservation across the Bilateria.
Retinogenesis and evolution of the vertebrate eye
The extensive knowledge of vertebrate eye development can be leveraged to better understand the evolution of complex organs. Exquisite anatomical data describing the visual organs in sister taxa, coupled to connectomic and molecular studies, have substantially enhanced our ability to assess homology across species. Here, we compare retinal structure and development between vertebrates, jawless fishes (lamprey and hagfish) and two invertebrate subphyla within Chordata, Cephalochordates and Urochordates (Suzuki and Grillner, 2018; Fain, 2019).
Retinal development in vertebrates
Retinal neurogenesis occurs in a stereotyped manner whereby neuroblasts are specified and differentiate into the seven cell types of the mature retina (Cepko, 2014). Retinal ganglion cells (RGCs; see Glossary, Box 1) are generated first, followed by cones, horizontal cells, amacrine cells, rods, bipolar cells, photoreceptors and Müller glia. These cells are arranged into the three laminae of the retina: the ganglion cell layer (GCL); the inner nuclear layer (INL); and the outer nuclear layer (ONL). The inner plexiform layer (IPL) separates RGCs and the INL, and the outer plexiform layer (OPL) separates the INL and ONL (Fig. 3A). Axons derived from RGCs make up the optic nerve, the sole output of the retina to visual centers of the brain. Rods and cones are the photoreceptive cells that receive and transmit visual information to RGCs via bipolar cells. Amacrine and horizontal cells serve as interneurons that modulate the activity of intraretinal circuits. The RPE is a melanin-containing cell monolayer that separates the retina from the choroidal blood supply. The RPE prevents light scatter and serves a number of crucial developmental and physiological roles in retinal homeostasis (Strauss, 2005). The transcription factor MITF is a key regulator of RPE development (Ma et al., 2019).
The molecular underpinnings of retinogenesis have been characterized in many vertebrate species, with key regulatory networks specifying retinal neuron classes largely conserved (Seritrakul and Gross, 2019; Bassett and Wallace, 2012; Cepko, 2014). Several of these are mentioned in descriptions of retinogenesis below, including Pax6, Pax2, Rx and Otx.
Lamprey and Hagfish
Lamprey possess cephalic eyes that develop biphasically: larvae possess immature eyes that develop into the adult image-forming camera-type eyes during metamorphic stages. Developmentally, the optic vesicles evaginate from the diencephalon (Murakami et al., 2001) and a rudimentary lens is formed in larvae (Kleerekoper, 1972; Meléndez-Ferro et al., 2002). Adult lamprey possess an ellipsoid multifocal lens, indicating that they can generate well-focused images, suggesting that a multifocal lens is ancestral in vertebrates (Bantseev et al., 2005; Gustafsson et al., 2008).
The larval (primary) retina is composed of only photoreceptors, RGCs and bipolar cells (Fig. 4A) (Dickson and Collard, 1979; Rubinson and Cain, 1989; Villar-Cerviño et al., 2006). This simple retinal circuitry, combined with the subdermal location of the eye, suggest that it is not image forming. Embryonic phases last ∼1 month and larvae then burrow into the sand for several years until they metamorphose into adults. In mid-stage larvae, the secondary retina proliferates and generates numerous neuroblasts that, with the exception of a few RGCs, remain undifferentiated (Fig. 4B) (Rubinson and Cain, 1989; Meléndez-Ferro et al., 2002; Villar-Cerviño et al., 2006; Villar-Cheda et al., 2008). This undifferentiated tissue encompasses ∼99% of the retinal area and gives rise to the adult retina (Villar-Cheda et al., 2008). The maintenance of this long-lived progenitor population in the secondary retina is not well understood.
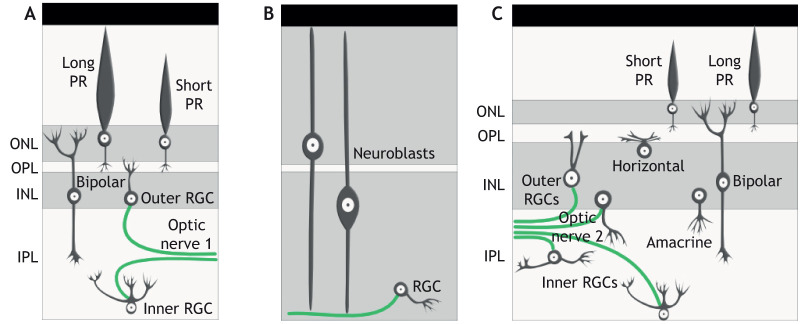
Fig. 4.
Organization of the larval and adult lamprey retina. (A) The central region of the larval retina (the primary retina) is composed of photoreceptors (PRs), retinal ganglion cells (RGCs) and bipolar cells. RGCs generate the first optic nerve, which maps to thalamic and pretectal areas. (B) The lateral region of the larval retina (the secondary retina) is filled with neuroblasts that, with the exception of a few RGCs, remain undifferentiated until late larval stages. (C) The adult retina contains all vertebrate retinal neuron types and generates the second optic nerve that maps to the tectum. Based on Suzuki and Grillner, 2018. INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer.
Differentiation commences with RGCs in the secondary retina during late larval stages. During metamorphosis, amacrine and horizontal cells form first, photoreceptors next and bipolar cells last (Rubinson and Cain, 1989). The adult lamprey retina resembles that of other vertebrates, with the exception of the morphology and organization of RGCs (Fig. 4C; Fain, 2019). Lamprey possess inner RGCs and outer RGCs, the cell bodies of which are located in the IPL and the INL, respectively (Fritzsch and Collin, 1990; Jones et al., 2009). To generate the optic nerve, axons from these RGCs come together between the INL and IPL rather than along the vitreal surface of the eye, as with other vertebrates. There are two distinct retinotectal projections (optic nerves) that emerge at late larval stages; RGCs of the primary retina map to thalamic and pretectal areas, whereas many of those of the secondary retina map to the tectum (De Miguel et al., 1989; Jones et al., 2009).
Hagfish are the sister taxon to lamprey, but lack lens-containing eyes. It is likely that the eyes of extant hagfish are degenerate and have adapted to life in deep sea habitats. Evidence from a fossilized hagfish (Myxinikela) supports this model, with eyes appearing to contain a lens and a melanin-containing RPE-like structure (Bardack, 1991; Gabbott et al., 2016). This highlights one confounding aspect of using comparative embryology to understand the evolution of complexity: the incidence of loss in the phylogeny (Box 2).
Box 2. Plesiomorphy and eye regression.
Loss in the phylogeny can obscure plesiomorphic traits or the ancestral character state. When an animal has a simple visual system one must ask whether the lineage never evolved complexity or whether it was lost. Hagfish are an example of this type of eye regression. The eyes of extant hagfish are buried under a layer of skin, fat and/or muscle and are unlikely to receive much light. The hagfish eye also lacks a lens (Fernholm and Holmberg, 1975), although it has been suggested that a placode might form (Stockard, 1906). The hagfish retina is laminated and contains two nuclear layers. The outer layer contains photoreceptors and the inner layer contains projection neurons that send axons to the hypothalamus (Kusunoki and Amemiya, 1983). The hagfish eye also contains an unpigmented epithelium on the scleral side of photoreceptors that likely functions as a non-pigmented RPE. No interneurons have been identified histologically in the hagfish retina and it is thought that photoreceptors synapse directly onto ganglion-like cells in the inner retina. Thus, the hagfish eye superficially resembles that of the embryonic lamprey. It also bears similarity to the pineal organ of non-mammalian vertebrates.
Loss is a fundamental part of evolutionary change. If recognized, in the context of visual organs, eye regression can reveal mechanisms of how an eye is built or dismantled and can also be a mechanism through which loci underlying ocular disorders can be identified (Partha et al., 2017). Eye regression or loss has occurred many times in vertebrates, and this process has been most well-studied in cavefish (Jeffery, 2009; Krishnan and Rohner, 2017). However, there are many other interesting examples of eye regression in animals, including in subterranean mammals (Emerling, 2018), diving beetles (Tierney et al., 2018) and marine snails (Sumner-Rooney et al., 2016).
Cephalochordates
Amphioxi possess four distinct light-responsive organs: the frontal eye, lamellar body, Joseph cells and dorsal ocelli (Fig. 5A; Lacalli, 1996; Lacalli et al., 1994). Ultrastructural and molecular studies suggest that the frontal eye is homologous to the vertebrate eye (Pergner and Kozmik, 2017). Structurally, the frontal eye is organized into four rows of neuronal cells and a pigmented cup (Fig. 5B,C). Pigmented cells express mitf, otx and a pax2/5/8 ortholog, and contain melanin, suggesting homology with the vertebrate RPE (Kozmik et al., 1999; Vopalensky et al., 2012). Dorsal and posterior to the pigment cup lie six Row1 ciliary photoreceptor cells that express otx and pax4/6 (Lacalli, 1996; Vopalensky et al., 2012).
Fig. 5.
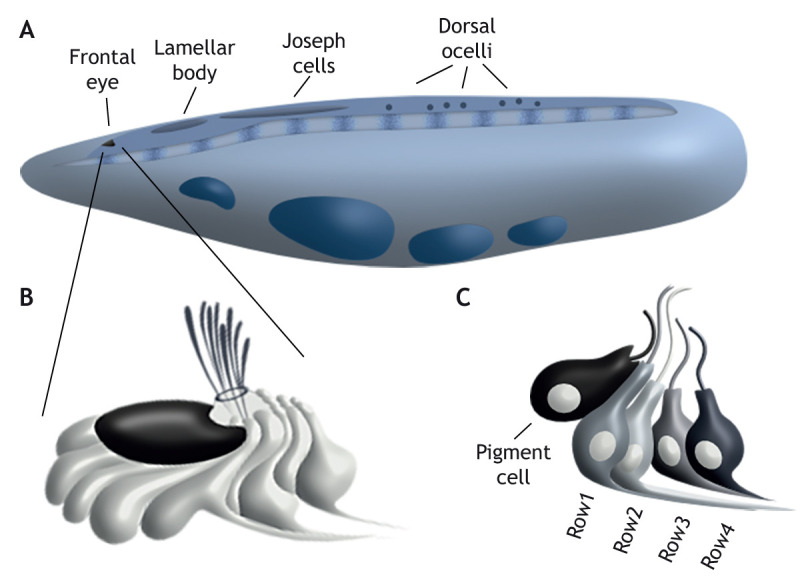
Structure of the amphioxus eye. (A) Cartoon of amphioxus larva showing the four light-responsive organs: the frontal eye; lamellar body; Joseph cells; and dorsal ocelli. (B) Enlarged view of the frontal eye showing the pigmented cup, neuronal cells and cilia emerging from the neuropore. (C) Cartoon showing the organization of the frontal eye; from anterior to posterior there is a pigment cell and then four rows of neurons. Row1 cells are opsin-expressing photoreceptors and Row2 cells extend axons to the cerebral vesicle. Panels A and C are based on Pergner and Kozmik (2017). Panel B is based on Lacalli (1996).
Row2 cells are posterior to and contact Row1 cells. These cells extend projections to the tegmental neuropil of the cerebral vesicle, a region thought to bear homology to the vertebrate diencephalon and mesencephalon (Lacalli, 1996; Vopalensky et al., 2012; Albuixech-Crespo et al., 2017). Row2 cells might be homologous to RGCs; however, this model requires additional molecular and functional evidence. Row3 and Row4 cells are the most posterior in the frontal eye; Row3 cells are characterized by reciprocal contacts between one another, whereas Row4 cells extend axons to the tegmental neuropil (Lacalli, 1996). Molecularly, less is known about Row3 and Row4 cells but they express pax4/6 and Rx (Vopalensky et al., 2012), and potentially use glutamate as a neurotransmitter (Pergner and Kozmik, 2017). When compared to the vertebrate retina, their position supports their identity as interneurons, but this requires experimental validation.
Urochordates
Within tunicates, most studies have been performed on ascidians (sea squirts). Although adults do not have a discrete eye-like structure, the larval ocellus mediates phototactic and light-dimming responses (Tsuda et al., 2003; Horie et al., 2008; Salas et al., 2018; Bostwick et al., 2020). The cellular composition and organization of the ocellus in Ciona intestinalis has been resolved (Ryan et al., 2016). The ocellus houses two photoreceptive systems: PR-I and PR-II. PR-I consists of 23 photoreceptors that project outer segments into a single cup-shaped pigment cell. PR-II consists of seven photoreceptors, is located anterior to PR-I and is not associated with the pigment cell; rather, PR-II photoreceptors send outer segments into the brain ventricle. There are three lens cells located ventral to PR-I and PR-II that direct light to photoreceptors of both systems. Photoreceptors of PR-I and PR-II synapse onto relay neurons, which connect directly to the motor ganglion. Evidence supports a role for PR-I in mediating phototactic responses and for PR-II in the light-dimming response, suggesting that PR-I serves the function of cephalic eyes (Salas et al., 2018; Jiang et al., 2005; Kourakis et al., 2019).
The cell lineage of the ocellus has been determined, as have molecular mechanisms leading to its specification (Esposito et al., 2015). The three lens cells and photoreceptors arise from the medial neural plate (Cole and Meinertzhagen, 2004; Taniguchi and Nishida, 2004; Oonuma et al., 2016). Pax-6 and Mitf are expressed in cells that give rise to the ocellus (Glardon et al., 1997; Mazet et al., 2003) and Rx is required for ocellus formation (D'Aniello et al., 2006). The pigment cell derives from a Mitf-expressing cephalic melanocyte lineage located at the lateral edge of the neural plate, thought to potentially represent a rudimentary neural crest cell (Abitua et al., 2012; Yajima et al., 2003). In contrast, the vertebrate RPE is not neural crest-derived, indicating that the embryonic origins of these structures are different.
Cell type composition, anatomy and basic development of the visual systems within the chordate lineage have been fairly well resolved. However, even within vertebrates, there are many questions remaining. For example, we know almost nothing about the formation of cone and RGC-dense specializations that enable high-acuity vision. In primates, this is called the fovea, and similar high-acuity regions are found in species of birds and lizards. Expanding on recent progress (da Silva and Cepko, 2017), studying these high-acuity areas across species is interesting evolutionarily but also important for regenerative approaches in vision restoration. The cell biology of neurogenesis across species and its role in generating cell type diversity is another frontier in evolutionary developmental biology. Recent descriptions of neurogenesis in the squid retina have surprisingly shown a strikingly vertebrate-like neuroepithelium, which might be important to the generation of neural complexity (Box 3) (Koenig et al., 2016).
