Abstract
Background: Papillary thyroid cancer (PTC) is a very common malignant disease with high morbidity. We needed some pretreatment indicators to help us predict prognosis and guide treatment. We conducted a study about some pretreatment prognostic indicators.
Methods: This clinical study recruited 705 postoperative PTC patients (211 males, 494 females). Clinical data before radioactive iodine (RAI) treatment were collected. Patients’ response to therapy were classified into two categories: ‘Good Prognosis Group’ (GPG) and ‘Poor Prognosis Group’ (PPG), according to ‘2015 American Thyroid Association Guidelines’. Differences of indicators between different prognosis groups were compared. Odds ratios (ORs) were calculated by univariate/multiple binary logistic regression models. Difference of body mass index (BMI) changes before and after RAI treatment between different prognosis groups was also compared.
Results: A total of 546 (77.45%) belonged to GPG, and 159 (22.55%) belonged to PPG. Platelet (PLT), neutrophil (NEUT), PLT subgroups, and combination of red blood cell distribution width (RDW) and BMI (COR-BMI) were different between two prognosis groups. The significance of the difference between the two groups of BMI disappeared after the Bonferroni correction. PLT and PLT subgroups had detrimental effects on the risk of PPG; T stage had a positive effect on the risk of PPG. PLT subgroup showed a detrimental effect on the risk of PPG when we included additional covariates.
Conclusions: We found that lower pretreatment PLT levels may indicate a poor prognosis for PTC. The relationship between platelet-derived growth factor (PDGF) and radiation sensitivity may be the key to this association.
Keywords: Papillary thyroid cancer (PTC), Prognosis, Platelet (PLT)
Introduction
Thyroid cancer is the most common endocrine system malignant disease, accounting for 90% of all endocrine cancer cases [1,2]. In the past few decades, the incidence of thyroid cancer has risen rapidly in many countries in the world, including China [3,4]. The incidence in Korea has increased the most: the incidence among people 15–79 years of age (standardized to the world population) increased from 12.2 per 100000 in 1993–1997 to 59.9 per 100000 in 2003–2007 [1]. This rapid growth has caused widespread public concern about thyroid cancer. Thyroid cancer can be classified according to its histopathological characteristics, mainly including papillary thyroid cancer (PTC), follicular thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer (ATC) [5]. Among all pathological types of thyroid cancer, PTC is the most common and least invasive type of histology, contributing to the highest morbidity increase [1,3,4,6]. For most patients, total thyroidectomy, ablation of tumor remnants by radioactive iodine (RAI) therapy and thyroid stimulating hormone (TSH) suppression therapy are the main steps in the treatment of PTC [7]. After this standardized treatment process, patients presented different outcomes: some patients were cured or stable; some patients’ disease progressed, and some patients were even distant metastasized. For the difference in patient prognosis, some indicators that were predictive of prognosis needed to be proposed to help us predict prognosis and guide treatment [8,9]. The goal of this strategy was to detect recurrent disease early, identify patients who would benefit from further treatment, and reduce over-investigation of low-risk patients.
Several factors can predict the prognosis of PTC patients [10,11]. In clinical practice, the most commonly used prognostic predictor of PTC patients is the 2015 American Thyroid Association (ATA) staging system, but its predictive power is still far from perfect [12,13]. The initial risk stratification system may not be able to accurately predict persistent diseases and recurrence during the follow-up. The prognosis of cancer patients is closely related to the response to treatment. Some gene mutations, such as proto-oncogene B-Raf (BRAF) and telomerase reverse transcriptase (TERT), have been found in more invasive subsets of PTC [14,15]. However, these gene mutations are rarely used in routine clinical practice because of their high cost and limited availability. Therefore, it is necessary to determine effective and reliable clinical prognostic parameters.
Chronic inflammation usually occurs in malnourished patients and is associated with poor prognosis [16,17]. Nutritional index and inflammatory markers or inflammation-based prognostic scores may be reliable, practical prognostic tools for a variety of malignancies, including PTC. Studies have shown that higher red blood cell distribution width (RDW) and lower body mass index (BMI) are markers of malnutrition and chronic inflammation in cancer patients [18,19]. Combination of RDW and BMI (COR-BMI) has been shown to be an important independent prognostic factor affecting tumor-specific survival in patients with laryngeal squamous cell carcinoma and nasopharyngeal carcinoma (NPC) [18,19]. Recent publications have suggested that platelet (PLT) count is related to prognosis in many cancers [20,21]. In addition, some inflammatory markers, such as neutrophil–lymphocyte ratio (NLR), macrophages, and tumor-infiltrating mast cells, have been reported to be associated with a poor prognosis in PTC [22–24].
However, the ability of some predictions in PTC has not been well studied. Therefore, the purpose of the present study was to investigate the ability of these indicators to predict prognosis in patients with PTC.
Materials and methods
Design
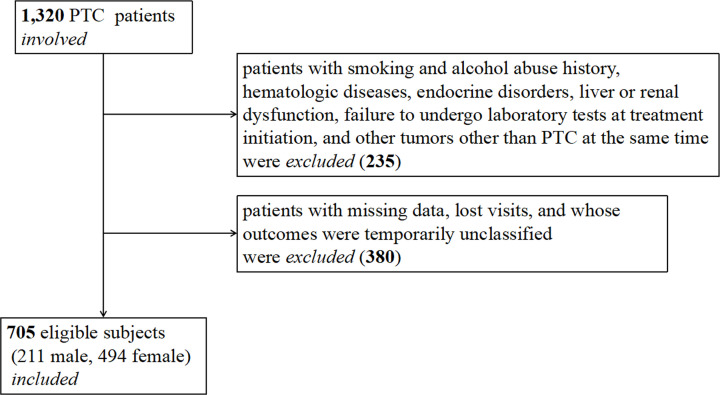
This clinical study was conducted at the Department of Nuclear Medicine, General Hospital of Tianjin Medical University in China. From August 2008 to August 2018, a total of 1320 PTC postoperative patients, who were scheduled to receive RAI therapy in the nuclear medicine ward, participated in the present study; refer to our previous researches [25–30]. All participants were asked to complete a questionnaire about medical history, lifestyle, alcohol intake, and smoking. To avoid the influence of confounding factors, the exclusion criteria were smoking and alcohol abuse history, hematologic diseases, endocrine disorders, liver or renal dysfunction, failure to undergo laboratory tests before RAI therapy, and tumors other than PTC at the same time. In addition, patients with missing data, lost visits, and whose outcomes were temporarily unclassified were excluded. Finally, 705 eligible subjects (211 males, 494 females) were included. The screening process of the patients is shown in Figure 1.
