Abstract
Chronic viral infections cause deterioration of our immune system. However, since persistent infections rarely can be eliminated, the reinvigoration capacity of an exhausted immune system has remained largely elusive. Chronic hepatitis C virus (HCV) infection can since some years be effectively cured with novel direct acting antiviral agents. Thus, it is now possible to study reversal of immunity in patients that are cured from a long-lasting chronic infection. We here highlight recent developments in the analysis of various immune cell populations during and after clearance of HCV infection. Surprisingly, whereas reinvigoration of certain immune traits clearly can be seen, many features of immune exhaustion persist over time after viral elimination. Thus, a long-term chronic insult might result in irreversible damage to our immune system. This will be important to consider in therapeutic vaccination efforts against chronic infection and in the development of immunotherapy based strategies against cancer.
Keywords: hepatitis C, chronic infection, direct acting antivirals, soluble inflammatory mediators, natural killer cell, MAIT cell, T cell
Introduction
Chronic viral infections have a profound impact on the immune system. In humans, it is well established that patients with chronic hepatitis viruses and/or HIV infection have an impaired adaptive immunity with dysfunctional CD4+ and CD8+ T cells contributing to the inability to clear the infection (1, 2). In addition, the natural killer (NK) cell repertoire and function are altered in patients with prolonged viremia from different chronic infections (3–5). Similarly, the mucosal-associated invariant T (MAIT) cell compartment is severely diminished with impaired function in many chronic infections (6), whereas atypical memory B cell accumulates (7). However, the capacity for immune system reinvigoration after elimination of a chronic pathogen remains less well understood. Here, we first review insights related to immune system restoration in chronic infections where virus can be suppressed but not cleared. After that we focus on new results into the possible reversal of immunity after clearance of chronic hepatitis C virus (HCV) infection. This latter research has been possible to perform because of recent paradigm-shifting development in treatment possibilities for chronic hepatitis C, where the vast majority of patients now can clear their chronic infection.
Evidence for Reversal of Immunity After Suppression, but Not Clearance, of a Chronic Viral Infection in Humans
It has been debated over the years to what extent the profound immune alterations observed in persistent infections could be reversed upon control or elimination of the underlying infection. This has been addressed to some extent in patients with chronic hepatitis B virus (HBV), hepatitis delta (HDV) or HIV infections receiving treatment. HBV replication can be controlled by potent nucleoside or nucleotide analogous (NA) but the infection is not cleared (8). Of note, suppression of viral replication with undetectable HBV viral load did not lead to major functional restoration of HBV-specific T cells (9). HBV-specific immunity only improved in the few patients that managed to clear infection after long-term antiviral therapy (functional cure, HBsAg seroconversion) (10). Although some earlier studies have shown transient restoration of HBV-specific T cells, this short-lived nature of immune restitution represents a favorable condition for virus reactivation (11, 12). Similarly, no full restoration of HIV-specific T cell responses was observed even after the virus had been suppressed for several years with antiviral treatment (13). With respect to NK cells, they are activated and functionally altered in hepatitis B and D virus infection (14, 15). The NK cell phenotype seems to be altered by viral suppression with NA (16), but functional consequences remain unclear. However, the phenotype of NK cells did predict long-term control of hepatitis B after stopping antiviral therapy (17). In chronic HIV infection, the NK cell population is dysregulated in several ways including in their capacity to interact with dendritic cells (18) and with the appearance of dysfunctional CD56neg NK cells (19). However, upon antiviral treatment, some of these alterations are reversed, although it takes two years or longer for them to be normalized (20). Similar to NK cells, also MAIT cells are affected by both chronic HBV and hepatitis D virus infections as well as by HIV with severely reduced numbers in circulation and diminished responsiveness to bacterial challenge or innate cytokine stimulation (21–23). Whereas partial reversal of NK cell immunity was observed upon suppression of HBV, HDV, or HIV infections, no such reversal has been described for MAIT (21–23). However, common for chronic HBV, HDV, and HIV infections is that antiviral treatment only suppresses viral replication and rarely (HBV, HDV) or never (HIV) leads to actual clearance of infection. Thus, although evidence for partial reversal of immunity exists in studies of these infections, the full reinvigoration capacity of the immune system is not possible to gauge since the infections de facto are not eliminated.
The Unique Model of Chronic HCV to Study Reversal of Immunity
In contrast to chronic HBV or HIV, chronic HCV infection can now be efficiently cured by antiviral therapies. Thus, chronic HCV infection represents a unique model to study host–pathogen interaction in humans and to investigate the effects of clearance of a persistent long-term infection on the immune system. As a background, infection with HCV becomes chronic in 50–90% of adults where it manifests as chronic liver disease including development of cirrhosis, liver failure, and hepatocellular carcinoma (24). Treatment of hepatitis C virus underwent fundamental changes in late 2013. Before then, antiviral therapy was based on administration of pegylated interferon alfa in combination with ribavirin curing approximately half of the patients but with severe side effects (25–27). In November 2013, the first interferon-free treatment option was approved for the treatment of chronic hepatitis C. Since then many additional direct acting antivirals (DAAs) became available. These DAAs are either targeting the HCV protease, the HCV NS5A protein which is involved in HCV replication and packaging of virions, or the HCV polymerase. Importantly, these regimens have basically no side effects and response rates have been shown to exceed 97%, not only in clinical trials, but also in real world treatment settings, and successful treatment leads to regression of clinical symptoms and complications of liver disease (28, 29).
Thus, with this remarkable development, it is now possible, for the first time, to study immune system function in well-controlled large cohorts of patients that become cured from a chronic infection. This is of both basic immunological and clinical relevance. In more detail, new basic knowledge on the inherent capacity of immune system reinvigorated after a prolonged chronic insult will be important for exposure to other heterologous pathogens, development of immune mediated diseases, immune control of malignancies, and also for vaccine design. Furthermore, and in the HCV context, the previously infected patients may become re-exposed to HCV, and it is currently unclear if those patients need to be re-treated or if they have a chance to spontaneously control HCV due to restored antiviral immunity. Indeed, successfully treated chronic hepatitis C patients still have a risk to develop hepatocellular carcinoma (30). In this setting, HCV clearance may interfere with immune surveillance of malignant cells, and thus a better insight into the effects of rapid HCV removal on innate and adaptive immunity is of interest.
