Abstract
A 59-year-old man with type 1 diabetes presented with heart failure. Echocardiography showed large vegetations on the mitral and aortic valves. Blood bacterial culture was positive for Staphylococcus warneri, a coagulase-negative staphylococcus (CoNS) family member. He was diagnosed with native valve endocarditis (NVE) induced by the resident bacteria and ultimately underwent double valve replacement. Retrospectively, slight laboratory data abnormalities and weight loss beginning four months before may have been signs of NVE. He had no history of immunosuppressive therapies or medical device implantation. Thus, CoNS can cause NVE after a long asymptomatic course in patients with poorly controlled diabetes.
Keywords: infective endocarditis (IE), native valve endocarditis (NVE), coagulase-negative staphylococcus (CoNS), Staphylococcus warneri, diabetes mellitus, bicuspid aortic valve
Introduction
Hyperglycemia is a well-known risk factor for infectious diseases, such as pneumonia, influenza, and urinary tract infection (1-5). Infective endocarditis (IE) is a critical infectious disease, and its prevalence is increased in patients with diabetes (6-8). The bacteria usually causing native valve endocarditis (NVE) are Staphylococcus aureus (S. aureus) and viridans group streptococci (9-11). However, resident bacteria, including coagulase-negative staphylococci (CoNS), are often isolated as causative pathogens in nosocomial infections. For IE, CoNS are thus recognized as common pathogens in prosthetic valve endocarditis (PVE) while being quite rare in NVE (9-13). IE induced by CoNS usually occurs in immunocompromised hosts and follows a chronic and indolent course, making an early diagnosis difficult. Therefore, it often becomes severe and has a serious outcome (13).
Staphylococcus warneri (S. warneri) is a member of the CoNS family and is also isolated from body surfaces (14, 15). S. warneri typically gives rise to clinical problems in patients with a compromised immune system or implanted prosthetic devices (16, 17). IE, especially NVE, caused by S. warneri is very rare, and there are few reports in the literature (16-25).
We herein report NVE caused by S. warneri in a patient with type 1 diabetes mellitus. We also conducted a literature review to identify cases of IE caused by S. warneri. Mild laboratory abnormalities and transient body weight loss were seen, but he had experienced no other symptoms until the onset of acute heart failure. When he underwent diagnostic examinations for the heart failure, large vegetations were detected on both the mitral and aortic valves, necessitating double valve replacement.
Case Report
The patient was a 59-year-old Japanese man with a 28-year history of type 1 diabetes. He visited our hospital monthly for management of diabetes with intensive therapy employing multiple-dose insulin injections. His height and body weight were 168 cm and 52 kg (body mass index: 18.4 kg/m2), respectively. He showed depleted insulin secretion (serum C-peptide level was below the limit of detection), such that his blood glucose levels fluctuated severely, and his hemoglobin A1c (HbA1c) level was around 9.0% despite intensive insulin therapy. He had been diagnosed with asymptomatic chronic severe (grade III) aortic regurgitation (AR) 16 years before the current presentation but had declined follow-up for the AR. He had never undergone surgery nor the implantation of any prosthetic devices.