Figure 1. Patient screening process.
Measurements
Demographics, anthropometric measurements, and peripheral blood indicators were collected from all subjects on the first day of their hospitalization, before RAI therapy. Fasting blood samples were obtained between 7 and 10 a.m. Height and weight were measured in centimeters and kilograms, respectively. BMI was calculated by dividing weight (kg) by height squared (m2). TSH and thyroglobulin (Tg) were analyzed on a fully automated ADVIA Centaur Analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany) based on a chemiluminescent reaction principle. Hemoglobin (Hg), Neutrophil (NEUT), lymphocyte (LBC), PLT, plateletcrit (PCT), mean platelet volume (MPV), platelet distribution width (PDW), RDW, and albumin (ALB) were determined enzymatically by an auto-analyzer (Hitachi Model 7600 analyzer, Hitachi, Tokyo, Japan). These variables were selected for potential relationship to the prognosis of PTC based on previous studies. Follow-up data on patient BMI changes were collected based on patient outpatient review.
Surgical specimens were microscopically examined by two or more experienced pathologists who assessed the following histopathological factors: the histological type of the primary lesion, the size of the primary tumor (measuring the longest diameter of the largest lesion), location, multifocal, extrathyroidal extension, lymphatic invasion, marginal involvement, lymph node metastasis, and potential thyroid disease such as chronic lymphocytic thyroiditis. The pathological variants other than conventional PTC included: follicular variant of PTC, oncocytic variant of PTC, diffuse sclerosing variant of PTC, tall cell variant of PTC, columnar cell variant of PTC, and solid variant of PTC. Post-treatment/Diagnostic whole-body RAI scans were performed by SPECT/CT (Discovery NM/CT 670, GE Healthcare, Chicago, America).
Definitions
For the RDW, a cutoff of 13.25 was generated according to the receiver operating characteristic (ROC) analysis in the training set for CSS (sensitivity 71.8%, specificity 33.3%, area under the curve [AUC] 0.554, 95% confidence interval [CI] 0.504–0.64, P<0.001). RDW values were categorized into two groups: RDW ≤ 13.25 and RDW > 13.25 (%). Similarly, NLR values were categorized into two groups: NLR ≤ 2.23 and NLR > 2.23. PLT values were categorized into two groups: PLT ≤ 302 and PLT > 302 (×109/l). It has been suggested that the BMI cut-off point for overweight or obesity in the Chinese population should be lower than WHO standards. Therefore, we adopted the Chinese BMI cut-off values proposed by the Working Group on Obesity in China described in the Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults to define overweight or obesity [31,32]. In our study, BMI was categorized into three groups: BMI ≤ 18.5, 18.5 < BMI ≤ 24, and BMI > 24 (kg/m2). For the COR-BMI, patients with RDW > 13.25 and BMI ≤ 18.5 were defined as COR-BMI 1. Patients with RDW ≤ 13.25 and BMI ≤ 18.5 or 18.5 < BMI ≤ 24, and patients with RDW > 13.25 and 18.5 < BMI ≤ 24 or BMI > 24 were defined as COR-BMI 2. Patients with RDW ≤ 13.25 and BMI > 24 were defined as COR-BMI 3. According to ‘2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer’ [7], patients’ response to therapy can be classified into two categories: Excellent response and Indeterminate response were defined as ‘Good Prognosis Group’ (GPG). Biochemical incomplete response and Structural incomplete response were defined as ‘Poor Prognosis Group’ (PPG) (Table 1). BMI changes were compared between the first and last times they were hospitalized.
Table 1. Clinical implications of response to therapy reclassification in patients with differentiated thyroid cancer treated with total thyroidectomy and radioiodine remnant ablation.
| Binary variable | Category | Definitions | Clinical outcomes |
|---|---|---|---|
| GPG | Excellent response | Negative imaging and either suppressed Tg < 0.2 ng/ml1 or TSH-stimulated Tg < 1 ng/ml1 |
1–4% recurrence <1% disease-specific death |
| Indeterminate response | Non-specific findings on imaging studies Faint uptake in thyroid bed on RAI scanning Non-stimulated Tg detectable, but <1 ng/ml Stimulated Tg detectable, but <10 ng/ml or Anti-Tg antibodies stable or declining in the absence of structural or functional disease |
15%–20% will have structural disease identified during follow-up In the remainder, the non-specific changes are either stable, or resolve <1% disease-specific death |
|
| PPG | Biochemical incomplete response | Negative imaging And Suppressed Tg ≥ 1 ng/ml1 Or Stimulated Tg ≥ 10 ng/ml1 Or Rising anti-Tg antibody levels |
At least 30% spontaneously evolve to NED 20% achieve NED after additional therapy 20% develop structural disease <1% disease-specific death |
| Structural incomplete response | Structural or functional evidence of disease With any Tg level With or without anti-Tg antibodies |
50–85% continue to have persistent disease despite additional therapy Disease-specific death rates as high as 11% with loco-regional metastases and 50% with structural distant metastases |
In the absence of anti-Tg antibodies.