In the sections below, we discuss recent insights that have been gained in the last couple of years with respect to immune restoration following removal of chronic hepatitis C. In addition, we summarized various recent studies on immune cells and their fate after HCV clearance in Table 1.
Table 1.
Summary of different immune cell populations and their fate after antiviral therapy.
| Authors | Type of patient | No. of patients | Treatment(follow up-EOT)Weeks | Main alterations observed upon HCV clearance | |
|---|---|---|---|---|---|
| Soluble immune mediators | |||||
| Carlin et al. (31) | CHC (cirrhotic and non- cirrhotic) | 131 | SOF/RBV (16 or 20) |
Four inflammatory markers were measured, IP-10, MCP-1, MIP-1β, and IL-18, and all decline during therapy but display different dynamics after therapy. MIP-1β and IP-10 displayed significant difference based on treatment outcome. | |
| Hengst et al. (32) | CHC (cirrhotic & NASH) |
53 | SOF/RBV (36) |
IP-10, IL-12 p40, IFN alpha 2a, IL-18, and TRAIL were upregulated in comparison to NASH and healthy controls. The changes in SIM were not fully reversible upon clearance of viral infection. | |
| Debes et al. (33) | CHC & NASH | 13 | SOF/NS5A/PI (+/−RBV) 96 |
Normalization of innate immunity after viral clearance. | |
| Gorin et al. (34) | CHC & AC | 28 | SOF+PI+3D+NS5A/NA(+/−RBV) (36 or 48) |
Rapid restoration of plasma cytokine milieu observed. Macrophage activation marker s CD163 remained elevated. In addition elevated levels for CSCL10 and sCD14 were observed whereas CCL5 and IL-4 remained suppressed. | |
| T cells | |||||
| CD8 | Martin et al. (35) | CHC | 51 | NA+PI +/−RBV (24) |
Specific phenotypic changes were observed on CD8 T cell but expression of CD127 and PD-1 on global CD8 T cells were not altered by therapy. Specific restoration of CD8 T cell proliferation. |
| Weiland et al. (36) | CHC | 24 | NS5A SOF/3D +/− RBV (8–12) |
HCV-specific CD8T cells were analyzed that displayed T cell exhaustion and memory like characteristic both before and after therapy. Only CD127+/PD1+ subset maintained after clearance. The subset characterized by high expression of transcription factor TCF1. | |
| Aregay et al. (37) | CHC | 40 | SOF/PI/3D/NS5A +/−RBV (24) |
Surface expression of co-regulatory receptors on exhausted HCV-specific CD8 T cells remained unaltered. Mitochondrial dysfunction of exhausted HCV-specific CD8 T cells was not restored. HCV-specific CD8 T cells remained functionally impaired after clearance. | |
| CD4 | Smits et al. (38) | CHC | 248 | SOF/PI/3D/NS5A +/−RBV (24) | HCV-specific CD4 T cells skewed towards a follicular T helper cell phenotype maintained after clearance. |
| Tregs | Langhans et al. (39) | CHC | 14 | SOF+PI/NS5A (54) |
Increased frequency and activation status of Tregs that do not normalize even after long term follow-up. |
| γδ T cells | Ravens et al. (40) | CHC & non cirrhotic | 23 | SOF+NS5A (48) |
NGS and flow cytometeric sorting was performed to monitor the peripheral γδ TCR repertoire and their clonal distribution. Overall clonality and complexity of TCR γδ was comparable to healthy. The γδ T cell compartment and their associated TCR repertoires were highly stable at a long term follow up. |
| Ghosh et al. (41) | CHC | 24 | SOF+NS5A/PI (12) |
Peripheral Vγ9Vδ2 γδ T cells showed phenotypic and functional alterations despite cure. CD38 expression in SVR group was not different from healthy but declined at the EOT but relapsers had higher CD38+ frequencies. | |
| MAIT cells | Hengst et al. (42) | CHC | 26 | SOF+ RBV (72) |
MAIT cells present in lower frequencies, circulating MAITs display altered phenotype, impaired in MR1 dependent function. Function and cell frequency not restored after elimination of virus, no correlation with clinical parameters and liver disease. |
| Spaan et al. (43) | CHC | 22 | PI/NS5A (24) SOF/NS5A+/−RBV (24) |
MAIT cell frequencies decreased in all cohorts. No association between the frequency of MAIT cells and ALT level, HCV RNA, and liver fibrosis score. Patients with differential fibrosis stage showed similar MAIT frequencies. | |
| Bolte et al. (44) | CHC | 42 | SOF+NS5A (24) |
Paired liver biopsies and blood samples were studied. MAIT cell frequency was lower in blood and liver compared to healthy. Liver MAIT cells displayed higher activation and cytotoxicity than MAITs from blood. Impaired MR1 dependent cell function. | |
| Cannizzo et al. (45) | HCV/HIV co-infected, IFN non responders, cART treated | 5 | NS5A/PI/SOF/3D +/− RBV (24) |
At baseline diminished total CD3 and CD8 MAITs compared to healthy and no recovery. Longitudinally. MAIT subsets showed higher CD69, PD-1, and granzyme expression but no difference in CD39 and Il-18R and perforin expression. | |
| Natural killer (NK) | |||||
| Serti et al. (46) | CHC | 13 | NS5A/PI (24) |
Post therapy decrease in NK cell activation and a normalization of NK cell cytotoxic effector functions to healthy. Paired liver biopsies showed similar normalization trend. | |
| Spaan et al. (47) | CHC | 12 | NS5A/PI (12) |
NK cell frequencies altered to levels comparable to healthy. NK cell functions (IFNγ and perforin) not modulated. | |
| Strunz et al. (48) | CHC | 35 | SOF/+ RBV (96) |
NK cells from patients with chronic HCV maintained their functional capacity. Chronic infection reduced NK cell diversity and this reduction persisted long after viral clearance. | |
| Wang et al. (49) | CHC | 26 | SOF/NS5A (24 or 36) |
Significant decline in CD56bright NK cell frequencies that normalize after EOT but no difference in CD56dim NK cells observed. Expression levels of NKG2A, NKp30, and CD94 were high at baseline but recovered to levels those of healthy after EOT. | |
| Jiang et al. (50) | CHC | 13 | SOF/NS5A (24 or 36) |
Expression of functional markers were downregulated after treatment but the potential activity of NK cells gets upregulated. Amongst the NK markers, NKp46 normalized at EOT. | |
| Golden-Mason et al. (51) | Prospective cohort | 22 | LDV/SOF (24) |
Transient activation followed by dampening of NK cell activity to pre- treatment levels | |
| Mele et al. (52) | CHC | 59 | DAA (24) |
Restoration of normal adaptive NK phenotype (activation markers normalized) and restored ADCC ability. | |
CHC, chronic hepatitis C; NASH, non-alcoholic steatohepatitis; AC, alcoholic cirrhotic; SOF, sofosbovir; RBV, ribavirin; PI, Protease inhibitor; NA, nucleot(s)ide analogs; 3D-Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir; EOT, end of treatment; NK, natural killer cells; MAIT, mucosal associated invariant T cells; TCF1, transcription factor; cART, combination antiretroviral therapy.