Box 3. Cephalopod neurogenesis.
Significant work has described the cell biology of neurogenesis in the vertebrate retina and central nervous system (Götz and Huttner, 2005; Amini et al., 2018), a process historically considered unique to vertebrates. This process is characterized by a pseudostratified neuroepithelium composed of cells undergoing interkinetic nuclear migration (IKNM) correlated with their cell division cycles. Mitosis occurs on the apical side of the epithelium. The progressive differentiation of retinal cell types is regulated by Notch signaling (Perron and Harris, 2000). Cells delaminate from the epithelium and differentiate, extending processes and synapsing appropriately. It has been hypothesized that this epithelial cell biology is essential for maintaining organization of highly dense progenitor cell populations (Strzyz et al., 2016).
Interestingly, a similar cell biological context is found in the cephalopod nervous system (see Koenig et al., 2016 for details). During squid development, the retina is also a pseudostratified epithelium, with nuclei undergoing IKNM and mitoses occurring apically. Eventually, photoreceptor cell bodies segregate to the posterior, behind the basal membrane, and no longer incorporate BrdU (a marker of proliferation). Cell bodies anterior of the basal membrane remain in the cell cycle, eventually forming support cells. Previous work has also shown that when Notch signaling is inhibited, the retina becomes disorganized, cells exit the cell cycle prematurely and differentiate improperly. However, significant work remains to understand the process of cell division, delamination and differentiation in the squid retina to understand how similar this cell biological process is to vertebrate neurogenesis.
Homology and the visual organs in the Ecdysozoa
The euarthropod visual system is thought to have had a pair of lateral-faceted (compound) eyes and two pairs of median ocelli-type eyes, which have diversified significantly across arthropod lineages. With the expansion of molecular and genetic analyses of visual organs across arthropod species, we can identify molecular signatures that have been maintained and those that have changed to better understand how these changes impact morphology and function. Here, we look at three examples: Drosophila, Tribolium and arachnids.
Drosophila
Drosophila have seven visual organs, which include two compound eyes, three ocelli and a pair of Bolwig organs or larval eyes (Fig. 6A; Friedrich, 2006). The primary adult visual organs are the compound eyes, each composed of ∼750 ommatidia (see Glossary, Box 1). Their development has been extensively reviewed (Charlton-Perkins and Cook, 2010; Roignant and Treisman, 2009; Kumar, 2012; Quan et al., 2012; Treisman, 2013; Wolff and Ready, 1993; Kumar, 2010, 2011). Each ommatidium possesses eight photoreceptors and twelve supporting cells, including cone cells, pigment cells and mechanosensory bristles. The compound eyes and ocelli are lens-containing and are derived from the eye-antennal imaginal disc during larval development. RDN genes ey (Pax6), toy (Pax6), dac, eya, so (Six1/2) and eyg (Pax6) are required for eye development. Many of these genes can also induce eye formation if ectopically expressed in other parts of the embryo.
Fig. 6.
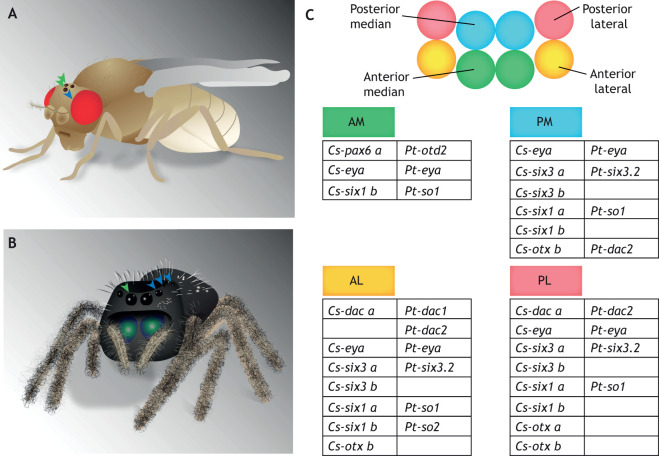
Eye homology in the Ecdysozoa. (A) Cartoon of Drosophila melanogaster with the ocelli (green arrowheads) and compound eyes (blue arrowhead) identified. (B) Cartoon of the jumping spider. The green arrowhead identifies the principal eye, homologous to the Drosophila ocelli; the blue arrowhead identifies the secondary eyes, homologous to the Drosophila compound eye. (C) Combinatorial gene expression in the developing principal [anterior median eye (AM)] and secondary eyes [posterior median (PM)], [anterior lateral (AL)] and [posterior lateral (PL)] in two species of spider, P. tepidariorum and C. salei, as reported by Samadi et al. (2015) and Schomburg et al. (2015).
Drosophila are holometabolous insects (undergoing complete metamorphosis). The Bolwig organs are homologous to elaborate stemmata found in other holometabolous insects, such as fireflies and scorpionflies (Gilbert, 1994; Buschbeck, 2014). They are also considered homologous to the earliest-born photoreceptor cells in the adult eye of hemimetabolous species (lacking a pupal stage), such as grasshoppers (Liu and Friedrich, 2004). A small population of cells from the Drosophila embryo in the dorsal head neuroectoderm generates the larval Bolwig organs, adult compound eye and ocelli (Friedrich, 2006). After embryonic development, these cells invaginate to generate the eye-antennal disc. The three ocelli are derived from the dorsal anterior region of the disc. One ocellus is derived from each disc, and the third ocellus is a fusion of anlagen from each disc. After an early growth phase, the morphogenetic furrow initiates differentiation in the compound eyes, sweeping across the disc in a wave, posterior to anterior. The R8 photoreceptor cell is specified first, followed by R2 and R5, then R3 and R4, and ending with R1, R6 and R7. The Drosophila acellular lens, or pseudocone, is made in each ommatidium by four cone cells that differentiate after the photoreceptor cells. During pupal development, primary, secondary and tertiary pigment cells are added in sequence, ending with bristle cell differentiation. The primary refractive material in the Drosophila eye is the cuticular cornea, rather than the pseudocone. The cornea is made by cone and pigment cells (Cagan and Ready, 1989; Kimori et al., 1992; Janssens and Gehring, 1999; Stahl et al., 2017; Charlton-Perkins and Cook, 2010).
Tribolium
Tribolium, or the red flour beetle, is a member of the largest insect order, the Coleoptera, and diverged from Drosophila 350 million years ago (Klingler, 2004). Interest in Tribolium has grown because, unlike Drosophila, this genus has maintained more of its gene content relative to the urbilaterian ancestor, undergoes short germ-band development and has a larval head capsule. Tribolium have both larval and adult eyes. The larval eye is a simple eyespot composed of two photoreceptor cell populations that merge during embryonic development (Ho, 1961; Marshall, 1927; Liu et al., 2006; Friedrich et al., 1996). In total, the larval eye has ∼25 photoreceptor cells and the adult eye is composed of ∼80 ommatidia (Friedrich et al., 1996). Similar to Drosophila, Tribolium photoreceptor cells also differentiate in a wave, posterior to anterior, starting with R8 and ending with R7, followed by cone, pigment and bristle cells (Friedrich et al., 1996). Additionally, Tribolium also have a cuticular corneal lens. However, a survey of the Tribolium genome showed an absence of Drosocrystallin, the primary cuticle protein contributing to the Drosophila lens (Tribolium Genome Sequencing Consortium, 2008).
The first genetic work focused on eye development in Tribolium examined the RDN. Knockdown of Eyeless, Toy, Dac, Eya and So led to a reduction in size or loss of the adult eye (Schinko et al., 2008; Yang et al., 2009a,b; Luan et al., 2014; Posnien et al., 2011). In addition, Otd and Pph13 (Arx) might play similar roles in photoreceptor differentiation across insects. In Drosophila and Tribolium, orthologs of Otd and Pph13 are required for proper rhabdomere development (Mishra et al., 2010; Li et al., 1996). In both insects, Otd regulates short-wavelength opsin (see Glossary, Box 1) expression and Pph13 regulates long-wavelength opsin expression (Mahato et al., 2014; Li et al., 1996).
Although developmental homologies suggest that the mechanisms for generating photoreceptor diversity in insects might be conserved, the genetics of these eyes clearly differs (Friedrich et al., 2016). For example, in Drosophila, eyg, a Pax6 homolog, works downstream of Notch signaling to control cell proliferation and growth of the eye disc (Chao et al., 2004). In Tribolium, loss of Eyg makes the adult eye larger at the expense of the antennal region, suggesting a patterning or competence change (ZarinKamar et al., 2011).
Arachnids
The primary and secondary eyes found in arachnids have predicted homologies with Drosophila visual organs: the primary eyes are homologous to ocelli and the secondary eyes evolved from the ommatidium of lateral compound eyes (Fig. 6B). To address this hypothesis, two species of spider have been the focus of molecular investigation: the house spider, Parateatoda tepidariorum; and the wandering spider, Cupiennius salei. A whole-genome duplication in spiders has resulted in the generation of paralogs of many transcription factors likely involved in eye development, including atonal, dac, otd, pax6 and six3 (Fig. 6C) (Schwager et al., 2017). Gene expression in P. tepidariorum suggests that the primary and secondary eyes emerge from different areas. The six secondary eyes develop from two bilateral eye fields, with gene expression suggesting that each field splits into three individual secondary eyes over time (Schomburg et al., 2015). This supports the hypothesis that secondary eyes might have evolved from dispersed ommatidia of an ancestral compound eye and that the principal eyes evolved from the insect ocellus (Schomburg et al., 2015; Samadi et al., 2015; Morehouse et al., 2017). RDN gene expression in P. tepadariorum and C. salei shows varying combinations in primary and secondary eyes. Expression is also inconsistent across species (Schomburg et al., 2015; Samadi et al., 2015; Turetzek et al., 2016; Schacht et al., 2020) (Fig. 6C). These patterns might be evidence of neofunctionalization or subfunctionalization of these duplicated genes in the developing visual system. Current functional investigations suggest that six3 paralogs are redundant in P. tepidariorum but that soA/Pt-so1 is necessary for the development of both primary and secondary eyes (Schacht et al., 2020; Gainett et al., 2020 preprint)
The evolution of complex non-cephalic visual systems and the role of developmental constraint
Developmental constraint is the concept that the lineage of a tissue can impact or limit the phenotypes that can evolve (Smith et al., 1985). One of the shared developmental aspects of vertebrate and Drosophila eyes is their association with cephalization of the nervous system. Many complex visual organs are found in the head or associated in development with the central nervous system. However, there are multiple examples of non-cephalic or distributed image-forming eyes. To highlight the role of developmental context in the evolution of complexity, we describe three examples found in the Spiralia: Chiton, Scallop and Fan worms.
Chiton
Polyplacophora (chitons) are a group of marine molluscs with eight overlapping shell plates made of aragonite. These plates are covered with thousands of pores containing sensory and secretory cells called aesthetes that lead to a canal system within the aragonite (Moseley, 1885; Sturrock and Baxter, 1993; Boyle, 1974; Baxter et al., 1987; Fernandez et al., 2007) (Fig. 7A,B). The aesthetes of chitons are thought to be multimodal sensors, but some species have evolved a subset into small lens-containing camera-type shell eyes (Moseley, 1885; Boyle, 1969a,b, 1976) (Fig. 7C). The lens in these eyes is composed of the aragonite shell material (Speiser et al., 2011; Li et al., 2015). This eye has the capacity to form an image and the animals show negative light responses (Speiser et al., 2011; Li et al., 2015; Kingston et al., 2018). The girdle tissue surrounding the shell plates is also photosensitive and lens-type tissue has been found in the sensory papilla of some species (Checa et al., 2017).
Fig. 7.
Shell eyes found in the adult chiton and scallop pallial eyes. (A) Cartoon of the polyplacophoran Acanthopleura granulata. These chiton species have hundreds of aragonite lens-containing eyes embedded in their shells. (B) Cartoon rendering of a magnified view of the chiton shell surface. Sensory pores (aesthetes) are evident in the shell surface. The blue arrow shows a lens-containing eye; the red arrowhead shows non-eye aesthetes. (C) High magnification cartoon rendering of the lens-containing eyes found in Acanthopleura. Figure based on Speiser et al. (2011). (D) Cartoon of a bay scallop with dozens of blue pallial eyes. A single eye is highlighted by a red arrowhead. (E) Cross-section of a single pallial eye. Proximal and distal retina, and corresponding axon tracts, cornea, lens, pigment cell layer and argentea (mirror) are shown. Anatomy based on Dakin (1928), Barber et al. (1967) and McReynolds and Gorman (1970).
Although lineage tracing of adult tissues has not been performed in lens-containing chitons, evidence from other species suggests that both adult sensory cells and shell-making cells are ectodermally derived (Henry et al., 2004). In adult chiton, eyes on the shell plates are eroded over time, often losing lenses and becoming non-functional (Moseley, 1885). The mantle continually secretes shell at the periphery, adding new aesthetes (Haas, 1981; McDougall and Degnan, 2018; Checa et al., 2017).
No developmental work has been endeavored on the lens-containing chiton visual system. Recent studies in the eyeless polyplacophoran, Acanthochitona crinita, identified a dispersed population of Pax2/5/8-expressing cells in the developing shell field. They are labeled by the FMRFamide neuropeptide antibody and hypothesized to be the sensory cells contributing to the adult aesthetes (Wollesen et al., 2015a). The character of the shell that makes the lenses in chitons is more uniform in its alignment of crystalline (see Glossary, Box 1) components than the rest of the shell (Li et al., 2015); however, how these lenses are made remains unknown. Gbx is expressed in the developing shell field and possibly expressed in shell-secreting cells (Wollesen et al., 2015b). Surveys in many species have also shown SoxB1 and Engrailed expressed in the neuroectoderm and Hox genes expressed co-linearly in the developing shell field (Huan et al., 2020; Fritsch et al., 2015; Jacobs et al., 2000; Wollesen et al., 2018). These genes might also play a role in the development of adult sensory structures.