Abbreviation: NED, a patient having no evidence of disease at final follow-up.
Statistical analysis
Continuous variables with normal distribution were presented as mean with standard deviation (SD), and the t test was used to compare between-group difference. Continuous variables with skewed distribution were presented as median with interquartile range (IQR), and Mann–Whitney U test was used to compare between-group difference. Categorical variables were presented as frequencies with percentages, and Chi-square test and Fisher’s exact test was used to compare between-group difference. For the pairwise comparison between groups greater than two, we adjusted the significance level according to Bonferroni’s method to reduce the risk of type I errors. After stratifying data by COR-BMI, NLR subgroups and PLT subgroups, odds ratios (ORs) for PPG with 95% CIs were calculated by univariate binary logistic regression. Further, multiple binary logistic regression calculated ORs for PPG with 95% CIs after adding other covariates. Difference of BMI changes before and after RAI treatment between different prognosis groups was also compared by Mann–Whitney U test. The analyses were performed with Statistical Package for Social Sciences (SPSS version 22.0; SPSS Inc., Chicago, IL). Differences were considered significant at P<0.05.
Results
Baseline characters between patients in different prognosis groups
The clinical characteristics of participants were summarized in Table 2. Those 705 eligible subjects included 211 males and 494 females. Among them, 546 (77.45%) belonged to GPG, and 159 (22.55%) belonged to PPG. PLT was higher in GPG than PPG (265.36 ± 63.62 vs. 251.20 ± 63.25, P<0.05). NEUT was lower in GPG than PPG (3.85 [2.95–3.85] vs. 4.47 [3.16–5.83], P<0.05). Lower PLT subgroups had a higher prevalence of PPG (24.4% vs. 18.0%, P<0.05). The distribution of COR-BMI between two prognosis groups was different (P<0.05). We further compared the differences between each two groups using the Bonferroni’s method. The significance level was adjusted to P<0.017. We found no significant differences between each of the two groups (differences between COR-BMI 1 and 2, 1 and 3, and 2 and 3: P>0.017). There was no significant difference in other variables between two prognosis groups (P>0.05).
Table 2. Comparison of clinical characteristics of patients with different prognosis groups.
| Characteristics | Total | Good curative effect | Poor curative effect | P-value |
|---|---|---|---|---|
| Number | 705 | 546 (77.45%) | 159 (22.55%) | |
| Continuous variables with normal distribution | ||||
| Age | 45.02 ± 11.46 | 44.87 ± 10.86 | 45.53 ± 13.36 | 0.573 |
| Hg | 139.61 ± 16.04 | 139.44 ± 16.36 | 140.19 ± 14.92 | 0.609 |
| ALB | 46.20 ± 3.17 | 46.26 ± 3.27 | 45.97 ± 2.77 | 0.279 |
| PLT | 261.95 ± 63.81 | 265.36 ± 63.62 | 251.20 ± 63.25 | 0.0141 |
| PDW | 12.32 ± 1.65 | 12.20 ± 2.12 | 12.48 ± 2.06 | 0.135 |
| MPV | 10.49 ± 0.75 | 10.47 ± 0.92 | 10.58 ± 0.91 | 0.177 |
| RDW | 13.37 ± 2.73 | 13.59 ± 5.72 | 13.11 ± 1.26 | 0.293 |
| BMI | 25.44 ± 3.19 | 25.45 ± 5.04 | 25.42 ± 4.21 | 0.945 |
| Continuous variables with skewed distribution | ||||
| NEUT | 3.99 (3.00–5.23) | 3.85 (2.95–3.85) | 4.47 (3.16–5.83) | 0.0081 |
| LBC | 2.02 (1.57–2.92) | 1.95 (1.54–2.65) | 2.06 (1.51–6.15) | 0.119 |
| PCT | 0.28 (0.24–0.33) | 0.28 (0.24–0.33) | 0.29 (0.23–0.33) | 0.159 |
| NLR | 2.11 (1.56–2.31) | 2.11 (1.54–3.32) | 2.11 (1.71–2.19) | 0.448 |
| Categorical variables | ||||
| Gender | 0.781 | |||
| Male | 211 | 162 (76.8%) | 49 (23.2%) | |
| Female | 494 | 384 (77.7%) | 110 (22.3%) | |
| Variants | 0.674 | |||
| Yes | 48 | 36 (75.0%) | 12 (25.0%) | |
| No | 657 | 510 (77.6%) | 147 (22.4%) | |
| PLT subgroups | 0.0011 | |||
| 1 | 550 | 411 (74.7%) | 139 (25.3%) | |
| 2 | 155 | 135 (87.1%) | 20 (12.9%) | |
| NLR subgroups | 0.069 | |||
| 1 | 505 | 382 (75.6%) | 123 (24.4%) | |
| 2 | 200 | 164 (82.0%) | 36 (18.0%) | |
| T stage | 0.1102 | |||
| 1a | 207 | 170 (82.1%) | 37 (17.9%) | |
| 1b | 273 | 210 (76.9%) | 63 (23.1%) | |
| 2 | 51 | 42 (82.4%) | 9 (17.6%) | |
| 3 | 99 | 71 (71.7%) | 28 (28.3%) | |
| 4a | 58 | 43 (74.1%) | 15 (25.9%) | |
| 4b | 17 | 10 (58.8%) | 7 (41.2%) | |
| N stage | 0.168 | |||
| 0 | 99 | 82 (82.8%) | 17 (17.2%) | |
| 1a | 369 | 289 (78.3%) | 80 (21.7%) | |
| 1b | 237 | 175 (73.8%) | 62 (26.2%) | |
| COR-BMI | 0.0471,2 | |||
| 1 | 9 | 6 (66.7%) | 3 (33.3%) | 0.3862,3 |
| 2 | 371 | 300 (80.9%) | 71 (19.1%) | 0.7033,4 |
| 3 | 325 | 240 (73.8%) | 85 (26.2%) | 0.0275 |
3,4,5Adjusted the significance level according to Bonferroni’s method. P<0.017 is considered significant.