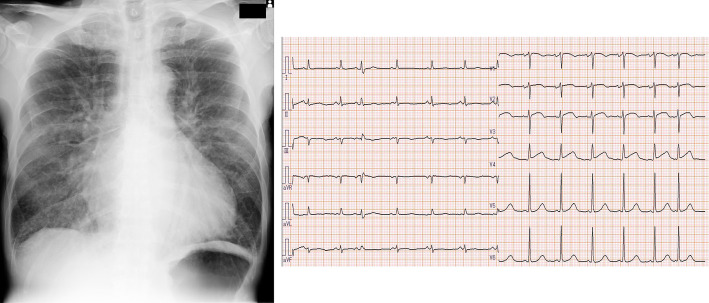
Eight days after his regular hospital visit, he visited an emergency clinic complaining of breathing difficulty and had a fever above 38℃. Until that day, he had not noticed any fever, chills, weakness, or any other symptoms. His blood pressure and pulse rate were 192/82 mmHg and 118/min, respectively. He showed orthopnea, and his oxygen saturation (SpO2) was 80%. He was transported to the emergency department of our hospital. A physical examination revealed a Levine 3/6 systolic murmur, although his cardiac murmur had not been checked at regular hospital visits. No physical findings suggesting IE, such as Osler nodes, Janeway lesions, or conjunctival petechiae, were recognized. His white blood cell (WBC) count was markedly increased to 20,800 /μL, and his C-reactive protein (CRP) was elevated to 6.06 mg/dL. Serum creatine phosphokinase MB was within the normal range, at 6.0 IU/L, and troponin T was negative (Table 1). Chest X-ray (Fig. 1) showed pulmonary congestion with cardiac enlargement (cardiothoracic ratio: 55%). Electrocardiography (Fig. 1) revealed ST elevation on V1-V4, but emergency echocardiography showed no dysfunction of cardiac contractility. He was diagnosed with acute heart failure due to valvular disease, and treatment with non-invasive positive pressure ventilation and nitrates was initiated.
Table 1.
Laboratory Data on Admission to Our Hospital.
| WBC | 20,800 | cells/µL | TP | 6.7 | g/dL | ||
| Neutrophils | 86.6 | % | Alb | 3.1 | g/dL | ||
| Eosinophils | 0.8 | % | CK | 66 | U/L | ||
| Basophils | 0.5 | % | CK-MB | 6 | U/L | ||
| Lymphocytes | 8.5 | % | Na | 135 | mmol/L | ||
| Monocytes | 3.6 | % | K | 4.7 | mmol/L | ||
| RBC | 4.32×106 | cells/µL | Cl | 102 | mmol/L | ||
| Hemoglobin | 13.7 | g/dL | Glucose | 171 | mg/dL | ||
| Hematocrit | 40.0 | % | Troponin T | (-) | |||
| PLT | 372×103 | cells/µL | BNP | 271.5 | pg/mL | ||
| CRP | 6.06 | mg/dL | |||||
| T-Bil | 1.2 | mg/dL | |||||
| AST | 178 | IU/L | PT | 82 | % | ||
| ALT | 93 | IU/L | APTT | 42.7 | seconds | ||
| LDH | 542 | IU/L | Fibrinogen | 383 | mg/dL | ||
| ALP | 576 | IU/L | FDP | 4.4 | µg/mL | ||
| γ-GTP | 173 | IU/L | D-dimer | 2.2 | µg/mL | ||
| Amylase | 49 | IU/L | |||||
| BUN | 16 | mg/dL | |||||
| Creatinine | 0.63 | mg/dL |
WBC: count of white blood cells, RBC: count of red blood cells, Hb: hemoglobin, PLT: count of platelets, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: gamma glutamyl transferase, BUN: blood urea nitrogen, TP: total protein, Alb: albumin, CK: creatine kinase, CK-MB: creatine phosphokinase MB, BNP: brain natriuretic peptide, CRP: C-reactive protein, PT: prothrombin time, APTT: activated partial thromboplastin time, FDP: fibrin degradation products
Figure 1.
Chest X-ray and electrocardiography findings on admission to our hospital.
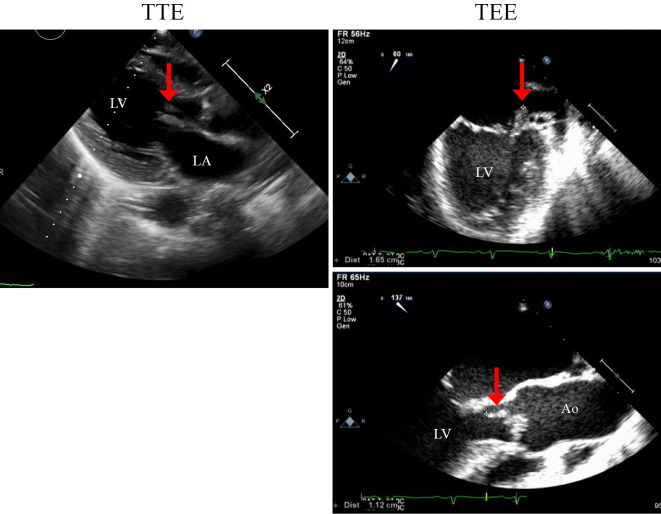
After hospital admission, a detailed examination by transthoracic echocardiography showed severe aortic regurgitation, severe mitral regurgitation, and a mobile vegetation on the mitral valve. Transesophageal echocardiography revealed a 16.5×6-mm mobile vegetation on the anterior leaflet of the mitral valve and an 11.2×5-mm nonmobile vegetation on the noncoronary cusp of the aortic valve (Fig. 2). These findings raised strong suspicion of NVE. In this case, head computed tomography (CT) and magnetic resonance imaging revealed no cerebral infarction or hemorrhaging, although a mobile vegetation was detected.