Scallop
Within bivalves, both cephalic eyes and pallial eyes are observed, with examples ranging in structure and complexity (Morton, 2008; Nilsson, 1994). The scallop has between 30 and 100 pallial eyes at the tips of tentacles emerging from the middle mantle fold (Fig. 7D). Remarkable for their complexity, each is image forming, containing a lens, two retinas and a mirror (Dakin, 1909; Patten, 1887; Barber et al., 1967). The lens has little refractive power; the mirror, which is composed of square plates of guanine crystals, and cornea act as the primary focusing agents (Land, 1966a,b; Palmer et al., 2017; Speiser et al., 2016). The eye is single chambered, with light entering through the lens and hitting the proximal retina in the posterior of the eye. The light then reflects off the mirror (argentea) behind the proximal retina toward the distal retina at the anterior (Land, 1965; Palmer et al., 2017) (Fig. 7E). The proximal retinal photoreceptors express a rhabdomeric opsin and depolarize in response to light, whereas the distal retinal photoreceptors express a ciliary opsin and hyperpolarize in response to light (Wang et al., 2017; Gorman and McReynolds, 1969; McReynolds and Gorman, 1970; Land, 1966a; Hartline, 1938). It has been difficult to assess the function of either retina but the current hypothesis is that the distal retina forms an image, whereas the proximal retina might be responsible for acute peripheral vision (Palmer et al., 2017).
Scallop trochophore larvae have eye spots, but their relation to adult visual organs is unknown (Cragg, 2016). Early descriptions of scallop eye development identified that the retina, pigmented cells and cornea are ectodermally derived and the lens is mesodermally derived (Dakin, 1909; Küpfer, 1916). The first evidence of the eye anlage is a thickening on the middle mantle fold. This extends to form an undifferentiated optic papilla. Some cells within the papilla segregate to become the optic ganglion and as the papilla grows, cells organize into the optic vesicle (Butcher, 1930; Patten, 1887). Cells in the anterior become pigmented and will form the iris and cornea. The posterior cells proliferate, generating an undifferentiated cellular mass that organizes into layers. The proximal retina differentiates first, followed by the distal retina (Audino et al., 2015). Behind these retinae is the argentea and a pigmented cell layer. The argentea is cellular but eventually these cells degrade their nuclei (Patten, 1887). The lens is cellular and the last tissue to differentiate, with the nuclei arranging themselves at the periphery (Audino et al., 2015). RNA-seq shows that Pax2/5/8, Brn3, Lmx1b and Six4/5 are enriched in the adult pallial visual system (Wang et al., 2017). Their contribution during ontogeny remains unknown.
Annelid
Annelids are a phylum of segmented worms found in the Spiralia that includes leeches, earthworms and fan worms, among others. Fan worms that live in tubes, including sabellid and serpulid annelids, have evolved a remarkable set of visual organs. They have long fan-like radiole tentacles used for feeding, with compound eyes along the tentacles to detect predators (Nilsson, 1994, 2013). These radiolar eyes show incredible diversity in placement and complexity (Bok et al., 2016; Bok and Nilsson, 2016). The most complex are found in Acromegalomma, which have two large compound eyes composed of hundreds of ocelli (Gil and Nishi, 2017). These animals have cerebral eyes as well, with rhabdomeric photoreceptors. The radiolar ocelli photoreceptors are ciliary (Eakin and Hermans, 1988; Verger-Bocquet, 1992; Purschke et al., 2006; Bok et al., 2017a). The use of this type of photoreceptor in image-forming eyes is unusual because in other annelid species, ciliary photoreceptors are associated with nonpigmented photoreception and the central nervous system (Bok et al., 2016). The radioles are constituents of the branchial crown, a specialized part of the annelid mouth (Bok et al., 2016, 2017b). Development of these visual organs has not been well documented, but the ocelli are derived from epidermal cells in the outer aboral corners of the radioles (Bok et al., 2017b). Transcriptomic data from the eyes of adult serpulid fan worms show expression of eya, six4, so, pax6, glass and dpp (Bok et al., 2017b).
Expression and function of shared regulatory factors in visual organs of the Spiralia
As discussed elsewhere, one of the ways we understand evolutionary relationships between organs, tissues and cells in development is the identification of characteristic patterns of gene expression and function. The RDN is the primary group of genes used to understand these relationships in visual organs; however, there are numerous genes and networks associated with eye development in animals. Here, we discuss some of these regulatory factors in the context of Spiralian visual organ development.
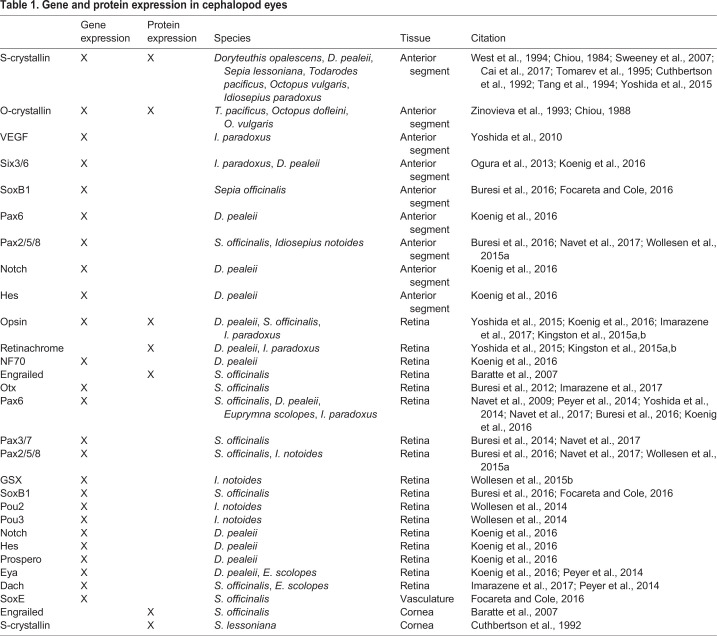
With the explosion of Spiralian genomic and transcriptomic sequencing, we can assess expression of canonical visual system-associated transcription factors in the third major branch of the Bilateria (Fig. 1). Early surveys show canonical eye-related gene expression during visual organ development and homeostasis; however, no exclusive combination of genes appears to be required. We see this in the eyeless chiton species, Leptochiton asellus. All larval eye spots express Six1/2, Eya, Dach, Lhx2/9, Irx, Ovo and FoxQ2; however, Pax6 and Six3/6 were only found in some, but not all, larval photoreceptor populations or associated cells (Vöcking et al., 2015). Cephalopods, a class of molluscs that does not undergo metamorphosis, have shown a significant number of canonical transcription factors expressed in the developing eyes (Table 1). However, without a clear lineage of these tissues, it is difficult to assess homology across these visual organs.
Table 1.
Gene and protein expression in cephalopod eyes
Within Annelids, the adult cerebral eye of Platynereis dumerilii is the most in-depth description of lens-containing eye development (Rhode, 1992). In Platynereis, the larval eye is composed of two cells and emerges early in development. The adult eye forms in the larva, both organs existing simultaneously (Rhode, 1992; Arendt et al., 2002). Molecular work in P. dumerilii demonstrated variation in gene expression between larval and adult cerebral eyes, with Pax6, eya, six1/2, and otx found in the larval eye and Atonal and Six1/2 found in the adult (Arendt et al., 2002; Steinmetz et al., 2010). In addition, recent studies in Capitella telata, an annelid species with eyes that do not contain a lens, showed that knockdown of Ct-pax6 leads to loss of the larval eye and downregulation of Ct-eya (Klann and Seaver, 2019).
Some of the most in-depth molecular descriptions of the eye in Spiralia is during regeneration in planaria (Lapan and Reddien, 2012, 2011). Eye regeneration is independent of Pax6 (Pineda et al., 2002), but Eya and Six1/2 are required (Mannini et al., 2004; Pineda et al., 2000). Access to embryos in planaria is difficult but expression studies have confirmed that eye development is also likely Pax6 independent (Martín-Durán et al., 2012).
These surveys are highlighting that no single gene appears to be required for visual system development across all species; yet, many canonical eye development genes are shared. With still incomplete data and little information about the function of any of these genes, the evolutionary implication(s) of this shared expression remains unclear. Much will be learned with the expansion of functional genomic methods paired with further genetic manipulations.
Visual system complexity outside the Bilateria
The transition to bilateral symmetry correlates with an explosion of innovation in the animal lineage, including the cephalization and increasing complexity of the central nervous system. However, interesting innovations in the nervous system found outside of Bilateria include multiple instances of lens-containing visual organs in the Cnidaria.
Cnidarians, including corals, sea anemones and jellyfish, are the sister phylum to bilaterally symmetric animals in the Metazoa. Cnidarians have a large range of visual organ complexity, from eye spots to lens-containing eyes (Martin, 2004). The majority of these photoreceptive organs are associated with the free-swimming medusa stage within the Medusazoan clade. Three classes of Medusazoa have evolved lens-containing eyes independently: Hydrozoa, Cubozoa and Scyphozoa (Picciani et al., 2018). The medusa stage in these animals emerges from a sessile polyp and the visual organs develop during this metamorphosis, a process that differs in each group. Cubozoa and Scyphozoa have evolved specialized sensory structures called rhopalia at the bell margin of the medusa. Depending on the species, the sensory modality and complexity of the rhopalia varies, but can include a statocyst and photoreceptive organs. Hydrozoans do not have rhopalia; their visual organs are usually found at the base of the tentacle (Martin, 2002).
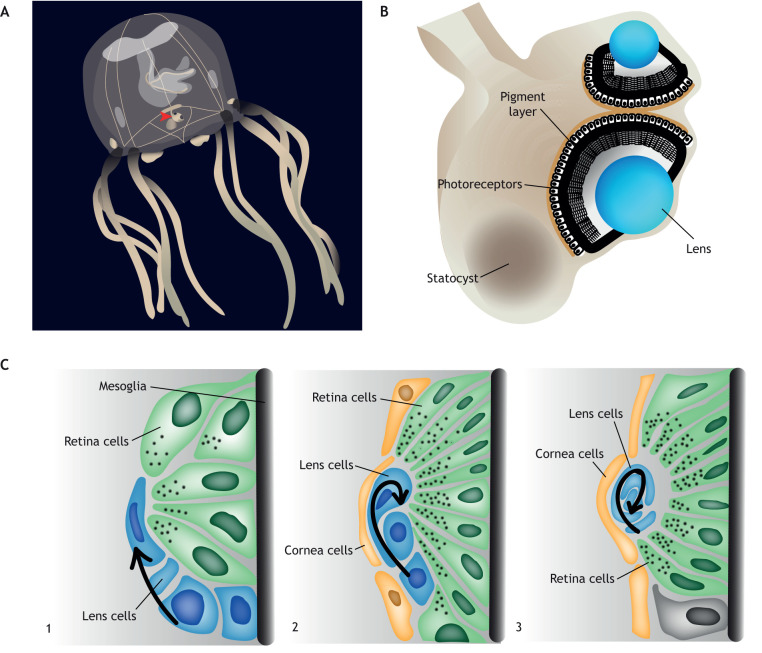
The Cubozoa or box jellyfish have the most sophisticated visual system of any cnidarian. In each of the four corners of the medusa, the associated rhopalium has six visual organs, each animal having 24 in total (Nilsson et al., 2005) (Fig. 8A). Two eyes in each corner have a lens, the others are simple pigmented eye cups. The two complex eyes have a cornea, iris, lens, retina and pigmented layer (Conant, 1898; Yamasu and Yoshida, 1976; Martin, 2004; Nilsson et al., 2005; O'Conner et al., 2009; Laska, 1982) (Fig. 8B). The upper eye is used for navigating mangrove forests of the cnidarian habitat and the lower eye is used for viewing the underwater environment (Nilsson et al., 2005; Hamner et al., 1995; Garm et al., 2007, 2011).
Fig. 8.
Cubozoan lens-containing eyes. (A) Cartoon of the box jellyfish Tripedalia cystophora. Red arrowhead shows the location of one of the four eye-containing rhopalia. (B) Cross-section of T. cystophora rhopalia. The small lens-containing eye points upward and is dorsal to the large lens-containing eye. Pigment is found within the photoreceptor cells and the cell bodies are found behind the pigment. The outer segment of the photoreceptor cells is anterior to the cell bodies and pigment. (C) Stages in the morphogenesis of the small lens-containing eye in T. cystophora. (1) Lens cells divide in line with the surface epithelia, start elongating and pigment begins to form in the retinal cells. (2) Lens cells turn toward the retina and begin wrapping around one another. Corneal cells are apparent. (3) Lens cells have made a round core and continue this rolling behavior. Figure based on Laska-Mehnert (1985).
The medusa in Cubozoa is formed from a whole-body metamorphosis of the polyp stage. Early in development, the polyp tentacles move from radial symmetry to tetraradial symmetry (Werner, 1983, 1975; Werner et al., 1971; Stangl et al., 2002). These polyp tentacles form the developing rhopalia, which are highly proliferative, and the lens and retina emerge from transdifferentiated tentacle muscle (Laska-Mehnert, 1985; Piatigorsky et al., 1989; Gurska and Garm, 2014). Shortly thereafter, a cup primordium and melanin are apparent (Stangl et al., 2002; Valley, 2011). The lens is cellular and, early in development, cells that will make the lens divide in parallel with the epithelial plane. One cell changes its orientation, turning toward the developing retina, becoming wrapped by the other epithelial cells (Fig. 8C). This ‘roll morphogenesis’ continues until a circular lens is formed (Laska-Mehnert, 1985). During this period, pigment granules appear in the photoreceptor cells and the morphology of these cells elongates. These eyes are functional once the animal has budded from the polyp base (Laska-Mehnert, 1985). Molecular analysis of the Cubozoan eye is limited. The adult Tripedelia retina expresses a Pax homolog, PaxB, Mitf and two opsins, as well as three crystallins in the lens (Liegertová et al., 2015; Piatigorsky et al., 1989; Kozmik, 2005; Kozmik et al., 2008).