P<0.05.
Fisher’s exact test.
Differences between COR-BMI 1 and 2.
Differences between COR-BMI 1 and 3.
Differences between COR-BMI 2 and 3.
Risks of PGP
We used binary logistic regression to model the risks of PPG (Table 3). PLT had a detrimental effect on the risk of PPG (OR = 0.996, 95% CI = 0.993–0.999, P<0.05). T stage had a positive effect on the risk of PPG (OR = 1.239, 95% CI = 1.084–1.417, P<0.05). PLT subgroups had a detrimental effect on the risk of PPG (OR = 0.438, 95% CI = 0.264–0.728, P<0.05). Among the other variables, there was no significant difference in the risk of PPG (P>0.05).
Table 3. Risk of PGP with different variables.
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Age | 1.005 (0.990–1.021) | 0.526 |
| Hg | 1.003 (0.992-1.014) | 0.609 |
| PLT | 0.996 (0.993–0.999) | 0.0091 |
| ALB | 0.973 (0.323–0.973) | 0.323 |
| NEUT | 0.988 (0.966–1.012) | 0.331 |
| LBC | 1.008 (0.985–1.032) | 0.483 |
| NLR | 1.097 (0.951–1.267) | 0.204 |
| Gender | 1.056 (0.720–1.549) | 0.781 |
| T stage | 1.239 (1.084–1.417) | 0.0021 |
| N stage | 1.299 (0.990–1.704) | 0.060 |
| Variants | 1.156 (0.587–1.156) | 0.675 |
| NLR subgroups | 0.682 (0.451–1.031) | 0.070 |
| PLT subgroups | 0.438 (0.264–0.728) | 0.0011 |
| COR-BMI | 1.380 (0.981–1.941) | 0.065 |
P<0.05.
Furthermore, we calculated risks for PPG with multiple binary logistic regressions (Table 4). Different models were stratified by COR-BMI, NLR subgroups, and PLT subgroups. Table 4 shows the confounding factors for each model. We found that COR-BMI 2 subgroups had a detrimental effect on the risk of PPG when COR-BMI 3 subgroups were used as reference (P<0.05). This effect still existed when we included additional covariates (P<0.05). Higher PLT subgroups showed a detrimental effect on the risk of PPG compared with the lower subgroup (P<0.05), when confounding factors were added or no confounding factors were added. While NLR subgroups showed no significant differences on the risk of PPG in both models (P>0.05).
Table 4. Risk of PGP.
| Variables | Crude OR | Adjusted OR | ||
|---|---|---|---|---|
| COR-BMI2 | OR (95% CI) | P-value | OR (95% CI) | P-value |
| 1 | 1.412 (0.345–5.770) | 0.631 | 1.326 (0.319–5.517) | 0.698 |
| 2 | 0.668 (0.467–0.956) | 0.0271 | 0.632 (0.437–0.915) | 0.0151 |
| 3 | Reference | Reference | ||
| NLR subgroups3 | ||||
| NLR ≤ 2.23 | Reference | Reference | ||
| NLR> 2.23 | 0.682 (0.451–1.031) | 0.070 | 0.698 (0.455–1.070) | 0.099 |
| PLT subgroups4 | (×109/l) | |||
| PLT ≤ 302 | Reference | Reference | ||
| PLT > 302 | 0.438 (0.264–0.728) | 0.0011 | 0.426 (0.254–0.714) | 0.0011 |
Crude ORs were calculated by univariate binary logistic regressions; adjusted ORs were calculated by multiple binary logistic regressions.
P<0.05.
Confounding factors in the multiple binary logistic regression included PLT, T stage, N stage, and NLR subgroups.
Confounding factors in the multiple binary logistic regression included COR-BMI, PLT, T stage, and N stage.
Confounding factors in the multiple binary logistic regression included COR-BMI, T stage, N stage, and NLR subgroups.
BMI changes before and after treatment between patients in different prognosis group
Overall, BMI changes in all eligible subjects ranged from −8.8 to 6.25, with mean and SD of −0.50 ± 2.32. Difference between two BMI changes curative effect groups was not significant (−0.55 ± 2.17 vs. −0.48 ± 2.39, P>0.05). Furthermore, we used binary logistic regression to calculate the risk of PPG. The result was not significant (OR = 1.014, 95% CI = 0.802–1.282, P>0.05).
Discussion
A lot of research on the predictive effect of indicators on the prognosis of malignant tumors has been done in other tumors. For example, the prognostic nutritional index have been reported to predict prognosis in NPC [33] and hepatocellular carcinoma [17]; NLR have been reported in NPC [34] and colorectal cancers [35]; mast cells have been reported in prostate cancer [36]; serum C-reactive protein, platelet–lymphocyte ratio, and lymphocyte–monocyte ratio were important predictors of prognosis of NPC [33,34]. High RDW presents a prognostic value of lower survival in oncological patients with gastric, lung, renal, and hematological neoplasia [37–39]. However, prognostic indicators of PTC are understudied. To the best of our knowledge, this is the first study to investigate the predictive role of COR-BMI and PLT in PTC patients. It is also the first study to evaluate PLT as an independent prognostic indicator for PTC patients.