HCV Clearance and Effect on Systemic Pro-Inflammation
Chronic HCV causes a distinct inflammatory milieu by inducing IFN stimulated genes (ISGs) which impacts clinical manifestations of HCV infection and even tumor development. Upon chronic HCV infection, hepatocytes are triggered to produce type I and III IFNs which induce production of ISGs with antiviral activity (5). Despite this induction of the interferon system, the majority of patients establish chronic infection. In patients with chronic hepatitis C, a variety of systemic pro-inflammatory cytokines and chemokines are elevated (32, 34). In addition, the systemic inflammatory repertoire is different in HCV-infected patients compared to patients with non-alcoholic steatohepatitis (NASH) a non-viral chronic disease (32). This elevation affects different pro-inflammatory cytokines, chemokines, and growth factors including CXCL10 (IP-10), IL-12, IFN-α, IL-18, and TRAIL (32, 34). An obvious question is if these changes are driven by HCV infection or just secondary to underlying liver inflammation and/or liver disease. Indeed, elevated levels of VCAM-1 and ICAM-1 have been shown to be associated with the degree of liver fibrosis (32). In long-term follow-up studies after clearance of infection with DAA treatment, many of the pro-inflammatory cytokines and chemokines returned to normal levels albeit a residual signature with elevated levels of IFN-α and TRAIL persisted months after viral clearance (32, 34). Overall, persistent HCV infection is associated with profound alterations in levels of soluble inflammatory mediators which are related with liver disease progression, treatment outcome and viral presence. Importantly, these changes were not fully reversible upon viral clearance (Table 1, Figure 1).
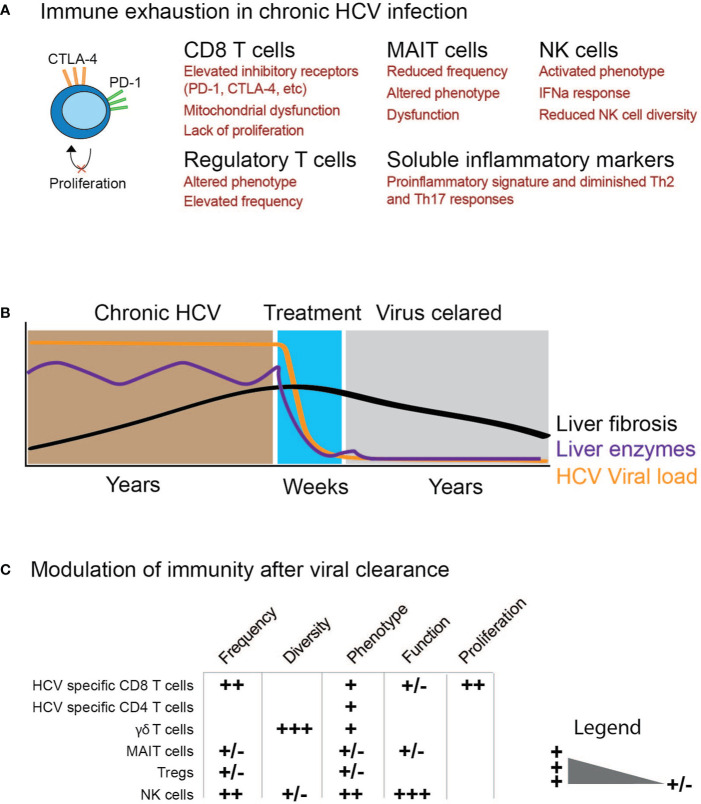
Figure 1.
Schematic illustration of immune cell function before and after elimination of chronic HCV infection. (A) Examples of possible immune exhaustion as a consequence of chronic HCV infection. (B) Time course for studying the impact of HCV clearance on immune cells. (C) Modulation of immunity after viral clearance with the degree of change ranging from (+/−) to (+++).
Partial Reinvigoration of CD4+ and CD8+ T Cells Upon Viral Clearance
Initial studies investigating HCV-specific T cells in patients receiving DAA therapies suggested a partial restoration of effector functions, in particular of antigen-specific T cell proliferation (35). Still, the level of restoration was heterogeneous, and not all patients normalized T cell function. These findings were supported by other studies showing that suppression of HCV replication led to a decline in T cell exhaustion marker expression and an increase in HCV-specific IFNγ responses after treatment (53–55). However, HCV-specific CD8+ T cells with phenotypic features of exhaustion and memory potential can survive in an antigen-independent manner, both during and after DAA therapy and HCV clearance (36). This survival might be mediated by expression of the transcription factor TCF-1 (36). However, a restoration of HCV-specific CD8+ T cell exhausted surface phenotype does not, per se, translate to full functional reinvigoration. Indeed, our group recently reported that the mitochondrial and metabolic dysfunction of virus-specific CD8+ T cells persisted despite viral clearance (37). However, other data suggests that mitochondrial function may partially improve in some patients (but not in all patients) during antiviral therapy (56).