Scyphozoa lens-containing species have been identified, but the development of their eyes has not been described (Maas, 1903; Hertwig and Hertwig, 1878). However, we do have some knowledge of nervous system development in the related moon jellyfish, Aurelia sp.1. Aurelia do not have lenses, but have pigmented eye cups (Nakanishi et al., 2009). The pigmented cells are derived from endoderm and the sensory cells from ectoderm (Nakanishi et al., 2009; Yamasu and Yoshida, 1976; Hyman, 1940). Pit1, Brn3 and Otx are expressed during early neurogenesis in the rhopalia (Nakanishi et al., 2010). Later, Brn3, Six1/2 and Eya are found in the developing optic cup (Nakanishi et al., 2010). Pax genes and Six3/6 are not expressed during the stages surveyed in the developing visual organ, supporting its independent evolution (Nakanishi et al., 2015).
The last group, Hydrozoa, bud off multiple medusae from a sessile polyp, and many lens-containing eyes have been described in these species. In Cladonema radiatum, the ocellus is ectodermal, with the retina composed of a layer of photoreceptor cells and a layer of pigmented cells (Martin, 2002; Weber, 1981). The lens is cellular and composed of two units. Each unit is a distal cytoplasmic extension of a pigmented cell. No developmental surveys of gene expression have been performed, but Six1/2, Six3/6, Six4/5, Eya and PaxA are expressed in adult eyes (Suga et al., 2010; Stierwald et al., 2004; Graziussi et al., 2012).
Cnidarians independently evolved the eye multiple times (Picciani et al., 2018) and a greater understanding of these organs may reveal the developmental basis of elaborate neural tissues and optical structures in all animals. Furthermore, the regenerative capacity of cnidarians make them a particularly attractive model to understand stem-cell biology and the regeneration of sensory structures in an evolutionary context.
Summary and future prospects
In this Review, we have discussed the visual organs of animals across broad phylogenetic distances, with a focus on ontogeny. We have highlighted six areas in which existing comparative studies and future work will help us understand fundamental questions in evolutionary biology. Although gene expression analyses in development have suggested homologies and potential evolutionary relationships between the eyes of diverse animals, it is now evident that no single gene regulates eye formation in all animals. In fact, the diversity found within the visual organs of animals has much more to offer us than an understanding of homology. Although we must keep gene expression in mind, we should expand our studies to include genetic function, cell biology and the morphogenetic mechanisms underlying visual organ formation across the Metazoa. The ability to study (and appreciate) this diversity is a powerful new and unbiased approach to understand the emergence of phenotype.
The areas discussed here are just a miniscule sampling of what the visual system has to offer. An additional example includes the developmental diversity of lens formation across animals, and how this can pattern the refractive index (Land, 2012). Another is the generation of reflective materials, such as a tapetum, to enhance photoreceptor sensitivity, or the retinal mirror found within scallops (Schwab et al., 2002; Land, 1966b). These materials have evolved multiple times but how they are generated is largely unknown. We know astonishingly little about the developmental basis of iris diversity or the cell biology of intracellular filters in photoreceptors, such as pigmented oil droplets in the avian retina (Banks et al., 2015; Toomey and Corbo, 2017). The list of newly accessible questions is endless.
In conclusion, much like we should celebrate the diversity among ourselves, so too should we celebrate the diversity throughout the Metazoa. We are in an era in which ‘model systems’ are no longer limited to those few that were once tractable to molecular techniques. We should leverage this diversity to uncover shared and unique developmental mechanisms underlying complex organ evolution and, in doing so, shed light on cell and developmental processes that are beyond our current imagination.
Acknowledgements
We thank Ana Gabriel for generating several figures; Katie Crutchfield and Andy Gill for editorial support; and Dave McClay, Susana da Silva and the anonymous reviewers for helpful comments on this Review. We apologize to any authors whose contributions to this broad and diverse field have been omitted from this Review due to space constraints.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health (NEI R01-EY18005, R01-EY29031 and R01-EY18005 to J.M.G.; 1DP5OD023111-01 to K.M.K.; and P30-EY08098 to the Department of Ophthalmology, University of Pittsburgh); the Eye and Ear Foundation of Pittsburgh; Research to Prevent Blindness; and Harvard University. Deposited in PMC for release after 12 months.
References
- Abitua P. B., Wagner E., Navarrete I. A. and Levine M. (2012). Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature 492, 104-107. 10.1038/nature11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuixech-Crespo B., López-Blanch L., Burguera D., Maeso I., Sánchez-Arrones L., Moreno-Bravo J. A., Somorjai I., Pascual-Anaya J., Puelles E., Bovolenta P. et al. (2017). Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol. 15, e2001573 10.1371/journal.pbio.2001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini R., Rocha-Martins M. and Norden C. (2018). Neuronal migration and lamination in the vertebrate retina. Front. Neurosci. 11, 742 10.3389/fnins.2017.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D., Tessmar K., de Campos-Baptista M. I. M., Dorresteijn A. and Wittbrodt J. (2002). Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development 129, 1143-1154. [DOI] [PubMed] [Google Scholar]
- Arendt D., Hausen H. and Purschke G. (2009). The ‘division of labour’ model of eye evolution. Philos. Trans. R. Soc. B Biol. Sci. 364, 2809-2817. 10.1098/rstb.2009.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D., Musser J. M., Baker C. V. H., Bergman A., Cepko C., Erwin D. H., Pavlicev M., Schlosser G., Widder S., Laubichler M. D. et al. (2016). The origin and evolution of cell types. Nat. Rev. Genet. 17, 744-757. 10.1038/nrg.2016.127 [DOI] [PubMed] [Google Scholar]
- Arnold J. M. (1966). On the occurrence of microtubules in the developing lens of the squid Loligo pealii. J. Ultrastruct. Res. 14, 534-539. 10.1016/S0022-5320(66)80080-1 [DOI] [PubMed] [Google Scholar]
- Arnold J. M. (1967). Fine structure of the development of the cephalopod lens. J. Ultrastruct. Res. 17, 527-543. 10.1016/S0022-5320(67)80139-4 [DOI] [PubMed] [Google Scholar]
- Audino J. A., Marian J. E. A. R., Wanninger A. and Lopes S. G. B. C. (2015). Development of the pallial eye in Nodipecten nodosus (Mollusca: Bivalvia): insights into early visual performance in scallops. Zoomorphology 134, 403-415. 10.1007/s00435-015-0265-8 [DOI] [Google Scholar]
- Banks M. S., Sprague W. W., Schmoll J., Parnell J. A. Q. and Love G. D. (2015). Why do animal eyes have pupils of different shapes? Sci. Adv. 1, e1500391 10.1126/sciadv.1500391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantseev V., Auclair F., Dubuc R. and Sivak J. G. (2005). Optical quality of the ocular lens of the sea lamprey (Petromyzon marinus) during the mature and transformer periods of life. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 191, 505-509. 10.1007/s00359-005-0611-2 [DOI] [PubMed] [Google Scholar]
- Baratte S., Andouche A. and Bonnaud L. (2007). Engrailed in cephalopods: a key gene related to the emergence of morphological novelties. Dev. Genes Evol. 217, 353-362. 10.1007/s00427-007-0147-2 [DOI] [PubMed] [Google Scholar]
- Barber V. C., Evans E. M. and Land M. F. (1967). The fine structure of the eye of the mollusc Pecten maximus. Z. Zellforsch. Mikrosk. Anat. 76, 295-312. 10.1007/BF00339290 [DOI] [PubMed] [Google Scholar]
- Bardack D. (1991). First fossil hagfish (myxinoidea): a record from the pennsylvanian of illinois. Science 254, 701-703. 10.1126/science.254.5032.701 [DOI] [PubMed] [Google Scholar]
- Bassett E. A. and Wallace V. A. (2012). Cell fate determination in the vertebrate retina. Trends Neurosci. 35, 565-573. 10.1016/j.tins.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Baxter J. M., Jones A. M. and Sturrock M. G. (1987). The infrastructure of aesthetes in Tonicella marmorea (Polyplacophora; Ischnochitonina) and a new functional hypothesis. J. Zool. 211, 589-604. 10.1111/j.1469-7998.1987.tb04473.x [DOI] [Google Scholar]
- Bernstein C. S., Anderson M. T., Gohel C., Slater K., Gross J. M. and Agarwala S. (2018). The cellular bases of choroid fissure formation and closure. Dev. Biol. 440, 137-151. 10.1016/j.ydbio.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blest A. D. (1983). Ultrastructure of secondary retinae of primitive and advanced jumping spiders (Araneae, Salticidae). Zoomorphology 102, 125-141. 10.1007/BF00363805 [DOI] [Google Scholar]
- Blest A. D., Williams D. S. and Kao L. (1980). The posterior median eyes of the dinopid spider Menneus. Cell Tissue Res. 211, 391-403. 10.1007/BF00234395 [DOI] [PubMed] [Google Scholar]
- Bok M. J. and Nilsson D.-E. (2016). Fan worm eyes. Curr. Biol. 26, R907-R908. 10.1016/j.cub.2016.06.032 [DOI] [PubMed] [Google Scholar]
- Bok M. J., Capa M. and Nilsson D.-E. (2016). Here, there and everywhere: the radiolar eyes of fan worms (Annelida, Sabellidae). Integr. Comp. Biol. 56, 784-795. 10.1093/icb/icw089 [DOI] [PubMed] [Google Scholar]
- Bok M. J., Porter M. L. and Nilsson D.-E. (2017a). Phototransduction in fan worm radiolar eyes. Curr. Biol. 27, R698-R699. 10.1016/j.cub.2017.05.093 [DOI] [PubMed] [Google Scholar]
- Bok M. J., Porter M. L., Ten Hove H. A., Smith R. and Nilsson D. E. (2017b). Radiolar eyes of serpulid worms (Annelida, Serpulidae): structures, function, and phototransduction. Biol. Bull. 233, 39-57. 10.1086/694735 [DOI] [PubMed] [Google Scholar]
- Bostwick M., Smith E. L., Borba C., Newman-Smith E., Guleria I., Kourakis M. J. and Smith W. C. (2020). Antagonistic inhibitory circuits integrate visual and gravitactic behaviors. Curr. Biol. 30, 600-609.e2. 10.1016/j.cub.2019.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. R. (1969a). Rhabdomeric ocellus in a chiton. Nature 222, 895-896. 10.1038/222895a0 [DOI] [Google Scholar]
- Boyle P. R. (1969b). Fine structure of the eyes of Onithochiton neglectus (Mollusca: Polyplacophora). Z. Zellforsch. Mikrosk. Anat. 102, 313-332. 10.1007/BF00335443 [DOI] [PubMed] [Google Scholar]
- Boyle P. R. (1974). The aesthetes of chitons. II. Fine structure in Lepidochitona cinereus (L.). Cell Tissue Res. 153, 383-398. 10.1007/BF00229166 [DOI] [PubMed] [Google Scholar]
- Boyle P. R. (1976). The aesthetes of chitons. III. Shell surface observations. Cell Tissue Res. 172, 379-388. 10.1007/BF00399520 [DOI] [PubMed] [Google Scholar]
- Buresi A., Baratte S., Da Silva C. and Bonnaud L. (2012). orthodenticle/otx ortholog expression in the anterior brain and eyes of Sepia officinalis (Mollusca, Cephalopoda). Gene Expr. Patterns 12, 109-116. 10.1016/j.gep.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Buresi A., Croll R. P., Tiozzo S., Bonnaud L. and Baratte S. (2014). Emergence of sensory structures in the developing epidermis in Sepia officinalis and other coleoid cephalopods. J. Comp. Neurol. 522, 3004-3019. 10.1002/cne.23562 [DOI] [PubMed] [Google Scholar]
- Buresi A., Andouche A., Navet S., Bassaglia Y., Bonnaud-Ponticelli L. and Baratte S. (2016). Nervous system development in cephalopods: how egg yolk-richness modifies the topology of the mediolateral patterning system. Dev. Biol. 415, 143-156. 10.1016/j.ydbio.2016.04.027 [DOI] [PubMed] [Google Scholar]
- Buschbeck E. K. (2014). Escaping compound eye ancestry: the evolution of single-chamber eyes in holometabolous larvae. J. Exp. Biol. 217, 2818-2824. 10.1242/jeb.085365 [DOI] [PubMed] [Google Scholar]
- Butcher E. O. (1930). The formation, regeneration, and transplantation of eyes in Pecten (Gibbus borealis). Biol. Bull. 59, 154-164. 10.2307/1536984 [DOI] [Google Scholar]
- Cagan R. L. and Ready D. F. (1989). The emergence of order in the Drosophila pupal retina. Dev. Biol. 136, 346-362. 10.1016/0012-1606(89)90261-3 [DOI] [PubMed] [Google Scholar]
- Cai J., Townsend J. P., Dodson T. C., Heiney P. A. and Sweeney A. M. (2017). Eye patches: protein assembly of index-gradient squid lenses. Science 357, 564-569. 10.1126/science.aal2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. B. (2008). Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25-36. 10.1016/j.cell.2008.06.030 [DOI] [PubMed] [Google Scholar]
- Cavodeassi F. and Wilson S. W. (2019). Looking to the future of zebrafish as a model to understand the genetic basis of eye disease. Hum. Genet. 138:993-1000. 10.1007/s00439-019-02055-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. Nat. Rev. Neurosci. 15, 615-627. 10.1038/nrn3767 [DOI] [PubMed] [Google Scholar]
- Chao J.-L., Tsai Y. C., Chiu S. J. and Sun Y. H. (2004). Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development 131, 3839-3847. 10.1242/dev.01258 [DOI] [PubMed] [Google Scholar]
