Abstract
Recently, immune checkpoint inhibitors (iCIs) have been used to treat cancers. Once some of the iCIs for the treatment of hepatocellular carcinoma (HCC) are certified in clinical trials, they are likely be administered to HCC patients with hepatitis C virus (HCV). However, the immunopathogenesis of HCV after the administration of iCIs has not been clarified. We experienced a lung cancer patient with HCV infection treated by nivolumab, programmed cell death 1 (PD-1) antibody. HCV-RNA gradually decreased after the start of nivolumab treatment. However, no increase in transaminase was observed during the decline of HCV-RNA. It was thought that HCV-specific cytotoxic T lymphocytes (CTLs) were activated by iCIs.
Keywords: iCIs, nivolumab, PD-1, CTLs
Introduction
Recently, immune checkpoint inhibitors (iCIs) have been used for the treatment of various kinds of cancers (1-11). In addition, clinical trials of various kinds of iCIs for hepatocellular carcinoma (HCC) are underway. It has been reported that some of them may have good anti-tumor effects (12). Once some of the iCIs for the treatment of HCC are verified in clinical trials, iCIs are likely to be administered to HCC patients with viral hepatitis B (HBV) and/or C. However, there have been few patients with chronic viral hepatitis, since patients with viral hepatitis have been considered ineligible due to the exclusion criteria for iCIs clinical trials (13).
The immunopathogenesis of hepatitis virus after the administration of iCIs has not been clarified. Recently, another group reported that hepatitis C virus (HCV)-RNA could be decreased by iCIs (12). However, the relationship between the anti-tumor effects and the anti-viral effects after the administration of iCIs has not been clarified.
We herein report the case of a chronic hepatitis C (CH-C) patient who achieved a reduction of the viral load without alanine transaminase (ALT) elevation after receiving iCIs.
Case Report
A 75-year-old man with advanced squamous cell lung cancer, cStageIVB [T2aN3M1c (BRA)] was found to have HCV infection at the diagnosis of lung cancer. However, since we considered the treatment of HCV infection not to affect the prognosis of this patient, we decided to start treatment while carefully monitoring the liver damage and HCV viral load. The HCV-RNA titer was first evaluated in June 20XX, and the patient showed 4.5 log IU/mL HCV RNA. The genotype of HCV was 1b.
The patient started receiving nivolumab as a third-line treatment in October 20XX+1 (first-line was carboplatin+nab-paclitaxcel). Transaminase levels before nivolumab treatment were within the normal range. Among the tumor markers, CYFRA was high at 5.1 ng/mL. The clinical data of this case are summarized in Table. HCV-RNA gradually decreased after the start of nivolumab treatment.
Table.
Blood Test at First Visit.
| Factor (normal range) | Values | |
|---|---|---|
| WBC (3,000-9,500) | 6,200 | /μL |
| Hb (13-17) | 16.3 | g/dL |
| Plt (15-38) | 12.4×104 | /μL |
| T-Bil (0.23-1.28) | 0.51 | mg/dL |
| AST (8-40) | 28 | U/L |
| ALT (4-42) | 34 | U/L |
| ALP (105-340) | 215 | U/L |
| γ-GTP (0-78) | 44 | U/L |
| BUN (8-20) | 11.6 | mg/dL |
| Cre (0.4-1.1) | 0.69 | mg/dL |
| ALB (3.8-5.3) | 3.5 | g/dL |
| PT-INR | 0.94 | |
| M2BPGi (0-0.9) | 3.73 | |
| Cryoglobulin | (-) | |
| HCV antibody | (+) | |
| HCV-RNA (0-1.0) | 4.5 | log IU/mL |
| HCV genotype | 1b | |
| CYFRA (0-3.5) | 6.2 | ng/mL |
| AFP (0-10.0) | 2.3 | mg/mL |
| CEA (0.1-5.0) | 3.0 | |
The patient had 4.5 log IU/mL HCV RNA. The genotype of HCV was 1b. Transaminase before Nivolumab treatment were within normal range. Among the tumor markers, only CYFRA was high, which was 6.2 ng/mL.