With respect to CD4+ T cells, antiviral treatment of HCV led to a shift from a Th1 to a follicular helper T cell (Tfh) signature within HCV-specific cells (38). Similar to HCV-specific CD8+ T cells, Tfh cells are likely persisting in an antigen-independent manner (38). Moreover, regulatory T cells are usually found in higher numbers in chronic hepatitis C and, surprisingly, these increased Treg frequencies with an activated phenotype persisted also during and after DAA therapy of HCV infection (39) (Table 1).
In addition, it has been recently shown that the transcription factor TOX is crucial during appearance and maintenance of exhausted T cells in mice (57–59). Of note, in humans, it is clearly shown that HCV-specific CD8T cells remain TOX positive after DAA mediated elimination indicating a chronic scar (59). Whereas TOX was suggested to be a master regulator of exhausted T cells in mice, other recent work in humans has shown that, except for being expressed on exhausted T cells, also effector memory T cells express this transcription factor (60). Finally, the effect of HCV therapy on T cells specific to other antigens such as CMV and EBV (61) as well as on T cells recognizing tumor antigens (62) has been studied. Here, molecules indicating activation of T cells decreased in expression levels over time, but no major functional changes were observed in the majority of cases. Table 1 summarizes the effect of DAAs on T cells.
Overall, studies investigating T cell responses suggest that viral clearance and lack of ongoing antigen stimulation lead to a down-regulation of T cell activation and exhaustion markers but antigen-specific dysfunction is not restored—even when patients are followed for up to a year after HCV elimination. Antigen-independent survival of distinct subsets of virus-specific CD4+ and CD8+ T cells has been reported, and these populations constitute potential targets for immunotherapy to prevent HCV re-infection (36). Long lived T cell memory is often observed during spontaneous resolution of acute hepatitis C infection both in humans and chimpanzees. These memory CD4 and CD8 T cells appear important for rapid control of secondary infection. In a recent study the data suggested that CD8T cell memory was rather narrow after successful treatment with DAA, and the authors suggested that vaccination maybe one option to induce the broader memory response which may provide protective immunity (63).
Unconventional T Cells in Clearance of Chronic HCV
Compared to conventional CD4 or CD8 T cells, unconventional T cells, such as γδ T cells and MAIT cells are typically rapid effector cells that respond within hours towards foreign antigens and/or other innate signals exhibiting important functions during viral infection (6, 64, 65). In chronic hepatitis C, γδ T cells are less efficient in producing cytokines and exhibit an activated phenotype (41, 66). Whereas the activated phenotype vanished upon DAA-mediated HCV clearance, the dysfunction remained (41). This dysfunction was not due to a skewing in the T cell receptor repertoire as it was shown to be unaltered in patients with chronic hepatitis C and further remained stable after elimination of HCV (40). This is distinct compared to chronic HIV infection where the repertoire is heavily altered because of the infection but slowly returns to normal after prolonged antiviral treatment (67).
Compared to γδ T cells, MAIT cells have been more extensively studied in the context of chronic HCV infection. MAIT cells are highly enriched in the human liver and they efficiently respond to innate cytokines such as IL-12, IL-18, and IFN-α suggesting that they exhibit an important role in the antiviral immunity against HCV (68, 69). However, in chronic hepatitis C, MAIT cell numbers are reduced both in liver and peripheral blood (42–45). In fact, of all alterations in peripheral blood immune cell subsets in chronic hepatitis C, loss of MAIT cells was shown to be the major phenotype (42). Loss of MAIT cells in chronic hepatitis C appears to both be a consequence of the infection, per se, but also to the underlying liver disease as patients with liver cirrhosis tend to have reduced numbers of MAIT cells (70, 71). Loss of MAIT cells was accompanied with MAIT cell activation with increased expression of CD69, HLA-DR, PD-1, and granzyme B (42). Despite having an activated phenotype, MAIT cell function, in response to bacterial challenge but not innate cytokine stimulation, was hampered in chronic hepatitis C (42, 44) (Table 1). This phenotype of MAIT cells observed in chronic hepatitis C is similar to what has been described for infections with HBV, HDV, and HIV (21–23). Upon treatment and viral clearance, circulating MAIT cell numbers remain suppressed for years (42) whereas a certain restoration of intrahepatic MAIT cells following viral clearance have been noted (44). However, MAIT cell activation and dysfunction remained (42, 44), albeit with some reversal of the activated signature noted in one study (44). The inability for MAIT cells to become reinvigorated upon pathogen removal appears to be shared among chronic infections as similar findings have been reported for chronic HBV, HDV, and HIV infections (21–23). The long-term consequences of having this “gap” in the immune system are currently unknown. However, it is interesting to note that patients with chronic viral hepatitis infections progressing to end-stage liver disease have an increased risk for bacterial infections (72). This might partly be due to a dysfunctional MAIT cell compartment (71). Future research should focus on identifying the signals needed for restoring the MAIT cell compartment. Some insight into this came from a recent study showing that in vivo IL-7 administration significantly expanded the human MAIT cell compartment (73).