- Charlton-Perkins M. and Cook T. A. (2010). Building a fly eye: terminal differentiation events of the retina, corneal lens, and pigmented epithelia. Curr. Top. Dev. Biol. 93, 129-173. 10.1016/B978-0-12-385044-7.00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa A. G., Vendrasco M. J. and Salas C. (2017). Cuticle of Polyplacophora: structure, secretion, and homology with the periostracum of conchiferans. Mar. Biol. 164, 64 10.1007/s00227-017-3100-6 [DOI] [Google Scholar]
- Chiou S.-H. (1984). Physicochemical characterization of a crystallin from the squid lens and its comparison with vertebrate lens crystallins. J. Biochem. 95, 75-82. 10.1093/oxfordjournals.jbchem.a134605 [DOI] [PubMed] [Google Scholar]
- Chiou S.-H. (1988). A novel crystallin from octopus lens. FEBS Lett. 241, 261-264. 10.1016/0014-5793(88)81073-1 [DOI] [PubMed] [Google Scholar]
- Chow R. L. and Lang R. A. (2001). Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 17, 255-296. 10.1146/annurev.cellbio.17.1.255 [DOI] [PubMed] [Google Scholar]
- Cole A. G. and Meinertzhagen I. A. (2004). The central nervous system of the ascidian larva: mitotic history of cells forming the neural tube in late embryonic Ciona intestinalis. Dev. Biol. 271, 239-262. 10.1016/j.ydbio.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Conant F. S. (1898). The Cubomedusae. Johns Hopkins Press. [Google Scholar]
- Cragg S. M. (2016). Biology and ecology of scallop larvae. In Developments in Aquaculture and Fisheries Science, Vol. 40 (ed. Shumway S. E. and Jay Parsons G.), pp. 31-83. Elsevier. [Google Scholar]
- Cuthbertson R. A., Tomarev S. I. and Piatigorsky J. (1992). Taxon-specific recruitment of enzymes as major soluble proteins in the corneal epithelium of three mammals, chicken, and squid. Proc. Natl Acad. Sci. USA. 89, 4004-4008. 10.1073/pnas.89.9.4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A. and Ashery-Padan R. (2014). The cellular and molecular mechanisms of vertebrate lens development. Development 141, 4432-4447. 10.1242/dev.107953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A. and Callaerts P. (2017). PAX6: 25th anniversary and more to learn. Exp. Eye Res. 156, 10-21. 10.1016/j.exer.2016.04.017 [DOI] [PubMed] [Google Scholar]
- da Silva S. and Cepko C. L. (2017). Fgf8 expression and degradation of retinoic acid are required for patterning a high-acuity area in the retina. Dev. Cell 42, 68-81.e6. 10.1016/j.devcel.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin W. J. (1909). Pectens (No. 17). Williams & Norgate. [Google Scholar]
- Dakin W. J. (1928). The eyes of Pecten, Spondylus, Amussium and allied Lamellibranchs, with a short discussion on their evolution. Proc. R. Soc. Lond. Ser. B Contain. Papers Biol. Character 103, 355-365. 10.1098/rspb.1928.0047 [DOI] [Google Scholar]
- D'Aniello S., D'Aniello E., Locascio A., Memoli A., Corrado M., Russo M. T., Aniello F., Fucci L., Brown E. R. and Branno M. (2006). The ascidian homolog of the vertebrate homeobox gene Rx is essential for ocellus development and function. Differentiation 74, 222-234. 10.1111/j.1432-0436.2006.00071.x [DOI] [PubMed] [Google Scholar]
- Dash S., Dang C. A., Beebe D. C. and Lachke S. A. (2015). Deficiency of the RNA binding protein caprin2 causes lens defects and features of peters anomaly. Dev. Dyn. 244, 1313-1327. 10.1002/dvdy.24303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel E., Rodicio M. C. and Anadón R. (1989). Ganglion cells and retinopetal fibers of the larval lamprey retina: an HRP ultrastructural study. Neurosci. Lett. 106, 1-6. 10.1016/0304-3940(89)90192-4 [DOI] [PubMed] [Google Scholar]
- DeVoe R. D., Small R. J. W. and Zvargulis J. E. (1969). Spectral sensitivities of wolf spider eyes. J. Gen. Physiol. 54, 1-32. 10.1085/jgp.54.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. H. and Collard T. R. (1979). Retinal development in the lamprey (Petromyzon marinus L.): premetamorphic ammocoete eye. Am. J. Anat. 154, 321-336. 10.1002/aja.1001540303 [DOI] [PubMed] [Google Scholar]
- Eakin R. M. and Hermans C. O. (1988). Eyes. In The Ultrastructure of Polychaeta, Vol. 4 Microfauna Marina (ed. Westheide W. and Hermans C. O.), pp. 135-156. Gustav Fischer Verlag. [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T. and Sasai Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51-56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Emerling C. A. (2018). Regressed but not gone: patterns of vision gene loss and retention in subterranean mammals. Integr. Comp. Biol. 58, 441-451. 10.1093/icb/icy004 [DOI] [PubMed] [Google Scholar]
- Erclik T., Hartenstein V., McInnes R. R. and Lipshitz H. D. (2009). Eye evolution at high resolution: the neuron as a unit of homology. Dev. Biol. 332, 70-79. 10.1016/j.ydbio.2009.05.565 [DOI] [PubMed] [Google Scholar]
- Esposito R., Racioppi C., Pezzotti M. R., Branno M., Locascio A., Ristoratore F. and Spagnuolo A. (2015). The ascidian pigmented sensory organs: structures and developmental programs. Genesis 53, 15-33. 10.1002/dvg.22836 [DOI] [PubMed] [Google Scholar]
- Fain G. L. (2019). Lamprey vision: photoreceptors and organization of the retina. Semin. Cell Dev. Biol. 106, 5-11. 10.1016/j.semcdb.2019.10.008 [DOI] [PubMed] [Google Scholar]
- Fernandez C. Z., Vendrasco M. J. and Runnegar B. (2007). Aesthete canal morphology in twelve species of chiton (Polyplacophora). Veliger 25, 51-69. [Google Scholar]
- Fernholm B. and Holmberg K. (1975). The eyes in three genera of hagfish (Eptatretus, Paramyxine and Myxine)--a case of degenerative evolution. Vision Res. 15, 253-259. 10.1016/0042-6989(75)90215-1 [DOI] [PubMed] [Google Scholar]
- Focareta L. and Cole A. G. (2016). Analyses of sox-B and sox-E family genes in the cephalopod Sepia officinalis: revealing the conserved and the unusual. PLoS ONE 11, e0157821 10.1371/journal.pone.0157821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foelix R. (2011). Biology of Spiders. New York, USA: Oxford University Press. [Google Scholar]
- Friedrich M. (2006). Ancient mechanisms of visual sense organ development based on comparison of the gene networks controlling larval eye, ocellus and compound eye specification in Drosophila . Arthropod. Struct. Dev. 35, 357-378. 10.1016/j.asd.2006.08.010 [DOI] [PubMed] [Google Scholar]
- Friedrich M., Rambold I. and Melzer R. R. (1996). The early stages of ommatidial development in the flour beetle Tribolium castaneum (Coleoptera; Tenebrionidae). Dev. Genes Evol. 206, 136-146. 10.1007/s004270050039 [DOI] [PubMed] [Google Scholar]
- Friedrich M., Cook T. and Zelhof A. C. (2016). Ancient default activators of terminal photoreceptor differentiation in the pancrustacean compound eye: the homeodomain transcription factors Otd and Pph13. Curr. Opin. Insect Sci. 13, 33-42. 10.1016/j.cois.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch M., Wollesen T., De Oliveira A. L. and Wanninger A. (2015). Unexpected co-linearity of Hox gene expression in an aculiferan mollusk. BMC Evol. Biol. 15, 151 10.1186/s12862-015-0414-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. and Collin S. P. (1990). Dendritic distribution of two populations of ganglion cells and the retinopetal fibers in the retina of the silver lamprey (Ichthyomyzon unicuspis). Vis. Neurosci. 4, 533-545. 10.1017/S0952523800005745 [DOI] [PubMed] [Google Scholar]
- Fuhrmann S. (2010). Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93, 61-84. 10.1016/B978-0-12-385044-7.00003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott S. E., Donoghue P. C. J., Sansom R. S., Vinther J., Dolocan A. and Purnell M. A. (2016). Pigmented anatomy in Carboniferous cyclostomes and the evolution of the vertebrate eye. Proc. Biol. Sci. 283, 20161151 10.1098/rspb.2016.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. J., Rhoades W., Prucka S. K. and Hjalt T. (2005). Fate maps of neural crest and mesoderm in the mammalian eye. Invest. Ophthalmol. Vis. Sci. 46, 4200-4208. 10.1167/iovs.05-0691 [DOI] [PubMed] [Google Scholar]
- Gainett G., Ballesteros J. A., Kanzler C. R., Zehms J. T., Zern J. M., Aharon S., Gavish-Regev E. and Sharma P. P. (2020). How spiders make their eyes: Systemic paralogy and function of retinal determination network homologs in arachnids. bioRxiv. 10.1101/2020.04.28.067199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garm A., O'Connor M., Parkefelt L. and Nilsson D.-E. (2007). Visually guided obstacle avoidance in the box jellyfish Tripedalia cystophora and Chiropsella bronzie. J. Exp. Biol. 210, 3616-3623. 10.1242/jeb.004044 [DOI] [PubMed] [Google Scholar]
- Garm A., Oskarsson M. and Nilsson D.-E. (2011). Box jellyfish use terrestrial visual cues for navigation. Curr. Biol. 21, 798-803. 10.1016/j.cub.2011.03.054 [DOI] [PubMed] [Google Scholar]
- Gehring W. J. (2014). The evolution of vision. Wiley Interdiscipl. Rev. Dev. Biol. 3, 1-40. 10.1002/wdev.96 [DOI] [PubMed] [Google Scholar]
- Gil J. and Nishi E. (2017). Nomenclatural checklist for Acromegalomma species (Annelida, Sabellidae), a nomen novum replacement for the junior homonym Megalomma Johansson, 1926. ZooKeys 677, 131 10.3897/zookeys.677.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. (1994). Form and function of stemmata in larvae of holometabolous insects. Annu. Rev. Entomol. 39, 323-349. 10.1146/annurev.en.39.010194.001543 [DOI] [Google Scholar]
- Giribet G. and Edgecombe G. D. (2019). The phylogeny and evolutionary history of arthropods. Curr. Biol. 29, R592-R602. 10.1016/j.cub.2019.04.057 [DOI] [PubMed] [Google Scholar]
- Glardon S., Callaerts P., Halder G. and Gehring W. J. (1997). Conservation of Pax-6 in a lower chordate, the ascidian Phallusia mammillata. Development 124, 817-825. [DOI] [PubMed] [Google Scholar]
- Gorman A. L. F. and McReynolds J. S. (1969). Hyperpolarizing and depolarizing receptor potentials in the scallop eye. Science 165, 309-310. 10.1126/science.165.3890.309 [DOI] [PubMed] [Google Scholar]
- Götz M. and Huttner W. B. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777-788. 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- Graziussi D. F., Suga H., Schmid V. and Gehring W. J. (2012). The “eyes absent”(eya) gene in the eye-bearing hydrozoan jellyfish Cladonema radiatum: conservation of the retinal determination network. J. Exp. Zool. B Mol. Dev. Evol. 318, 257-267. 10.1002/jez.b.22442 [DOI] [PubMed] [Google Scholar]
- Grusch M., Barth F. G. and Eguchi E. (1997). Fine structural correlates of sensitivity in the eyes of the ctenid spider, Cupiennius salei Keys. Tissue Cell 29, 421-430. 10.1016/S0040-8166(97)80028-6 [DOI] [PubMed] [Google Scholar]
- Gurska D. and Garm A. (2014). Cell proliferation in cubozoan jellyfish Tripedalia cystophora and Alatina moseri. PLoS ONE 9, e102628 10.1371/journal.pone.0102628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson O. S. E., Collin S. P. and Kröger R. H. H. (2008). Early evolution of multifocal optics for well-focused colour vision in vertebrates. J. Exp. Biol. 211, 1559-1564. 10.1242/jeb.016048 [DOI] [PubMed] [Google Scholar]
- Haas W. (1981). Evolution of calcareous hardparts in primitive molluscs. Malacologia 21, 403-418. [Google Scholar]
- Hamner W. M., Jones M. S. and Hamner P. P. (1995). Swimming, feeding, circulation and vision in the Australian box jellyfish, Chironex fleckeri (Cnidaria: Cubozoa). Mar. Freshw. Res. 46, 985-990. 10.1071/MF9950985 [DOI] [Google Scholar]
- Hartline H. K. (1938). The discharge of impulses in the optic nerve of Pecten in response to illumination of the eye. J. Cell. Comp. Physiol. 11, 465-478. 10.1002/jcp.1030110311 [DOI] [Google Scholar]
- Henry J. Q., Okusu A. and Martindale M. Q. (2004). The cell lineage of the polyplacophoran, Chaetopleura apiculata: variation in the spiralian program and implications for molluscan evolution. Dev. Biol. 272, 145-160. 10.1016/j.ydbio.2004.04.027 [DOI] [PubMed] [Google Scholar]
- Hertwig O. and Hertwig R. (1878). Das Nervensystem und die Sinnesorgane der Medusen: Vogel. [Google Scholar]
- Ho F. K. (1961). Optic organs of Tribolium confusum and T. castaneum and their usefulness in age determination (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 54, 921-925. 10.1093/aesa/54.6.921 [DOI] [Google Scholar]
- Homann H. (1971). Die Augen der Araneae: Anatomie, Ontogenese und Bedeutung für die Systematik (Chelicerata, Arachnida). Z. Morph. Tiere 69, 201-272. 10.1007/BF00277623 [DOI] [Google Scholar]
- Horie T., Sakurai D., Ohtsuki H., Terakita A., Shichida Y., Usukura J., Kusakabe T. and Tsuda M. (2008). Pigmented and nonpigmented ocelli in the brain vesicle of the ascidian larva. J. Comp. Neurol. 509, 88-102. 10.1002/cne.21733 [DOI] [PubMed] [Google Scholar]
- Huan P., Wang Q., Tan S. and Liu B. (2020). Dorsoventral decoupling of Hox gene expression underpins the diversification of molluscs. Proc. Natl Acad. Sci. USA 117, 503-512. 10.1073/pnas.1907328117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L. H. (1940). The Invertebrates: Protozoa Through Ctenophora. New York, NY: McGraw-Hill. [Google Scholar]
- Imarazene B., Andouche A., Bassaglia Y., Lopez P.-J. and Bonnaud-Ponticelli L. (2017). Eye development in Sepia officinalis embryo: what the uncommon gene expression profiles tell us about eye evolution. Front. Physiol. 8, 613 10.3389/fphys.2017.00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. R. and Pollard S. D. (1996). Predatory behavior of jumping spiders. Annu. Rev. Entomol. 41, 287-308. 10.1146/annurev.en.41.010196.001443 [DOI] [PubMed] [Google Scholar]