WBC: White Blood Cell, Hb: Hemoglobin, PLT: platelet, T-Bil: total billirubin, AST: Aspartate transaminase, ALT: alanine aminotransferase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, Cre: Creatinine, ALB: albumin, PT-INR: prothrombin time-International normalized ratio, M2BPGi: Mac-2 binding protein, HCV: hepatitis C virus, CYFRA: cytokeratin subunit 19 fragment, AFP: alpha fetoprotein, CEA: Carcinoembryonic antigen
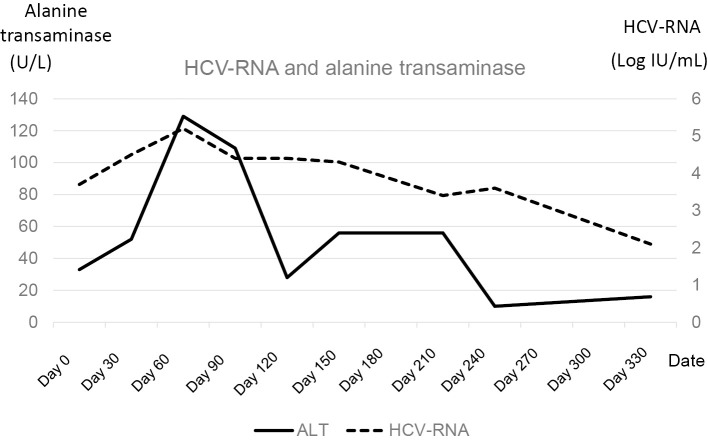
However, no increase in transaminase levels was observed during the decline of HCV-RNA (Fig. 1). Two months after starting nivolumab monotherapy, the patient suffered from radiation pneumonitis. After that, his general condition worsened, and nivolumab treatment was discontinued.
Figure 1.
The sequential data of HCV-RNA and transaminase. The dotted line indicates the titer of HCV-RNA. The solid line indicates the serum amount of ALT. HCV-RNA declined without ALT elevation. After two months of administration, nivolumab was discontinued. HCV: hepatitis C virus, ALT: alanine transaminase
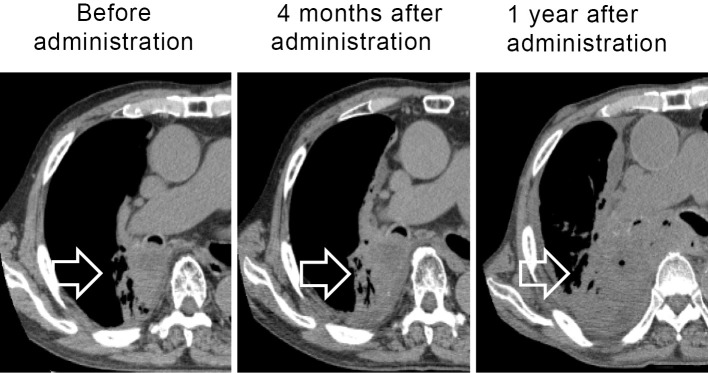
HCV-RNA titers decreased after the discontinuation of nivolumab treatment, reaching 2.1 log IU/mL at 1 year after the start of nivolumab administration. The patient did not suffer any adverse effects from nivolumab. The primary lesion was stable until four months after the start of nivolumab administration (two months after discontinuation of nivolumab administration). However, tumor progression was observed at one year after the start of nivolumab administration (Fig. 2). HCV-RNA titers continued to decrease, regardless of the tumor progression.
Figure 2.
Plain computed tomography (CT) scanning of lung cancer (primary lesion). Three time points of CT scanning for lung cancer are shown. The arrow indicates the main tumor in the right S6 lesion extending to the right main bronchus. The primary lesion was stable until four months after starting nivolumab administration (two months after the discontinuation of nivolumab administration). One year after starting treatment, the tumor was judged to be progressive disease.
Discussion
In this case, there were two important findings. First, HCV-RNA titers were decreased without liver damage after the start of nivolumab treatment. Second, the decline in the HCV-RNA titers was observed regardless of the anti-tumor effect. An increase in ALT can reportedly achieve good response during interferon/ribavirin treatment in CH-C patients, since the immune response might contribute to the eradication of HCV-infected hepatocytes (14). In contrast, an excessive immune response to HCV might induce liver damage. Some patients infected with viruses, such as with HCV, HBV and human immunodeficiency virus (HIV), might have exhausted cytotoxic T lymphocytes (CTLs) and therefore be unable to eradicate viruses (15-17). One important mechanism underlying CTL exhaustion is the programmed cell death 1 (PD-1)/programmed cell death ligand 1 pathway (18). In the present case, nivolumab, anti-PD-1 antibody, might recover the exhausted HCV-specific CTLs. It has been reported that the administration of immune checkpoint blockers to HIV-infected individuals on antiretroviral therapy might facilitate latency disruption (19). Another group also reported the safety with an efficacy signal during treatment of PD-1 inhibitor for cancers with HIV (20). In a metastatic melanoma patient, nivolumab might induce inflammation of seborrheic keratoses by PD-1 inhibition reactivating virally driven T lymphocytes (21). Furthermore, CTLs can control viruses non-cytotoxically via interferon-γ production (22). Therefore, the viral load may have been decreased without liver damage by HCV-specific CTLs in this case.