Impact of Chronic HCV and Clearance Thereof on NK Cells
Similar to MAIT cells, also NK cells are highly enriched in the human liver (74) and thus have been extensively studied in the context of chronic HCV infection (5, 75). Both genetic and cellular studies have revealed an important role for NK cells in the control of HCV infection (5, 75–77). However, in chronic hepatitis C, NK cell phenotype and function are compromised at multiple levels (15, 78, 79). Upon antiviral treatment and rapid clearance of HCV, several groups have in recent years assessed whether the compromised NK cell compartment recovers (Table 1 and Figure 1). Interestingly, when measuring single parameters of NK cell “health”, both phenotype and function seem to partly, or fully, normalize upon viral clearance. This includes reversal of an aberrant phenotype with normalized expression of activation and inhibitory receptors such as NKp30, NKp46, TRAIL, and NKG2A (46, 47, 49, 50, 80). This reversal in NK cell phenotype happened within months after viral clearance and was also associated with restored NK cell function (46, 49, 50, 81). The signal responsible for this restoration currently remains unknown. Future work should determine whether this is an active reinvigoration via certain signaling pathways or rather the removal of the virus and possibly the ensuing resolution of inflammation that leads to a seemingly restored NK cell compartment.
Diversity is an essential feature of our immune system. While this term has been mostly associated with adaptive immune responses, recent work has also shown that NK cells represent a highly diverse population of immune cells (82, 83). A recent study performed a high-dimensional analysis of NK cells in chronic HCV and treatment thereof (48). It revealed that chronic HCV infection increased inter-individual, but decreased intra-individual, NK cell diversity. This occurred independent of underlying CMV infection, a potent influencer of NK cell repertoire formation and NK cell diversity (84, 85) but could partly be linked to the degree of underlying liver disease (48). Intriguingly, the altered NK cell diversity appeared irreversible since it persisted for at least two years after clearance of chronic HCV. Thus, distinct from single measurements of NK cell function, that appears to normalize upon clearance of a chronic pathogen (46, 47, 49, 50, 51, 80) global affection on the NK cell compartment still remain for years (52). The impact of altered NK cell diversity on an individual’s immunological health in the longer perspective should now be the focus in future studies.
Important Unanswered Questions
Despite a plethora of recent studies investigating the capacity of the immune system to reset after removal of chronic hepatitis C, several important questions remain. More detailed studies on exhausted HCV-specific CD4+ and CD8+ T cells in chronic HCV are warranted since there might be a degree of heterogeneity with subpopulations of exhausted HCV-specific cells becoming fully reinvigorated after DAA-mediated clearance of the virus. Additionally, various other environmental and host factors may influence the evolution of HCV-specific T cells before, during, and after antiviral therapy including stage of liver disease, sex, and age. These factors may also explain differences between different cohorts. Furthermore, several additional arms of the immune system still remain to be studied in the context of DAA treatment of hepatitis C patients including myeloid immune cells and HCV-specific B cells. Out of necessity, most of the above described work have focused on immune cells in peripheral blood. However, researchers should in the future also strive towards finding means to access and interrogate the intrahepatic immune environment in relation to rapid clearance of chronic HCV (44, 86). Additionally, studies on the epigenetic imprint of immune cells after successful treatment are also warranted. The growing understanding of epigenetic gene regulation as it relates to both the stability and malleability of T cell memory may offer the potential to selectively modify T cell memory in disease by targeting epigenetic mechanisms (87). Underlying this are alterations at the chromatin level that regulate constitutive and inducible gene expression including histone modification and DNA methylation (88). Some studies have demonstrated that HCV infection modifies the position of histone modifications, thereby inducing an epigenetic signature that persists following the cure with DAAs and these changes can be reverted by specific drugs. This may further provide an opportunity for prevention of HCC progression (87, 89). It is well accepted that HCV cure does not eliminate the short term risk to develop hepatocellular carcinoma. Moreover, there has been concern that HCC recurrence rates may even be higher in patients who had received curative first line therapies for HCC and who subsequently received DAA therapy against chronic hepatitis C. In a recent paper from our group we showed that HCC surveillance may indeed be affected by DAA therapy of chronic HCV infection and identified that IL-12 could be a key player in the regulation of HCC-specific CD8+T cell responses (62). Finally, although some of the published studies have longitudinally characterized patients for up to almost 2.5 years after viral clearance (48), with the large number of chronic hepatitis C patients now be cured, longitudinal studies aiming at following patient cohorts for at least 5, or even 10 years, are now feasible and will provide an even better estimate of our immune systems inherent capacity to recover.
Conclusions
In this brief review, we highlight recent development in the analysis of various immune cell populations during and after clearance of chronic HCV infection. This represents the first human model where a pathogen successfully can be eliminated after years of chronic infection allowing us to determine long-term consequences of immunity following resolution of this insult. Although there has been evidence on antigen-independent survival mechanisms, including a role for TCF-1, of HCV-specific T cells which could represent targets for immune interventions. However, surprisingly many imprints of chronic HCV infection on distinct immune compartments persist for years despite antigen elimination.
Author Contributions
TK and BS drafted the figure and table and wrote parts of the review. HW and NB drafted the layout for the review and wrote the discussion. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Projektnummer 158989968—SFB 900, SFB738 of the German Research Foundation (DFG), German Centre for Infection Research (DZIF), internal funds from the university of Duisburg-Essen, Swedish Research Council, the Swedish Cancer Society, the Swedish Foundation for Strategic Research, Knut and Alice Wallenberg Foundation, the Novo Nordisk Foundation, the Center for Innovative Medicine at Karolinska Institutet, the Stockholm County Council, Karolinska Institutet.
Conflict of Interest
HW reports grants and personal fees from Abbvie, grants, personal fees, and non-financial support from Abbott, grants, personal fees, and non-financial support from Roche Diagnostics, personal fees from Siemens, grants and personal fees from BMS, grants and personal fees from Gilead, grants and personal fees from Novartis, grants and personal fees from Roche, personal fees from Janssen, grants and personal fees from Merck/MSD, grants and personal fees from Eiger, grants and personal fees from Falk and Falk Foundation, other from Transgene, non-financial support and other from Myr-GmbH, all outside the submitted work; and HW received honoraria for consulting and research support by companies developing diagnostic tools and antiviral therapies for hepatitis B and C.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CMV, cytomegalovirus; DAA, direct acting antiviral; EBV, Epstein bar virus; ICAM-1, intercellular cell adhesion molecule-1; γδT cells, gamma-delta T cells; MAIT, mucosal-associated invariant T cells; NA, nucleos(t)ide analog; NK cells, natural killer cells; VCAM-1, vascular cell adhesion molecule.