- Jacobs D. K., Wray C. G., Wedeen C. J., Kostriken R., DeSalle R., Staton J. L., Gates R. D. and Lindberg D. R. (2000). Molluscan engrailed expression, serial organization, and shell evolution. Evol. Dev. 2, 340-347. 10.1046/j.1525-142x.2000.00077.x [DOI] [PubMed] [Google Scholar]
- Janssens H. and Gehring W. J. (1999). Isolation and characterization of drosocrystallin, a lens crystallin gene of Drosophila melanogaster. Dev. Biol. 207, 204-214. 10.1006/dbio.1998.9170 [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. (2009). Regressive evolution in astyanax cavefish. Annu. Rev. Genet. 43, 25-47. 10.1146/annurev-genet-102108-134216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Tresser J. W., Horie T., Tsuda M. and Smith W. C. (2005). Pigmentation in the sensory organs of the ascidian larva is essential for normal behavior. J. Exp. Biol. 208, 433-438. 10.1242/jeb.01420 [DOI] [PubMed] [Google Scholar]
- Jonasova K. and Kozmik Z. (2008). Eye evolution: lens and cornea as an upgrade of animal visual system. Semin. Cell Dev. Biol. 19, 71-81. 10.1016/j.semcdb.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Jones M. R., Grillner S. and Robertson B. (2009). Selective projection patterns from subtypes of retinal ganglion cells to tectum and pretectum: distribution and relation to behavior. J. Comp. Neurol. 517, 257-275. 10.1002/cne.22154 [DOI] [PubMed] [Google Scholar]
- Kayal E., Bentlage B., Sabrina Pankey M., Ohdera A. H., Medina M., Plachetzki D. C., Collins A. G. and Ryan J. F. (2018). Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 18, 139 10.1186/s12862-018-1142-030208839 [DOI] [Google Scholar]
- Kimori N., Usukura J. and Matsumoto H. (1992). Drosocrytallin, a major 52 kDa glycoprotein of the Drosophila melanogaster corneal lens. Purification, biochemical characterization, and subcellular localization. J. Cell Sci. 102, 191-201. [DOI] [PubMed] [Google Scholar]
- Kingston A. C. N., Kuzirian A. M., Hanlon R. T. and Cronin T. W. (2015a). Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. J. Exp. Biol. 218, 1596-1602. 10.1242/jeb.117945 [DOI] [PubMed] [Google Scholar]
- Kingston A. C. N., Wardill T. J., Hanlon R. T. and Cronin T. W. (2015b). An unexpected diversity of photoreceptor classes in the longfin squid, Doryteuthis pealeii. PLoS ONE 10, e0135381 10.1371/journal.pone.0135381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A. C. N., Chappell D. R. and Speiser D. I. (2018). Evidence for spatial vision in Chiton tuberculatus, a chiton with eyespots. J. Exp. Biol. 221, jeb183632 10.1242/jeb.183632 [DOI] [PubMed] [Google Scholar]
- Klann M. and Seaver E. C. (2019). Functional role of pax6 during eye and nervous system development in the annelid Capitella teleta. Dev. Biol. 456, 86-103. 10.1016/j.ydbio.2019.08.011 [DOI] [PubMed] [Google Scholar]
- Kleerekoper H. T. (1972). The sense organs. In Biology of Lampreys, Vol. 2 (ed. Potter M. H. A. I. C.), pp. 373-404. London: Academic Press. [Google Scholar]
- Klingler M. (2004). Tribolium. Curr. Biol. 14, R639-R640. 10.1016/j.cub.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Kocot K. M., Poustka A. J., Stöger I., Halanych K. M. and Schrödl M. (2020). New data from Monoplacophora and a carefully-curated dataset resolve molluscan relationships. Sci. Rep. 10, 101 10.1038/s41598-019-56728-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K. M., Sun P., Meyer E. and Gross J. M. (2016). Eye development and photoreceptor differentiation in the cephalopod Doryteuthis pealeii. Development 143, 3168-3181. 10.1242/dev.134254 [DOI] [PubMed] [Google Scholar]
- Kourakis M. J., Borba C., Zhang A., Newman-Smith E., Salas P., Manjunath B. and Smith W. C. (2019). Parallel visual circuitry in a basal chordate. eLife 8, e44753 10.7554/eLife.44753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z. (2005). Pax genes in eye development and evolution. Curr. Opin. Genet. Dev. 15, 430-438. 10.1016/j.gde.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Holland N. D., Kalousova A., Paces J., Schubert M. and Holland L. Z. (1999). Characterization of an amphioxus paired box gene, AmphiPax2/5/8: developmental expression patterns in optic support cells, nephridium, thyroid-like structures and pharyngeal gill slits, but not in the midbrain-hindbrain boundary region. Development 126, 1295-1304. [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Ruzickova J., Jonasova K., Matsumoto Y., Vopalensky P., Kozmikova I., Strnad H., Kawamura S., Piatigorsky J., Paces V. et al. (2008). Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc. Natl. Acad. Sci. USA 105, 8989-8993. 10.1073/pnas.0800388105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan J. and Rohner N. (2017). Cavefish and the Basis for Eye Loss. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20150487 10.1098/rstb.2015.0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J. P. (2010). Retinal determination: the beginning of eye development. Curr. Top. Dev. Biol. 93, 1-28. 10.1016/B978-0-12-385044-7.00001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J. P. (2011). My what big eyes you have: how the Drosophila retina grows. Dev. Neurobiol. 71, 1133-1152. 10.1002/dneu.20921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J. P. (2012). Building an ommatidium one cell at a time. Dev. Dyn. 241, 136-149. 10.1002/dvdy.23707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpfer M. (1916). Entwicklungsgeschichtliche und neuro-histologische Beiträge zur Kenntnis der Sehorgane am Mantelrande der Pecten-Arten: mit anschliessen vergleichend-anatomischen Betrachtungen. Fischer. [Google Scholar]
- Kusunoki T. and Amemiya F. (1983). Retinal projections in the hagfish, Eptatretus burgeri. Brain Res. 262, 295-298. 10.1016/0006-8993(83)91021-1 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Otsuna H., Kidokoro H., Carney K. R., Saijoh Y. and Chien C.-B. (2012). A complex choreography of cell movements shapes the vertebrate eye. Development 139, 359-372. 10.1242/dev.071407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalli T. C. (1996). Frontal eye circuitry, rostral sensory pathways and brain organization in amphioxus larvae: evidence from 3D reconstructions. Philos. Trans. Biol. Sci. 351, 243-263. 10.1098/rstb.1996.0022 [DOI] [Google Scholar]
- Lacalli T. C., Holland N. D. and West J. E. (1994). Landmarks in the anterior central nervous system of amphioxus larvae. Philos. Trans. Biol. Sci. 344, 165-185. 10.1098/rstb.1994.0059 [DOI] [Google Scholar]
- Lamb T. D., Collin S. P. and Pugh E. N. (2007). Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 8, 960-976. 10.1038/nrn2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M. F. (1965). Image formation by a concave reflector in the eye of the scallop, Pecten maximus. J. Physiol. 179, 138-153. 10.1113/jphysiol.1965.sp007653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M. F. (1966a). Activity in the optic nerve of Pecten maximus in response to changes in light intensity, and to pattern and movement in the optical environment. J. Exp. Biol. 45, 83-99. [DOI] [PubMed] [Google Scholar]
- Land M. F. (1966b). A multilayer interference reflector in the eye of the scallop, Pecten maximus. J. Exp. Biol. 45, 433-447. [Google Scholar]
- Land M. F. (1985). The morphology and optics of spider eyes. In Neurobiology of Arachnids (ed. Barth F. G.), pp. 53-78. Berlin, Heidelberg: Springer. [Google Scholar]
- Land M. F. (2012). The evolution of lenses. Ophthalmic Physiol. Opt. 32, 449-460. 10.1111/j.1475-1313.2012.00941.x [DOI] [PubMed] [Google Scholar]
- Land M. F. (2018). Eyes to See: The Astonishing Variety of Vision in Nature. USA: Oxford University Press. [Google Scholar]
- Land M. F. and Nilsson D. E. (2012). Animal Eyes. Oxford University Press. [Google Scholar]
- Lapan S. W. and Reddien P. W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genet. 7, e1002226 10.1371/journal.pgen.1002226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan S. W. and Reddien P. W. (2012). Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell reports 2, 294-307. 10.1016/j.celrep.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska G. and Hündgen M. (1982). Morphologie und Ultrastuktur der Lichtsinnesorgane von Tripedalia cystophora Conant (Cnidaria, Cubozoa). Zool. Jb. Anat. 108, 107-123. [Google Scholar]
- Laska-Mehnert G. (1985). Cytologische Veränderungen während der Metamorphose des CubopolypenTripedalia cystophora (Cubozoa, Carybdeidae) in die Meduse. Helgoländer Meeresuntersuchungen 39, 129-164. 10.1007/BF01997447 [DOI] [Google Scholar]
- Li Y., Brown S. J., Hausdorf B., Tautz D., Denell R. E. and Finkelstein R. (1996). Two orthodenticle-related genes in the short-germ beetle Tribolium castaneum. Dev. Genes Evol. 206, 35-45. 10.1007/s004270050028 [DOI] [PubMed] [Google Scholar]
- Li L., Connors M. J., Kolle M., England G. T., Speiser D. I., Xiao X., Aizenberg J. and Ortiz C. (2015). Multifunctionality of chiton biomineralized armor with an integrated visual system. Science 350, 952-956. 10.1126/science.aad1246 [DOI] [PubMed] [Google Scholar]
- Liegertová M., Pergner J., Kozmiková I., Fabian P., Pombinho A. R., Strnad H., Pačes J., Vlček Č., Bartůněk P. and Kozmik Z. (2015). Cubozoan genome illuminates functional diversification of opsins and photoreceptor evolution. Sci. Rep. 5, 11885 10.1038/srep11885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. and Friedrich M. (2004). The Tribolium homologue of glass and the evolution of insect larval eyes. Dev. Biol. 269, 36-54. 10.1016/j.ydbio.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Liu Z., Yang X., Dong Y. and Friedrich M. (2006). Tracking down the “head blob”: comparative analysis of wingless expression in the developing insect procephalon reveals progressive reduction of embryonic visual system patterning in higher insects. Arthropod. Struct. Dev. 35, 341-356. 10.1016/j.asd.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Locy W. A. (1886). Observations on the Development of Agelena naevia, Vol. 12, No. 3 Museum. [Google Scholar]
- Luan Q., Chen Q. and Friedrich M. (2014). The Pax6 genes eyeless and twin of eyeless are required for global patterning of the ocular segment in the Tribolium embryo. Dev. Biol. 394, 367-381. 10.1016/j.ydbio.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Ma X., Li H., Chen Y., Yang J., Chen H., Arnheiter H. and Hou L. (2019). The transcription factor MITF in RPE function and dysfunction. Prog. Retin. Eye Res. 73, 100766 10.1016/j.preteyeres.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Maas O. (1903). Die scyphomedusen der Siboga-expedition. EJ Brill. [Google Scholar]
- Mahato S., Morita S., Tucker A. E., Liang X., Jackowska M., Friedrich M., Shiga Y. and Zelhof A. C. (2014). Common transcriptional mechanisms for visual photoreceptor cell differentiation among Pancrustaceans. PLoS Genet. 10, e1004484 10.1371/journal.pgen.1004484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannini L., Rossi L., Deri P., Gremigni V., Salvetti A., Saló E. and Batistoni R. (2004). Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Dev. Biol. 269, 346-359. 10.1016/j.ydbio.2004.01.042 [DOI] [PubMed] [Google Scholar]
- Marlétaz F., Peijnenburg K. T. C. A., Goto T., Satoh N. and Rokhsar D. S. (2019). A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr. Biol. 29, 312-318. 10.1016/j.cub.2018.11.042 [DOI] [PubMed] [Google Scholar]
- Marshall W. S. (1927). The development of the compound eye of the Confused Flour Beetle, Tribolium confusum Jacq. Trans. Wise. Acad. Sci. Arts Lett 23, 611-630. [Google Scholar]
- Martin V. J. (2002). Photoreceptors of cnidarians. Can. J. Zool. 80, 1703-1722. 10.1139/z02-136 [DOI] [Google Scholar]
- Martin V. J. (2004). Photoreceptors of cubozoan jellyfish. Hydrobiologia 530-531, 135-144. 10.1007/s10750-004-2674-4 [DOI] [Google Scholar]
- Martín-Durán J. M., Monjo F. and Romero R. (2012). Morphological and molecular development of the eyes during embryogenesis of the freshwater planarian Schmidtea polychroa. Dev. Genes Evol. 222, 45-54. 10.1007/s00427-012-0389-5 [DOI] [PubMed] [Google Scholar]
- Mazet F., Hutt J. A., Millard J. and Shimeld S. M. (2003). Pax gene expression in the developing central nervous system of Ciona intestinalis. Gene Expr. Patterns 3, 743-745. 10.1016/S1567-133X(03)00137-6 [DOI] [PubMed] [Google Scholar]
- McDougall C. and Degnan B. M. (2018). The evolution of mollusc shells. Wiley Interdiscipl. Rev. Dev. Biol. 7, e313 10.1002/wdev.313 [DOI] [PubMed] [Google Scholar]
- McReynolds J. S. and Gorman A. L. F. (1970). Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians. J. Gen. Physiol. 56, 376-391. 10.1085/jgp.56.3.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen I. A. (1990). Development of the squid's visual system. In Squid as Experimental Animals (ed. Gilbert D. L., Adelman W. J. and Arnold J. M.), pp. 399-419. Boston, MA: Springer. [Google Scholar]