The other important finding was that the HCV-RNA still decreased even as the lung cancer progressed. It was previously reported that adverse events appeared regardless of cancer progression. Furthermore, adverse events can appear at any time after the start of iCI administration (23). It was believed that HCV-specific CTLs were activated by iCIs, and this activation was sustained even after the discontinuation of iCI administration. In the present case, lung cancer-antigen specific CTLs might not have been activated, or else the activation of the lung cancer-antigen specific CTLs was not sufficient to suppress the cancer progression due to various kinds of immune suppressive reactions. However, HCV-specific CTLs were able to be activated by nivolumab, and the activation of HCV-specific CTLs persisted even after the discontinuation of nivolumab administration.
Our literature search failed to turn up any papers describing the changes in liver enzyme levels and tumor progression during the decline in the HCV-RNA titer. iCIs reduced HCV-RNA titers without causing liver damage in this case. Furthermore, the HCV-RNA also continued to decrease even as the lung cancer progressed. When administering iCIs to HCV-infected patients with cancer, periodic testing of the RNA level should be done. The above phenomenon might also occur in chronic viral diseases other than HCV. When we administer iCIs for such viral antigens as HBV, we should evaluate the liver damage carefully.
In conclusion, we experienced a case of CH-C with a decline in HCV-RNA after the administration of anti-PD-1 antibody. The characteristics of such cases and the progression of other chronic viral diseases need to be explored through the accumulation of further cases.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sport, Science and Technology of Japan (Y.K. #16K09335 and #19K08374) and Grant-in-Aid from the Ministry of Health, Labour and Welfare.
Ryo Fukuda and Yasuteru Kondo contributed egually to this work.
References
- 1. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 123: 1904-1911, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolchok JD. PD-1 Blockers. Cell 162: 937, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315: 1600-1609, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711-723, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823-1833, 2016. (in eng). [DOI] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627-1639, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803-1813, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372: 311-319, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17: 1590-1598, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol 35: 1542-1549, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med 374: 2542-2552, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389: 2492-2502, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davar D, Wilson M, Pruckner C, Kirkwood JM. PD-1 blockade in advanced melanoma in patients with hepatitis C and/or HIV. Case Rep Oncol Med 2015: 737389, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thurairajah PH, Thorburn D, Hubscher S, et al. Incidence and characterization of serum transaminases elevations in pegylated interferon and ribavirin treated patients with chronic hepatitis C. Aliment Pharmacol Ther 25: 1293-1300, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Luxenburger H, Neumann-Haefelin C, Thimme R, Boettler T. HCV-specific T cell responses during and after chronic HCV infection. Viruses 10: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondo Y, Machida K, Liu HM, et al. Hepatitis C virus infection of T cells inhibits proliferation and enhances fas-mediated apoptosis by down-regulating the expression of CD44 splicing variant 6. J Infect Dis 199: 726-736, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kondo Y, Ueno Y, Kakazu E, et al. Lymphotropic HCV strain can infect human primary naive CD4+ cells and affect their proliferation and IFN-gamma secretion activity. J Gastroenterol 46: 232-241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahn E, Youngblood B, Lee J, Lee J, Sarkar S, Ahmed R. Demethylation of the PD-1 promoter is imprinted during the effector phase of CD8 T cell exhaustion. J Virol 90: 8934-8946, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fromentin R, DaFonseca S, Costiniuk CT, et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4(+) T cells from ART-suppressed individuals. Nat Commun 10: 814, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spano JP, Veyri M, Gobert A, et al. Immunotherapy for cancer in people living with HIV: safety with an efficacy signal from the series in real life experience. Aids 33: F13-f19, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Rambhia PH, Honda K, Arbesman J. Nivolumab induced inflammation of seborrheic keratoses: a novel cutaneous manifestation in a metastatic melanoma patient. Melanoma Res 28: 475-477, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 5: 215-229, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51-60, 2016. [DOI] [PubMed] [Google Scholar]