References
- 1. Rehermann B. HCV in 2015: Advances in hepatitis C research and treatment. Nat Rev Gastroenterol Hepatol (2016) 13:70–2. 10.1038/nrgastro.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones RB, Walker BD. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest (2016) 126:455–63. 10.1172/JCI80566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol (2016) 64:S60–s70. 10.1016/j.jhep.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 4. Florez-Alvarez L, Hernandez JC, Zapata W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front Immunol (2018) 9:2290. 10.3389/fimmu.2018.02290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol (2014) 61:S14–25. 10.1016/j.jhep.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 6. Ussher JE, Willberg CB, Klenerman P. MAIT cells and viruses. Immunol Cell Biol (2018) 96:630–41. 10.1111/imcb.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest (2018) 128:4588–603. 10.1172/JCI121960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol (2017) 66:398–411. 10.1016/j.jhep.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 9. Hong M, Bertoletti A. Tolerance and immunity to pathogens in early life: insights from HBV infection. Semin Immunopathol (2017) 39:643–52. 10.1007/s00281-017-0641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrari C, Boni C, Rossi M, Vecchi A, Barili V, Laccabue D, et al. T cell regulation in HBV-related chronic liver disease. J Hepatol (2017) 66:1096–8. 10.1016/j.jhep.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 11. Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest (1998) 102:968–75. 10.1172/JCI3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology (2012) 143:963–73.e969. 10.1053/j.gastro.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 13. Gaiha GD, Walker BD. CD8(+) T Cells and cART: A Dynamic Duo? Immunity (2016) 45:466–8. 10.1016/j.immuni.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 14. Lunemann S, Malone DF, Grabowski J, Port K, Beziat V, Bremer B, et al. Effects of HDV infection and pegylated interferon alpha treatment on the natural killer cell compartment in chronically infected individuals. Gut (2015) 64:469–82. 10.1136/gutjnl-2014-306767 [DOI] [PubMed] [Google Scholar]
- 15. Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis (2014) 209:1362–73. 10.1093/infdis/jit561 [DOI] [PubMed] [Google Scholar]
- 16. Boni C, Lampertico P, Talamona L, Giuberti T, Invernizzi F, Barili V, et al. Natural killer cell phenotype modulation and natural killer/T-cell interplay in nucleos(t)ide analogue-treated hepatitis e antigen-negative patients with chronic hepatitis B. Hepatol (Baltimore Md (2015) ) 62:1697–709. 10.1002/hep.28155 [DOI] [PubMed] [Google Scholar]
- 17. Zimmer CL, Rinker F, Honer Zu Siederdissen C, Manns MP, Wedemeyer H, Cornberg M, et al. Increased NK Cell Function After Cessation of Long-Term Nucleos(t)ide Analogue Treatment in Chronic Hepatitis B Is Associated With Liver Damage and HBsAg Loss. J Infect Dis (2018) 217:1656–66. 10.1093/infdis/jiy097 [DOI] [PubMed] [Google Scholar]
- 18. Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol (2005) 5:835–43. 10.1038/nri1711 [DOI] [PubMed] [Google Scholar]
- 19. Bjorkstrom NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol (2010) 31:401–6. 10.1016/j.it.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 20. Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood (2009) 114:3822–30. 10.1182/blood-2009-06-226332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dias J, Hengst J, Parrot T, Leeansyah E, Lunemann S, Malone DFG, et al. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of MAIT cells. J Hepatol (2019) 71:301–12. 10.1016/j.jhep.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood (2013) 121:1124–35. 10.1182/blood-2012-07-445429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood (2013) 121:951–61. 10.1182/blood-2012-06-436436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guidelines E. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol (2018) 69:461–511. 10.1016/j.jhep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 25. Guidelines ECP. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol (2011) 55:245–64. 10.1016/j.jhep.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 26. Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol (2015) 62:S87–99. 10.1016/j.jhep.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 27. Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut (2006) 55:1350–9. 10.1136/gut.2005.076646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol (2017) 67:1204–12. 10.1016/j.jhep.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 29. Herzer K, Gerken G, Kroy D, Tacke F, Plewe J, Eurich D, et al. Impact of direct-acting antiviral therapy on the need for liver transplantation related to hepatitis C in Germany. J Hepatol (2018) 69:982–4. 10.1016/j.jhep.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 30. Mettke F, Schlevogt B, Deterding K, Wranke A, Smith A, Port K, et al. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther (2018) 47:516–25. 10.1111/apt.14427 [DOI] [PubMed] [Google Scholar]
- 31. Carlin AF, Aristizabal P, Song Q, Wang H, Paulson MS, Stamm LM, et al. Temporal dynamics of inflammatory cytokines/chemokines during sofosbuvir and ribavirin therapy for genotype 2 and 3 hepatitis C infection. Hepatology (2015) 62(4):1047–58. 10.1002/hep.27971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hengst J, Falk CS, Schlaphoff V, Deterding K, Manns MP, Cornberg M, et al. Direct-Acting Antiviral-Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients With Chronic Hepatitis C. J Infect Dis (2016) 214:1965–74. 10.1093/infdis/jiw457 [DOI] [PubMed] [Google Scholar]