As we all know, PTC has a high morbidity but most patients have a good prognosis [1,6]. GPG has a good prognosis and a high propensity to reach long-term remission [7]. In our study, GPG accounted for 77.45% and PPG accounted for 22.55%, similar to other studies [2,7]. We found PLT, NEUT, and COR-BMI were different in two prognosis groups (P<0.05). However, we adjusted the significance levels of COR-BMI according to the Bonferroni’s method and compared the two groups. We did not find significant differences between the two groups (P>0.017). Even though we found COR-BMI 2 subgroups had a detrimental effect on the risk of PPG when COR-BMI 3 was used as reference (P<0.05). This may be an error that is not corrected by statistics. Therefore, we cannot conclude that there is a correlation between COR-BMI and PTC prognosis based on the above results.
COR-BMI is a new indicator of the combination of inflammation and nutritional status. The predictive role of this indicator on the prognosis of malignant tumors has only been proposed recently [18,19]. In our study, we cannot get a definitive conclusion whether there is a correlation between COR-BMI and PTC prognosis. This may be related to the disease state of PTC different from other malignant tumors. PTC is a relatively indolent malignancy [7,13]. No previous studies have reported the relationship between RDW and prognosis of PTC. There are not many researches on the inflammation status of PTC. Kari et al. proposed that autoimmune disease, such as Hashimoto’s thyroiditis, may be related to PTC oncogenesis [23]. There is also literature suggesting the role of macrophages and mast cell in the prognosis of PTC tumors [24]. In addition, we found no significant difference in BMI changes between the two prognosis groups during follow-up. This is also consistent with our previous understanding that PTC, unlike other malignant tumors, usually does not experience weight loss (cachexia). In contrast, many studies suggested that obesity is a risk factor for PTC [40,41]. Our study provided new evidence for the insignificant changes in BMI in PTC patients. Previous studies have shown that NLR is associated with poor prognosis of PTC [22]. However, we did not find a significant association between them. There are previous studies that are consistent with our views [42,43]. Increased NLR is associated with inflammatory disease, whereas in non-inflammatory diseases, NLR is within normal range [43]. NLR in our study is within the low and narrow range (median 2.11, IQR 1.56–2.31). This may be the cause of NLR negative results. Further prospective studies will be needed to be performed to validate these conflicting results.
Unexpectedly, we found a significant effect of lower PLT on poor prognosis. Although PLT displayed a detrimental effect on the risk of PPG (OR = 0.996, 95% CI = 0.993–0.999, P<0.05). We cannot say that this statistically significant result is clinically significant because both OR and 95% CI are too close to 1. We further divided the PLT values into two groups, of which 302 × 109/l was the cut-off value. After that, PLT subgroups showed a detrimental effect on the risk of PPG (OR = 0.438, 95% CI = 0.264–0.728, P<0.05). This statistical difference still existed after we added other covariates that may affect prognosis (P<0.05). Therefore, we can conclude that the lower PLT group of patients with PTC has a worse prognosis than the higher PLT group. This result is different from the mainstream view of other solid tumors [20,21], including lung cancer, renal cell carcinoma, gallbladder cancer, rectal cancer, gastric cancer, esophageal squamous cell carcinoma, and ATC.
There are several possible explanations for the pro-tumor effect of PLT in malignancies [20,44]. First, tumor cells can directly induce the activation and aggregation of PLT, promote tumor cell–platelet thrombosis, thereby preventing the immune system from detecting and preventing natural killer cells from clearing intravascular tumor cells, thereby promoting metastasis. This seems to be the main mechanism by which platelets protect tumor cells. Second, thrombin-activated platelets release a variety of growth factors to promote angiogenesis and tumor cell proliferation [20,44]. However, as we discussed earlier, PTC is less invasive and different from other malignant tumors [7,13]. We found the opposite relationship in PTC. We believe this may be related to the association of PLT with radiation sensitivity. Most PTC patients receive ablation of tumor remnants by RAI therapy after thyroidectomy. RAI is not only a key means to remove residual thyroid and metastatic foci, but also an important means to reduce tumor recurrence in patients [5,7]. Therefore, the radiation sensitivity of tumor cells to RAI is important for prognosis. In the course of treatment, some PTC tumor cells will dedifferentiate and become resistant to RAI, and finally become radioiodine-refractory thyroid cancer [2,45]. Resistance to RAI therapy is a central feature of disease recurrence and poor outcomes in thyroid cancer. Studies have shown that platelet-derived growth factor (PDGF), which is mainly synthesized, stored, and released through platelets [46], has a special role in radiation sensitivity of tumors [47,48]. However, the role of PDGF in radiation sensitivity of tumor cells is controversial. On one hand, Studies have shown that increasing the PDGF-receptor can increase the radiation sensitivity of NPC [48]. On the other hand, inhibition of PDGF suppresses tumor growth and enhances tumor radiation response in fibrosarcoma and prostate cancer [47]. Furthermore, McMullen et al. demonstrated that PDGFR-α can induce thyroid follicular cell dedifferentiation [52].Most studies support the view that PDGF promotes resistance to radiation therapy. However, our findings may suggest the possibility of another potential mechanism. Further and more complete research on the relationship between PLT/PDGF and radiation sensitivity is urgently needed.
We noticed that gender did not affect the patient’s prognosis in our results, which is different from previous works’ mainstream viewpoints [49,50]. We thought that the reasons for this difference may be as follows: (1) The deviation of patient selection caused by insufficient sample size. (2) Different hospitals have different references for choosing the dose of RAI. Our department may intentionally impose a higher dose on male patients when giving RAI treatment. In our study, the dose of RAI received by patients was not included in the statistical analysis. We also found that there are also some studies showed that gender is not an independent prognostic factor for survival in PTC [9]. To validate this controversy, we need further research.