Figure 2.
Transthoracic (TTE) and transesophageal (TEE) echocardiograms. Red arrows indicate the vegetations on the valves.
On reviewing the clinical course until hospitalization (Table 2), we noted that at the visit four months before admission, his WBC count had been slightly elevated. The following month, his albumin (Alb) level decreased to 3.0 g/dL, and his hemoglobin (Hb) level had shown a gradual decline over the 2 months prior to admission. During this period, he had experienced a 4-kg weight loss. Esophagogastroduodenoscopy and whole-body CT were performed, but no abnormalities were detected. One month later, he had regained some weight, and the laboratory findings had nearly normalized, except for a slightly elevated CRP level (0.54 mg/dL). At the last visit (8 days before admission), his WBC count had again risen to 9,300 /μL, while his Hb and Alb levels had again decreased to 13.1 g/dL and 3.0 g/dL, respectively. Furthermore, his CRP level had increased to 4.18 mg/dL. At that time, his diastolic blood pressure has shown an obvious decrease (Table 2). Thus far, he had not experienced a fever or any symptoms other than weight loss. We suspected diseases of infectious and/or malignant origin and initiated comprehensive examinations to identify the source of his clinical findings.
Table 2.
Time Courses of Body Weight, Blood Pressure, and Laboratory Data before Admission.
| Months | -7 | -6 | -5 | -4 | -3 | -2 | -1 | 0 (last regular visit before admission) |
|---|---|---|---|---|---|---|---|---|
| Body weight (kg) | 53 | 54 | 52 | 52 | 52 | 48 | 52 | 53 |
| Blood Pressure (mmHg) | 113/67 | 132/64 | 118/77 | 126/70 | - | 126/69 | - | 127/53 |
| WBC (cells/µL) | 4,100 | 5,700 | 5,400 | 9,300 | 10,600 | 8,300 | 6,600 | 9,300 |
| CRP (mg/dL) | - | - | - | - | - | - | 0.54 | 4.18 |
| Hb (g/dL) | 15.0 | 14.5 | 15.2 | 14.5 | 13.1 | 12.8 | 14.0 | 13.1 |
| Alb (g/dL) | 3.6 | 3.7 | 3.8 | 3.6 | 3.0 | 3.1 | 3.5 | 3.0 |
| HbA1c (%) | 9.7 | 9.4 | 9.0 | 8.6 | 8.5 | 8.6 | 8.3 | 8.7 |
WBC: count of white blood cells, CRP: C-reactive protein, Hb: hemoglobin, Alb: albumin, HbA1c: hemoglobin A1c
After heart failure treatment had been started, his clinical symptoms showed rapid improvement, and his hemodynamic stability was maintained during the first six hours. He initially received empirical intravenous antibiotic therapy consisting of 12 g/day of ampicillin sulbactam (ABPC/S) and 120 mg/day of gentamycin (GM). Three blood culture sets were obtained on the admission, and all were positive for S. warneri [minimum inhibitory concentration (MIC) to ABPC/S ≤8 μg/mL; MIC to GM ≤1 μg/mL; MIC to cefazolin (CEZ) ≤2 μg/mL]. Thus, IE caused by this organism was diagnosed.