- 33. Debes JD, van Tilborg M, Groothuismink ZMA, Hansen BE, Schulze Zur Wiesch J, de Knegt RJ, et al. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology (2018) 154(3):515–7.e513. 10.1053/j.gastro.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 34. Gorin JB, Malone DFG, Strunz B, Carlsson T, Aleman S, Bjorkstrom NK, et al. Plasma FABP4 is associated with liver disease recovery during treatment-induced clearance of chronic HCV infection. Sci Rep (2020) 10:2081. 10.1038/s41598-020-58768-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol (2014) 61:538–43. 10.1016/j.jhep.2014.05.043 [DOI] [PubMed] [Google Scholar]
- 36. Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, et al. TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun (2017) 8:15050. 10.1038/ncomms15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aregay A, Owusu Sekyere S, Deterding K, Port K, Dietz J, Berkowski C, et al. Elimination of hepatitis C virus has limited impact on the functional and mitochondrial impairment of HCV-specific CD8+ T cell responses. J Hepatol (2019) 71:889–99. 10.1016/j.jhep.2019.06.025 [DOI] [PubMed] [Google Scholar]
- 38. Smits M, Zoldan K, Ishaque N, Gu Z, Jechow K, Wieland D, et al. Follicular T helper cells shape the HCV-specific CD4 T cell repertoire after viral elimination. J Clin Invest (2019) 130:998–1009. 10.1172/JCI129642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Langhans B, Nischalke HD, Kramer B, Hausen A, Dold L, van Heteren P, et al. Increased peripheral CD4(+) regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol (2017) 66:888–96. 10.1016/j.jhep.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 40. Ravens S, Hengst J, Schlapphoff V, Deterding K, Dhingra A, Schultze-Florey C, et al. Human gammadelta T Cell Receptor Repertoires in Peripheral Blood Remain Stable Despite Clearance of Persistent Hepatitis C Virus Infection by Direct-Acting Antiviral Drug Therapy. Front Immunol (2018) 9:510. 10.3389/fimmu.2018.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghosh A, Mondal RK, Romani S, Bagchi S, Cairo C, Pauza CD, et al. Persistent gamma delta T-cell dysfunction in chronic HCV infection despite direct-acting antiviral therapy induced cure. J Viral Hepat (2019) 29(9):1105–16. 10.1111/jvh.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hengst J, Strunz B, Deterding K, Ljunggren HG, Leeansyah E, Manns MP, et al. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur J Immunol (2016) 46:2204–10. 10.1002/eji.201646447 [DOI] [PubMed] [Google Scholar]
- 43. Spaan M, Hullegie SJ, Beudeker BJ, Kreefft K, van Oord GW, Groothuismink ZM, et al. Frequencies of Circulating MAIT Cells Are Diminished in Chronic HCV, HIV and HCV/HIV Co-Infection and Do Not Recover during Therapy. PloS One (2016) 11:e0159243. 10.1371/journal.pone.0159243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bolte FJ, O’Keefe AC, Webb LM, Serti E, Rivera E, Liang TJ, et al. Intra-Hepatic Depletion of Mucosal-Associated Invariant T Cells in Hepatitis C Virus-Induced Liver Inflammation. Gastroenterology (2017) 153:1392–1403.e1392. 10.1053/j.gastro.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cannizzo ES, Cerrone M, Merlini E, van Wilgenburg B, Swadling L, Ancona G, et al. Successful direct-acting antiviral therapy in HIV/HCV co-infected patients fails to restore circulating mucosal-associated invariant T cells. Eur J Immunol (2019) 49:1127–9. 10.1002/eji.201948152 [DOI] [PubMed] [Google Scholar]
- 46. Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology (2015) 149:190–200.e192. 10.1053/j.gastro.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, et al. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis (2016) 213:216–23. 10.1093/infdis/jiv391 [DOI] [PubMed] [Google Scholar]
- 48. Strunz B, Hengst J, Deterding K, Manns MP, Cornberg M, Ljunggren HG, et al. Chronic hepatitis C virus infection irreversibly impacts human natural killer cell repertoire diversity. Nat Commun (2018) 9:2275. 10.1038/s41467-018-04685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang XX, Luo BF, Jiang HJ, Cong X, Jin Q, Ma DL, et al. Recovery of natural killer cells is mainly in post-treatment period in chronic hepatitis C patients treated with sofosbuvir plus ledipasvir. World J Gastroenterol (2018) 24:4554–64. 10.3748/wjg.v24.i40.4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang HJ, Wang XX, Luo BF, Cong X, Jin Q, Qin H, et al. Direct antiviral agents upregulate natural killer cell potential activity in chronic hepatitis C patients. Clin Exp Med (2019) 19:299–308. 10.1007/s10238-019-00564-9 [DOI] [PubMed] [Google Scholar]
- 51. Golden-Mason L, McMahan RH, Kriss MS, Kilgore AL, Cheng L, Dran RJ, et al. Early and late changes in natural killer cells in response to ledipasvir/sofosbuvir treatment. Hepatol Commun (2018) 2:364–75. 10.1002/hep4.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mele D, Oliviero B, Mantovani S, Ludovisi S, Lombardi A, Genco F, et al. Adaptive Natural Killer Cell Functional Recovery in Hepatitis C Virus Cured Patients. Hepatol (Baltimore Md) (2020). 10.1002/hep.31273 [DOI] [PubMed]
- 53. Shrivastava S, Bhatta M, Ward H, Romani S, Lee R, Rosenthal E, et al. Multitarget Direct-Acting Antiviral Therapy Is Associated With Superior Immunologic Recovery in Patients Coinfected With Human Immunodeficiency Virus and Hepatitis C Virus. Hepatol Commun (2018) 2:1451–66. 10.1002/hep4.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shrivastava S, Wilson E, Poonia B, Tang L, Osinusi A, Kohli A, et al. Augmentation of hepatitis C virus-specific immunity and sustained virologic response. J Viral Hepat (2017) 24:742–9. 10.1111/jvh.12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barili V, Fisicaro P, Montanini B, Acerbi G, Filippi A, Forleo G, et al. Targeting p53 and histone methyltransferases restores exhausted CD8+ T cells in HCV infection. Nat Commun (2020) 11:604. 10.1038/s41467-019-14137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat (2015) 22:983–91. 10.1111/jvh.12465 [DOI] [PubMed] [Google Scholar]