- Meléndez-Ferro M., Villar-Cheda B., Abalo X. M., Pérez-Costas E., Rodríguez-Muñoz R., Degrip W. J., Yáñez J., Rodicio M. C. and Anadón R. (2002). Early development of the retina and pineal complex in the sea lamprey: comparative immunocytochemical study. J. Comp. Neurol. 442, 250-265. 10.1002/cne.10090 [DOI] [PubMed] [Google Scholar]
- Mishra M., Oke A., Lebel C., McDonald E. C., Plummer Z., Cook T. A. and Zelhof A. C. (2010). Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development 137, 2895-2904. 10.1242/dev.051722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T., Coates M. I., Farrar R., Larson P., Manning P. L., Wogelius R. A., Edwards N. P., Anné J., Bergmann U., Palmer A. R. et al. (2019). Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proc. Natl. Acad. Sci. USA 116, 2146-2151. 10.1073/pnas.1814794116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehouse N. I., Buschbeck E. K., Zurek D. B., Steck M. and Porter M. L. (2017). Molecular evolution of spider vision: new opportunities, familiar players. Biol. Bull. 233, 21-38. 10.1086/693977 [DOI] [PubMed] [Google Scholar]
- Morton B. (2008). The evolution of eyes in the Bivalvia: new insights. Am. Malacol. Bull. 26, 35-45. 10.4003/006.026.0205 [DOI] [Google Scholar]
- Moseley H. N. (1885). Memoirs: on the presence of eyes in the shells of certain Chitonidæ, and on the structure of these organs. J. Cell Sci. 2, 37-60. [Google Scholar]
- Murakami Y., Ogasawara M., Sugahara F., Hirano S., Satoh N. and Kuratani S. (2001). Identification and expression of the lamprey Pax6 gene: evolutionary origin of the segmented brain of vertebrates. Development 128, 3521-3531. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Hartenstein V. and Jacobs D. K. (2009). Development of the rhopalial nervous system in Aurelia sp.1 (Cnidaria, Scyphozoa). Dev. Genes Evol. 219, 301-317. 10.1007/s00427-009-0291-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Yuan D., Hartenstein V. and Jacobs D. K. (2010). Evolutionary origin of rhopalia: insights from cellular–level analyses of Otx and POU expression patterns in the developing rhopalial nervous system. Evol. Dev. 12, 404-415. 10.1111/j.1525-142X.2010.00427.x [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Camara A. C., Yuan D. C., Gold D. A. and Jacobs D. K. (2015). Gene expression data from the moon jelly, Aurelia, provide insights into the evolution of the combinatorial code controlling animal sense organ development. PLoS ONE 10, e0132544 10.1371/journal.pone.0132544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navet S., Andouche A., Baratte S. and Bonnaud L. (2009). Shh and Pax6 have unconventional expression patterns in embryonic morphogenesis in Sepia officinalis (Cephalopoda). Gene Expr. Patterns 9, 461-467. 10.1016/j.gep.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Navet S., Buresi A., Baratte S., Andouche A., Bonnaud-Ponticelli L. and Bassaglia Y. (2017). The Pax gene family: Highlights from cephalopods. PLoS ONE 12, e0172719 10.1371/journal.pone.0172719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D. E. (1994). Eyes as optical alarm systems in fan worms and ark clams. Philos. Trans. R. Soc. Lond. B Biol. Sci. 346, 195-212. 10.1098/rstb.1994.0141 [DOI] [Google Scholar]
- Nilsson D.-E. (2004). Eye evolution: a question of genetic promiscuity. Curr. Opin. Neurobiol. 14, 407-414. 10.1016/j.conb.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Nilsson D.-E. (2009). The evolution of eyes and visually guided behaviour. Philos. Trans. R. Soc. B Biol. Sci. 364, 2833-2847. 10.1098/rstb.2009.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D.-E. (2013). Eye evolution and its functional basis. Vis. Neurosci. 30, 5-20. 10.1017/S0952523813000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D.-E., Gislén L., Coates M. M., Skogh C. and Garm A. (2005). Advanced optics in a jellyfish eye. Nature 435, 201-205. 10.1038/nature03484 [DOI] [PubMed] [Google Scholar]
- Oakley T. H. and Speiser D. I. (2015). How complexity originates: the evolution of animal eyes. Annu. Rev. Ecol. Evol. Syst. 46, 237-260. 10.1146/annurev-ecolsys-110512-135907 [DOI] [Google Scholar]
- O'Connor M., Garm A. and Nilsson D.-E. (2009). Structure and optics of the eyes of the box jellyfish Chiropsella bronzie. J. Comp. Physiol. A 195, 557-569. 10.1007/s00359-009-0431-x [DOI] [PubMed] [Google Scholar]
- Ogura A., Yoshida M.-A., Moritaki T., Okuda Y., Sese J., Shimizu K. K., Sousounis K. and Tsonis P. A. (2013). Loss of the six3/6 controlling pathways might have resulted in pinhole-eye evolution in Nautilus. Sci. Rep. 3, 1432 10.1038/srep01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonuma K., Tanaka M., Nishitsuji K., Kato Y., Shimai K. and Kusakabe T. G. (2016). Revised lineage of larval photoreceptor cells in Ciona reveals archetypal collaboration between neural tube and neural crest in sensory organ formation. Dev. Biol. 420, 178-185. 10.1016/j.ydbio.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Palmer B. A., Taylor G. J., Brumfeld V., Gur D., Shemesh M., Elad N., Osherov A., Oron D., Weiner S. and Addadi L. (2017). The image-forming mirror in the eye of the scallop. Science 358, 1172-1175. 10.1126/science.aam9506 [DOI] [PubMed] [Google Scholar]
- Partha R., Chauhan B. K., Ferreira Z., Robinson J. D., Lathrop K., Nischal K. K., Chikina M. and Clark N. L. (2017). Subterranean mammals show convergent regression in ocular genes and enhancers, along with adaptation to tunneling. eLife 6, e25884 10.7554/eLife.25884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten W. (1887). Eyes of molluscs and arthropods. J. Morphol. 1, 67-92. 10.1002/jmor.1050010105 [DOI] [Google Scholar]
- Paulus H. F. (1979). Eye structure and the monophyly of the Arthropoda. In Arthropod Phylogeny (ed. Gupta A. P.), pp. 299-383. New York, NY: Van Nostrand Reinhold. [Google Scholar]
- Pergner J. and Kozmik Z. (2017). Amphioxus photoreceptors - insights into the evolution of vertebrate opsins, vision and circadian rhythmicity. Int. J. Dev. Biol. 61, 665-681. 10.1387/ijdb.170230zk [DOI] [PubMed] [Google Scholar]
- Perron M. and Harris W. A. (2000). Determination of vertebrate retinal progenitor cell fate by the Notch pathway and basic helix-loop-helix transcription factors. Cell. Mol. Life Sci. 57, 215-223. 10.1007/PL00000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyer S. M., Pankey M. S., Oakley T. H. and McFall-Ngai M. J. (2014). Eye-specification genes in the bacterial light organ of the bobtail squid Euprymna scolopes, and their expression in response to symbiont cues. Mech. Dev. 131, 111-126. 10.1016/j.mod.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., Horwitz J., Kuwabara T. and Cutress C. E. (1989). The cellular eye lens and crystallins of cubomedusan jellyfish. J. Comp. Physiol. A 164, 577-587. 10.1007/BF00614500 [DOI] [PubMed] [Google Scholar]
- Picciani N., Kerlin J. R., Sierra N., Swafford A. J. M., Ramirez M. D., Roberts N. G., Cannon J. T., Daly M. and Oakley T. H. (2018). Prolific origination of eyes in cnidaria with co-option of non-visual opsins. Curr. Biol. 28, 2413-2419.e4. 10.1016/j.cub.2018.05.055 [DOI] [PubMed] [Google Scholar]
- Pineda D., Gonzalez J., Callaerts P., Ikeo K., Gehring W. J. and Salo E. (2000). Searching for the prototypic eye genetic network: Sine oculis is essential for eye regeneration in planarians. Proc. Natl. Acad. Sci. USA 97, 4525-4529. 10.1073/pnas.97.9.4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda D., Rossi L., Batistoni R., Salvetti A., Marsal M., Gremigni V., Falleni A., Gonzalez-Linares J., Deri P. and Saló E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423-1434. [DOI] [PubMed] [Google Scholar]
- Porter M. L., Blasic J. R., Bok M. J., Cameron E. G., Pringle T., Cronin T. W. and Robinson P. R. (2012). Shedding new light on opsin evolution. Proc. Biol. Sci. 279, 3-14. 10.1098/rspb.2011.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnien N., Koniszewski N. and Bucher G. (2011). Insect Tc-six4 marks a unit with similarity to vertebrate placodes. Dev. Biol. 350, 208-216. 10.1016/j.ydbio.2010.10.024 [DOI] [PubMed] [Google Scholar]
- Purschke G., Arendt D., Hausen H. and Müller M. C. M. (2006). Photoreceptor cells and eyes in Annelida. Arthropod. Struct. Dev. 35, 211-230. 10.1016/j.asd.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Quan X.-J., Ramaekers A. and Hassan B. A. (2012). Transcriptional control of cell fate specification: lessons from the fly retina. Curr. Top. Dev. Biol. 98, 259-276. 10.1016/B978-0-12-386499-4.00010-0 [DOI] [PubMed] [Google Scholar]
- Quiring R., Walldorf U., Kloter U. and Gehring W. J. (1994). Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265, 785-789. 10.1126/science.7914031 [DOI] [PubMed] [Google Scholar]
- Rhode B. (1992). Development and differentiation of the eye in Platynereis dumerilii (Annelida, Polychaeta). J. Morphol. 212, 71-85. 10.1002/jmor.1052120108 [DOI] [PubMed] [Google Scholar]
- Roignant J.-Y. and Treisman J. E. (2009). Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 53, 795 10.1387/ijdb.072483jr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson K. and Cain H. (1989). Neural differentiation in the retina of the larval sea lamprey (Petromyzon marinus). Vis. Neurosci. 3, 241-248. 10.1017/S0952523800009998 [DOI] [PubMed] [Google Scholar]
- Ryan K., Lu Z. and Meinertzhagen I. A. (2016). The CNS connectome of a tadpole larva of Ciona intestinalis (L.) highlights sidedness in the brain of a chordate sibling. eLife 5, e16962 10.7554/eLife.16962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H. R. (1990). Structure and Function of the Squid Eye. In Squid as Experimental Animals (ed. Gilbert D. L., Adelman W. J. Jr and Arnold J. M.), pp. 371-397. Springer US. [Google Scholar]
- Salas P., Vinaithirthan V., Newman-Smith E., Kourakis M. J. and Smith W. C. (2018). Photoreceptor specialization and the visuomotor repertoire of the primitive chordate Ciona. J. Exp. Biol. 221, jeb177972 10.1242/jeb.177972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvini-Plawen L. and Mayr E. (1977). On the evolution of photoreceptors and eyes. In Evolutionary Biology, Vol. 10 (ed. Hecht M.K.), pp. 207-263. Plenum Press. [Google Scholar]
- Samadi L., Schmid A. and Eriksson B. J. (2015). Differential expression of retinal determination genes in the principal and secondary eyes of Cupiennius salei Keyserling (1877). EvoDevo 6, 16 10.1186/s13227-015-0010-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. and Zipursky S. L. (2010). Design principles of insect and vertebrate visual systems. Neuron 66, 15-36. 10.1016/j.neuron.2010.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht M. I., Schomburg C. and Bucher G. (2020). six3 acts upstream of foxQ2 in labrum and neural development in the spider Parasteatoda tepidariorum. Dev. Genes Evol. 230, 95-104. 10.1007/s00427-020-00654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinko J. B., Kreuzer N., Offen N., Posnien N., Wimmer E. A. and Bucher G. (2008). Divergent functions of orthodenticle, empty spiracles and buttonhead in early head patterning of the beetle Tribolium castaneum (Coleoptera). Dev. Biol. 317, 600-613. 10.1016/j.ydbio.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Schomburg C., Turetzek N., Schacht M. I., Schneider J., Kirfel P., Prpic N.-M. and Posnien N. (2015). Molecular characterization and embryonic origin of the eyes in the common house spider Parasteatoda tepidariorum. EvoDevo 6, 15 10.1186/s13227-015-0011-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröer W.-D. (2017). Fine structure of the anterior median eyes of the funnel-web spider Agelena labyrinthica (Araneae: Agelenidae). Arthropod. Struct. Dev. 46, 196-214. 10.1016/j.asd.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Schwab I. R., Yuen C. K., Buyukmihci N. C., Blankenship T. N. and Fitzgerald P. G. (2002). Evolution of the tapetum. Trans. Am. Ophthalmol. Soc. 100, 187-199. [PMC free article] [PubMed] [Google Scholar]
- Schwager E. E., Sharma P. P., Clarke T., Leite D. J., Wierschin T., Pechmann M., Akiyama-Oda Y., Esposito L., Bechsgaard J., Bilde T. et al. (2017). The house spider genome reveals an ancient whole-genome duplication during arachnid evolution. BMC Biol. 15, 1-27. 10.1186/s12915-017-0399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seritrakul P. and Gross J. M. (2019). Genetic and epigenetic control of retinal development in zebrafish. Curr. Opin. Neurobiol. 59, 120-127. 10.1016/j.conb.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin N., Tabin C. and Carroll S. (2009). Deep homology and the origins of evolutionary novelty. Nature 457, 818-823. 10.1038/nature07891 [DOI] [PubMed] [Google Scholar]
- Smith J. M., Burian R., Kauffman S., Alberch P., Campbell J., Goodwin B., Lande R., Raup D. and Wolpert L. (1985). Developmental constraints and evolution: a perspective from the Mountain Lake conference on development and evolution. Q Rev. Biol. 60, 265-287. 10.1086/414425 [DOI] [Google Scholar]
- Speiser D. I., Eernisse D. J. and Johnsen S. (2011). A chiton uses aragonite lenses to form images. Curr. Biol. 21, 665-670. 10.1016/j.cub.2011.03.033 [DOI] [PubMed] [Google Scholar]