According to the clinical guideline established by the Japanese Circulation Society, emergency surgery is generally recommended for heart failure of NYHA III to IV or urgent surgery for NVE mobile vegetation exceeding 10 mm and severe valve dysfunction (26). In this case, however, his heart failure was successfully improved. Based on the guideline (26), the risk of embolism was considered to have been reduced by the administration of appropriate antibiotic therapy. In addition, the patient had type 1 diabetes, and his glycemic control was so poor that we were concerned that double-valve surgery would be a high-risk procedure. Therefore, we planned elective surgery after sufficient control of both infection and diabetes.
Based on the blood culture results, the antibiotic regimen was switched to 6 g/day of CEZ. A detailed dental examination revealed no abnormalities, such as periodontitis. After four weeks of antibiotic therapy, he underwent surgical therapy. His aortic valve was found to be bicuspid, and the aortic and mitral annuli were intact without abscess formation. Large vegetations were exenterated, and the mitral and aortic valves were both replaced with mechanical valves. He experienced no postoperative complications and was discharged on the 22nd day after the operation without apparent embolism. He has not had any recurrence in over two years since the operation.
Discussion
We encountered a case with NVE caused by S. warneri, a member of the CoNS family. Prior to the manifestation of acute heart failure in this patient, he had had no obvious symptoms suggesting IE, such as a fever, chills, sweating, or chest pain, aside from mild and temporary weight loss and laboratory abnormalities. Two chronic factors-blood glucose fluctuation due to poorly controlled type 1 diabetes and regurgitation caused by a bicuspid aortic valve (BAV)-may have contributed to the development of NVE by low-virulence resident bacteria, which rarely cause this disease.
The pathogens commonly causing NVE are S. aureus and viridans group streptococci (14). However, while CoNS are a common cause of PVE (14), they are only rarely associated with NVE (9-11). CoNS, represented by Staphylococcus epidermidis (S. epidermidis), colonize the skin and mucous membranes of humans and animals. Notably, making a diagnosis of NVE caused by CoNS often takes longer than diagnosing NVE due to S. aureus because of the indolent course of this disease, at least in the early stage, and frequent failure to recognize blood culture results as true positives rather than as contaminants (13). Furthermore, CoNS, as a pathogen of IE, is associated with higher rates of requiring surgical treatments than S. aureus and viridans group streptococci, and of severe outcomes, such as persistent bacteremia, congestive heart failure, and mortality, than viridans group streptococci (12, 13). Therefore, it is important to recognize this disease early in the clinical course and to initiate therapies promptly.
In the present case, slight laboratory data abnormalities and weight loss had been noted four months before the heart failure symptoms manifested. We cannot rule out the possibility that our patient had contracted the S. warneri infection immediately prior to hospitalization. However, considering that CoNS-induced IE was reported to usually show an asymptomatic exacerbation over time, we speculate that a chronic S. warneri infection had been present during the four-month period.
S. warneri is a member of the CoNS family and initially colonizes the human skin surface during the first few days or weeks of life, as does S. epidermidis (14). Compared with S. epidermidis, S. warneri very rarely causes clinical issues and is usually considered to be disease-causing only in patients with a compromised immune system or with prosthetic devices (16, 17). Indeed, IE, especially NVE, caused by S. warneri is very rare. We searched the PubMed database for cases of IE caused by S. warneri and found only 10 cases with IE due to this organism (16-25) (Table 3). Three cases had prosthetic valves (20, 22, 24), and another four had a history of undergoing surgery (18), medical device implantations (19, 21), or trauma (23). The case reported by Kamath et al. had liver cirrhosis (16). Two cases did not have any apparent infection route, as in our case (17, 25). One had degenerative valve disease (25), while the other had no known background diseases (17). In these two cases, age was considered to have been a risk factor for NVE caused by S. warneri. Our case was not of highly advanced age (59 years old), and he had no history suggesting an invasive bacterial route. Therefore, severe blood glucose fluctuation due to type 1 diabetes and regurgitation associated with BAV were considered to have been the risk factors for the rare and severe infectious disease of NVE due to CoNS in this patient.
Table 3.