- 57. Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature (2019) 571:211–8. 10.1038/s41586-019-1325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature (2019) 571:270–4. 10.1038/s41586-019-1324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature (2019) 571:265–9. 10.1038/s41586-019-1326-9 [DOI] [PubMed] [Google Scholar]
- 60. Sekine T, Perez-Potti A, Nguyen S, Gorin JB, Wu VH, Gostick E, et al. TOX is expressed by exhausted and polyfunctional human effector memory CD8(+) T cells. Sci Immunol (2020) 5(49):eaba7918. 10.1126/sciimmunol.aba7918 [DOI] [PubMed] [Google Scholar]
- 61. Owusu Sekyere S, Suneetha PV, Hardtke S, Falk CS, Hengst J, Manns MP, et al. Type I Interferon Elevates Co-Regulatory Receptor Expression on CMV- and EBV-Specific CD8 T Cells in Chronic Hepatitis C. Front Immunol (2015) 6:270. 10.3389/fimmu.2015.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Owusu Sekyere S, Schlevogt B, Mettke F, Kabbani M, Deterding K, Wirth TC, et al. HCC Immune Surveillance and Antiviral Therapy of Hepatitis C Virus Infection. Liver Cancer (2019) 8:41–65. 10.1159/000490360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Callendret B, Eccleston HB, Hall S, Satterfield W, Capone S, Folgori A, et al. T-cell immunity and hepatitis C virus reinfection after cure of chronic hepatitis C with an interferon-free antiviral regimen in a chimpanzee. Hepatol (Baltimore Md (2014) ) 60:1531–40. 10.1002/hep.27278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Godfrey D, II, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol (2015) 16:1114–23. 10.1038/ni.3298 [DOI] [PubMed] [Google Scholar]
- 65. Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol (2014) 32:121–55. 10.1146/annurev-immunol-032713-120216 [DOI] [PubMed] [Google Scholar]
- 66. Yin W, Tong S, Zhang Q, Shao J, Liu Q, Peng H, et al. Functional dichotomy of Vdelta2 gammadelta T cells in chronic hepatitis C virus infections: role in cytotoxicity but not for IFN-gamma production. Sci Rep (2016) 6:26296. 10.1038/srep26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chaudhry S, Cairo C, Venturi V, Pauza CD. The gammadelta T-cell receptor repertoire is reconstituted in HIV patients after prolonged antiretroviral therapy. AIDS (Lond Engl) (2013) 27:1557–62. 10.1097/QAD.0b013e3283611888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood (2011) 117:1250–9. 10.1182/blood-2010-08-303339 [DOI] [PubMed] [Google Scholar]
- 69. van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, et al. MAIT cells are activated during human viral infections. Nat Commun (2016) 7:11653. 10.1038/ncomms11653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut (2018) 67:918–30. 10.1136/gutjnl-2017-314458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Niehaus CE, Strunz B, Cornillet M, Falk CS, Schnieders A, Maasoumy B, et al. MAIT cells are enriched and highly functional in ascites of patients with decompensated liver cirrhosis. Hepatol (Baltimore Md) (2020). 10.1002/hep.31153 [DOI] [PubMed]
- 72. Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2011) 9:727–38. 10.1016/j.cgh.2011.02.031 [DOI] [PubMed] [Google Scholar]
- 73. Sortino O, Richards E, Dias J, Leeansyah E, Sandberg JK, Sereti I. IL-7 treatment supports CD8+ mucosa-associated invariant T-cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS (Lond Engl) (2018) 32:825–8. 10.1097/QAD.0000000000001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol (2016) 16:310–20. 10.1038/nri.2016.34 [DOI] [PubMed] [Google Scholar]
- 75. Park SH, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity (2014) 40:13–24. 10.1016/j.immuni.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khakoo S, II, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Sci (New York NY (2004) ) 305:872–4. 10.1126/science.1097670 [DOI] [PubMed] [Google Scholar]
- 77. Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology (2010) 138:1885–97. 10.1053/j.gastro.2010.01.051 [DOI] [PubMed] [Google Scholar]
- 78. Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut (2006) 55:869–77. 10.1136/gut.2005.076463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S, et al. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatol (Baltimore Md (2012) 56:841–9. 10.1002/hep.25723 [DOI] [PubMed] [Google Scholar]
- 80. Li Y, Zeng Y, Zeng G, Li J, Zhang X, Cai Q, et al. The effects of direct-acting antiviral agents on the frequency of myeloid-derived suppressor cells and natural killer cells in patients with chronic hepatitis C. J Med Virol (2019) 91:278–86. 10.1002/jmv.25302 [DOI] [PubMed] [Google Scholar]
- 81. Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, et al. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNalpha. Gut (2017) 66:724–35. 10.1136/gutjnl-2015-310033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Trans Med (2013) 5:208ra145. 10.1126/scitranslmed.3006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Filipovic I, Sonnerborg I, Strunz B, Friberg D, Cornillet M, Hertwig L, et al. 29-Color Flow Cytometry: Unraveling Human Liver NK Cell Repertoire Diversity. Front Immunol (2019) 10:2692. 10.3389/fimmu.2019.02692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med (2011) 208:13–21. 10.1084/jem.20100762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood (2013) 121:2678–88. 10.1182/blood-2012-10-459545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hengst J, Theorell J, Deterding K, Potthoff A, Dettmer A, Ljunggren HG, et al. High-resolution determination of human immune cell signatures from fine-needle liver aspirates. Eur J Immunol (2015) 45:2154–7. 10.1002/eji.201445369 [DOI] [PubMed] [Google Scholar]
- 87. Perez S, Kaspi A, Domovitz T, Davidovich A, Lavi-Itzkovitz A, Meirson T, et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PloS Genet (2019) 15:e1008181. 10.1371/journal.pgen.1008181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut (2016) 65:1895–905. 10.1136/gutjnl-2015-311292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology (2019) 156:2313–2329.e2317. 10.1053/j.gastro.2019.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]