Although we have gained some new discoveries, our research still has some limitations. First limitation is retrospective nature and single-center of our study. Second, our study only divided the patient’s prognosis into two groups according to the treatment response, and did not include the patient’s survival time in the study. Further studies with patients’ survival time will access more information about the prognosis. However, PTC patients typically have longer survival times. Survival analysis is difficult to carry out and standardized follow-up will be a big problem. Third, some covariates that influence prognosis should also be included in the study, such as family history, surgical method/scope of surgery [51], the time of the first RAI after surgery, RAI dose, gene mutations etc. Obviously, information on the quality and extent of neck dissection is notably not available. The molecular markers such as BRAF point mutation and TERT promoter point mutation should also be included. Fourth, dynamics of post-treatment changes in these biomarkers should be considered. The prognosis of PTC patients is closely related to the treatment response. These clinical indicators are constantly changing during the course of treatment. We need to further understand the changes of these prognostic indicators. In view of the above limitations, larger multicenter and preferably prospective studies are warranted to validate our findings in the future.
In conclusion, we studied the predictive role of some indicators in the prognosis of patients with PTC. We did not find that COR-BMI and NLR are significant predictors of PTC prognosis. No significant difference in BMI changes was found between the two prognosis groups during follow-up. We found that lower PLT levels may indicate a poor prognosis for PTC. The relationship between PDGF and radiation sensitivity may be the key to this association. Further prospective studies will need to be performed to validate these preliminary results.
Abbreviations
- ATA
American Thyroid Association
- ATC
anaplastic thyroid cancer
- BMI
body mass index
- BRAF
proto-oncogene B-Raf
- CI
confidence interval
- COR-BMI
combination of RDW and BMI
- GPG
Good Prognosis Group
- IQR
interquartile range
- LBC
lymphocyte
- NEUT
neutrophil
- NLR
neutrophil–lymphocyte ratio
- NPC
nasopharyngeal carcinoma
- OR
odds ratio
- PDGF
platelet-derived growth factor
- PLT
platelet
- PPG
Poor Prognosis Group
- PTC
papillary thyroid cancer
- RAI
radioactive iodine
- RDW
red blood cell distribution width
- SD
standard deviation
- SPSS
Statistical Package for Social Sciences
- TERT
telomerase reverse transcriptase
- Tg
thyroglobulin
- TSH
thyroid stimulating hormone
Contributor Information
Cen Lou, Email: 3194110@zju.edu.cn.
Zhaowei Meng, Email: zmeng@tmu.edu.cn.
Data Availability
We will provide the original data for non-commercial needs of the journal without breaching participant confidentiality.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Key Clinical Specialty Project (awarded to the Departments of Nuclear Medicine and Radiology); the Tianjin Medical University General Hospital New Century Excellent Talent Program; the Young and Middle-aged Innovative Talent Training Program from Tianjin Education Committee; the Talent Fostering Program (the 131 Project) from Tianjin Education Committee, Tianjin Human Resources and Social Security Bureau (to Zhaowei Meng)]; the China National Natural Science Foundation [grant number 81571709 (to Zhaowei Meng)]; the Key Project of Tianjin Science and Technology Committee Foundation [grant number 16JCZDJC34300 (to Zhaowei Meng)]; and the Tianjin Science and Technology Committee Foundation [grant number 17JCYBJC25400 (to Yaguang Fan)].
Author Contribution
Xiangxiang Liu: Methodology, Software, Formal analysis, Data Curation, Writing - Original Draft, Visualization. Zhongke Huang: Conceptualization, Writing - Original Draft, Writing - Review & Editing. Xianghui He: Conceptualization. Xiangqian Zheng: Conceptualization. Qiang Jia: Conceptualization, Supervision. Jian Tan: Conceptualization, Supervision. Yaguang Fan: Methodology, Software, Funding acquisition. Cen Lou: Conceptualization, Validation, Writing - Review & Editing. Zhaowei Meng: Conceptualization, Validation, Writing - Review & Editing, Project administration, Funding acquisition.
Ethics Approval
This article does not involve any human or animal health-related interventions to evaluate health effects (such as drugs, surgical procedures, equipment, behavioral therapy, dietary interventions, and changes in nursing procedures etc.). This is a retrospective cross-sectional article. The institutional review board and ethics committee of Tianjin Medical University General Hospital approved the ethical, methodologic, and protocol aspects of this investigation. We confirm that all methods in the current study were carried out in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
References
- 1.Vaccarella S., Franceschi S., Bray F., Wild C.P., Plummer M. and Dal Maso L. (2016) Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 375, 614–617 10.1056/NEJMp1604412 [DOI] [PubMed] [Google Scholar]
- 2.Tesselaar M.H., Smit J.W., Nagarajah J., Netea-Maier R.T. and Plantinga T.S. (2017) Pathological processes and therapeutic advances in radioiodide refractory thyroid cancer. J. Mol. Endocrinol. 59, R141–R154 10.1530/JME-17-0134 [DOI] [PubMed] [Google Scholar]
- 3.Du L., Wang Y., Sun X., Li H., Geng X., Ge M. et al. (2018) Thyroid cancer: trends in incidence, mortality and clinical-pathological patterns in Zhejiang Province, Southeast China. BMC Cancer 18, 291 10.1186/s12885-018-4081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim H., Devesa S.S., Sosa J.A., Check D. and Kitahara C.M. (2017) Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 317, 1338–1348 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabanillas M.E., McFadden D.G. and Durante C. (2016) Thyroid cancer. Lancet 388, 2783–2795 10.1016/S0140-6736(16)30172-6 [DOI] [PubMed] [Google Scholar]