- Speiser D. I., Gagnon Y. L., Chhetri R. K., Oldenburg A. L. and Johnsen S. (2016). Examining the effects of chromatic aberration, object distance, and eye shape on image-formation in the mirror-based eyes of the bay scallop Argopecten irradians. Integr. Comp. Biol. 56, 796-808. 10.1093/icb/icw099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A. L., Charlton-Perkins M., Buschbeck E. K. and Cook T. A. (2017). The cuticular nature of corneal lenses in Drosophila melanogaster. Dev. Genes Evol. 227, 271-278. 10.1007/s00427-017-0582-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl K., Salvini-Plawen L. V. and Holstein T. W. (2002). Staging and induction of medusa metamorphosis in Carybdea marsupialis (Cnidaria, Cubozoa). Vie et milieu (1980) 52, 131-140. [Google Scholar]
- Steinmetz P. R. H., Urbach R., Posnien N., Eriksson J., Kostyuchenko R. P., Brena C., Guy K., Akam M., Bucher G. and Arendt D. (2010). Six3 demarcates the anterior-most developing brain region in bilaterian animals. EvoDevo 1, 14 10.1186/2041-9139-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierwald M., Yanze N., Bamert R. P., Kammermeier L. and Schmid V. (2004). The Sine oculis/Six class family of homeobox genes in jellyfish with and without eyes: development and eye regeneration. Dev. Biol. 274, 70-81. 10.1016/j.ydbio.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Stockard C. R. (1906). The embryonic history of the lens in bdellostoma stouti in relation to recent experiments. Am. J. Anat. 6, 511-515. 10.1002/aja.1000060114 [DOI] [Google Scholar]
- Strausfeld N. J. and Barth F. G. (1993). Two visual systems in one brain: neuropils serving the secondary eyes of the spider Cupiennius salei. J. Comp. Neurol. 328, 43-62. 10.1002/cne.903280104 [DOI] [PubMed] [Google Scholar]
- Strauss O. (2005). The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845-881. 10.1152/physrev.00021.2004 [DOI] [PubMed] [Google Scholar]
- Strzyz P. J., Matejcic M. and Norden C. (2016). Heterogeneity, cell biology and tissue mechanics of pseudostratified epithelia: coordination of cell divisions and growth in tightly packed tissues. Int. Rev. Cell Mol. Biol. 325, 89-118. 10.1016/bs.ircmb.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Sturrock M. G. and Baxter J. M. (1993). The ultrastructure of the aesthetes of Leptochiton asellus (Polyplacophora: Lepidopleurina). J. Zool. 230, 49-61. 10.1111/j.1469-7998.1993.tb02671.x [DOI] [Google Scholar]
- Suga H., Tschopp P., Graziussi D. F., Stierwald M., Schmid V. and Gehring W. J. (2010). Flexibly deployed Pax genes in eye development at the early evolution of animals demonstrated by studies on a hydrozoan jellyfish. Proc. Natl Acad. Sci. USA 107, 14263-14268. 10.1073/pnas.1008389107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner-Rooney L., Sigwart J. D., McAfee J., Smith L. and Williams S. T. (2016). Repeated eye reduction events reveal multiple pathways to degeneration in a family of marine snails. Evolution 70, 2268-2295. 10.1111/evo.13022 [DOI] [PubMed] [Google Scholar]
- Suzuki D. G. and Grillner S. (2018). The stepwise development of the lamprey visual system and its evolutionary implications. Biol. Rev. Camb. Philos. Soc. 93, 1461-1477. 10.1111/brv.12403 [DOI] [PubMed] [Google Scholar]
- Sweeney A. M., Des Marais D. L., Andrew Ban Y.-E. and Johnsen S. (2007). Evolution of graded refractive index in squid lenses. J. R. Soc. Interface 4, 685-698. 10.1098/rsif.2006.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.-S., Lin C.-C. and Chang G.-G. (1994). Isolation and characterization of octopus hepatopancreatic glutathione S-transferase. Comparison of digestive gland enzyme with lens S-crystallin. J. Protein Chem. 13, 609-618. 10.1007/BF01890459 [DOI] [PubMed] [Google Scholar]
- Taniguchi K. and Nishida H. (2004). Tracing cell fate in brain formation during embryogenesis of the ascidian Halocynthia roretzi. Dev. Growth Differ. 46, 163-180. 10.1111/j.1440-169X.2004.00736.x [DOI] [PubMed] [Google Scholar]
- Tierney S. M., Langille B., Humphreys W. F., Austin A. D. and Cooper S. JB. (2018). Massive parallel regression: a précis of genetic mechanisms for vision loss in diving beetles. Integr. Comp. Biol. 58, 465-479. 10.1093/icb/icy035 [DOI] [PubMed] [Google Scholar]
- Tomarev S. I., Chung S. and Piatigorsky J. (1995). Glutathione S-transferase and S-crystallins of cephalopods: evolution from active enzyme to lens-refractive proteins. J. Mol. Evol. 41, 1048-1056. 10.1007/BF00173186 [DOI] [PubMed] [Google Scholar]
- Toomey M. B. and Corbo J. C. (2017). Evolution, development and function of vertebrate cone oil droplets. Front. Neural Circuits 11, 97 10.3389/fncir.2017.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J. E. (2013). Retinal differentiation in Drosophila. Wiley Interdiscipl. Rev. Dev. Biol. 2, 545-557. 10.1002/wdev.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribolium Genome Sequencing Consortium (2008). The genome of the model beetle and pest Tribolium castaneum. Nature 452, 949 10.1038/nature06784 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Sakurai D. and Goda M. (2003). Direct evidence for the role of pigment cells in the brain of ascidian larvae by laser ablation. J. Exp. Biol. 206, 1409-1417. 10.1242/jeb.00235 [DOI] [PubMed] [Google Scholar]
- Turetzek N., Pechmann M., Schomburg C., Schneider J. and Prpic N.-M. (2016). Neofunctionalization of a duplicate dachshund gene underlies the evolution of a novel leg segment in arachnids. Mol. Biol. Evol. 33, 109-121. 10.1093/molbev/msv200 [DOI] [PubMed] [Google Scholar]
- Uehara A., Uehara K. and Ogawa K. (1994). Fine structure of the anteromedial eye of the liphistiid spider, Heptathela kimurai. Anat. Rec. 240, 141-147. 10.1002/ar.1092400115 [DOI] [PubMed] [Google Scholar]
- Valley J. R. (2011). Eye Development in the Cubozoan Jellyfish Carybdea Marsupialis: A Thesis. Master's thesis, Appalachian State University, Boone, NC, USA. [Google Scholar]
- Verger-Bocquet M. (1992). Polychaeta: sensory structures. Microsc. Anat. Invertebr. 7, 181-196. [Google Scholar]
- Villar-Cerviño V., Abalo X. M., Villar-Cheda B., Meléndez-Ferro M., Pérez-Costas E., Holstein G. R., Martinelli G. P., Rodicio M. C. and Anadón R. (2006). Presence of glutamate, glycine, and γ-aminobutyric acid in the retina of the larval sea lamprey: comparative immunohistochemical study of classical neurotransmitters in larval and postmetamorphic retinas. J. Comp. Neurol. 499, 810-827. 10.1002/cne.21136 [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Abalo X. M., Villar-Cerviño V., Barreiro-Iglesias A., Anadón R. and Rodicio M. C. (2008). Late proliferation and photoreceptor differentiation in the transforming lamprey retina. Brain Res. 1201, 60-67. 10.1016/j.brainres.2008.01.077 [DOI] [PubMed] [Google Scholar]
- Vöcking O., Kourtesis I. and Hausen H. (2015). Posterior eyespots in larval chitons have a molecular identity similar to anterior cerebral eyes in other bilaterians. EvoDevo 6, 40 10.1186/s13227-015-0036-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vopalensky P., Pergner J., Liegertova M., Benito-Gutierrez E., Arendt D. and Kozmik Z. (2012). Molecular analysis of the amphioxus frontal eye unravels the evolutionary origin of the retina and pigment cells of the vertebrate eye. Proc. Natl. Acad. Sci. USA 109, 15383-15388. 10.1073/pnas.1207580109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang J., Jiao W., Li J., Xun X., Sun Y., Guo X., Huan P., Dong B., Zhang L. et al. (2017). Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat. Ecol. Evol. 1, 1-12. 10.1038/s41559-017-0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. (1981). Structure, histochemistry, ontogenetic development, and regeneration of the ocellus of Cladonema radiatum Dujardin (Cnidaria, Hydrozoa, Anthomedusae). J. Morphol. 167, 313-331. 10.1002/jmor.1051670306 [DOI] [PubMed] [Google Scholar]
- Werner B. (1975). Bau und Lebensgeschichte des Polypen von Tripedalia cystophora (Cubozoa, class. nov., Carybdeidae) und seine Bedeutung für die Evolution der Cnidaria. Helgoländer wissenschaftliche Meeresuntersuchungen 27, 461 10.1007/BF01611150 [DOI] [Google Scholar]
- Werner B. (1983). Die Metamorphose des Polypen vonTripedalia cystophora (Cubozoa, Carybdeidae) in die Meduse. Helgoländer Meeresuntersuchungen 36, 257-276. 10.1007/BF01983630 [DOI] [Google Scholar]
- Werner B., Cutress C. E. and Studebaker J. P. (1971). Life cycle of Tripedalia cystophora Conant (cubomedusae). Nature 232, 582-583. 10.1038/232582a0 [DOI] [PubMed] [Google Scholar]
- West J. A., Sivak J. G., Pasternak J. and Piatigorsky J. (1994). Immunolocalization of S–crystallins in the developing squid (Loligo opalescens) lens. Dev. Dyn. 199, 85-92. 10.1002/aja.1001990202 [DOI] [PubMed] [Google Scholar]
- West J. A., Sivak J. G. and Doughty M. J. (1995). Microscopical evaluation of the crystalline lens of the squid (Loligo opalescens) during embryonic development. Exp. Eye Res. 60, 19-35. 10.1016/S0014-4835(05)80080-6 [DOI] [PubMed] [Google Scholar]
- Williams L. W. (1909). Anatomy of Loligo pealii. Leiden-Holland, Library and Printing-Office late E.J. Brill. [Google Scholar]
- Williams A. L. and Bohnsack B. L. (2015). Neural crest derivatives in ocular development: discerning the eye of the storm. Birth Defects Res. C Embryo Today 105, 87-95. 10.1002/bdrc.21095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T. and Ready D. F. (1993). Pattern Formation in the Drosophila Retina. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Wollesen T., McDougall C., Degnan B. M. and Wanninger A. (2014). POU genes are expressed during the formation of individual ganglia of the cephalopod central nervous system. EvoDevo 5, 41 10.1186/2041-9139-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollesen T., Monje S. V. R., Todt C., Degnan B. M. and Wanninger A. (2015a). Ancestral role of Pax2/5/8 in molluscan brain and multimodal sensory system development. BMC Evol. Biol. 15, 231 10.1186/s12862-015-0505-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollesen T., Monje S. V. R., McDougall C., Degnan B. M. and Wanninger A. (2015b). The ParaHox gene Gsx patterns the apical organ and central nervous system but not the foregut in scaphopod and cephalopod mollusks. Evodevo 6, 41 10.1186/s13227-015-0037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollesen T., Rodríguez Monje S. V., Luiz de Oliveira A. and Wanninger A. (2018). Staggered Hox expression is more widespread among molluscs than previously appreciated. Proc. R. Soc. B 285, 20181513 10.1098/rspb.2018.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima I., Endo K., Sato S., Toyoda R., Wada H., Shibahara S., Numakunai T., Ikeo K., Gojobori T., Goding C. R. et al. (2003). Cloning and functional analysis of ascidian Mitf in vivo: insights into the origin of vertebrate pigment cells. Mech. Dev. 120, 1489-1504. 10.1016/j.mod.2003.08.009 [DOI] [PubMed] [Google Scholar]
- Yamasu T. and Yoshida M. (1976). Fine structure of complex ocelli of a cubomedusan, Tamoya bursaria Haeckel. Cell Tissue Res. 170, 325-339. 10.1007/BF00219415 [DOI] [PubMed] [Google Scholar]
- Yang X., ZarinKamar N., Bao R. and Friedrich M. (2009a). Probing the Drosophila retinal determination gene network in Tribolium (I): The early retinal genes dachshund, eyes absent and sine oculis. Dev. Biol. 333, 202-214. 10.1016/j.ydbio.2009.02.040 [DOI] [PubMed] [Google Scholar]
- Yang X., Weber M., Zarinkamar N., Posnien N., Friedrich F., Wigand B., Beutel R., Damen W. G. M., Bucher G., Klingler M. et al. (2009b). Probing the Drosophila retinal determination gene network in Tribolium (II): The Pax6 genes eyeless and twin of eyeless. Dev. Biol. 333, 215-227. 10.1016/j.ydbio.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Yoshida M.-A., Shigeno S., Tsuneki K. and Furuya H. (2010). Squid vascular endothelial growth factor receptor: a shared molecular signature in the convergent evolution of closed circulatory systems. Evol. Dev. 12, 25-33. 10.1111/j.1525-142X.2009.00388.x [DOI] [PubMed] [Google Scholar]
- Yoshida M.-A., Yura K. and Ogura A. (2014). Cephalopod eye evolution was modulated by the acquisition of Pax-6 splicing variants. Sci. Rep. 4, 4256 10.1038/srep04256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. A., Ogura A., Ikeo K., Shigeno S., Moritaki T., Winters G. C., Kohn A. B. and Moroz L. L. (2015). Molecular evidence for convergence and parallelism in evolution of complex brains of cephalopod molluscs: insights from visual systems. Integr. Comp. Biol. 55, 1070-1083. 10.1093/icb/icv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura M. (1955). Embryological Studies on the Liphistiid Spider, Heptathela kimurai Part II. J. Sci Ser. B 2, 1-86. [Google Scholar]
- Young H. C., Reid T. G., Randall L. A., Lachowski L. E., Foster D. J., Pengelly C. J., Latty T. and and Reid M. L. (2013). Influences of movement behavior on animal distributions at edges of homogeneous patches. Int. J. Zool. 2013, 602845 10.1155/2013/602845 [DOI] [Google Scholar]
- ZarinKamar N., Yang X., Bao R., Friedrich F., Beutel R. and Friedrich M. (2011). The Pax gene eyegone facilitates repression of eye development in Tribolium. EvoDevo 2, 8 10.1186/2041-9139-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinovieva R. D., Tomarev S. I. and Piatigorsky J. (1993). Aldehyde dehydrogenase-derived omega-crystallins of squid and octopus. Specialization for lens expression. J. Biol. Chem. 268, 11449-11455. [PubMed] [Google Scholar]