Review of Previous Cases with Endocarditis Caused by Staphylococcus Warneri.
| Reference | Age/Gender | Background disease | Prosthetic valve | Past history indicating possible bacterial invasion route | Time from contributing factor to diagnosis | Valve involved |
|---|---|---|---|---|---|---|
| 18 | 32/M | - | - | Vasectomy, Epididymitis | 2 months | Aortic |
| 19 | 66/M | - | - | Hip replacement | 1 year | Aortic and Mitral |
| 16 | 64/M | Liver cirrhosis | - | No information | No information | Aortic, Mitral and Pulmonary |
| 20 | 71/M | Rheumatic aortic stricture | + | AVR | 5 days | Aortic |
| 21 | 48/M | - | - | Disc prosthesis | 2 years | Aortic |
| 17 | 78/F | - | - | - | - | Mitral |
| 22 | 43/F | AR | + | AVR, Dental extraction, Mammaplasty, IE | 3 months | Aortic |
| 23 | 59/M | Right-sided nephrectomy | - | Scalp laceration | 2 weeks | Mitral |
| 24 | 67/M | Ischemic stroke | + | AVR, CABG | 7 months | Aortic |
| 25 | 79/M | Degenerative valvular disease | - | - | - | Mitral |
| current case | 59/M | T1DM BAV | - | - | 4 months (time from signs of IE) | Aortic and Mitral |
M: male, F: female, AVR: aortic valve replacement, AR: aortic regurgitation, IE: infective endocarditis, CABG: coronary artery bypass grafting, T1DM: type 1 diabetes mellitus, BAV: bicuspid aortic valve
Diabetes mellitus is well known to be a risk factor for infectious disease development (1-5). Patients using insulin reportedly have a higher risk of hospitalization for infectious disease (27) and higher mortality due to IE (28). A recent cohort study showed patients with type 1 diabetes to be at an increased risk for serious infections like endocarditis (8). These findings suggest that the severity of diabetes is involved in the outcomes of serious infectious diseases. BAV is the most common congenital heart defect, with an estimated prevalence between 0.5% and 2% (29, 30). Earlier case series showed the prevalence of IE in patients with BAV to range from 10% to 30% (31). A recent population-based follow-up study also demonstrated the relative risk of IE in patients with BAV to be 16.9 as compared with the general population (32). However, two cohort studies showed the prevalence of IE in patients with BAV to be only about 2%, which was similar to that in normal subjects (29, 33). Although the IE risk with BAV remains a controversial topic, regurgitation caused by BAV might have been involved in the IE development in our present case, as aortic regurgitation is a risk factor for IE (34).
This case illustrates two clinically important lessons. First, low-virulence resident bacteria can cause NVE. The chronic factors of severe blood glucose fluctuations due to poorly controlled type 1 diabetes and regurgitation caused by BAV may both have contributed to the development of NVE, a disease rarely induced by S. warneri. The second lesson is that the prodromal symptoms were very mild but likely started four months before overt heart failure manifested. Since patients with IE induced by such resident bacteria reportedly have poor outcomes, we must carefully follow patients with poorly controlled diabetes in terms of the development of infectious diseases, including IE. Careful physical examinations, including chest auscultation and dermal and conjunctival findings, may prompt clinicians to recognize signs of pathological progression. In particular, mild prolonged symptoms and laboratory data abnormalities that do not necessarily suggest infectious diseases might be signs of impending IE.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Lepper PM, Ott S, Nuesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344: e3397, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allard R, Leclerc P, Tremblay C, Tannenbaum TN. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 33: 1491-1493, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hulme KD, Gallo LA, Short KR. Influenza Virus and Glycemic Variability in Diabetes: A Killer Combination? Front Microbiol 8: 861, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fu AZ, Iglay K, Qiu Y, Engel S, Shankar R, Brodovicz K. Risk characterization for urinary tract infections in subjects with newly diagnosed type 2 diabetes. J Diabetes Complications 28: 805-810, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 23: 3-13, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Hoen B, Duval X. Clinical practice. Infective endocarditis. N Engl J Med 368: 1425-1433, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of infectious endocarditis in patients with type II diabetes mellitus. J Diabetes Complications 21: 403-406, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 41: 513-521, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA 320: 72-83, 2018. [DOI] [PubMed] [Google Scholar]