- 6.Vaccarella S., Dal Maso L., Laversanne M., Bray F., Plummer M. and Franceschi S. (2015) The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid 25, 1127–1136 10.1089/thy.2015.0116 [DOI] [PubMed] [Google Scholar]
- 7.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E. et al. (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D. and Weinberg R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 9.Liu C., Chen T., Zeng W., Wang S., Xiong Y., Liu Z. et al. (2017) Reevaluating the prognostic significance of male gender for papillary thyroid carcinoma and microcarcinoma: a SEER database analysis. Sci. Rep. 7, 11412 10.1038/s41598-017-11788-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censi S., Barollo S., Grespan E., Watutantrige-Fernando S., Manso J., Iacobone M. et al. (2019) Prognostic significance of TERT promoter and BRAF mutations in TIR-4 and TIR-5 thyroid cytology. Eur. J. Endocrinol. 181, 1–11 10.1530/EJE-19-0073 [DOI] [PubMed] [Google Scholar]
- 11.Choi Y.M., Kim W.G., Kwon H., Jeon M.J., Lee J.J., Ryu J.S. et al. (2016) Early prognostic factors at the time of diagnosis of bone metastasis in patients with bone metastases of differentiated thyroid carcinoma. Eur. J. Endocrinol. 175, 165–172 10.1530/EJE-16-0237 [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y., Zhao Q., Liu C., Wang S., Liu Z. and Huang T. (2018) Prognosis of patients with TX stage differentiated thyroid cancer: propensity scored matching analysis of the SEER database 2004-2013. Am. J. Transl. Res. 10, 2004–2014 [PMC free article] [PubMed] [Google Scholar]
- 13.Perrier N.D., Brierley J.D. and Tuttle R.M. (2018) Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 68, 55–63 10.3322/caac.21439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melo M., Gaspar da Rocha A., Batista R., Vinagre J., Martins M.J., Costa G. et al. (2017) TERT, BRAF, and NRAS in primary thyroid cancer and metastatic disease. J. Clin. Endocrinol. Metab. 102, 1898–1907 10.1210/jc.2016-2785 [DOI] [PubMed] [Google Scholar]
- 15.Vuong H.G., Altibi A.M.A., Duong U.N.P. and Hassell L. (2017) Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. 87, 411–417 10.1111/cen.13413 [DOI] [PubMed] [Google Scholar]
- 16.Oh S.Y., Kim Y.B. and Suh K.W. (2017) Prognostic significance of systemic inflammatory response in stage II colorectal cancer. J. Surg. Res. 208, 158–165 10.1016/j.jss.2016.08.100 [DOI] [PubMed] [Google Scholar]
- 17.Pinato D.J., North B.V. and Sharma R. (2012) A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br. J. Cancer 106, 1439–1445 10.1038/bjc.2012.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y., Mao Y., Chen S., Yang A. and Zhang Q. (2016) A novel inflammation- and nutrition-based prognostic system for patients with laryngeal squamous cell carcinoma: Combination of Red Blood Cell Distribution Width and Body Mass Index (COR-BMI). PLoS ONE 11, e0163282 10.1371/journal.pone.0163282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., He S.S., Cai X.Y., Chen H.Y., Yang X.L., Lu L.X. et al. (2018) The novel prognostic score Combining Red Blood Cell Distribution Width and Body Mass Index (COR-BMI) has prognostic impact for survival outcomes in nasopharyngeal carcinoma. J. Cancer 9, 2295–2301 10.7150/jca.24838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou C., Jiang F., Ma H., Zhu Q., Wang Z., Zhao B. et al. (2019) Prognostic role of preoperative platelet, fibrinogen, and D-dimer levels in patients with non-small cell lung cancer: a multicenter prospective study. Thorac. Cancer 10, 304–311 10.1111/1759-7714.12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R.T., Zhang L.Q., Mu Y.P., Li J.B., Xu X.S., Pang Q. et al. (2015) Prognostic significance of preoperative platelet count in patients with gallbladder cancer. World J. Gastroenterol. 21, 5303–5310 10.3748/wjg.v21.i17.5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong W., Yang S., Yang X. and Guo F. (2016) Blood preoperative neutrophil-to-lymphocyte ratio is correlated with TNM stage in patients with papillary thyroid cancer. Clinics (Sao Paulo) 71, 311–314 10.6061/clinics/2016(06)04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kari S., Flynn J.C., Zulfiqar M., Snower D.P., Elliott B.E. and Kong Y.C. (2013) Enhanced autoimmunity associated with induction of tumor immunity in thyroiditis-susceptible mice. Thyroid 23, 1590–1599 10.1089/thy.2013.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liotti F., Visciano C. and Melillo R.M. (2012) Inflammation in thyroid oncogenesis. Am. J. Cancer Res. 2, 286–297 [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., Liang C., Zhao L., Meng Z., Zhang C., Jia Q. et al. (2018) Influence of radioactive iodine therapy on liver function in patients with differentiated thyroid cancer. Nucl. Med. Commun. 39, 1113–1120 10.1097/MNM.0000000000000919 [DOI] [PubMed] [Google Scholar]
- 26.Li N., Zhang C., Meng Z., Xu K., He X., Yu Y. et al. (2018) Changes of serum midkine as a dynamic prognostic factor to monitor disease status in papillary thyroid cancer. Medicine (Baltimore) 97, e12242 10.1097/MD.0000000000012242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upadhyaya A., Zhou P., Meng Z., Wang P., Zhang G., Jia Q. et al. (2017) Radioprotective effect of vitamin E on salivary glands after radioiodine therapy for differentiated thyroid cancer: a randomized-controlled trial. Nucl. Med. Commun. 38, 891–903 10.1097/MNM.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 28.Jia Q., Meng Z., Xu K., He X., Tan J., Zhang G. et al. (2017) Serum midkine as a surrogate biomarker for metastatic prediction in differentiated thyroid cancer patients with positive thyroglobulin antibody. Sci. Rep. 7, 43516 10.1038/srep43516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu N., Meng Z., Jia Q., Tan J., Zhang G., Zheng W. et al. (2016) Multiple-factor analysis of the first radioactive iodine therapy in post-operative patients with differentiated thyroid cancer for achieving a disease-free status. Sci. Rep. 6, 34915 10.1038/srep34915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng Z., Tan J., Zhang G., Tian W., Fu Q., Li W. et al. (2015) Evaluation of serum midkine as a biomarker in differentiated thyroid cancer. Life Sci. 130, 18–24 10.1016/j.lfs.2015.02.028 [DOI] [PubMed] [Google Scholar]