- 10. Vogkou CT, Vlachogiannis NI, Palaiodimos L, Kousoulis AA. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis 35: 1227-1245, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 387: 882-893, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Chu VH, Cabell CH, Abrutyn E, et al. Native valve endocarditis due to coagulase-negative staphylococci: report of 99 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 39: 1527-1530, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Chu VH, Woods CW, Miro JM, et al. Emergence of coagulase-negative staphylococci as a cause of native valve endocarditis. Clin Infect Dis 46: 232-242, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev 27: 870-926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hira V, Kornelisse RF, Sluijter M, et al. Colonization dynamics of antibiotic-resistant coagulase-negative staphylococci in neonates. J Clin Microbiol. J Clin Microbiol 51: 595-597, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamath U, Singer C, Isenberg HD. Clinical significance of Staphylococcus warneri bacteremia. J Clin Microbiol 30: 261-264, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kini GD, Patel K, Parris AR, Tang JS. An unusual presentation of endocarditis caused by Staphylococcus warneri. Open Microbiol J 4: 103-105, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dan M, Marien GJ. Goldsand G. Endocarditis caused by Staphylococcus warneri on a normal aortic valve following vasectomy. Can Med Assoc J 131: 211-213, 1984. [PMC free article] [PubMed] [Google Scholar]
- 19. Wood CA, Sewell DL, Strausbaugh LJ. Vertebral osteomyelitis and native valve endocarditis caused by Staphylococcus warneri. Diagn Microbiol Infect Dis 12: 261-263, 1989. [DOI] [PubMed] [Google Scholar]
- 20. Abgrall S, Meimoun P, Buu-Hoi A, Couetil JP, Gutmann L, Mainardi JL. Early prosthetic valve endocarditis due to Staphylococcus warneri with negative blood culture. J Infect 42: 166, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Stollberger C, Wechsler-Fordos A, Geppert F, et al. Staphylococcus warneri endocarditis after implantation of a lumbar disc prosthesis in an immunocompetent patient. J Infect 52: e15-e18, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Arslan F, Saltoglu N, Mete B, Mert A. Recurrent Staphylococcus warnerii prosthetic valve endocarditis: a case report and review. Ann Clin Microbiol Antimicrob 10: 14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhardwaj B, Bhatnagar UB, Conaway DG. An Unusual presentation of native valve endocarditis caused by Staphylococcus warneri. Rev Cardiovasc Med 17: 140-143, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Kuvhenguhwa MS, Belgrave KO, Shah SU, Bayer AS, Miller LG. A case of early prosthetic valve endocarditis caused by Staphylococcus warneri in a patient presenting with congestive heart failure. Cardiol Res 8: 236-240, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diaconu R, Golumbeanu E, Constantin A, Donoiu I. Native valve endocarditis with Staphylococcus warneri. BMJ Case Rep 12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakatani S, Ohara T, Ashihara K, et al. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ J 83: 1767-1809, 2019. [DOI] [PubMed] [Google Scholar]
- 27. Donnelly JP, Nair S, Griffin R, et al. Association of diabetes and insulin therapy with risk of hospitalization for infection and 28-day mortality risk. Clin Infect Dis 64: 435-442, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duval X, Alla F, Doco-Lecompte T, et al. Diabetes mellitus and infective endocarditis: the insulin factor in patient morbidity and mortality. Eur Heart J 28: 59-64, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 117: 2776-2784, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 55: 2789-2800, 2010. [DOI] [PubMed] [Google Scholar]
- 31. Ward C. Clinical significance of the bicuspid aortic valve. Heart 83: 81-85, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michelena HI, Katan O, Suri RM, Baddour LM, Enriquez-Sarano M. Incidence of infective endocarditis in patients with bicuspid aortic valves in the community. Mayo Clin Proc 91: 122-123, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 300: 1317-1325, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Michel PL, Acar J. Native cardiac disease predisposing to infective endocarditis. Eur Heart J 16(Suppl B): 2-6, 1995. [DOI] [PubMed] [Google Scholar]