- 31.Chen C.M. (2008) Overview of obesity in Mainland China. Obesity Rev. 9, 14–21 10.1111/j.1467-789X.2007.00433.x [DOI] [PubMed] [Google Scholar]
- 32.Liang Y.J., Xi B., Song A.Q., Liu J.X. and Mi J. (2012) Trends in general and abdominal obesity among Chinese children and adolescents 1993-2009. Pediatr. Obes. 7, 355–364 10.1111/j.2047-6310.2012.00066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L., Xia L., Wang Y., Hong S., Chen H., Liang S. et al. (2016) Low prognostic nutritional index (PNI) predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. PLoS ONE 11, e0158853 10.1371/journal.pone.0158853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X.H., Chang H., Xu B.Q., Tao Y.L., Gao J., Chen C. et al. (2017) An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 6, 310–319 10.1002/cam4.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang J., Lin G., Ye M., Tong D., Zhao J., Zhu D. et al. (2019) Decreased mean platelet volume predicts poor prognosis in metastatic colorectal cancer patients treated with first-line chemotherapy: results from mCRC biomarker study. BMC Cancer 19, 15 10.1186/s12885-018-5252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khazaie K., Blatner N.R., Khan M.W., Gounari F., Gounaris E., Dennis K. et al. (2011) The significant role of mast cells in cancer. Cancer Metastasis Rev. 30, 45–60 10.1007/s10555-011-9286-z [DOI] [PubMed] [Google Scholar]
- 37.Życzkowski M., Rajwa P., Gabrys E., Jakubowska K., Jantos E. and Paradysz A. (2018) The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin. Genitourin. Cancer 16, e677–e683 10.1016/j.clgc.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 38.Ai L., Mu S. and Hu Y. (2018) Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int. 18, 61 10.1186/s12935-018-0558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S., Han F., Wang Y., Xu Y., Qu T., Ju Y. et al. (2017) The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol. 17, 163 10.1186/s12876-017-0685-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitahara C.M., McCullough M.L., Franceschi S., Rinaldi S., Wolk A., Neta G. et al. (2016) Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid 26, 306–318 10.1089/thy.2015.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid D., Ricci C., Behrens G. and Leitzmann M.F. (2015) Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes. Rev. 16, 1042–1054 10.1111/obr.12321 [DOI] [PubMed] [Google Scholar]
- 42.Yaylaci S., Tosun O., Sahin O., Genc A.B., Aydin E., Demiral G. et al. (2016) Lack of variation in inflammatory hematological parameters between benign nodular goiter and papillary thyroid cancer. Asian Pac. J. Cancer Prev. 17, 2321–2323 10.7314/APJCP.2016.17.4.2321 [DOI] [PubMed] [Google Scholar]
- 43.Lang B.H., Ng C.P., Au K.B., Wong K.P., Wong K.K. and Wan K.Y. (2014) Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma. World J. Surg. 38, 2605–2612 10.1007/s00268-014-2630-z [DOI] [PubMed] [Google Scholar]
- 44.Peterson J.E., Zurakowski D., Italiano J.E., Michel L.V., Connors S., Oenick M. et al. (2012) VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 15, 265–273 10.1007/s10456-012-9259-z [DOI] [PubMed] [Google Scholar]
- 45.Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R. et al. (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- 46.Östman A. (2017) PDGF receptors in tumor stroma: biological effects and associations with prognosis and response to treatment. Adv. Drug Deliv. Rev. 121, 117–123 10.1016/j.addr.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 47.Christensen M., Najy A.J., Snyder M., Movilla L.S. and Kim H.R. (2014) A critical role of the PTEN/PDGF signaling network for the regulation of radiosensitivity in adenocarcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 88, 151–158 10.1016/j.ijrobp.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mu X., Ma J., Zhang Z., Zhou H., Xu S., Qin Y. et al. (2015) Famitinib enhances nasopharyngeal cancer cell radiosensitivity by attenuating radiation-induced phosphorylation of platelet-derived growth factor receptor and c-kit and inhibiting microvessel formation. Int. J. Radiat. Biol. 91, 771–776 10.3109/09553002.2015.1062574 [DOI] [PubMed] [Google Scholar]
- 49.Guo K. and Wang Z. (2014) Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int. J. Clin. Exp. Pathol. 7, 5393–5403 [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D., Tang J., Kong D., Cui Q., Wang K., Gong Y. et al. (2018) Impact of gender and age on the prognosis of differentiated thyroid carcinoma: a retrospective analysis based on SEER. Hormones Cancer 9, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Guan Q. and Xiang J. (2019) Nomogram for predicting level V lymph node metastases in papillary thyroid carcinoma with clinically lateral lymph node metastases: a large retrospective cohort study of 1037 patients from FDUSCC. J. Cancer 10, 772–778 10.7150/jca.28527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Campistrous A., Adewuyi E.E., Benesch M.G.K., Ko Y.M., Lai R., Thiesen A., Dewald J., Wang P., Chu K., Ghosh S. et al. (2016) PDGFRα Regulates Follicular Cell Differentiation Driving Treatment Resistance and Disease Recurrence in Papillary Thyroid Cancer. EBioMedicine 12, 86–97 10.1016/j.ebiom.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will provide the original data for non-commercial needs of the journal without breaching participant confidentiality.